Bio-Based Flame Retardant Superhydrophobic Coatings by Phytic Acid/Polyethyleneimine Layer-by-Layer Assembly on Nylon/Cotton Blend Fabrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
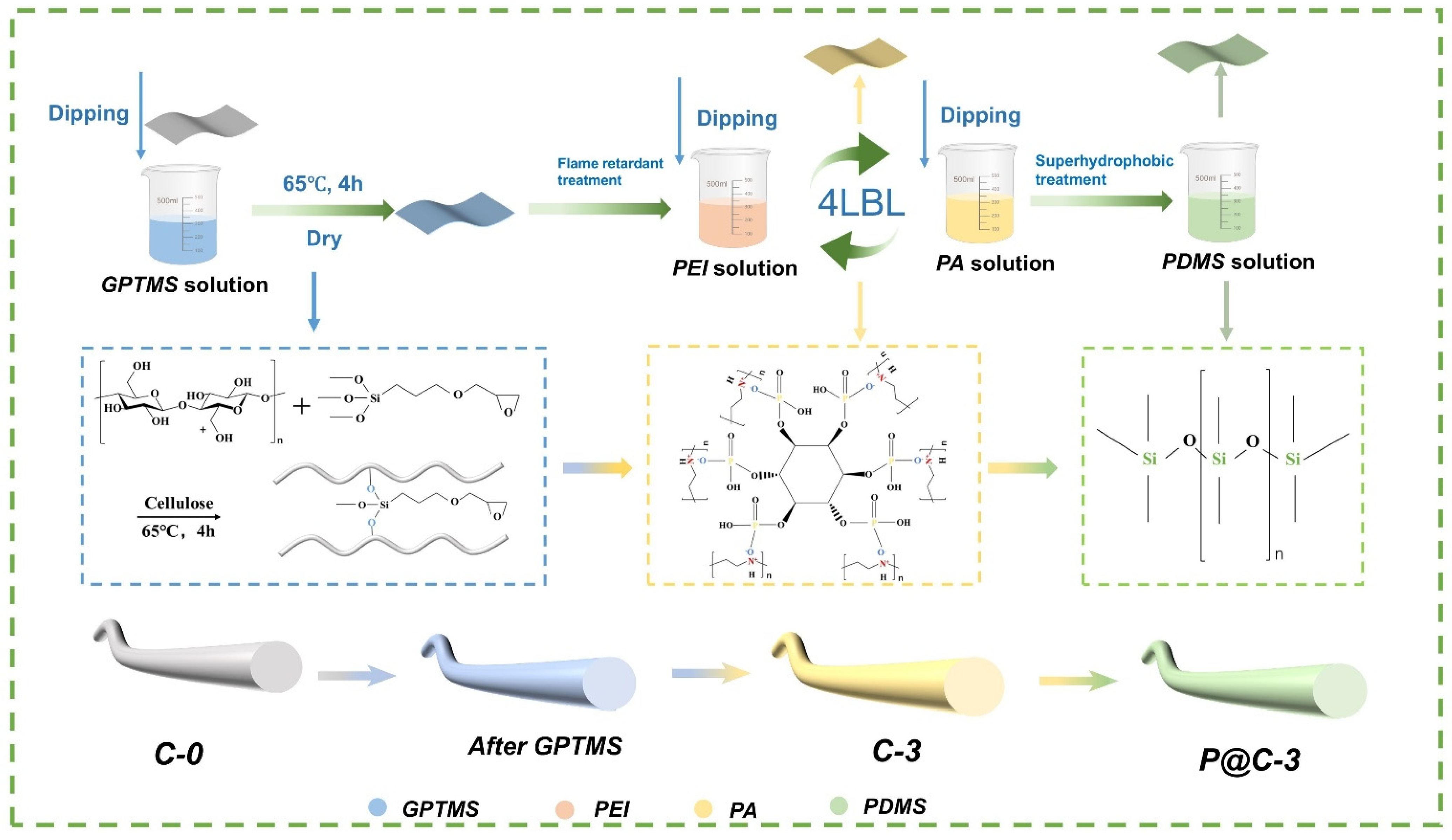
2.2. Preparation of Flame Retardant Superhydrophobic NC Fabrics
2.2.1. Pretreatment of NC Fabrics
2.2.2. Preparation of Flame Retardant Fabrics
Preparation of PA and PEI Solutions
Preparation of Flame Retardant NC Fabrics
2.2.3. Preparation of Flame Retardant Superhydrophobic Fabrics
2.3. Characterizations
3. Results
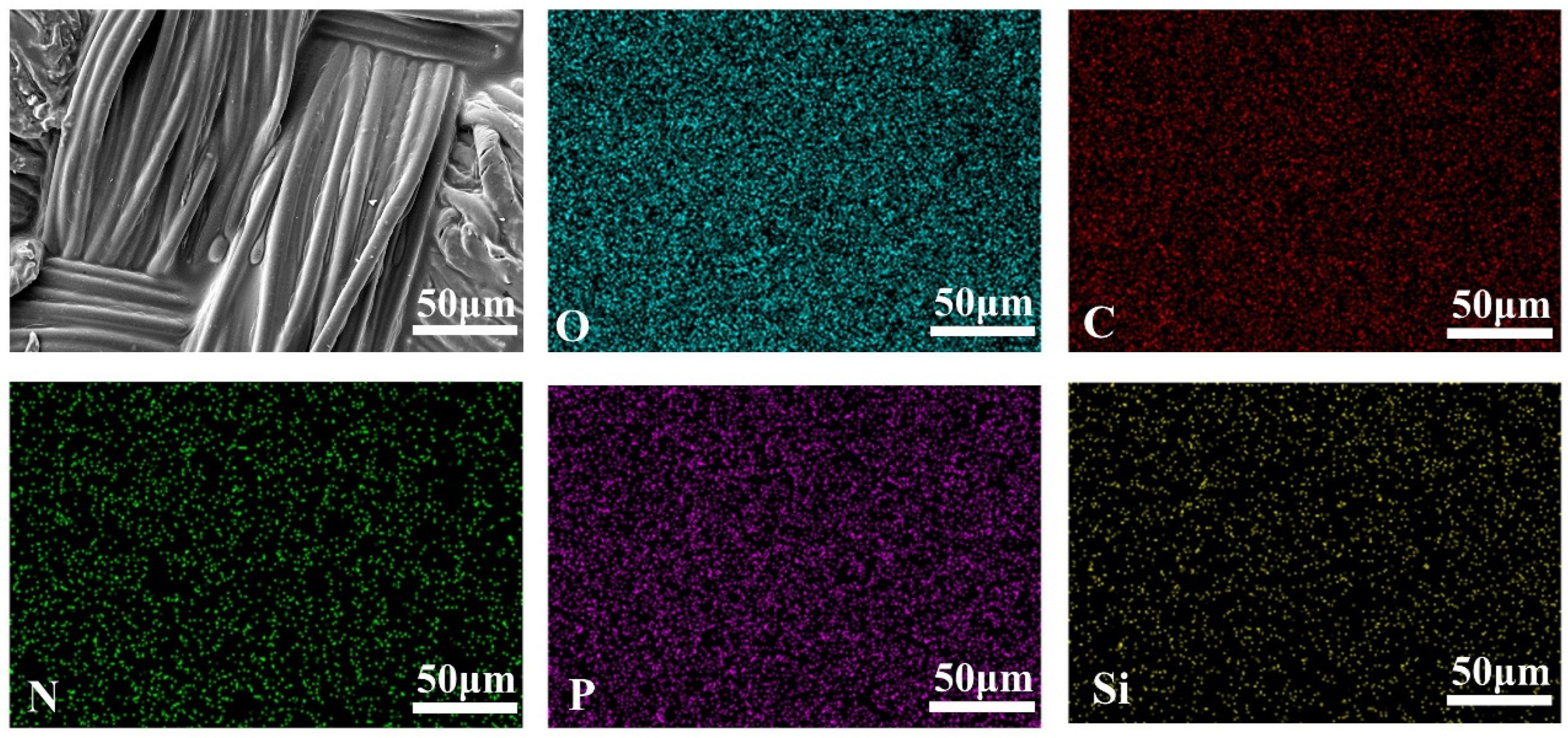
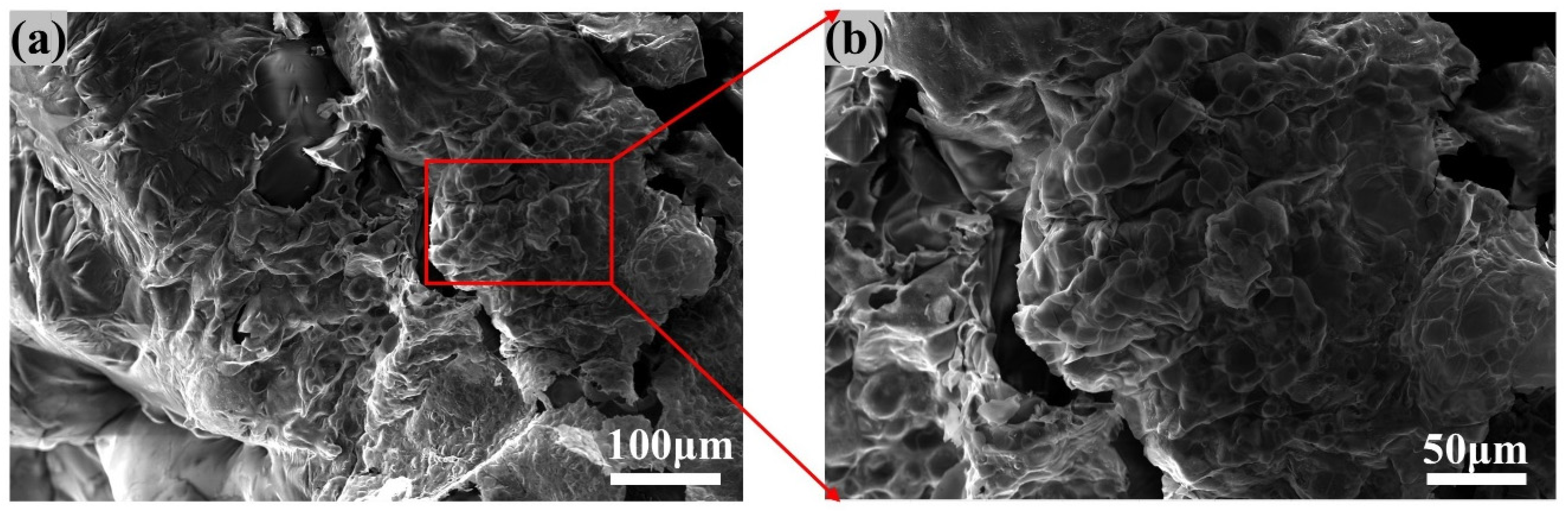
3.1. Surface Morphology Analysis
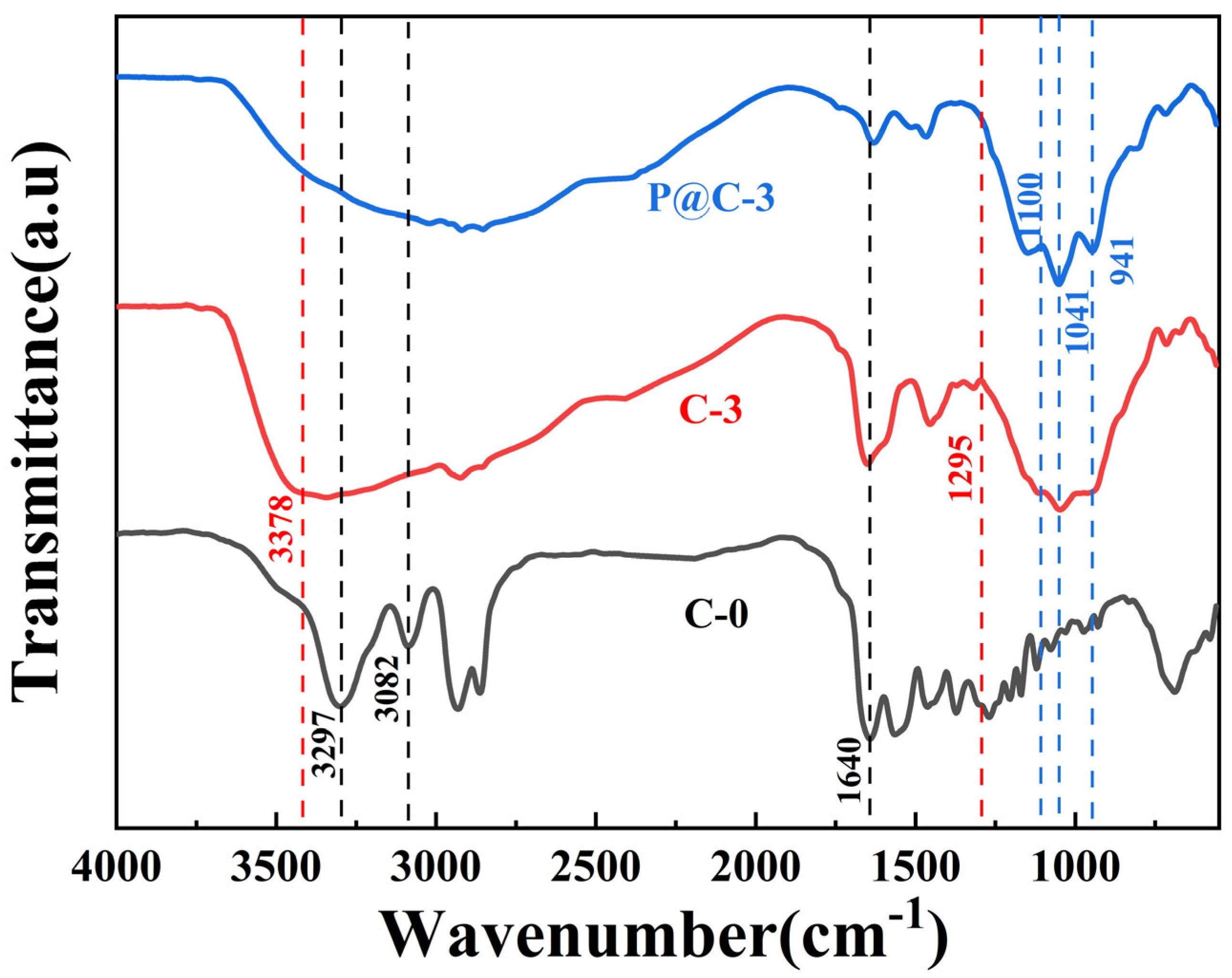
3.2. FTIR Analysis
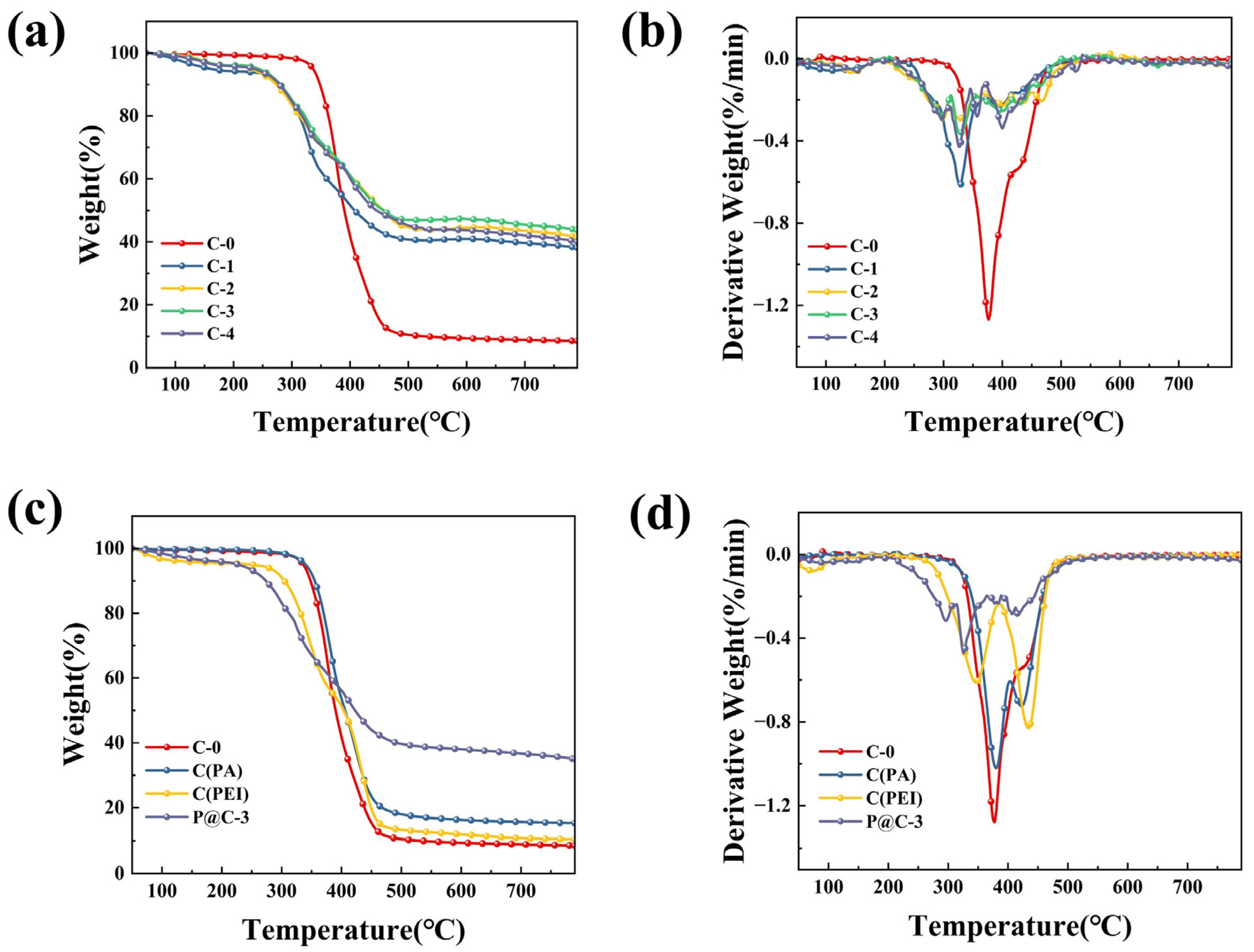
3.3. Thermal Stability Property Analysis
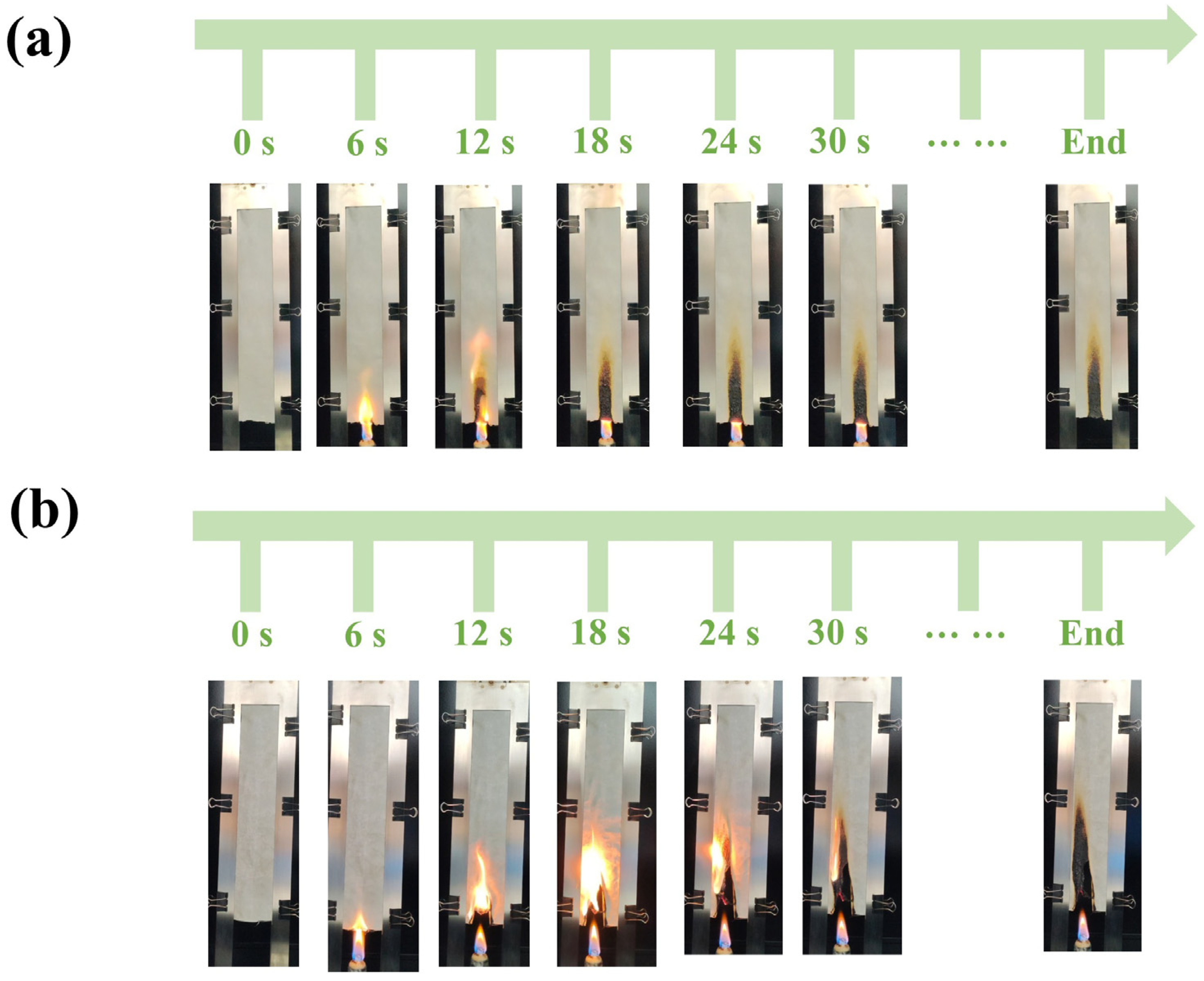
3.4. Flame Retardancy
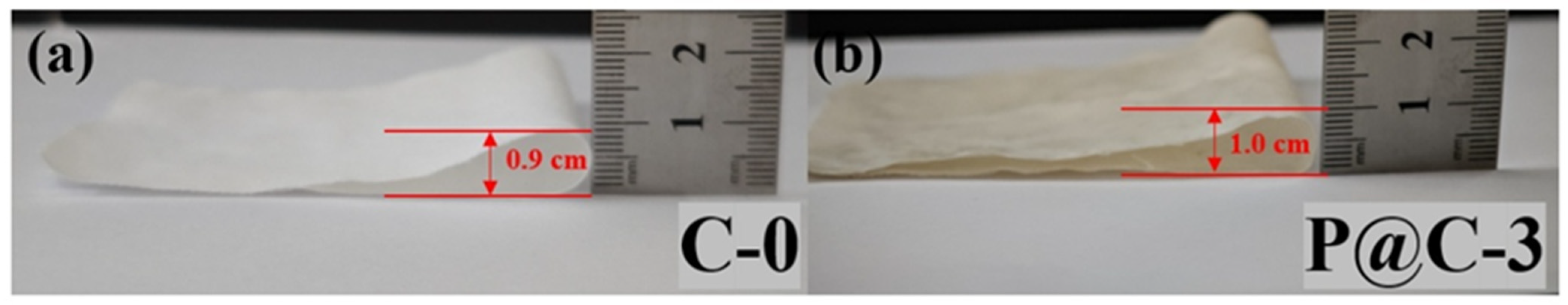
3.5. Analysis of Fabric Softness
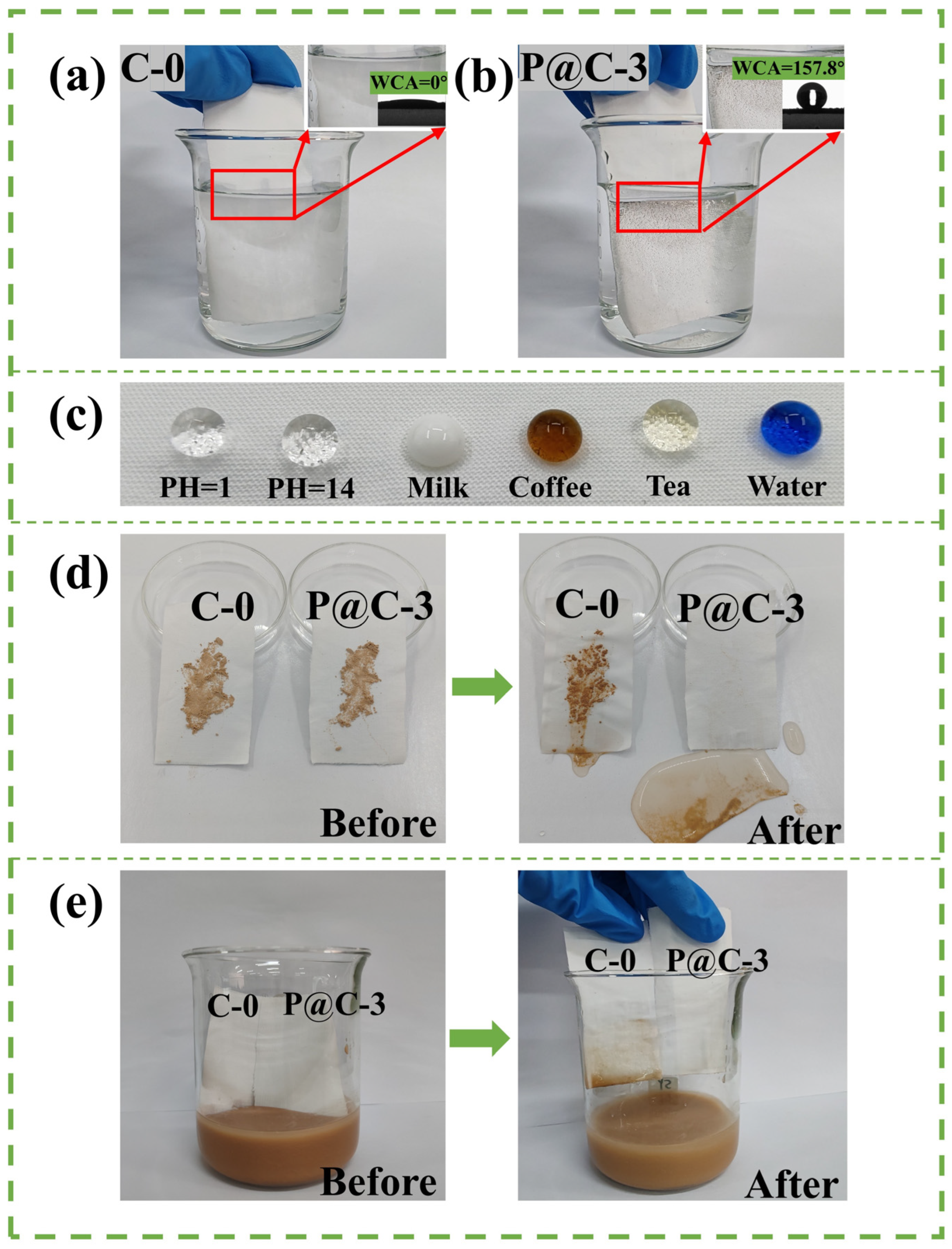
3.6. Superhydrophobicity and Self-Cleaning Performance
3.7. Durability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Tang, R.C. Adsorption and flame retardant properties of potassium diphenyl sulfonate on nylon 6 fabric. React. Funct. Polym. 2018, 126, 36–43. [Google Scholar] [CrossRef]
- Wankhede, B.; Bisaria, H.; Ojha, S.; Dakre, V. A review on cotton fibre-reinforced polymer composites and their applications. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 1347–1362. [Google Scholar] [CrossRef]
- Abd El-Hady, M.M.; Sharaf, S.; Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 2020, 27, 1099–1110. [Google Scholar] [CrossRef]
- Qi, L.; Qiu, S.; Xi, J.; Yu, B.; Hu, Y.; Xing, W. Construction of super-hydrophobic, highly effective flame retardant coating for cotton fabric with superior washability and abrasion resistance. J. Colloid Interface Sci. 2022, 607, 2019–2028. [Google Scholar] [CrossRef]
- Zhang, A.N.; Zhao, H.B.; Cheng, J.B.; Li, M.E.; Li, S.L.; Cao, M.; Wang, Y.Z. Construction of durable eco-friendly biomass-based flame-retardant coating for cotton fabrics. Chem. Eng. J. 2021, 410, 128361. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, L.; Zhang, C.; Li, S.; Tian, P.; Zhang, F.; Zhang, G. Eco-friendly synthesis of a highly efficient phosphorus flame retardant based on xylitol and application on cotton fabric. Cellulose 2019, 26, 2123–2138. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Yu, H.Y.; Jia, B.; Wang, X.; Chen, X.; Yang, X. Enhancing flame retardancy, antibacterial activity and UV resistance of polyamide 66 fabric by fully biobased intumescent flame-retardant nanocellulose coating. Int. J. Biol. Macromol. 2024, 283, 137994. [Google Scholar] [CrossRef]
- Qi, P.; Chen, F.; Li, Y.; Li, H.; Gu, X.; Sun, J.; Zhang, S. A review of durable flame-retardant fabrics by finishing: Fabrication strategies and challenges. Adv. Fiber Mater. 2023, 5, 731–763. [Google Scholar] [CrossRef]
- Fu, J.; Yang, F.; Chen, G.; Zhang, G.; Huang, C.; Guo, Z. A facile coating with water-repellent and flame-retardant properties on cotton fabric. New J. Chem. 2019, 43, 10183–10189. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Huang, J.; Zhou, Y.; Cai, W.; Wang, J.; Song, L.; Hu, Y. Construction of durable flame-retardant and robust superhydrophobic coatings on cotton fabrics for water-oil separation application. Chem. Eng. J. 2020, 398, 125661. [Google Scholar] [CrossRef]
- Cui, S.; Zhai, H.M.; Li, W.S.; Tong, W.; Li, X.S.; Cai, A.H.; Fan, X.J.; Li, X.Q.; Xiong, D.S. Superhydrophobic Fe-based amorphous coating fabricated by detonation spraying with excellent anti-corrosion and self-cleaning properties. Rare Met. 2023, 42, 629–644. [Google Scholar] [CrossRef]
- Lin, D.; Zeng, X.; Li, H.; Lai, X.; Wu, T. One-pot fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via sol-gel reaction. J. Colloid Interface Sci. 2019, 533, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Song, W.M.; Sun, Y.; Yang, J.X.; Liu, Y. A higher durability flame retardant for regenerated cellulose fabrics based on fully bio-based phytic acid and L-arginine. Int. J. Biol. Macromol. 2025, 308, 142377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, M.; Wang, Q.; Li, B. A novel durable flame retardant cotton fabric produced by surface chemical grafting of phosphorus-and nitrogen-containing compounds. Cellulose 2017, 24, 4069–4081. [Google Scholar] [CrossRef]
- Peng, S.; Zhao, C.; Li, Z.; Lian, Y.; Ren, S.; Zhang, L.; Zhang, J.; Chen, X.; Wu, Z.; Qu, H. Facile manufacturing process of durable superamphiphobic and flame-retardant coatings based on layer-by-layer assembly. Surf. Interfaces 2022, 31, 102109. [Google Scholar] [CrossRef]
- Cai, T.; Wang, J.; Zhang, C.; Cao, M.; Jiang, S.; Wang, X.; Wang, B.; Hu, W.; Hu, Y. Halogen and halogen-free flame retarded biologically-based polyamide with markedly suppressed smoke and toxic gases releases. Compos. Part B Eng. 2020, 184, 107737. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Y.; Zhang, F. A biological reactive flame retardant for flame retardant modification of cotton fabric. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127601. [Google Scholar] [CrossRef]
- Zhao, B.; Kolibaba, T.J.; Lazar, S.; Grunlan, J.C. Environmentally-benign, water-based covalent polymer network for flame retardant cotton. Cellulose 2021, 28, 5855–5866. [Google Scholar] [CrossRef]
- Liu, J.; Qi, P.; Zhang, J.; Liu, X.; Chen, F.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Bio-based phytic acid-induced polypyrrole/silver nanowires coating towards multifunctional nylon/cotton blend fabrics. Chem. Eng. J. 2023, 476, 146837. [Google Scholar] [CrossRef]
- Li, J.; Cao, Q.; Zhao, Y.; Gu, C.; Liu, B.; Fan, Q.; Zhang, C.; Huang, Y.; Jiang, S.; Jian, X.; et al. Bio-based flame retardant for manufacturing fire safety, strong yet tough versatile epoxy resin. Compos. Part B Eng. 2024, 276, 111362. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.Y.; Guan, J.P.; Cheng, X.W.; Chen, G. In-situ polymerization of phosphorus/nitrogen flame-retardant coating for polyester/cotton blend fabrics with superior durability. Int. J. Biol. Macromol. 2024, 277, 134458. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Zhang, L.Y.; Li, P.; Liu, Y. High-efficient flame-retardant finishing of cotton fabrics based on phytic acid. Int. J. Mol. Sci. 2023, 24, 1093. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qi, P.; Peng, A.; Sun, J.; Cui, Z.; Liu, W.; Li, H.; Gu, X.; Zhang, S. Preparation of durable flame retardant nylon-cotton blend fabrics by 3-glycidyloxypropyl trimethoxy silane associated with polyethyleneimine and phytic acid. Cellulose 2022, 29, 7413–7430. [Google Scholar] [CrossRef]
- Barbalini, M.; Bertolla, L.; Toušek, J.; Malucelli, G. Hybrid silica-phytic acid coatings: Effect on the thermal stability and flame retardancy of cotton. Polymers 2019, 11, 1664. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Fang, Y. High-efficiency and durable flame retardant cotton fabric with good physical and mechanical properties by layer-by-layer assembly polyethylenimine/phytic acid coating. J. Nat. Fibers 2022, 19, 11790–11801. [Google Scholar] [CrossRef]
- Zilke, O.; Plohl, D.; Opwis, K.; Mayer-Gall, T.; Gutmann, J.S. A flame-retardant phytic-acid-based LbL-coating for cotton using polyvinylamine. Polymers 2020, 12, 1202. [Google Scholar] [CrossRef]
- Zheng, X.T.; Dong, Y.Q.; Liu, X.D.; Xu, Y.L.; Jian, R.K. Fully bio-based flame-retardant cotton fabrics via layer-by-layer self assembly of laccase and phytic acid. J. Clean. Prod. 2022, 350, 131525. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, L.; Fan, Z.; Yu, Y.; Liu, R. Bio-based coating of phytic acid, chitosan, and biochar for flame-retardant cotton fabrics. Polym. Degrad. Stab. 2022, 199, 109898. [Google Scholar] [CrossRef]
- Qiu, X.; Li, Z.; Li, X.; Zhang, Z. Flame retardant coatings prepared using layer by layer assembly: A review. Chem. Eng. J. 2018, 334, 108–122. [Google Scholar] [CrossRef]
- Ling, C.; Guo, L.; Wang, Z. A review on the state of flame-retardant cotton fabric: Mechanisms and applications. Ind. Crops Prod. 2023, 194, 116264. [Google Scholar] [CrossRef]
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog. Org. Coat. 2022, 171, 107027. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular firefighting—How modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Kumar, A.; Bhushan, B. Self-cleaning, stain-resistant and anti-bacterial superhydrophobic cotton fabric prepared by simple immersion technique. J. Colloid Interface Sci. 2019, 535, 66–74. [Google Scholar] [CrossRef]
- Cheng, Q.Y.; Guan, C.S.; Wang, M.; Li, Y.D.; Zeng, J.B. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Fu, X.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Preparation of fluorine-free superhydrophobic and wear-resistant cotton fabric with a UV curing reaction for self-cleaning and oil/water separation. RSC Adv. 2021, 11, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, H.; Guan, H.; Zheng, L.; Lai, X.; Chen, W.; Zeng, X. Facile fabrication of superhydrophobic, flame-retardant and conductive cotton fabric for human motion detection. Cellulose 2022, 29, 605–617. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Mahmood, K.; Jin, Y.; Huang, C.; Huang, Z.; Zhang, S.; Ming, W. Fabrication of multifunctional PET fabrics with flame retardant, antibacterial and superhydrophobic properties. Prog. Org. Coat. 2021, 157, 106296. [Google Scholar] [CrossRef]
- Galvão, F.M.F.; Cabral, R.L.B.; Santos, J.E.L.; Santos, E.V.; Kim, S.; Souza, D.F.S.; Silva, K.K.O.S.; Nascimento, J.H.O. Superhydrophobic and Supercapacitive Reduced Graphene Oxide/Fluoropolymer Nanocoating on Polyester Fabric via Spray Mist Coating. Colloids Surf. A Physicochem. Eng. Asp. 2025, 716, 136696. [Google Scholar] [CrossRef]
- Wang, J.; He, J.; Ma, L.; Zhang, Y.; Shen, L.; Xiong, S.; Li, K.; Qu, M. Multifunctional conductive cellulose fabric with flexibility, superamphiphobicity and flame-retardancy for all-weather wearable smart electronic textiles and high-temperature warning device. Chem. Eng. J. 2020, 390, 124508. [Google Scholar] [CrossRef]
- Chen, F.F.; Zhu, Y.J.; Xiong, Z.C.; Dong, L.Y.; Chen, F.; Lu, B.Q.; Yang, R.L. Hydroxyapatite nanowire-based all-weather flexible electrically conductive paper with superhydrophobic and flame-retardant properties. ACS Appl. Mater. Interfaces 2017, 9, 39534–39548. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Macintyre, C.R.; Wen, X.; Bahl, P.; Kumar, U.; Chughtai, A.A.; Joshi, R. Nanoparticles incorporated graphene-based durable cotton fabrics. Carbon 2020, 166, 148–163. [Google Scholar] [CrossRef]
- Kale, K.H.; Palaskar, S.; Bhat, N.V. Effect of Plasma Polymerization of Hexamethyldisiloxane on Polyester/Cotton Blended Fabrics Properties. AATCC Rev. 2012, 12, 53. [Google Scholar]
- Liu, W.; Shi, R.; Zhang, Z.; Yan, M.; Chen, X.; Chen, Y. Coordination driven layer-by-layer deposition technology used for fabrication of flame retardant polyamide 66 fabric. Polym. Adv. Technol. 2021, 32, 3232–3241. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Y.; Song, T.; Bao, M.; Li, Y.; Lu, J.; Li, Y. An environmentally benign approach to prepare superhydrophobic magnetic melamine sponge for effective oil/water separation. Sep. Purif. Technol. 2020, 236, 116308. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Deng, S.; Fu, X.; Du, Z.; Cheng, X.; Wang, H. A polydopamine-bridged hierarchical design for fabricating flame-retarded, superhydrophobic, and durable cotton fabric. Cellulose 2019, 26, 7009–7023. [Google Scholar] [CrossRef]
- Grancaric, A.M.; Colleoni, C.; Guido, E.; Botteri, L.; Rosace, G. Thermal behaviour and flame retardancy of monoethanolamine-doped sol-gel coatings of cotton fabric. Prog. Org. Coat. 2017, 103, 174–181. [Google Scholar] [CrossRef]
- Yin, C.; Lu, Q.; Yu, X.; Yang, S.; Gao, Q.; Zhang, X.; He, P.; Zhang, J.; Wang, R. Fabrication of eco friendly, durable fire retardant and hydrophobic cotton via layer-by-layer assembly. Prog. Org. Coat. 2025, 200, 108937. [Google Scholar] [CrossRef]
- Martin, C.; Hunt, B.J.; Ebdon, J.R.; Ronda, J.C.; Cadiz, V. Synthesis, crosslinking and flame retardance of polymers of boron-containing difunctional styrenic monomers. React. Funct. Polym. 2006, 66, 1047–1054. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Gao, M.; Chai, W.; Su, X.; Zheng, Z.; Liu, Y. Bio-inspired and facile fabrication strategy of bio-based, halogen-free and superhydrophobic cotton fabrics with multi-functionality and durability. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130586. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.T. Large-Scale Fabrication of Graded Convex Structure for Superhydrophobic Coating Inspired by Nature. Materials 2022, 15, 2179. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Wu, X.; You, H.; Yang, G.; Zhang, K.; Sun, C.; Liu, S. A Degradable Photo-Thermal Aerogel with Biomimetic Structure and Selective Wettability for High-Throughput Recovery of Viscous Crude Oil. Adv. Funct. Mater. 2025, 2425886. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, Y.; Chen, H.; Wei, L.; Zhu, G.; Liu, L.; Yao, J. Efficient, durable, and breathable flame retardant cotton fabric via a feasible surface finishing. Appl. Surf. Sci. 2023, 615, 156314. [Google Scholar] [CrossRef]





| Sample | PEI (wt%) | PA (wt%) |
|---|---|---|
| C-0 | -- | -- |
| C-1 | 6.0% | 5.0% |
| C-2 | 6.0% | 5.5% |
| C-3 | 6.0% | 6.0% |
| C-4 | 6.0% | 6.5% |
| Sample | T-10%/°C | T-50%/°C | T-max/% | Vmax (%/min) | Residue at 800 °C (wt%) |
|---|---|---|---|---|---|
| C-0 | 348 | 390 | 378 | 1.269 | 8.6 |
| C-1 | 278 | 407 | 329 | 0.608 | 37.9 |
| C-2 | 274 | 456 | 328 | 0.283 | 41.1 |
| C-3 | 282 | 458 | 327 | 0.356 | 43.9 |
| C-4 | 281 | 445 | 328 | 0.412 | 39.9 |
| P@C-3 | 277 | 425 | 324 | 0.452 | 35.1 |
| C(PA) | 356 | 405 | 382 | 1.008 | 15.2 |
| C(PEI) | 307 | 404 | 435 | 0.828 | 10.3 |
| Sample | Add-On (wt%) | Vertical Burning Test | ||
|---|---|---|---|---|
| Afterflame Time (s) | Afterglow Time (s) | Damaged Length (mm) | ||
| C-0 | -- | 26 | 9 | 300 ± 0 |
| C-1 | 22.6 | 24 | 11 | 300 ± 0 |
| C-2 | 25.3 | 0 | 0 | 55 ± 2 |
| C-3 | 27.1 | 0 | 0 | 35 ± 2 |
| C-4 | 29.4 | 0 | 0 | 99 ± 3 |
| P@C-3 | 31.6 | 0 | 0 | 78 ± 1 |
| C(PA) | 15.3 | 20 | 7 | 300 ± 0 |
| C(PEI) | 18.6 | 18 | 9 | 300 ± 0 |
| C(PDMS) | 5.3 | 23 | 12 | 300 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Zheng, H.; Cao, J.; Guo, X. Bio-Based Flame Retardant Superhydrophobic Coatings by Phytic Acid/Polyethyleneimine Layer-by-Layer Assembly on Nylon/Cotton Blend Fabrics. Coatings 2025, 15, 699. https://doi.org/10.3390/coatings15060699
Shen Y, Zheng H, Cao J, Guo X. Bio-Based Flame Retardant Superhydrophobic Coatings by Phytic Acid/Polyethyleneimine Layer-by-Layer Assembly on Nylon/Cotton Blend Fabrics. Coatings. 2025; 15(6):699. https://doi.org/10.3390/coatings15060699
Chicago/Turabian StyleShen, Yue, Haiyan Zheng, Jiqiang Cao, and Xinyun Guo. 2025. "Bio-Based Flame Retardant Superhydrophobic Coatings by Phytic Acid/Polyethyleneimine Layer-by-Layer Assembly on Nylon/Cotton Blend Fabrics" Coatings 15, no. 6: 699. https://doi.org/10.3390/coatings15060699
APA StyleShen, Y., Zheng, H., Cao, J., & Guo, X. (2025). Bio-Based Flame Retardant Superhydrophobic Coatings by Phytic Acid/Polyethyleneimine Layer-by-Layer Assembly on Nylon/Cotton Blend Fabrics. Coatings, 15(6), 699. https://doi.org/10.3390/coatings15060699






