Construction of Silane-Modified Diatomite-Magnetic Nanocomposite Superhydrophobic Coatings Using Multi-Scale Composite Principle
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ODEM and OFe3O4
2.3. Preparation of EP/ODEM@OFe3O4
2.4. Preparation of Superhydrophobic Cotton Fabrics
2.5. Characterization
3. Results
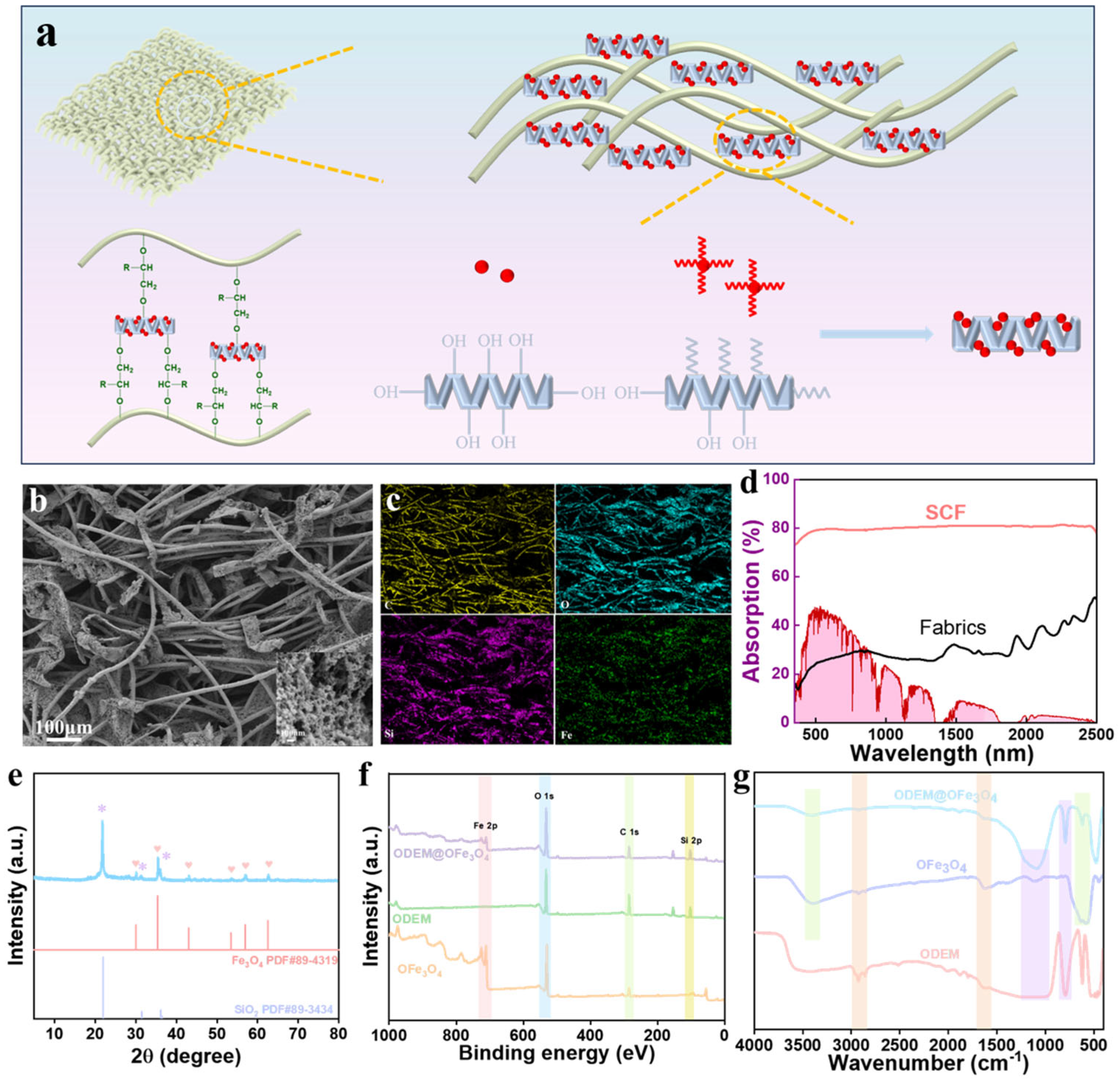
3.1. Physicochemical Characterization of ODEM and OFe3O4 Flakes
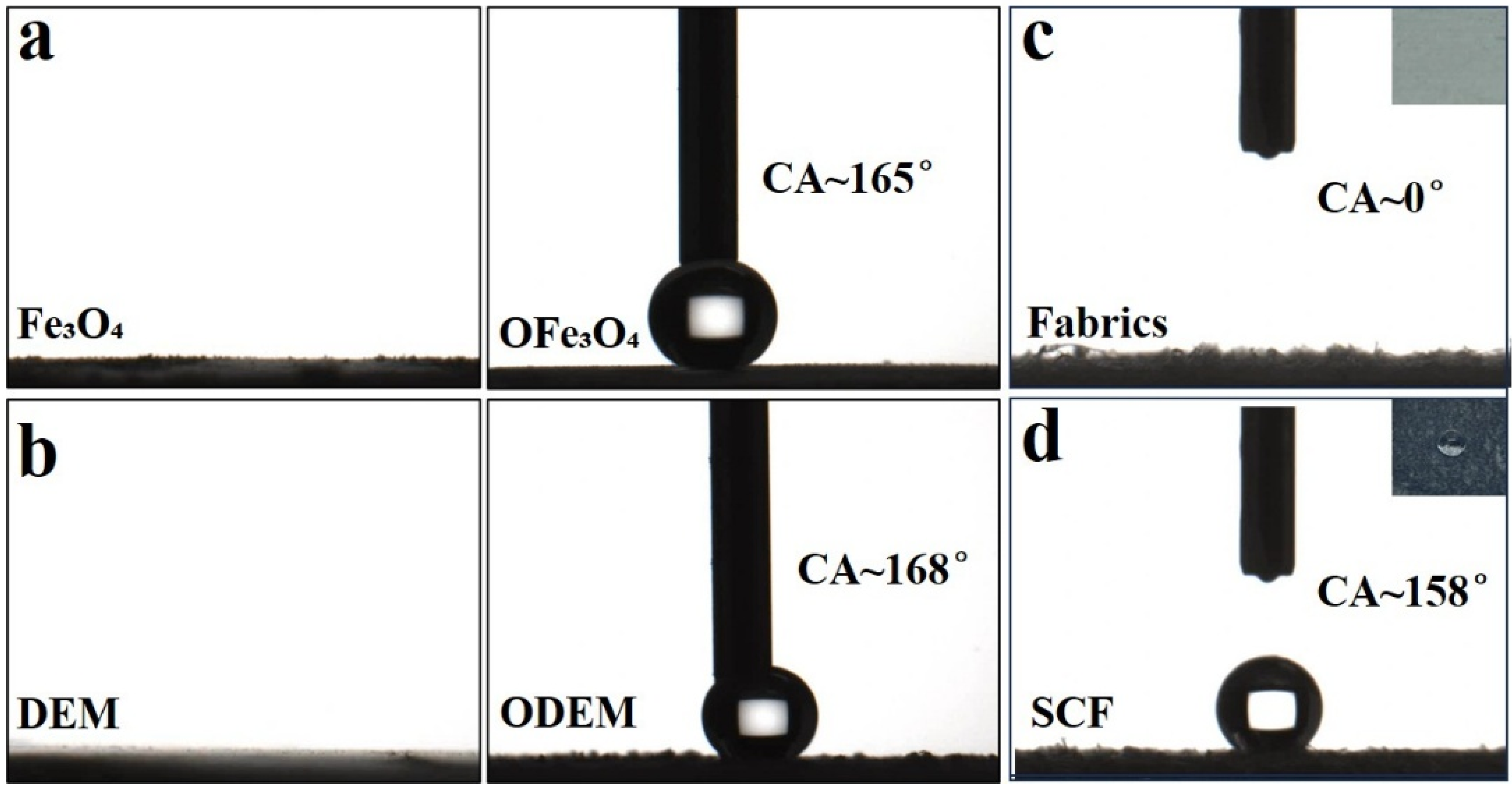
3.2. Surface Wettability of the Coatings
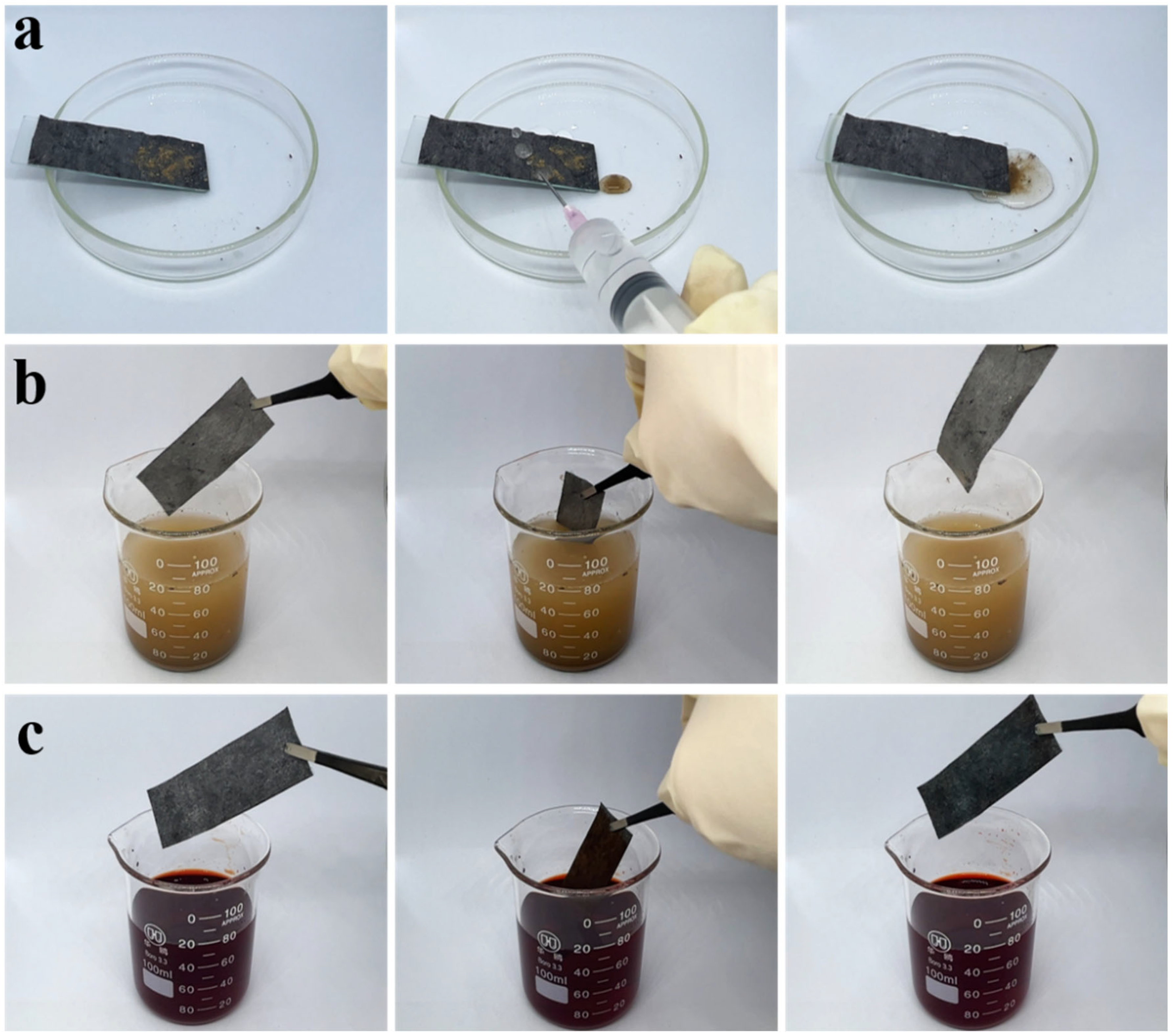
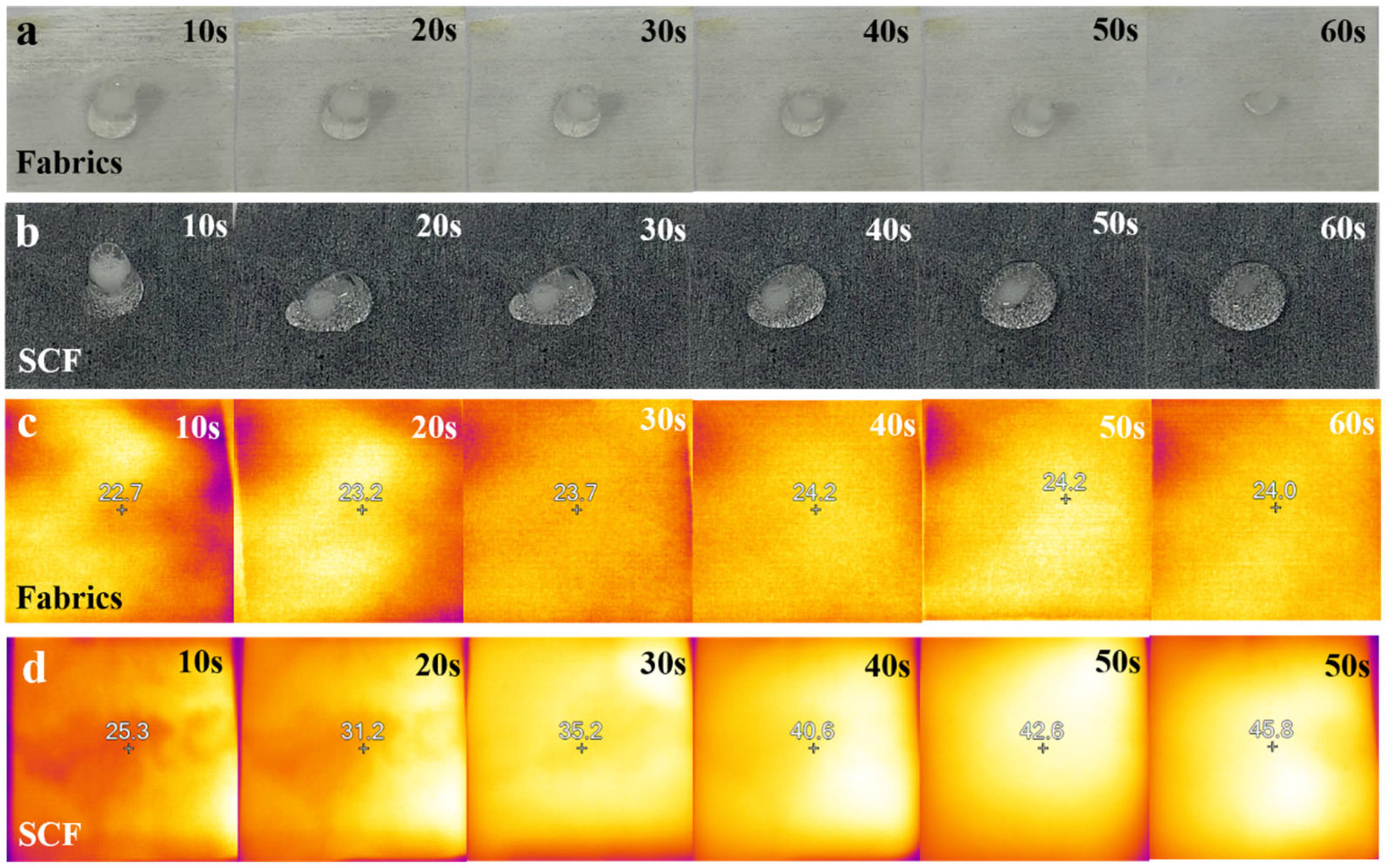
3.3. Self-Cleaning Test of Superhydrophobic Coatings
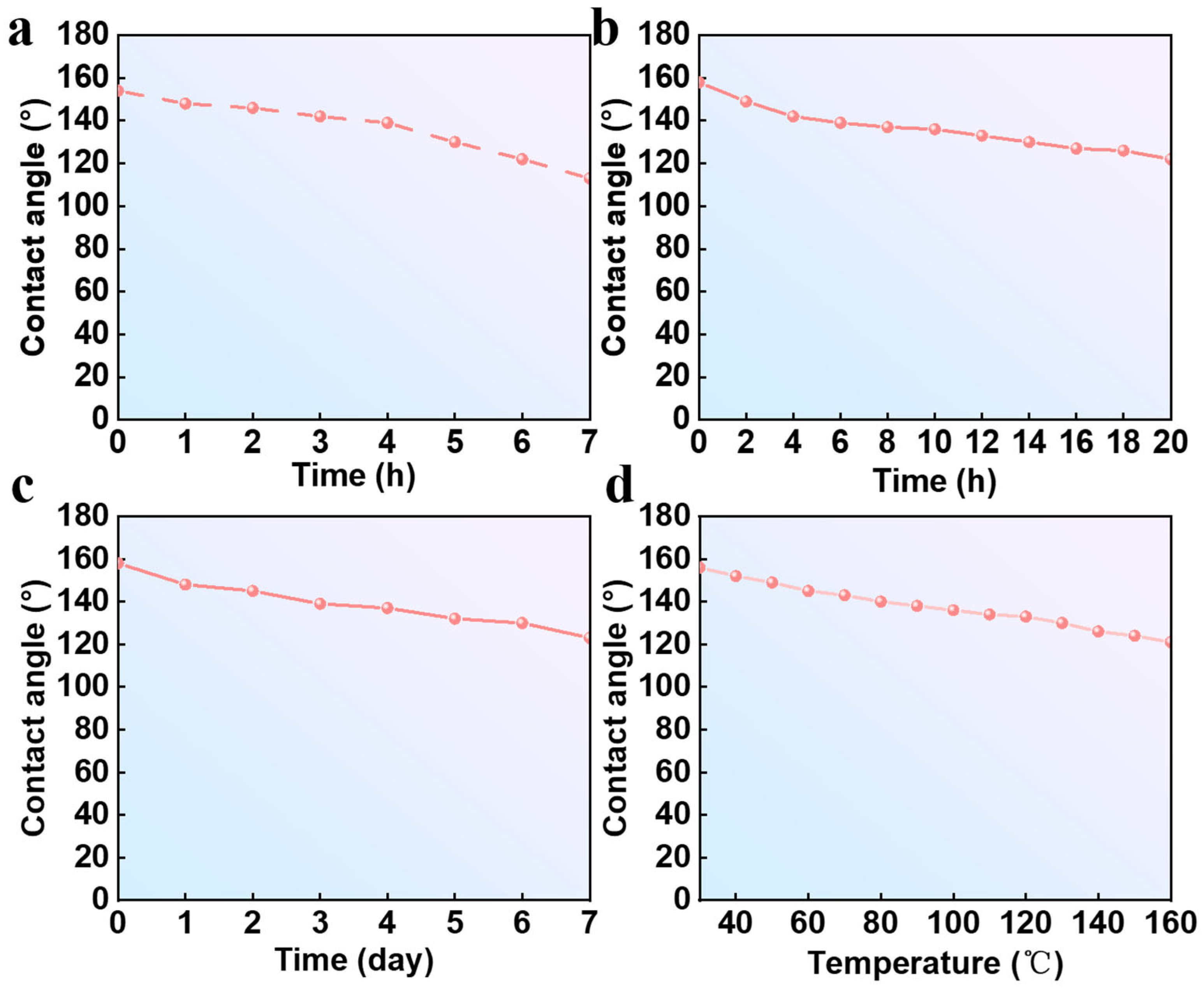
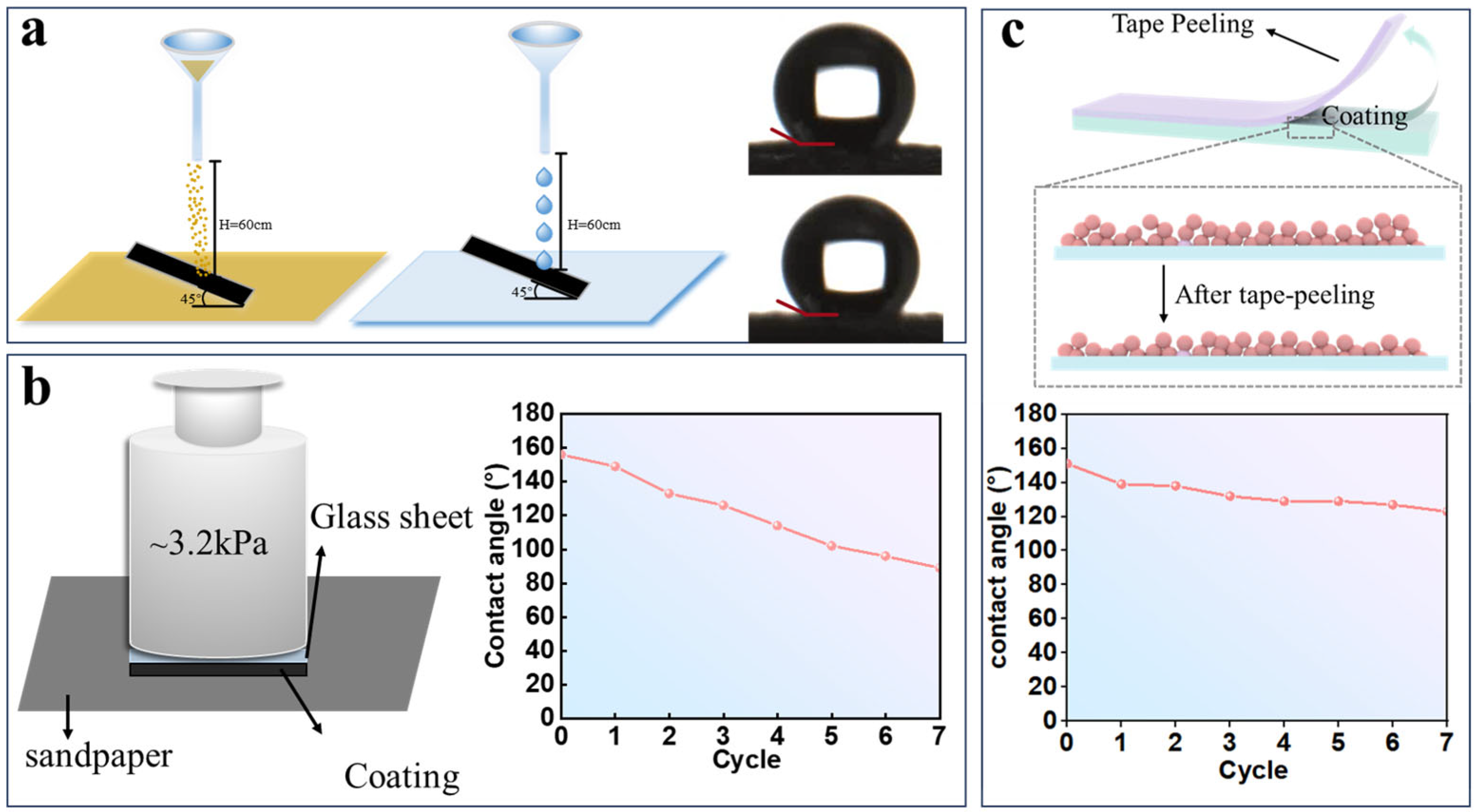
3.4. Stability and Feasibility Analysis of Superhydrophobic Coatings
3.5. The Photothermal Properties of Superhydrophobic Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Rasitha, T.P.; Krishna, N.G.; Anandkumar, B.; Vanithakumari, S.C.; Philip, J. A comprehensive review on anticorrosive/antifouling superhydrophobic coatings: Fabrication, assessment, applications, challenges and future perspectives. Adv. Colloid Interface Sci. 2024, 324, 103090. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Xiong, Z.; He, Y.; Wang, X. Superhydrophobic lotus-leaf-like surface made from reduced graphene oxide through soft-lithographic duplication. RSC Adv. 2020, 10, 5478–5486. [Google Scholar] [CrossRef]
- Liu, P.; Gao, Y.; Wang, F.; Yang, J.; Yu, X.; Zhang, W.; Yang, L. Superhydrophobic and self-cleaning behavior of Portland cement with lotus-leaf-like microstructure. J. Clean. Prod. 2017, 156, 775–785. [Google Scholar] [CrossRef]
- Xiao, Y.; Qi, Y.; Shen, X.; Zhu, M.; Li, S. Preparation of a Lotus-Leaf-Like Coating with Robust Super-Hydrophobicity and UV-Resistant Ability. J. Inorg. Organomet. Polym. Mater. 2023, 33, 579–590. [Google Scholar] [CrossRef]
- Wang, W.; Feng, L.; Song, B.; Wang, L.; Shao, R.; Xia, Y.; Liu, D.; Li, T.; Liu, S.; Wang, L.; et al. Fabrication and application of superhydrophobic nonwovens: A review. Mater. Today Chem. 2022, 26, 101227. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Modin, E.B.; Sayfutdinova, A.R.; Emelyanenko, K.A.; Vasiliev, A.L.; Emelyanenko, A.M. Combination of Functional Nanoengineering and Nanosecond Laser Texturing for Design of Superhydrophobic Aluminum Alloy with Exceptional Mechanical and Chemical Properties. ACS Nano 2017, 11, 10113–10123. [Google Scholar] [CrossRef]
- Latthe, S.S.; Sutar, R.S.; Kodag, V.S.; Bhosale, A.K.; Kumar, A.M.; Sadasivuni, K.K.; Xing, R.; Liu, S. Self—Cleaning superhydrophobic coatings: Potential industrial applications. Prog. Org. Coat. 2019, 128, 52–58. [Google Scholar] [CrossRef]
- Xue, X.; Yang, Z.; Li, Y.; Sun, P.; Feng, Y.; He, Z.; Qu, T.; Dai, J.-G.; Zhang, T.; Qin, J.; et al. Superhydrophobic self-cleaning solar reflective orange-gray paint coating. Sol. Energy Mater. Sol. Cells 2018, 174, 292–299. [Google Scholar] [CrossRef]
- Wang, G.; Li, A.; Li, K.; Zhao, Y.; Ma, Y.; He, Q. A fluorine-free superhydrophobic silicone rubber surface has excellent self-cleaning and bouncing properties. J. Colloid Interface Sci. 2021, 588, 175–183. [Google Scholar] [CrossRef]
- Moghadam, S.G.; Parsimehr, H.; Ehsani, A. Multifunctional superhydrophobic surfaces. Adv. Colloid Interface Sci. 2021, 290, 102397. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Guo, Z. Biomimetic super durable and stable surfaces with superhydrophobicity. J. Mater. Chem. A 2018, 6, 16731–16768. [Google Scholar] [CrossRef]
- Jiao, X.; Li, M.; Yu, X.; Yang, S.; Zhang, Y. Mechanically robust superamphiphobic ceramic coatings with releasable nanoparticle-capsules. Chem. Eng. J. 2022, 446, 137336. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, Y.; Li, L.; Dai, C.; Xu, Z.; Dong, Y.; Zeng, H. Nanofluids with superhydrophobic nanoparticles for improved depressurization mechanism in low permeability reservoirs. Chem. Eng. J. 2024, 495, 153467. [Google Scholar] [CrossRef]
- Naderizadeh, S.; Athanassiou, A.; Bayer, I.S. Interfacing superhydrophobic silica nanoparticle films with graphene and thermoplastic polyurethane for wear/abrasion resistance. J. Colloid Interface Sci. 2018, 519, 285–295. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Zheng, Q. Dopamine/Silica Nanoparticle Assembled, Microscale Porous Structure for Versatile Superamphiphobic Coating. ACS Nano 2016, 10, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.-X.; Luo, L.-Y.; Li, M.-T.; Xu, X.-K.; Ren, J.-R. Robust high-surface-insulating and superhydrophobic materials by constructing nanoparticles decorated porous structures. Compos. Sci. Technol. 2025, 261, 111033. [Google Scholar] [CrossRef]
- Selim, M.S.; Fatthallah, N.A.; Higazy, S.A.; Wu, Z.; Hao, Z. Superhydrophobic silicone/graphene oxide-silver-titania nanocomposites as eco-friendly and durable maritime antifouling coatings. Ceram. Int. 2024, 50, 452–463. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Chaushev, T.A.; Mohammadi, R.; Vladkova, T.G. Silver-doped superhydrophobic carbon soot coatings with enhanced wear resistance and anti-microbial performance. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123880. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, H.; Peng, J.; Ni, X.; Pei, L.; Ye, P.; Lu, R.; Yuan, S.; Bai, Z.; Zhu, Y.; et al. A multifunctional superhydrophobic coating with efficient anti-adhesion and synergistic antibacterial properties. Prog. Org. Coat. 2024, 186, 108028. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; McHale, G.; Atherton, S.; Newton, M.I. An introduction to superhydrophobicity. Adv. Colloid Interface Sci. 2010, 161, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sun, F.; Qian, H.; Wang, H.; Mu, L.; Zhu, J. A biomimetic spherical cactus superhydrophobic coating with durable and multiple anti-corrosion effects. Chem. Eng. J. 2018, 338, 670–679. [Google Scholar] [CrossRef]
- Gong, L.; Yang, W.; Sun, Y.; Zhou, C.; Wu, F.; Zeng, H. Fabricating Tunable Superhydrophobic Surfaces Enabled by Surface-Initiated Emulsion Polymerization in Water. Adv. Funct. Mater. 2023, 33, 2214747. [Google Scholar] [CrossRef]
- Zhang, R.-G.; Feng, R.; Wang, F.; Li, H.-L.; Sun, R.-Y.; Gao, H.-H.; Li, C.-B.; Wang, Y.-Z.; Song, F. A surfactant-mediated dynamic protection strategy for 100% waterborne fabrication of durable superhydrophobic coatings. Chem. Eng. J. 2024, 499, 156683. [Google Scholar] [CrossRef]
- Wang, P.; Sun, B.; Liang, Y.; Han, H.; Fan, X.; Wang, W.; Yang, Z. A stretchable and super-robust graphene superhydrophobic composite for electromechanical sensor application. J. Mater. Chem. A 2018, 6, 10404–10410. [Google Scholar] [CrossRef]
- Long, M.; Peng, S.; Deng, W.; Miao, X.; Wen, N.; Zhou, Q.; Yang, X.; Deng, W. A robust superhydrophobic PDMS@ZnSn(OH)6 coating with under-oil self-cleaning and flame retardancy. J. Mater. Chem. A 2017, 5, 22761–22771. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Liu, F.; Han, E.-H. Multi-stimuli-triggered and self-repairable fluorocarbon organic coatings with urea-formaldehyde microcapsules filled with fluorosilane. J. Mater. Sci. Technol. 2020, 45, 70–83. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, W.; Zheng, H.; Zhao, C.; Liu, D. Laser-Engineered Superhydrophobic Polydimethylsiloxane for Highly Efficient Water Manipulation. ACS Appl. Mater. Interfaces 2021, 13, 48163–48170. [Google Scholar] [CrossRef]
- Elzaabalawy, A.; Meguid, S.A. Development of novel superhydrophobic coatings using siloxane-modified epoxy nanocomposites. Chem. Eng. J. 2020, 398, 125403. [Google Scholar] [CrossRef]
- Arukalam, I.O.; Oguzie, E.E.; Li, Y. Nanostructured superhydrophobic polysiloxane coating for high barrier and anticorrosion applications in marine environment. J. Colloid Interface Sci. 2018, 512, 674–685. [Google Scholar] [CrossRef]
- Wang, H.; Geng, Y.; Xie, Z.; Li, M.; Deng, Q.; Tian, Y.; Chen, R.; Zhu, X.; Liao, Q. Carbon-based photothermal superhydrophobic materials with hierarchical structure enhances the anti-icing and photothermal deicing properties. ACS Appl. Mater. Interfaces 2021, 13, 48308–48321. [Google Scholar]
- Lin, Y.; Zhang, H.; Zou, Y.; Lu, K.; Li, L.; Wu, Y.; Cheng, J.; Zhang, Y.; Chen, H.; Yu, Q. Superhydrophobic photothermal coatings based on candle soot for prevention of biofilm formation. J. Mater. Sci. Technol. 2023, 132, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Guo, S. Sandwich-Structured Surface Coating of a Silver-Decorated Electrospun Thermoplastic Polyurethane Fibrous Film for Excellent Electromagnetic Interference Shielding with Low Reflectivity and Favorable Durability. ACS Appl. Mater. Interfaces 2022, 14, 40351–40360. [Google Scholar] [CrossRef]
- Peng, X.; Yuan, Z.; Zhao, H.; Wang, H.; Wang, X. Preparation and mechanism of hydrophobic modified diatomite coatings for oil-water separation. Sep. Purif. Technol. 2022, 288, 120708. [Google Scholar] [CrossRef]
- Li, A.; Li, G.; Xu, Y.; Jia, Y.; Liu, Y. Superhydrophobic surface with good anti-icing properties and high durability. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134539. [Google Scholar] [CrossRef]
- Sutar, R.S.; Kodag, S.G.; Ekunde, R.A.; Sawant, A.S.; Ekunde, T.A.; Nagappan, S.; Kim, Y.H.; Saji, V.S.; Liu, S.; Latthe, S.S. Durable self-cleaning superhydrophobic cotton fabrics for wearable textiles. Ind. Crops Prod. 2024, 222, 119717. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, Q.; Li, Y.; Yuan, Z.; He, W.; Zhang, L.; Cheng, Z. Durable superhydrophobic cotton fabrics based on fluorinated alternating copolymer and silica nanoparticle: Preparation and oil-water separation. Eur. Polym. J. 2024, 211, 113029. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I.; Gyoshev, S.D.; Lazarov, Y.; Chaushev, T.A.; Grakov, T. On the Development of Ultradurable Extremely Water-Repellent and Oleophobic Soot-Based Fabrics with Direct Relevance to Sperm Cryopreservation. ACS Appl. Bio Mater. 2022, 5, 3519–3529. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wu, M.; Xia, R.; Hu, J.; Huang, F. Construction of Silane-Modified Diatomite-Magnetic Nanocomposite Superhydrophobic Coatings Using Multi-Scale Composite Principle. Coatings 2025, 15, 786. https://doi.org/10.3390/coatings15070786
Li D, Wu M, Xia R, Hu J, Huang F. Construction of Silane-Modified Diatomite-Magnetic Nanocomposite Superhydrophobic Coatings Using Multi-Scale Composite Principle. Coatings. 2025; 15(7):786. https://doi.org/10.3390/coatings15070786
Chicago/Turabian StyleLi, Dan, Mei Wu, Rongjun Xia, Jiwen Hu, and Fangzhi Huang. 2025. "Construction of Silane-Modified Diatomite-Magnetic Nanocomposite Superhydrophobic Coatings Using Multi-Scale Composite Principle" Coatings 15, no. 7: 786. https://doi.org/10.3390/coatings15070786
APA StyleLi, D., Wu, M., Xia, R., Hu, J., & Huang, F. (2025). Construction of Silane-Modified Diatomite-Magnetic Nanocomposite Superhydrophobic Coatings Using Multi-Scale Composite Principle. Coatings, 15(7), 786. https://doi.org/10.3390/coatings15070786






