UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
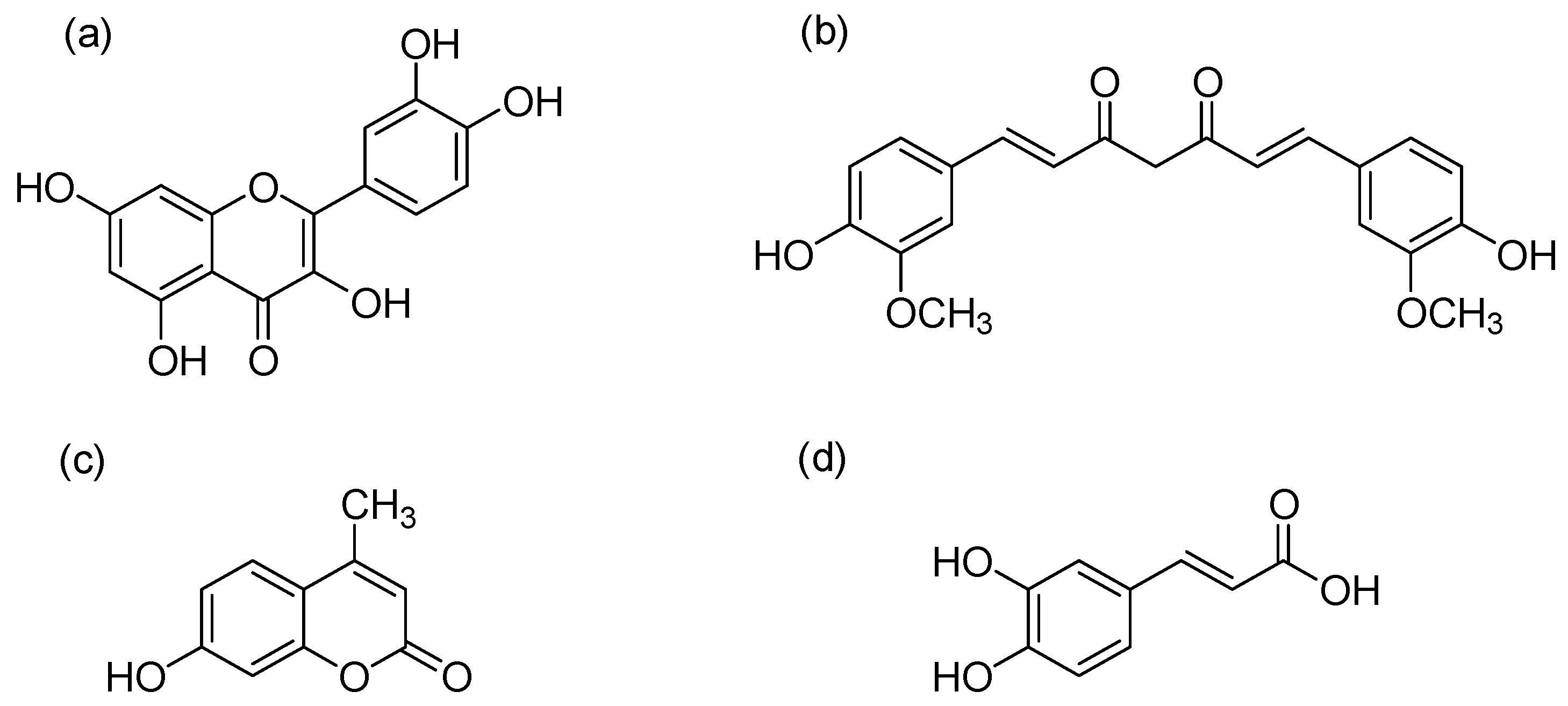
2.1. Raw Materials and Chemicals
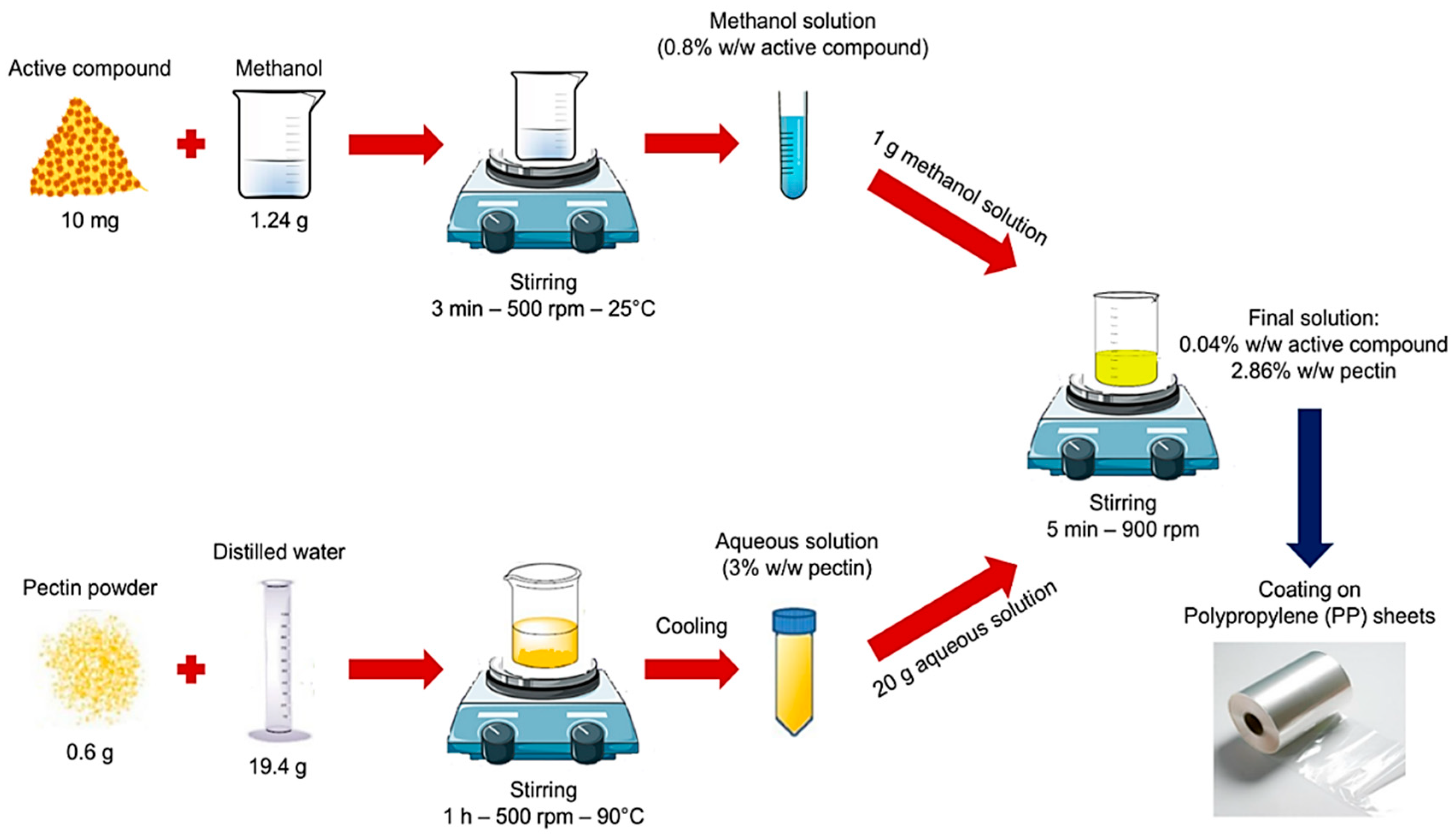
2.2. Preparation of Coating Solutions and Application to Plastic Films
2.3. Characterization of Films
2.4. Statistical Analysis
3. Results and Discussion
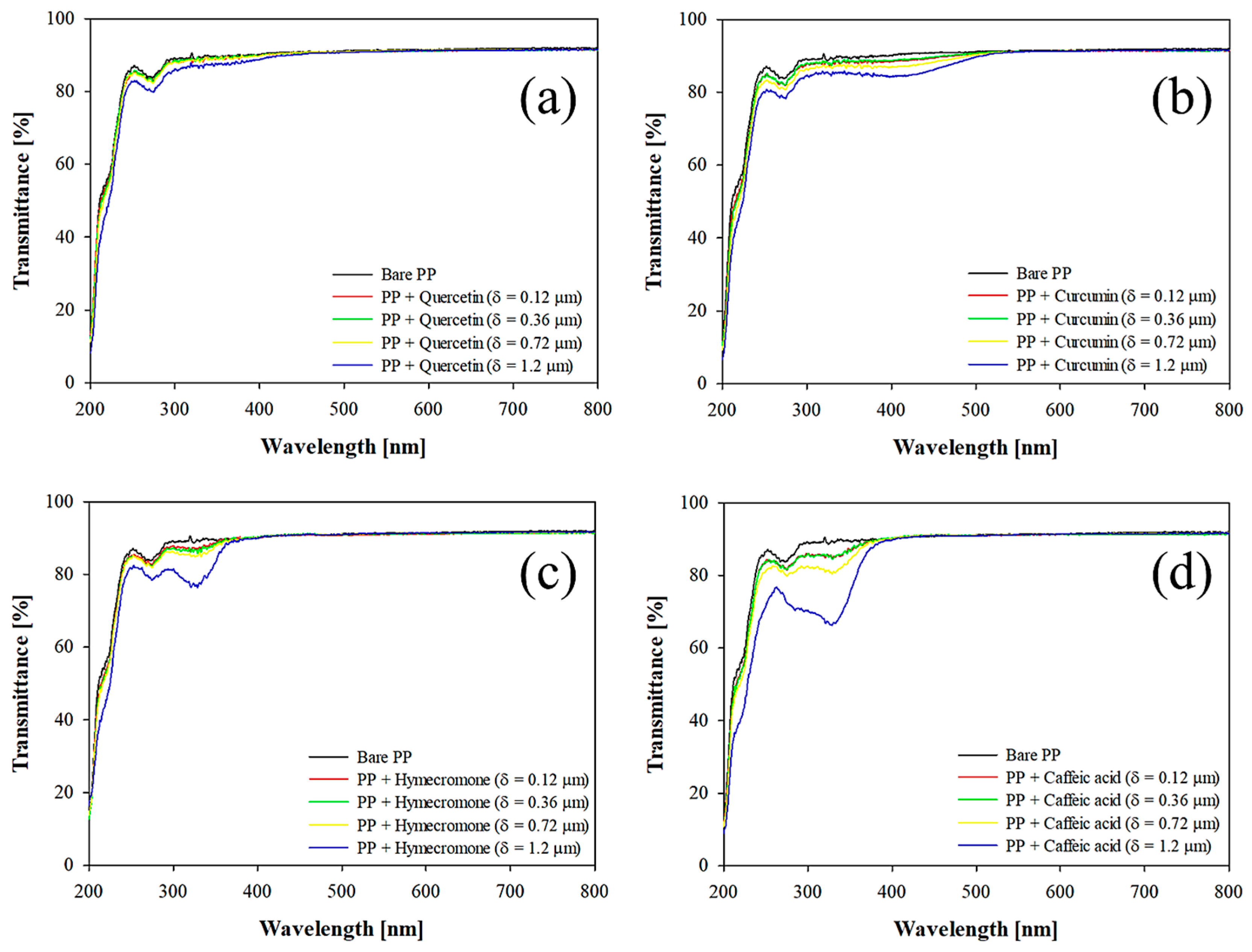
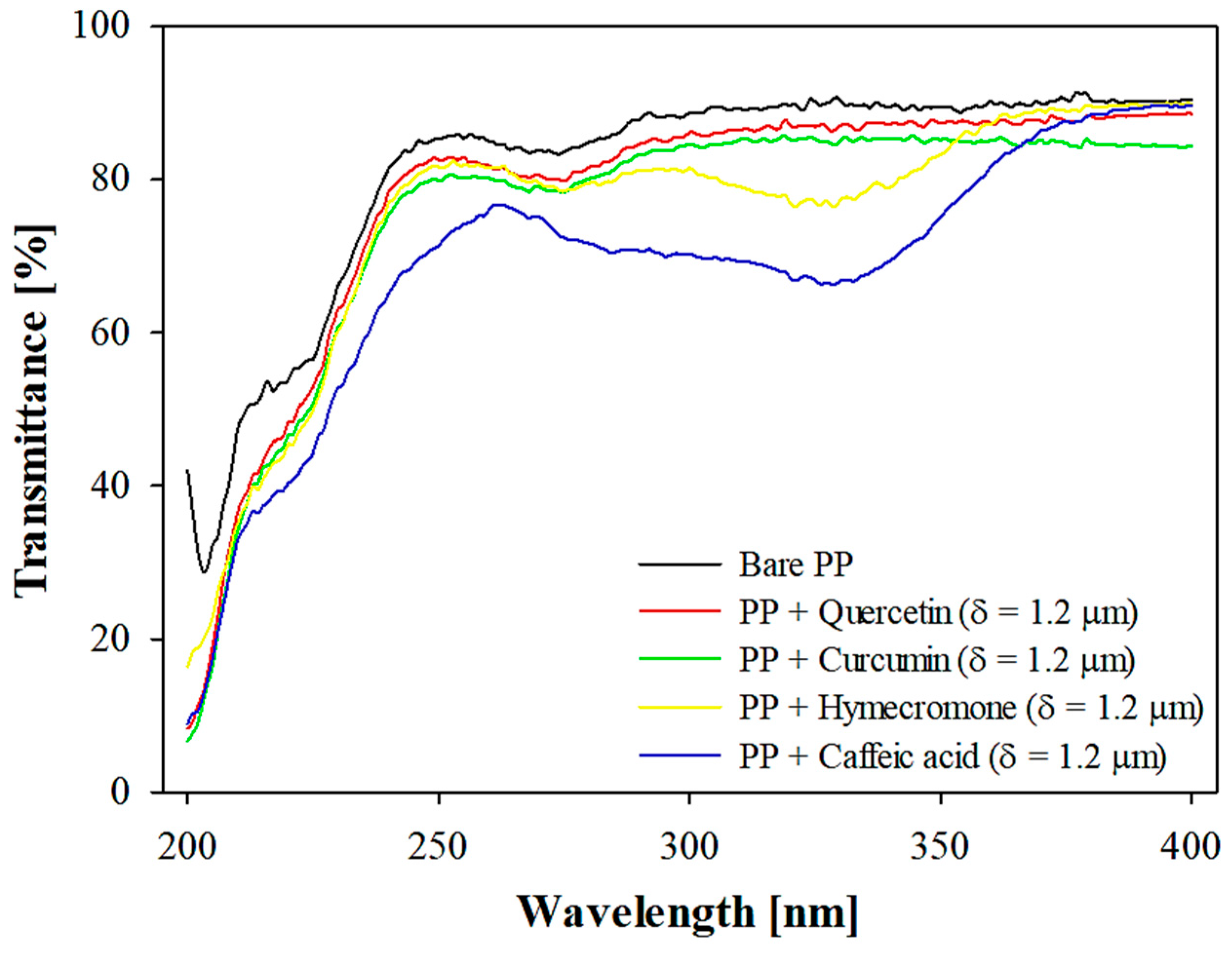
3.1. The Effect of Coating Thickness on the Optical Properties of the Plastic Substrate
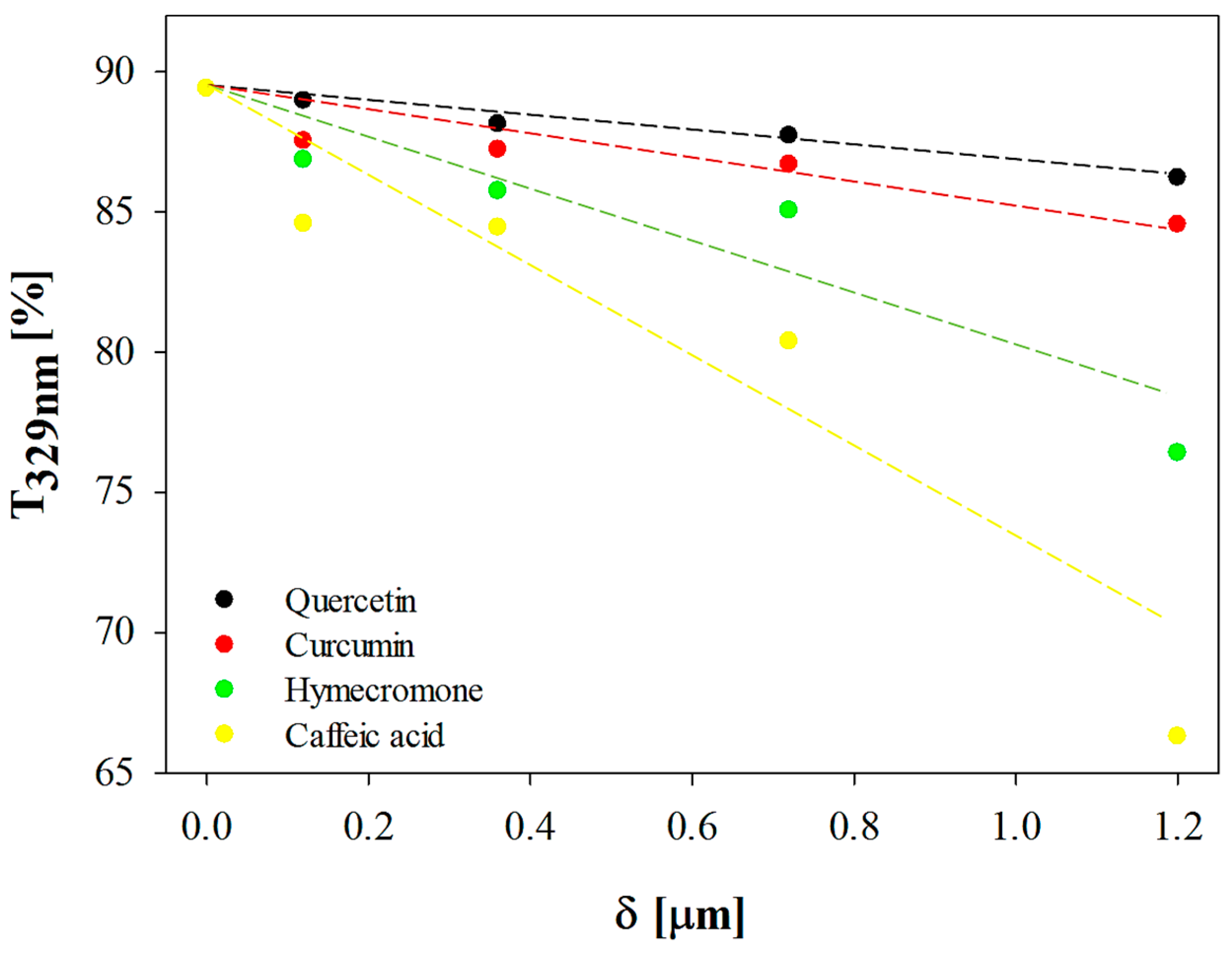
| Active Compound | DR [% μm−1] | R2 | δT329nm = 50% [μm] | CB Ratio [%] |
|---|---|---|---|---|
| Quercetin | 2.63 | 0.98 | 14.98 | 27.24 |
| Curcumin | 4.17 | 0.90 | 9.45 | 19.11 |
| Hymecromone | 9.67 | 0.90 | 4.07 | 9.23 |
| Caffeic acid | 18.71 | 0.92 | 2.10 | 4.98 |
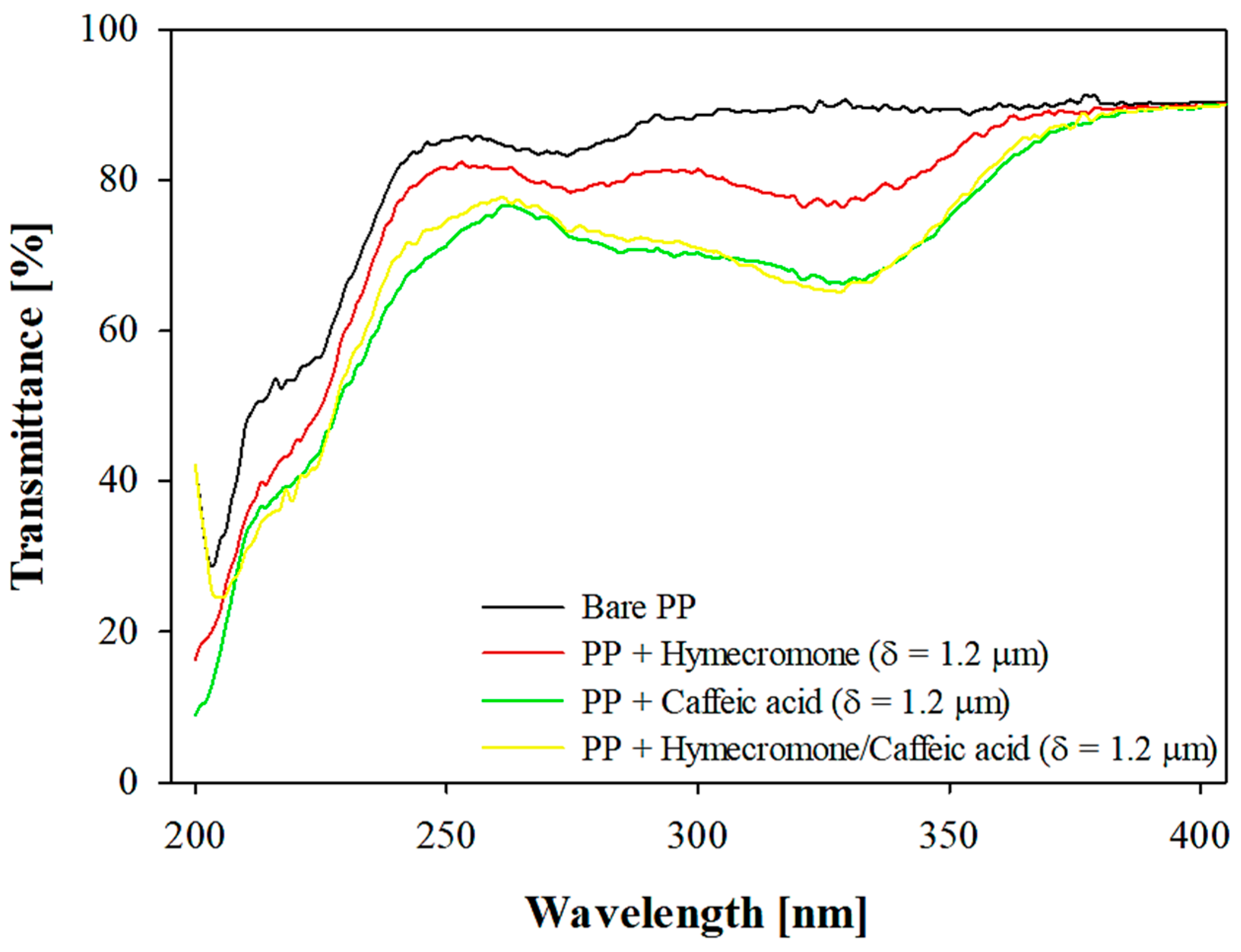
3.2. Pectin-Coated OPP Films vs. Commercial Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Versino, F.; Ortega, F.; Monroy, Y.; Rivero, S.; López, O.V.; García, M.A. Sustainable and bio-based food packaging: A review on past and current design innovations. Foods 2023, 12, 1057. [Google Scholar] [CrossRef]
- Sani, M.A.; Khezerlou, A.; Tavassoli, M.; Abedini, A.H.; McClements, D.J. Development of sustainable UV-screening food packaging materials: A review of recent advances. Trends Food Sci. Technol. 2024, 145, 104366. [Google Scholar] [CrossRef]
- Aydar, A.Y.; Ozbek, Z.A. Alterations in lipid attributes upon irradiation. In Non-thermal Processing of Major Food Macromolecules; Falsafi, S.R., Rostamabadi, H., Rastogi, N.K., Eds.; Academic Press: New York, NY, USA, 2025; pp. 347–360. [Google Scholar]
- Geng, L.; Liu, K.; Zhang, H. Lipid oxidation in foods and its implications on proteins. Front. Nutr. 2023, 10, 1192199. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Chandran, G.U.; Kumar, A.A.; Menon, S.K.; Sambhudevan, S.; Shankar, B. The potential role of flavonoids in cellulose-based biopolymeric food packaging materials for UV radiation protection. Cellulose 2024, 31, 4733–4773. [Google Scholar] [CrossRef]
- Galgano, F.; Caruso, M.C.; Ventura, N.M.; Magno, C.; Favati, F. Effects of anti-UV film and protective atmosphere on fresh-cut iceberg lettuce preservation. Acta Aliment. 2017, 46, 35–42. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer nanocomposites for sustainable UV protective packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Zayat, M.; Garcia-Parejo, P.; Levy, D. Preventing UV-light damage of light sensitive materials using a highly protective UV-absorbing coating. Chem. Soc. Rev. 2007, 36, 1270–1281. [Google Scholar] [CrossRef]
- Loste, J.; Lopez-Cuesta, J.M.; Billon, L.; Garay, H.; Save, M. Transparent polymer nanocomposites: An overview on their synthesis and advanced properties. Progr. Polym. Sci. 2019, 89, 133–158. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
- del Rosario Herrera-Rivera, M.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Perez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Lopusiewicz, L. Recent progress on UV-light barrier food packaging films–a systematic review. IFSET 2023, 103550. [Google Scholar] [CrossRef]
- Dai, K.; Cao, S.; Yuan, J.; Wang, Z.; Li, H.; Yuan, C.; Yan, X.; Xing, R. Recent Advances of Sustainable UV Shielding Materials: Mechanisms and Applications. ACS Appl. Mater. Interfaces 2025, 17, 30402–30422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Grabska-Zielińska, S.; Michalska-Sionkowska, M. The Application of Phenolic Acids in The Obtainment of Packaging Materials Based on Polymers—A Review. Foods 2023, 12, 1343. [Google Scholar] [CrossRef]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montava-Jordà, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the use of gallic acid as a potential natural antioxidant and ultraviolet light stabilizer in cast-extruded bio-based high-density polyethylene films. Polymers 2019, 12, 31. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly (lactide)/poly (butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Peng, Z.Y.; Peng, M.J.; Yan, W.B.; Ouyang, Y.Z.; Zhu, H.L. Flavonoids health benefits and their molecular mechanism. Mini Rev. Med. Chem. 2011, 11, 169–177. [Google Scholar] [CrossRef]
- Gao, W.; Mu, B.; Yang, F.; Li, Y.; Wang, A. Green preparation of licorice flavonoids/ZnO/attapulgite nanocomposites for multifunctional chitosan-based food packaging films. LWT 2024, 201, 116273. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Hassan, K.R.; Ahsa, H.M.; Hussain, M.; Wang, J.; Zou, X.-G.; Bu, T.; Rayan, A.M.; Yang, K. Development of chitosan-based edible film incorporated with purified flavonoids from Moringa oleifera: Structural, thermal, antibacterial activity and application. Food Chem. 2024, 457, 140059. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bai, J.; Jin, X.; Liu, H.; Wu, D.; Gan, R.; Gao, H. Innovative edible films for food preservation: Combining pectin and flavonoids from citrus peels with soy protein isolates. LWT 2024, 214, 117102. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefin. 2024, 14, 4419–4440. [Google Scholar] [CrossRef]
- Carullo, D.; Vergani, L.; Franzoni, G.; Mapelli, F.; Ferrante, A.; Borin, S.; Farris, S. Pectin-Based Films for Applications in the Horticultural Sector: A Preliminary Characterization. Chem. Eng. Trans. 2024, 110, 283–288. [Google Scholar]
- Farris, S.; Mora, L.; Capretti, G.; Piergiovanni, L. Charge Density Quantification of Polyelectrolyte Polysaccharides by Conductometric Titration: An Analytical Chemistry Experiment. J. Chem. Educ. 2012, 89, 121–124. [Google Scholar] [CrossRef]
- He, J.; Yang, S.; Goksen, G.; Cong, X.; Khan, M.R.; Zhang, W. Functionalized pectin/alginate food packaging films based on metal-phenol networks. Food Biosci. 2024, 58, 103635. [Google Scholar] [CrossRef]
- Bhatia, S.; Jawad, M.; Chinnam, S.; Al-Harrasi, A.; Shah, Y.A.; Khan, T.S.; Al-Azri, M.S.; Koca, E.; Aydemir, L.Y.; Diblan, S.; et al. Development and Characterization of Potato Starch–Pectin-Based Active Films Enriched With Juniper Berry Essential Oil for Food Packaging Applications. Food Sci. Nutr. 2025, 13, e4688. [Google Scholar] [CrossRef]
- De Brito Ribeiro, T.T.; Barbosa, A.M.; Nunes, T.P.; da Costa, A.S.G.; Oliveira, M.B.P.P.; Borges, G.R.; Padilha, F.F.; Dariva, C.; Santos, K.S. Development of Antifungal Packaging Based on Pectin/Gelatin Containing Azadirachta indica Bioactive Extracts for Carica papaya L. Fruit Coating. Appl. Sci. 2025, 15, 4423. [Google Scholar] [CrossRef]
- Jesus, G.A.M.; Castro, M.C.; Souza, P.R.; Monteiro, J.P.; Sabino, R.M.; Sablani, S.S.; Martins, A.F.; Bonafé, E.G. Antioxidant, UV-blocking, and biodegradable pectin films containing selenium nanoparticles for sustainable food packaging. Food Hydrocoll. 2025, 167, 111449. [Google Scholar] [CrossRef]
- Akoueson, F.; Paul-Pont, I.; Tallec, K.; Huvet, A.; Doyen, P.; Dehaut, A.; Duflos, G. Additives in polypropylene and polylactic acid food packaging: Chemical analysis and bioassays provide complementary tools for risk assessment. Sci. Total Environ. 2023, 857, 159318. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.M.; Khan, S.A.; Hossain, M.D. Research and application of polypropylene: A review. Discov. Nano 2024, 19, 1–21. [Google Scholar] [CrossRef]
- Ma, R.; Tang, P.; Feng, Y.; Li, D. UV absorber co-intercalated layered double hydroxides as efficient hybrid UV-shielding materials for polypropylene. Dalton Trans. 2019, 48, 2750–2759. [Google Scholar] [CrossRef]
- ASTM D823-18(2022); Standard Practices for Producing Films of Uniform Thickness of Paint, Coatings and Related Products on Test Panels. ASTM International: West Conshohocken, PA, USA, 2022.
- Ghaani, M.; Soltanzadeh, M.; Carullo, D.; Farris, S. Development of a Biopolymer-Based Anti-Fog Coating with Sealing Properties for Applications in the Food Packaging Sector. Polymers 2024, 16, 1745. [Google Scholar] [CrossRef]
- ASTM D1003-10; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Carullo, D.; Rovera, C.; Bellesia, T.; Büyüktaş, D.; Ghaani, M.; Santo, N.; Romano, D.; Farris, S. Acid-derived bacterial cellulose nanocrystals as organic filler for the generation of high-oxygen barrier bio-nanocomposite coatings. Sustain. Food Technol. 2023, 1, 941–950. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D3985-17; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM F1927-20; Standard Test Method for Determination of Oxygen Gas Transmission Rate, Permeability and Permeance at Controlled Relative Humidity Through Barrier Materials Using a Coulometric Detector. ASTM International: West Conshohocken, PA, USA, 2020.
- Aleixandre-Tudo, J.L.; Du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solis-Oviedo, R.L., De La Cruz Pech-Canul, A., Eds.; IntechOpen: London, UK, 2019; Chapter 3. [Google Scholar]
- Farris, S.; Introzzi, L.; Piergiovanni, L. Evaluation of a bio-coating as a solution to improve barrier, friction and optical properties of plastic films. Packag. Tech. Sci. 2009, 22, 69–83. [Google Scholar] [CrossRef]
- Verduin, J.; den Uijl, M.J.; Peters, R.J.B.; van Bommel, M.R. Photodegradation Products and their Analysis in Food. J. Food Sci. Nutr. 2020, 6, 067. [Google Scholar] [CrossRef]
- Regulation (EU) 2025/40 of the European Parliament and of the Council on Packaging and Packaging Waste. Available online: https://eur-lex.europa.eu/eli/reg/2025/40/oj/eng (accessed on 10 April 2025).
- Cazón, P.; Vázquez, M.; Velazquez, G. Cellulose-glycerol-polyvinyl alcohol composite films for food packaging: Evaluation of water adsorption, mechanical properties, light-barrier properties and transparency. Carbohydr. Polym. 2018, 195, 432–443. [Google Scholar] [CrossRef]
- Cozzolino, C.A.; Castelli, G.; Trabattoni, S.; Farris, S. Influence of colloidal silica nanoparticles on pullulan-coated BOPP film. Food Packag. Shelf Life 2016, 8, 50–55. [Google Scholar] [CrossRef]
- Carullo, D.; Casson, A.; Rovera, C.; Ghaani, M.; Bellesia, T.; Guidetti, R.; Farris, S. Testing a coated PE-based mono-material for food packaging applications: An in-depth performance comparison with conventional multi-layer configurations. Food Packag. Shelf Life 2023, 39, 101143. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, S.M.; Hong, S.I. Characterization of protein-coated polypropylene films as a novel composite structure for active food packaging application. J. Food Eng. 2008, 86, 484–493. [Google Scholar] [CrossRef]
- Kaloper, S.; Plohl, O.; Mozina, S.S.; Vesel, A.; Simat, V.; Zemljic, L.F. Exploring chitosan-plant extract bilayer coatings: Advancements in active food packaging via polypropylene modification. Int. J. Biol. Macromol. 2024, 270, 132308. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yang, X.; Sun, J.; Gao, H.; Bai, Y.; Shao, L. Polyacrylate Decorating Poly(ethylene terephthalate) (PET) Film Surface for Boosting Oxygen Barrier Property. Coatings 2021, 11, 1451. [Google Scholar] [CrossRef]
- Sharbafian, F.; Tosic, K.; Schmiedt, R.; Novak, M.; Krainz, M.; Rainer, B.; Apprich, S. Investigation and Comparison of Alternative Oxygen Barrier Coatings for Flexible PP Films as Food Packaging Material. Coatings 2024, 14, 1086. [Google Scholar] [CrossRef]

| Bar No° | Wire Diameter [mm] | Wet Coating Thickness [μm] | Nominal Coating Thickness [μm] | Actual Coating Thickness [μm] | Active Compound Content [mg/m2] * |
|---|---|---|---|---|---|
| 1 | 0.05 | 4 | 0.12 | 0.11 ± 0.01a | 0.048 |
| 2 | 0.15 | 12 | 0.36 | 0.35 ± 0.04b | 0.144 |
| 3 | 0.30 | 24 | 0.72 | 0.70 ± 0.06c | 0.288 |
| 4 | 0.51 | 40 | 1.2 | 1.1 ± 0.09d | 0.48 |
| Material | Haze [%] |
|---|---|
| Bare PP | 3.9 ± 0.1ab |
| PP + Quercetin | 4.3 ± 0.1b |
| PP + Curcumin | 3.8 ± 0.2a |
| PP + Hymecromone | 3.6 ± 0.1a |
| PP + Caffeic acid | 5.4 ± 0.1b |
| Material | Mechanical Properties | Oxygen Transmission Rate (OTR) | |||
|---|---|---|---|---|---|
| E [GPa] | εB [%] | TS [MPa] | (0% RH) [cm3 m−2 day−1] | (80% RH) [cm3 m−2 day−1] | |
| Bare PP | 2.1 ± 0.2a | 100 ± 24a | 104 ± 16a | 1026.25 ± 104.31b | 1040.42 ± 82.37b |
| PP + Quercetin | 2.3 ± 0.3a | 85 ± 17a | 110 ± 20a | 0.52 ± 0.06a | 316.61 ± 20.16a |
| PP + Curcumin | 2.1 ± 0.2a | 82 ± 25a | 115 ± 15a | 0.64 ± 0.07a | 325.22 ± 15.84a |
| PP + Hymecromone | 2.1 ± 0.1a | 78 ± 32a | 108 ± 12a | 0.55 ± 0.08a | 330.35 ± 23.72a |
| PP + Caffeic acid | 2.2 ± 0.2a | 82 ± 27a | 112 ± 8a | 0.58 ± 0.07a | 318.19 ± 19.61a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gennaro, M.; Büyüktaş, D.; Carullo, D.; Pinto, A.; Dallavalle, S.; Farris, S. UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications. Coatings 2025, 15, 741. https://doi.org/10.3390/coatings15070741
Gennaro M, Büyüktaş D, Carullo D, Pinto A, Dallavalle S, Farris S. UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications. Coatings. 2025; 15(7):741. https://doi.org/10.3390/coatings15070741
Chicago/Turabian StyleGennaro, Matteo, Duygu Büyüktaş, Daniele Carullo, Andrea Pinto, Sabrina Dallavalle, and Stefano Farris. 2025. "UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications" Coatings 15, no. 7: 741. https://doi.org/10.3390/coatings15070741
APA StyleGennaro, M., Büyüktaş, D., Carullo, D., Pinto, A., Dallavalle, S., & Farris, S. (2025). UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications. Coatings, 15(7), 741. https://doi.org/10.3390/coatings15070741










