Ni-Co Electrodeposition Improvement Using Phenylsalicylimine Derivatives as Additives in Ethaline-Based Deep Eutectic Solvents (DES)
Abstract
1. Introduction
2. Materials and Methods
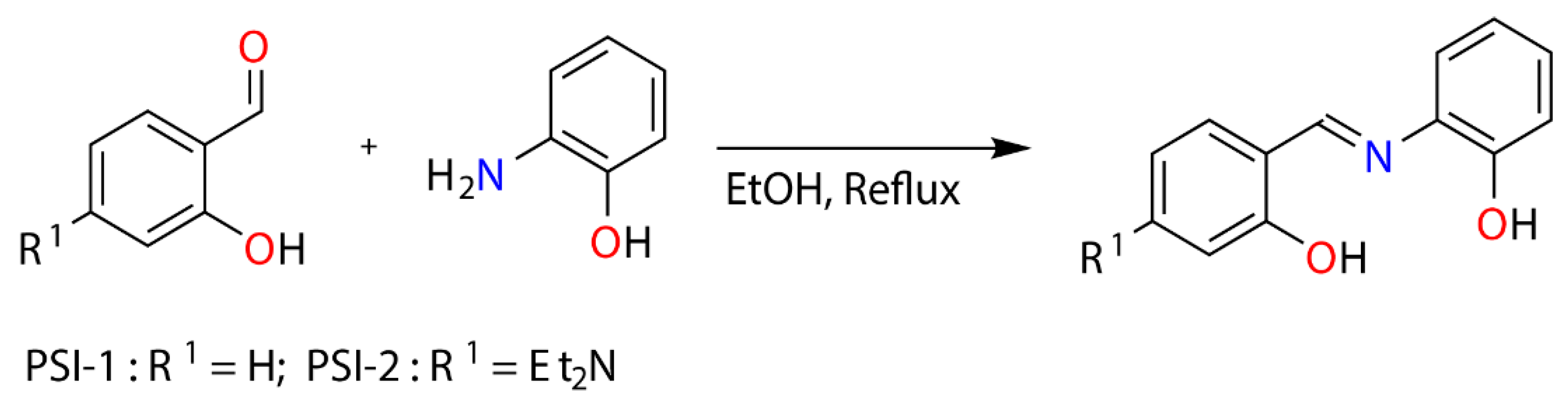
2.1. Synthesis of Phenylsalicylimines
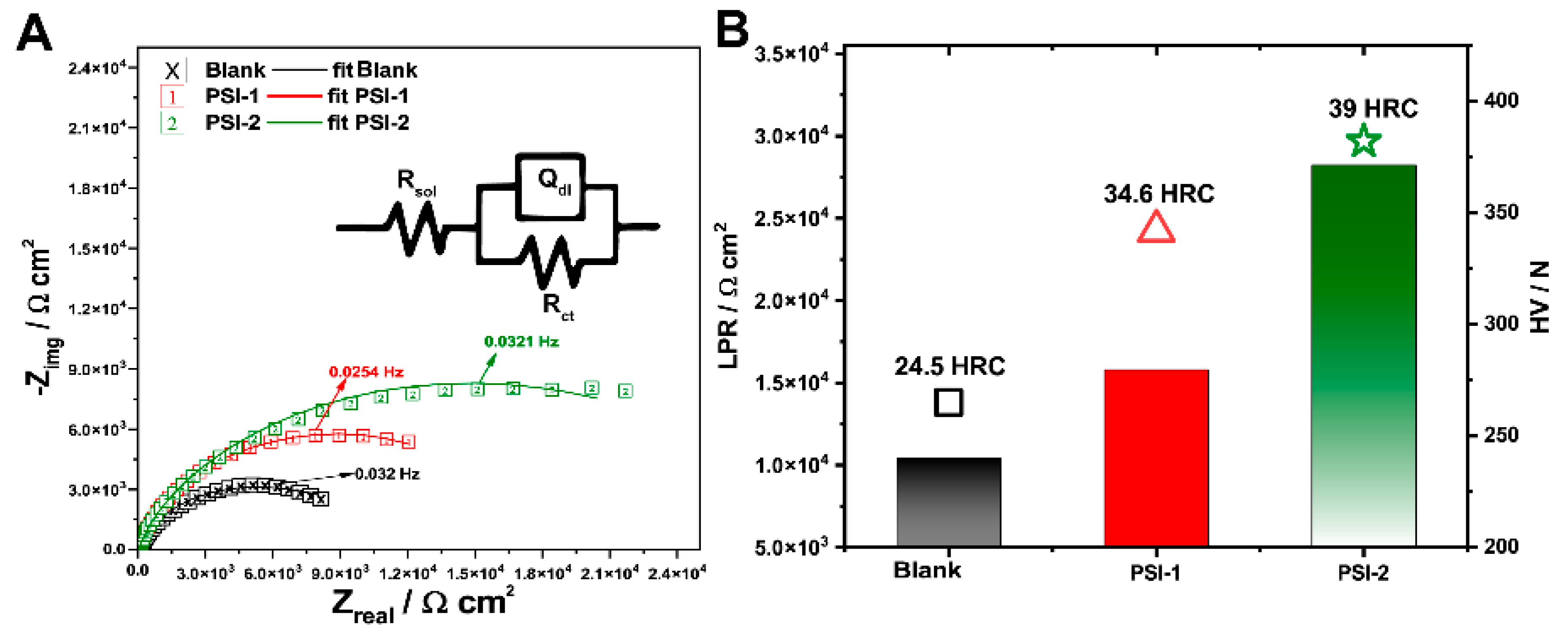
2.2. Electrochemical Evaluation
2.3. Optical Evaluation
2.4. Cell Parameters
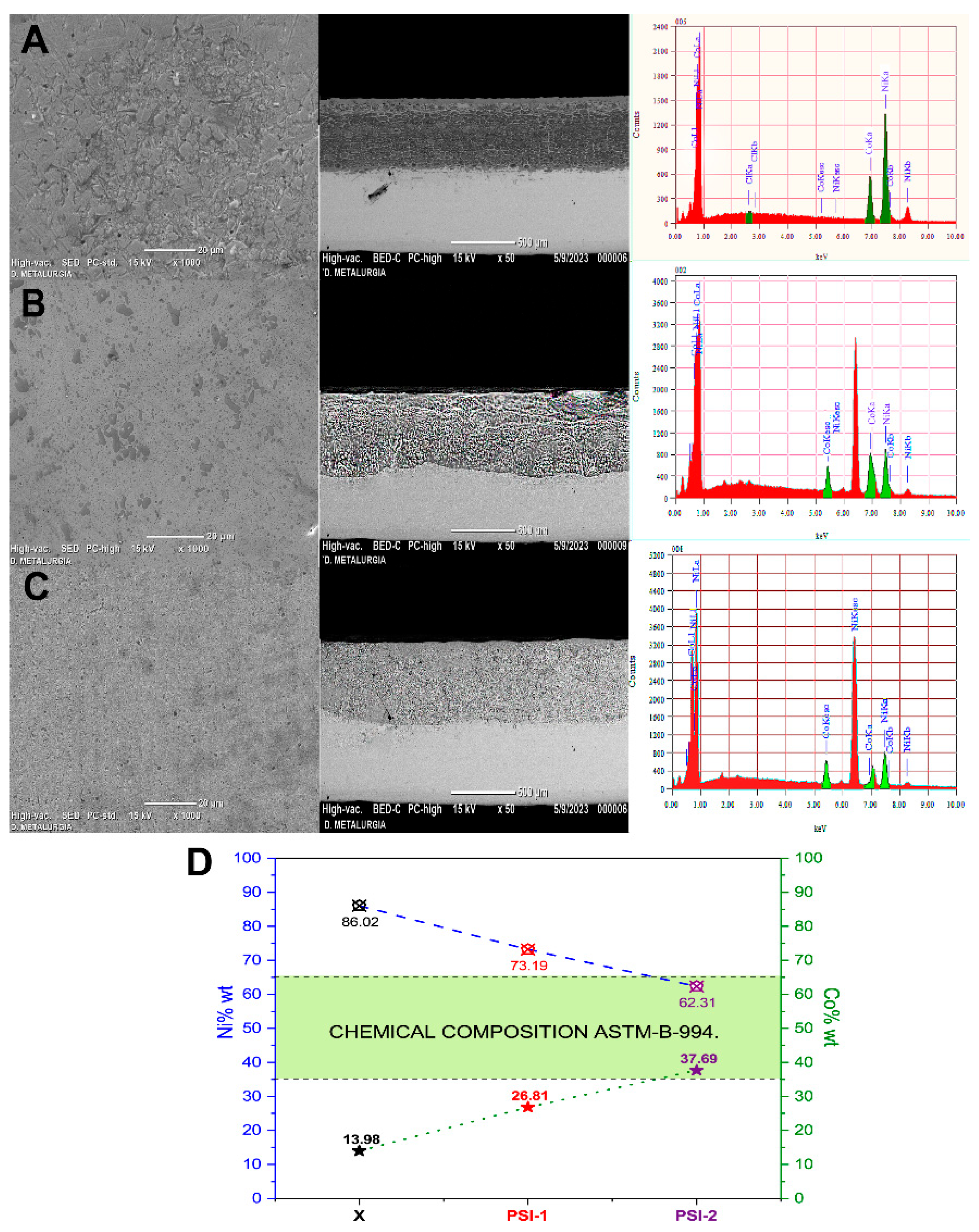
2.5. Metallic Coating Assessment
2.6. Computational Evaluation
3. Results and Analysis
3.1. Synthesis and Characterization of Ligands
3.2. Cyclic Voltammetry Study
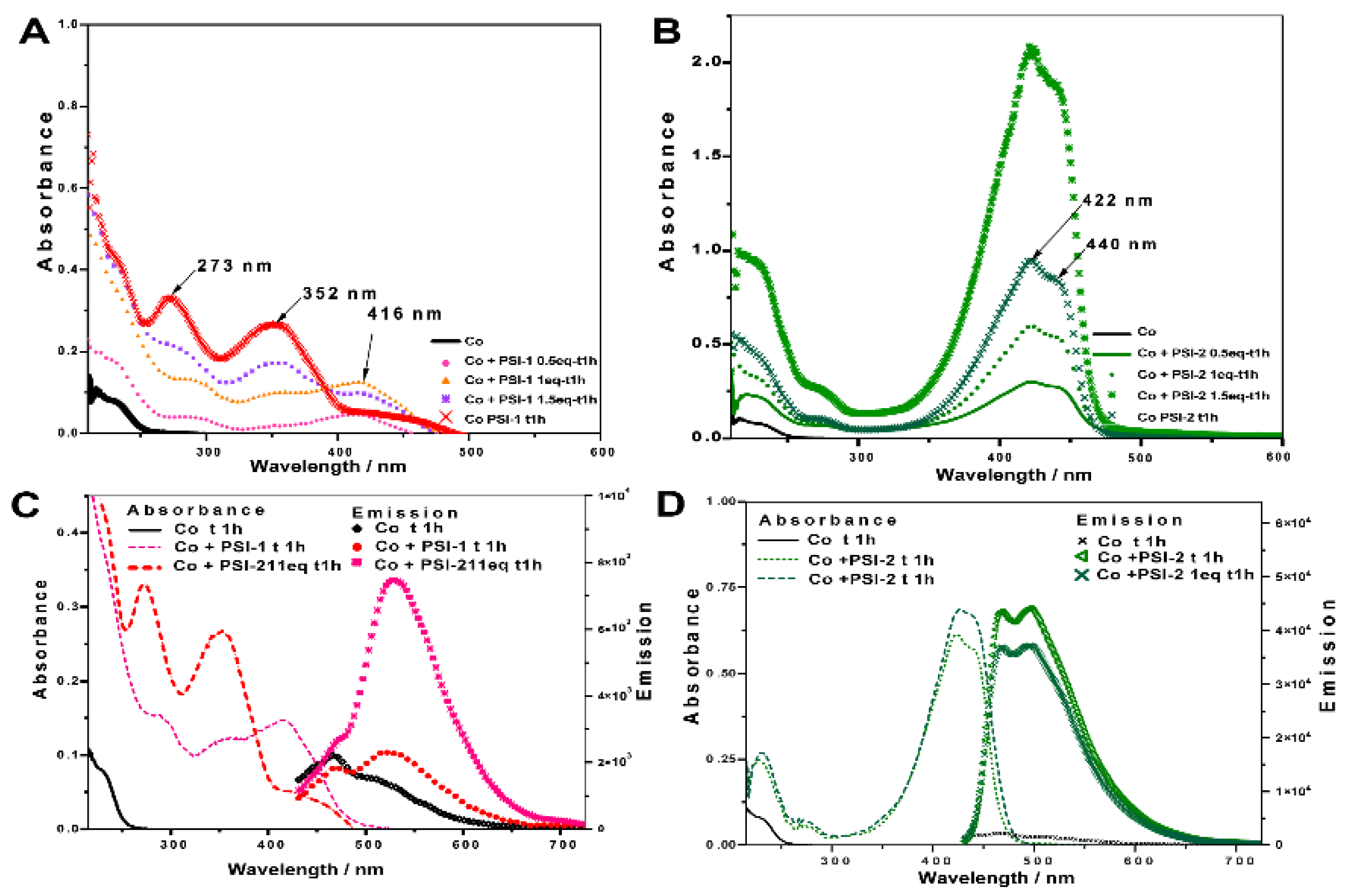
3.3. UV–Vis
3.4. Cell Parameters
3.5. Ni-Co Coating Assessments
3.6. Theoretical-Computational Analysis
3.7. Mechanism of PSI Additives of Ni-Co Deposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akande, I.G.; Oluwole, O.O.; Fayomi, O.S.I.; Odunlami, O.A. Overview of mechanical, microstructural, oxidation properties and high-temperature applications of superalloys. Mater Today-Proc. 2021, 43, 2222–2231. [Google Scholar] [CrossRef]
- Dawood, N.M.; Salim, A.M. A review on characterization, classifications, and applications of super alloys. J. Univ. Babylon Eng. Sci. 2021, 29, 53–62. [Google Scholar]
- Cormier, J. Ni-and Co-based superalloys and their coatings. Metals 2018, 8, 1055. [Google Scholar] [CrossRef]
- Dahms, H.; Croll, I. The anomalous codeposition of iron-nickel alloys. J Electrochem. Soc. 1965, 112, 771–775. [Google Scholar] [CrossRef]
- Ganji, D.K.; Rajyalakshmi, G. Influence of Alloying Compositions on the Properties of Nickel-Based Superalloys: A Review; Springer: Singapore, 2020; pp. 537–555. [Google Scholar] [CrossRef]
- Gloria, A.; Montanari, R.; Richetta, M.; Varone, A. Alloys for Aeronautic Applications: State of the Art and Perspectives. Metals 2019, 9, 662. [Google Scholar] [CrossRef]
- You, Y.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Electrodeposition of Ni-Co alloys from a deep eutectic solvent. Surf. Coat. Technol. 2012, 206, 3632–3638. [Google Scholar] [CrossRef]
- Zamani, M.; Amadeh, A.; Baghal, S.M.L. Effect of Co content on electrodeposition mechanism and mechanical properties of electrodeposited Ni-Co alloy. Trans. Nonferr. Metal Soc. 2016, 26, 484–491. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Kityk, A.A.; Shaiderov, D.A.; Danilov, F.I. Effect of water content on physicochemical properties and electrochemical behavior of ionic liquids containing choline chloride, ethylene glycol and hydrated nickel chloride. J. Mol. Liq. 2015, 212, 716–722. [Google Scholar] [CrossRef]
- Li, X.H.; Deng, R.R.; Li, Y.; Xu, C.Y.; Hua, Y.X.; Zhang, Q.X. Application of additives for the electrodeposition of metals and alloys from ionic liquids. Chin. J. Eng. 2024, 46, 657–675. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, J.; Wang, W.; Ming, Z.; Wu, M.; Qin, S.; Wang, P.; Mitsuzaki, N.; Chen, Z. Effect of ANSA as an additive on electrodeposited Sn-Ag-Cu alloy coatings in deep eutectic solvent. J. Mol. Liq. 2024, 414, 126161. [Google Scholar] [CrossRef]
- Lobato de Faria, M. Impact of Coordination on Ion Conduction and Electroplating. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2023. [Google Scholar]
- Hu, Y.; Wang, C. Electrodeposition of nickel–cobalt alloys from metal chloride-l-serine deep eutectic solvent for the hydrogen evolution reaction. J. Mater. Chem. A 2024, 12, 16769–16779. [Google Scholar] [CrossRef]
- Alesary, H.F.; Cihangir, S.; Ballantyne, A.D.; Harris, R.C.; Weston, D.P.; Abbott, A.P.; Ryder, K.S. Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim. Acta 2019, 304, 118–130. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S. Bright metal coatings from sustainable electrolytes: The effect of molecular additives on electrodeposition of nickel from a deep eutectic solvent. J. Phys. Chem. Chem. Phys. 2017, 19, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Huheey, J.E.; Keiter, E.A.; Keiter, R.L.; Medhi, O.K. Inorganic Chemistry: Principles of Structure and Reactivity; Pearson Education India: Chennai, India, 2006. [Google Scholar]
- Pototschnig, G.; Maulide, N.; Schnurch, M. Direct Functionalization of C-H Bonds by Iron, Nickel, and Cobalt Catalysis. Chemistry 2017, 23, 9206–9232. [Google Scholar] [CrossRef]
- Sebastian J.K., J.; Muraleedharan Nair, M.K.; Sasi, S. Cobalt(Ii) and Nickel(Ii) Complexes of a Schiff Base: Synthesis, Characterisation and Antimicrobial Investigations. Rasayan J. Chem. 2022, 15, 1202–1211. [Google Scholar] [CrossRef]
- Škugor Rončević, I.; Vladislavić, N.; Chatterjee, N.; Sokol, V.; Oliver, C.L.; Kukovec, B.-M. Structural and Electrochemical Studies of Cobalt(II) and Nickel(II) Coordination Polymers with 6-Oxonicotinate and 4,4′-Bipyridine. Chemosensors 2021, 9, 352. [Google Scholar] [CrossRef]
- Tas, E.; Kara, H.; Durgun, M.; Kilic, A.; Yilmaz, I. Synthesis, Spectral Characterization and Electrochemical Investigations of Mononuclear Cu (II), Ni (II) and Co (II) Metal Complexes Containing Different New vic-dioxime Groups. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2009, 39, 379–387. [Google Scholar] [CrossRef]
- Rachid, T.; Abdelkrim, R.; Sghir, E.K. ChemInform Abstract: Synthesis and Applications. 10 Years of Experience in Monodentate, Bidentate, Tridentate, and Macrocycle Pyrazole Heterocyclic Chemistry. ChemInform 2010, 41, 906–912. [Google Scholar] [CrossRef]
- Malinowski, J.; Zych, D.; Jacewicz, D.; Gawdzik, B.; Drzezdzon, J. Application of Coordination Compounds with Transition Metal Ions in the Chemical Industry-A Review. Int. J. Mol. Sci. 2020, 21, 5443. [Google Scholar] [CrossRef]
- Kumar, S.; Devi, J.; Dubey, A.; Kumar, D.; Jindal, D.K.; Asija, S.; Sharma, A. Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff base ligands: Synthesis, characterization, DFT, in vitro antimicrobial activity and molecular docking studies. Res. Chem. Intermediat. 2023, 49, 939–965. [Google Scholar] [CrossRef]
- Chandra, S.; Jain, D.; Sharma, A.K.; Sharma, P. Coordination modes of a schiff base pentadentate derivative of 4-aminoantipyrine with cobalt(II), nickel(II) and copper(II) metal ions: Synthesis, spectroscopic and antimicrobial studies. Molecules 2009, 14, 174–190. [Google Scholar] [CrossRef]
- Singh, H.L.; Kulhari, P.; Choudhary, G.; Khaturia, S. Synthesis, spectral, DFT, and antibacterial studies of Nickel(II) and Cobalt (II) complexes with aminoantipyrine based Schiff base ligands. Inorg. Chem. Commun. 2024, 162, 112192. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Yamada, S. Metal-Complexes with Schiff-Bases Obtained from Salicylaldehyde Derivatives and 2-Aminoethylpyridine. Inorg. Chim. Acta 1975, 12, 109–112. [Google Scholar] [CrossRef]
- Vusak, D.; Smrecki, N.; Muratovic, S.; Zilic, D.; Prugovecki, B.; Matkovic-Calogovic, D. Structural diversity in the coordination compounds of cobalt, nickel and copper with N-alkylglycinates: Crystallographic and ESR study in the solid state. RSC Adv. 2021, 11, 23779–23790. [Google Scholar] [CrossRef]
- Rai, N.; Malik, B.A. Studies of Some Novel Coordination Compounds and Their Application in Nanoparticle Synthesis; Grin Verlag: Munchen, Germany, 2018. [Google Scholar]
- Mederos, A.; Domínguez, S.; Hernández-Molina, R.; Sanchiz, J.; Brito, F. Coordinating ability of ligands derived from phenylenediamines. Coordin. Chem. Rev. 1999, 193-195, 857–911. [Google Scholar] [CrossRef]
- El-Ajaily, M.; Abou-Krisha, M.M.; Etorki, A.; Alassbaly, F.; Maihub, A. Schiff base derived from phenylenediamine and salicylaldehyde as precursor techniques in coordination chemistry. Chem. Pharm. Res. 2013, 5, 933–938. [Google Scholar]
- Elango, G.A.; Guhanathan, S. Co (II), Ni (II) and Cu (II) Complexes with Schiff Base Ligand: Syntheses, Characterization, Antimicrobial Studies and Molecular Docking Studies. SOJ Mater. Sci. Eng. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Zakrzewski, G.; Sacconi, L. Four-, five and six-coordinated nickel (II) and cobalt (II) complexes of Schiff bases derived from pyridine-2-carboxaldehyde and N, N-substituted ethylenediamines. Inorg. Chem. 1968, 7, 1034–1036. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Chakraborty, P.; Drew, M.G.B.; Ghosh, A. Nickel(II) complexes of terdentate or symmetrical tetradentate Schiff bases: Evidence of the influence of the counter anions in the hydrolysis of the imine bond in Schiff base complexes. Inorg. Chim. Acta 2009, 362, 502–508. [Google Scholar] [CrossRef]
- Mocanu, M.I.; Patrascu, A.A.; Hillebrand, M.; Shova, S.; Lloret, F.; Julve, M.; Andruh, M. Trinuclear Nickel(II) and Cobalt(II) Complexes Constructed from Mannich-Schiff-Base Ligands: Synthesis, Crystal Structures, and Magnetic Properties. Eur. J. Inorg. Chem. 2019, 2019, 4773–4783. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Abo-Aly, M.M.; Lasheen, A.A.M. Molecular structural, vibrational assignments, electronic structure and DFT calculations, and molecular docking of N-benzylideneaniline and N-salicylidene-o-aminoaphenol Schiff bases. Inorg. Nano-Met. Chem. 2021, 53, 1306–1315. [Google Scholar] [CrossRef]
- Roncagliolo-Barrera, P.; Rodriguez, C.E.; Gomez, F.J.R. Comparative assessment of Ni-Co coatings obtained from a deep eutectic solvent, choline chloride-ethylene glycol, and water by electroplating. J. Solid State Electr. 2023, 27, 3075–3089. [Google Scholar] [CrossRef]
- ASTM B254-92(2020)e1; Standard Practice for Preparation of and Electroplating on Stainless Steel. American Society for Testing and Materials: West Conshohocken, PA, USA, 2020. [CrossRef]
- ASTM B578-21; Standard Test Method for Microindentation Hardness of Electroplated Coatings. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021. [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision A. 03, Gaussian. 2016. Available online: https://www.scienceopen.com/book?vid=cdeb68d5-cf23-457a-bc65-84001255a903 (accessed on 7 July 2025).
- Neeraj; Kumar, A.; Kumar, V.; Prajapati, R.; Asthana, S.K.; Upadhyay, K.; Zhao, J. A remarkable effect of N, N-diethylamino functionality on the optoelectronic properties of a salicylimine-based probe for Al3+. Dalton Trans. 2014, 43, 5831–5839. [Google Scholar] [CrossRef]
- Muñoz, B.M.; Santillan, R.; Rodriguez, M.; Méndez, J.M.; Romero, M.; Farfan, N.; Lacroix, P.G.; Nakatani, K.; Ramos-Ortíz, G.; Maldonado, J.L. Synthesis, crystal structure and non-linear optical properties of boronates derivatives of salicylideniminophenols. J. Organomet. Chem. 2008, 693, 1321–1334. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; Bucak, E.; Karabulut, S. In situ modification of the Grubbs first generation catalyst: A highly controllable metathesis catalyst bearing tridentate Schiff base ligands. J. Mol. Catal. A Chem. 2013, 376, 53–62. [Google Scholar] [CrossRef]
- Lamère, J.F.; Lacroix, P.G.; Farfán, N.; Rivera, J.M.; Santillan, R.; Nakatani, K.J. Synthesis, characterization and nonlinear optical (NLO) properties of a push–pull bisboronate chromophore with a potential electric field induced NLO switch. J. Mater. Chem. 2006, 16, 2913–2920. [Google Scholar] [CrossRef]
- Wang, W.L.; Guerrero, T.; Merecias, S.R.; García-Ortega, H.; Santillan, R.; Daran, J.C.; Farfán, N.; Agustin, D.; Poli, R. Substituent effects on solvent-free epoxidation catalyzed by dioxomolybdenum(VI) complexes supported by ONO Schiff base ligands. Inorg. Chim. Acta 2015, 431, 176–183. [Google Scholar] [CrossRef]
- Juma, J.A.; Karim, W.O.; Aziz, S.B. UV-VIS, Surface Morphology and Electrochemical Study of Cobalt Electrodeposition from Choline Chloride-ethylene Glycol Liquid Mixture. Int. J. Electrochem. Sci. 2021, 16, 21104. [Google Scholar] [CrossRef]
- Cui, J.N.; Sun, H.J.; Tan, Y.; Zhou, X.; Wang, B.J.; Sun, J. Electrodeposition of Ni from Choline Chloride/Ethylene Glycol Deep Eutectic Solvent and Pure Ethylene Glycol. JOM 2024, 76, 2178–2188. [Google Scholar] [CrossRef]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef]
- Bigos, A.; Beltowska-Lehman, E.; Kot, M. Studies on electrochemical deposition and physicochemical properties of nanocrystalline Ni-Mo alloys. Surf. Coat. Technol. 2017, 317, 103–109. [Google Scholar] [CrossRef]
- Pan, B.; Zhang, Q.; Liu, Z.; Yang, Y. Influence of butynediol and tetrabutylammonium bromide on the morphology and structure of electrodeposited cobalt in the presence of saccharin. Mater. Chem. Phys. 2019, 228, 37–44. [Google Scholar] [CrossRef]
- Fukui, R.; Katayama, Y.; Miura, T. The effect of organic additives in electrodeposition of Co from an amide-type ionic liquid. Electrochim. Acta 2011, 56, 1190–1196. [Google Scholar] [CrossRef]
- Landa-Castro, M.; Sebastián, P.; Giannotti, M.I.; Serrà, A.; Gómez, E. Electrodeposition of nanostructured cobalt films from a deep eutectic solvent: Influence of the substrate and deposition potential range. Electrochim. Acta 2020, 359, 136928. [Google Scholar] [CrossRef]
- Beiginejad, H.; Amani, A.; Nematollahi, D.; Khazalpour, S. Thermodynamic study of the electrochemical oxidation of some aminophenol derivatives: Experimental and theoretical investigation. Electrochim. Acta 2015, 154, 235–243. [Google Scholar] [CrossRef]
- Senft, L.; Moore, J.L.; Franke, A.; Fisher, K.R.; Scheitler, A.; Zahl, A.; Puchta, R.; Fehn, D.; Ison, S.; Sader, S.; et al. Quinol-containing ligands enable high superoxide dismutase activity by modulating coordination number, charge, oxidation states and stability of manganese complexes throughout redox cycling. Chem. Sci. 2021, 12, 10483–10500. [Google Scholar] [CrossRef] [PubMed]
- Picchio, M.L.; Minudri, D.; Mantione, D.; Criado-Gonzalez, M.; Guzman-Gonzalez, G.; Schmarsow, R.; Muller, A.J.; Tome, L.C.; Minari, R.J.; Mecerreyes, D. Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors. Acs Sustain. Chem. Eng. 2022, 10, 8135–8142. [Google Scholar] [CrossRef]
- Protsenko, V. Using Deep Eutectic Solvent-Assisted Plating Baths to Electrodeposit Composite Coatings: A Review. Coatings 2024, 14, 375. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, S.; Li, F.; Zhang, M.; Ren, X.; Li, M. Tailoring crystalline orientation of electrodeposited cobalt by alkynol additives. Electrochim. Acta 2024, 497, 144593. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Impact of ionic liquid [FPIM]Br on the electrodeposition of Ni and Co from an aqueous sulfate bath. J. Mater. Res. Technol. 2021, 12, 170–185. [Google Scholar] [CrossRef]
- Tułodziecki, M.; Tarascon, J.-M.; Taberna, P.-L.; Guéry, C. Importance of the double layer structure in the electrochemical deposition of Co from soluble Co2+-based precursors in Ionic Liquid media. Electrochim. Acta 2014, 134, 55–66. [Google Scholar] [CrossRef]
- Verdoorn, D.S.; Zuliani, A.; Ranjan, P.; Holgado, J.P.; Khiar, N.; Saya, J.M.; Carrillo-Carrión, C.; Maes, B.U.W.; Orru, R.V.A. Unveiling the Potential of a Cobalt-Based Metal-Organic Framework in Carbodiimide Synthesis. Adv. Synth. Catal. 2025, 367, e202401540. [Google Scholar] [CrossRef]
- Jayadev, V.; Pradhan, S.C.; Nitha, P.; John, J.; Unni, K.N.; Soman, S. Investigating mass transport and recombination as a function of structural variation in dye-sensitized solar cells employing indole fused heterocyclic organic sensitizers and cobalt electrolytes. J. Photochem. Photobiol. 2024, 19, 100223. [Google Scholar] [CrossRef]
- Sivasankaran, L.; Pradhan, S.C.; Mishra, R.K.; Soman, S.; Ajayaghosh, A. Role of alkyl groups regulating recombination and mass transport at cobalt electrolyte-dye interface in dye sensitized solar cells. Sol. Energy 2022, 236, 182–194. [Google Scholar] [CrossRef]
- Robertson, T.I.; Nelson, P.N. A DFT and experimental study of the spectroscopic and hydrolytic degradation behaviour of some benzylideneanilines. J. Mol. Struct. 2022, 1249, 131625. [Google Scholar] [CrossRef]
- Belal, A.A.; El-Deen, I.M.; Farid, N.Y.; Zakaria, R.; Refat, M.S. Synthesis, spectroscopic, coordination and biological activities of some transition metal complexes containing ONO tridentate Schiff base ligand. Spectrochim. Acta A 2015, 149, 771–787. [Google Scholar] [CrossRef]
- Szlyk, E.; Surdykowski, A.; Barwiolek, M.; Larsen, E. Spectroscopy and stereochemistry of the optically active copper(II), cobalt(II) and nickel(II) complexes with Schiff bases N,N′-(1R,2R)-(−)-1,2-cyclohexylenebis(3-methylbenzylideneiminato) and N,N′-(1R,2R)-(−)-1,2-cyclohexylenebis(5-methylbenzylideneiminato). Polyhedron 2002, 21, 2711–2717. [Google Scholar] [CrossRef]
- Bufarwa, S.M.; El-Sefait, R.M.; Thbayh, D.K.; Belaidi, M.; Al-Shemary, R.K.; Abdusamea, R.M.; El-Ajaily, M.M.; Fiser, B.; Bader, H.A.; Saleh, A.A.; et al. Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: Structural elucidation, computational aspects, and biological evaluation. Rev. Inorg. Chem. 2025, 45, 105–124. [Google Scholar] [CrossRef]
- Selvi, M.S.; Rajendran, L.; Abukhaled, M. Analytical study and parameter-sensitivity analysis of catalytic current at a rotating disk electrode. J. Phys. Commun. 2020, 4, 105017. [Google Scholar] [CrossRef]
- Gamboa, S.A.; Gonzalez-Rodríguez, J.G.; Valenzuela, E.; Campillo, B.; Sebastian, P.J.; Reyes-Rojas, A. Evaluation of the corrosion resistance of Ni-Co-B coatings in simulated PEMFC environment. Electrochim. Acta 2006, 51, 4045–4051. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.N. Surface pretretament by phosphate conversion coatings—A review. Rev. Adv. Mat. Sci. 2005, 9, 130–177. Available online: http://www.ipme.ru/e-journals/RAMS/ (accessed on 7 July 2025).
- ASTM B994/B994M-22; Standard Specification for Nickel-Cobalt Alloy Coating. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022. [CrossRef]
- Bekish, Y.N.; Grabchikov, S.; Tsybul’skaya, L.S.; Kukareko, V.A.; Perevoznikov, S.S. Electroplated cobalt-boron alloys: Formation and structure features. Prot. Met. Phys. Chem. Surf. 2013, 49, 319–324. [Google Scholar] [CrossRef]
- Qiao, G.Q.; Wang, S.C.; Wang, X.H.; Chen, X.Y.; Wang, X.Z.; Cui, H.Z. Ni/Co/black phosphorus nanocomposites for Q235 carbon steel corrosion-resistant coating. Adv. Compos. Hybrid. Mater. 2022, 5, 438–449. [Google Scholar] [CrossRef]
- Rasooli, A.; Safavi, M.S.; Kasbkar Hokmabad, M. Cr2O3 nanoparticles: A promising candidate to improve the mechanical properties and corrosion resistance of Ni-Co alloy coatings. Ceram. Int. 2018, 44, 6466–6473. [Google Scholar] [CrossRef]
- ASTM G59-97(2020); Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM G102-89(2015)e1; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. American Society for Testing and Materials: West Conshohocken, PA, USA, 2004. [CrossRef]
- Matsumoto, T.; Takahashi, K.; Kitagishi, K.; Shinoda, K.; Huaman, J.L.C.; Piquemal, J.Y.; Jeyadevan, B. Dissolution and reduction of cobalt ions in the polyol process using ethylene glycol: Identification of the active species and its role. New J. Chem. 2015, 39, 5008–5018. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, structural characterization, thermal studies, catalytic efficiency and antimicrobial activity of some M(II) complexes with ONO tridentate Schiff base N-salicylidene-o-aminophenol (saphH2). J. Mol. Struct. 2012, 1010, 130–138. [Google Scholar] [CrossRef]




| Co (II) | Ni (II) | |||||
|---|---|---|---|---|---|---|
| Compound | E vs Ag | Ag (I)/V | ιlim/µA cm−2 | DCo/cms−2 10−6 | E vs Ag | Ag (I)/V | ιlim/µA cm−2 | DNi/cm s−2 10−6 |
| X | −0.105 | 15.849 | 2.56656 | −0.106 | 2.4996 | 0.29442 |
| PSI-1 | −0.131 | 10.471 | 1.9523 | −0.112 | 2.4667 | 0.29186 |
| PSI-2 | −0.117 | 2.5119 | 1.6268 | −0.114 | 2.0798 | 0.26078 |
| Coating | Rsol/Ω∙cm2 | Rct/Ω∙cm2 | Qdl/µF cm−2 | α1 |
|---|---|---|---|---|
| X | 31.91 ± 1.18 | 10 349 ± 1.03 | 15.542 ± 1.89 | 0.779 |
| PSI-1 | 36.53 ± 2.12 | 16 632 ± 3.08 | 8.931 ± 2.04 | 0.722 |
| PSI-2 | 31.55 ± 1.96 | 21 805 ± 4.09 | 0.145 ± 1.31 | 0.731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordaz-Romero, E.; Roncagliolo-Barrera, P.; Ballinas-Indili, R.; González-Antonio, O.; Farfán, N. Ni-Co Electrodeposition Improvement Using Phenylsalicylimine Derivatives as Additives in Ethaline-Based Deep Eutectic Solvents (DES). Coatings 2025, 15, 814. https://doi.org/10.3390/coatings15070814
Ordaz-Romero E, Roncagliolo-Barrera P, Ballinas-Indili R, González-Antonio O, Farfán N. Ni-Co Electrodeposition Improvement Using Phenylsalicylimine Derivatives as Additives in Ethaline-Based Deep Eutectic Solvents (DES). Coatings. 2025; 15(7):814. https://doi.org/10.3390/coatings15070814
Chicago/Turabian StyleOrdaz-Romero, Enrique, Paola Roncagliolo-Barrera, Ricardo Ballinas-Indili, Oscar González-Antonio, and Norberto Farfán. 2025. "Ni-Co Electrodeposition Improvement Using Phenylsalicylimine Derivatives as Additives in Ethaline-Based Deep Eutectic Solvents (DES)" Coatings 15, no. 7: 814. https://doi.org/10.3390/coatings15070814
APA StyleOrdaz-Romero, E., Roncagliolo-Barrera, P., Ballinas-Indili, R., González-Antonio, O., & Farfán, N. (2025). Ni-Co Electrodeposition Improvement Using Phenylsalicylimine Derivatives as Additives in Ethaline-Based Deep Eutectic Solvents (DES). Coatings, 15(7), 814. https://doi.org/10.3390/coatings15070814






