Enhanced Corrosion Resistance and Cytocompatibility of Magnesium Alloys with Mg(OH)2/Polydopamine Composite Coatings for Orthopedic Applications
Abstract
1. Introduction
2. Material and Methods
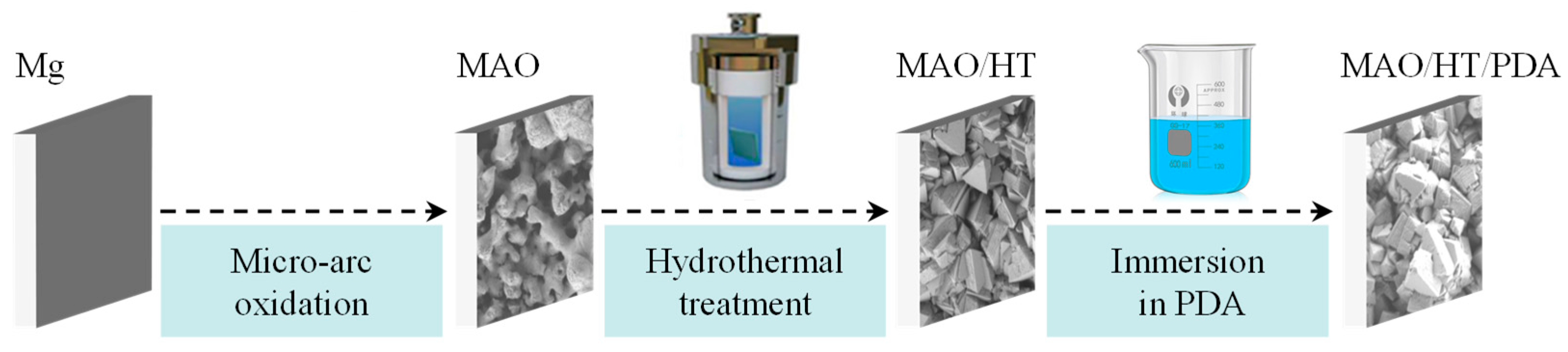
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Corrosion Resistance Evaluation
2.4. In Vitro Cytocompatibility
3. Results and Discussion
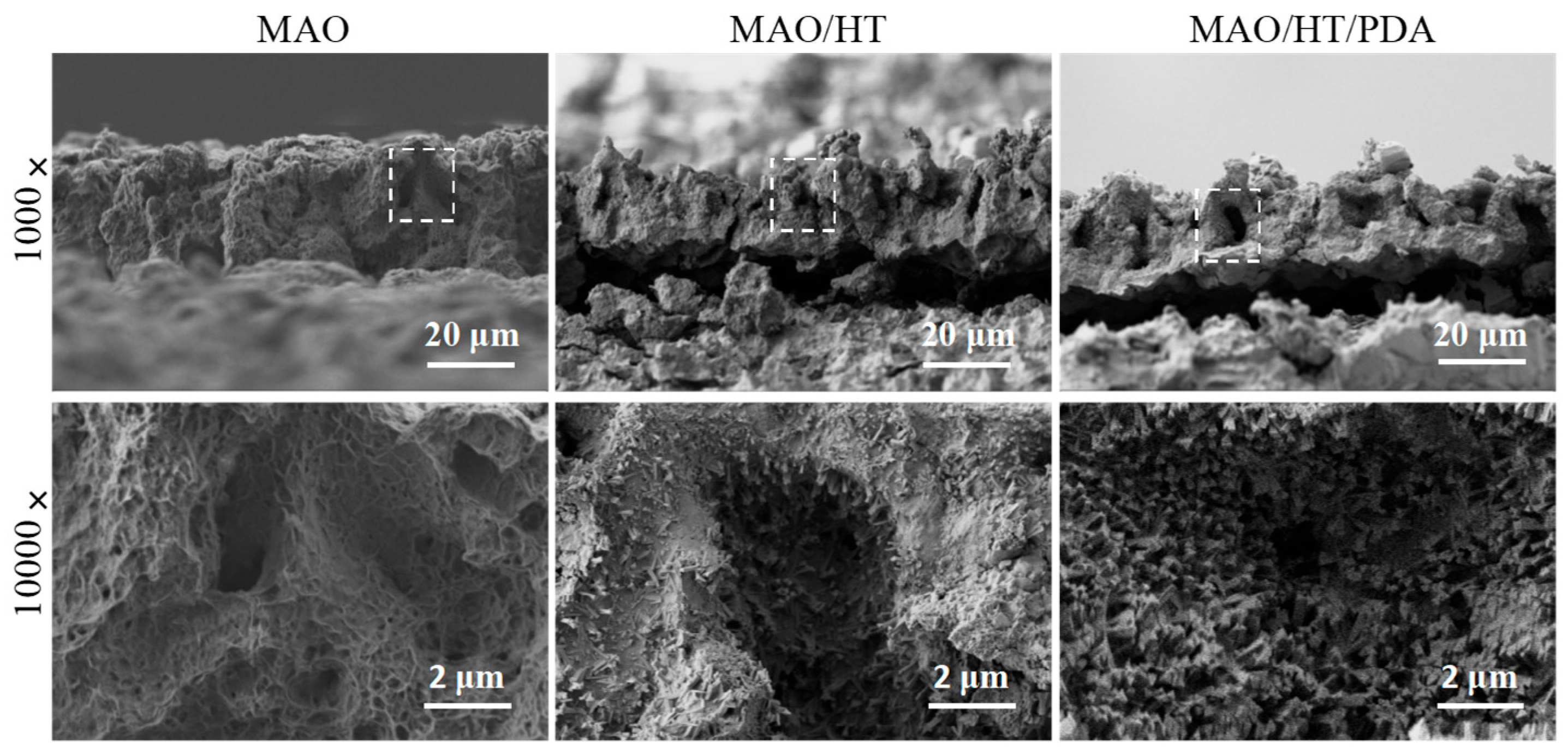
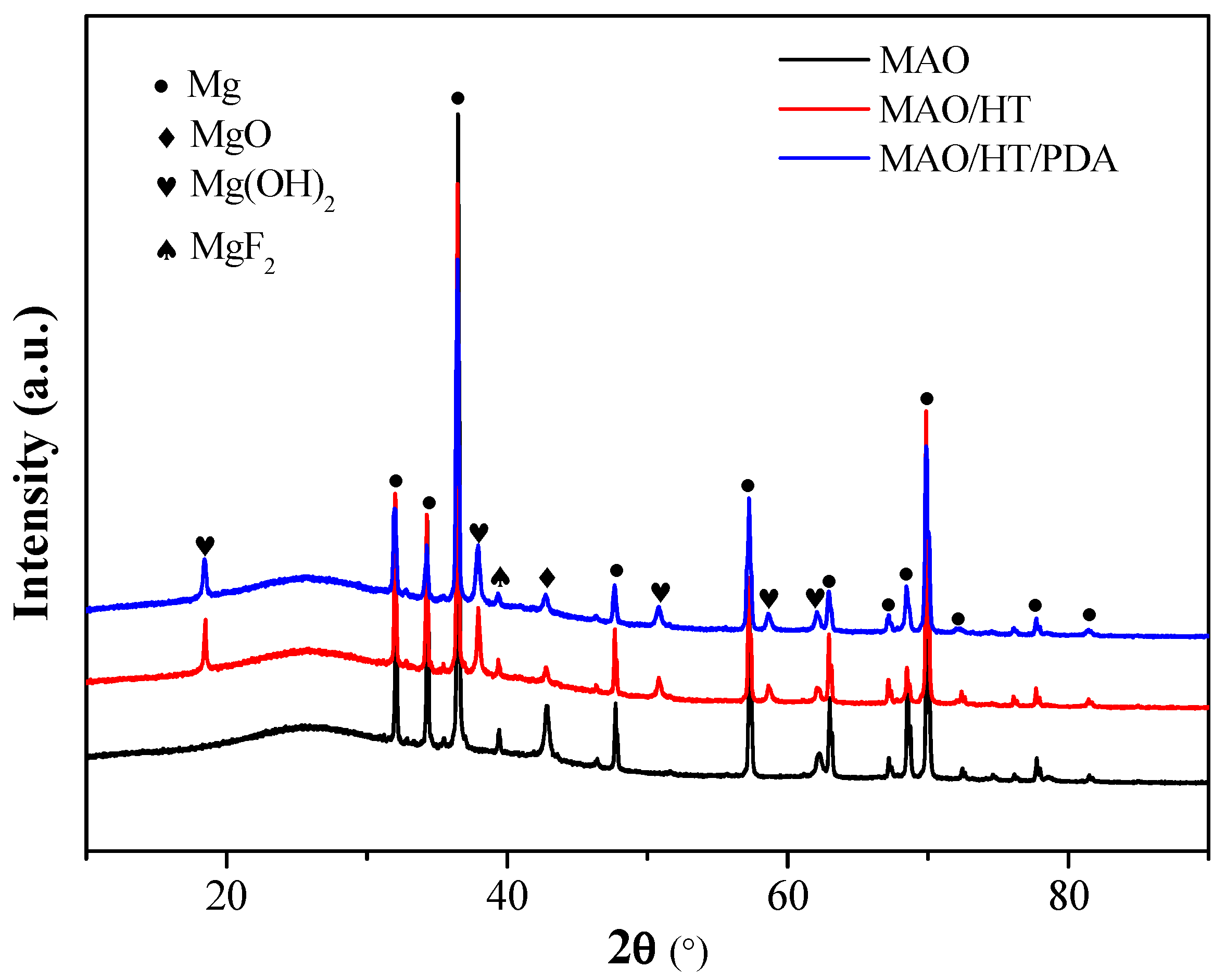
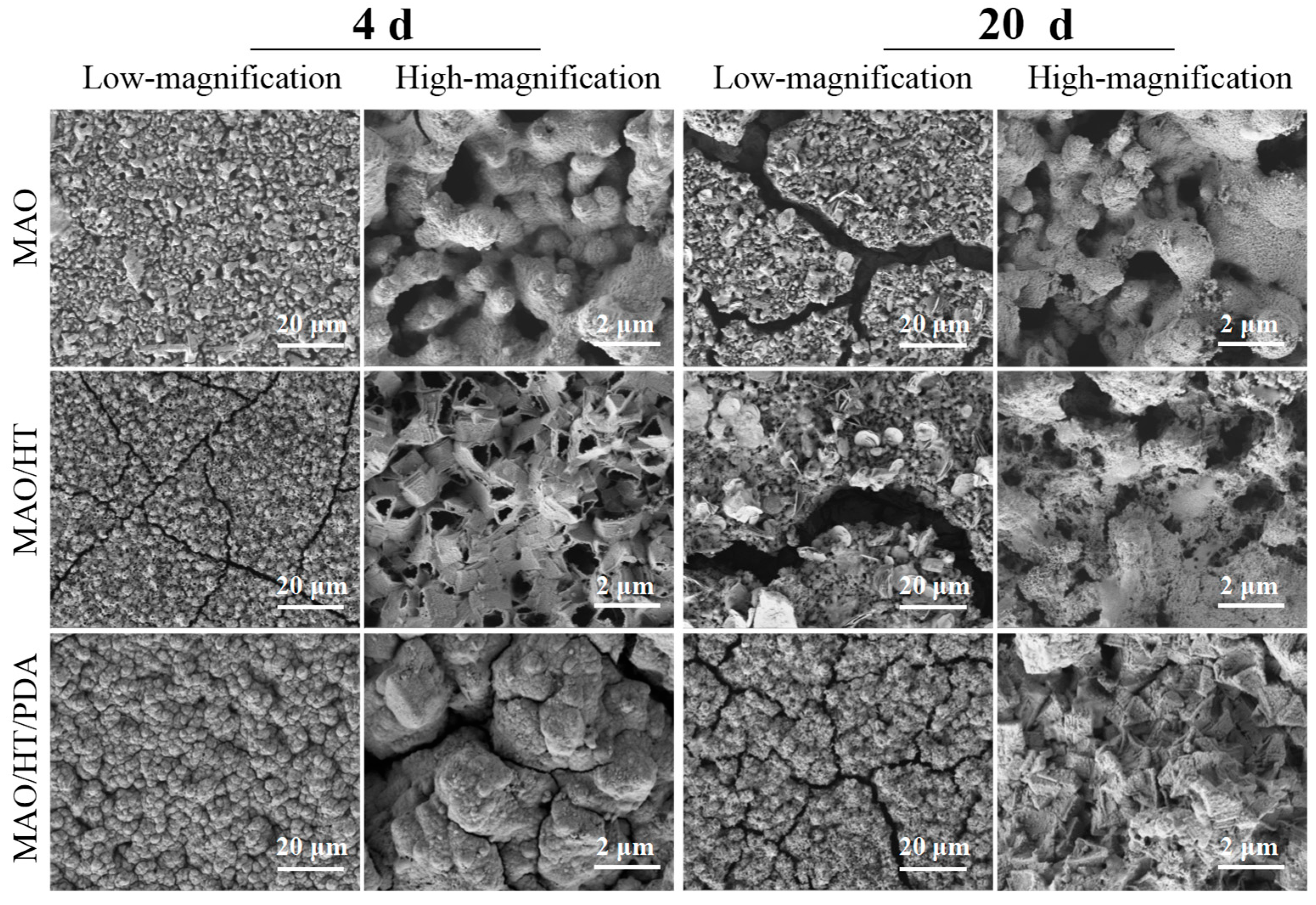
3.1. Coating Character
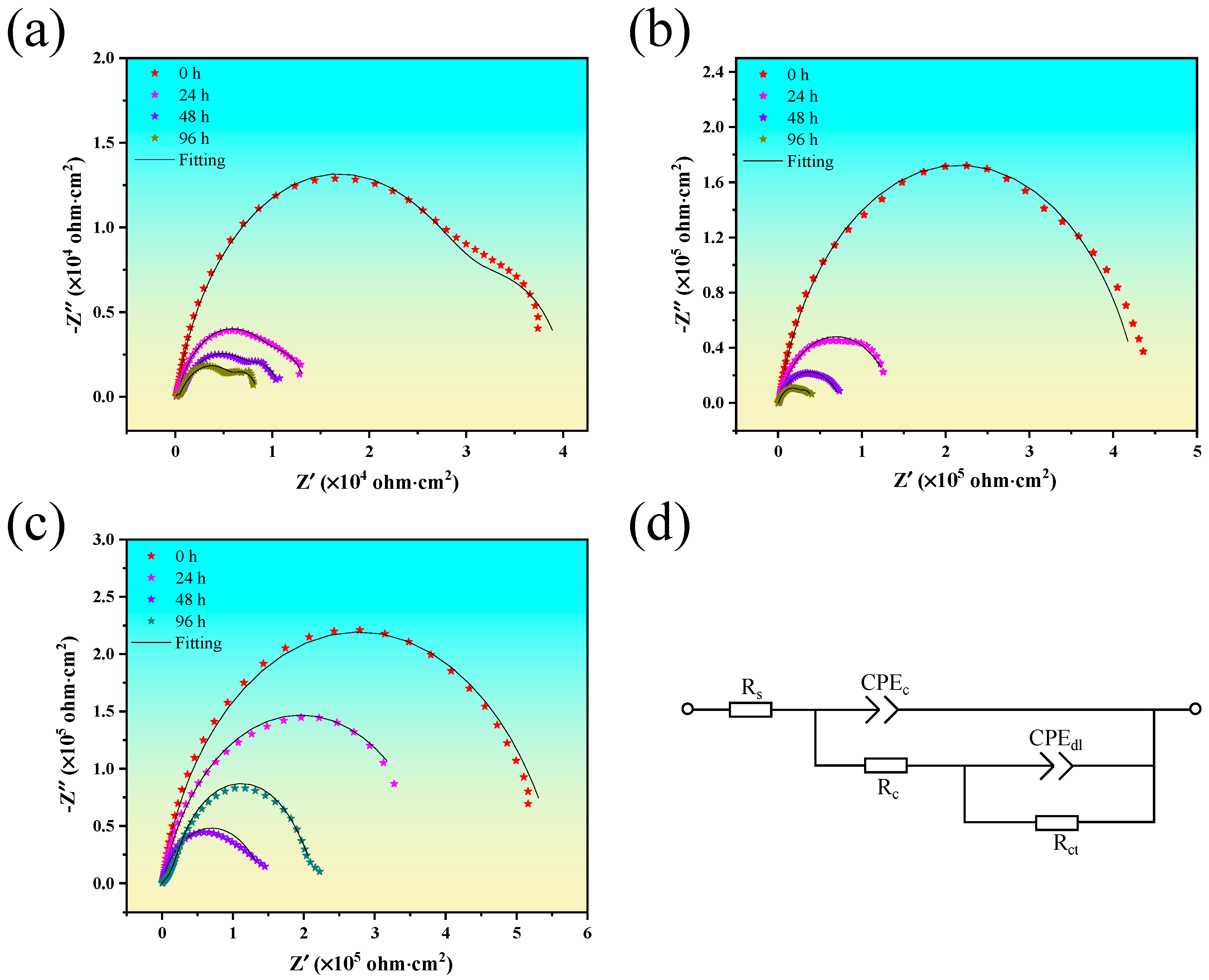
3.2. Corrosion Resistance
3.3. Degradation Behavior In Vitro
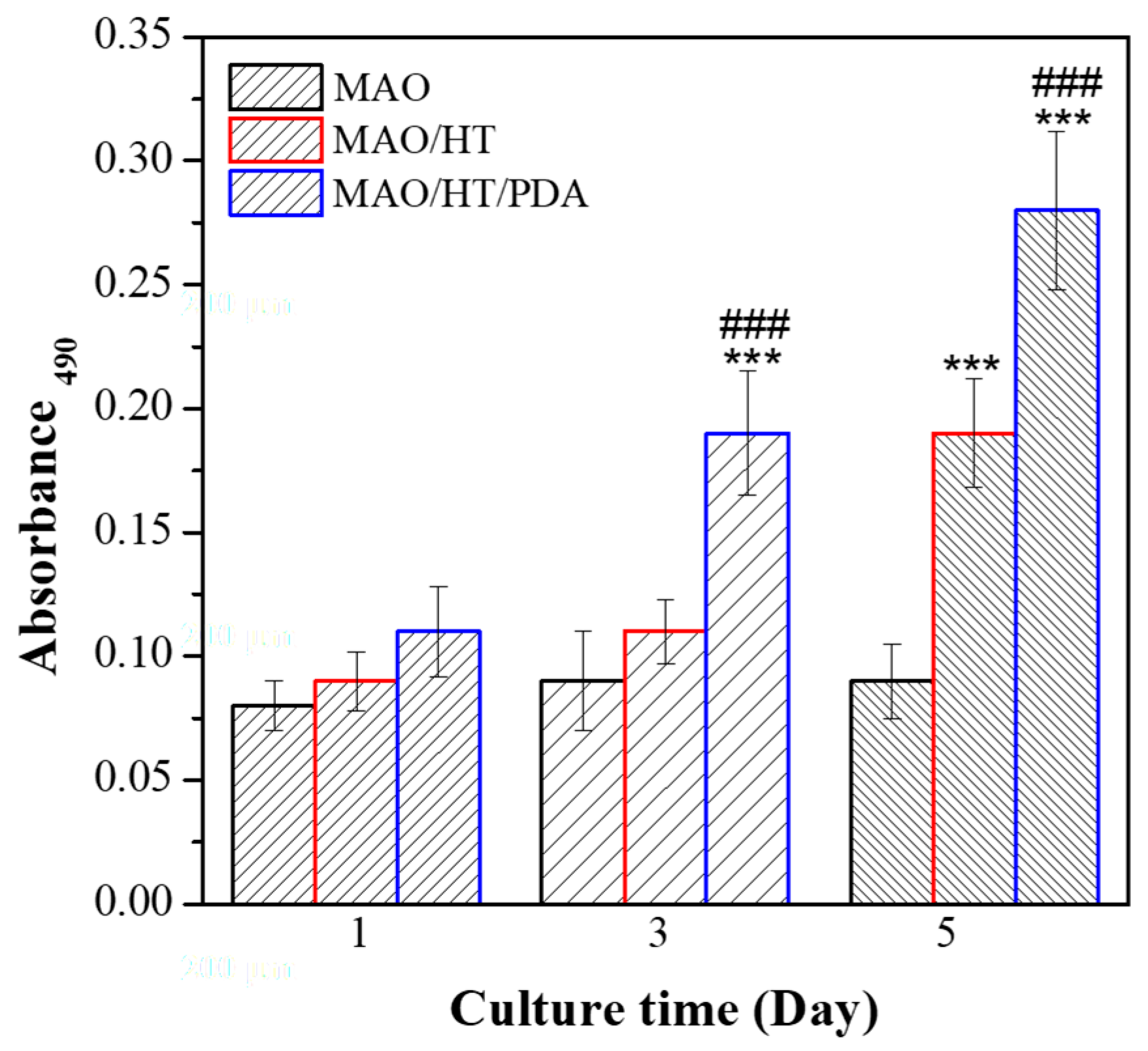
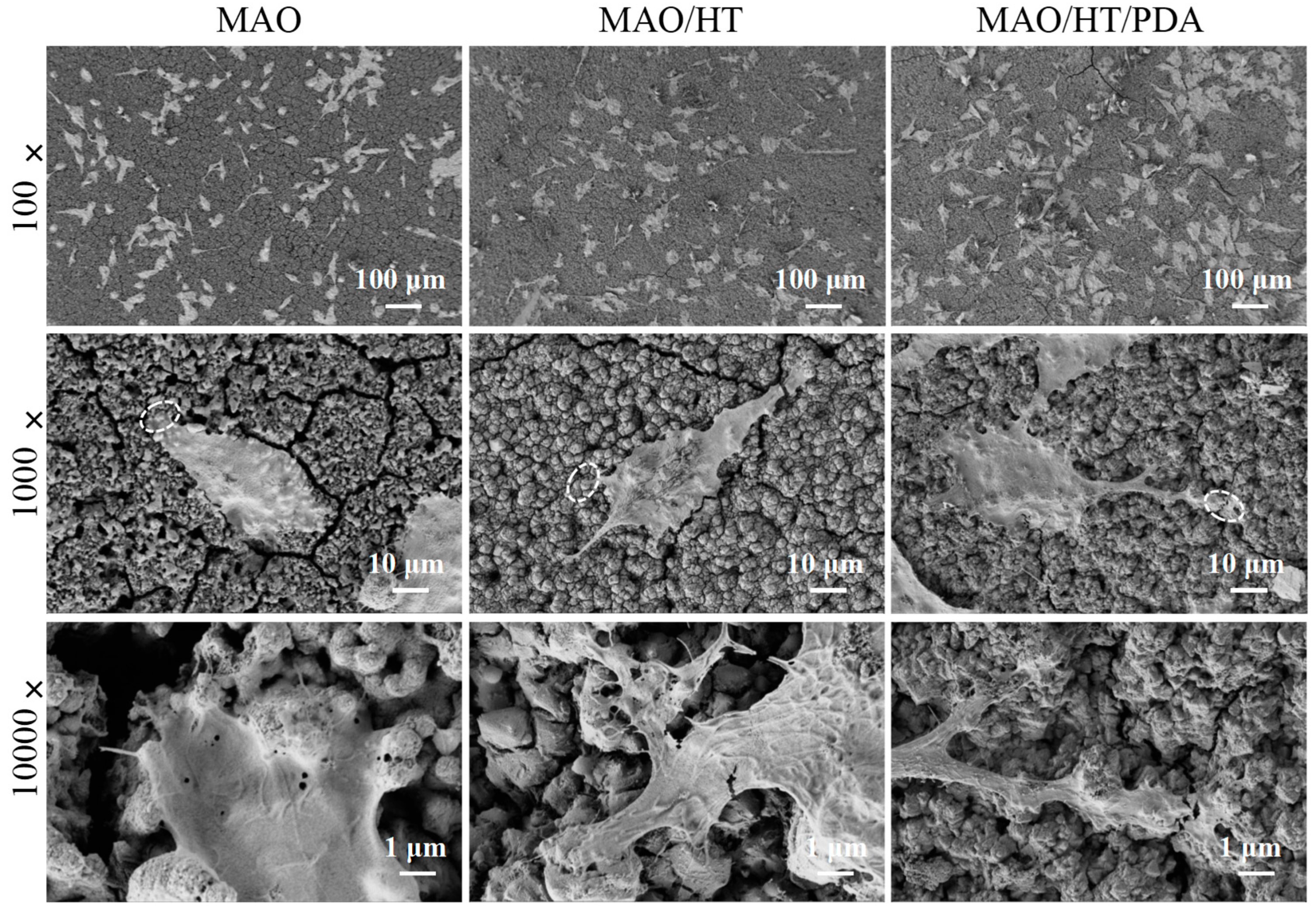
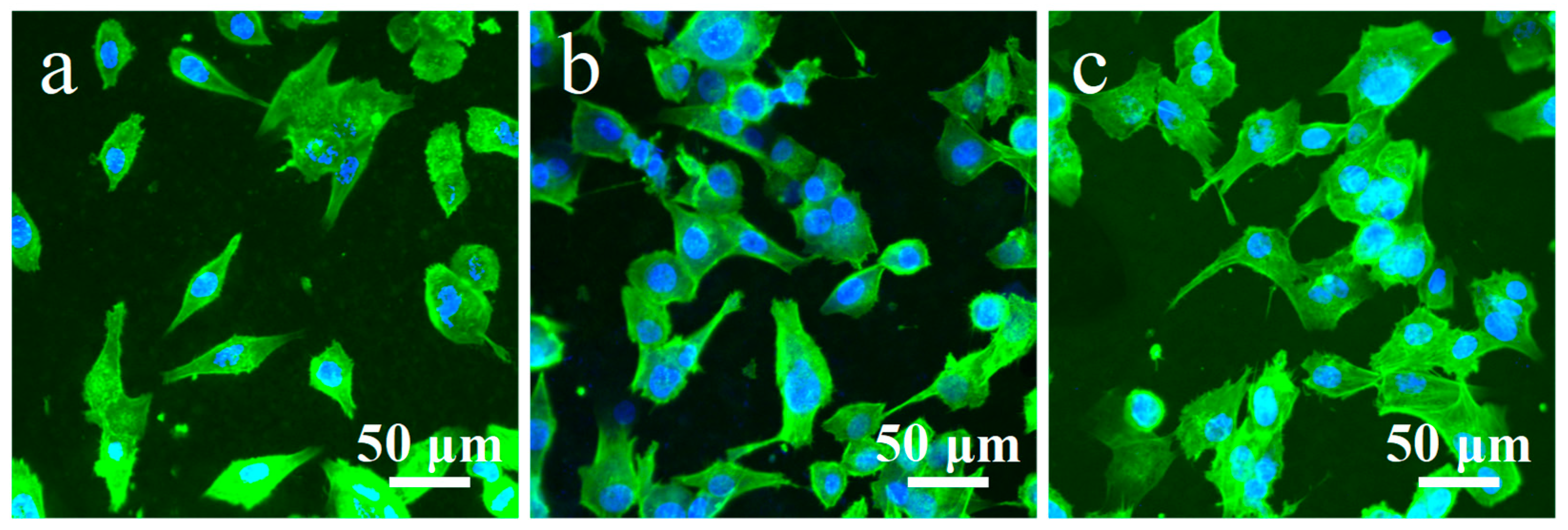
3.4. Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Hu, J.; Yu, L.; Wang, K.; Zhang, W.; Fan, H.; Deng, Z.; Wu, J.; Wang, K. Corrosion behavior investigation of gallium coating on magnesium alloy in simulated body fluid. J. Mater. Res. Technol. 2023, 27, 225–236. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Rabiee, S.M.; Hosseinipour, S.J.; Jamaati, R. Effect of microstructure and texture of AZ31 magnesium alloy substrate on nucleation and growth of biomimetic calcium phosphate coating. J. Mater. Res. Technol. 2023, 27, 5154–5164. [Google Scholar] [CrossRef]
- Iqbal, F.; Ali, A.; Naveed, M.; Ikram, F.; Fatima, H. Hydrothermal deposition of high strength biocompatible magnesium phosphate coating through in situ conversion of AZ91D-3Ca magnesium substrate. Surf. Coat. Tech. 2023, 457, 129301. [Google Scholar] [CrossRef]
- Li, X.; Hu, J.; Yu, Z.; Liu, M.; Xiao, X.; Qin, G.; Yang, L.; Zhang, E. A study on the in vitro and in vivo degradation behaviour and biocompatibility of a Mg-Mn-Zn alloy with PLLA and Micro arc oxidation composite coating. Surf. Coat. Tech. 2023, 471, 129894. [Google Scholar] [CrossRef]
- Nachtsheim, J.; Ma, S.Y.; Burja, J.; Batič, B.Š.; Markert, B. Tuning the long-term corrosion behaviour of biodegradable WE43 magnesium alloy by PEO coating. Surf. Coat. Tech. 2023, 474, 130115. [Google Scholar] [CrossRef]
- Peng, Y.F.; Wan, S.; Liao, B.K.; Guo, X. Preparation and properties of multi-function composite coating on AZ91D magnesium alloy with wave-assimilation and anti-corrosion performance. Surf. Coat. Tech. 2024, 478, 130464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.L.; Liu, S.B.; Wei, Z.; Zhang, T.C.; Yuan, S. Bilayer diatomite-based composite coatings with superhydrophobic and self-healing properties for enhanced anticorrosion of AZ31B magnesium alloys. Surf. Coat. Tech. 2024, 489, 131151. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Dai, Y.; She, J.; Zhang, D.; Qi, F.; Wei, W.; Ouyang, X. A novel Ca-Mg-P/PDA composite coating of Mg alloys to improve corrosion resistance for orthopedic implant materials. Surf. Coat. Tech. 2023, 471, 129920. [Google Scholar] [CrossRef]
- Witecka, A.; Valet, S.; Basista, M.; Boccaccini, A.R. Electrophoretically deposited high molecular weight chitosan/bioactive glass composite coatings on WE43 magnesium alloy. Surf. Coat. Tech. 2021, 418, 127232. [Google Scholar] [CrossRef]
- Zhao, Z.; Zong, L.S.; Liu, C.D.; Li, X.; Wang, C.; Liu, W.; Cheng, X.; Wang, J.; Jian, X. A novel Mg(OH)2/MgFx(OH)1−x composite coating on biodegradable magnesium alloy for coronary stent application. Corros. Sci. 2022, 208, 110627. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, K.; Xu, W.; Wang, C.; Qian, K.; Shao, Y.; Jiang, G.; Liu, H.; Ju, J.; Höche, D.; et al. Localized accelerated degradation of magnesium: A new insight into the mechanism of its biomedical degradation. Corros. Sci. 2024, 237, 112335. [Google Scholar] [CrossRef]
- Barzegari, M.; Mei, D.; Lamaka, S.; Geris, L. Computational modeling of degradation process of biodegradable magnesium biomaterials. Corros. Sci. 2021, 190, 109674. [Google Scholar] [CrossRef]
- Yang, C.; Cai, H.; Cui, S.H.; Huang, J.; Zhu, J.; Wu, Z.; Ma, Z.; Fu, R.K.; Sheng, L.; Tian, X.; et al. A zinc-doped coating prepared on the magnesium alloy by plasma electrolytic oxidation for corrosion protection. Surf. Coat. Tech. 2022, 433, 128148. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wu, L.; Yao, W.H.; Wu, J.; Xie, Z.; Yuan, Y.; Jiang, B.; Pan, F. In situ growth of Mg-Zn-Al LDHs by ZIF-8 carrying Zn source and micro-arc oxidation integrated coating for corrosion and protection of magnesium alloys. Surf. Coat. Tech. 2022, 451, 129032. [Google Scholar] [CrossRef]
- He, H.; Li, K.; Luo, W.; Jiao, Z.; Ai, F.; Zhou, K.; Cao, C. Structure design, preparation and performance of a novel composite coating on medical magnesium-zinc alloy. Surf. Coat. Tech. 2022, 443, 128643. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, L.; He, X.X.; Yao, W.; Wu, J.; He, Y.; Zhou, Y.; Yuan, Y.; Wang, J.; Xie, Z.; et al. Corrosion resistance study of ZIF-67/Co-Fe LDHs composite coating on the surface of AZ31 magnesium alloy micro-arc oxidation film. J. Alloys Compd. 2024, 989, 174278. [Google Scholar] [CrossRef]
- Liu, Y.X.; Duan, J.Q.; Zhang, J.L.; Guo, X.G. Active corrosion protection of micro-arc oxidation-based composite coating on magnesium alloy: Multiple roles of ionic liquid modified layered double hydroxide. Ceram. Int. 2024, 50, 26160–26170. [Google Scholar] [CrossRef]
- Tang, C.; Pan, J.; Wu, J.; Zhao, X.; Lei, J.; Li, L.; Pan, F. Surroundings-adaptive coating enabling robustness of magnesium alloys. Colloid Surf. A 2024, 696, 134385. [Google Scholar] [CrossRef]
- Yao, W.H.; Wu, L.; Wang, J.F.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.L.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.H.; Wang, Z.C.; Hu, C.; Liu, J.; Yu, H.; Chen, C. Corrosion resistance and biodegradability of micro-arc oxidation coatings with the variable sodium fluoride concentration on ZM21 magnesium alloys. J. Alloys Compd. 2023, 962, 171172. [Google Scholar] [CrossRef]
- Fischerauer, S.F.; Kraus, T.; Wu, X.; Tangl, S.; Sorantin, E.; Hänzi, A.C.; Löffler, J.F.; Uggowitzer, P.J.; Weinberg, A.M. In vivo degradation performance of micro-arc-oxidized magnesium implants: A micro-CT study in rats. Acta Biomater. 2013, 9, 5411–5420. [Google Scholar] [CrossRef]
- Li, C.L.; Yao, X.H.; Hang, R.Q.; Zhang, X.Y. Facile preparation of nanostructured octacalcium phosphate coatings on micro-arc oxidized magnesium with different functionalities for bone repair application. Colloid Surf. B 2021, 197, 111426. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Yao, X.H.; Hang, R.Q.; Huang, X.B.; Hang, R.Q. Corrosion behavior and cytocompatibility of nanostructured hydroxyapatite hydrothermally grown on porous MgO coatings with different P contents on magnesium. Mater. Lett. 2020, 264, 127136. [Google Scholar] [CrossRef]
- Liu, Y.P.; Cheng, X.; Wang, X.Y.; Sun, Q.; Wang, C.X.; Di, P.; Lin, Y. Micro-arc oxidation-assisted sol-gel preparation of calcium metaphosphate coatings on magnesium alloys for bone repair. Mat. Sci. Eng. C 2021, 131, 112491. [Google Scholar] [CrossRef]
- Wu, W.X.; Wang, W.P.; Lin, H.C. A study on corrosion behavior of micro-arc oxidation coatings doped with 2-aminobenzimidazole loaded halloysite nanotubes on AZ31 magnesium alloys. Surf. Coat. Tech. 2021, 416, 127116. [Google Scholar] [CrossRef]
- Wang, T.X.; Xu, Y.C.; Liu, Z.Q.; Li, G.Y.; Guo, Y.T.; Lian, J.S.; Zhang, Z.H.; Ren, L.Q. A chitosan/polylactic acid composite coating enhancing the corrosion resistance of the bio-degradable magnesium alloy. Prog. Org. Coat. 2023, 178, 107469. [Google Scholar] [CrossRef]
- Alabbasi, A.; Liyanaarachchi, S.; Bobby Kannan, M. Polylactic acid coating on a biodegradable magnesium alloy: An in vitro degradation study by electrochemical impedance spectroscopy. Thin Solid Films 2012, 520, 6841–6844. [Google Scholar] [CrossRef]
- Beyzavi, A.H.; Azadi, M.; Azadi, M.; Dezianian, S.; Talebsafa, V. Bio-polymer coatings fabricated on AM60 magnesium alloys by fused deposition modeling 3D-printing to investigate electrochemical behavior. Mat. Lett. 2023, 337, 133935. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Qi, K. Formation mechanism, degradation behavior, and cytocompatibility of a nanorod-shaped HA and pore-sealed MgO bilayer coating on magnesium. ACS Appl. Mater. Interfaces 2014, 6, 18258–18274. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Zeng, R.C.; Cui, L.Y.; Li, S.Q.; Zhang, F.; He, Q.K.; Bobby Kannan, M.; Jiang, H.W.; Chen, D.C.; et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31-Influence of EDTA. J. Mater. Sci. Technol. 2019, 35, 1088–1098. [Google Scholar] [CrossRef]
- Sareło, P.; Sobieszczańska, B.; Wysokińska, E.; Gąsior-Głogowska, M.; Kałas, W.; Podbielska, H.; Wawrzyńska, M.; Kopaczyńska, M. In vitro examinations of the anti-inflammatory interleukin functionalized polydopamine based biomaterial as a potential coating for cardiovascular stents. Biocybern. Biomed. Eng. 2023, 43, 369–385. [Google Scholar] [CrossRef]
- Peng, M.X.; Zhang, Q.C.; Zhang, M.N.; Yang, Z.W.; Wan, Y.Z.; Deng, X.Y.; Luo, H.L. Dealloying and polydopamine/silver coating on NiTi alloy for improved antibacterial activity. Mater. Chem. Phys. 2023, 305, 127939. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jin, G.C.; Miao, H.; Xu, S.A. Preparation and characterization of polydopamine and n-butyl methacrylate copolymer coatings on titanium-nickel alloy stents. RSC Adv. 2024, 14, 15240–15248. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Zheng, B.; Gu, Y.P.; Shen, C.; Wen, J.; Meng, Z.B.; Chen, S.P.; Ou, J.; Qin, A.M. New approach for improving anticorrosion and biocompatibility of magnesium alloys via polydopamine intermediate layer-induced hydroxyapatite coating. Surf. Interfaces 2020, 19, 100501. [Google Scholar] [CrossRef]
- Chung, J.J.; Chen, C.C.; Ding, S. Strontium-polydopamine hybrid coating to improve antibacterial ability and osseointegration of titanium implants. Mater. Chem. Phys. 2024, 312, 128629. [Google Scholar] [CrossRef]
- Wu, J.P.; Liu, Y.T.; Cao, Q.D.; Yu, T.; Zhang, J.; Liu, Q.Y.; Yang, X.Y. Growth factors enhanced angiogenesis and osteogenesis on polydopamine coated titanium surface for bone regeneration. Mater. Des. 2020, 196, 109162. [Google Scholar] [CrossRef]
- ASTM G31-72(2004); Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2004.
- Carangelo, A.; Acquesta, A.; Monetta, T. In-vitro corrosion of AZ31 magnesium alloys by using a polydopamine coating. Bioact. Mater. 2019, 4, 71–78. [Google Scholar] [CrossRef]
- Jiang, W.S.; Tian, Q.M.; Vuong, T.; Shashaty, M.; Gopez, C.; Sanders, T.; Liu, H.N. Comparison study on four biodegradable polymer coatings for controlling magnesium degradation and human endothelial cell adhesion and spreading. ACS Biomater.-Sci. Eng. 2017, 3, 936–950. [Google Scholar] [CrossRef]
- Guarise, C.; Maglio, M.; Sartori, M.; Galesso, D.; Barbera, C.; Pavan, M.; Martini, L.; Giavaresi, G.; Sambri, V.; Fini, M. Titanium implant coating based on dopamine-functionalized sulphated hyaluronic acid: In vivo assessment of biocompatibility and antibacterial efficacy. Mat. Sci. Eng. C 2021, 128, 112286. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wang, C.X.; Zhang, Y.; Lin, Y. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mat. Sci. Eng. C 2021, 129, 112376. [Google Scholar] [CrossRef] [PubMed]

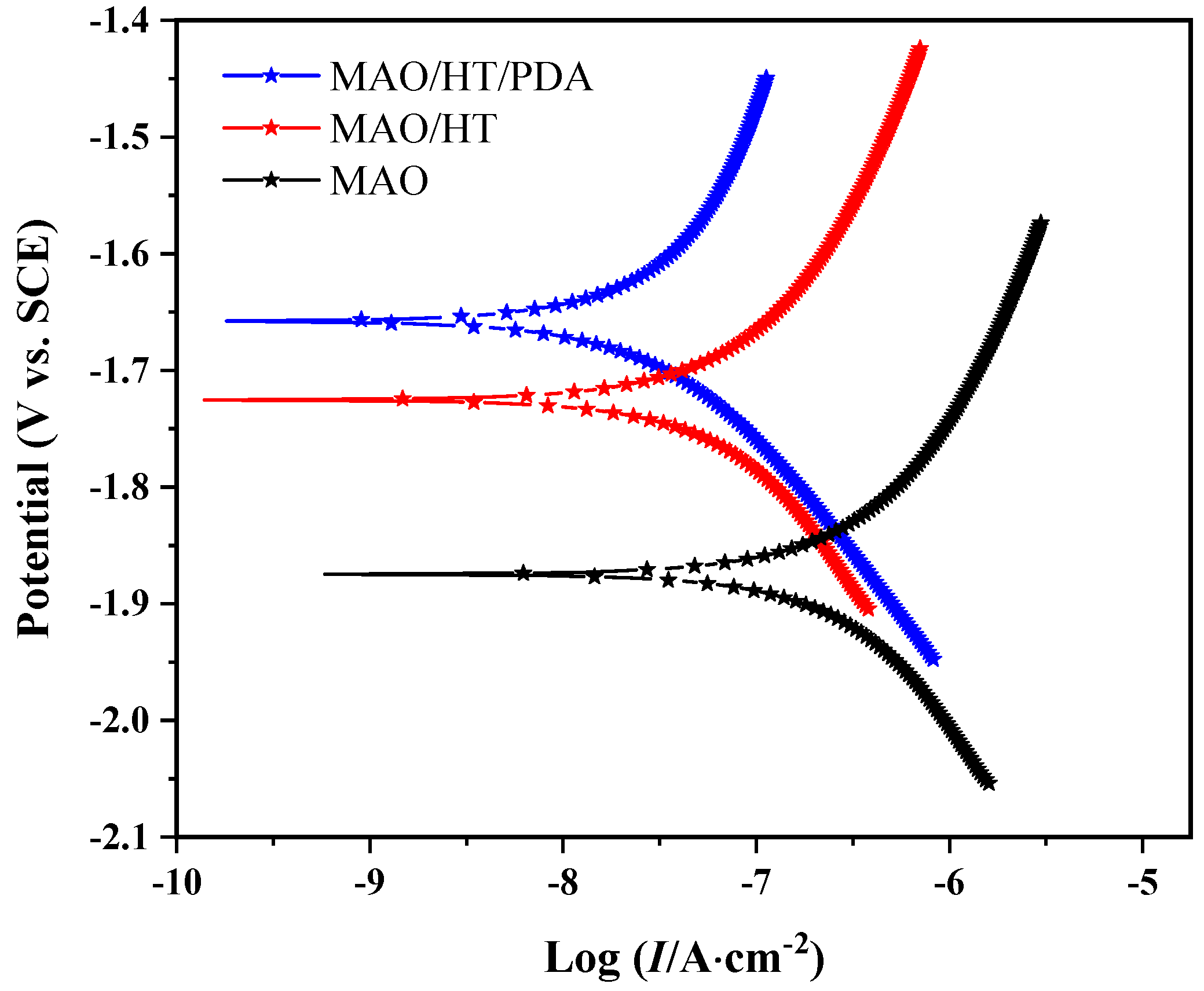
| Samples | MAO | MAO/HT | MAO/HT/PDA |
|---|---|---|---|
| Ecorr (V vs. SCE) | −1.87 | −1.73 | −1.65 |
| Icorr (A·cm−2) | 1.99 × 10−5 | 1.28 × 10−6 | 2.51 × 10−7 |
| Time | MAO | MAO/HT | MAO/HT/PDA | |||
|---|---|---|---|---|---|---|
| Rc (Ω·cm2) | Rct (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | |
| 0 | 5.98 × 103 | 7.11 × 103 | 7.25 × 104 | 4.32 × 104 | 8.56 × 104 | 4.82 × 104 |
| 24 | 4.72 × 103 | 2.27 × 103 | 6.53 × 104 | 3.75 × 104 | 6.31 × 104 | 4.04 × 104 |
| 48 | 3.25 × 103 | 1.72 × 103 | 3.75 × 104 | 1.78 × 104 | 5.38 × 104 | 2.80 × 104 |
| 96 | 2.55 × 103 | 1.53 × 103 | 1.02 × 104 | 9.85 × 103 | 4.21 × 104 | 2.01 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, B.; Yan, W. Enhanced Corrosion Resistance and Cytocompatibility of Magnesium Alloys with Mg(OH)2/Polydopamine Composite Coatings for Orthopedic Applications. Coatings 2025, 15, 729. https://doi.org/10.3390/coatings15060729
Li C, Li B, Yan W. Enhanced Corrosion Resistance and Cytocompatibility of Magnesium Alloys with Mg(OH)2/Polydopamine Composite Coatings for Orthopedic Applications. Coatings. 2025; 15(6):729. https://doi.org/10.3390/coatings15060729
Chicago/Turabian StyleLi, Chunlin, Boqiong Li, and Wenxia Yan. 2025. "Enhanced Corrosion Resistance and Cytocompatibility of Magnesium Alloys with Mg(OH)2/Polydopamine Composite Coatings for Orthopedic Applications" Coatings 15, no. 6: 729. https://doi.org/10.3390/coatings15060729
APA StyleLi, C., Li, B., & Yan, W. (2025). Enhanced Corrosion Resistance and Cytocompatibility of Magnesium Alloys with Mg(OH)2/Polydopamine Composite Coatings for Orthopedic Applications. Coatings, 15(6), 729. https://doi.org/10.3390/coatings15060729






