Protective ALD Thin Films for Morphologically Diverse Types of Limestone
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Reflectance and Colorimetry
2.3. Acqueous Acid Immersion and pH Tracking
2.4. Optical Microscopy
2.5. SEM-EDS
3. Results and Discussion
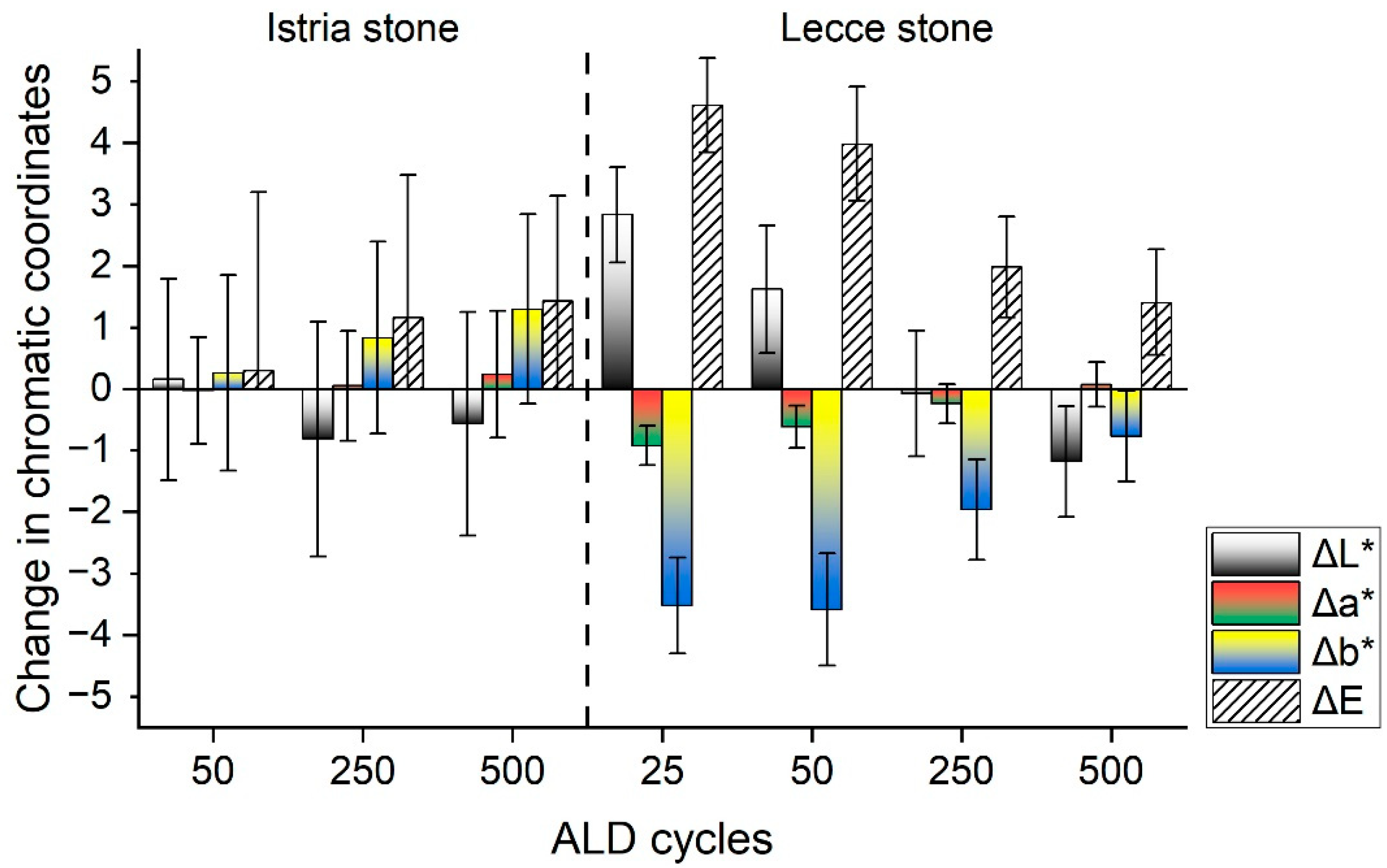
3.1. Appearance Changes
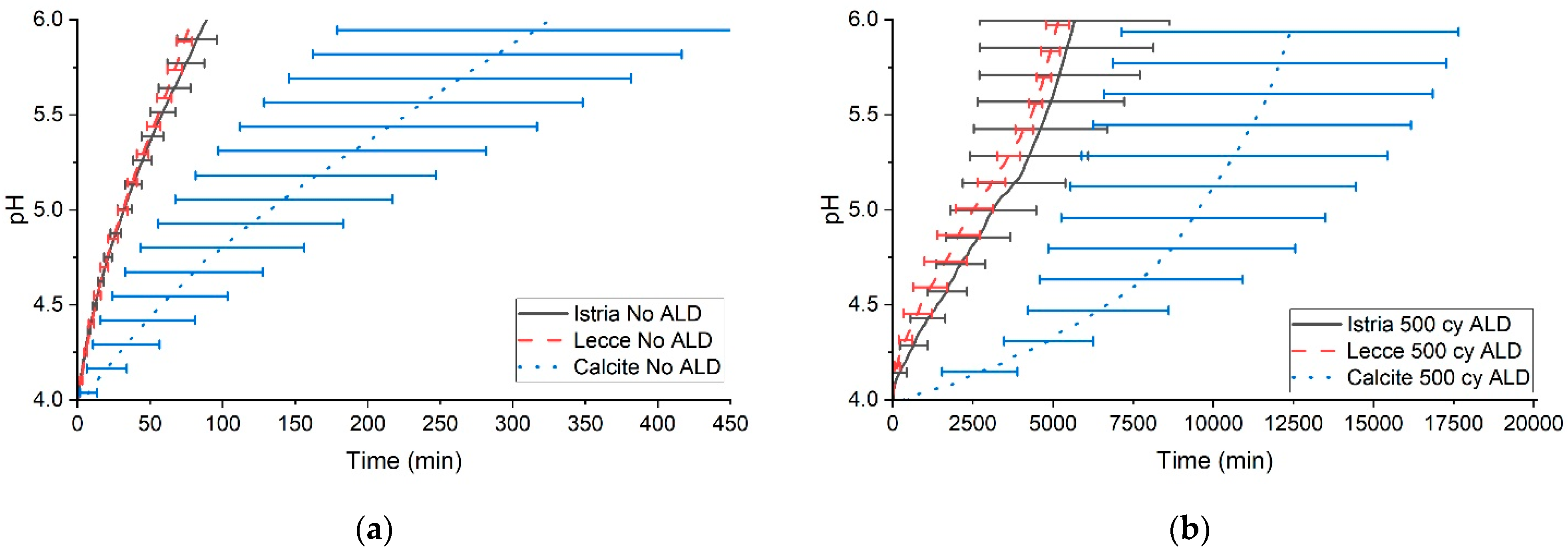
3.2. pH Evolution for Measuring Protection Against Acid Attack
3.3. pH Evolution to Measure Effect of ALD Thickness
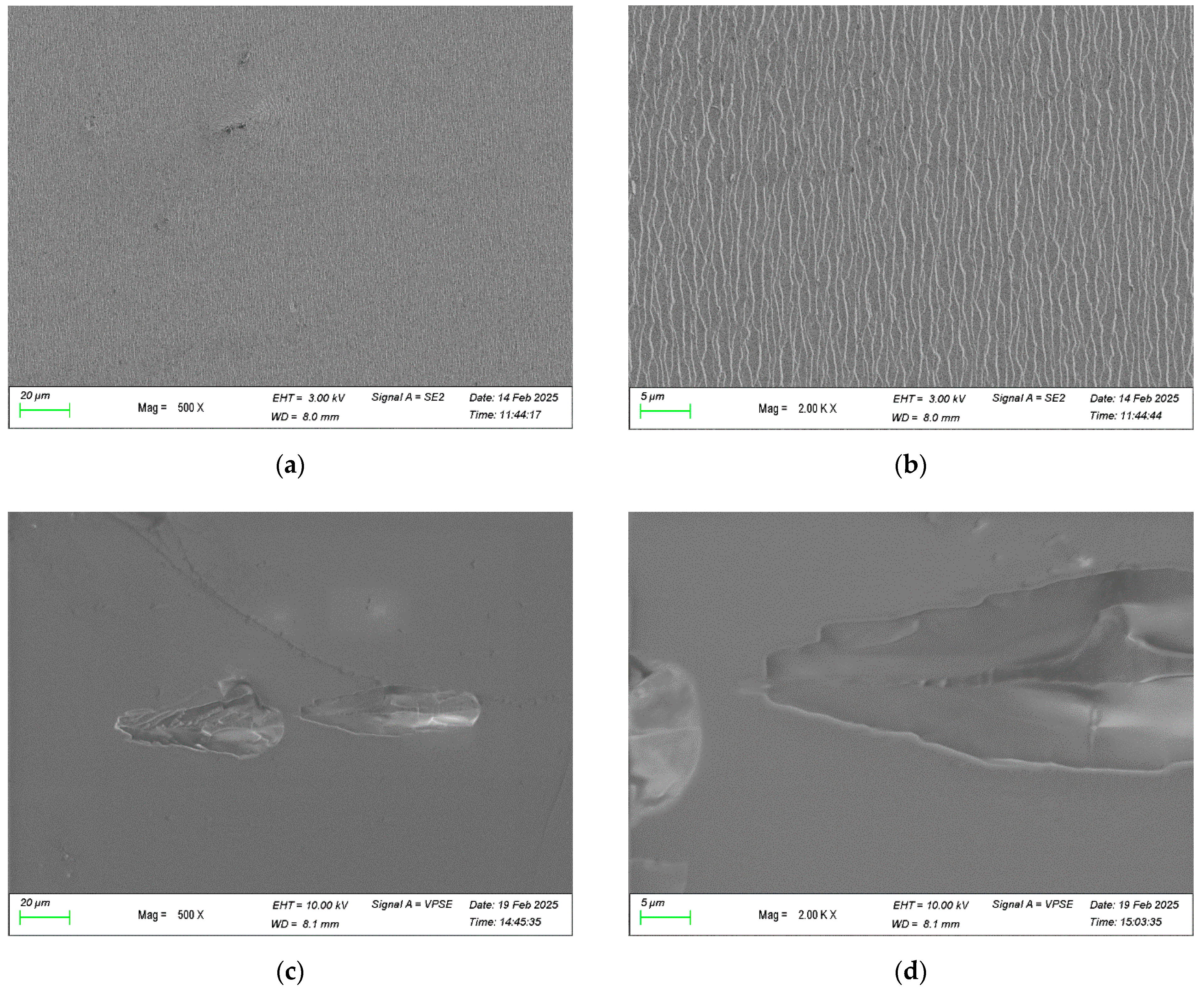
3.4. Morphology of Limestone After Acid Immersion
3.5. Optical Imaging, Area Fraction of Damage, and pH Evolution
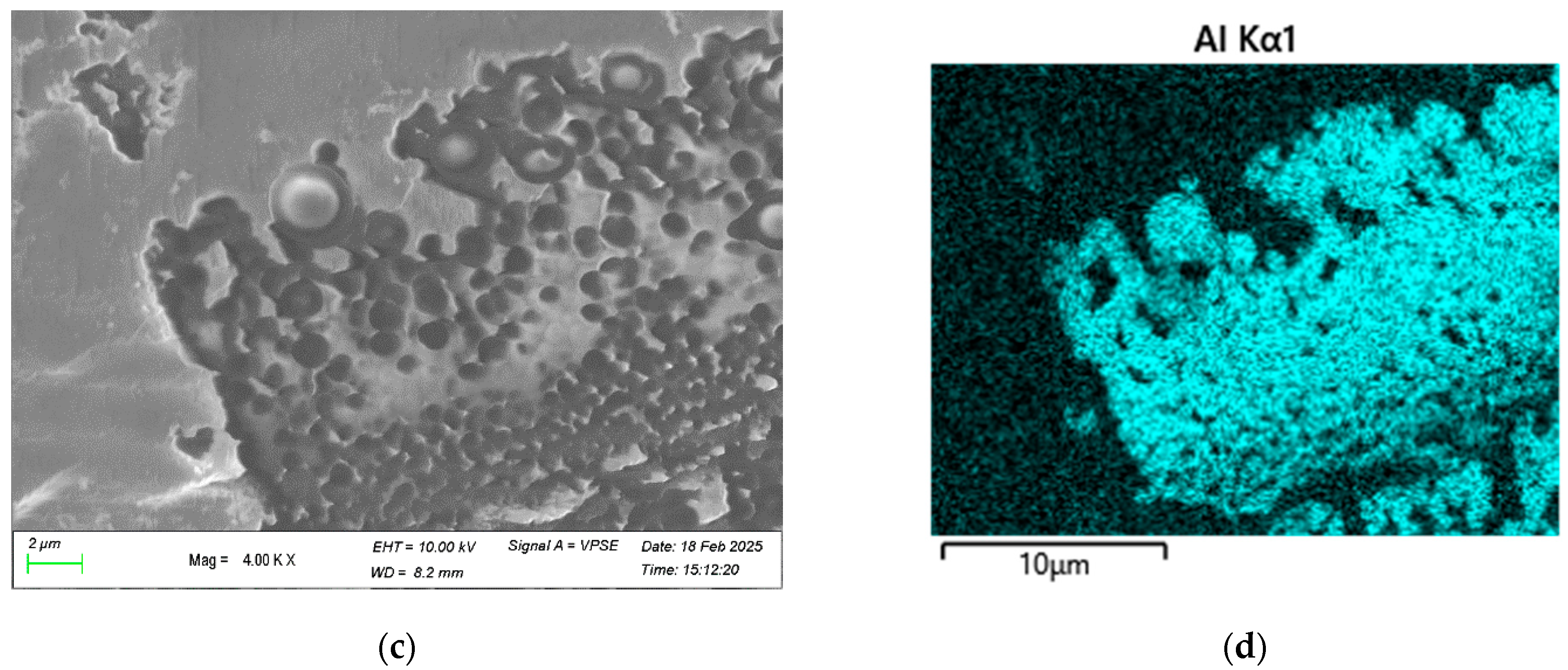
3.6. Reaction Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brimblecombe, P. The Effects of Air Pollution on the Built Environment; Imperial College Press: London, UK; World Scientific Pub. Co.: River Edge, NJ, USA, 2003; ISBN 978-1-84816-128-3. [Google Scholar]
- Sabbioni, C. Mechanisms of Air Pollution Damage to Stone. In Air Pollution Reviews; Imperial College Press: London, UK, 2003; Volume 2, pp. 63–106. ISBN 978-1-86094-291-4. [Google Scholar]
- Vidal, F.; Vicente, R.; Mendes Silva, J. Review of Environmental and Air Pollution Impacts on Built Heritage: 10 Questions on Corrosion and Soiling Effects for Urban Intervention. J. Cult. Herit. 2019, 37, 273–295. [Google Scholar] [CrossRef]
- Vidović, K.; Hočevar, S.; Menart, E.; Drventić, I.; Grgić, I.; Kroflič, A. Impact of Air Pollution on Outdoor Cultural Heritage Objects and Decoding the Role of Particulate Matter: A Critical Review. Environ. Sci. Pollut. Res. 2022, 29, 46405–46437. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, P.; Grossi, C.M. Millennium-Long Damage to Building Materials in London. Sci. Total Environ. 2009, 407, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Ghedini, N.; Ozga, I.; Bonazza, A.; Dilillo, M.; Cachier, H.; Sabbioni, C. Atmospheric Aerosol Monitoring as a Strategy for the Preventive Conservation of Urban Monumental Heritage: The Florence Baptistery. Atmos. Environ. 2011, 45, 5979–5987. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Barca, D.; Bonazza, A.; Comite, V.; La Russa, M.F.; Pezzino, A.; Ruffolo, S.A.; Sabbioni, C. Application of Spectrometric Analysis to the Identification of Pollution Sources Causing Cultural Heritage Damage. Environ. Sci. Pollut. Res. 2013, 20, 8848–8859. [Google Scholar] [CrossRef]
- Maas, R.; Grennfelt, P.; Amann, M.; Harnett, B.; Kerr, J.; Berton, E.; Pritula, D.; Reiss, I.; Almodovar, P.; Héroux, M.-E.; et al. Towards Cleaner Air. Scientific Assessment Report 2016; Narayana Press: Gylling, Denmark, 2016. [Google Scholar]
- Grennfelt, P.; Engleryd, A.; Forsius, M.; Hov, Ø.; Rodhe, H.; Cowling, E. Acid Rain and Air Pollution: 50 Years of Progress in Environmental Science and Policy. Ambio 2020, 49, 849–864. [Google Scholar] [CrossRef]
- Gnemmi, M.; Falchi, L.; Zendri, E. Non-Invasive-Monitoring Methodology for the Evaluation of Environmental Impacts on Istrian Stone Surfaces in Venice. Atmosphere 2022, 13, 1036. [Google Scholar] [CrossRef]
- Annual Mean NO2 Concentrations Observed at (Sub) Urban Background Stations. Available online: https://www.eea.europa.eu/data-and-maps/daviz/annual-mean-no2-concentration-observed-7#tab-googlechartid_chart_21 (accessed on 9 June 2024).
- Trends in Atmospheric Concentrations of CO2 (Ppm), CH4 (Ppb) and N2O (Ppb), Between 1800 and 2017. Available online: https://www.eea.europa.eu/data-and-maps/daviz/atmospheric-concentration-of-carbon-dioxide-5 (accessed on 9 June 2024).
- Marquardt, A.E.; Breitung, E.M.; Drayman-Weisser, T.; Gates, G.; Phaneuf, R.J. Protecting Silver Cultural Heritage Objects with Atomic Layer Deposited Corrosion Barriers. Herit. Sci. 2015, 3, 37. [Google Scholar] [CrossRef]
- Hiebert, M.E.; Weaver, J.; Lam, T.; Little, N.; Hyde, E.; Vicenzi, E.P.; Phaneuf, R.J.; Smithsonian Institution; National Institute of Standards and Technology. ALD Deposited Amorphous Alumina Coatings Can Slow Glass Alteration. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. B 2022, 63, 97–110. [Google Scholar] [CrossRef]
- Boyce, G.P.; Sreenilayam, S.; Balliana, E.; Zendri, E.; Phaneuf, R.J. Amorphous Alumina ALD Coatings for the Protection of Limestone Cultural Heritage Objects. Coatings 2024, 14, 931. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Biscontin, G. Origin, Characteristics and Morphology of Weathering Crusts on Istria Stone in Venice. Atmos. Environ. 1999, 33, 1699–1709. [Google Scholar] [CrossRef]
- Biscontin, G.; Fassina, V.; Miaravelaki, P.; Zendri, E. Venice: Stone Material Behaviour in Connection with the Environment. MRS Proc. 1990, 185, 253. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M. Performances and Coating Morphology of a Siloxane-Based Hydrophobic Product Applied in Different Concentrations on a Highly Porous Stone. Coatings 2016, 6, 60. [Google Scholar] [CrossRef]
- Charola, E. Salts in the Deterioration of Porous Materials: An Overview. J. Am. Inst. Conserv. 2000, 39, 327–342. [Google Scholar] [CrossRef]
- Charola, A.E.; Wendler, E. An Overview of the Water-Porous Building Materials Interactions. Restor. Build. Monum. 2015, 21, 55–65. [Google Scholar] [CrossRef]
- Puurunen, R.L.; Vandervorst, W. Island Growth as a Growth Mode in Atomic Layer Deposition: A Phenomenological Model. J. Appl. Phys. 2004, 96, 7686–7695. [Google Scholar] [CrossRef]
- Ritala, M.; Leskelä, M.; Dekker, J.-P.; Mutsaers, C.; Soininen, P.J.; Skarp, J. Perfectly Conformal TiN and Al2O3 Films Deposited by Atomic Layer Deposition. Chem. Vap. Depos. 1999, 5, 7–9. [Google Scholar] [CrossRef]
- Shkondin, E.; Takayama, O.; Lindhard, J.M.; Larsen, P.V.; Mar, M.D.; Jensen, F.; Lavrinenko, A.V. Fabrication of High Aspect Ratio TiO2 and Al2O3 Nanogratings by Atomic Layer Deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 031605. [Google Scholar] [CrossRef]
- Conserving Stone Heritage: Traditional and Innovative Materials and Techniques; Gherardi, F., Maravelaki, P.N., Eds.; Cultural Heritage Science; Springer International Publishing: Cham, Switzerland, 2022; ISBN 978-3-030-82941-4. [Google Scholar]
- Maravelaki-Kalaitzaki, P.; Bertoncello, R.; Biscontin, G. Evaluation of the Initial Weathering Rate of Istria Stone Exposed to Rain Action, in Venice, with X-Ray Photoelectron Spectroscopy. J. Cult. Herit. 2002, 3, 273–282. [Google Scholar] [CrossRef]
- Bugani, S.; Camaiti, M.; Morselli, L.; Van De Casteele, E.; Janssens, K. Investigating Morphological Changes in Treated vs. Untreated Stone Building Materials by x-Ray Micro-CT. Anal. Bioanal. Chem. 2008, 391, 1343–1350. [Google Scholar] [CrossRef]
- Costantini, R.; Balliana, E.; Dalla Torre, D.; Aricò, F.; Zendri, E. Evaluating the Impacts of Alcohol-Based Solutions on Silk: Chemical, Mechanical and Wettability Changes before and after Artificial Ageing. Heritage 2022, 5, 3588–3604. [Google Scholar] [CrossRef]
- Izzo, F.C.; Balliana, E.; Perra, E.; Zendri, E. Accelerated Ageing Procedures to Assess the Stability of an Unconventional Acrylic-Wax Polymeric Emulsion for Contemporary Art. Polymers 2020, 12, 1925. [Google Scholar] [CrossRef] [PubMed]
- Chartier, G. Introduction to Optics; Advanced texts in physics; Springer: New York, NY, USA, 2005; ISBN 978-0-387-40346-5. [Google Scholar]
- Johnston-Feller, R. Color Science in the Examination of Museum Objects: Nondestructive Procedures. Color Res. Appl. 2002, 27, 456–457. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. Calcite Dissolution Kinetics: Surface Speciation and the Origin of the Variable pH Dependence. Chem. Geol. 1984, 42, 119–136. [Google Scholar] [CrossRef]
- Addo, T.S. Effect of Sample Miscut on Dissolution Kinetics of Calcite (104) Cleavage Surfaces. Master’s Thesis, Wright State University, Dayton, OH, USA, 2013. [Google Scholar]
- Garcia, S.P.; Bao, H.; Hines, M.A. Etchant Anisotropy Controls the Step Bunching Instability in KOH Etching of Silicon. Phys. Rev. Lett. 2004, 93, 166102. [Google Scholar] [CrossRef]
- Ryan, R.L.; McCafferty, E. Rupture of an Oxide Blister. J. Electrochem. Soc. 1995, 142, 2594–2597. [Google Scholar] [CrossRef]
- Arvidson, R.S.; Collier, M.; Davis, K.J.; Vinson, M.D.; Amonette, J.E.; Luttge, A. Magnesium Inhibition of Calcite Dissolution Kinetics. Geochim. Cosmochim. Acta 2006, 70, 583–594. [Google Scholar] [CrossRef]
- Vinson, M.D.; Arvidson, R.S.; Luttge, A. Kinetic Inhibition of Calcite (104) Dissolution by Aqueous Manganese(II). J. Cryst. Growth 2007, 307, 116–125. [Google Scholar] [CrossRef]
- Sweegers, C.; De Coninck, H.C.; Meekes, H.; Van Enckevort, W.J.P.; Hiralal, I.D.K.; Rijkeboer, A. Morphology, Evolution and Other Characteristics of Gibbsite Crystals Grown from Pure and Impure Aqueous Sodium Aluminate Solutions. J. Cryst. Growth 2001, 233, 567–582. [Google Scholar] [CrossRef]
- Schoen, R.; Roberson, C.E. Structures of Aluminum Hydroxide and Geochemical Implications. Am. Mineral. 1970, 55, 43–77. [Google Scholar]
- Willis, S.A.; McGuinness, E.K.; Li, Y.; Losego, M.D. Re-Examination of the Aqueous Stability of Atomic Layer Deposited (ALD) Amorphous Alumina (Al2O3) Thin Films and the Use of a Postdeposition Air Plasma Anneal to Enhance Stability. Langmuir 2021, 37, 14509–14519. [Google Scholar] [CrossRef] [PubMed]
- Parsons, R. Atlas of Electrochemical Equilibria in Aqueous Solutions. J. Electroanal. Chem. Interfacial Electrochem. 1967, 13, 471. [Google Scholar] [CrossRef]
- Kardar, M.; Detmold, W. Diffusion Within a Sphere: Lecture in Mathematical Methods for Aspiring Physicists. Available online: https://web.mit.edu/8.Math/www/lectures/lec13/3.5.3.pdf (accessed on 25 March 2025).






| Calcium Carbonate Type | Initial Mass Loss Rate (No ALD) (mg/min) | Total Mass Loss Rate (No ALD) (mg/min) | Initial Mass Loss Rate (500 cy ALD) (mg/min) | Total Mass Loss Rate (500 cy ALD) (mg/min) |
|---|---|---|---|---|
| Lecce Stone | (1.86 ± 0.21) × 10−2 | (4.16 ± 0.54) × 10−2 | (2.11 ± 0.32) × 10−4 | (6.32 ± 0.82) × 10−4 |
| Istria Stone | (1.77 ± 0.20) × 10−2 | (3.61± 0.81) × 10−2 | (1.43 ± 0.19) × 10−4 | (5.73 ± 3.35) × 10−4 |
| Calcite | (4.04 ± 0.45) × 10−3 | (1.00 ± 0.49) × 10−2 | (3.46 ± 0.45) × 10−5 | (2.59 ± 1.24) × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyce, G.P.; Sreenilayam, S.; Balliana, E.; Zendri, E.; Phaneuf, R.J. Protective ALD Thin Films for Morphologically Diverse Types of Limestone. Coatings 2025, 15, 698. https://doi.org/10.3390/coatings15060698
Boyce GP, Sreenilayam S, Balliana E, Zendri E, Phaneuf RJ. Protective ALD Thin Films for Morphologically Diverse Types of Limestone. Coatings. 2025; 15(6):698. https://doi.org/10.3390/coatings15060698
Chicago/Turabian StyleBoyce, Gillian P., Suveena Sreenilayam, Eleonora Balliana, Elisabetta Zendri, and Raymond J. Phaneuf. 2025. "Protective ALD Thin Films for Morphologically Diverse Types of Limestone" Coatings 15, no. 6: 698. https://doi.org/10.3390/coatings15060698
APA StyleBoyce, G. P., Sreenilayam, S., Balliana, E., Zendri, E., & Phaneuf, R. J. (2025). Protective ALD Thin Films for Morphologically Diverse Types of Limestone. Coatings, 15(6), 698. https://doi.org/10.3390/coatings15060698








