The Potential Application of AZ31-Mg(OH)2/CeO2 as Temporary Medical Implants: Evaluation of the Corrosion Resistance and Biocompatibility Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. CeO2 Coating
2.3. Characterization Techniques
2.3.1. Structural Characterization
2.3.2. Roughness
2.3.3. Electrochemical Testing
2.3.4. In Vitro Cell Culture Assays
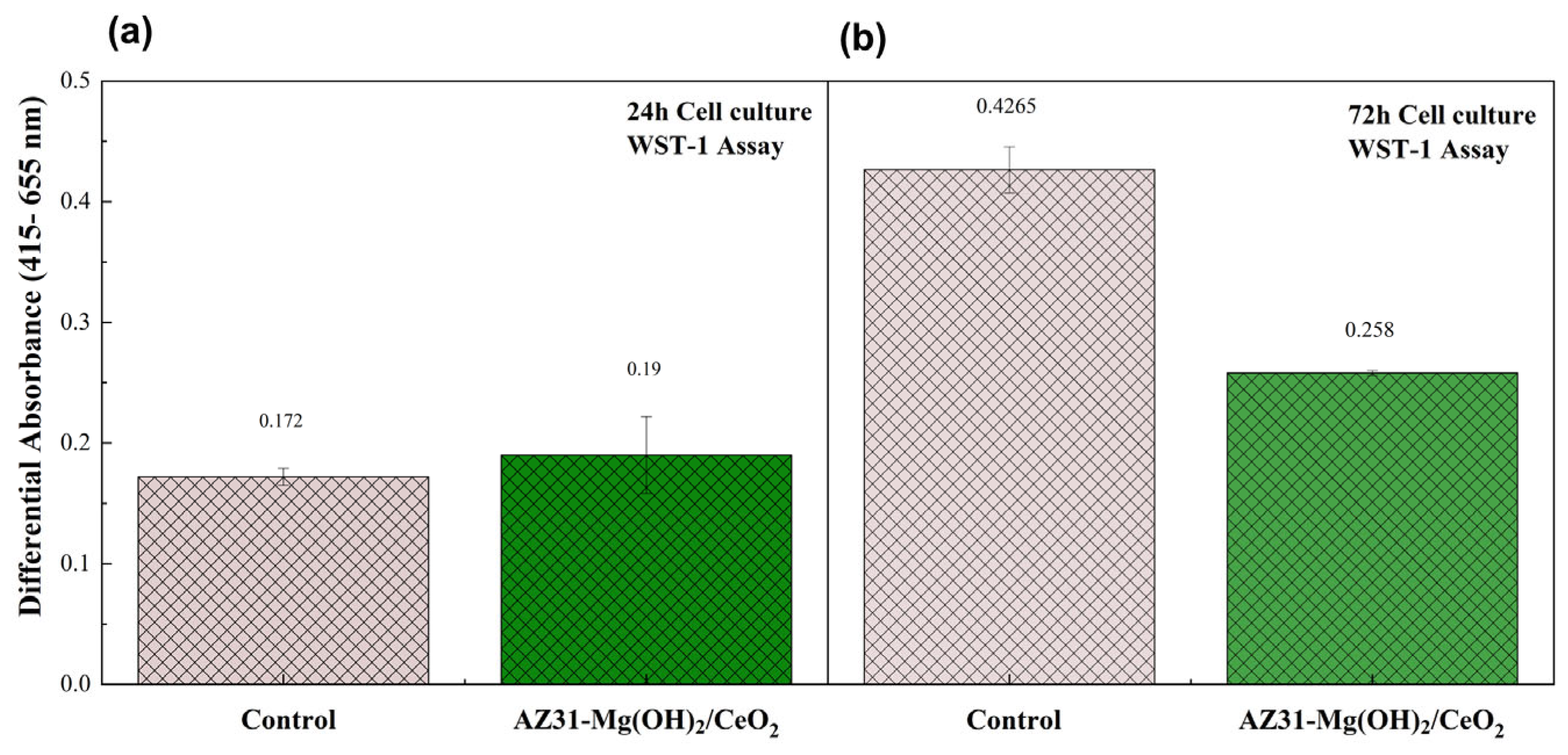
2.3.5. Cell Proliferation Assay
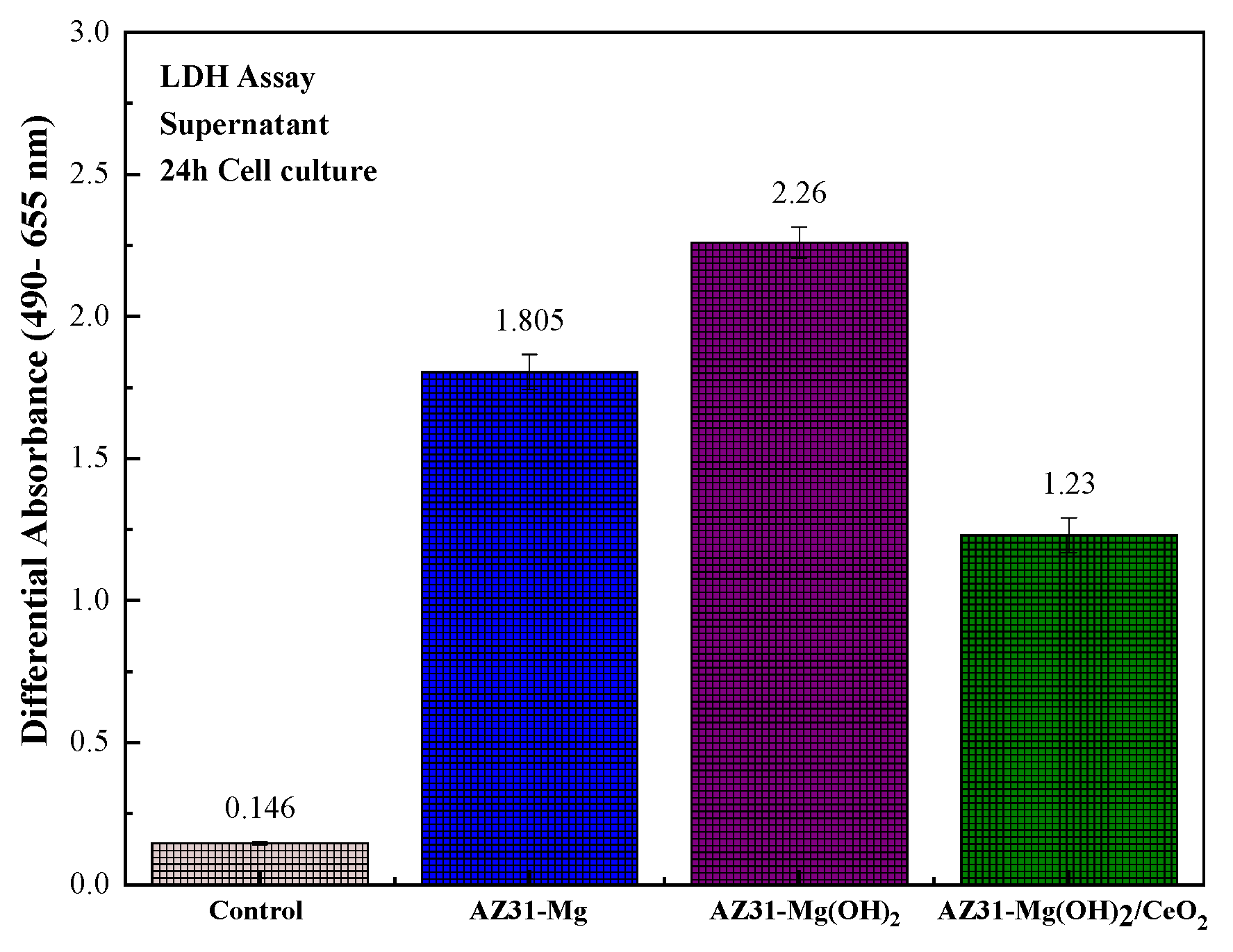
2.3.6. LDH Assay
2.3.7. Live/Dead Assay
3. Results and Discussion
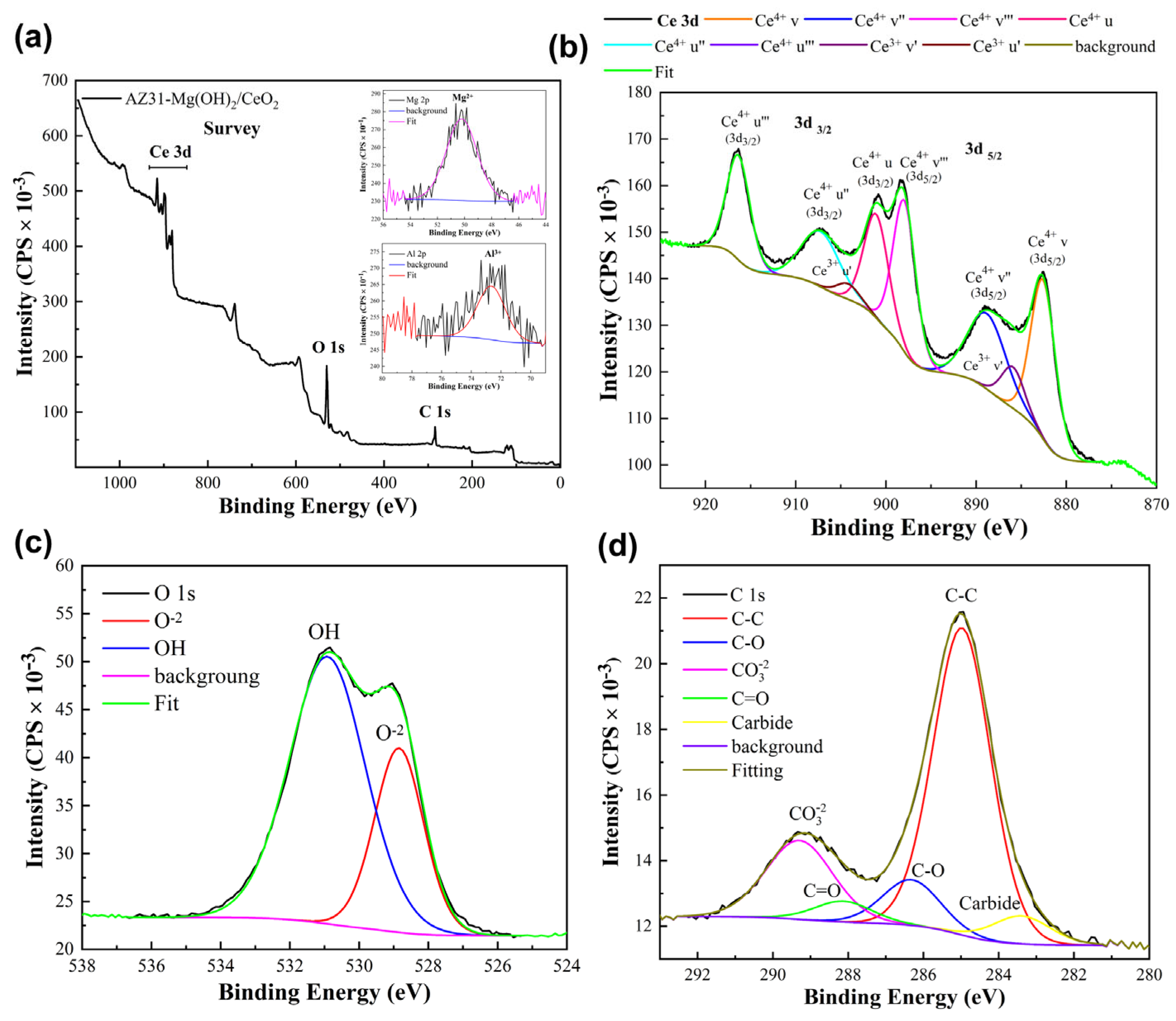
3.1. Structural Characterization
X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Roughness
Atomic Force Microscopy
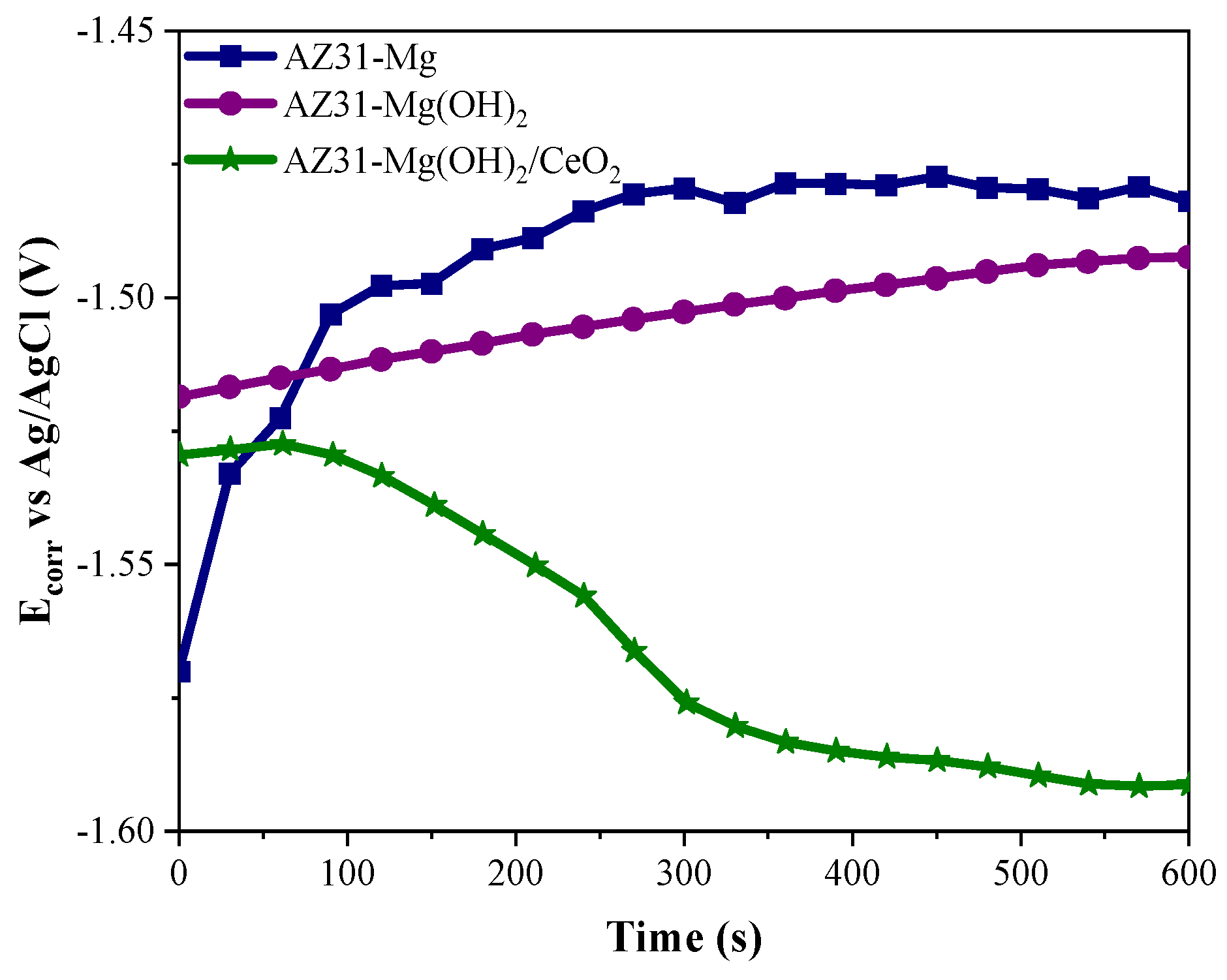
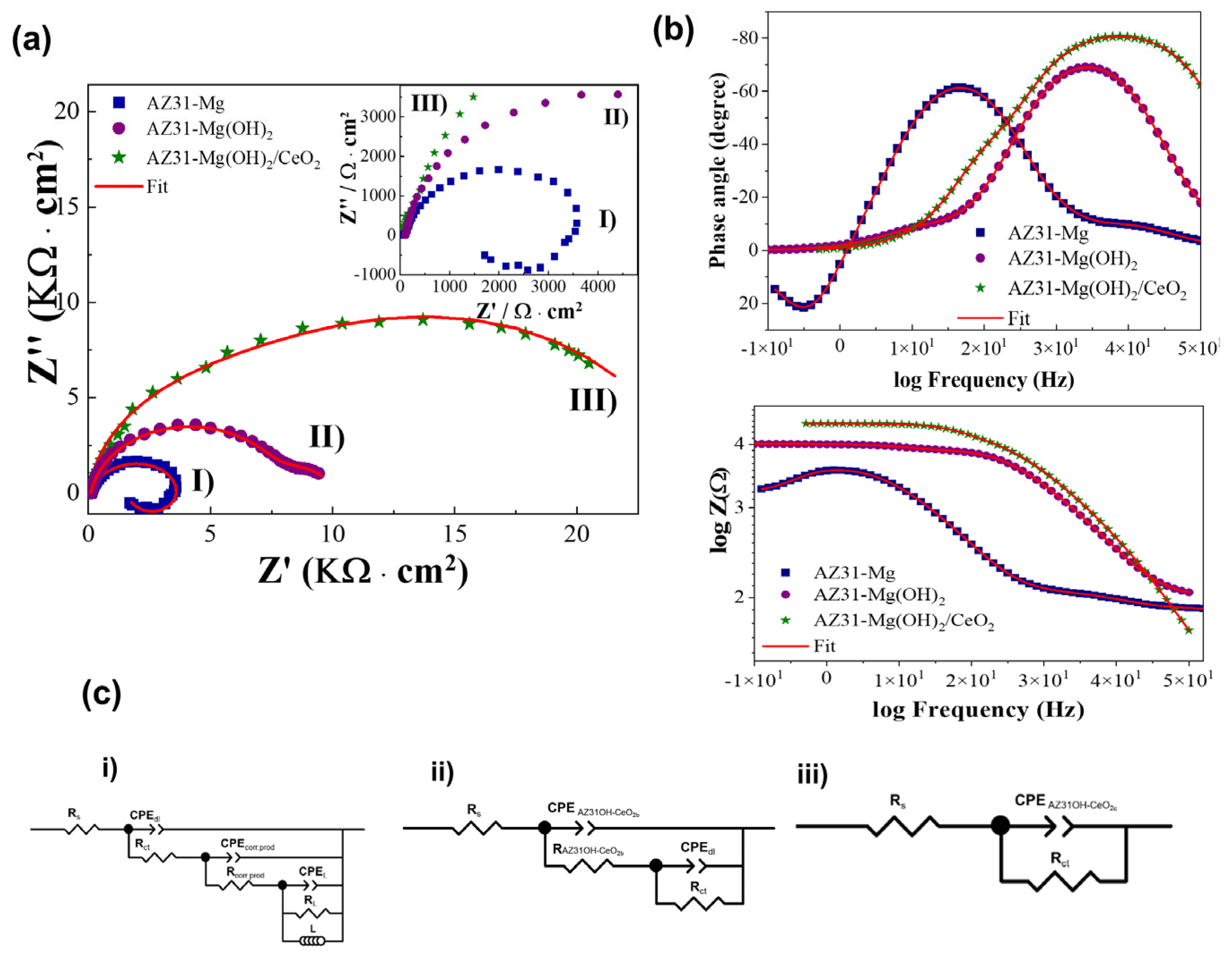
3.3. Electrochemical Results
3.3.1. Open Circuit Potential (OCP)
3.3.2. EIS
3.3.3. Polarization Curves
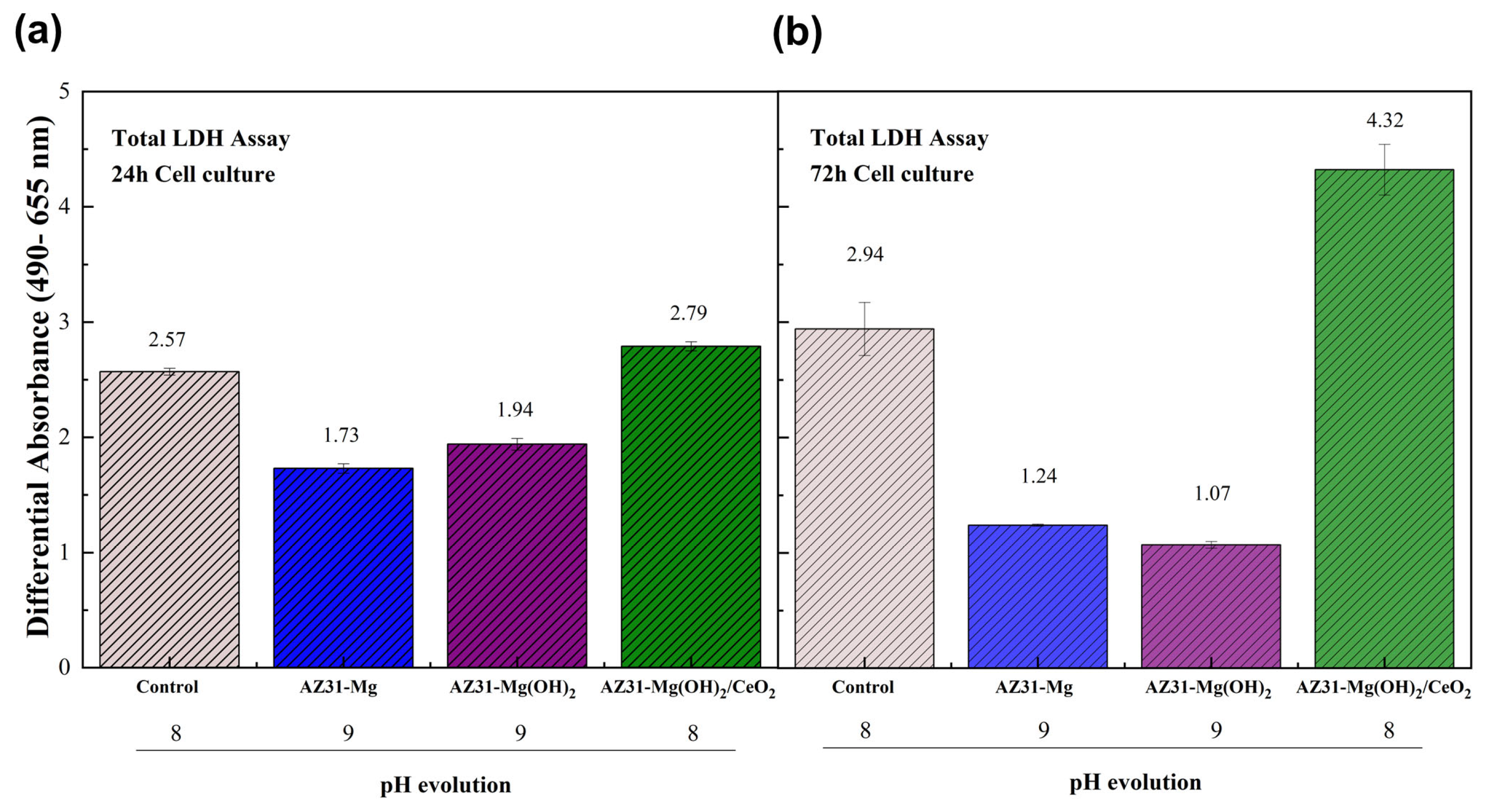
3.4. Biocompatibility
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seelig, M.G. A study of magnesium wire as an absorbable suture and ligature material. Arch. Surg. 1924, 8, 669–680. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Badkoobeh, F.; Mostaan, H.; Rafiei, M.; Bakhsheshi-Rad, H.R.; RamaKrishna, S.; Chen, X. Additive manufacturing of biodegradable magnesium-based materials: Design strategies, properties, and biomedical applications. J. Magnes. Alloys 2023, 11, 801–839. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Priya, A.K.; Balakrishnan, D.; Dutta, K.; Rajendran, S.; Soto-Moscoso, M. Insight on recent development in metallic biomaterials: Strategies involving synthesis, types and surface modification for advanced therapeutic and biomedical applications. Biochem. Eng. J. 2022, 187, 108522. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Yamagami, R.; Sieg, J.P.; Bevilacqua, P.C. Functional Roles of Chelated Magnesium Ions in RNA Folding and Function. Biochemistry 2021, 60, 2374–2386. [Google Scholar] [CrossRef]
- Gray-Munro, J.E.; Seguin, C.; Strong, M. Influence of surface modification on the in vitro corrosion rate of magnesium alloy AZ31. J. Biomed. Mater. Res. Part A 2009, 91 Pt A, 221–230. [Google Scholar] [CrossRef]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 Pt B, 58–67. [Google Scholar] [CrossRef]
- Abraham, A.M.; Subramani, V. Effect of Magnesium as Biomaterial in Biodegrdation. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Buxbaum, R.E.; Rajachar, R.M.; Goldman, J. New approaches in evaluating metallic candidates for bioabsorbable stents. Emerg. Mater. Res. 2012, 1, 237–255. [Google Scholar] [CrossRef]
- Liu, F.; Chen, C.; Niu, J.; Pei, J.; Zhang, H.; Huang, H.; Yuan, G. The processing of Mg alloy micro-tubes for biodegradable vascular stents. Mater. Sci. Eng. C 2015, 48, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Deshmukh, S.; Jones, I.; Chiu, Y.-L. Biodegradable magnesium alloys for orthopaedic applications. BMT 2021, 2, 214–235. [Google Scholar] [CrossRef]
- Zhou, W.; Xue, F.; Li, M. Corrosion Behavior of Al-Mg Alloys with Different Alloying Element Contents in 3.5% NaCl Solution. Metals 2025, 15, 327. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Sunil, B.R.; Kumar, R.S. Microstructure, mechanical properties and corrosion behavior of Rare Earths (RE) containing Mg-Zn alloy for biomedical applications. Mater. Today Proc. 2023, 102, 6–10. [Google Scholar] [CrossRef]
- Kim, J.; Pan, H. Effects of magnesium alloy corrosion on biological response—Perspectives of metal-cell interaction. Prog. Mater. Sci. 2023, 133, 101039. [Google Scholar] [CrossRef]
- Kumar, S.; Katyal, P.; Chaudhary, R.N.; Singh, V. Assessment of factors influencing bio-corrosion of magnesium based alloy implants: A review. Mater. Today Proc. 2022, 56, 2680–2689. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Púa, L.D.C.G.; Montenegro, J.C.R.; Reyes, A.M.F.; Rodríguez, H.Z.; Méndez, V.N.P. Biomaterials for orthopedic applications and techniques to improve corrosion resistance and mechanical properties for magnesium alloy: A review. J. Mater. Sci. 2023, 58, 3879–3908. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, W.; Zhang, T.; Wang, F. Effect of variations of Al content on microstructure and corrosion resistance of PEO coatings on Mg Al alloys. J. Alloys Compd. 2017, 690, 195–205. [Google Scholar] [CrossRef]
- Jahan, U.M.; Blevins, B.; Minko, S.; Reukov, V.V. Advancing biomedical applications: Antioxidant and biocompatible cerium oxide nanoparticle-integrated poly-ε-caprolactone fibers. Biomed. Phys. Eng. Express 2025, 11, 015020. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Heydari, M.; Khodaei, M. Cerium oxide nanoparticles: Synthesis methods and applications in wound healing. Mater. Today Bio 2023, 23, 100823. [Google Scholar] [CrossRef]
- Chen, D.; Mei, D.; Li, Y.; Chen, L.; Wang, H.; Huang, W.; Wang, L.; Zhu, S.; Guan, S. Protective nature of cerium-based oxides coating against Mg corrosion in Hanks’ balanced salt solution. Corros. Sci. 2023, 219, 111255. [Google Scholar] [CrossRef]
- Petrova, V.A.; Poshina, D.N.; Golovkin, A.S.; Mishanin, A.I.; Zhuravskii, S.G.; Yukina, G.Y.; Naumenko, M.Y.; Sukhorukova, E.G.; Savin, N.A.; Erofeev, A.S.; et al. Electrospun Composites of Chitosan with Cerium Oxide Nanoparticles for Wound Healing Applications: Characterization and Biocompatibility Evaluation In Vitro and In Vivo. Polymers 2024, 16, 1787. [Google Scholar] [CrossRef]
- Fagali, N.S.; Madrid, M.A.; Maceda, B.T.P.; Fernández, M.E.L.; Puerto, R.M.L.; de Mele, M.F.L. Effect of degradation products of iron-bioresorbable implants on the physiological behavior of macrophages in vitro. Metallomics 2020, 12, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nakata, Y.; Shida, S. Electron beam induced orientation selective epitaxial growth of CeO2(100) layers on Si(100) substrates by dc reactive sputtering. J. Phys. Conf. Ser. 2008, 100, 082014. [Google Scholar] [CrossRef]
- Shyu, J.Z.; Otto, K.; Watkins, W.L.H.; Graham, G.W.; Belitz, R.K.; Gandhi, H.S. Characterization of Pd/γ-alumina catalysts containing ceria. J. Catal. 1988, 114, 23–33. [Google Scholar] [CrossRef]
- Allahgholi, A.; Flege, J.I.; Thieß, S.; Drube, W.; Falta, J. Oxidation-State Analysis of Ceria by X-Ray Photoelectron Spectroscopy. Available online: www.chemphyschem.org (accessed on 15 November 2024).
- Benito-Santiago, S.E.; Onofre-Bustamante, E.; Lozano-Puerto, R.M. Synthesis and Characterisation of CeO2 Coatings on the AZ31 Alloy for Corrosion Protection and In Vitro Biocompatibility of MC3T3-E1 Pre-Osteoblasts. Metals 2023, 13, 653. [Google Scholar] [CrossRef]
- Anjum, M.J.; Zhao, J.; Ali, H.; Tabish, M.; Murtaza, H.; Yasin, G.; Malik, M.U.; Khan, W.Q. A Review on Self-Healing Coatings Applied to Mg Alloys and Their Electrochemical Evaluation Techniques. Int. J. Electrochem. Sci. 2020, 15, 3040–3053. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce4+/organic inhibitor for AA2024 in 3.5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef]
- Yu, J.; Lv, Y.; Duan, T.; Meng, F.; Cheng, Q.; Xu, Y.; Zhou, K.; Xue, L.; Leng, Z. Discharge and Corrosion Behaviors of Mg-Li and Mg-Li-La Alloys as Anodes for Seawater Battery. Int. J. Electrochem. Sci. 2020, 15, 10922–10935. [Google Scholar] [CrossRef]
- Singh, I.B.; Singh, M.; Das, S. A comparative corrosion behavior of Mg, AZ31 and AZ91 alloys in 3.5% NaCl solution. J. Magnes. Alloys 2015, 3, 142–148. [Google Scholar] [CrossRef]
- Kumar, R.; Katyal, P.; Gupta, M.; Singh, V. Electrochemical Corrosion Behaviour Analysis of Mg-Alloys Used for Orthopaedics and Vascular Implants. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1225, 012063. [Google Scholar] [CrossRef]
- Luo, Y.; Sun, Y.; Lv, J.; Wang, X.; Li, J.; Wang, F. Transition of interface oxide layer from porous Mg(OH)2 to dense MgO induced by polyaniline and corrosion resistance of Mg alloy therefrom. Appl. Surf. Sci. 2015, 328, 247–254. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, X.; Guo, W.; Rohwerder, M. Growth Behavior of Initial Product Layer Formed on Mg Alloy Surface Induced by Polyaniline. J. Electrochem. Soc. 2015, 162, C294–C301. [Google Scholar] [CrossRef]
- Brito-Franco, A.; Vazquez-Velez, E.; Lopez-Sesenes, R.; Larios-Gálvez, A.K.; Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G. Use of CeO2 nanoparticles as CO2-corrosion inhibitors of a duplex stainless steel. Green Chem. Lett. Rev. 2024, 17, 2360497. [Google Scholar] [CrossRef]
- Feliu, S., Jr. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Song, G. Recent Progress in Corrosion and Protection of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 563–586. [Google Scholar] [CrossRef]
- Cao, F.; Song, G.-L.; Atrens, A. Corrosion and passivation of magnesium alloys. Corros. Sci. 2016, 111, 835–845. [Google Scholar] [CrossRef]
- Jamali, S.S.; Moulton, S.E.; Tallman, D.E.; Forsyth, M.; Weber, J.; Wallace, G.G. Evaluating the corrosion behaviour of Magnesium alloy in simulated biological fluid by using SECM to detect hydrogen evolution. Electrochimica Acta 2015, 152, 294–301. [Google Scholar] [CrossRef]
- Atrens, A.; Chen, X.; Shi, Z. Mg Corrosion—Recent Progress. Corros. Mater. Degrad. 2022, 3, 566–597. [Google Scholar] [CrossRef]
- Benbouzid, A.Z.; Gomes, M.P.; Costa, I.; Gharbi, O.; Pébère, N.; Rossi, J.L.; Tran, M.T.; Tribollet, B.; Turmine, M.; Vivier, V. A new look on the corrosion mechanism of magnesium: An EIS investigation at different pH. Corros. Sci. 2022, 205, 110463. [Google Scholar] [CrossRef]
- Gomes, M.P.; Costa, I.; Pébère, N.; Rossi, J.L.; Tribollet, B.; Vivier, V. On the corrosion mechanism of Mg investigated by electrochemical impedance spectroscopy. Electrochimica Acta 2019, 306, 61–70. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.-L.; Liu, M.; Dargusch, M.S.; Atrens, A. Influence of casting porosity on the corrosion behaviour of Mg0.1Si. Corros. Sci. 2015, 94, 255–269. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wei, Q.; Wang, N.; Hou, B. Electrochemical behavior of Mg-Al-Zn-In alloy as anode materials in 3.5 wt.% NaCl solution. Electrochim. Acta 2017, 238, 156–167. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Shan, D.; Han, E.-H. Formation mechanism of a self-sealing pore micro-arc oxidation film on AM60 magnesium alloy. Surf. Coat. Technol. 2015, 266, 188–196. [Google Scholar] [CrossRef]
- Jiang, D.; Zhou, H.; Wan, S.; Cai, G.-Y.; Dong, Z.-H. Fabrication of superhydrophobic coating on magnesium alloy with improved corrosion resistance by combining micro-arc oxidation and cyclic assembly. Surf. Coat. Technol. 2018, 339, 155–166. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, F.; Song, L.; Zeng, R.-C.; Li, S.-Q.; Han, E.-H. Corrosion resistance of ceria/polymethyltrimethoxysilane modified magnesium hydroxide coating on AZ31 magnesium alloy. Surf. Coat. Technol. 2017, 328, 121–133. [Google Scholar] [CrossRef]
- Chaudry, U.M.; Farooq, A.; bin Tayyab, K.; Malik, A.; Kamran, M.; Kim, J.-G.; Li, C.; Hamad, K.; Jun, T.-S. Corrosion behavior of AZ31 magnesium alloy with calcium addition. Corros. Sci. 2022, 199, 110205. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Shi, L.; Zhang, F.; Li, S.; Zeng, R. Corrosion resistance of a self-healing multilayer film based on SiO2 and CeO2 nanoparticles layer-by-layer assembly on Mg alloys. Mater. Lett. 2019, 237, 14–18. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2013, 2, 218–240. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, Z.; Jiang, C.; Yang, W.; Chen, Y.; Yin, X.; Liu, Y. Design of the double-layer biocompatible coating on AZ31 magnesium alloy for highly effective corrosion resistance. Surf. Coat. Technol. 2021, 428, 127897. [Google Scholar] [CrossRef]
- Zhang, J.; Hou, S.; Zhang, M.; Zhang, S.; Li, W. Corrosion resistance and biocompatibility of silica coatings on AZ31 magnesium alloy via magnetron sputtering. Mater. Today Commun. 2024, 41, 110890. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Puliyalil, H.; Filipič, G.; Kumar, K.C.; Pottathara, Y.B.; Rudolf, R.; Fuchs-Godec, R.; Mohan, M.K.; Cvelbar, U. Corrosion studies of plasma modified magnesium alloy in simulated body fluid (SBF) solutions. Surf. Coat. Technol. 2020, 385, 125434. [Google Scholar] [CrossRef]
- Wang, J.; Deng, S.; Meng, M.; Tu, W.; Ou, J. Improved corrosion resistance and biocompatibility of AZ31 alloy by acid pickling pretreatment and (H+) hydroxyapatite/chitosan composite coating. Surf. Coat. Technol. 2023, 454, 129157. [Google Scholar] [CrossRef]
- Carter, S.-S.D.; Barbe, L.; Tenje, M.; Mestres, G. Exploring microfluidics as a tool to evaluate the biological properties of a titanium alloy under dynamic conditions. Biomater. Sci. 2020, 8, 6309–6321. [Google Scholar] [CrossRef]
- Sammons, R. Biological responses to hydroxyapatite. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 53–83. [Google Scholar] [CrossRef]
- Peron, M.; Skaret, P.C.; Fabrizi, A.; Varone, A.; Montanari, R.; Roven, H.J.; Ferro, P.; Berto, F.; Torgersen, J. The effect of Equal Channel Angular Pressing on the stress corrosion cracking susceptibility of AZ31 alloy in simulated body fluid. J. Mech. Behav. Biomed. Mater. 2020, 106, 103724. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lamaka, S.V.; Mei, D.; Scharnagl, N.; Feyerabend, F.; Zheludkevich, M.L.; Willumeit-Römer, R. Mg Biodegradation Mechanism Deduced from the Local Surface Environment under Simulated Physiological Conditions. Adv. Healthc. Mater. 2021, 10, 2100053. [Google Scholar] [CrossRef]
- Hsu, C.; Nazari, M.H.; Li, Q.; Shi, X. Enhancing degradation and corrosion resistance of AZ31 magnesium alloy through hydrophobic coating. Mater. Chem. Phys. 2019, 225, 426–432. [Google Scholar] [CrossRef]
- Alvarez, F.; Puerto, R.M.L.; Pérez-Maceda, B.; Grillo, C.A.; de Mele, M.F.L. Time-Lapse Evaluation of Interactions Between Biodegradable Mg Particles and Cells. Microsc. Microanal. 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 2018, 465. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, M.; Kawakabe, S.; Arimura, Y. A comparative study of colorimetric cell proliferation assays in immune cells. Cytotechnology 2016, 68, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Soltani, M.; Karami-Mohajeri, S.; Mohadesi, A.; Ranjbar, M.; Oghabian, Z.; Mehrpour, O.; Khosravi, F. An interdisciplinary approach to assessing the toxicity reduction of cerium oxide nanoparticles coated with polyethylene glycol and polyvinylpyrrolidone polymers: An in vitro study. Toxicol. In Vitro 2025, 105, 106022. [Google Scholar] [CrossRef]
- Filippova, A.D.; Sozarukova, M.M.; Baranchikov, A.E.; Kottsov, S.Y.; Cherednichenko, K.A.; Ivanov, V.K. Peroxidase-like Activity of CeO2 Nanozymes: Particle Size and Chemical Environment Matter. Molecules 2023, 28, 3811. [Google Scholar] [CrossRef]
- Zhang, S.; Ruan, H.; Xin, Q.; Mu, X.; Wang, H.; Zhang, X.-D. Modulation of the biocatalytic activity and selectivity of CeO2 nanozymes via atomic doping engineering. Nanoscale 2023, 15, 4408–4419. [Google Scholar] [CrossRef]
- Mukherjee, A.; Ashrafi, A.M.; Bytesnikova, Z.; Svec, P.; Richtera, L.; Adam, V. An investigation on the multiple roles of CeO2 nanoparticle in electrochemical sensing: Biomimetic activity and electron acceptor. J. Electroanal. Chem. 2023, 935, 117301. [Google Scholar] [CrossRef]
- Rzigalinski, B.A.; Carfagna, C.S.; Ehrich, M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1444. [Google Scholar] [CrossRef]
- Pota, G.; Silvestri, B.; Vitiello, G.; Gallucci, N.; Di Girolamo, R.; Scialla, S.; Raucci, M.G.; Ambrosio, L.; Di Napoli, M.; Zanfardino, A.; et al. Towards nanostructured red-ox active bio-interfaces: Bioinspired antibacterial hybrid melanin-CeO2 nanoparticles for radical homeostasis. Biomater. Biomater. Advances 2023, 153, 213558. [Google Scholar] [CrossRef]



| Alloy | Chemical Composition (wt. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Zn% | Ca% | Al% | Si% | Cu% | Mn% | Fe% | Ni% | Total Others | Mg% Balance | |
| AZ31-Mg | 1.14 | 0.00 | 3.1 | 0.001 | 0.00 | 0.20 | 0.004 | 0.0004 | ˂0.3 | Remainder |
| HBSS Solution Composition (g/L) | |
|---|---|
| NaCl | 8 |
| KCl | 0.4 |
| CaCl2 | 0.14 |
| MgSO4·7H2O | 0.1 |
| MgCl2·6H2O | 0.1 |
| Na2HPO4·2H2O | 0.06 |
| KH2PO4 | 0.06 |
| D-glucose | 1 |
| NaHCO3 | 0.35 |
| Sample | Rs (cm2) | CPEAZ31OH (−1 sn/cm2) | η | CPEcorr.prod (−1 sn/cm2) | η | CPEAZ31OH-CeO2 (−1 sn/cm2) | η | Rcorr.prod (cm2) | CPEdl (−1sn/cm2) | η | Rct (cm2) | CPEL (−1 sn/cm2) | η | RL (cm2) | L (H cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ31-Mg | 78 | − | − | 2 × 10−6 | 0.75 | − | − | 40 | 1 × 10−6 | 0.98 | 3690 | −5 × 10−6 | 0.4 | −2000 | 0.05 |
| AZ31O-Mg(OH)2 | 100 | 8 × 10−7 | 0.85 | − | − | − | − | 10,000 | 2 × 10−7 | 0.93 | 20,000 | − | − | − | − |
| AZ31O-Mg(OH)2/CeO2 | 20 | − | − | − | − | 1.7 × 10−7 | 0.96 | 5200 | 1.3 × 10−7 | 0.93 | 24,500 | − | − | − | − |
| Materials/System | Ecorr (V) | Icorr (A·cm2) | R (Ω·m2) | Reference |
|---|---|---|---|---|
| PA@PCL@Ce-HA | −1.34 | 4.56 × 10−7 | 7000 | [53] |
| AZ31/Si-3 | −1.59 | 6.56 × 10−6 | 2947 | [54] |
| AZ1(G) | −1.470 | 2.07 × 10−3 | 30 | [55] |
| AZ31-(H+)HAp | −1.30 | 6.26 × 10−6 | 2.75 × 104 | [56] |
| AZ31-Mg(OH)2/CeO2 | −1.59 | 2.37 × 10−6 | 2.45 × 104 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofre-Bustamante, E.; Lozano, R.M.; Escudero, M.L.; Espíndola-Flores, A.C.; Benito-Santiago, S.E. The Potential Application of AZ31-Mg(OH)2/CeO2 as Temporary Medical Implants: Evaluation of the Corrosion Resistance and Biocompatibility Properties. Coatings 2025, 15, 450. https://doi.org/10.3390/coatings15040450
Onofre-Bustamante E, Lozano RM, Escudero ML, Espíndola-Flores AC, Benito-Santiago SE. The Potential Application of AZ31-Mg(OH)2/CeO2 as Temporary Medical Implants: Evaluation of the Corrosion Resistance and Biocompatibility Properties. Coatings. 2025; 15(4):450. https://doi.org/10.3390/coatings15040450
Chicago/Turabian StyleOnofre-Bustamante, Edgar, Rosa M. Lozano, María L. Escudero, Ana C. Espíndola-Flores, and Sandra E. Benito-Santiago. 2025. "The Potential Application of AZ31-Mg(OH)2/CeO2 as Temporary Medical Implants: Evaluation of the Corrosion Resistance and Biocompatibility Properties" Coatings 15, no. 4: 450. https://doi.org/10.3390/coatings15040450
APA StyleOnofre-Bustamante, E., Lozano, R. M., Escudero, M. L., Espíndola-Flores, A. C., & Benito-Santiago, S. E. (2025). The Potential Application of AZ31-Mg(OH)2/CeO2 as Temporary Medical Implants: Evaluation of the Corrosion Resistance and Biocompatibility Properties. Coatings, 15(4), 450. https://doi.org/10.3390/coatings15040450







