Abstract
In this study, chitooligosaccharides (COS) were introduced into black soybean protein (BSP) using transglutaminase (TG) as a biocatalyst. The film-processing properties and physiological activities of the enzymatically glycosylated black soybean protein (EGBSP) were studied. The results showed that glycosylation decreased the surface hydrophobicity, absolute value of the zeta potential, its minimum solubility, and film permeability of BSP by 69.86%, 6.04%, 36.68%, and 14.91%, respectively, while increasing the tensile strength and elongation at break of its protein film by 56.57% and 172.68%, respectively. The gel time was shortened, and the acid-induced gel properties of EGBSP were similar to those of BSP. The anticancer effect of EGBSP was evaluated by the tumor inhibition rate, flow cytometry, and morphology observation of an ascites tumor in H22 tumor-bearing mice. The immune organs (spleen, thymus), immune cells (lymphocytes, NK cells), and immune factors (IL-2, IL-12) of H22 tumor-bearing mice were detected to evaluate the immunomodulatory effects of EGBSP. The results showed that medium and high doses of BSP had positive effects on immune enhancement and anti-cancer activity of H22 tumor-bearing mice, while almost all doses of EGBSP showed significant effects. These results indicated that glycosylation significantly improved the anti-cancer effect and immunomodulatory activity of H22 tumor-bearing mice while prolonging their overall survival. In conclusion, the glycosylation method using microbial transglutaminase to catalyze the introduction of chitooligosaccharides into black bean protein can improve the film-processing properties and biological activities of BSP more effectively than the enzyme crosslinking method.
1. Introduction
Black soybean (Glycine max) is a kind of soybean (whose production in China was 20.65 million tons in 2024) with black seeds and is widely planted in China, especially in the northeastern area [1]. Black soybeans have a higher content of proteins (crude protein contents 92.01%) and amino acids compared to common soybeans [2]. Therefore, the spherical structure of black soybean protein (BSP), which is marked by restricted molecular flexibility, somewhat limits the properties that are required in forming functional films. Glycosylation is a protein modification method that has attracted the attention of researchers in recent years, among which the Maillard reaction is the most well-known. However, the efficiency of the Maillard reaction is non-ideal, with very strict reaction conditions [3]. It has been reported that some of its products may cause oxidative stress, toxic neurons, and inflammation and promote connective tissue aging [4]. On the other side, enzymatic glycosylation has been found to potentially have higher reaction specificity and poses a lower risk of generating these toxic products [5,6].
Transglutaminase (TG), whose structure is shown in Figure 1, is commonly used to induce protein crosslinking in the food industry. Transglutaminase is an acyl transferase that catalyzes the transamide reaction. It uses the γ-amide group of the glutamine residue in the protein polypeptide chain as the acyl donor and the ε-amino group of lysine as the acyl acceptor. Catalyzation of the formation of ε-(γ-glutamyl) lysine isopeptide bonds are intramolecular and/or intermolecular, causing the crosslinking of protein molecules and thereby improving protein solubility, foaming, emulsification, rheology, and so on. Among them, lysine can be replaced by several compounds containing primary amine groups to produce a variety of protein-(γ-glutamyl) derivatives, and some restrictive amino acids can be introduced into the protein to improve its nutritional value. Or, it can be imported into the physiological activity of small-molecule amine-modified peptides or proteins. In summary, transglutaminase can catalyze two nucleophilic substitution reactions on protein substrates, as illustrated in Figure 2. Reaction A produces a γ-glutamyl-ε-lysine side-chain peptide, which leads to protein crosslinking. In Reaction B, a small-molecule amine is introduced onto the protein substrate, and there is competition between these two reactions [7].
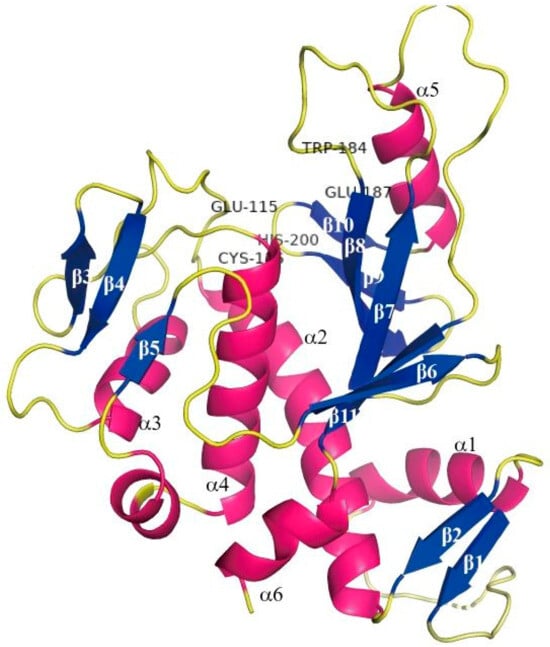
Figure 1.
Overall structure of microbial TGase (MTG): schematic ribbon drawing of the MTG molecule viewed from above the plate face. The secondary structure is numbered.
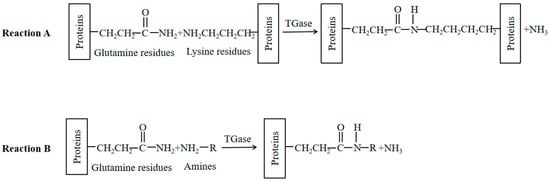
Figure 2.
Two typical reactions catalyzed by transglutaminase.
In our previous studies, BSP was employed as an acyl donor, TG as a biocatalyst, and chitooligosaccharides (COS) as an acyl receptor. It has been confirmed that TG could catalyze the reaction of glutamine residues in BSP with the primary amines offered by COS [8,9].
In this research, the film-processing properties and physiological activities of the glycosylation product were systematically studied in order to improve the functional properties of the protein modification.
2. Materials and Sample Preparation
Black soybean meal was kindly provided by Baishibei Green Food Co., Ltd. (Jilin, China). COS (M = 2000 Da, degree of deacetylation 75%) was purchased from Golden Shell Pharmaceutical Co., Ltd. (Yuhuan, China). And TG was purchased from Yiming Fine Chemical Industry Co., Ltd. (Taixing, China).
NaH2PO4, Na2HPO4 (Taiyang Chemistry Co., Ltd., Taixing, China); NaCl (Haiwang Chemical Industry Co., Ltd., Weifang, China); NaOH (Yongrun Chemical Industry Co., Ltd., Changzhou, China); HCl (Zhongyuan Chemical Industry Co., Ltd., Nanyang, China); acetic acid (No.3 Chemical reagent factory, Tianjin, China); methanol (Tian Da reagent factory, Tianjin, China); 8-Anilino-1-naphthalenesulfonic Acid(Ans-) (Sigma-Aldrich Co., Louis, MO, USA); glucono-delta-lactone (GDL) (Nanjing Chemical reagent Co., Ltd., Nanjing, China); Acridine orange (AO:Beyotime biotechnology Co., Ltd., Shanghai, China); ethidium bromide (EB:Beyotime biotechnology Co., Ltd., Shanghai, China); IL-2, IL-12 ELISA Kit for Detecting (Sigma-Aldrich Co., Louis, MO, USA); citric acid (Gibco Life Technologies, Grand Island, NY, USA); DMSO (Gibco Life Technologies, USA); RPMI 1640 culture medium (Gibco Life Technologies, USA); pancreatin (Gibco Life Technologies, USA); ConA (Gibco Life Technologies, USA); fetal bovine serum:Sigma-Aldrich Co., USA); MTT (Sigma-Aldrich Co., Louis, MO, USA); medical ethanol (Beyotime biotechnology Co., Ltd., Shanghai, China); PBS (Beyotime biotechnology Co., Ltd., Shanghai, China); diethyl ether (Beyotime biotechnology Co., Ltd., Shanghai, China); ACK cell lysate (Beyotime biotechnology Co., Ltd., Shanghai, China); trypan blue (Gibco Life Technologies, Grand Island, NY, USA).
All reagents without special announcement are analytical pure.
2.1. Preparation of Black Soybean Protein Isolated (BSP)
The BSP sample was prepared according to Supplementary File Figure S1. One hundred grams of black soybean meal was ground to 40–60 mesh, stirred for 120 min with 1.2 L of water at 30–60 °C, and centrifuged at 1710× g (adjusted to pH 8.0 by 1.0 mol/L of sodium hydroxide) [10,11]. The extract was centrifuged at 1710× g for 10 min. The supernatant was collected, and the pH was adjusted to 4.4–4.6 using 1.0 M HCl (stirred until the isoelectric point). The supernatant was left undisturbed for 30 min, and the precipitate was collected and washed twice with dilute water. The protein was homogenized, and the pH was adjusted to 6.5–7.0 using 5% sodium hydroxide, followed by freeze-drying to obtain the BSP samples.
2.2. Preparation of Enzymatically Glycosylated Black Soybean Protein (EGBSP)
The COS solution was added with glucosamine to the BSP solution (60 g·L−1) until the COS reached 4 mmol·g·L−1 (as each gram of BSP contains about 1 mmol of amide groups, while each gram of COS could supply about 4.19 mmol primary amine). TG solution was added (addition: 10 U·g−1 BSP) to the mixture until the BSP concentration reached 40 g·L−1 in the reaction system [12]. The molar ratio of the acyl donors supplied by BSP and acyl acceptors supplied by COS should be about 1:3. After the reaction system is fully mixed, it was kept in a thermostatically controlled water bath shaker at 37 °C for 3 h, and then, the samples were water-bathed at 85 °C for enzyme deactivation. After cooling, the pH of the samples was adjusted to 4.5 and then centrifuged at 3040× g for 10 min [13]. The glycosylation was 16.46 g/kg.
2.3. Preparation of Enzymatic Crosslinked Black Soybean Protein Isolated (ECBSP)
TG solution was added (addition: 10 U·g−1 BSP) until the BSP concentration came to 40 g·L−1. The reaction system was kept in a thermostatically controlled water bath shaker at 37 °C for 3 h, and then the samples were water-bathed at 85 °C for enzyme deactivation. After cooling, the pH of the samples was adjusted to 4.5 and centrifuged at 4000 r·m−1 for 10 min. The precipitate was collected and washed twice with diluted water. The unbound COS was removed (tested by HPLC) by dialysis at 4 °C and then freeze-dried to preserve it. The same amount of TG was used in order to conduct the comparison.
FT-IR spectra of EGBSP and ECBSP are shown in Figure S2 in the Supplementary File.
2.4. Experimental Animals and Tumor Strain
The eighty SPF Kunming mice (certification number SCXK-2011-0004, Yisi Experimental Animal Technology Co., Ltd., Changchun, China), with half being male and half being female, weighed 20 ± 2 g and were of the Mice H22 ascitic tumor strain (Chinese Academy of Medical Sciences).
2.5. Instruments
Thermostatic Bath (Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China); analytical balance (Mettler Toledo, Zurich, Switzerland); electron balance (Shanghai Renhe Scientific Instrument Co., Ltd., Shanghai, China); H-1650 centrifuge (Xiangyi Instrument Co., Ltd., Xiangtan, China); Nano-ZS90 (Malvern Instruments, Westborough, MA, USA); TA.XT.PLUS texture analysis (Stable Micro Systems, London, UK); Discovery HR-1 rotary rheometer (TA Instruments Inc., New Castle, DE, USA); PHS-3C Rex laboratory pH meter (Precision Scientific Instrument Co., Ltd., Shanghai, China); CO2 incubator (New Brunswick Scient, Edison, NJ, USA); super-clean worktable (Donglian Electronic & Technology Development Co., Ltd., Harbin, China), CX41-FS inverted microscope (Olympus Corporation, Tokyo, Japan); enzyme mark instrument (Bio-Rad Laboratories, Hercules, CA, USA); micro- Oscillator (Jiangsu Tianli Medical Instrument Co., Ltd., Taizhou, China); transferpettor (Gilson, Inc., Middleton, WI, USA); stainless steel cell sieve (Nanjing Jiancheng Bioengineering Institute, Nanjing, China); cell culture plate (Gibco Life Technologies, Grand Island, NY, USA); cell culture flask (Gibco Life Technologies, Grand Island, NY, USA); BSC-130011AZ biosafety cabinet (Suzhou Antai Airtech Co., Ltd., Suzhou, China); FACS Calibur (BD Biosciences, Franklin Lake, NJ, USA).
3. Experimental Methods
3.1. Effect of Enzymatic Glycosylation on Film Processing Functions of BSP
The ECBSP was used as the control group in studies on processing properties for EGBSP.
3.1.1. Determination of Zeta Potential of BSP and Its Modified Products
The sample was added to 0.2% phosphate buffer (10 mmol/L, pH 7.0), and 1 mL of the sample was taken at 25 °C to determine the zeta potential.
3.1.2. Surface Hydrophobicity Analysis of BSP and Its Modified Products
The surface hydrophobicity was determined by an ANS probe [14]. The sample solution was prepared with 0.01 mol·L−1 phosphate buffer (pH 7.0) in the range of 0.05–0.5 g·L−1 (protein-based). Twenty μL of ANS solution were added to 4 mL of sample (8.0 mmol·L−1 in the same buffer). The fluorescence intensity of the sample was measured at an excitation wavelength of 390 nm (excitation) and an emission wavelength of 470 nm (excitation). The fluorescence intensity and the linear slope of the protein content map were employed to evaluate the surface hydrophobicity of the sample.
3.1.3. Determination of Solubility of BSP and Its Modified Products
A certain amount of sample was added to a buffer solution with a pH value of 2–11, mixed using a vortex, stored at 4 °C for 12 h, and centrifuged at 8500 r·min−1 for 20 min to obtain the supernatant. The absorption value of the sample at 280 nm was measured by an ultraviolet spectrophotometer. The preparation of the buffer solutions was as follows: 0.05 mol·L−1 citric acid buffer (pH 2–3); 0.05 mol·L−1 acetate buffer (pH 4–5); 0.05 mol·L−1 phosphate buffer (pH 6–8); and 0.05 mol·L−1 carbonate buffer (pH 9–11).
3.1.4. Rheology of Acid-Induced Gel Formation of BSP and Its Modified Products
A sample protein solution of 6% (w/v) (pH = 7.0) was prepared. Gluconolactone (GDL) was added at 0.1 g·mL−1 1BSP and stirred at room temperature for 2 min. The dynamic rheological properties of protein acid-induced gel formation were observed using a rotating rheometer with a 60 mm diameter parallel plate. The linear viscoelastic region is determined by a low amplitude oscillation test, and the parameters are as follows. The gap was 1 mm; the strain value was 0.2%. The mode was time sweep. The change in elastic modulus (G′) of the protein sample over 0–4.5 h within the linear viscoelastic region was monitored. The gel time was defined as the time corresponding to G′ ≥ 1 Pa.
3.1.5. Film Preparation of BSP and Its Modified Products
The 30.% w/w sample was mixed with glycine for 15 min and then stirred continuously in PBS (5 g/100 mL with pH of 10.0) at 70 °C for 30 min to obtain the film-forming liquid. The liquid was de-foamed using ultrasound (160 W, 40 kHz) for 15 min and then poured into neutral-coated petri dishes. The expected film thickness was 1.50 mm, and the amount of film-forming liquid was calculated so that the solid content of film-forming liquid was the same (about 35 mL/150 cm2, about 30% glycerol). The samples were dried in an air-circulating oven at 25 °C until a constant moisture content was achieved (approximately 24 h) and then separated from the petri dish. Then, the films were stored at 25 ± 1 °C and 50 ± 1% for 48 h [15].
3.1.6. Mechanical Properties of Films Made of BSP and Its Modified Products
The mechanical properties of the protein film are mainly characterized by the tensile strength (TS) and elongation (E) of the sample protein film. For the sample-thickness measurement, the thicknesses of five positions on the protein film of the sample were randomly measured with a micrometer, and the average value was considered as the film thickness. Before measuring the mechanical properties of the sample protein film with a physical property tester, it is necessary to cut the film to a size of 2 cm × 10 cm. The initial distance is set at 50 mm, and the drawing rate is 1 mm/s. TS and E can be calculated by Formulas (1) and (2) [16]:
where N refers to the maximum load force (N) of the sample film at the tensile fracture, and S refers to the initial cross-sectional area (m2). ΔL refers to the increase in the length of the sample film at the tensile fracture (mm), and L0 refers to the initial length (mm).
3.1.7. Permeability of Films Made of BSP and Its Modified Products
The water vapor permeability (WVP) of the protein sample film was determined by the ASTME96-95 gravimetric method [17]. The mouth of the P2O5 (0%RH) bottle was sealed with a sample film, and the vial was placed in a drying chamber filled with a saturated NaCl solution (75% RH). The weight of the vial was measured until it reached equilibrium. The WVP of the film is calculated according to Formula (3):
where Δm is the increased weight of the vial after Δt (s) time, x is the film thickness, A is the film area (1.49 × 10−4 m2), and Δp (Pa) is the pressure difference of water vapor on both sides of the sample film.
3.2. Effect of Enzymatic Glycosylation on Biological Activities of BSP
Since the ECBSP did not show significant physiological activities in our previous studies, it was not used as the control group in the following part.
3.2.1. The Grouping and Administration of Mice
A mouse solid tumor model was established with H22 ascites tumor cells with a density of 1 × 107/mL. Each mouse was disinfected with alcohol at the right subaxillary, and hypodermic inoculation with 0.2 mL was conducted.
The mice were randomly divided into seven groups 24 h after inoculation, with each group containing 10 mice. The scheme was as follows: the control group used an equal volume of physiological saline, the low-dose group with BSP-100 mg/(kg·d), middle-dose group with BSP-200 mg/(kg·d), high-dose group with BSP-400 mg/(kg·d), low-dose group with EGBSP-100 mg/(kg·d), middle-dose group with EGBSP-200 mg/(kg·d), and high-dose group with EGBSP-400 mg/(kg·d). The mice were given injections for 10 days from the first day after vaccination.
After these treatments, the mice were weighed, and cervical dislocation was carried out. The survival statuses of the H22 tumor-bearing mice were under daily observation after inoculation, and the deaths were recorded. The tumor tissues were carefully weighed [18]. The tumor inhibitory rate was determined by Equation (4).
TIR represents the tumor inhibitor rate;
TWC represents the tumor weight of the control group;
TWA represents the tumor weight of the administration group.
3.2.2. Morphological Observation of Mice Ascites Tumor
The H22 ascitic tumor cells, along with the cells that had undergone intraperitoneal passage, were collected, and their density was adjusted to 1 × 107 cells/mL, as determined by hemocytometer count. The peritoneal skin of each mouse was disinfected with alcohol, followed by a subcutaneous injection of 0.2 mL. Treatment proceeded for 5 days, based on the solid tumor model groups and administration methods, starting 3 days post-inoculation. Ascitic fluid from the mice was extracted to adjust the cell density to 1 × 106 cells/mL. Samples from the treatment group were diluted at the same ratio. An AO-EB fluorescent staining solution was prepared by mixing equal volumes of AO (250 μg/mL) and EB (250 μg/mL). The cell suspension samples were then mixed evenly with the fluorescent staining solution in a 1:5 ratio and immediately examined under a fluorescence microscope.
3.2.3. Flow Cytometry of Apoptosis of H22 Hepatocellular Carcinoma Cells in Tumor-Bearing Mice
For the complete medium preparation, 89% DMEM medium +10% fetal bovine serum +1% Penicillin–Streptomycin Solution was combined. The H22 mice’s peritoneal tumor cells were taken under an aseptic environment, and collagenase was used to prepare the single-cell suspension. Cultivation in vitro was simulated according to the physiological conditions in vivo.
The cells were collected and prepared as a single-cell suspension. The cells were washed with PBS twice and centrifuged for 5 min at 2000 r/min to collect 1–5 × 105 cells; 500 μL PBS were added to the suspension of the cells. Five μL Annexin V-FITC and 5 μL propidium iodide (PI) solution were added. The suspension was mixed thoroughly and incubated in the dark at room temperature for 5–15 min. The flow cytometry test should be carried out within 1 h.
3.2.4. Research on Lymphocyte Proliferation Activity
Mouse spleens were prepared under aseptic conditions and immersed in serum-free I 1640 medium on ice. A single-cell suspension was prepared by gently grinding through a 200 mesh cytoscreener and washing twice with RPMI 1640 medium [19]. After adding 3 mL pre-heated ACK lysate, the spleen cell precipitation was kept away from light at room temperature for 3 min and then centrifuged at 100× g for 3 min. The precipitation was washed twice with an RPMI 1640 medium (centrifugation at 90× g for 5 min) after the supernatant was discarded. Finally, the spleen cells were resuspended with 10% FBS (fetal bovine serum), and the living cells were stained with 0.4% trypan blue and counted. The cell density was adjusted to 1.0 × 106/mL. The spleen cells were inoculated into a 96-well culture plate at a concentration of 1 × 107/L. There were six parallel wells for each sample. For each well, the amount of medium was 200 μL. Three wells were designed as an experimental group, and ConA was added to 5 mg/L for each well. The other three wells were used as a control group, and no ConA was added to the wells. The culture plates were cultured at 37 °C in a 5% CO2 incubator for 72 h, and then, the supernatant was discarded. Ten μL MTT (5 mg/mL) and 90 μL serum-free medium were added into each well, and the samples were cultured at 37 °C in a 5% CO2 incubator for another 4 h. Then, the samples were oscillated adequately with a microplate oscillator for 15 min at 37 °C, and 150 μL 10% DMSO were added into each well. The OD value for the samples was obtained by a microplate reader at 570 nm. Finally, the lymphocyte proliferation activity was estimated with the stimulus index (SI) according to Formula (5).
EWO represents experimental well OD;
CWO represents control well OD.
3.2.5. Research on Killing Activity of Mouse NK Cells
Mouse lymphoma cells (YAC-1) were cultured in a 37 °C 5% CO2 incubator using a complete medium containing 10% fetal bovine serum, with subculturing every 2–3 days until the cells reached the logarithmic growth phase [20]. Spleen cells at 1 × 107/L were used as effector cells, and mouse lymphoma cells YAC-1 at 1 × 105/L were used as target cells. And effector-target ratio was 100:1. The experiments were designed as follows: ① natural release group: 100 μL target cells and 100 μL medium for each well; ② maximum release group: 100 μL target cells and 100 μL of 1% NP40 for each well; and ③ experimental group: 100 μL target cells and 100 μL effector cells for each well. There were three parallel wells at every dosage group. Then, the culture plate was cultured at 37 °C in a 5% CO2 incubator for 16 h. After the cultivation, 100 μL of the culture supernatant in each well was transferred to a new culture plate in order and put into a temperature box at 37 °C for 10 min. Sequentially, 0.1 mL of the substrate solution (which should be formulated immediately before its clinical application) was added for each well and kept still at room temperature for 10–15 min, and then, 30 μM citric acid were added for each well. The OD value for each sample was obtained with an enzyme mark instrument at 570 nm. The killing activity of the mouse NK cells was calculated according to Equation (6).
KRNK represents the killing ratio of the NK cells;
EGOD represents the experimental group OD;
NRGOD represents the natural release group OD;
MRGOD represents the maximum release group OD.
3.2.6. Research on Immune Factors
The contents of IL-2 and IL-12 in serum were detected by the double antibody sandwich ELISA method exactly according to the instructions for the ELISA kits [21].
3.3. Statistical Analysis
The experiments in Section 3.1 were performed in triplicate, and the experiments in Section 3.2 were performed in quintuplicate. The data are represented as mean ± standard deviation. An analysis of variance (ANOVA) of the data was performed using LSD combined with Waller–Duncan through the SPSS v.17.0 statistical software (IBM, Armonk, NY, USA), and OriginPro (OriginLab Corporation, Northampton, MA, USA) was used for drawing. For Section 3.1, the same letters in the chart represent no significant differences (p > 0.05), and the different letters represent significant differences (p < 0.05). For Section 3.2, * represents significant differences (p < 0.05), and ** represents very significant differences (p < 0.01).
4. Results and Discussion
4.1. Results of Effect of Enzymatic Glycosylation on Film Processing Functions of BSP
4.1.1. Results of Zeta Potential of BSP and Its Modified Products
As shown in Figure 3, the zeta potentials of BSP, ECBSP, and EGBSP were −21.5 mV, −23.8 mV, and −20.2 mV, respectively. San et al. reported that the zeta potential of heated black bean protein isolate and transglutaminase crosslinked heated black bean protein isolate were −33.0 mV and −39.0 mV, respectively [22] which is similar to the result for ECBSP in this research. Li Y et al. found that lactose glycosylation of BSP through the Maillard reaction increased the zeta potential of BSP [23]. In this study, however, some differences were observed. The increased zeta potential values of the EGBSP might be due to the neutralization reaction between the negative charge on the BSP surface and the positive charge on the COS surface. Additionally, a more open secondary structure can expose hydrophobic amino acids, which will also lead to a lower protein surface potential.
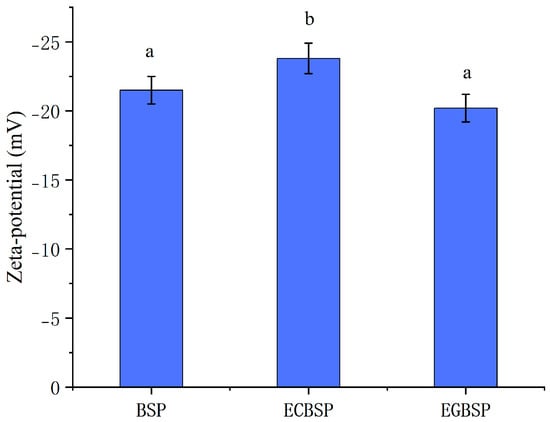
Figure 3.
Zeta potential of BSP and its modified products. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein (EGBSP). Different letters (a, b) indicate significant differences between groups (p < 0.05).
4.1.2. Results of Surface Hydrophobicity Analysis of BSP and Its Modified Products
The surface hydrophobicity of BSP and its modified products is influenced by the spatial conformation and the exposure of hydrophobic residues contained in BSP. According to the results in Table 1, when compared with the surface hydrophobicity of BSP, the surface hydrophobicity of EGBSP decreased, which was consistent with the research results of Gu X et al. The surface hydrophobicity of glycosylation products of soy protein isolate decreased [24]. The surface hydrophobicity of ECBSP increases, which is similar to the report of Hiller B [25]. Yuan F et al. also observed that glycogroup introduction catalyzed by transglutaminase reduced the surface hydrophobicity of proteins [26]. The introduction of glycosyl in BSP can reduce surface hydrophobicity, while transglutaminase increases the surface hydrophobicity of BSP during self-crosslinking by exposing hydrophobic amino acids embedded within the protein molecule.

Table 1.
Surface hydrophobicity of BSP and its modified products.
4.1.3. Results of Solubility of BSP and Its Modified Products
The solubility of BSP and its modified products at pH 2–11 is shown in Figure 4. Among the three samples, BSP exhibited the highest solubility, while ECBSP had the lowest. Since protein solubility can be considered as the balance between the interactions between protein–solvent (hydrophilic) and protein–protein (hydrophobic) [27], the significant decrease in solubility caused by the crosslinking of black bean protein isolates is due to the production of protein copolymers, which can be observed in the SDS-PAGE results of our previous research [8]. The solubility of EGBSP was better than ECBSP, mainly due to the process of glycosylation inserting the hydrophilic residue of the protein into the protein and introducing the hydrophilic hydroxyl group into the protein, thus enhancing the hydrophilicity of the protein.
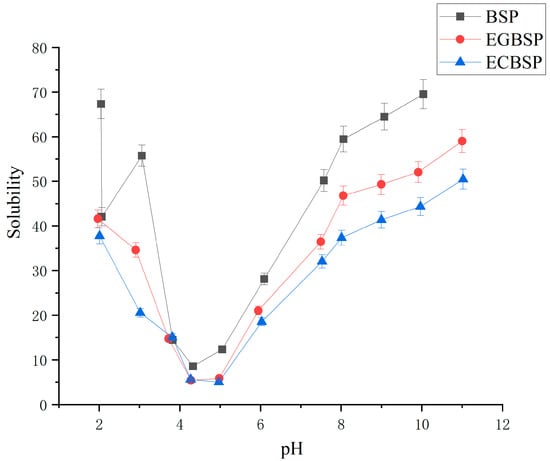
Figure 4.
Solubility of BSP and its modified products in pH range of 2–11. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein isolated (EGBSP).
4.1.4. Results of Rheology of Acid-Induced Gel Formation of BSP and Its Modified Products
The addition of GDL to the protein sample dispersion induces gelation through the hydrophobic aggregation of proteins [28]. As shown in Figure 5, the energy storage modulus (G′) of each protein sample dispersion remains almost unchanged before 8000 s, but G′ increases with time after 8000 s. The method of preliminary determination of the protein gel is G′ ≥ 1 Pa. It can be seen from the experimental results that EGBSP dispersion starts with gelatin first, followed by BSP and ECBSP. The experimental results showed that the gel time of the protein would lag after TG-catalyzed protein crosslinking. The introduction of the sugar group significantly shortens the gelation time. The chemical bond of BSP changed after modification, and the rheological and gel properties of BSP changed after modification. The large molecular weight polymerization formed during glycosylation partially forms a network structure. It has better water retention. The structure of GDL-induced gels is largely maintained by hydrophobic interaction and hydrogen bonding. Under heat treatment, the formation of both covalent and non-covalent bonds leads to a sharp increase in the acid-induced gel storage modulus.
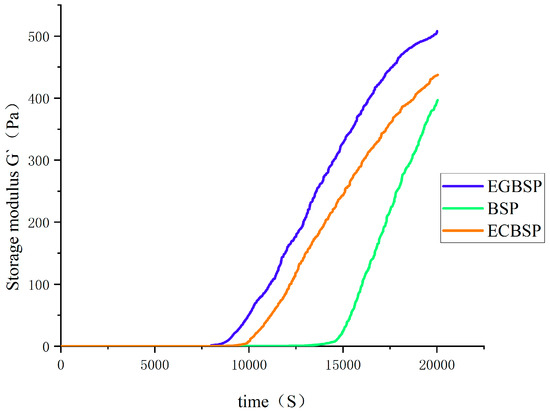
Figure 5.
Time sweep of GDL-induced gel process of BSP and its modified products’ dispersion solution. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein isolated (EGBSP).
4.1.5. Results of Mechanical Properties of Films Made of BSP and Its Modified Products
The mechanical properties of dried BSP and its modified products are related to the intermolecular forces involved in forming protein films [29]. Hydrophobic interaction and hydrogen bonding are the dominant factors. As shown in Figure 6, the TS value of BSP-modified products is higher than that of unmodified BSP, indicating that the increase in crosslinking will increase the strength of the BSP protein film. Among them, the strength of the EGBSP protein film improved most obviously. The effect of hygrothermal glycosylation on the TS value of the film was relatively insignificant. As illustrated in Figure 7, the E value of the ECBSP and EGBSP protein film is higher than that of the BSP protein. The results are consistent with the results of Xu et al. [30]. The E value of EGBSP is higher than that of ECBSP, which may be due to its more flexible secondary structure exposed to more hydrophobic groups in the proteins. In the process of film formation, these hydrophobic groups would bind through hydrophobic interaction, which would change the spatial structure of the protein film and result in a more flexible film.
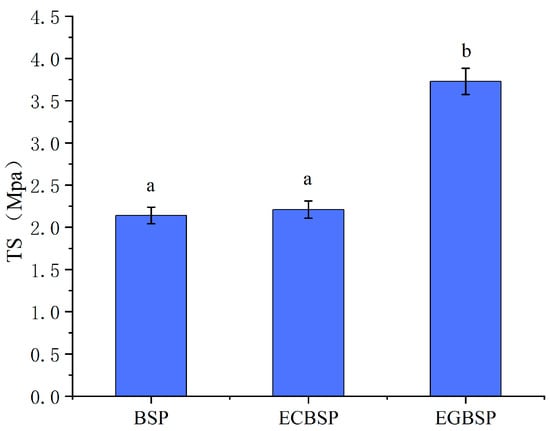
Figure 6.
Effect of modification on TS value of BSP protein film. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Different letters (a, b) indicate significant differences between groups (p < 0.05).
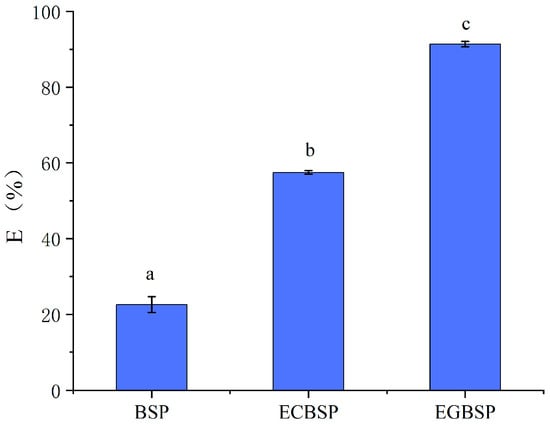
Figure 7.
Effect of modification on E value of BSP protein film. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Different letters (a–c) indicate significant differences between groups (p < 0.05).
4.1.6. Results of Permeability of Films Made of BSP and Its Modified Products
As shown in Figure 8 BSP crosslinking with TG can significantly reduce the WVP value of the film. Due to its relatively compact molecular structure, BSP has high strength and water vapor barrier ability, which is because water molecules have more difficulty penetrating the film when the crosslinking degree increases. The WVP value of EGBSP is even lower than that of BSP, which is mainly due to the fact that the crosslinking degree of glycosylation makes it more difficult for water molecules to penetrate the film.
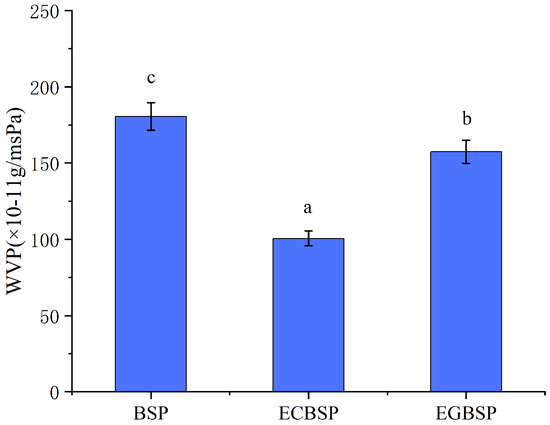
Figure 8.
Effect of modification on WVP value of BSP protein film. Notes: black soybean protein isolated (BSP); enzymatic crosslinked black soybean protein isolated (ECBSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Different letters (a–c) indicate significant differences between groups (p < 0.05).
4.2. Results of Effect of Enzymatic Glycosylation on Biological Activities of BSP
According to our preliminary results of this research, which are shown in Supplementary File Tables S1–S5, the concentrations of the research were selected according to Gao et al. [31], with some modifications. The results showed that the BSP was considered to have oxidation resistance, and antioxidation was introduced to BSP in glycosylation. Meanwhile, antioxidant intake enhances the body’s immunity and decreases tumor occurrence.
4.2.1. Results of Tumor Weight of Mice with Hepatoma H22
The tumors of the control group mice grew rapidly, with disease progression and loss of appetite, as well as decreased activity. The tumor growth in the BSP group mice was slightly slower than that in the control group mice, with no significant improvement in their survival status. The tumor growth in the EGBSP group mice was significantly inhibited. The survival status of the EGBSP group mice was markedly better than that of the BSP group mice. The survival status of each experimental mice group was shown in Supplementary File Figure S3.
As shown in Table 2, compared to the control group, the middle dose group of BSP had significantly reduced weight and size of the tumor tissues, and each group of the EGBSP had very significant inhibition on the transplanted hepatoma of H22 mice. These consist of the survival status of the experimental mice.

Table 2.
Effect of BSP and EGBSP on tumor weight of mice with hepatoma H22 (±S, n = 10).
4.2.2. Results of Morphological Observation of Mice Ascites Tumor
Apoptosis of cells is a continuous process. The AO/EB double immunofluorescent method is an important method for identifying necrosis and apoptosis of cells. After fluorescence staining, cells with normal nuclear chromatin and the whole membrane showed homogeneous green. Cells that turned light green and appeared to have dense plaque and fragments after nucleuses staining treatment were regarded as early apoptotic cells. Cells that appeared light green with condensed plaques and fragments following nuclear staining were identified as early apoptotic cells. Cells with broken films, whole nuclear films, and homogeneous red chromatin were considered necrotic cells [32].
According to Figure 9, the ascites tumor’s cell concentration in the BSP group was lower than that of the control group. However, it was still higher than that of the EGBSP. The results showed that both the BSP and EGBSP have anti-tumor function in vivo. Cell apoptosis is a basic cellular activity to maintain the physiological balance of the body [33]. There is evidence that the anticancer activity of some functional components is related to the induction of cell apoptosis. The mitochondria-dependent pathway is one of the most classical apoptosis pathways [34,35]. Based on the results of in vitro antioxidant experiments on BSP and EGBSP shown in Supplementary File Tables S1–S5, they may induce tumor cell apoptosis by quenching free radicals on the mitochondria [36].
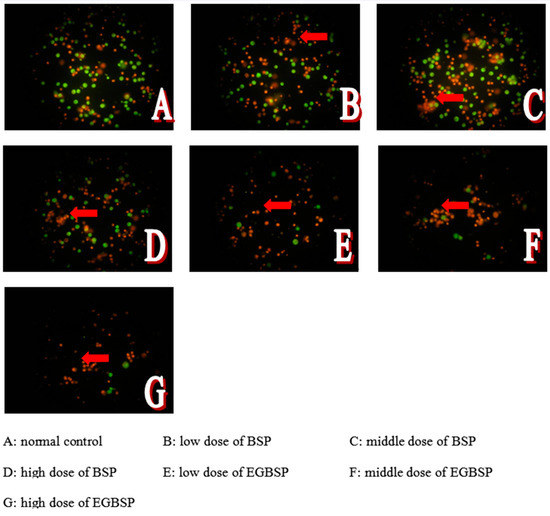
Figure 9.
Effect of BSP and EGBSP on cellular morphology of mice with H22 ascites tumor. Notes: black soybean protein isolated (BSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Red arrows point to the cells with broken films.
4.2.3. Results of Flow Cytometry of Mice Ascites Tumor
In a normal state, phosphatidylserine (PS) is located exclusively on the inner side of the lipid bilayer of the cell membrane. In the early stage of apoptosis, the PS in the cell membrane reversed from the inside to the outside of the lipid membrane. The molecular weight of Annexin V is 35–36 kD, and it has calcium ion dependence. Annexin V is a kind of phospholipid-binding protein, which has a strong affinity to PS. Therefore, it can bind to the cell membrane of early apoptotic cells through their overturned PS outside. Researchers often use Annexin V as a marker to detect early apoptosis cells. When labeled with a fluorescent dye, Annexin V serves as a fluorescent probe to detect apoptosis via flow cytometry. Propidium iodide (PI) is a nucleic acid dye that cannot penetrate the intact cell membrane. But when the cells are in the intermediate and advanced apoptosis and death stages, PI could penetrate the cell membrane to dye the nucleus. Therefore, a combination of Annexin V and PI could distinguish the cells in different apoptosis stages. The scatter diagrams were set as FITC vs. PI dot plots and were divided into four regions: Q1 (left upper quadrant) shows damaged cells FITC−/PI+; Q4 (left lower quadrant) shows normal cells FITC/PI−; Q3 (right lower quadrant) shows earlier apoptotic cells FITC+/PI−; and Q2 (right upper quadrant) shows later apoptotic and necrotic cells FITC−/PI+.
As shown in Figure 10, the ability of EGBSP and BSP to induce apoptosis in H22 tumor cells was enhanced compared to the control group. The number of early apoptotic cells in the middle- and high-dose group of EGBSP is significantly higher than that in the BSP group. This suggests that the introduction of COS can significantly enhance BSP’s ability to induce early apoptosis of H22 tumor cells during the enzymatic glycosylation process. Many studies have shown that the anti-tumor activity of functional components is also mediated by enhancing immune responses. The results of Nura R et al. show that antioxidants are a potential choice for the prevention and treatment of cancer that is related to reactive oxygen species (ROS) [37]. The results are consistent with our research. The antioxidant activity of EGBSP is higher than that of BSP, and the ability of EGBSP to induce apoptosis is also higher than that of BSP.
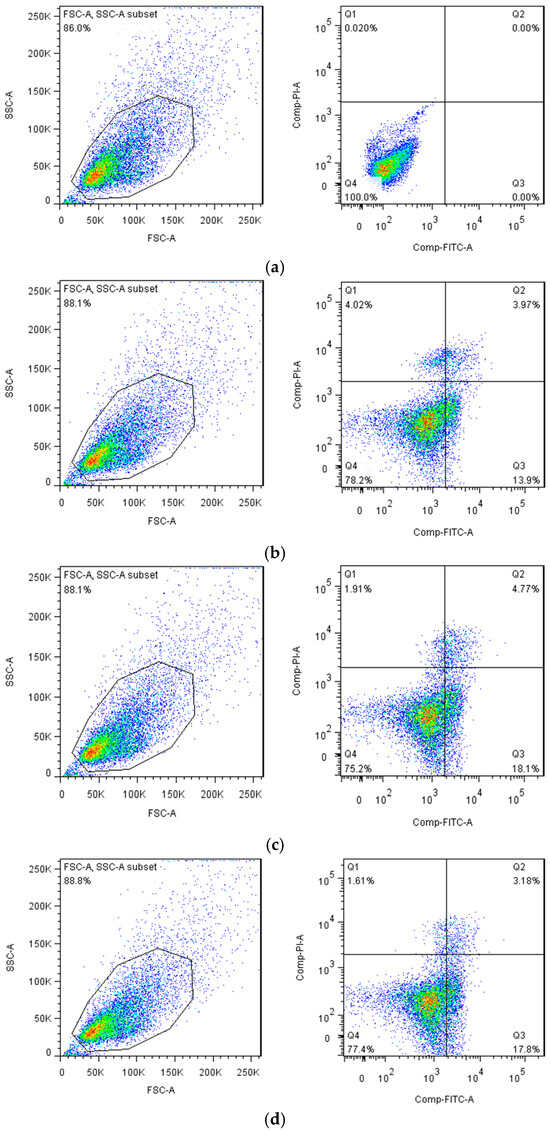
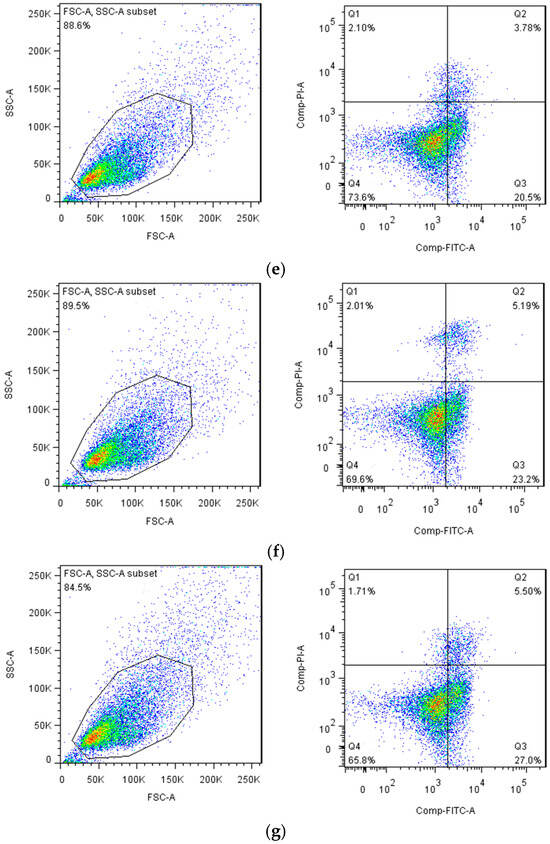
Figure 10.
Effect of BSP and EGBSP on flow cytometry of mice with H22 ascites tumor. (a) Normal control; (b) low dose of BSP; (c) middle dose of BSP; (d) high dose of BSP; (e) low dose of EGBSP; (f) middle dose of EGBSP; (g) high dose of EGBSP. Notes: black soybean protein isolated (BSP); enzymatically glycosylated black soybean protein isolated (EGBSP).
4.2.4. Results of Lymphocyte Proliferation Activity
According to the results in Table 3, both the middle and high doses of BSP could significantly enhance the spleen index in mice with H22 ascites tumors compared to the control group (p < 0.05), which showed that BSP had a certain immune enhancement. Each dose of EGBSP could very significantly improve the spleen index and thymus index of mice with H22 ascites tumor (p < 0.01), which verified the excellent biological detoxication and immune enhancement. The thymus, as an important lymphoid organ and central immune organ of human beings, is the place for the differentiation and maturation of T cells [38]. It has a regulatory effect on peripheral immune organs and immune cells in the establishment and maintenance of self-tolerance. The atrophy and decline of the thymus might cause a decrease in cellular immunity, weakening the ability to monitor and clear a tumor. The state of the thymus directly shows the cellular immune function. The spleen, being the largest peripheral immune organ, serves as a repository for T and B cells and is also a key site for initiating immune responses. In this study, the spleen and thymus indexes indicated that the enzymatic glycosylation products of BSP had a protective effect on these organs in tumor-bearing mice. However, in the control group, the malignant proliferation of tumor cells seriously damaged the immune systems of mice [39,40].

Table 3.
Effect of BSP and EGBSP on immune organs index of mice with H22 (±S, n = 10).
4.2.5. Results of Effect of BSP and EGBSP on Immune Cells of Mice with H22
According to Table 4, compared to the control group, both the middle and high doses of BSP could significantly enhance the killing activity of mouse NK cells (p < 0.05), while the middle dose of BSP could very significantly promote the lymphocyte (p < 0.01). Both low and high doses of EGBSP have significantly enhanced the killing activity of mouse NK cells and enhanced significantly the lymphocytes of mice with H22. Meanwhile, the middle dose of EGBSP has a highly significant effect on the improvement of both the killing activity of mouse NK cells and lymphocytes in mice with H22 (p < 0.01).

Table 4.
Effect of BSP and EGBSP on immune cells of mice with H22 (±S, n = 10).
T lymphocytes include CD4+ helper T cells and CD8+ cytotoxic T cells (CTL) [41]. CD8+ could secrete cytokines, such as INF-γ and TNF-α, and directly combine tumor antigens to kill the tumor. CD4+ can secrete cytokines, such as IL-2 and NF-γ and assist in the activation of CD8+, NK cells, and macrophages [42].
NK cells, as the first line for the body to defend against tumors, could directly kill the tumor without activation. And it does not depend on antibodies [43]. NK cells have no tumor specificity and MHC restriction and can kill tumors through the ADCC mechanism [44].
4.2.6. Results of Effect of BSP and EGBSP on Serum IL-2 and IL-12 of Mice with Tumor H22
Figure 11 shows the effect of BSP and EGBSP on the serum IL-2 of mice with tumor H22. Figure 12 shows the effect of BSP and EGBSP on the serum IL-12 of mice with tumor H22. According to Figure 11 and Figure 12, compared with the control group, a high dose of BSP significantly improved IL-12 (p < 0.01), and a high dose of EGBSP significantly improved both IL-2 and IL-12. These results are consistent with the results on the killing activity of mice NK cells [45].
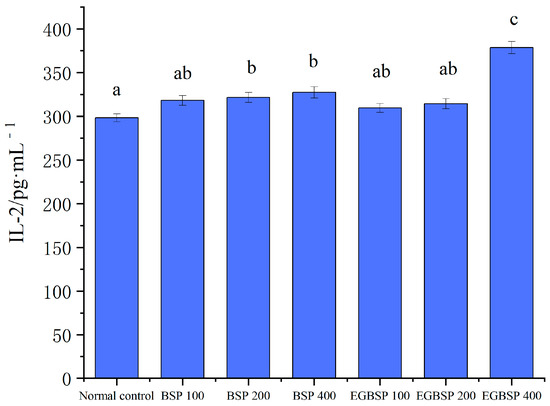
Figure 11.
Effect of BSP and EGBSP on serum IL-2 of mice with tumor H22. Notes: black soybean protein isolated (BSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Values are the mean ±SD for 10 mice. Different letters (a–c) indicate significant differences between groups (p < 0.05).
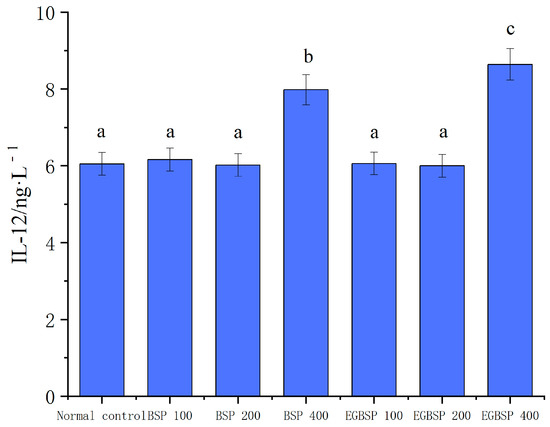
Figure 12.
Effect of BSP and EGBSP on serum IL-12 of mice with tumor H22. Notes: black soybean protein isolated (BSP); enzymatically glycosylated black soybean protein isolated (EGBSP). Values are the mean ± SD for 10 mice. Different letters (a–c) indicate significant differences between groups (p < 0.05).
5. Conclusions
Compared with BSP, the zeta potential and surface hydrophobicity of EGBSP decreased, while those of ECBSP increased. All the modified products can form large aggregations during the dispersion process. The solubility of EGBSP is better than that of ECBSP. The enzymatic glycosylation process inserts the hydrophilic residue of the protein into the protein and introduces the hydrophilic hydroxyl group into the protein, thus enhancing the hydrophilicity of the protein. After TG-catalyzed protein crosslinking, the gel time of the protein would lag. The introduction of a sugar group can shorten the gel time to a great extent. The enzymatic glycosylation also enhanced the mechanical properties of the protein membrane and decreased its membrane transmissibility. Most middle and high doses of BSP had a certain positive effect on immuno-enhancement and anti-cancer activity in mice with the tumor H22, while almost every dose of EGBSP had a very significant effect.
The modification method of introducing COS into BSP by TG catalysis could better improve the film-processing properties of black bean protein isolate compared with enzyme crosslinking modification. It also has a better effect in enhancing immunity and inducing tumor cell apoptosis. It could be considered as a new and efficient modification technology for BSP. EGBSP shows good performance in most functional properties. In the future, the selective permeability and self-healing properties of modified protein films will be further explored to effectively expand the application of plant protein isolates in the fields of functional edible films and medical film-forming materials and provide a reference for the modification of other proteins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings15020238/s1, Figure S1: Preparation process of black soybean protein isolated; Figure S2: FT-IR spectra of BSP and its modified products; Figure S3: The survival curves of experimental mice; Table S1: DPPH free radicals scavenging activity; Table S2: ABTS free radicals scavenging activity; Table S3: Total reducing ability; Table S4: Total antioxidant ability; Table S5: Nitroso free radicals activity.
Author Contributions
Conceptualization, Y.Z.; Data curation, D.L.; Formal analysis, L.H.; Methodology, B.D.; Project administration, B.D. and J.C.; Resources, W.X.; Software, X.G.; Supervision, X.G.; Writing—original draft, Y.Z.; Writing—review and editing, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Doctoral research start-up support program (Funding number: 22BQ68) and by the 2023 Cultivation Plan of Innovative Talents, Harbin University of Commerce (Funding number: 2023-KYYWF-1014).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors are grateful to the School of Light Industry and the School of Food Engineering at Harbin University of Commerce for providing the necessary facilities to carry out this research work. The authors express their sincere thanks to the reviewers for their valuable comments and suggestions on this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mitharwal, S.; Saini, A.; Chauhan, K.; Taneja, N.K.; Oberoi, H.S. Unveiling the nutrient-wealth of black soybean: A holistic review of its bioactive compounds and health implications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70001. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Hao, X.; Ji, X.; Zhu, Y.; Chen, X.; Yao, Y. A systematic review of black soybean (Glycine max (L.) Merr.): Nutritional composition, bioactive compounds, health benefits, and processing to application. Food Front. 2024, 5, 1188–1211. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Guo, Y.R.; Hou, T.Y.; Ning, Q.R.; Han, W.Y.; Zhao, X.Y.; Cui, F.; Li, H. Formation of advanced glycation end products in glucose–amino acid models of Maillard reaction under dry- and wet-heating conditions. J. Sci. Food Agric. 2024, 105, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Mondaca-Navarro, B.A.; Torres-Arreola, W.; Ávila-Villa, L.A.; Villa-Lerma, A.G.; Hernández-Mendoza, A.; Wall-Medrano, A.; Ramírez, R.R. Obtaining glycoconjugates of marine origin via Maillard reaction and their cytotoxic effect: An alternative for the use of animal byproducts. J. Sci. Food Agric. 2024, 100, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Akharume, F.; Aluko, R.; Adedeji, A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Pourmohammadi, K. Chemical modifications and their effects on gluten protein: An extensive review. Int. J. Food Chem. 2021, 343, 128398. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Fu, Y.; Ma, L.; Dai, H.; Wang, H.; Chen, H.; Zhu, H.; Yu, Y.; Zhang, Y. Collagen glycopeptides from transglutaminase-induced glycosylation exhibit a significant salt taste-enhancing effect. J. Agric. Food Chem. 2023, 71, 8558–8568. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Y.; Lu, S.; Yao, X.; Zheng, X.; Zhao, R.; Li, Z.; Shen, H.; Zhang, S. Effects of Modified Processing Methods on Structural Changes of Black Soybean Protein Isolate. Molecules 2018, 23, 2127. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Jo, Y.H.; Han, Y.S.; Jung, W.J. Production of chitin- and chitosan-oligosaccharide using the edible insect, Tenebrio molitor. Entomol. Res. 2022, 52, 207–213. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Q.; Li, R.; Hao, L.; Liu, H.; Wang, X.; Xu, N.; Zhao, X. Structural and emulsifying properties of soybean protein isolate glycated with glucose based on pH treatment. J. Sci. Food Agric. 2022, 102, 4462–4472. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, J.; Zhao, L.; He, W.; Liu, T.; Liu, B. Analysis of the gel properties, microstructural characteristics, and intermolecular forces of soybean protein isolate gel induced by transglutaminase. Food Sci. Nutr. 2022, 10, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Cui, Q.; Yu, Z.; Wang, X.; Zhao, X.H. Effects of transglutaminase glycosylated soy protein isolate on its structure and interfacial properties. J. Sci. Food Agric. 2021, 101, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Cao, Y.; Chang, S.K.C. Characteristics of soy films as affected by transglutaminase cross-linking and inclusions of pectin and protein enhancers. J. Food Sci. 2024, 89, 4389–4402. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, N.; Wang, Z.; Wang, X. Wheat bran dietary fibre-induced changes in gluten aggregation and conformation in a dough system. Int. J. Food Sci. 2021, 56, 86–92. [Google Scholar] [CrossRef]
- Deshpande, M.; Sathe, S.K. Interactions with 8-Anilinonaphthalene-1-sulfonic Acid (ANS) and Surface Hydrophobicity of Black Gram (Vigna mungo) Phaseolin. J. Food Sci. 2018, 83, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Lai, S.; Wang, H.; Yang, H. Impact of soybean protein isolate-chitosan edible coating on the softening of apricot fruit during storage. LWT 2018, 96, 604–611. [Google Scholar] [CrossRef]
- González, A.; Gastelú, G.; Barrera, G.N.; Ribotta, P.D.; Igarzabal, C.I.Á. Preparation and characterization of soy protein films reinforced with cellulose nanofibers obtained from soybean by-products. Food Hydrocoll. 2019, 89, 758–764. [Google Scholar] [CrossRef]
- Waldmann, T.A. The IL-2/IL-2 receptor system: A target for rational immune intervention. Trends Pharmacol. Sci. 1993, 14, 159–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Wang, F.; Li, Q.; Fan, Y.; Zhao, X.; Hao, L.; Hou, H. Stability of oil-in-water emulsion and immunomodulating activity in S180 tumor-bearing mice. J. Food Sci. 2024, 89, 5884–5899. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Y.; Jing, X.; Liu, Y.; Liu, A. Structural Characterization of an Alkali-Soluble Polysaccharide from Angelica sinensis and Its Antitumor Activity in Vivo. Chem. Biodivers. 2021, 18, e2100089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Ma, T.; Zhang, X.; Zhao, Q.; Zhu, K.; Cao, J.; Liu, Z.; Shen, X.; Li, C. Holothuria leucospilota Polysaccharides Improve Immunity and the Gut Microbiota in CyclophosphamideTreated Immunosuppressed Mice. Nutr. Food Res. 2023, 67, 2200317. [Google Scholar] [CrossRef] [PubMed]
- San, Y.; Xing, Y.; Li, B.; Zheng, L. Effect of transglutaminase cross-linking on the structure and emulsification performance of heated black bean protein isolate. J. Sci. Food Agric. 2025, 105, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhong, F.; Ji, W.; Yokoyama, W.; Shoemaker, C.F.; Zhu, S.; Xia, W. Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll. 2013, 30, 53–60. [Google Scholar] [CrossRef]
- Gu, X.; Campbell, L.J.; Euston, S.R. Influence of sugars on the characteristics of glucono-δ-lactone-induced soy protein isolate gels. Food Hydrocoll. 2009, 23, 314–326. [Google Scholar] [CrossRef]
- Hiller, B.; Lorenzen, P.C. Surface Hydrophobicity of Physicochemically and Enzymatically Treated Milk Proteins in Relation to Techno-functional Properties. J. Agric. Food Chem. 2008, 56, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Lv, L.; Li, Z.; Mi, N.; Chen, H.; Lin, H. Effect of transglutaminase-catalyzed glycosylation on the allergenicity and conformational structure of shrimp (Metapenaeus ensis) tropomyosin. Food Chem. 2017, 219, 215–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Zhang, X.; Xia, S.; Jia, C.; Karangwa, E.; Shabbar, A.; Feng, B.; Zhong, F. Effects of maltodextrin glycosylation following limited enzymatic hydrolysis on the functional and conformational properties of soybean protein isolate. Eur. Food Res. Technol. 2014, 238, 957–968. [Google Scholar] [CrossRef]
- Chang, Y.; Su, H.; Shiau, S. Rheological and textural characteristics of black soybean touhua (soft soybean curd) prepared with glucono-δ-lactone. Food Chem. 2009, 115, 585–591. [Google Scholar] [CrossRef]
- Ciannamea, E.M.; Stefani, P.M.; Ruseckaite, R.A. Physical and mechanical properties of compression molded and solution casting soybean protein concentrate based films. Food Hydrocoll. 2014, 38, 193–204. [Google Scholar] [CrossRef]
- Xu, Y.P.; Wang, Y.; Zhang, T.; Mu, G.Q.; Jiang, S.J.; Zhu, X.M.; Tuo, Y.F.; Qian, F. Evaluation of the properties of whey protein films with modifications. J. Food Sci. 2021, 86, 923–931. [Google Scholar] [CrossRef]
- Gao, C.; Wang, F.; Yuan, L.; Liu, J.; Sun, D.; Li, X. Physicochemical property, antioxidant activity, and cytoprotective effect of the germinated soybean proteins. Food Sci. Nutr. 2019, 7, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimarães, J.E. Critical evaluation of techniques to detect and measure cell death--study in a model of uv radiation of the leukaemic cell line hl60. Anal. Cell. Pathol. Cell. Oncol. 1999, 19, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Hou, X.; Su, D.; He, Z.; Zhao, J.; Liu, T. Effect of PhenylEthanol Glycosides from Cistanche Tubulosa on Autophagy and Apoptosis in H22 Tumor-Bearing Mice. Evid. Based Complement. Altern. Med. 2022, 14, 3993445. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Zhu, M.; Chen, K.; Xie, H.; Bai, H.; Chen, Q. Z-Guggulsterone Induces Apoptosis in Gastric Cancer Cells through the Intrinsic Mitochondria-Dependent Pathway. Sci. World J. 2021, 6, 3152304. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, S.; Luo, X.; Hu, M.; Ma, F.; Wang, Y.; Lai, X.; Zhou, L. Polysaccharides from Chinese Herbal Lycium barbarum Induced Systemic and Local Immune Responses in H22 Tumor-Bearing Mice. J. Immunol. Res. 2018, 12, 3431782. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, C.; Zhao, S.; Wang, M.; Shang, L.; Zhou, J.; Ma, Y. The role of tumor-associated macrophages in hepatocellular carc inoma progression: A narrative review. Cancer Med. 2023, 12, 22109–22129. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Azlan, A.; Yusof, H.M.; Sarbon, N.M. Antioxidant and anticancer activities of enzymatic eel (monopterus sp.) protein hydrolysate as influenced by different molecular weight. Biocatal. Agric. Biotechnol. 2018, 16, 10–16. [Google Scholar] [CrossRef]
- Paglia, P.; Chiodoni, C.; Rodolfo, M.; Colombo, M.P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic t lymphocytes against tumor antigen in vivo. Clin. Exp. Immunol. 1996, 183, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liu, W.; Huang, L.; Wang, L.; Yu, J.; Wang, Y.; Wu, D.T.; Wang, S. Quality evaluation of immunomodulatory polysaccharides from Agaricus bisporus by an integrated fingerprint technique. Food Front. 2023, 4, 474–490. [Google Scholar] [CrossRef]
- Kan, Y.; Liu, Y.; Huang, Y.; Zhao, L.; Jiang, C.; Zhu, Y.; Pang, Z.; Hu, J.; Pang, W.; Lin, W. The regulatory effects of Pseudostellaria heterophylla polysaccharide on immune function and gut flora in immunosuppressed mice. Food Sci. Nutr. 2022, 10, 3828–3841. [Google Scholar] [CrossRef]
- Wang, C.; Tang, J.; Crowley-Nowick, P.A.; Wilson, C.M.; Kaslow, R.A.; Geisler, W.M. Interleukin (IL)-2 and IL-12 responses to Chlamydia trachomatis infection in adolescents. J. Exp. Med. 2005, 142, 548–554. [Google Scholar] [CrossRef]
- Thomas, G.R.; Chen, Z.; Enamorado, I.; Bancroft, C.; Van Waes, C. IL-12- and IL-2-induced tumor regression in a new murine model of oral squamous-cell carcinoma is promoted by expression of the CD80 co-stimulatory molecule and interferon-γ. Int. J. Cancer 2000, 86, 368–374. [Google Scholar] [CrossRef]
- Bagheri, Y.; Barati, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Yousefi, M. Current Progress in cancer Immunotherapy Based on Natural Killer Cells. Cell. Biol. Int. 2021, 45, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dou, L.; Sui, L.; Xue, Y.; Xu, S. Natural killer cells in cancer immunotherapy. MedComm 2024, 5, 626–656. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Lee, H.Y.; Shin, D.Y.; Jeong, H.N.; Hwang, H.M.; Park, H.Y.; Kim, S.H.; Kim, M.J.; Kang, H.J.; Kim, J.H.; et al. Immune-Enhancing Activity of Vitis coignetiae Extract via Increasing Cytokine and Natural Killer Cell Activity in Splenocytes and Cyclophosphamide-Induced Immunosuppressed Rats. J. Food Biochem. 2024, 12, 5010095. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).