3.2. Ceramic Pigments and Metallic Oxides Used in the Experimental Section
The compatibility of metal oxides and ceramic pigments used for recycled and crushed glass is a crucial factor in achieving durable and aesthetically pleasing materials. When recycled glass is used in combination with metal oxides and ceramic pigments, it is essential to consider the chemical and physical interactions between these substances to ensure color stability, material durability and material behavior under industrial and environmental conditions of use.
Metal oxides, such as iron (Fe2O3), copper (CuO), chromium (Cr2O3), cobalt (CoO) and manganese (MnO), are commonly used to color glass and to modify certain optical properties of glass. These substances can interact with recycled glass components during the melting and heat treatment process.
- -
Melting temperature: Metal oxides must be compatible with the melting temperature of the recycled glass. Glass has a variable melting temperature, typically between 750 °C and 1000 °C, and metal oxides must be stable at these temperatures in order not to cause undesirable reactions or the formation of unstable secondary phases.
- -
Formation of crystalline phases: Some chemical reactions between metal oxides and glass compounds can lead to the formation of crystalline phases instead of amorphous phases. This may affect the transparency or texture of the glass and may influence the strength of the material.
Ceramic pigments, which are used to give recycled glass intense and durable colors, are often combined with metal oxides to produce specific visual effects. Ceramic pigments are often high-temperature stable metal oxides and may include oxides of chromium, copper, cobalt or iron.
- -
Color stability: Ceramic pigments need to be stabilized in the glass matrix to prevent color change during heat treatment. This can be achieved by choosing pigments that have a stable crystalline structure and do not react with the glass components at high temperatures.
- -
Thermal compatibility: Ceramic pigments must have thermal expansion coefficients compatible with recycled glass. If the coefficient of expansion of the pigments is too different from that of the glass, this can lead to cracks or warpage in the final material.
In combination with recycled glass, metal oxides and ceramic pigments can influence the mechanical properties of the final material, such as hardness, impact strength and abrasion resistance.
- -
Hardness: Iron and chromium oxides can contribute to the hardness of recycled glass, making it more resistant to scratching and abrasion.
- -
Shock resistance: In contrast, some transition metals, such as copper and manganese, can introduce internal stresses into the glass, which can affect shock resistance, especially at varying temperatures.
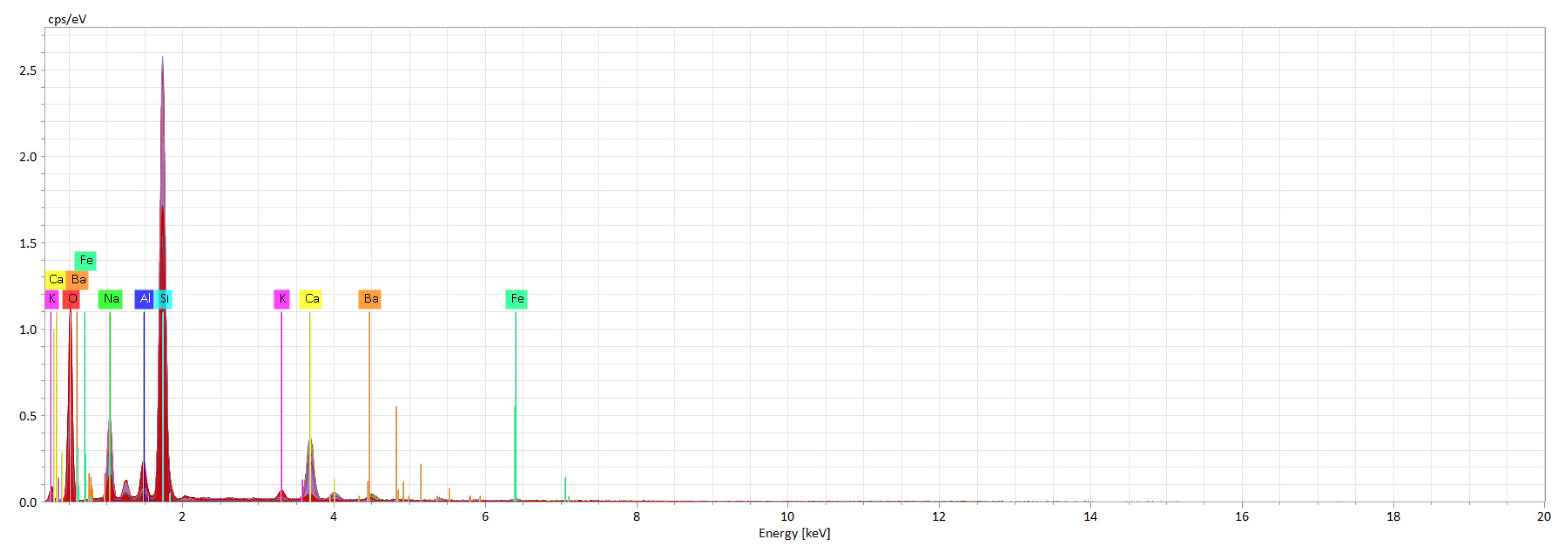
Recycled glass usually contains sodium silicate (Na2O-SiO2), calcium oxides (CaO) and other compounds that can react with metal oxides and ceramic pigments.
- -
Reaction with alkalines: Metal oxides containing aluminum or calcium can react with alkaline compounds in the glass (such as Na2O or K2O), resulting in the formation of insoluble silicate that can affect the clarity and transparency of the glass.
- -
Formation of secondary compounds: Under certain temperature conditions, metal oxides can form secondary compounds that affect the aesthetic quality or physical properties of recycled glass.
The compatibility of metal oxides and ceramic pigments with recycled and crushed glass is essential to obtain a quality end-material with improved physico-chemical and optical properties. Their correct choice can ensure color stability, durability of the material and its performance in industrial applications such as the production of mosaics, ceramic tiles or other decorative products. It is also important to monitor chemical reactions and thermal compatibility to prevent deficiencies or defects in the final product.
In this study, three types of oxides were used to evaluate the compatibility and effects on recycled and crushed glass, especially in the process of synthesis and heat treatment. Titanium oxide (TiO2) and two ceramic oxides, CK11020 and CK33100, were chosen due to their specific properties that influence both the coloring and stability of the final material.
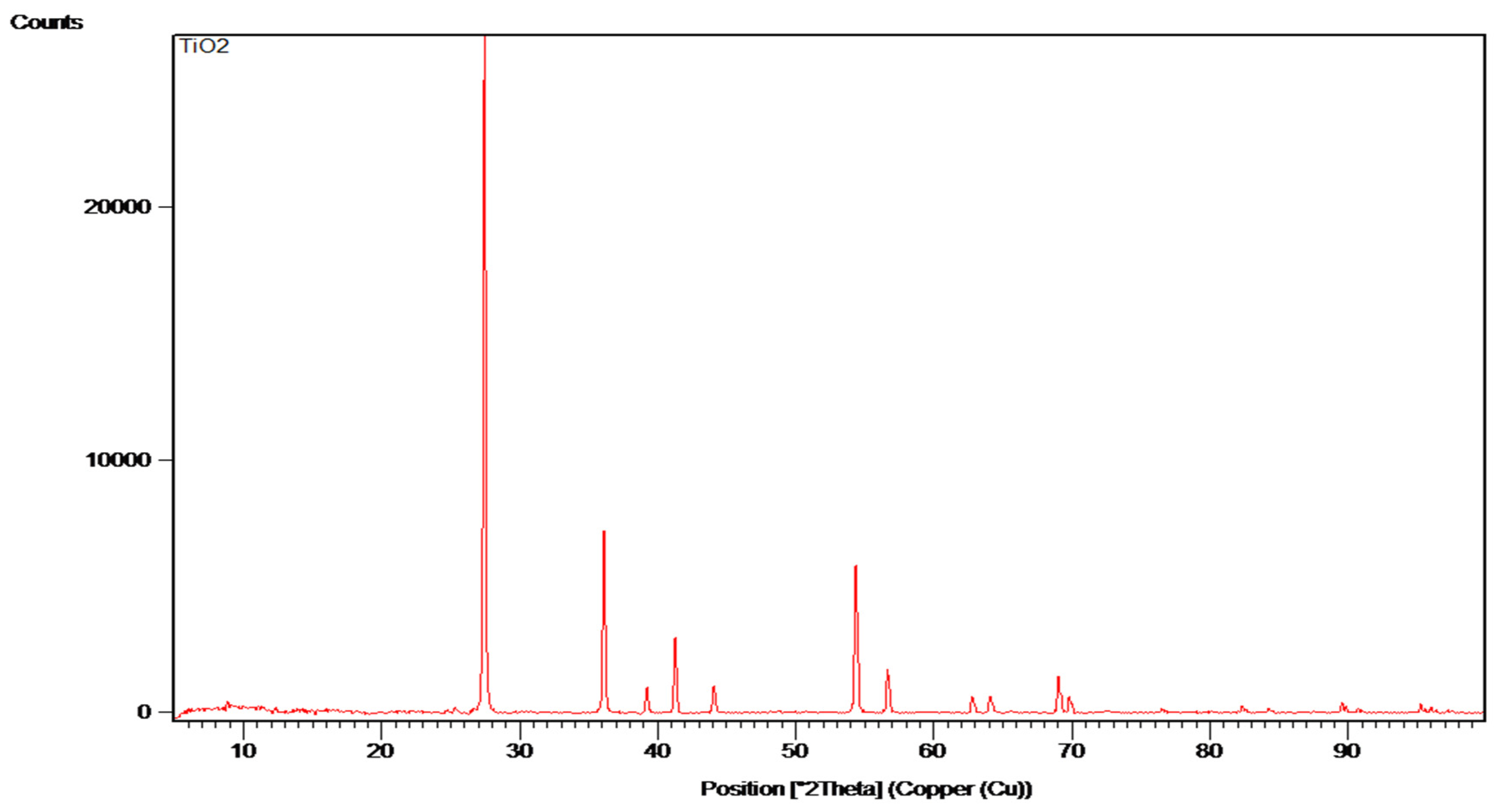
Titanium oxide (TiO
2) is a ceramic pigment known for its high thermal stability and ability to produce intense and durable colors, particularly in shades of white and opacity. In this context, TiO
2 was used to evaluate its ability to improve the optical properties of recycled and crushed glass. TiO
2 is also often used to impart a high degree of opacity to materials, which can be beneficial in design and architectural applications, such as mosaics or handicrafts (
Figure 14).
In the heat treatment process, TiO2 is relatively inert and stable at high temperatures, making it ideal for use in combination with recycled glass without negatively influencing its structure. This oxide can also help improve the UV resistance of the material, preventing premature degradation of the glass’s color and texture.
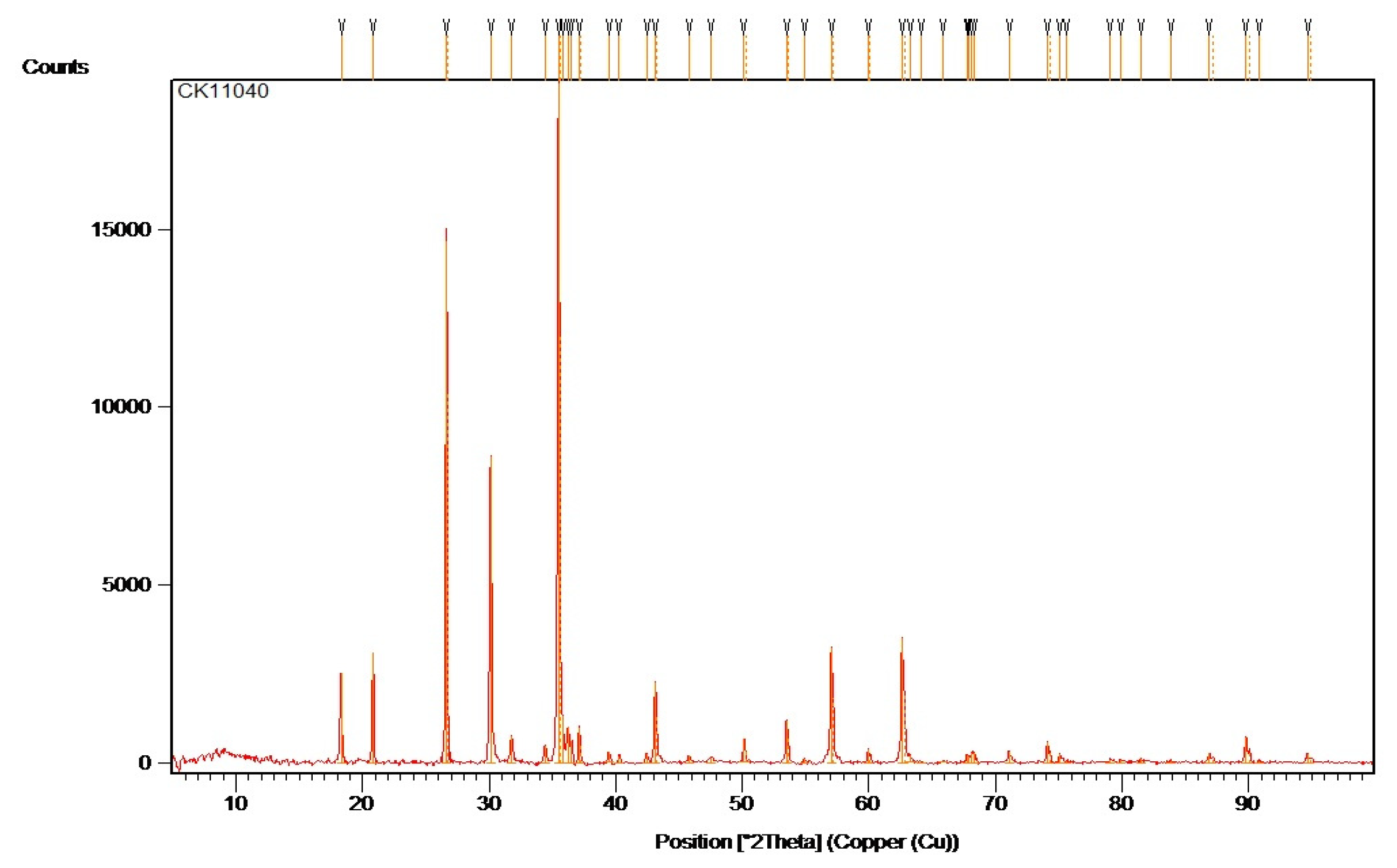
Ceramic Oxide CK11040 is a pigment that belongs to the category of ceramic metal oxides and is commonly used to achieve vibrant colors and stability in ceramics and glass. Depending on the concentration and sintering temperature, CK11020 often provides a specific color effect.
We chose CK11040 in the present study to observe its effects on recycled glass, particularly in terms of color stability following heat treatment. This ceramic oxide is very stable chemically, and we looked at how it interacts with the recycled glass to see how it behaves during the high temperatures involved in glass sintering and crystallization.
CK11040 can also contribute to the formation of fine crystals, which improve the texture of the material and can provide greater durability in glass end-use applications such as decorative mosaics or building materials (
Figure 15).
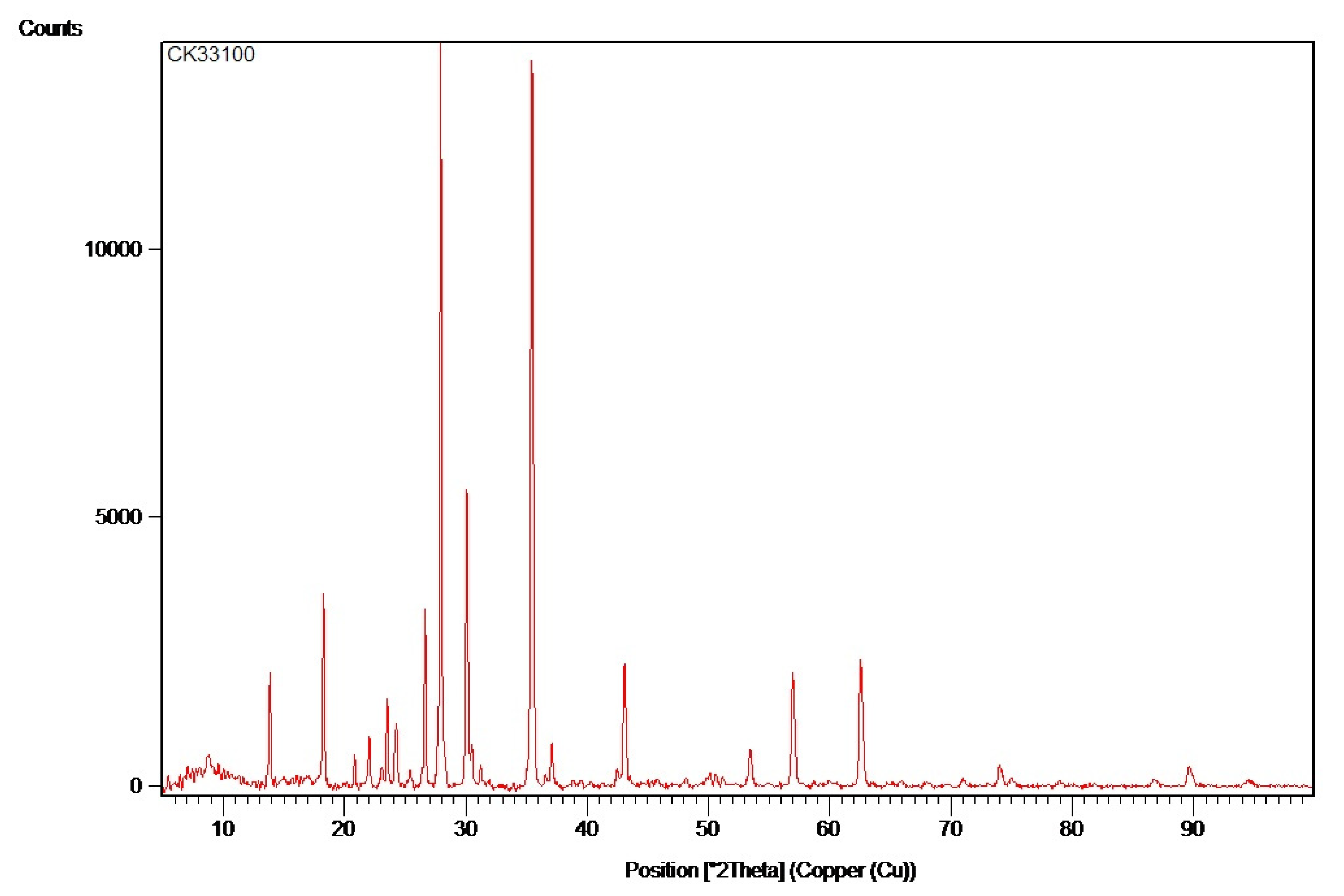
Ceramic oxide CK33100 is another important material in this study, used to evaluate the compatibility with recycled glass and its ability to influence its crystalline structure during heat treatment. CK33100 is recognized for its stability at high temperatures and its ability to control the crystallization process in glass, which is essential in obtaining materials with improved mechanical performance.
This study used CK33100 to observe how this oxide contributes to the sintering process of recycled glass. The study also looked at how this oxide affects the strength of the final material, including its ability to withstand pressure and impacts, due to the formation of stronger and more uniform crystal structures. The application of CK33100 in heat treatments demonstrated an improvement in the material structure, making it stronger and more durable under intense use.
In summary, using titanium oxide (TiO
2) and the ceramic oxides CK11020 and CK33100 when making and heating recycled and crushed glass led to better materials, particularly in terms of color stability, durability, and strength. These oxides worked well with the recycled glass, helping to create stable crystal structures and keeping the final material strong when heated. Thus, these combinations of oxides can be successfully used in the production of mosaics, building materials, or decorative products, having a positive impact on their quality and performance (
Figure 16).
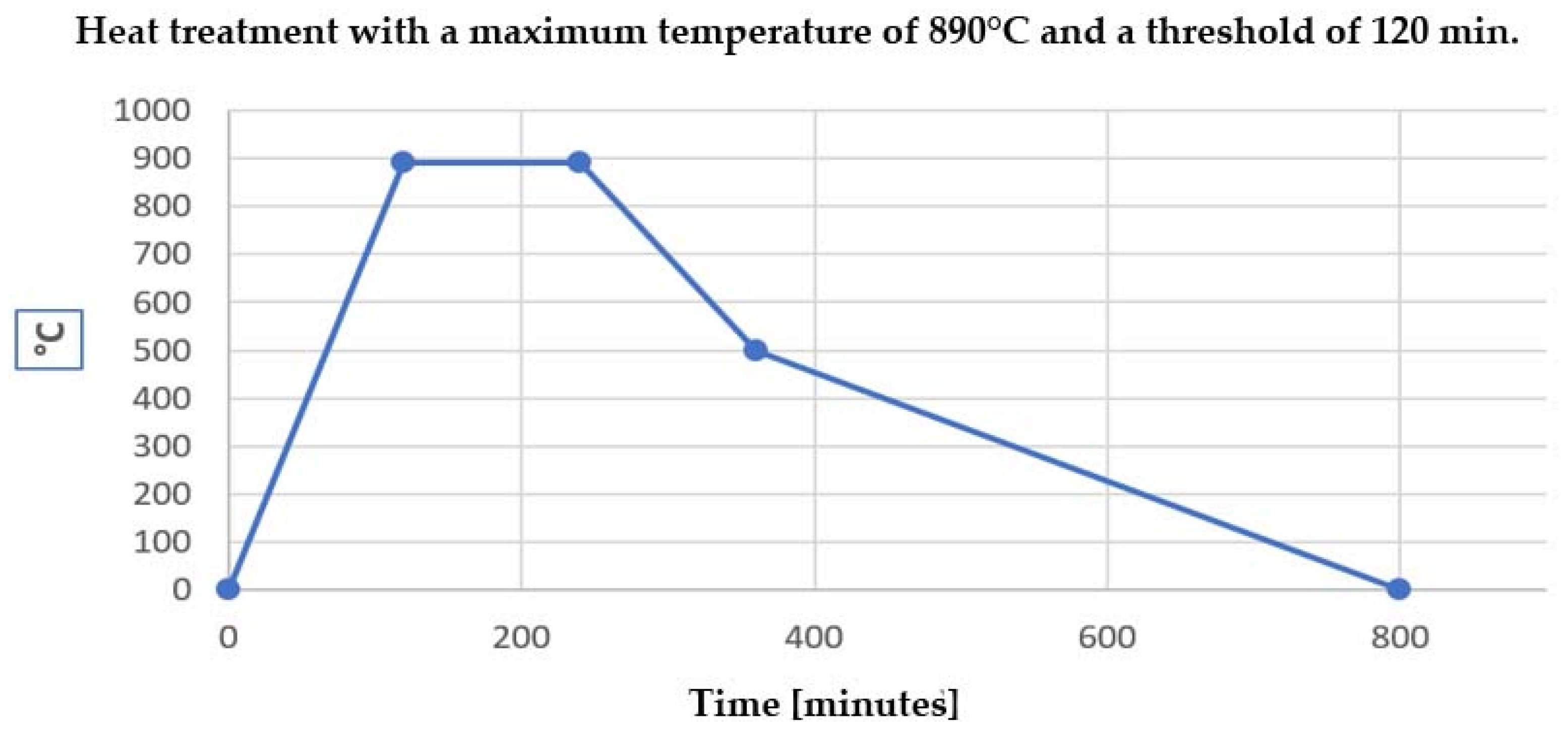
For X-ray diffraction (XRD) examination of glass (especially heat-treated recycled glass), the typical range of angles (2θ) is chosen depending on the objective of the analysis—identification of crystalline phases or confirmation of amorphous structure. The standard XRD scanning range for glass is 2θ = 5°–80° → 2θ = 5°–80° → it is the most common range used for general characterization, allowing the detection of most common crystalline phases (silicates, oxides, carbonates, etc.), covering the areas where the most relevant diffraction maxima occur.
The X-ray diffraction (XRD) tests on the four samples of heat-treated recycled glass showed that they all have the same mixed structure, which includes both non-crystal and crystal parts, with no major differences among them.
Analysis parameters were as follows: scanning range: 2θ = 5°–80°; constant analysis conditions: 40 kV, 30 mA, 0.02° step, time/step 1 s. All diffractograms show a broad amorphous halo centered around 2θ ≈ 28°, characteristic of the vitreous structure of the glass. Sharp diffraction peaks are superimposed on this amorphous background, indicating the presence of well-defined crystalline phases. Peaks were repeatedly identified at positions 2θ ≈ 22°, 26.5°, 31°, and 50°, corresponding to calcium and sodium silicate, potentially identified as wollastonite, quartz, and other stable oxides. The heat treatment applied to recycled and crushed glass did not lead to significant crystallization differences. The results obtained by XRD analysis suggest that the heat-treated recycled glass possesses an identical composite structure consisting of an amorphous matrix and a stable crystalline phase. This structural constancy is a positive indicator of the uniformity of the material, essential for its applicability in industrial products such as mosaics, decorative elements, and façade components, where predictable mechanical and chemical behavior is required. This structural consistency confirms that the recycled glass-based material can be produced in a standardized way with repeatable properties, representing a sustainable and reliable alternative to traditional Murano or Dynasty Smalti Glass Mosaic (China, DGM) (
Figure 17).
3.4. Compressive Strength Analysis
After the heat treatment process, recycled glass can become strong enough to resist forces up to 16 MPa because of the way its internal structure is improved and crystals are formed. This heat treatment allows the material to become much more compact and homogeneous, reducing porosity and forming a stable crystal network. The compressive strength of 16 MPa indicates that this type of glass is much more resistant to mechanical stress compared to traditional glass or those used in artistic mosaics or industrial applications.
The compressive strength of traditional glass (such as that used in windows, glasses, or other household applications) is relatively high compared to other mechanical properties of this material. The compressive strength of traditional glass is about 1000–1500 MPa (megapascals, equivalent to 1000–1500 N/mm2). Glass has a very high compressive strength but is very brittle under stretching and impact due to its amorphous nature and lack of plasticizing mechanisms (it does not have dislocations like metals).
This strength value is significantly higher than that of other types of glass used in various industries, demonstrating that heat-treated recycled glass can successfully replace conventional materials in a wide range of applications, from civil construction to decorative mosaics, where durability and strength are essential.
Comparing these three types of glass, it is clear that heat-treated recycled glass is a superior choice in terms of mechanical strength. While Murano glass, with a strength of 2.5 MPa, and DSG glass, with 3.5 MPa, are particularly suitable for decorative uses or in applications where aesthetics are more important than physical strength, 16 MPa heat-treated recycled glass is much more robust and durable, suitable for a wide range of industrial and civil applications where shock and compression resistance are a decisive factor.
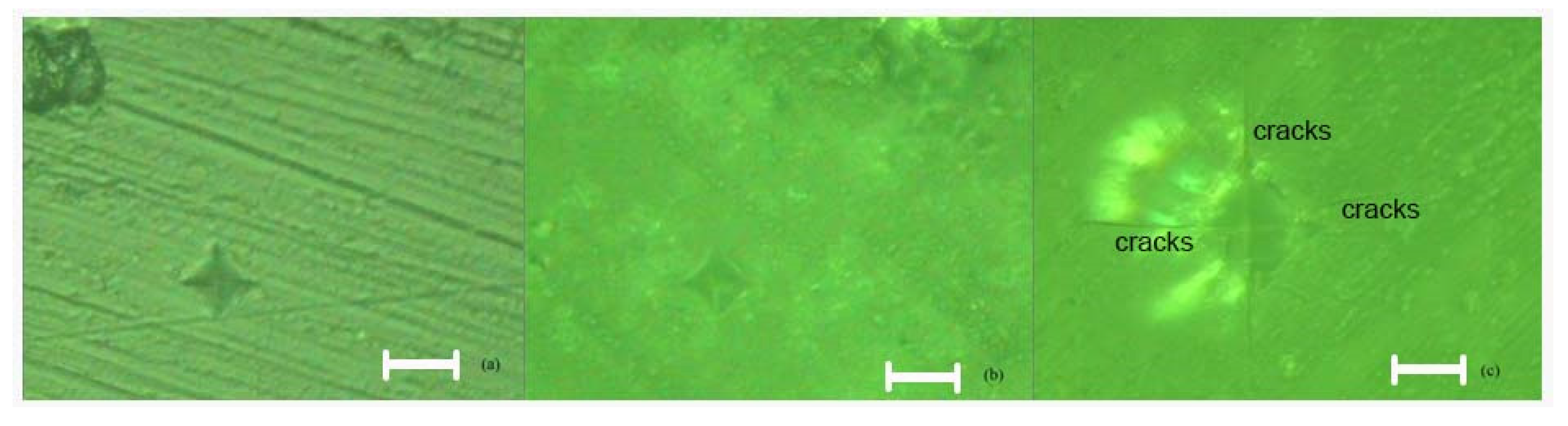
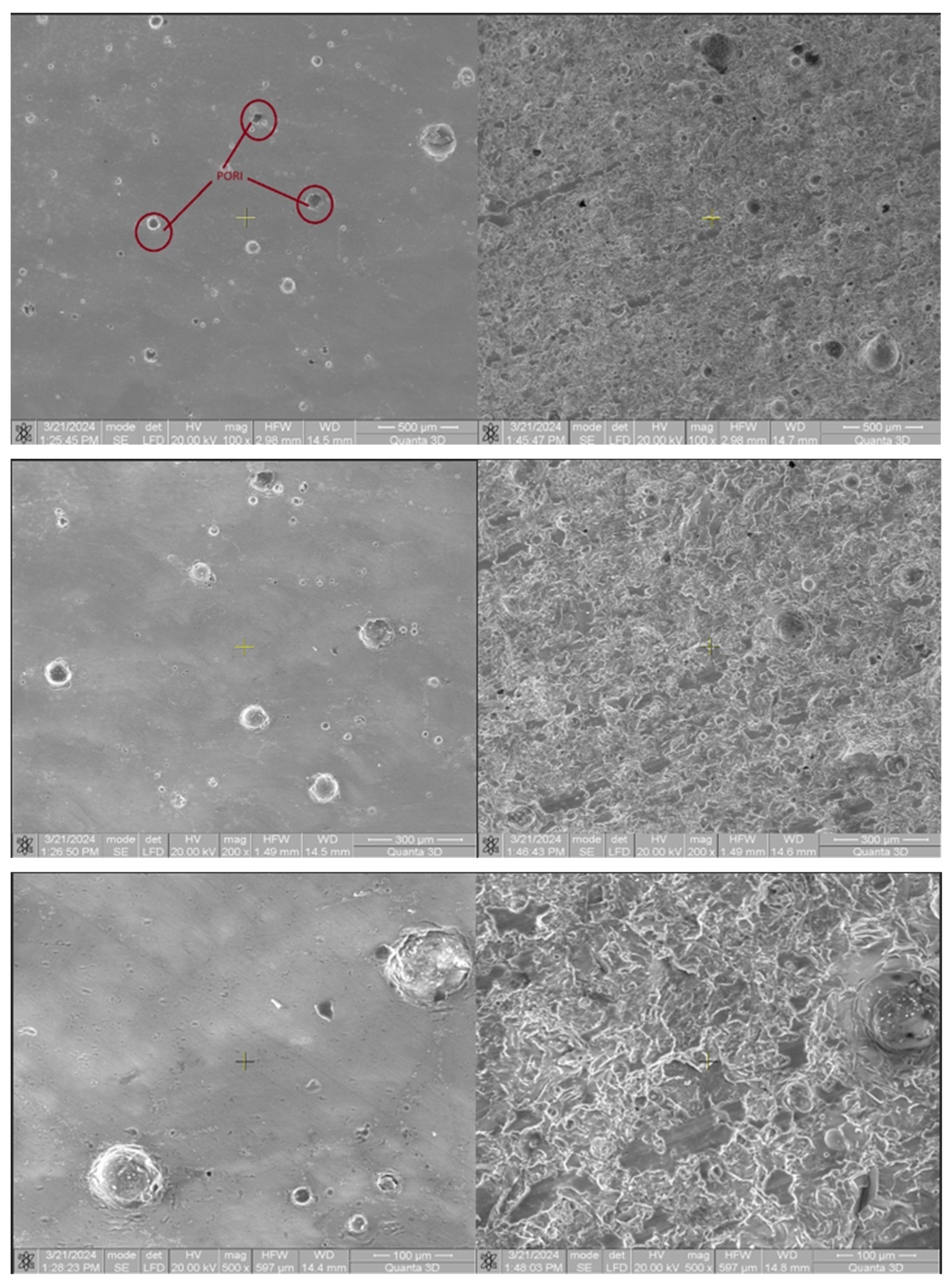
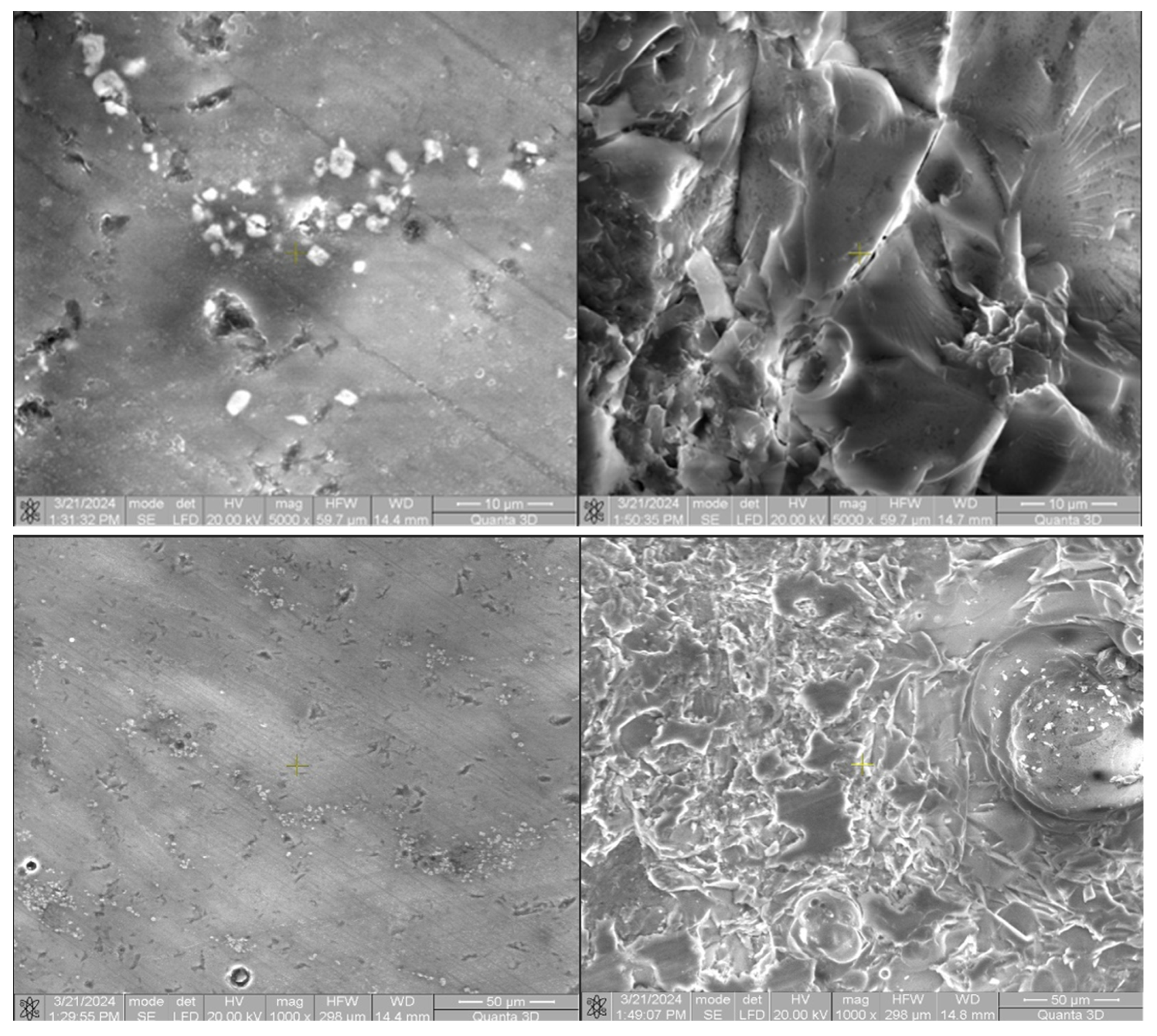
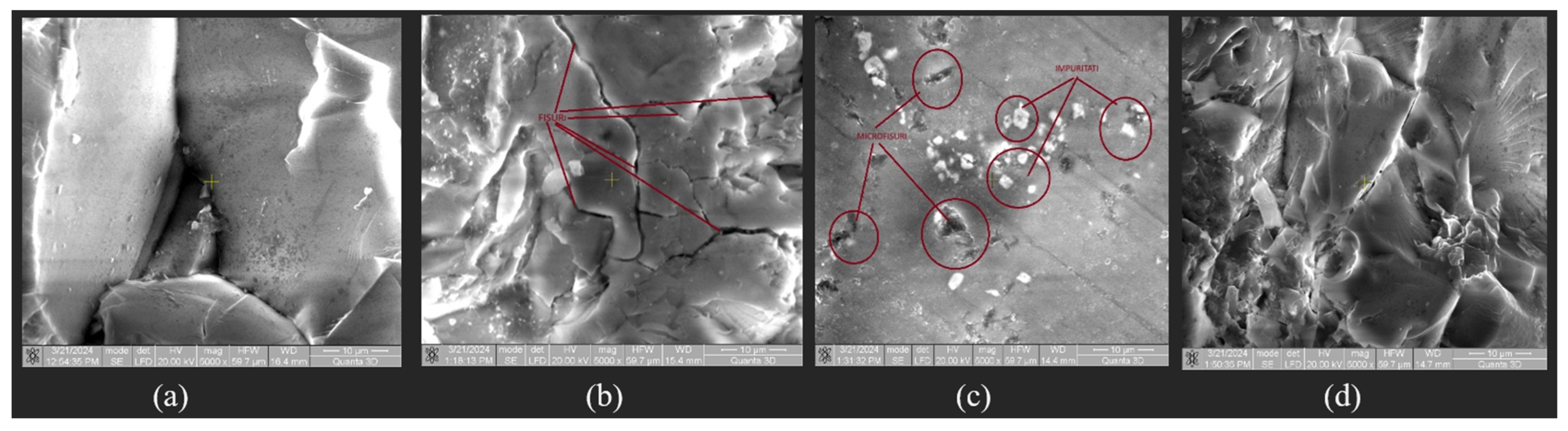
Following microscopic analysis of SRC_T compared to Murano and DGM-China, it is observed that it is a compact and homogeneous material that has a higher structural strength, making it better able to withstand mechanical loads and stresses. This property can be particularly important in applications where reliability and durability are essential. The absence of cracks and pores suggests that the composition of the material is uniform throughout its mass. Such characteristics can be crucial for ensuring consistency and predictability in manufacturing processes and in the final product’s performance. The absence of cracks and pores indicates an efficient and well-controlled manufacturing process. Such results can lead to reduced costs and more consistent and high-quality production. SEM images were taken at magnifications of 200×, 500×, 1000×, and 5000× (
Figure 23).
3.5. The Study Aims to Compare the Chemical Resistance of SRC_T Against Murano Glass and DGM-China
For the comparative study of chemical resistance, we used two solutions of HCl (hydrochloric acid) and KOH (potassium hydroxide). Each of them has distinct properties and key roles in various chemical processes, including materials treatment, cleaning, and chemical synthesis. HCl is a strong, colorless or slightly yellow acid with a characteristic odor. It dissolves completely in water, forming an acidic solution that is used in numerous industrial and laboratory applications. KOH is a strong, water-soluble base that forms alkaline solutions. It is an ionic compound containing potassium ions (K⁺) and hydroxide ions (OH⁻). HCl and KOH are both highly reactive chemicals but have opposite properties: HCl is an acid, and KOH is a base. In combination, they can neutralize each other in a typical neutralization reaction, forming water (H
2O) and salt (potassium chloride, KCl). This type of reaction is the basis of many chemical processes in industry and laboratories, including the pH adjustment of solutions or the synthesis of various chemicals (
Figure 24).
HCl and KOH are important for treating materials, including studying how well materials like recycled glass can resist chemical damage.
Regarding studies on recycled glass, HCl is frequently used to analyze the chemical behavior of glass and to clean its surfaces of impurities. On the other hand, KOH is used in certain chemical treatment processes to modify the.
Testing the chemical resistance of materials, especially against acidic and alkaline solutions, is an essential parameter for evaluating their durability in aggressive environments. In this study, we looked at how well recycled and thermally treated glass (SRC_T) can resist damage from hydrochloric acid (HCl) solutions at 30% and 18% concentrations, and potassium hydroxide (KOH) solutions at 30 g/L and 100 g/L. Comparing the results obtained with those of Murano glass and DGM-China (industrial-grade glass from China) helps establish the performance of SRC_T compared to traditional materials.
For testing how well they resist chemicals, SRC_T, Murano, and DGM-China samples were placed in HCl and KOH solutions at the specified strengths for 7 days. The tests were performed at room temperature, and the structural and compositional changes of the bottles were monitored by visual and microscopic analyses, but also by measuring weight changes and mechanical strength (
Table 7,
Table 8,
Table 9 and
Table 10).
3.5.1. Testing with 30% and 18% HCl
HCl solutions at concentrations of 30% and 18% were used to simulate the aggressive acidic conditions that could affect materials in various industrial environments. All three types of glass—SRC_T, Murano, and DGM-China—showed a similar reaction to these solutions. After 7 days of exposure, all materials underwent minor changes in the surface structure, with no visible signs of cracking or significant mass loss.
Even though the 30% HCl concentration had a slightly stronger effect on all three types of glass, no significant differences were observed in their behavior, and their chemical resistance remained relatively constant throughout the test. In the case of the 18% HCl solution, all materials remained stable, without showing any notable changes in physical or chemical structure.
3.5.2. Testing with KOH: 30 g/L and 100 g/L
Regarding testing with KOH solutions, both at the concentration of 30 g/L and 100 g/L, all three types of glass—SRC_T, Murano, and DGM-China—reacted in a similar way. After 7 days of exposure, all materials showed high resistance to alkaline solutions without suffering significant structural damage. Surface changes were minor, and mass losses were almost imperceptible.
In the case of the 100 g/L KOH solution, all three types of glass remained stable and did not show cracks or fissures, indicating a similar resistance to strong alkaline solutions. Also, at the KOH concentration of 30 g/L, no notable differences were observed between the tested materials, all remaining stable and unchanged.
Chemical resistance tests showed similar results for all three types of glass—SRC_T, Murano, and DGM-China—when using 30% and 18% HCl solutions and 30 g/L and 100 g/L KOH. All tested materials showed comparable resistance to aggressive chemical agents, with no significant differences in chemical behavior or structural changes. These results suggest that, in terms of chemical resistance, SRC_T, Murano, and DGM-China can be considered equivalent, having similar performances under conditions of exposure to strong acids and bases.