Tuning Electrical and Optical Properties of SnO2 Thin Films by Dual-Doping Al and Sb
Abstract
1. Introduction
2. Experimental
2.1. Chemistry and Equipment
2.2. Experimental Procedure
3. Results and Discussion
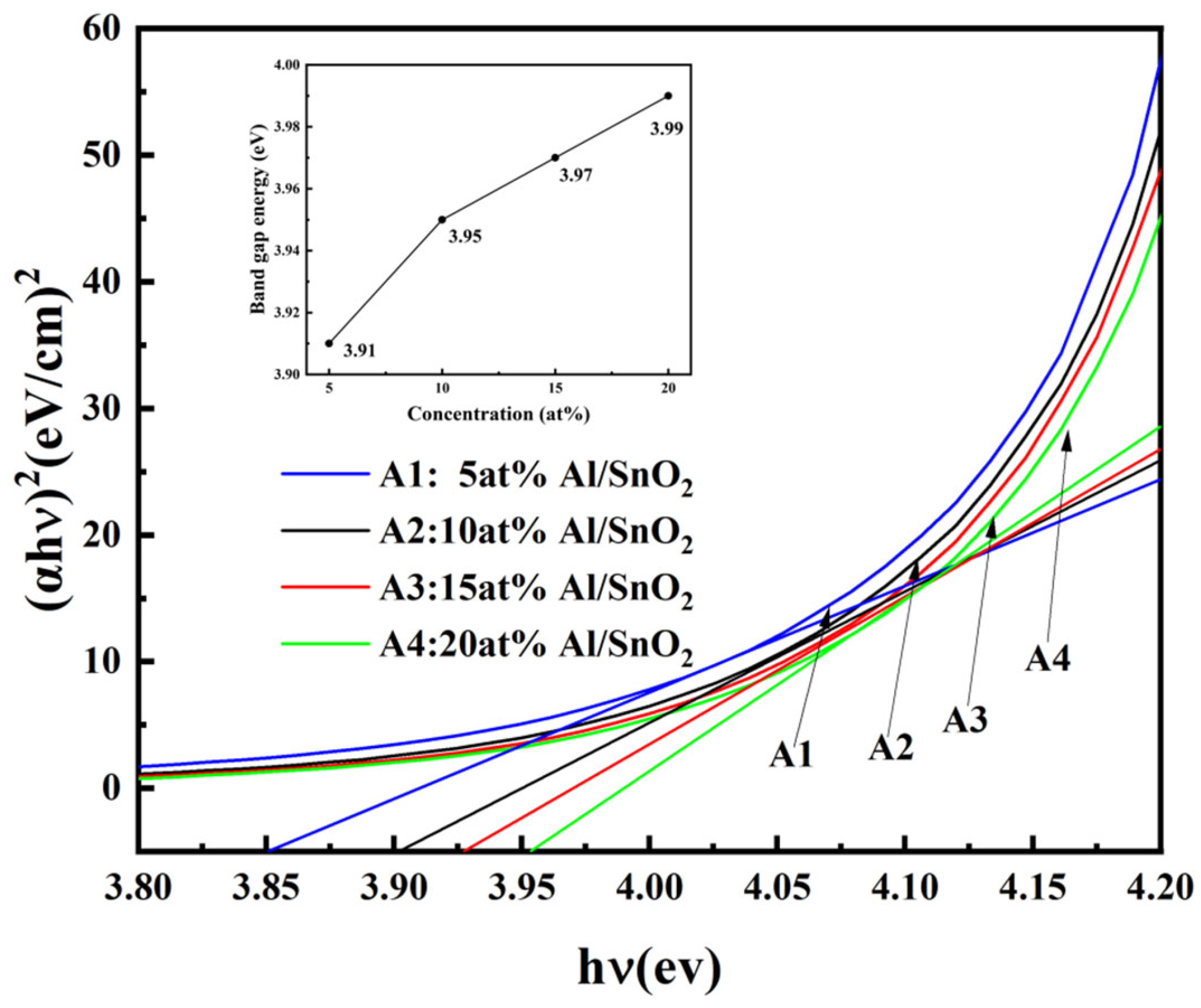
3.1. The Influence of Al Doping on the Optoelectronic Properties of SnO2 Thin Films
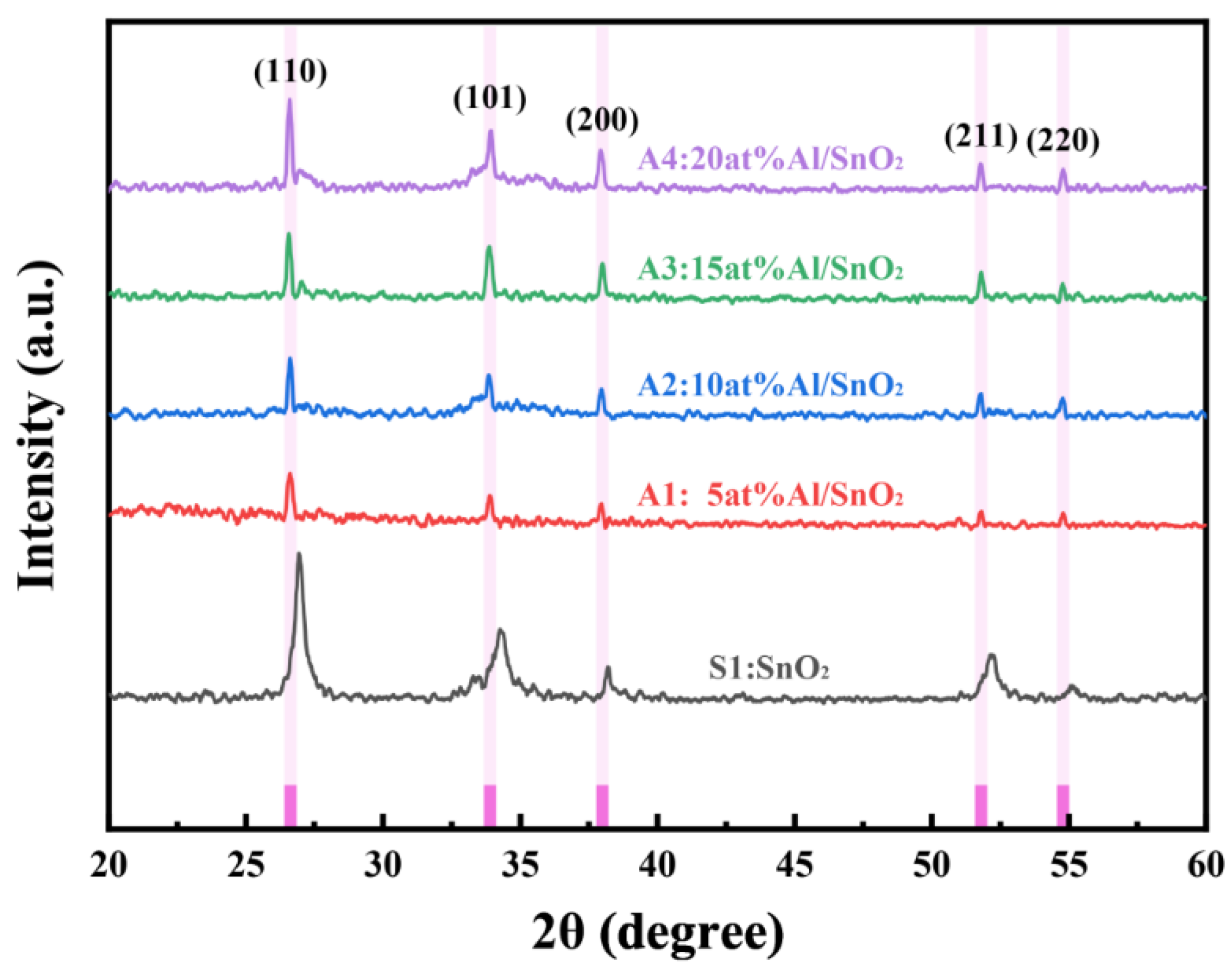
3.1.1. Analysis of the XRD Results
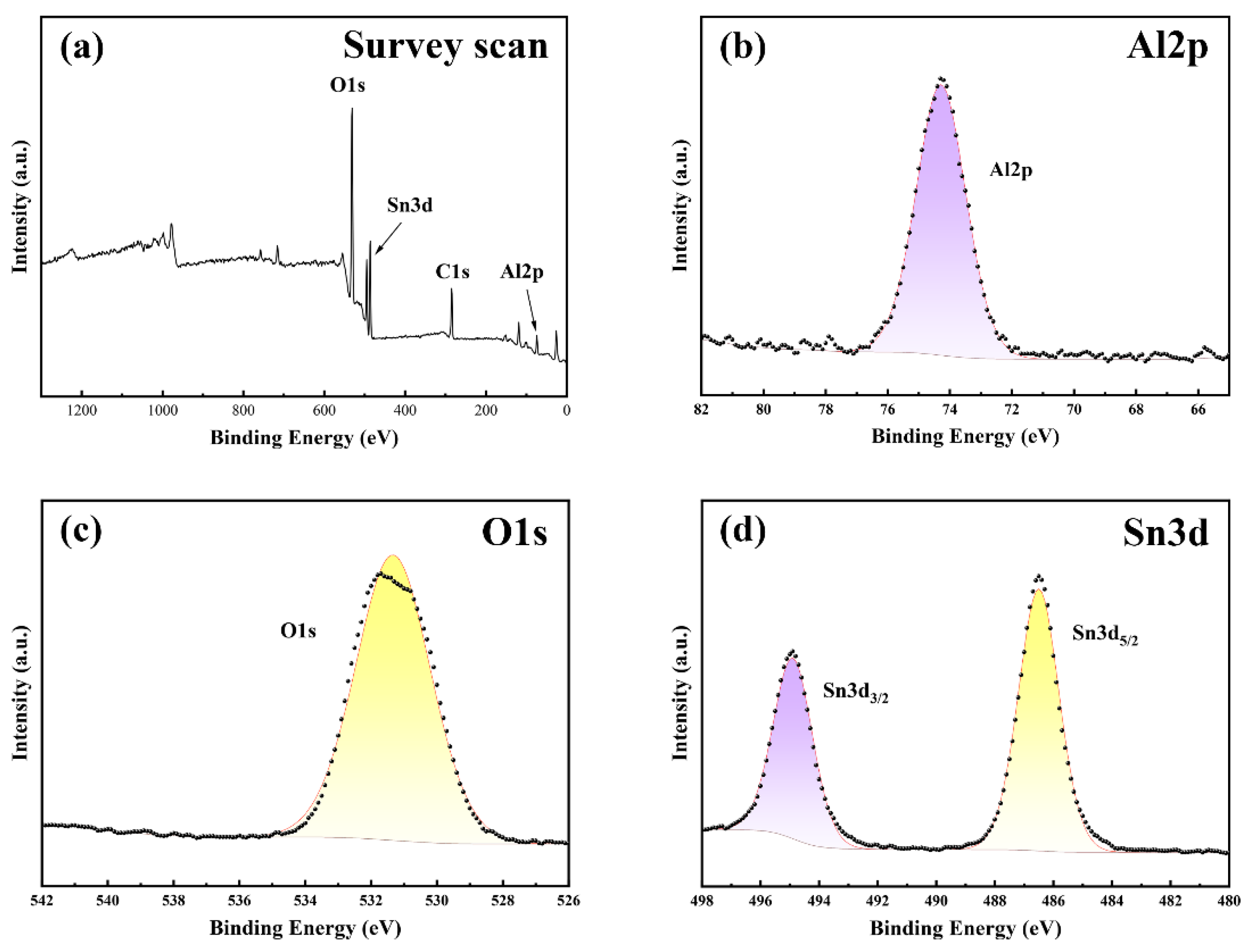
3.1.2. Analysis of the XPS Results
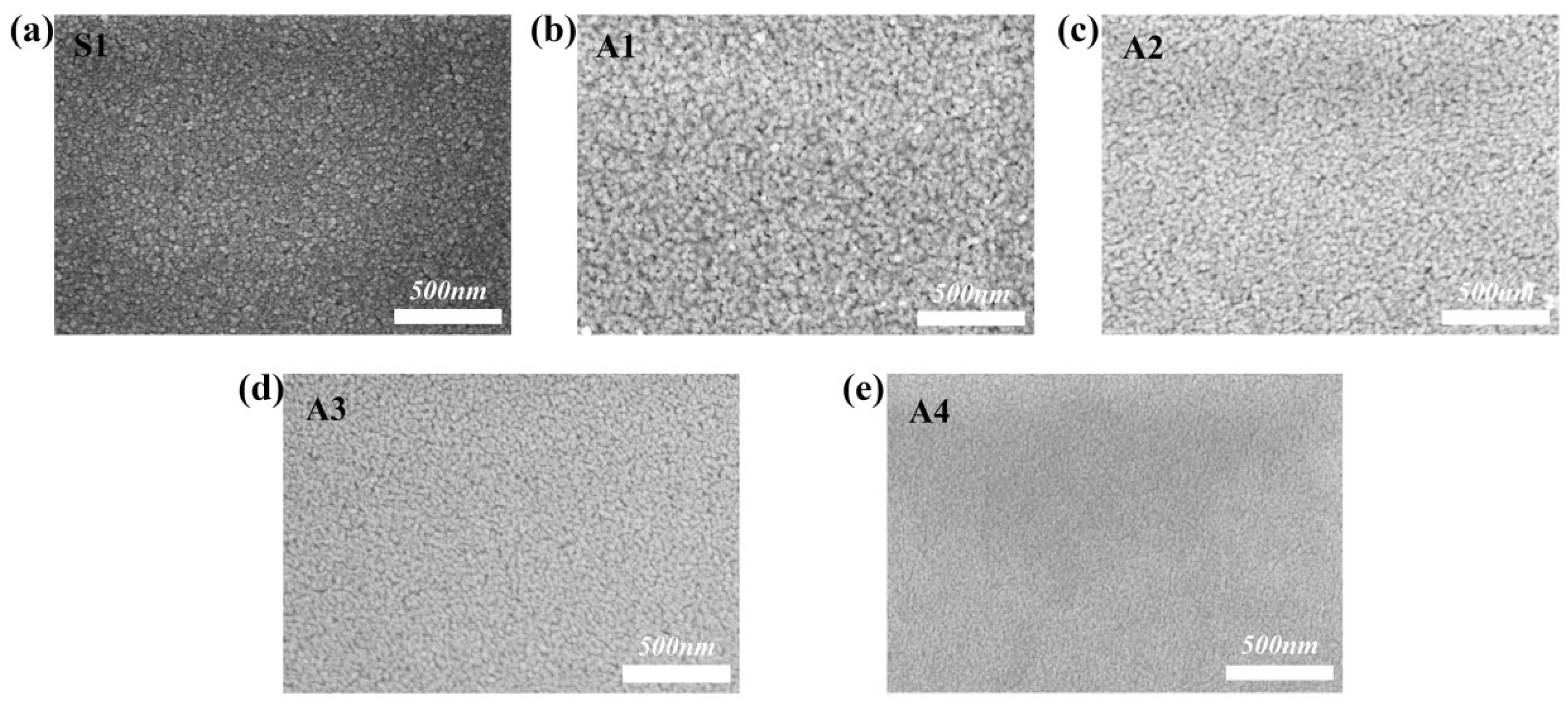
3.1.3. Analysis of the SEM Results
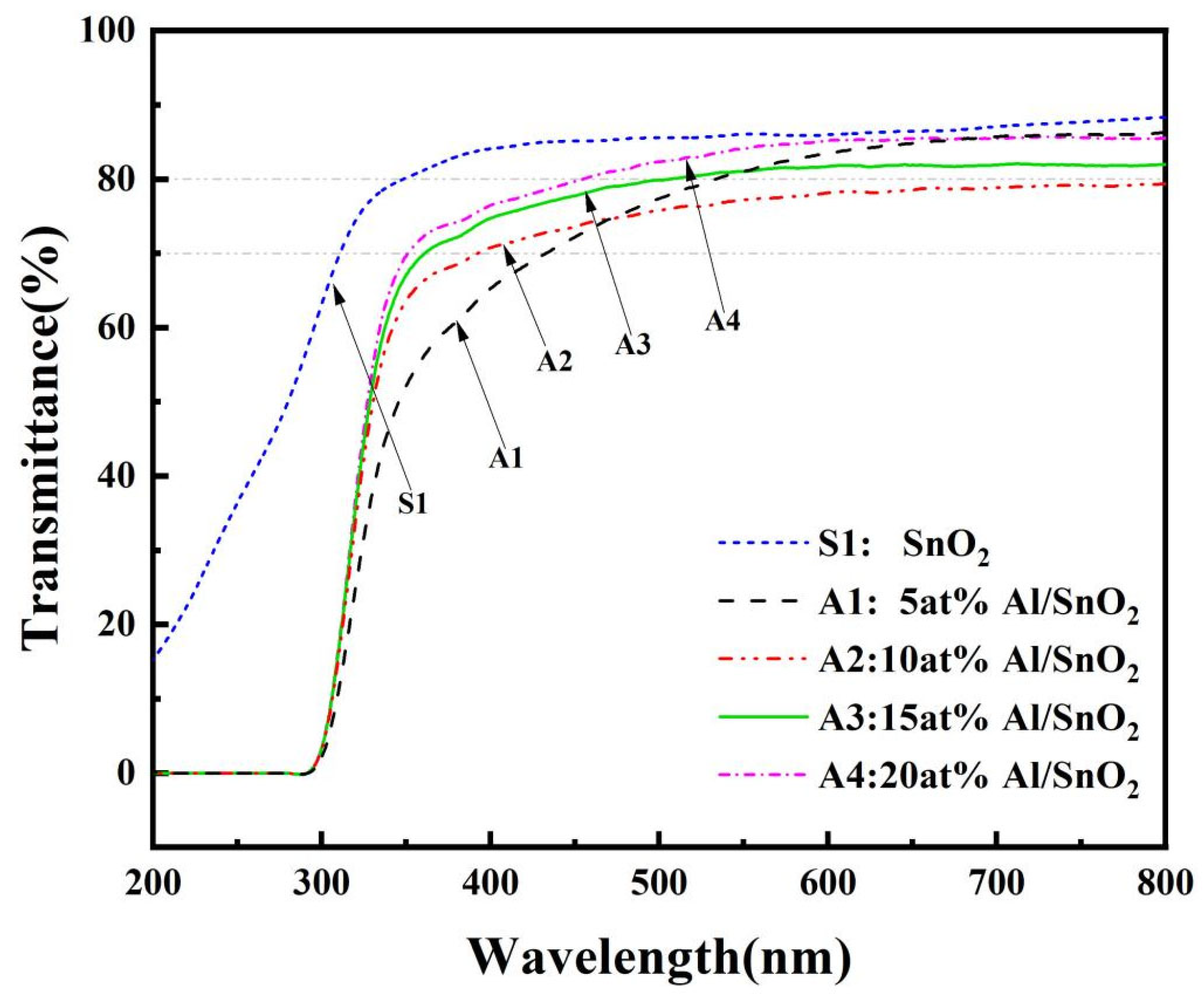
3.1.4. Analysis of the Optical Properties
3.1.5. Analysis of the Electrical Properties
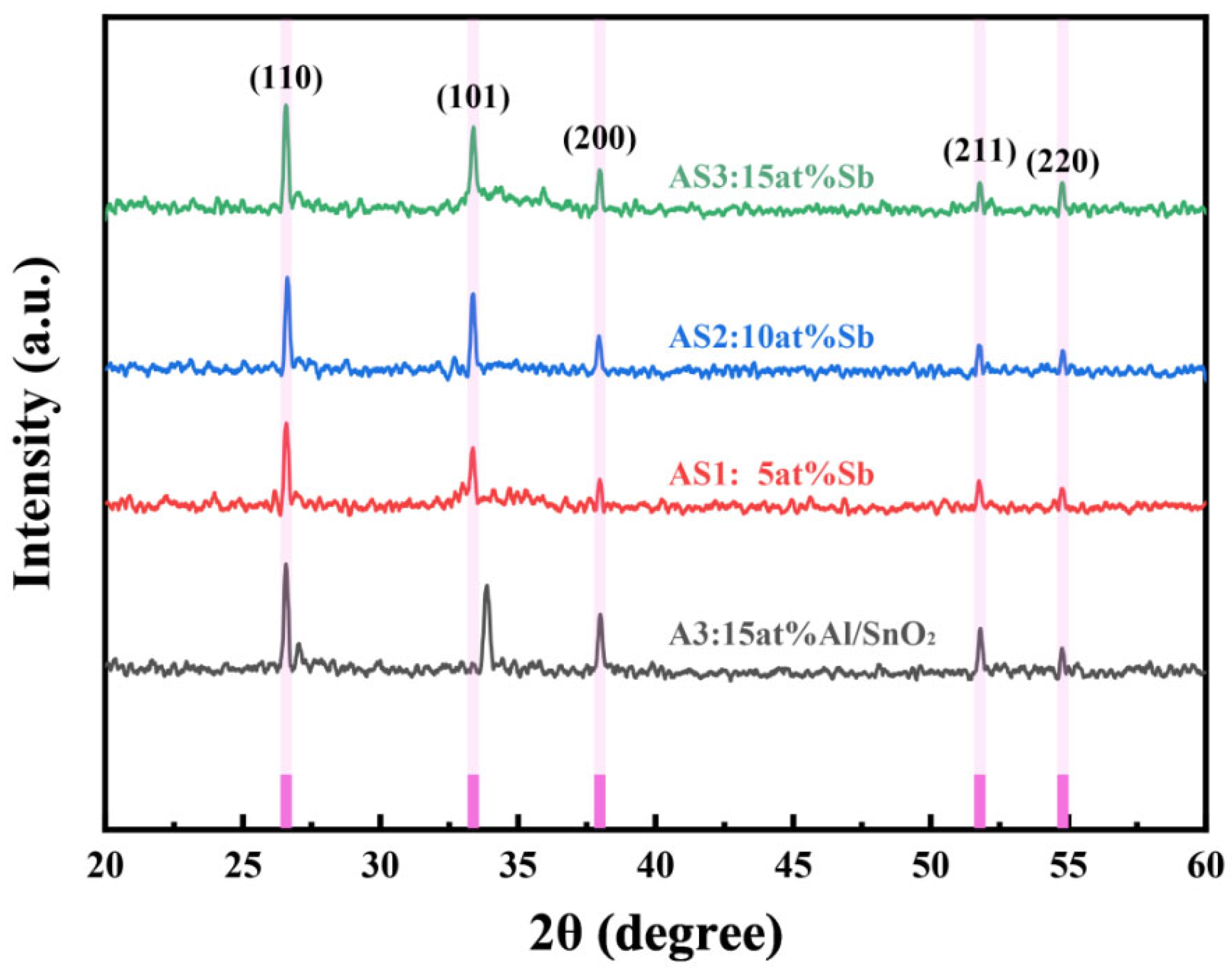
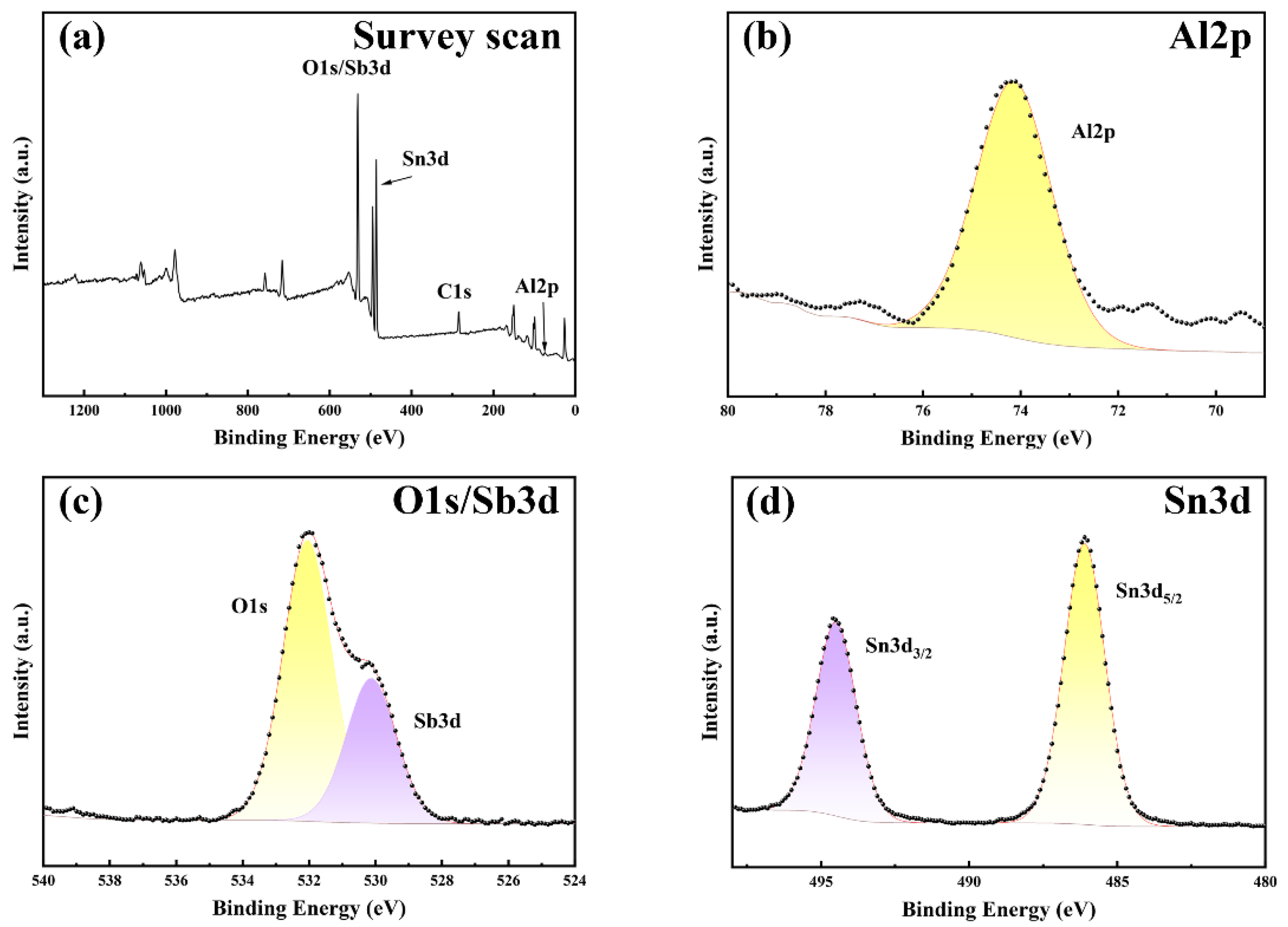
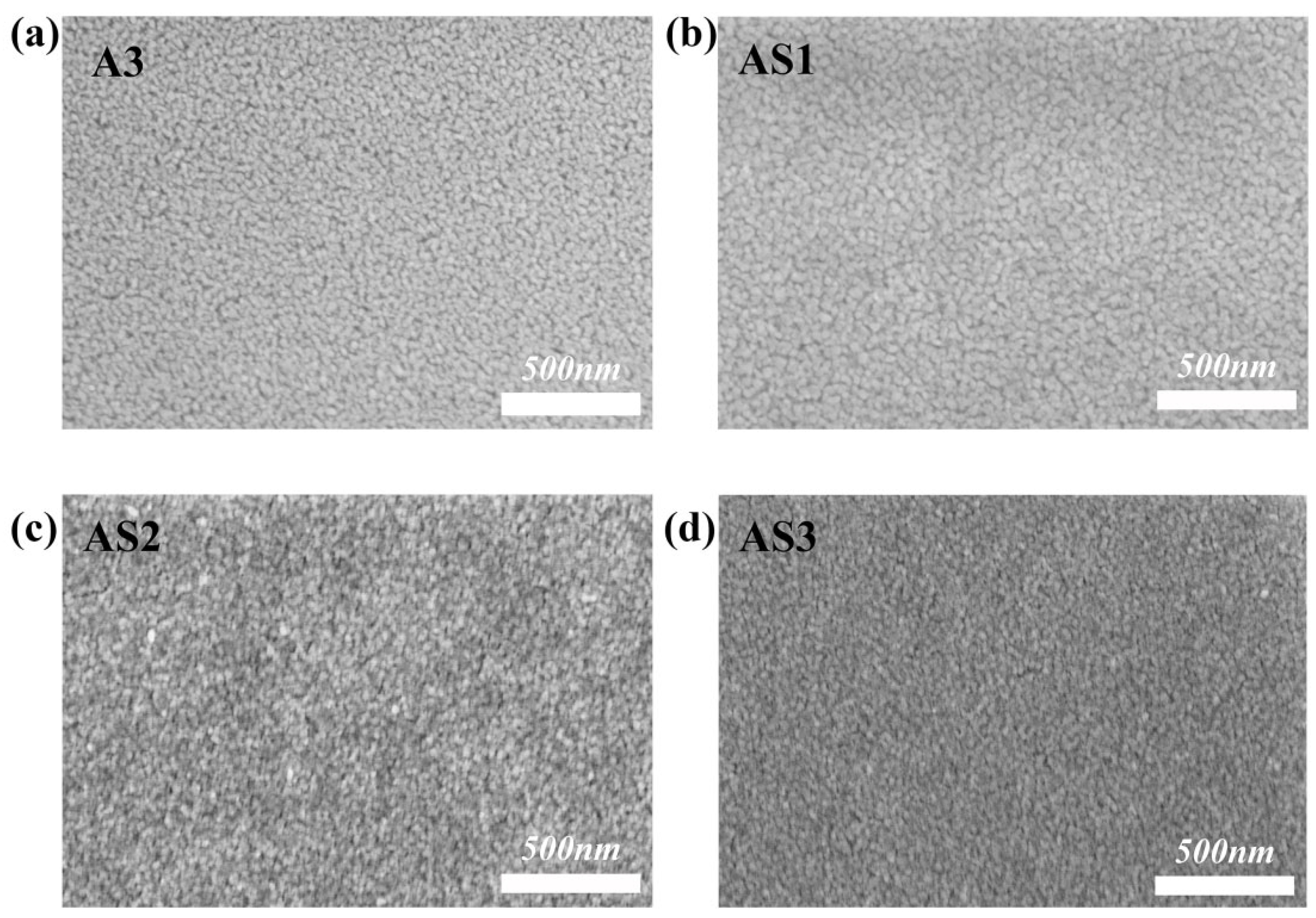
3.2. Influence of Sb Doping on the Photoelectric Properties of Al-SnO2 Thin Films
3.2.1. Analysis of the XRD Results
3.2.2. Analysis of the XPS Results
3.2.3. Analysis of the SEM Results
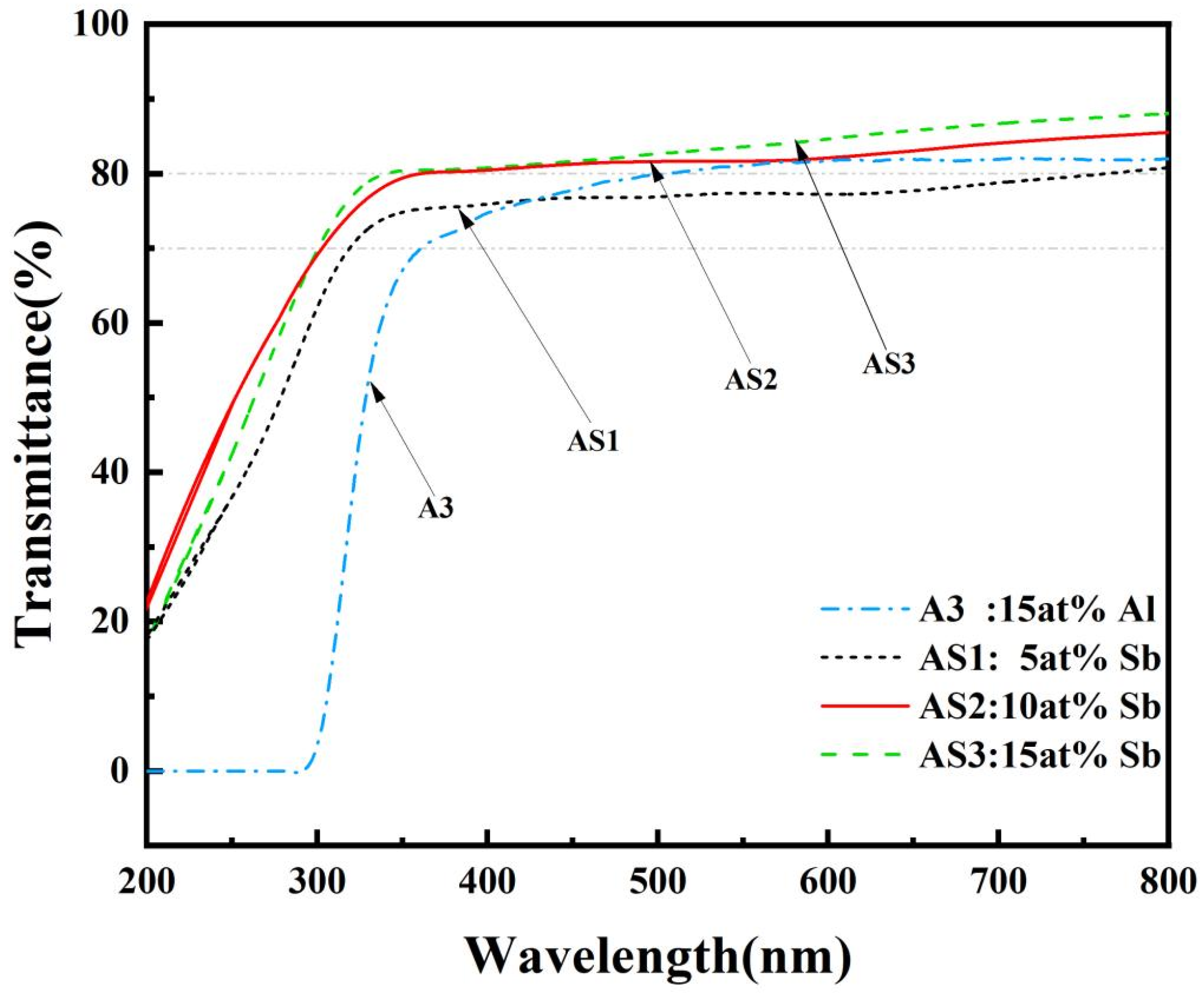
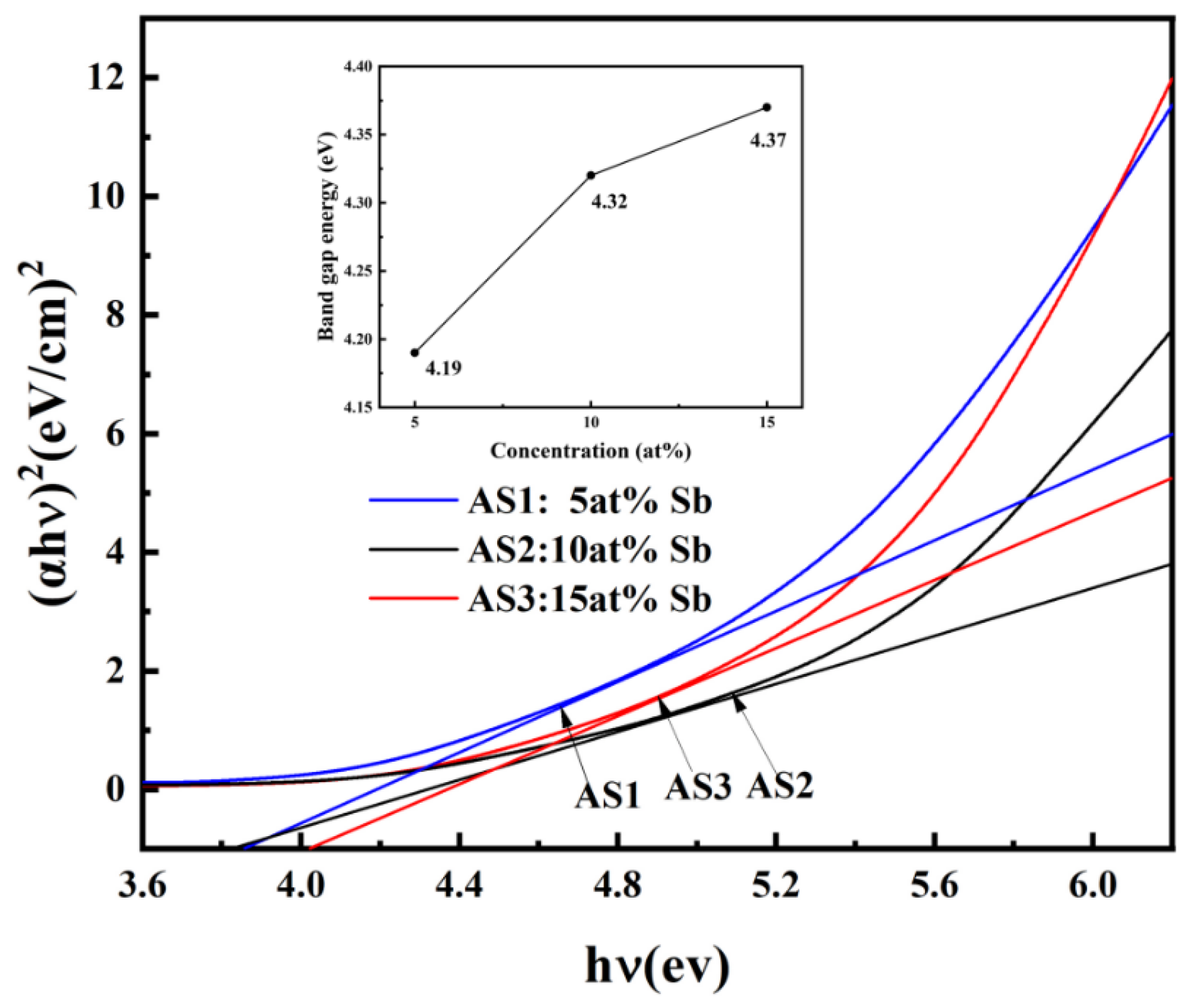
3.2.4. Analysis of the Optical Properties
3.2.5. Analysis of the Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huda, M.N.; Hashmi, G. Fabrication, characterization and performance analysis of sol–gel dip coated SnO2 thin film. Discov. Mater. 2025, 5, 10. [Google Scholar] [CrossRef]
- Kania, A.; Szindler, M.M.; Szindler, M.; Brytan, Z.; Łoński, W. Structure and Selected Properties of SnO2 Thin Films. Materials 2024, 17, 3348. [Google Scholar] [CrossRef] [PubMed]
- Joseph, D.P.; Radha, R.; Fernandes, J.M.; Muniramaiah, R.; Purushothamreddy, N.; Kovendhan, M.; Venkateswaran, C. Investigation of the transparent conducting properties of spray-pyrolyzed Li and F co-doped SnO2 thin film electrodes. J. Mater. Sci. Mater. Electron. 2022, 33, 8435–8445. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, C.; Yang, L.; Jiang, J.; Guo, Y. Optimizing the gas sensing characteristics of Co-doped SnO2 thin film based hydrogen sensor. J. Alloys Compd. 2019, 785, 819–825. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Muniramaiah, R.; Maharana, G.; Janbandhu, S.Y.; Jayavelu, Y.; Kovendhan, M.; Venkateswaran, C.; Sambasivam, S.; Joseph, D.P. Spray-pyrolyzed rare-earth dysprosium-doped SnO2 thin films for plausible photocatalytic application. J. Mater. Sci. Mater. Electron. 2023, 34, 2131. [Google Scholar] [CrossRef]
- Nath, A.; Salam, J.A.; Anand, A.M.; Raj, A.; Jayakrishnan, R. Solar cells using spray coated a-axis grown F: SnO2 thin films. Opt. Mater. 2025, 159, 116612. [Google Scholar] [CrossRef]
- Rahal, A.; Benramache, S.; Benhaoua, B. Preparation of n-type semiconductor SnO2 thin films. J. Semicond. 2013, 34, 083002. [Google Scholar] [CrossRef]
- Bendjedidi, H.; Attaf, A.; Saidi, H.; Aida, M.S.; Semmari, S.; Bouhdjar, A.; Benkhetta, Y. Properties of n-type SnO2 semiconductor prepared by spray ultrasonic technique for photovoltaic applications. J. Semicond. 2015, 36, 123002. [Google Scholar] [CrossRef]
- Mokaripoor, E.; Bagheri-Mohagheghi, M.M. Study of structural, electrical and photoconductive properties of F and P co-doped SnO2 transparent semiconducting thin film deposited by spray pyrolysis. Mater. Sci. Semicond. Process. 2015, 30, 400–405. [Google Scholar] [CrossRef]
- Tran, Q.P.; Fang, J.S.; Chin, T.S. Properties of fluorine-doped SnO2 thin films by a green sol–gel method. Mater. Sci. Semicond. Process. 2015, 40, 664–669. [Google Scholar] [CrossRef]
- Niu, Y.; Duan, L.; Zhao, X.; Han, C.; Guo, J.; Geng, W. Effect of Sb doping on structural and photoelectric properties of SnO2 thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 3289–3302. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Ning, H.; Yuan, W.; Deng, Y.; Zhang, X.; Wang, S.; Wang, J.; Yao, R.; Peng, J. Characterization studies of the structure and properties of Zr-doped SnO2 thin films by spin-coating technique. Superlattices Microstruct. 2018, 123, 330–337. [Google Scholar] [CrossRef]
- Soumya, S.S.; Xavier, T.S. Effect of cobalt doping on the microstructural, optical and electrical properties of SnO2 thin films by sol-gel spin coating technique. Phys. B Condens. Matter 2022, 624, 413432. [Google Scholar] [CrossRef]
- Sujatha Lekshmy, S.; Joy, K. Structural and optoelectronic properties of indium doped SnO2 thin films deposited by sol gel technique. J. Mater. Sci. Mater. Electron. 2014, 25, 1664–1672. [Google Scholar] [CrossRef]
- Che, L.; Song, J.; Yang, J.; Chen, X.; Li, J.; Zhang, N.; Yang, S.; Wang, Y. Fluorine, chlorine, and gallium co-doped zinc oxide transparent conductive films fabricated using the sol-gel spin method. J. Mater. 2023, 9, 745–753. [Google Scholar] [CrossRef]
- Houaidji, N.; Ajili, M.; Chouial, B.; Kamoun, N.T. First investigation of structural and optoelectronic properties of F and Ni co-doped SnO2 sprayed thin films. Optik 2020, 208, 164026. [Google Scholar] [CrossRef]
- Turgut, G.; Keskenler, E.F.; Aydın, S.; Yılmaz, M.; Doğan, S.; Düzgün, B. An investigation of the Nb doping effect on structural, morphological, electrical and optical properties of spray deposited F doped SnO2 films. Phys. Scr. 2013, 87, 035602. [Google Scholar] [CrossRef]
- Abed, C.; Fernández, S.; Aouida, S.; Elhouichet, H.; Priego, F.; Castro, Y.; Gómez-Mancebo, M.B.; Munuera, C. Processing and Study of Optical and Electrical Properties of (Mg, Al) Co-Doped ZnO Thin Films Prepared by RF Magnetron Sputtering for Photovoltaic Application. Materials 2020, 13, 2146. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, G.; Huang, R.; Wang, J. Study on Electrical and Optical Properties of Sb-Doped SnO2 Transparent Conducting Films. J. Inorg. Mater. 2002, 132–138. [Google Scholar]
- Ramarajan, R.; Kovendhan, M.; Thangaraju, K.; Joseph, D.P.; Babu, R.R.; Elumalai, V. Enhanced optical transparency and electrical conductivity of Ba and Sb co-doped SnO2 thin films. J. Alloys Compd. 2020, 823, 153709. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Magnetron sputtering. J. Vac. Sci. Technol. A 2020, 38, 060805. [Google Scholar] [CrossRef]
- Leng, D.; Wu, L.; Jiang, H.; Zhao, Y.; Zhang, J.; Li, W.; Feng, L. Preparation and Properties of SnO2 Film Deposited by Magnetron Sputtering. Int. J. Photoenergy 2012, 2012, 235971. [Google Scholar] [CrossRef]
- Vernardou, D. Advances in chemical vapor deposition. Materials 2020, 13, 4167. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Dong, L.; Li, Y.; Li, C.; Zhao, H. Studies on the structural and electrical properties of F-doped SnO2 film prepared by APCVD. Appl. Surf. Sci. 2011, 257, 10499–10502. [Google Scholar] [CrossRef]
- Faudoa-Arzate, A.; Morales-Rodriguez, H.; Sanchez-Carrillo, M.; González-Vázquez, A.; Realyvazquez-Guevara, P.; Rojas, J.; Chávez-Montes, W. SEM Study of Growth of SnO2 Nanoflowers in Thin films by Spray Pyrolysis. Microsc. Microanal. 2019, 25, 840–841. [Google Scholar] [CrossRef]
- Soussi, L.; Garmim, T.; Karzazi, O.; Rmili, A.; El Bachiri, A.; Louardi, A.; Erguig, H. Effect of (Co, Fe, Ni) doping on structural, optical and electrical properties of sprayed SnO2 thin film. Surf. Interfaces 2020, 19, 100467. [Google Scholar] [CrossRef]
- Deyu, G.K.; Muñoz-Rojas, D.; Rapenne, L.; Deschanvres, J.-L.; Klein, A.; Jiménez, C.; Bellet, D. SnO2 Films Deposited by Ultrasonic Spray Pyrolysis: Influence of Al Incorporation on the Properties. Molecules 2019, 24, 2797. [Google Scholar] [CrossRef]
- Kumar, S. Exploration of structural, morphological and magnetic properties of transition metal doped SnO2 films grown using pulsed laser deposition. Vacuum 2020, 182, 109725. [Google Scholar] [CrossRef]
- Sinha, S.K.; Ray, S.K.; Manna, I. Effect of Al doping on structural, optical and electrical properties of SnO2 thin films synthesized by pulsed laser deposition. Philos. Mag. 2014, 94, 3507–3521. [Google Scholar] [CrossRef]
- Haya, S.; Brahmia, O.; Halimi, O.; Sebais, M.; Boudine, B. Sol–gel synthesis of Sr-doped SnO2 thin films and their photocatalytic properties. Mater. Res. Express 2017, 4, 106406. [Google Scholar] [CrossRef]
- Turan, E.; Kul, M.; Akın, S. Structural and optical investigation of spray-deposited SnO2 thin films. J. Mater. Sci. Mater. Electron. 2022, 33, 15689–15703. [Google Scholar] [CrossRef]
- Lee, C.; Lee, W.-Y.; Lee, H.; Ha, S.; Bae, J.-H.; Kang, I.-M.; Kang, H.; Kim, K.; Jang, J. Sol-gel processed yttrium-doped SnO2 thin film transistors. Electronics 2020, 9, 254. [Google Scholar] [CrossRef]
| Sample Name | Resistivity (Ω·cm) | Mobility (cm2/V·S) | Carrier Concentration (cm−3) | Hall Coefficient |
|---|---|---|---|---|
| S1 | 6.806 × 10−3 | 8.753 × 102 | 1.049 × 1019 | −5.957 × 10−2 |
| A1 | 1.610 × 10−4 | 4.205 × 102 | 9.232 × 1019 | −6.770 × 10−2 |
| A2 | 2.785 × 10−4 | 5.354 × 102 | 8.657 × 1019 | −3.770 × 10−2 |
| A3 | 1.058 × 10−4 | 1.085 × 103 | 5.855 × 1020 | 1.070 × 10−3 |
| A4 | 3.581 × 10−4 | 6.205 × 102 | 7.813 × 1019 | 2.223 × 10−2 |
| Sample Name | Resistivity (Ω·cm) | Mobility (cm2/V·S) | Carrier Concentration (cm−3) | Hall Coefficient |
|---|---|---|---|---|
| A3 | 1.058 × 10−4 | 1.085 × 103 | 5.855 × 1020 | 1.070 × 10−3 |
| AS1 | 2.701 × 10−4 | 7.199 × 102 | 4.234 × 1019 | −1.476 × 10−2 |
| AS2 | 2.653 × 10−4 | 6.951 × 102 | 5.389 × 1019 | −1.844 × 10−2 |
| AS3 | 1.953 × 10−4 | 5.465 × 102 | 6.455 × 1020 | −4.295 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, H.; Zhang, X.; Zhou, Z.; Wang, L. Tuning Electrical and Optical Properties of SnO2 Thin Films by Dual-Doping Al and Sb. Coatings 2025, 15, 669. https://doi.org/10.3390/coatings15060669
Wang Y, Zhang H, Zhang X, Zhou Z, Wang L. Tuning Electrical and Optical Properties of SnO2 Thin Films by Dual-Doping Al and Sb. Coatings. 2025; 15(6):669. https://doi.org/10.3390/coatings15060669
Chicago/Turabian StyleWang, Yuxin, Hongyu Zhang, Xinyi Zhang, Zhengkai Zhou, and Lu Wang. 2025. "Tuning Electrical and Optical Properties of SnO2 Thin Films by Dual-Doping Al and Sb" Coatings 15, no. 6: 669. https://doi.org/10.3390/coatings15060669
APA StyleWang, Y., Zhang, H., Zhang, X., Zhou, Z., & Wang, L. (2025). Tuning Electrical and Optical Properties of SnO2 Thin Films by Dual-Doping Al and Sb. Coatings, 15(6), 669. https://doi.org/10.3390/coatings15060669







