Structural, Optical and Electrical Properties of the Flexible, Asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 Films Deposited via Magnetron Sputtering
Abstract
1. Introduction
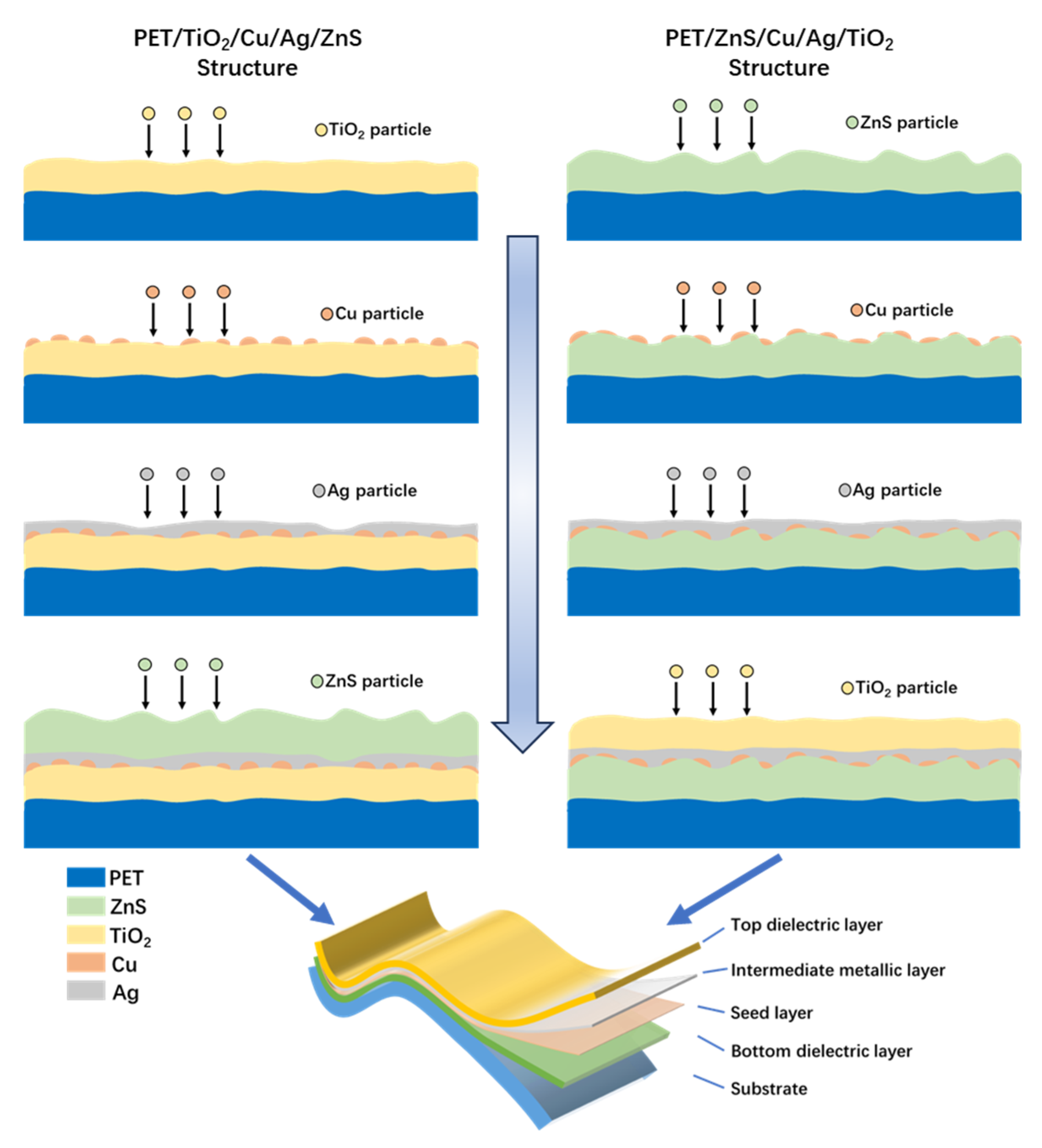
2. Materials and Methods
3. Results
3.1. Surface Characterization of the Substrate and Bottom Dielectric Layers
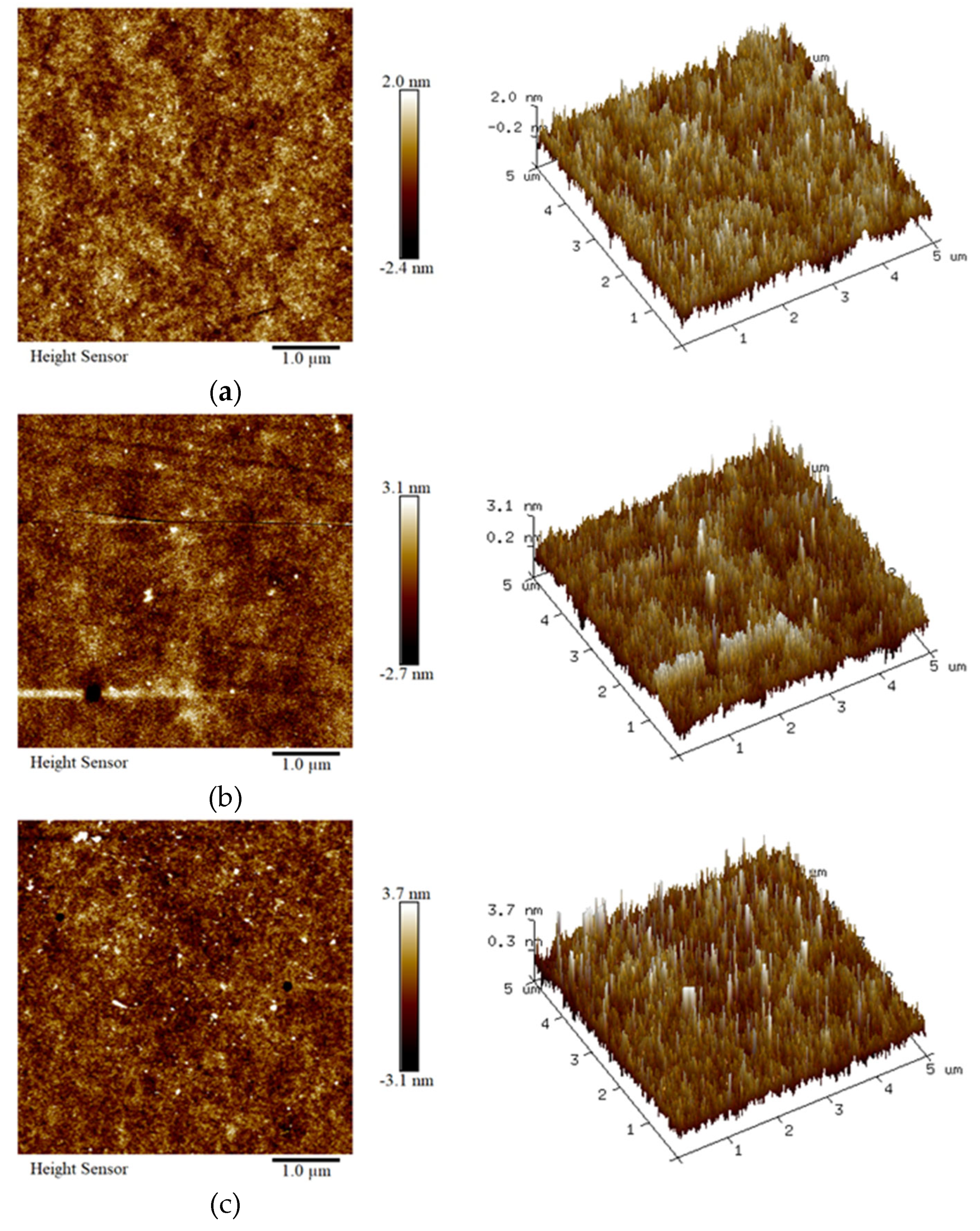
3.2. Surface Morphology of the Intermediate Layers and Top Dielectric Layers
3.3. Phase Structure Analysis for the TiO2/Cu/Ag/ZnS Multilayer
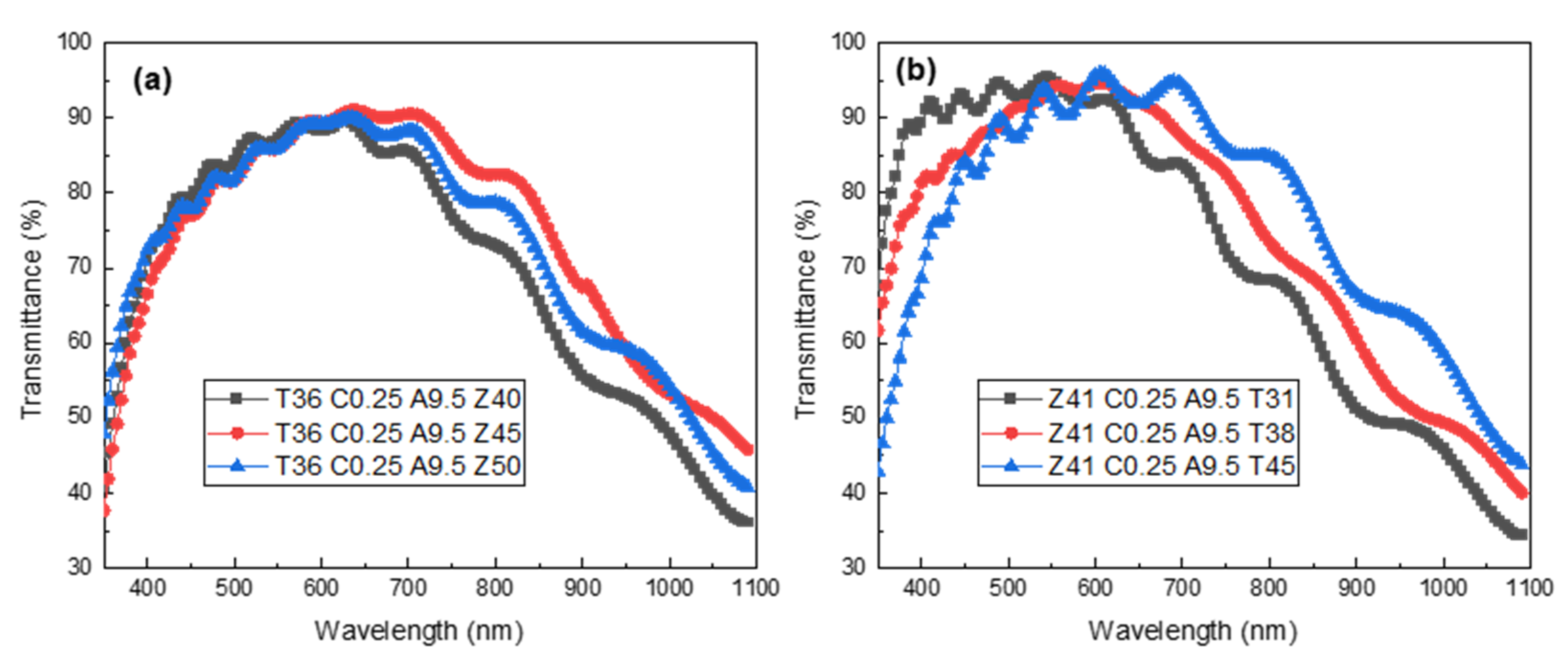
3.4. The Light Transmission of Asymmetric, Multilayered TCFs
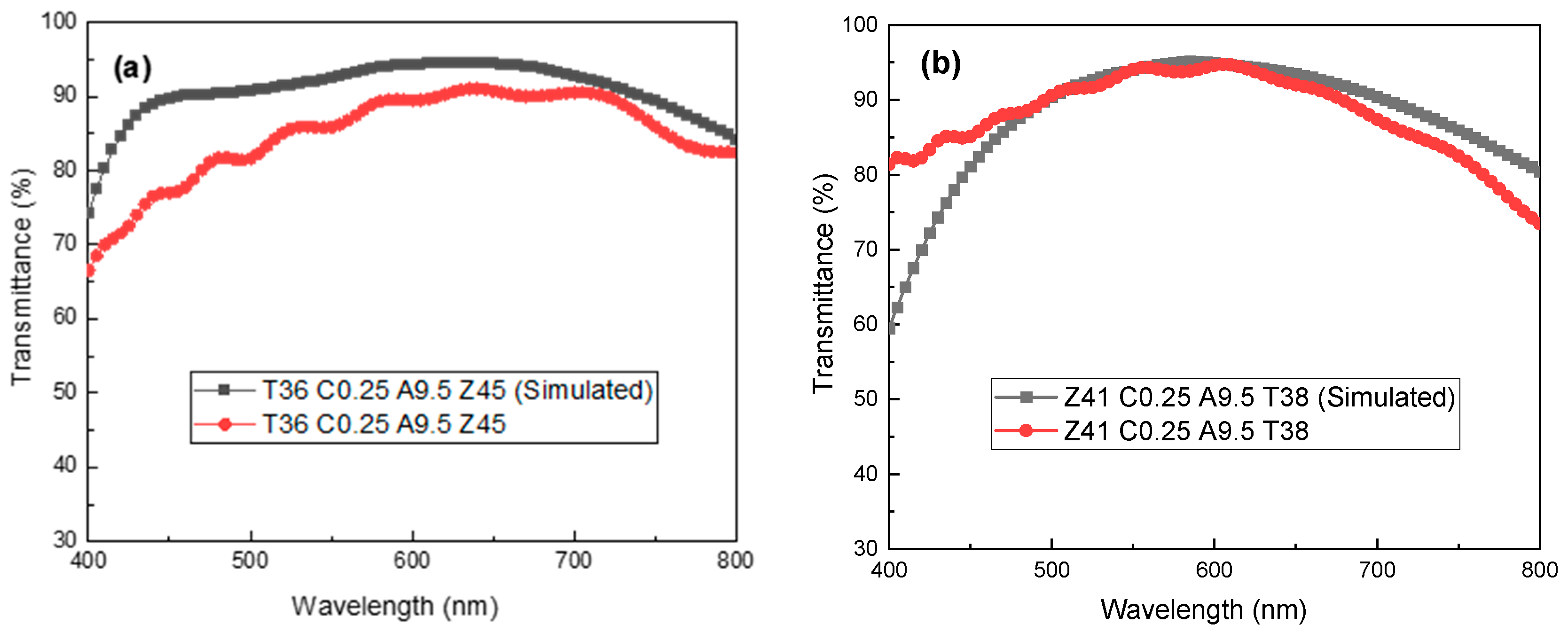
3.5. The Numerical Simulation of Light Transmission
3.6. The Electrical Properties of Multilayered TCFs with Different Structures
3.7. The Figure of Merit for the Asymmetric, Multilayered TCFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabizadeh, M.; Ehsani, M.H. Effect of heat treatment on optical, electrical and thermal properties of ZnO/Cu/ZnO thin films for energy-saving application. Ceram. Int. 2022, 48, 16108–16113. [Google Scholar] [CrossRef]
- Wu, J.; Qian, X.; Liu, C.; Ji, Y.; Yu, S. Design and realization of neutral-tinted low-E film. Vacuum 2022, 203, 111228. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, S.; Wa, P.; Yu, W.; Li, X.; Wu, L.; Ding, W.; Fu, G. Recent progress of multilayer composite transparent conductive film. Chin. Sci. Bull. 2017, 62, 372–384. [Google Scholar]
- Girtan, M.; Negulescu, B. A review on oxide/metal/oxide thin films on flexible substrates as electrodes for organic and perovskite solar cells. Opt. Mater. X 2022, 13, 100122. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, N.; Wang, L.; Li, Y.; Yan, Y.; Li, X.; Xie, A.; Sun, D.; Han, J. Atomic Layer-deposited flexible ZnO transparent electrodes with Zn-Al-O interface for electrochromic devices. Ceram. Int. 2025, 51, 13018–13027. [Google Scholar] [CrossRef]
- Gerlein, L.F.; Benavides-Guerrero, J.A.; Cloutier, S.G. Photonic post-processing of a multi-material transparent conductive electrode architecture for optoelectronic device integration. RSC Adv. 2024, 14, 4748–4758. [Google Scholar] [CrossRef]
- Shugang, J.; Yue, W.; Haixu, L.; Wanbing, L.; Wei, Y. Research Progress of Flexible Organic/Inorganic Hybrid Perovskite Solar Cells. Chin. J. Lumin. 2018, 39, 1687–1696. [Google Scholar]
- Zhang, J.; Zhang, W.; Cheng, H.-M.; Silva, S.R.P. Critical review of recent progress of flexible perovskite solar cells. Mater. Today 2020, 39, 66–88. [Google Scholar] [CrossRef]
- Guillén, C.; Herrero, J. TCO/metal/TCO structures for energy and flexible electronics. Thin Solid Film. 2011, 520, 1–17. [Google Scholar] [CrossRef]
- Lee, S.-M.; Koo, H.-W.; Kim, T.-W.; Kim, H.-K. Asymmetric ITO/Ag/ZTO and ZTO/Ag/ITO anodes prepared by roll-to-roll sputtering for flexible organic light-emitting diodes. Surf. Coat. Technol. 2018, 343, 115–120. [Google Scholar] [CrossRef]
- Han, Y.C.; Lim, M.S.; Park, J.H.; Choi, K.C. ITO-free flexible organic light-emitting diode using ZnS/Ag/MoO3 anode incorporating a quasi-perfect Ag thin film. Org. Electron. 2013, 14, 3437–3443. [Google Scholar] [CrossRef]
- Jin, J.; Liu, C.; Yang, Z.-j.; Wang, L. The influence of AZO films on morphology and surface plasmon resonance properties of Ag particles. Surf. Coat. Technol. 2019, 363, 135–141. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Maniyara, R.A.; Rodrigo, D.; Yu, R.; Canet-Ferrer, J.; Ghosh, D.S.; Yongsunthon, R.; Baker, D.E.; Rezikyan, A.; García de Abajo, F.J.; Pruneri, V. Tunable plasmons in ultrathin metal films. Nat. Photonics 2019, 13, 328–333. [Google Scholar] [CrossRef]
- Li, B.-J.; Yang, G.-Y.; Huang, L.-J.; Wang, T.-Y.; Ren, N.-F. Effects of BN layer on photoelectric properties and stability of flexible Al/Cu/ZnO multilayer thin film. Ceram. Int. 2020, 46, 14686–14696. [Google Scholar] [CrossRef]
- Dhar, A.; Alford, T.L. High quality transparent TiO2/Ag/TiO2 composite electrode films deposited on flexible substrate at room temperature by sputtering. APL Mater. 2013, 1, 12102. [Google Scholar] [CrossRef]
- Formica, N.; Mantillaperez, P.; Ghosh, D.S.; Janner, D.; Tong, L.C.; Huang, M.; Garner, S.; Martorell, J.; Pruneri, V. An ITO-free polymer solar cell on flexible glass. ACS Appl. Mater. Interfaces 2015, 7, 4541. [Google Scholar] [CrossRef]
- Jiang, S.; Feng, L.; Zhang, W.; Liu, H.; Liu, H.; Liu, Y.; Li, B.; Wu, L.; Liu, X.; Wang, X.; et al. Indium-free flexible perovskite solar cells with AZO/Cu/Ag/AZO multilayer transparent electrodes. Sol. Energy Mater. Sol. Cells 2022, 246, 111895. [Google Scholar] [CrossRef]
- Lim, S.; Han, D.; Kim, H.; Lee, S.; Yoo, S. Cu-based multilayer transparent electrodes: A low-cost alternative to ITO electrodes in organic solar cells. Sol. Energy Mater. Sol. Cells 2012, 101, 170–175. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Louarn, G.; Khelil, A.; Morsli, M.; Stephant, N.; Bou, A.; Abachi, T.; Cattin, L.; Makha, M.; Torchio, P.; et al. Broadening of the transmission range of dielectric/metal multilayer structures by using different metals. Vacuum 2015, 111, 32–41. [Google Scholar] [CrossRef]
- Guan, M.; Wang, L.; Zhang, Y.; Wang, D.; Wang, Q.; Liu, J. Effect of metal seed layer on the critical thickness and photoelectric properties of ultrathin Ag films. Mater. Today Commun. 2023, 37, 107540. [Google Scholar] [CrossRef]
- Meiss, J.; Riede, M.K.; Leo, K. Towards efficient tin-doped indium oxide (ITO)-free inverted organic solar cells using metal cathodes. Appl. Phys. Lett. 2009, 94, 013303. [Google Scholar] [CrossRef]
- Formica, N.; Ghosh, D.S.; Carrilero, A.; Chen, T.L.; Simpson, R.E.; Pruneri, V. Ultrastable and atomically smooth ultrathin silver films grown on a copper seed layer. ACS Appl. Mater. Interfaces 2013, 5, 3048–3053. [Google Scholar] [CrossRef]
- Liao, J.; Qian, R.; Wang, G.; Chen, N.; Xu, S.; Hu, S.; Zhu, W.; Luo, G. High performances multilayer transparent conductive films with manipulated Ag growth and layer thickness. J. Mater. Sci. Mater. Electron. 2023, 34, 795. [Google Scholar] [CrossRef]
- Kao, P.-C.; Hsu, C.-J.; Chen, Z.-H.; Chen, S.-H. Highly transparent and conductive MoO3/Ag/MoO3 multilayer films via air annealing of the MoO3 layer for ITO-free organic solar cells. J. Alloys Compd. 2022, 906, 164387. [Google Scholar] [CrossRef]
- Cho, H.; Yun, C.; Park, J.-W.; Yoo, S. Highly flexible organic light-emitting diodes based on ZnS/Ag/WO3 multilayer transparent electrodes. Org. Electron. 2009, 10, 1163–1169. [Google Scholar] [CrossRef]
- Kermani, H.; Fallah, H.R.; Hajimahmoodzadeh, M. Design and fabrication of nanometric ZnS/Ag/MoO3 transparent conductive electrode and investigating the effect of annealing process on its characteristics. Phys. E Low-Dimens. Syst. Nanostruct. 2013, 47, 303–308. [Google Scholar] [CrossRef]
- Peng, C.H.; Chen, P.S.; Lo, J.W.; Lin, T.W.; Lee, S.W. Indium-free transparent TiOx/Ag/WO3 stacked composite electrode with improved moisture resistance. J. Mater. Sci. Mater. Electron. 2016, 27, 12060–12066. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Han, Y.C.; Kim, H.C.; Jeong, E.G.; Choi, K.C. Highly Transparent and Flexible Organic Light-Emitting Diodes with Structure Optimized for Anode/Cathode Multilayer Electrodes. Adv. Funct. Mater. 2015, 25, 7145–7153. [Google Scholar] [CrossRef]
- Li, B.-J.; Li, Z.-X.; Huang, L.-J.; Wang, L.; Ren, N.-F. Preparation of AZO-based multilayer thin films with high comprehensive properties by introducing Cu/Ag bimetallic layers. J. Mater. Sci. Mater. Electron. 2023, 34, 832. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical Constants of the Noble Metals. Phys. Rev. B 1972, 6, 4370–4379. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, J.; Zhao, J.; Li, X.; Liu, L. Complex refractive indices measurements of polymers in infrared bands. J. Quant. Spectrosc. Radiat. Transf. 2020, 252, 107063. [Google Scholar] [CrossRef]
- Kamptner, A.; Scharber, M.C.; Schiek, M. Accurate Determination of the Uniaxial Complex Refractive Index and the Optical Band Gap of Polymer Thin Films to Correlate their Absorption Strength and Onset of Absorption. ChemPhysChem 2024, 25, e202400233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, J.; Chen, Z.; Hu, Y.; Li, H.; Zhang, R.; Tang, K.; Wang, L. Influence of Cu Thickness and Annealing on the Properties of ZnS/Cu/ZnS Multilayer Films. J. Electron. Mater. 2020, 49, 4498–4503. [Google Scholar] [CrossRef]
- Ren, N.-F.; Wang, W.-Z.; Li, B.-J.; Huang, L.-J.; Zhang, Y. Preparation and property optimization of silver-embedded FTO transparent conductive thin films by laser etching and coating AZO layer. J. Mater. Sci. Mater. Electron. 2021, 32, 10644–10661. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Zhao, L.; Nie, L.; Liu, J. Preparation of Ag-based transparent conductive films with high figure-of-merit from dielectric layers’ combination. Opt. Quantum Electron. 2024, 57, 40. [Google Scholar] [CrossRef]
- Haacke, G. New figure of merit for transparent conductors. J. Appl. Phys. 1976, 47, 4086–4089. [Google Scholar] [CrossRef]
- Yun, J. Ultrathin Metal films for Transparent Electrodes of Flexible Optoelectronic Devices. Adv. Funct. Mater. 2017, 27, 1606641. [Google Scholar] [CrossRef]
- Wan Shamsuddin, W.N.S.; Zuber, K.; Murphy, P.J.; Jane, M.L. Environmental durability of soft low-e coatings: A review. Sol. Energy Mater. Sol. Cells 2024, 266, 112673. [Google Scholar] [CrossRef]
- Yu, S.; Song, L.; Wu, C.; Li, L. Enhanced conductivity and stability of Cu-embedded zinc tin oxide flexible transparent conductive thin films. Ceram. Int. 2022, 48, 15925–15931. [Google Scholar] [CrossRef]


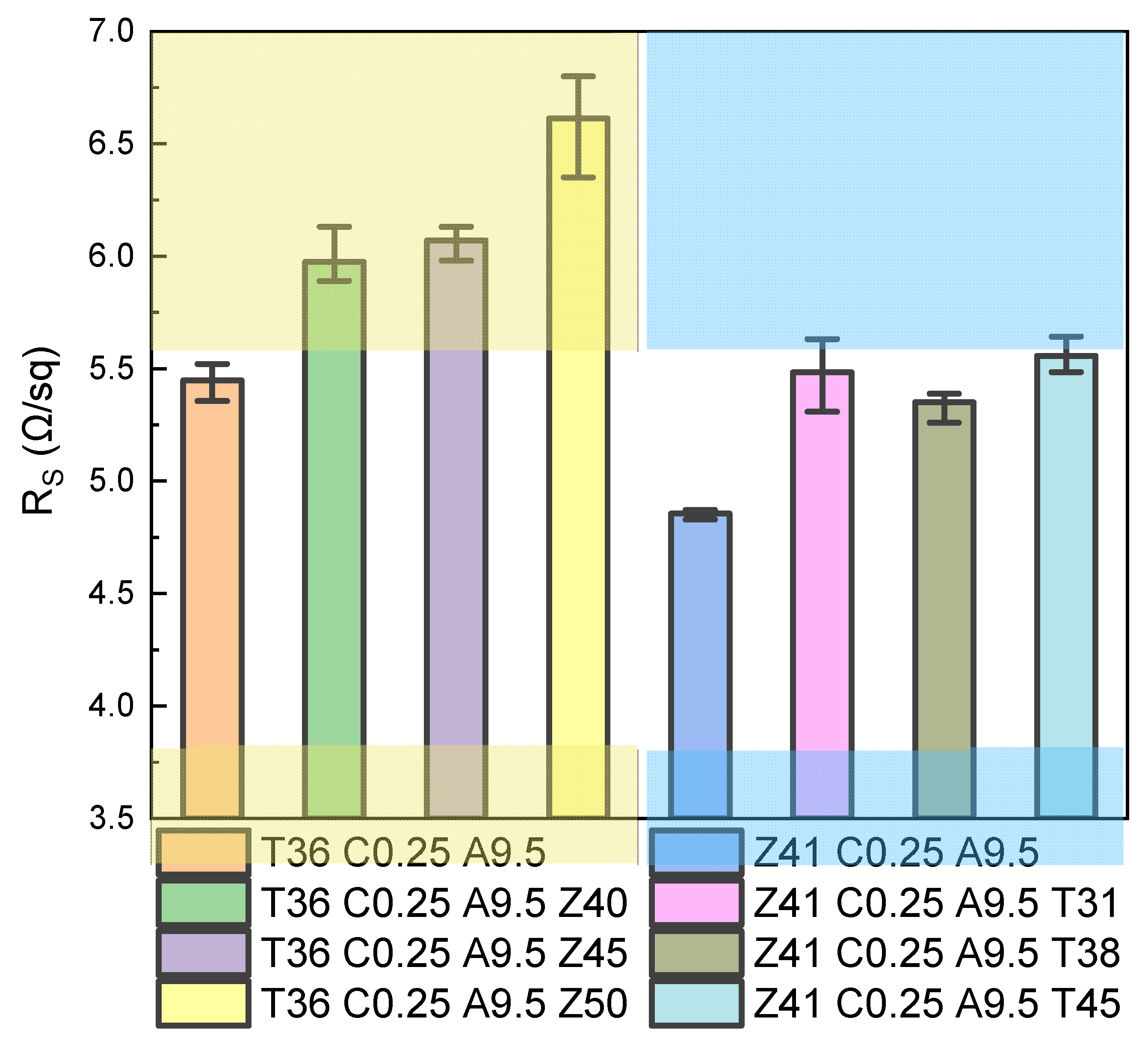
| TCF | (Ω/sq) | (%) | (%) | (10−3 Ω−1) | (10−3 Ω−1) |
|---|---|---|---|---|---|
| T36 C0.25 A9.5 Z40 | 5.98 | 87.20 | 82.99 | 42.52 | 25.93 |
| T36 C0.25 A9.5 Z45 | 6.07 | 85.83 | 84.75 | 35.74 | 31.50 |
| T36 C0.25 A9.5 Z50 | 6.64 | 85.98 | 83.91 | 33.26 | 26.07 |
| Z41 C0.25 A9.5 T31 | 5.48 | 95.21 | 86.65 | 111.66 | 43.52 |
| Z41 C0.25 A9.5 T38 | 5.35 | 94.04 | 87.90 | 101.15 | 51.47 |
| Z41 C0.25 A9.5 T45 | 5.56 | 92.70 | 88.46 | 84.34 | 52.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Tao, K.; Zhang, J.; Ren, Y.; Liu, Z. Structural, Optical and Electrical Properties of the Flexible, Asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 Films Deposited via Magnetron Sputtering. Coatings 2025, 15, 650. https://doi.org/10.3390/coatings15060650
Li Q, Tao K, Zhang J, Ren Y, Liu Z. Structural, Optical and Electrical Properties of the Flexible, Asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 Films Deposited via Magnetron Sputtering. Coatings. 2025; 15(6):650. https://doi.org/10.3390/coatings15060650
Chicago/Turabian StyleLi, Qingping, Kai Tao, Jiayi Zhang, Yazhe Ren, and Zhiyong Liu. 2025. "Structural, Optical and Electrical Properties of the Flexible, Asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 Films Deposited via Magnetron Sputtering" Coatings 15, no. 6: 650. https://doi.org/10.3390/coatings15060650
APA StyleLi, Q., Tao, K., Zhang, J., Ren, Y., & Liu, Z. (2025). Structural, Optical and Electrical Properties of the Flexible, Asymmetric TiO2/Cu/Ag/ZnS and ZnS/Cu/Ag/TiO2 Films Deposited via Magnetron Sputtering. Coatings, 15(6), 650. https://doi.org/10.3390/coatings15060650







