Abstract
Due to their excellent mechanical properties and good biocompatibility, titanium (Ti) and its alloys are widely used as biomaterials. However, when implanted in the body, metallic materials may cause serious complications such as wear and infection, leading to patient discomfort and, in some cases, the need for revision surgery. Micro-arc oxidation (MAO) is a surface modification technique that offers a promising strategy to overcome these challenges. This study investigated the impact of the microstructure of Ti-25 Ta-xNb alloys (x = 10, 20, and 30 wt%) and the variation in applied voltage during the MAO process on the characteristics of the TiO2 oxide coatings formed. The alloys were treated by MAO at 200, 250, and 300 V using a bioactive electrolyte containing Ca, P, Mg, and Ag. EDS, SEM, XRD, Raman spectroscopy, and adhesion tests performed characterization. Results indicated that Nb addition stabilized the β phase and anticipated the potentiostatic regime. Increasing the voltage supplied to the system provides greater energy, prolonging the galvanostatic regime and promoting the formation of larger and more uniform pores. The oxide coating thickness ranged from approximately 3 to 10 μm, with a tendency to decrease at higher voltages. The coatings exhibited low c, with anatase and rutile phases predominating, the applied voltage and Nb concentration influencing their relative proportions. Even in small amounts, all electrolyte elements (P, Mg, and Ag) were successfully incorporated into the coatings under all conditions. Raman and XRD analyses confirmed a decrease in anatase and an increase in rutile phases with increasing voltage and Nb content. Mechanical testing revealed good adhesion of the coatings in all samples, with the best results obtained at 200 V. The findings demonstrate that the developed coatings exhibit promising characteristics for future surface engineering strategies aimed at improving the performance of metallic biomaterials.
1. Introduction
The growing number of studies on biomaterials reflects the significant development in the area and the need to offer alternatives that improve the quality of life for patients requiring biomedical implants [1,2,3,4,5]. The estimated number of hip and knee arthroplasty revision surgeries by 2030 indicates an alarming increase of 174% and 673%, respectively. These projections underscore the importance of developing new strategies to reduce the risks of infection, discomfort, and rejection while promoting enhanced osseointegration and implant durability [6,7,8].
Among the fundamental mechanical properties associated with osseointegration, Young’s modulus (also known as the modulus of elasticity) refers to a material’s rigidity and is directly related to its mechanical properties. When there is a significant difference between the modulus of elasticity of the prosthesis and that of the cortical bone, load transfer is inadequate, resulting in excess weight on the prosthesis and a subsequent loss of bone mineral density. This phenomenon is known as the stress-shielding effect [9,10,11].
Titanium (Ti) alloys with a body-centered cubic crystalline structure (β phase) are promising for biomedical applications due to their low elastic modulus values, which help reduce the stress-shielding effect. Due to its allotropic condition, Ti can assume a β crystalline structure at temperatures above the β transus (883 °C). At the same time, below this limit, it adopts a hexagonal close-packed structure (α phase) [12,13,14].
In addition to its allotropic versatility, Ti possesses other characteristics that justify its extensive use in biomedical applications. The scientific literature emphasizes the high corrosion resistance, excellent mechanical strength-to-density ratio, biocompatibility, an elastic modulus closer to that of cortical bone, and its high reactivity with oxygen at elevated temperatures, which enables the formation of Ti dioxide (TiO2) [3,4,15,16,17].
TiO2 can be formed in three distinct crystalline forms: brookite (orthorhombic, metastable), anatase (tetragonal, metastable), and rutile (tetragonal, stable). The presence and proportion of these phases directly influence mechanical performance, wear resistance, and surface bioactivity [4,5,18,19]. The surface oxide layer acts as a barrier against corrosion and, when porous, promotes the adhesion of cells and proteins [19,20,21]. Thus, the functionalization of metallic surfaces has been widely employed as a strategy to enhance osseointegration.
In this context, the micro-arc oxidation (MAO) technique stands out for its easy operation, low cost, and good reproducibility, enabling the production of ceramic coatings with strong adhesion to the metallic substrate [4,22,23]. The composition and properties of these coatings can be controlled by parameters such as voltage, electric current, treatment time, electrolyte composition, and composition of the metal or alloy [4,5,24,25].
Elements such as tantalum (Ta) and niobium (Nb) are commonly added to Ti due to their ability to stabilize the β phase by lowering the transition temperature. In addition to their structural contribution, both elements exhibit high corrosion resistance and biocompatibility owing to their non-toxic nature [13,26,27,28]. Similar to the Ti oxidation process, both elements Ta and Nb can also form an oxide layer during the MAO technique.
Nb pentoxide (Nb2O5) is the oxide most likely to be produced during this process. The presence of this compound in the alloy reduces the current density [29,30]. In addition, Nb has a high resistance to the breakdown of the oxide layer in physiological solutions, surpassing that of the Ti alloys [31,32,33]. The Nb2O5 coating has shown positive results in both cellular responses and the incorporation of biological materials and drugs [30,34].
Similarly, Ta pentoxide has additional advantages, with researchers indicating that the presence of Ta pentoxide (Ta2O5) reinforces the TiO2 coating, improving corrosion resistance and promoting pore formation [35]. Although the primary aim of the present study was not specifically to promote the formation of Nb2O5 or Ta2O5, the high oxygen affinity of Nb and its tendency to form Nb2O5 under certain electrochemical conditions indicate that the formation of these oxides may occur, which could be beneficial for the coating properties.
Given this context, this study aims to develop bioactive and mechanically compatible oxide layers on Ti-25Ta-xNb alloys (x = 10, 20, 30 wt%) using the MAO technique to enhance implant performance. The effects of the different microstructures resulting from the addition of Nb on the growth of TiO2 layers will be investigated, as well as the influence of different applied voltages on the morphology, thickness, and properties of the coatings.
The purpose of both analyses is to understand the mechanism of oxide-layer growth and to identify the factors that have the greatest influence on this process. To enhance the biological response, bioactive elements such as calcium (Ca), phosphorus (P), magnesium (Mg), and silver (Ag) were strategically incorporated into the electrolyte to promote a functionalized surface with high potential for biomedical applications [23,35,36,37].
2. Materials and Methods
Preparation of Ti-25Ta-xNb alloys (10%, 20%, 30% by weight): Initially, the proportions of Ti (CP–Ti, Sandinox Inc., Sorocaba, Brazil), Ta (Goodfellow Ltd., Cambridge, UK), and Nb (Sigma-Aldrich Corp., St. Louis, MO, USA) precursors were calculated to obtain the chemical composition of the Ti-25Ta-xNb alloys (x = 10%, 20%, and 30% by weight). Although the metals used are highly pure (around 99%), their surfaces may contain impurities. To avoid contamination with other elements, chemical pickling was carried out in a Kroll solution (5 vol.% HF, 30 vol.% , and 65 vol.% ). Inside the vacuum chamber of the melting furnace, the Ti, Ta, and Nb metals were inserted into a water-cooled copper crucible. Once the metals were positioned, the high vacuum process began in the chamber, removing atmospheric air to insert argon. This process was carried out until a vacuum of approximately mBar was achieved. With a controlled argon atmosphere, the melting process involved the propagation of an electric arc generated by the potential difference between the alloying elements and a tungsten tip, resulting in high temperatures capable of reaching the melting point of the elements. To ensure the homogeneity of the composition, each alloy was remelted six times. Then, to obtain specimens with a final thickness of 1 mm, a hot rolling treatment with air cooling was carried out. The thickness reduction was achieved by heating the samples to 1000 °C in an EDG (BR) furnace, model 10PS 3000. The rolling process is analogous to the operation of a compressor roller, in which a reduction in thickness leads to an increase in the other dimensions while keeping the volume of the alloy constant. After rolling, the samples were subjected to a solubilization heat treatment in a high vacuum (~ mBar) to dissolve secondary phases and obtain a homogeneous microstructure, thereby favoring the retention of the β phase and increasing mechanical strength and ductility. To achieve this, a heat treatment furnace was used with a heating rate of 10 °C per minute until it reached 1000 °C, a temperature maintained for 6 h. Rapid cooling was performed by applying a mixture of ice and water to the quartz tube, which was still under vacuum, and positioning the samples within it.
Surface treatment: The MAO treatment was performed by applying a continuous, anodic electric voltage to substrates measuring 10 × 10 × 1 mm3, which were immersed in 200 mL of electrolyte solution. The electrolyte was mixed using a magnetic stirrer until a homogeneous solution was obtained. The system was powered by a N5751A DC power supply (Keysight Inc., Santa Rosa, CA, USA) connected to an Agilent digital multimeter. The data in Table 1 shows the parameters adopted. It is worth noting that the same procedure and conditions were applied to all specimens.

Table 1.
Parameters adopted for the MAO process.
The electrolyte consisted of 0.35 M calcium acetate monohydrate ; 0.02 M β-glycerophosphate disodium salt pentahydrate ·5; 0.1 M magnesium acetate tetrahydrate ; and 0.25 mM silver nitrate (), diluted in distilled water.
Chemical characterization: The procedure involved analyzing the elements using X-ray energy dispersive spectroscopy (EDS) and Raman spectroscopy. The EDS analysis aimed to verify the presence of impurities in the molten samples and quantify the elements located on the surface and in the bulk. For the EDS measurements, a Philips XL-30FEG (Field Emission Gun, Eindhoven, The Netherlands) scanning electron microscope (SEM) was used, equipped with an EDS detector (Bruker Corp., Xflash 6160 model, Billerica, MA, USA). The vibrational structures of the molecules present on the surfaces were also verified by Raman spectroscopy (Metrohm Raman spectrometer, model i-Raman Plus 532H, Newark, DE, USA), using a wavelength (λ) of 532 nm and a laser operating at 100% power.
Structural and microstructural characterization: Scanning electron micrographs of the samples in the bulk condition were obtained using a Carl Zeiss EVO 15 scanning electron microscope (SEM). Micrographs of the oxide layer were acquired using a Zeiss SEM model Sigma 300 (Carl Zeiss Corp., Oberkochen, Germany). X-ray diffraction (XRD) measurements were performed using a MiniFlex 600 diffractometer (Rigaku Corp., Tokyo, Japan) with Cu-Kα radiation (λ = 0.154 nm) over a range of angles (2 θ) from 10° to 100° at a collection speed of 5°/min. The radiation source operated at a potential difference of 40 kV and an electric current of 15 mA. The diffraction peaks were identified from the crystallographic records in the ICDD database. Based on the recognition and intensity of the peaks present in the X-ray diffractograms, it was possible to quantify the phases present in the oxide layer and the substrate using Equation (1) [38,39].
From the XRD profiles, it was possible to estimate the crystallinity of the coatings by using Equation (2) [40].
Mechanical characterization: The adhesion of the coatings to the substrate was tested using the VDI adhesion test. This classification is determined based on the penetration resistance of the Rockwell C tip on the coating and the substrate, providing information on the adhesion quality between the layers. A Sussen Wolpert Rockwell C indenter with a load of 150 kg was used for this analysis [39,41,42].
3. Results and Discussions
The following results present the chemical, structural, and microstructural characterization of the substrate, as well as the oxide layer, which was also analyzed in terms of its morphological and mechanical properties. All analyses were performed in triplicate (n = 3) to ensure the reliability and reproducibility of the data obtained.
3.1. Bulk Characterization
After the solubilization heat treatment, the samples were analyzed by EDS, which enabled verification of the chemical composition of the Ti-25Ta-xNb system alloys (x = 10%, 20%, and 30% by weight). The results presented in Table 2 indicate that the samples exhibit appropriate stoichiometry.

Table 2.
Semi-quantitative chemical analysis by EDS.
Figure 1 exhibits the XRD patterns of the substrates. By identifying the peaks, it was possible to quantify the phases using Equation (1). The results indicate that the Ti-25Ta-10Nb alloy has peaks characteristic of the orthorhombic (α″ = 65%) and body-centered cubic (β = 35%) crystal structures. The Ti-25Ta-20Nb alloy is biphasic, with the β phase comprising the majority (63%). The Ti-25Ta-30Nb alloy, conversely, consists exclusively of the β phase, revealing that the incorporation of Nb stabilized the β crystalline structure [13].
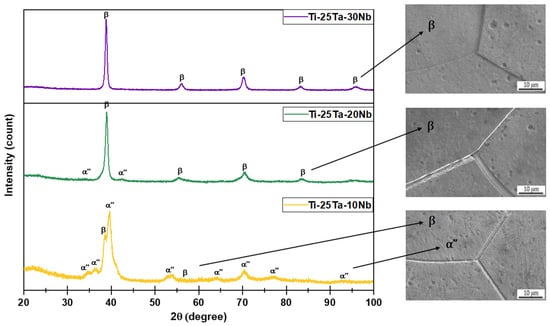
Figure 1.
XRD profiles and SEM images of the Ti-25Ta-xNb system alloys (x = 10%, 20%, and 30%).
Lee et al. investigated Ti-Nb alloys and concluded that for Nb contents below 10 wt%, only the hexagonal close-packed crystalline structure (α phase) was formed. When the Nb concentration reached 15 wt%, the microstructure showed the α″ and β phases. For Nb concentrations above 30 wt%, only the β structure was observed. These results corroborate the observations of this study, indicating that in the Ti-25Ta-10Nb alloy, Ta also acted as a stabilizing β element [43].
The SEM micrographs corroborate the data obtained from the XRD diffractograms. The Ti-25Ta-10Nb and Ti-25Ta-20Nb alloys exhibited grain contours characteristic of the β phase, along with finer needles corresponding to the α″ phase. The alloy with 20 wt% of Nb showed a reduced number of needles (α″ = 37%). In comparison, the Ti-25Ta-30Nb alloy displayed only grain boundaries of the β phase. Therefore, the MAO technique was performed on alloys with different chemical compositions and crystalline phase distributions, with Ti-25Ta-10Nb classified as α″ + β, Ti-25Ta-20Nb as β + α″, and Ti-25Ta-30Nb as the β phase.
3.2. Surface Characterization
3.2.1. Electrical Analysis
In this work, the MAO technique was carried out using a constant electric current of 2.5 A. The graphs in Figure 2 show that the surface modification process can be divided into two stages. The galvanostatic stage occurs under a constant electric current, and the potentiostatic stage begins the decay of the electric current [4,44,45].
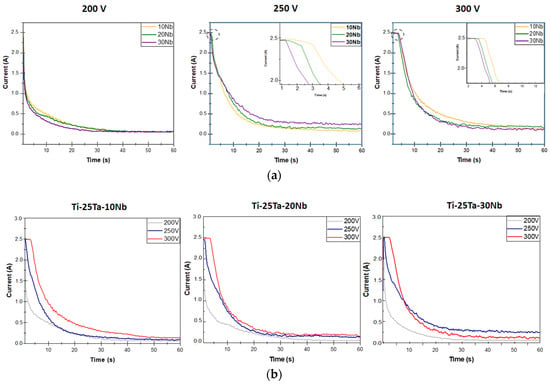
Figure 2.
Current curves as a function of time during the MAO process under different experimental conditions: (a) variation in the Nb content at a constant voltage (upper graphs) and (b) variation in the voltage (200, 250, and 300 V) with the Nb content kept constant (lower graphs).
The oxide layer begins to form in the galvanostatic stage. In this phase, high-energy electrical arcs are generated, which promote an increase in the coating thickness and the incorporation of the elements present in the electrolyte into the oxide layer [4,18,46]. However, for the electric current to remain constant, more energy must be supplied to the system, and this demand is met by increasing the voltage [4,18,46].
More energy is required because the oxide coating also acts as a resistor [18,30,46]. However, when the threshold voltage is reached, the oxide coating, which functions as a dielectric barrier, causes the electric current to decrease, initiating the transition to the potentiostatic stage. Therefore, the energy involved during this stage is reduced, which consequently lowers the number of electrical discharges, resulting in a more controlled surface modification and less incorporation of electrolyte elements into the oxide coating.
Figure 2a shows the behavior of the electric current as a function of time during the MAO process with the influence of Nb. The electrolyte used was the same for all conditions.
Except for the alloys obtained at 200 V, which did not reach the electric current limited of 2.5 A, increasing the percentage by weight of Nb advanced the start of the potentiostatic stage, indicating that alloys with higher amounts of Nb initially form layers with low electrical conductivity.
The samples subjected to voltages of 250 and 300 V exhibited similar behavior, indicating that the time interval of the galvanostatic stage tends to be longer in alloys with lower Nb concentrations. Namely, the lower the Nb concentration, the higher the dielectric barrier and the oxide layer formed.
Figure 2b also shows the behavior of the electric current as a function of time, but under the influence of voltage variation. Regardless of the amount of Nb, the samples prepared at a voltage of 200 V operated exclusively in the potentiostatic stage, with fewer electrical discharges and less energy, which may impair the incorporation of the elements present in the electrolyte into the oxide layer. For the galvanostatic stage to be visualized, it would be necessary to set a lower electric current value or increase the threshold voltage, as occurs in the 300 V condition.
The surface modification of the alloys with 10%, 20%, and 30% Nb by weight showed similar results [30,47,48]. The galvanostatic regime is greater when the voltage is set at 300 V. The time interval of this regime decreased as the voltage was reduced to 250 V. As a result, because they remained in the galvanostatic regime for a longer period, the samples produced at a voltage of 300 V were subjected to a high number of electrical discharges, which can lead to an increase in temperature during the process, favoring the formation and sintering of electrolyte elements in the oxide layer.
Another analysis that can be carried out involves the area of the graphs obtained by integrating the curves, which provides the value of the total electrical charge involved in the process [49]. The amount of electrical charge obtained at a voltage of 200 V is lower than that at the voltages of 250 and 300 V, justifying the lower amount of energy supplied to the process. The alloys prepared at 300 V showed higher electric charge values in each chemical composition.
3.2.2. Morphological Analysis
The SEM micrographs shown in Figure 3 reveal the topography and morphology of the samples. Under all conditions, pores with a volcanic appearance are visible, which is a characteristic microstructure of the surface modification process [4,50].
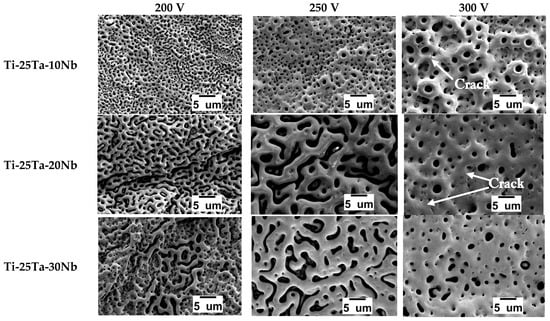
Figure 3.
SEM micrographs of the oxide layers produced by MAO.
In the MAO treatment, a thin coating, also called a dielectric barrier, is formed. When the critical voltage is reached, localized rupture occurs, and micro-arcs start to strike the oxide layer. At this stage, the elements that make up the coating are removed. At the same time, the high temperatures reached cause the electrolyte to melt and boil, creating bubbles, mainly of O2 and H2, which, when they burst, initiate the formation of cavities, that is, pores [46,51,52,53].
Figure 4 shows that the Ti-25Ta-10Nb alloy exhibited larger pore diameters as the voltage increased. At 300 V, the micro-arcs that collide with the oxide coating are more energetic, causing greater removal of coating material and generating larger bubbles, which results in pores of larger dimensions [39,47,54].
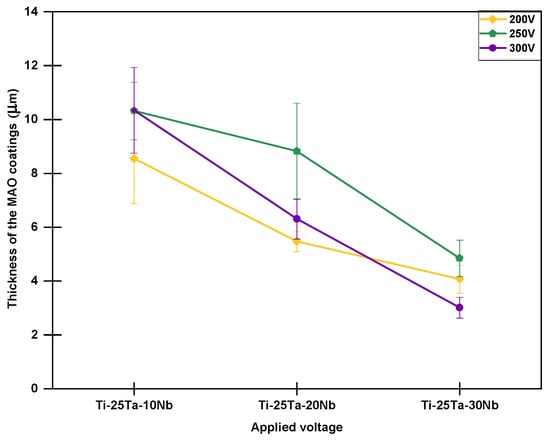
Figure 4.
Thickness of the coatings obtained by MAO as a function of applied voltage and Nb content.
The surface coatings of the Ti-25Ta-20Nb and Ti-25Ta-30Nb alloys exhibit irregular and elongated pores at 200 V. This characteristic intensifies at 250 V. When the voltage is increased to 300 V, the pore morphology becomes more uniform (Figure 3). Therefore, the increase in voltage influenced the microstructure of the coating. The irregular and unusual structure may be associated with the formation of preferential discharge channels, which alter the dynamics of local solidification of the oxide coating. This more extensive pore configuration can benefit applications that require a high surface area-to-volume ratio [50,55].
The study by Rossi et al. [56] reports a microstructure similar to that of alloys containing 20% and 30% Nb by weight. The Ti-33Nb-33Zr alloy, which exhibits a β structure, presents irregular and non-uniform pores after the MAO process at 300 V, using calcium acetate, magnesium acetate, and glycerophosphate as electrolyte components.
In addition to pores, cracks were observed in the Ti-25Ta-10Nb and Ti-25Ta-20Nb conditions produced at 300 V. These cracks occur due to the difference in expansion coefficients between the metals that make up the alloy and the elements present in the oxide layer, resulting in thermal shock [57]. Cracks on the surface of the coating can reduce its thickness and facilitate delamination of the oxide layer.
By shifting the focus to the increase in voltage, it is observed that the addition of Nb also leads to changes in the microstructure, particularly when there is a modification in the crystalline structure of the substrate. In the Ti-25Ta-10Nb alloy (mostly α″), there is an increase in pore diameter as the percentage by weight of Nb increases. However, the Ti-25Ta-20Nb and Ti-25Ta-30Nb alloys did not exhibit significant differences when compared, since both consist of the β phase. This behavior can be explained by the greater amount of Nb in the alloy, which contributes to an increase in the melting capacity of the pores due to the high energies released by the micro-arcs during the process [48].
Kaseem and Choe developed alloys in the binary Ti-xNb system and verified an increase in pore size in the coatings after the MAO process as the percentage of Nb by weight increased [58].
In summary, all conditions resulted in an oxide layer composed of pronounced, interconnected pores. The variation in voltage during the MAO technique, together with the addition of Nb, influenced the topography of the coating.
The presence of pores in the oxide layer is particularly relevant to the field of biomaterials. Pores facilitate cell adhesion and bone growth by promoting the proliferation of osteoblastic cells inside the pores. This porous structure also enhances resistance to wear and corrosion while favoring the osseointegration process [30,59,60].
Figure 4 shows the thickness of the oxide layers obtained from SEM micrographs of the cross-sectional views of the samples. However, to understand these results, it is necessary to relate them to the galvanostatic and potentiostatic stages corresponding to each condition.
Analysis of Figure 4 shows that, except for the samples produced at 300 V, the Ti-25Ta-10Nb alloy has the lowest oxide coating thickness values. However, returning to some of the discussions regarding the graphs in Figure 2a, it is clear that the sample with 10% by weight of Nb subjected to voltages of 250 and 300 V remained in the galvanostatic regime for a longer period, which is the main factor responsible for the formation of the coating. This behavior may be linked to changes in the crystal structure. According to Rossi et al. [56] and other researchers, the addition of Nb tends to increase the number of defects in the crystal lattice, favoring a greater incidence of localized electric arcs, which can lead to an increase in the thickness of the oxide layer.
At the same time, increasing the voltage led to a decrease in the thickness of the oxide layer, the samples subjected to voltages of 250 V (predominantly β) and 300 V (β phase) showed the lowest thickness values. This result partially corroborates previously published studies. According to Wang et al. [61], increasing the voltage does not necessarily result in a thicker coating due to irregularities at the coating–substrate interface, which can lead to asymmetric and uneven thicknesses.
Observe that although high voltages are associated with greater protection of the substrate, they can also promote the formation of cracks in the topography, as shown in Figure 4. These cracks act as deflectors for electric arcs and represent critical regions for the onset of the coating delamination, especially under high-temperature oxidation conditions [62,63,64].
In conclusion, the Ti-25Ta-30Nb alloy produced at 200 V exhibited the thickest oxide coating (~10 μm), whereas the same alloy processed at 300 V showed the least thickness (~3 μm). However, when considering the margin of error, there was little variation between the conditions due to the irregular morphology of the oxide coating [58]. Additionally, other studies have also reported slight changes in the coating thickness of alloys containing Nb.
3.2.3. Structural Analysis
Figure 5a shows the XRD patterns obtained from specimens with different concentrations of Nb. The addition of Nb influenced the alloy’s structure, resulting in the formation of an oxide coating.
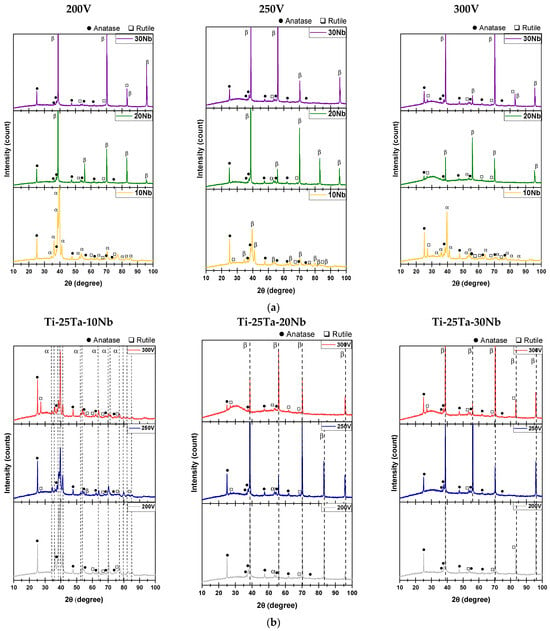
Figure 5.
XRD patterns of the oxide layers produced by MAO, based on different analyses: (a) variation in Nb content (upper graphs) and (b) variation in voltage (200, 250, and 300 V) (lower graphs).
In the Ti-25Ta-10Nb alloy, characteristic peaks of the α″ phase predominate. As the Nb concentration increases, the Ti-25Ta-20Nb alloy begins to exhibit the β crystalline structure in addition to the α″ phase. The Ti-25Ta-30Nb alloy, in turn, is exclusively composed of the β phase. The oxide coating in all three conditions exhibits TiO2 diffraction patterns, indicating the presence of both anatase and rutile crystalline phases. These results indicate that the addition of Nb promotes the stabilization of the β phase and that Ti is the most oxidizable element among the alloying components.
During surface modification, the incidence of electrical discharges generates localized heating inside the coating. This heating is intensified by the low thermal conductivity of the oxides, which hinders heat dissipation. As a result, the transformation of anatase into rutile is favored [65,66].
According to Jung et al. [67], the presence of anatase in the coating promotes greater integration of the implant with the bone tissue. Furthermore, anatase contributes to a porous and rough structure, increasing bone cell adhesion and proliferation [68]. In addition, anatase is also widely used in controlled drug release systems, where it can be modified to allow the gradual release of drugs [69].
Rutile also has benefits in the field of biomaterials. As the most stable form of TiO2, it maintains its chemical and mechanical properties over prolonged periods, which is an interesting option for implants that require longevity [70]. In addition, Chen et al. [67] observed that rutile has high hardness, making it ideal for applications in areas subject to high loads or friction.
Figure 5b also shows the X-ray diffractograms, but with a focus on the influence of increased voltage during the MAO process.
The alloys containing 20% and 30% Nb indicate that the voltage applied during the micro-arc oxidation process intensified diffraction peaks characteristic of the anatase and rutile crystalline phases. The increase in voltage implies that more energy was required to generate the electric arcs, resulting in high temperatures and pressures at these sites. These conditions promoted the partial transformation of the anatase phase into rutile [18].
It is important to note that, under none of the conditions produced were characteristic peaks of Ta pentoxide (Ta2O5) or Nb oxide (Nb2O5) detected in the XRD patterns. The absence of these oxides can be attributed to the high melting points of Ta and Nb, which require significantly more energy for oxide formation during the process. According to Kuroda et al. [71], the synthesis of Ta2O5 can be achieved through annealing heat treatments at temperatures of around 700 °C.
The crystalline phases in the coatings obtained by MAO were quantified to understand the effect of adding Nb to the Ti-25Ta-xNb alloys (x = 10%, 20%, and 30%). The results are presented in Figure 6. Increasing the Nb concentration promotes the formation of more rutile in the coatings. This behavior can be attributed to the intensification of surface exothermic reactions during the MAO process. Additionally, the presence of Nb interferes with the galvanostatic stage, reducing its duration and consequently increasing the energy associated with the electrical discharges. This increase in energy favors the transition from the anatase phase to the rutile phase, as reported in the literature [47].
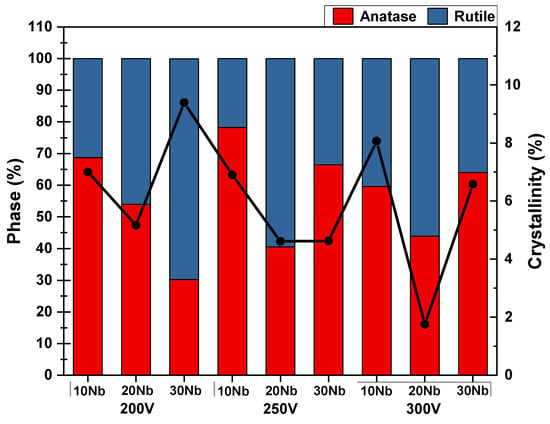
Figure 6.
Quantifying the phases that make up the oxide layer (Y-axis on the left, represented by the bar graph) and degree of crystallinity (Y-axis on the right, represented by the line graph).
Figure 6 also shows that the applied voltage influences phase formation. For the samples treated at 200 V, a reduction in the proportion of anatase and an increase in the amount of rutile is observed with the addition of Nb.
A similar behavior was observed in the treatments carried out at 250 V, where the addition of Nb resulted in a decrease in the anatase content. The phases present in the coatings were quantified using Equation (1), which is based on the relative intensities of the characteristic peaks identified in the XRD analyses.
In addition, all the coatings, regardless of the processing condition, exhibited an amorphous halo in the diffractograms, indicating low crystallinity. The crystallinity analysis presented in Figure 6 reinforces this observation, indicating that crystallinity remains low and varies with both the alloy composition and the applied voltage.
During the MAO process, the samples are subjected to intense electrical discharges, which can reach temperatures of up to 10,000 K [64]. However, once the oxide’s dielectric barrier is broken, there is a sudden reduction in the electric current and the amount of plasma generated, allowing the electrolyte to rapidly cool the surface. As a result, the localized melting of the coating is followed by almost instantaneous re-solidification, preventing the atoms from organizing into more orderly crystalline structures. This rapid solidification promotes the formation of an amorphous surface layer, a characteristic feature of the coatings obtained under the studied conditions [18].
3.2.4. Chemical Analysis
The quantifications of the elements obtained by SEM-EDS analysis are shown in Figure 7. The results indicate that all the samples are composed of the alloying elements (Ti, Ta, and Nb) and the elements present in the electrolyte (Ca, P, Mg, and Ag). Carbon (C) was also identified, having been incorporated into the oxide coating from calcium acetate, disodium glycerophosphate, and magnesium acetate. This result corroborates the data obtained from the XRD profiles, in which characteristic peaks of TiO2 were observed in the anatase and rutile crystalline phases.
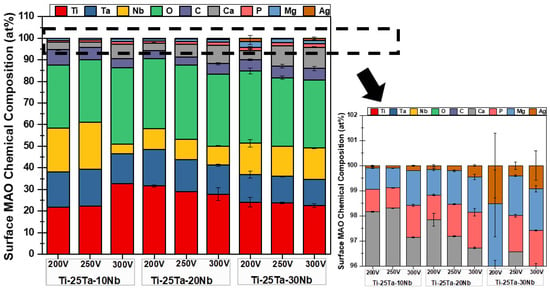
Figure 7.
Chemical composition of the substrate and the oxide layer of the Ti-25Ta-xNb alloys produced under different voltages.
The percentages by weight of Ta and Nb vary compared to the substrate. Ta showed values below 25% in all conditions, as did Nb and their respective proportions. This disagreement was expected due to possible oxidation processes, segregation, and volatilization of the elements during the MAO technique. In addition, EDS measurements are semi-quantitative and analyzed at a limited depth, so it is understood that the chemical composition shown in the graph in Figure 7 refers to the surface layers.
The elements Ca, P, Mg, and Ag were also identified in all analyzed conditions, although in small amounts. Ca, P, and Mg are widely used in the biofunctionalization of Ti alloys through the MAO process, as they contribute significantly to bone tissue mineralization and support the adhesion, differentiation, and proliferation of osteoblasts [72]. Coatings obtained by MAO that exhibit a porous topography and contain Ca and P tend to enhance cell adhesion. The porosity and roughness of the surface increase the contact area with the bone, promoting better integration. Moreover, Ca2+ and PO43− ions facilitate protein fixation, stimulating the growth and differentiation of bone cells [73].
Using Ca and P in the oxide layer is also beneficial, as these elements comprise the mineral hydroxyapatite, the primary inorganic component of bone tissue. Using the molar ratio between Ca and P, it is possible to check whether hydroxyapatite is formed by comparing it with the reference value of 1.67 [64].
The calculations for all conditions indicate that this mineral is not present in the formed oxide layer. Thermal treatments can be carried out to crystallize the amorphous part, thereby increasing the probability of obtaining hydroxyapatite [71].
Additionally, Ag plays a different role when added to the electrolyte. Since Ag confers antimicrobial properties, the aim is to reduce problems related to implant infections. Studies such as those by Shimabukuro et al. [74] have verified the antibacterial efficacy of Ag coatings against bacterial strains, including E. coli and S. aureus.
The increase in voltage and the addition of Nb had no significant influence on the results.
Figure 8 shows the results obtained through Raman spectroscopy analysis, which depend on the addition of Nb and the variation of the applied voltage. This technique, sensitive to molecular vibrations, allows us to observe that, regardless of the voltage applied during the MAO process [75], the Ti-25Ta-10Nb alloy exhibited the most intense peaks corresponding to the anatase crystalline phase. This finding corroborates the XRD analysis and confirms that the Ti-25Ta-10Nb alloy exhibits greater crystallinity among the alloys with the highest anatase content.
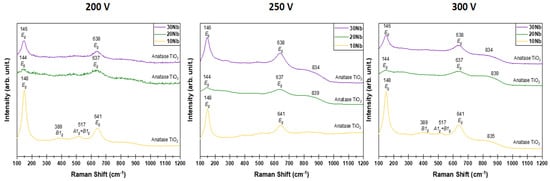
Figure 8.
Raman spectra of the samples produced by MAO under different voltages (200, 250, and 300 V) and different Nb compositions.
The intensity of the anatase peaks is reduced in alloys with 20% and 30% by weight of Nb. A new signal around is identified when the oxide layer is formed at 250 and 300 V. This peak seems to be related to the presence of rutile, also identified under these conditions using X-ray diffractograms (Figure 5a). Similarly, the addition of Nb contributes to a reduction in the amount of anatase.
No major changes were observed when the voltage was varied between 200 and 300 V. Table 3 presents the Raman peaks detected in comparison to the vibrational modes described in the literature [76,77,78].

Table 3.
Assignment of the Raman peaks detected as a function of the vibrational modes of TiO2.
3.2.5. Mechanical Analysis
When carrying out surface modification, it is important to understand the coating’s adhesion to the substrate. Daimler Benz proposed the Rockwell-C classification using the Verein Deutscher Ingenieure (VDI) standard. Micrographs labeled HF1 represent high coating adhesion to the substrate, while HF6 reflects low adhesion [79].
Figure 9 shows the optical micrographs of the indentations and their respective classifications. It can be seen that all the samples exhibit satisfactory adhesion, with HF3 being the highest index obtained among the sets. The alloys produced at a voltage of 200 V and the Ti-25Ta-20Nb alloy prepared at a voltage of 250 V show better adhesion between the substrates and the oxide layers and are classified as HF1. This result corroborates the scientific literature, indicating that lower voltages (200 V) generate low-energy electrical discharges and, consequently, lower temperatures, which favor more efficient adhesion of the coating to the substrate.
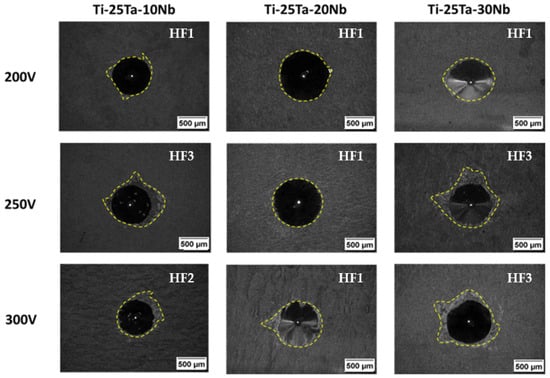
Figure 9.
Optical micrographs for analyzing the adhesion of biofunctionalized oxide coatings containing Ca, P, Mg, and Ag to the Ti-25Ta-xNb substrate produced by MAO at 200, 250, and 300 V.
The Ti-25Ta-10Nb and Ti-25Ta-30Nb alloys, produced at 250 and 300 V, showed indices between HF2 and HF3, which are still considered indicative of acceptable adhesion, as no substrate fragments were observed within the cracks around the indentation.
4. Conclusions
The elements Nb and Ta acted as β-phase stabilizers in the Ti-25Ta-xNb alloy system, directly influencing the formation of oxide layers.
The addition of Nb advanced the potentiostatic regime and resulted in oxide layers with lower electrical conductivity, highlighting the influence of alloy composition on coating performance.
The variation in applied voltage during the MAO process affected the morphology and thickness of the layers. Higher voltages increased the micro-arc energy, leading to thinner, more uniform layers with larger pores.
The alloy predominantly composed of the α″ phase (Ti-25Ta-10Nb) exhibited higher porosity compared to the alloys with a body-centered cubic structure (20% and 30% Nb).
Chemical characterization revealed that the TiO2 layers were predominantly composed of anatase and rutile phases, which may offer biological benefits.
An increase in voltage (from 200 to 300 V) causes a decrease in anatase content and an increase in rutile formation, which is associated with higher discharge energy during the process.
All samples exhibited low crystallinity, attributed to the presence of an amorphous phase formed during rapid solidification.
Strong adhesion of the coatings to the substrate was observed, highlighting the potential of the MAO technique for producing bioactive layers.
The oxide layers formed by MAO on the Ti-25Ta-xNb alloy system exhibited promising characteristics, including the presence of anatase and rutile phases, suitable porosity, and good adhesion, indicating their potential for biomedical applications.
Author Contributions
Conceptualization, F.d.F.Q., D.R.N.C., M.F. and J.V.R.; formal analysis, F.d.F.Q. and D.R.N.C.; resources, C.R.G. and J.V.R.; data curation, F.d.F.Q., D.R.N.C., M.O. and O.N.P.; writing—original draft preparation, F.d.F.Q., D.R.N.C. and J.V.R.; writing—review and editing, M.F., C.R.G. and J.V.R.; visualization, F.d.F.Q., M.O. and O.N.P.; supervision, C.R.G. and J.V.R.; project administration, J.V.R.; funding acquisition, D.R.N.C. and C.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Coordination of Superior Level Staff Improvement (CAPES; grant 88881.980553/2024-01), the National Council for Scientific and Technological Research (CNPq; grants #314.810/2021–8, #404020/2023–2 and #421.677/2023–6), and the São Paulo Research Foundation (FAPESP; grants #2022/15205–6, #2024/03148–3 and 2024/01132–2) funding agencies.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The results will be provided upon request.
Acknowledgments
The authors thank Fenelon Martinho Lima Pontes for the XRD measurements and Luiz Gustavo Possato for the Raman spectroscopy. The technical support of Luca Imperatori, Massimo Di Menno Di Bucchianico, Rover Belo (UFSCar), and Wilians Govedise is also gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pesode, P.; Barve, S. A Review—Metastable β Titanium Alloy for Biomedical Applications. J. Eng. Appl. Sci. 2023, 70, 25. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C 2020, 106, 110154. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S.; Singh, H.; Gepreel, M.A.-H. A Review on Alloy Design, Biological Response, and Strengthening of β-Titanium Alloys as Biomaterials. Mater. Sci. Eng. C 2021, 121, 111661. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Chen, C.; Zhao, Z. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Kamath, A.F.; Ong, K.L.; Lau, E.; Chan, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J.; Bozic, K.J. Quantifying the Burden of Revision Total Joint Arthroplasty for Periprosthetic Infection. J. Arthroplast. 2015, 30, 1492–1497. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Long, W.J.; Scott, W.N.; Old, A.B. Revision of Total Knee Arthroplasties Performed in Young, Active Patients with Posttraumatic Arthritis and Osteoarthritis. J. Knee Surg. 2017, 30, 905–908. [Google Scholar] [CrossRef]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.-Y.; Anderson, D.E. Bone and Cartilage Interfaces With Orthopedic Implants: A Literature Review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based alloys and composites for orthopedic implants Applications: A comprehensive review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- de Freitas Quadros, F.; de Oliveira, J.R.; dos Santos, J.F.; Bassi, A.P.F.; Jorge, J.H.; Tavares, D.C.; Grandini, C.R. Chemical, Structural, Microstructural, Mechanical, and Biological Characterization of Ti–25Ta-xNb System Alloys for Biomedical Applications. J. Mater. Res. Technol. 2024, 28, 3699–3706. [Google Scholar] [CrossRef]
- Bieler, T.R.; Zeng, L. Alloys: Titanium; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Vizureanu, P.; Bălțatu, M.S. Titanium-Based Alloys for Biomedical Applications; Materials Research Forum LLC: Millersville, PA, USA, 2020. [Google Scholar]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Hou, W.-T.; Lou, N.; Wang, H.-D.; Cai, Z.-H.; Xing, Z.-G.; Piao, Z.-Y. Research on the microstructure and corrosion-wear resistance mechanism of Ti–6Al–4V alloy under cryogenic burnishing. J. Mater. Res. Technol. 2025, 35, 5854–5871. [Google Scholar] [CrossRef]
- Oliveira, F.G.; Henriques, B.; Toptan, F.; Silva, F.S.; Zheludkevich, M.; Montemor, M.F. Understanding Growth Mechanisms and Tribocorrosion Behaviour of Porous TiO2 Anodic Films Containing Calcium, Phosphorus and Magnesium. Appl. Surf. Sci. 2015, 341, 1–12. [Google Scholar] [CrossRef]
- Alves, S.A.; Rossi, A.L.; Ribeiro, A.R.; Toptan, F.; Pinto, A.M.; Celis, J.-P.; Shokuhfar, T.; Rocha, L.A. Tribo-electrochemical behavior of bio-functionalized TiO2 nanotubes in artificial saliva: Understanding of degradation mechanisms. Wear 2017, 384–385, 28–42. [Google Scholar] [CrossRef]
- Vijayalakshmi, U.; Chellappa, M.; Anjaneyulu, U.; Manivasagam, G. Preparation and evaluation of the cytotoxic nature of TiO2 nanoparticles by direct contact method. Int. J. Nanomed. 2015, 10, 31–41. [Google Scholar] [CrossRef]
- Antonio, R.F.; Rangel, E.C.; Mas, B.A.; Duek, E.A.; Cruz, N.C. Growth of hydroxyapatite coatings on tantalum by plasma electrolytic oxidation in a single step. Surf. Coat. Technol. 2019, 357, 698–705. [Google Scholar] [CrossRef]
- Li, L.-H.; Kong, Y.-M.; Kim, H.-W.; Kim, Y.-W.; Kim, H.-E.; Heo, S.-J.; Koak, J.-Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef]
- Wen, X.; Liu, Y.; Xi, F.; Zhang, X.; Kang, Y. Micro-arc oxidation (MAO) and its potential for improving the performance of titanium implants in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1282590. [Google Scholar] [CrossRef] [PubMed]
- Quintero, D.; Galvis, O.; Calderón, J.; Castaño, J.; Echeverría, F. Effect of electrochemical parameters on the formation of anodic films on commercially pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2014, 258, 1223–1231. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Electrochemical and biological characterization of coatings formed on Ti–15Mo alloy by plasma electrolytic oxidation. Mater. Sci. Eng. C 2014, 43, 172–181. [Google Scholar] [CrossRef]
- Yılmaz, E.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; İyIbilgin, O. Mechanical properties and electrochemical behavior of porous Ti-Nb biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 87, 59–67. [Google Scholar] [CrossRef]
- Liu, J.; Chang, L.; Liu, H.; Li, Y.; Yang, H.; Ruan, J. Microstructure, mechanical behavior and biocompatibility of powder metallurgy Nb-Ti-Ta alloys as biomedical material. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Niinomi, M. Ti–25Ta alloy with the best mechanical compatibility in Ti–Ta alloys for biomedical applications. Mater. Sci. Eng. C 2009, 29, 1061–1065. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.A.; Haithcock, D.A.; Wieland, M.; Schwartz, Z.; Boyan, B.D. Biocompatibility of Niobium Coatings. Coatings 2011, 1, 72–87. [Google Scholar] [CrossRef]
- Babaei, K.; Fattah-Alhosseini, A.; Chaharmahali, R. A review on plasma electrolytic oxidation (PEO) of niobium: Mechanism, properties and applications. Surf. Interfaces 2020, 21, 100719. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in Niobium Oxide-Containing Coatings for Biomedical Applications: A Critical Review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; da Cruz, N.C.; Rangel, E.C.; Fais, L.M.; Vaz, L.G.; Barão, V.A. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloys Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Tao, X.; Li, S.; Zheng, C.; Fu, J.; Hao, Y.; Yang, R.; Guo, Z. Synthesis of a porous oxide layer on a multifunctional biomedical titanium by micro-arc oxidation. Mater. Sci. Eng. C 2009, 29, 1923–1934. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of Beta-Stabilizing Elements of Titanium Alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-P.; Kaseem, M.; Choe, H.-C. Plasma electrolytic oxidation of Ti-25Nb-xTa alloys in solution containing Ca and P ions. Surf. Coat. Technol. 2020, 395, 125916. [Google Scholar] [CrossRef]
- Qiao, L.; Lou, J.; Zhang, S.; Qu, B.; Chang, W.; Zhang, R. The entrance mechanism of calcium and phosphorus elements into micro arc oxidation coatings developed on Ti6Al4V alloy. Surf. Coat. Technol. 2016, 285, 187–196. [Google Scholar] [CrossRef]
- Costa, N.; Correa, D.; Lisboa-Filho, P.; Sousa, T.; Grandini, C.; Rocha, L. Influence of the molybdenum on characteristics of oxide films produced by micro-arc oxidation on Ti-15Zr-based alloys. Surf. Coat. Technol. 2021, 408, 126856. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.; Affonço, L.; Lisboa-Filho, P.; da Silva, J.; Rocha, L.; Pinto, A.; Toptan, F. Corrosion and tribocorrosion behaviour of titanium nitride thin films grown on titanium under different deposition times. Surf. Coat. Technol. 2019, 374, 878–888. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Oliveira, J.R.; Bassi, A.P.F.; dos Santos, J.F.; de Freitas Quadros, F.; Jorge, J.H.; Grandini, C.R. Assessment of Applied Voltage on the Structure, Pore Size, Hardness, Elastic Modulus, and Adhesion of Anodic Coatings in Ca-, P-, and Mg-Rich Produced by MAO in Ti–25Ta–Zr Alloys. J. Mater. Res. Technol. 2023, 26, 4656–4669. [Google Scholar] [CrossRef]
- Khan, H.; Matin, M.A.; Cho, D.-W.; Kim, J.H.; Kurniawan, N.A.; Abdullahi, A.A. Experimental Methods in Chemical Engineering: X-ray Diffraction Spectroscopy—XRD. Can. J. Chem. Eng. 2020, 98, 1255–1266. [Google Scholar] [CrossRef]
- Kayali, Y.; Taktak, S. Characterization and Rockwell-C adhesion properties of chromium-based borided steels. J. Adhes. Sci. Technol. 2015, 29, 2065–2075. [Google Scholar] [CrossRef]
- Heo, J.; Lee, J.; Kim, S.; Alfantazi, A.; Cho, S.O. Corrosion resistance of austenitic stainless steel using cathodic plasma electrolytic oxidation. Surf. Coat. Technol. 2023, 462, 129448. [Google Scholar] [CrossRef]
- Lee, C.; Ju, C.-P.; Lin, J.C. Structure–Property Relationship of Cast Ti–Nb Alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef]
- Pesode, P.; Barve, S. Surface modification of titanium and titanium alloy by plasma electrolytic oxidation process for biomedical applications: A review. Mater. Today: Proc. 2021, 46, 594–602. [Google Scholar] [CrossRef]
- Duarte, L.T.; Bolfarini, C.; Biaggio, S.R.; Rocha-Filho, R.C.; Nascente, P.A. Growth of aluminum-free porous oxide layers on titanium and its alloys Ti-6Al-4V and Ti-6Al-7Nb by micro-arc oxidation. Mater. Sci. Eng. C 2014, 41, 343–348. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Cardoso, G.C.; Grandini, C.R. The effect of Nb on the formation of TiO2 anodic coating oxide on Ti–Nb alloys through MAO treatment. J. Mater. Res. Technol. 2024, 29, 1165–1171. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; Grandini, C.R.; Afonso, C.R.M. Surface Characterization of New β Ti-25Ta-Zr-Nb Alloys Modified by Micro-Arc Oxidation. Materials 2023, 16, 2352. [Google Scholar] [CrossRef]
- Cardoso, G.C.; Souza, V.S.; dos Santos, F.C.; Alves, R.; Rocha, R.C.; de Oliveira, J.R.; Bassi, A.P.F.; Jorge, J.H. Antimicrobial Cu-Doped TiO2 Coatings on the β Ti-30Nb-5Mo Alloy by Micro-Arc Oxidation. Materials 2023, 17, 156. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Zhang, D.; Li, H.; Yang, L.; Liu, H. The Influence of Alloy Elements in Ti6Al4V and Ti35Nb2Ta3Zr on the Structure, Morphology and Properties of MAO Coatings. Vacuum 2018, 157, 229–236. [Google Scholar] [CrossRef]
- Yavari, S.A.; Necula, B.S.; Fratila-Apachitei, L.E.; Duszczyk, J.; Apachitei, I. Biofunctional surfaces by plasma electrolytic oxidation on titanium biomedical alloys. Surf. Eng. 2016, 32, 411–417. [Google Scholar] [CrossRef]
- Snezhko, L.A.; Erokhin, A.L.; Kalinichenko, O.A.; Misnyankin, D.A. Hydrogen Release on the Anode in the Course of Plasma Electrolytic Oxidation of Aluminum. Mater. Sci. 2016, 52, 421–430. [Google Scholar] [CrossRef]
- Snizhko, L.O. The nature of anodic gas at plasma electrolytic oxidation. Prot. Met. Phys. Chem. Surfaces 2014, 50, 705–708. [Google Scholar] [CrossRef]
- Rudnev, V.; Medkov, M.; Lukiyanchuk, I.; Steblevskaya, N.; Kilin, K.; Belobeletskaya, M. Ta-containing coatings formed on titanium and stainless steel by plasma electrolytic oxidation and/or extraction pyrolysis. Surf. Coat. Technol. 2014, 258, 1232–1238. [Google Scholar] [CrossRef]
- Yasui, T.; Hayashi, K.; Fukumoto, M. Behaviors of Micro-Arcs, Bubbles, and Coating Growth during Plasma Electrolytic Oxidation of β-Titanium Alloy. Materials 2022, 16, 360. [Google Scholar] [CrossRef]
- Rossi, M.C.; dos Santos, R.F.; Kuroda, P.A.B.; Afonso, C.R.M. Characteristics of ceramic-like coatings obtained by plasma electrolyte oxidation on different Ti alloys. Boletin Soc. Esp. Ceram. Vidr. 2023, 63, 33–46. [Google Scholar] [CrossRef]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.-C. Electrochemical and bioactive characteristics of the porous surface formed on Ti-xNb alloys via plasma electrolytic oxidation. Surf. Coat. Technol. 2019, 378, 125027. [Google Scholar] [CrossRef]
- Echeverry-Rendón, M.; Galvis, O.; Giraldo, D.Q.; Pavón, J.; López-Lacomba, J.L.; Jiménez-Piqué, E.; Anglada, M.; Robledo, S.M.; Castaño, J.G.; Echeverría, F. Osseointegration improvement by plasma electrolytic oxidation of modified titanium alloys surfaces. J. Mater. Sci. Mater. Med. 2015, 26, 72. [Google Scholar] [CrossRef]
- Xie, L.; Yin, G.; Yan, D.; Liao, X.; Huang, Z.; Yao, Y.; Kang, Y.; Liu, Y. Structure, morphology and fibroblasts adhesion of surface-porous titanium via anodic oxidation. J. Mater. Sci. Mater. Med. 2009, 21, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cheng, Z.; Zhang, Y.; Shi, X.; Rao, M.; Wu, S. Effect of Voltage on the Microstructure and High-Temperature Oxidation Resistance of Micro-Arc Oxidation Coatings on AlTiCrVZr Refractory High-Entropy Alloy. Coatings 2022, 13, 14. [Google Scholar] [CrossRef]
- Kuromoto, N.K.; Simão, R.A.; Soares, G.A. Titanium oxide films produced on commercially pure titanium by anodic oxidation with different voltages. Mater. Charact. 2007, 58, 114–121. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Huang, T.-Y.; Zhai, D.-J.; Wang, H.-B.; Feng, K.-Q.; Xiang, L. Study on strontium doped bioactive coatings on titanium alloys surfaces by micro-arc oxidation. Surf. Coat. Technol. 2022, 451, 129045. [Google Scholar] [CrossRef]
- Chen, K.-T.; Huang, J.-W.; Lin, W.-T.; Kuo, T.-Y.; Chien, C.-S.; Chang, C.-P.; Lin, Y.-D. Effects of Micro-Arc Oxidation Discharge Parameters on Formation and Biomedical Properties of Hydroxyapatite-Containing Flower-like Structure Coatings. Materials 2022, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mater. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Jung, Y.C.; Shin, K.R.; Ko, Y.G.; Shin, D.H. Surface characteristics and biological response of titanium oxide layer formed via micro-arc oxidation in K3PO4 and Na3PO4 electrolytes. J. Alloys Compd. 2014, 586, S548–S552. [Google Scholar] [CrossRef]
- Yu, W.Q.; Liu, X.B.; Chen, C.; Yang, S.; Li, D.; Wu, S.; Wei, S. The Effect of Anatase TiO2 Nanotube Layers on MC3T3-E1 Preosteoblast Adhesion, Proliferation, and Differentiation. J. Biomed. Mater. Res. Part A 2010, 94, 1012–1022. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Staroń, A.; Pulit-Prociak, J.; Banach, M. Methods for Reducing the Toxicity of Metal and Metal Oxide NPs as Biomedicine. Materials 2020, 13, 279. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Kuroda, P.A.B.; de Mattos, F.N.; Grandini, C.R.; Afonso, C.R.M. Effect of heat treatment on the phases, pore size, roughness, wettability, hardness, adhesion, and wear of Ti-25Ta MAO coatings for use as biomaterials. J. Mater. Sci. 2023, 58, 15485–15498. [Google Scholar] [CrossRef]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- Wu, S.-D.; Zhang, H.; Dong, X.-D.; Ning, C.-Y.; Fok, A.S.; Wang, Y. Physicochemical properties and in vitro cytocompatibility of modified titanium surfaces prepared via micro-arc oxidation with different calcium concentrations. Appl. Surf. Sci. 2015, 329, 347–355. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Yamada, R.; Ashida, M.; Chen, P.; Doi, H.; Nozaki, K.; Nagai, A.; Hanawa, T. Investigation of Realizing Both Antibacterial Property and Osteogenic Cell Compatibility on Titanium Surface by Simple Electrochemical Treatment. ACS Biomater. Sci. Eng. 2019, 5, 5623–5630. [Google Scholar] [CrossRef]
- Yuferov, Y.; Borodianskiy, K. Ca/P in situ introduction for enhancing coating biocompatibility via plasma electrolytic oxidation in low-temperature molten salt. Open Ceram. 2024, 18, 100602. [Google Scholar] [CrossRef]
- Arsov, L.D.; Kormann, C.; Plieth, W. Electrochemical synthesis and in situ Raman spectroscopy of thin films of titanium dioxide. J. Raman Spectrosc. 1991, 22, 573–575. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Dai, Y.; Zhang, Y.; Lu, B.; Ma, G. Raman study of phase transformation of TiO2 rutile single crystal irradiated by infrared femtosecond laser. Appl. Surf. Sci. 2007, 253, 7497–7500. [Google Scholar] [CrossRef]
- Rodríguez-Castro, G.A.; Jiménez-Tinoco, L.F.; Méndez-Méndez, J.V.; Arzate-Vázquez, I.; Meneses-Amador, A.; Martínez-Gutiérrez, H.; Campos-Silva, I. Damage Mechanisms in AISI 304 Borided Steel: Scratch and Daimler-Benz Adhesion Tests. Mater. Res. 2015, 18, 1346–1353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).