Corrosion and Wear Behavior of 17-4PH Stainless Steel Manufactured by Selective Laser Melting and Bulk Material After Solution Treatment
Abstract
1. Introduction
2. Experimental
2.1. 17-4PH Stainless Steel Bulk Samples and Selective Laser Melting (SLM) Parameters
2.2. Solution Treatment Conditions
2.3. Electrochemical Analysis, Corrosion, and Corrosive Wear Testing
2.4. XRD and DSC Analytical Parameters
2.5. Quantitative Analysis and Calculation of Tribocorrosion
3. Results
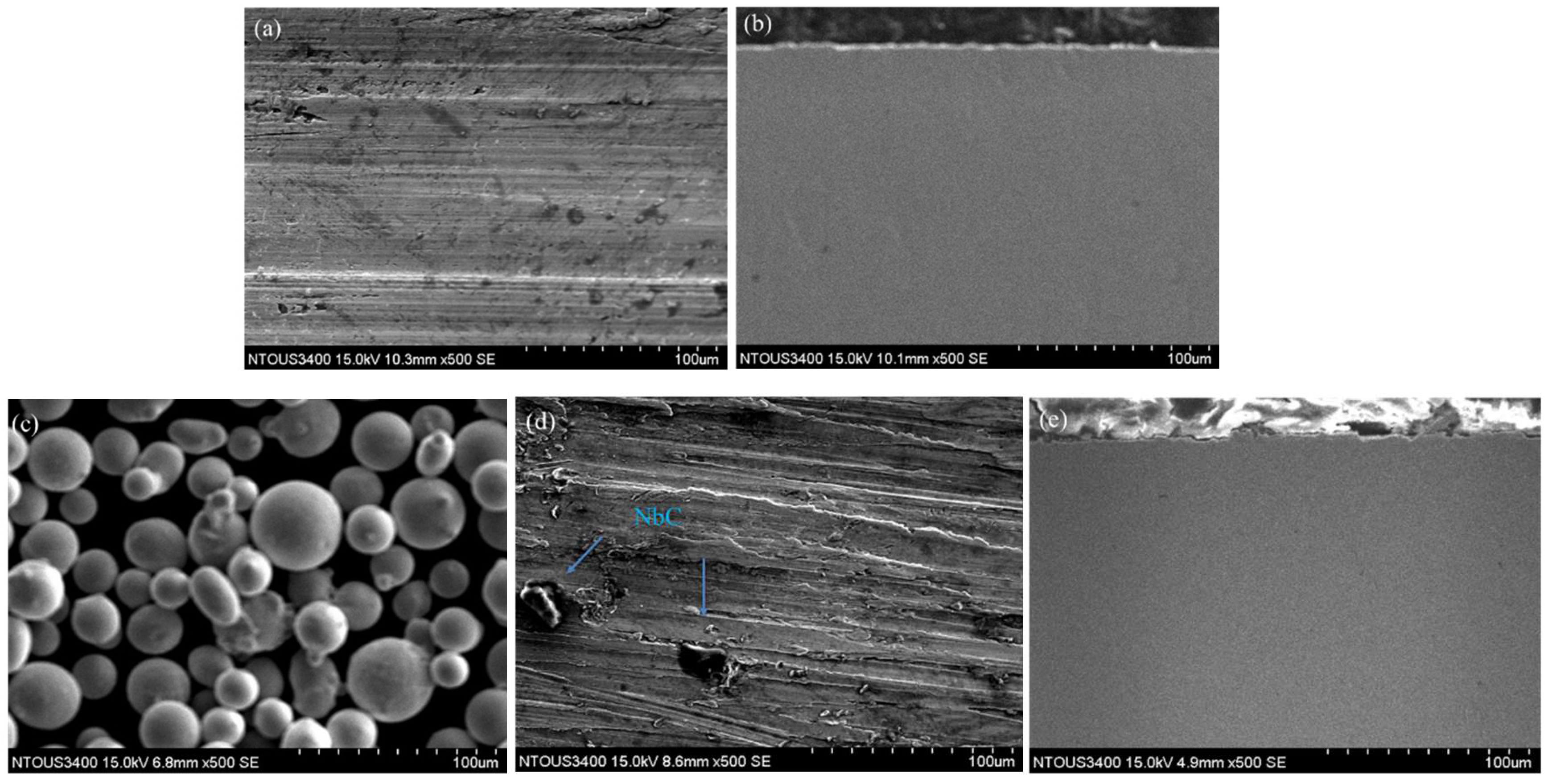
3.1. Observation of the As-Built Microstructure
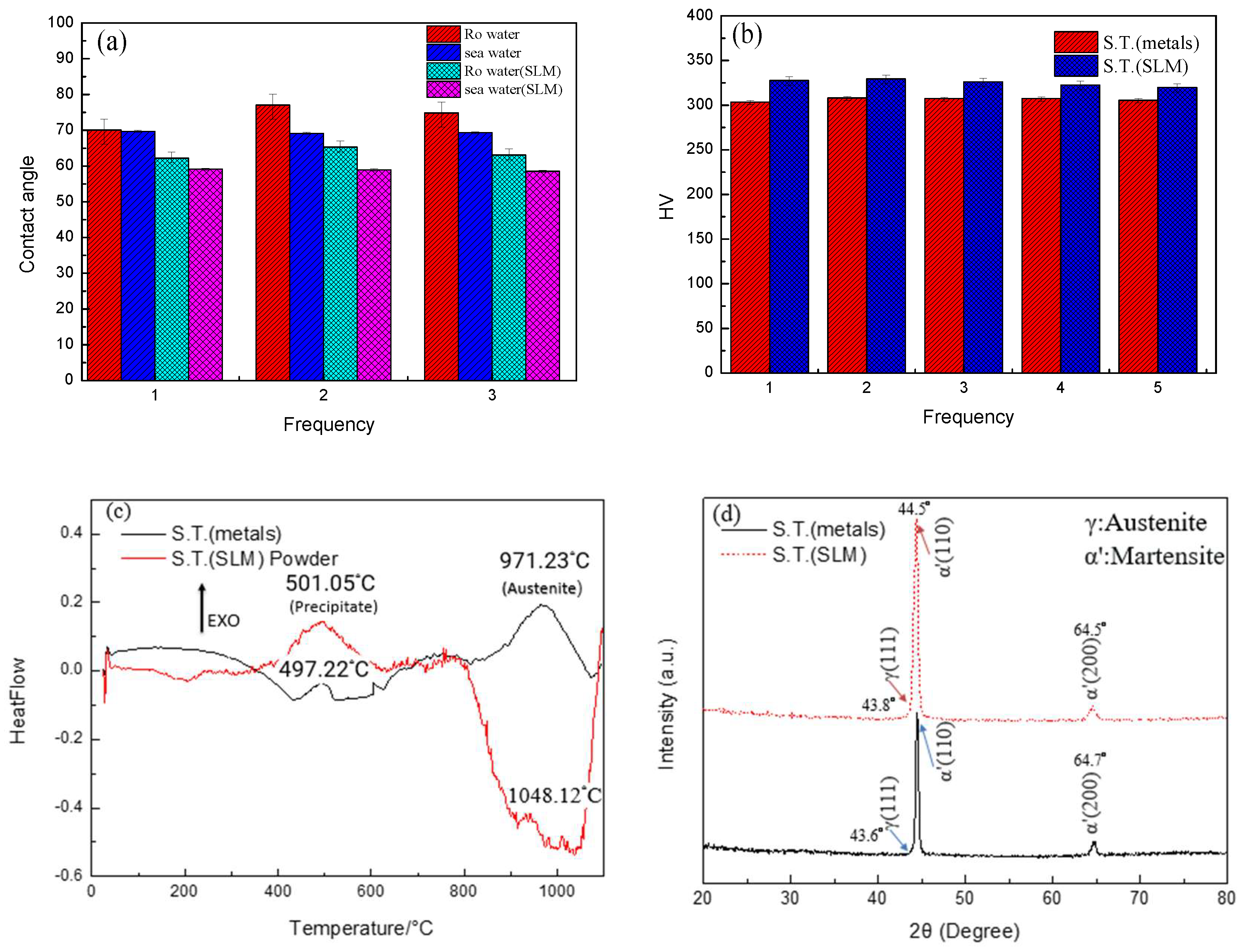
3.2. Hardness Measurement, XRD, and DSC Analysis Results
3.3. Corrosion Testing and Dry/Wet Wear Test Results
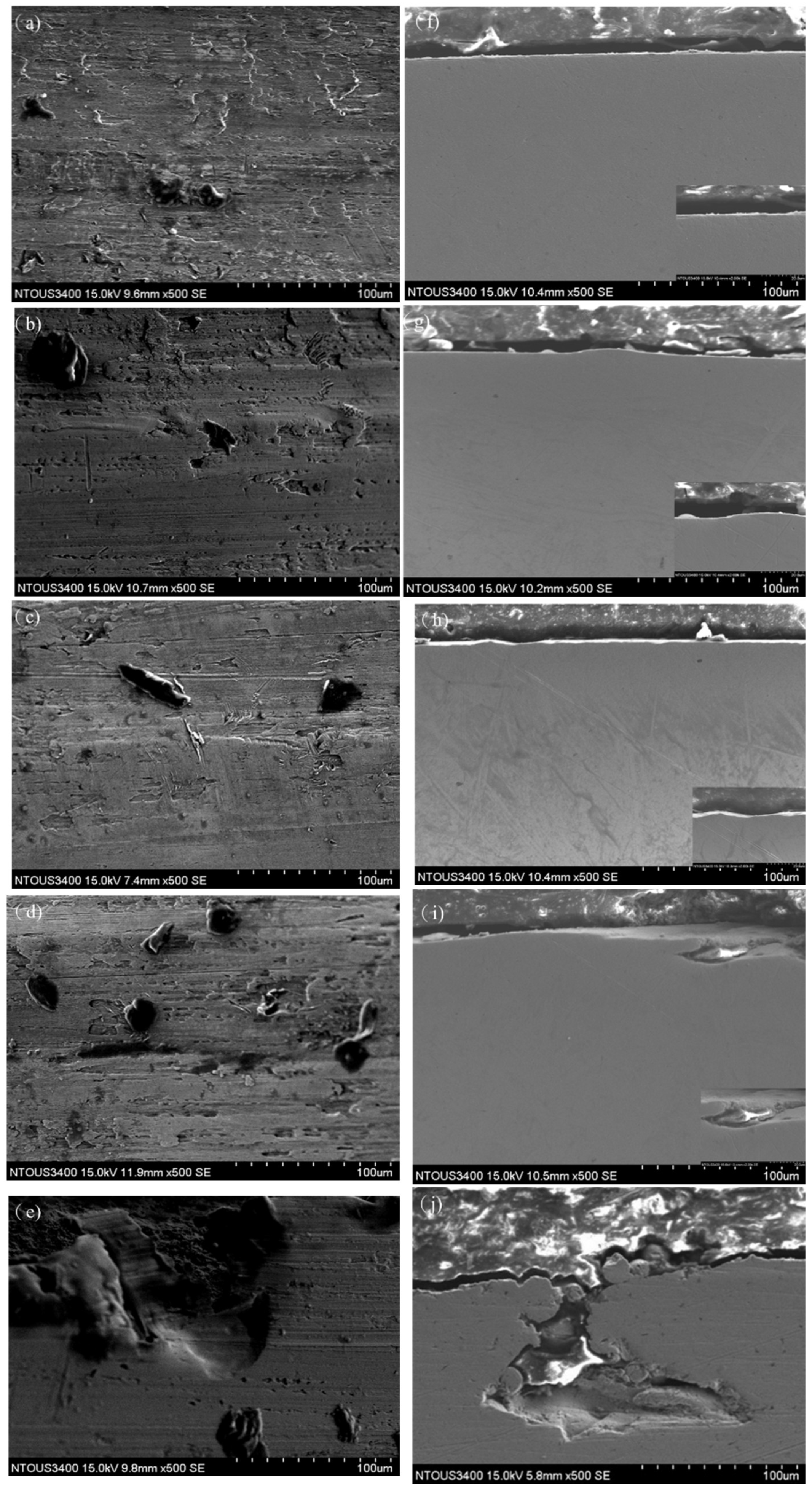
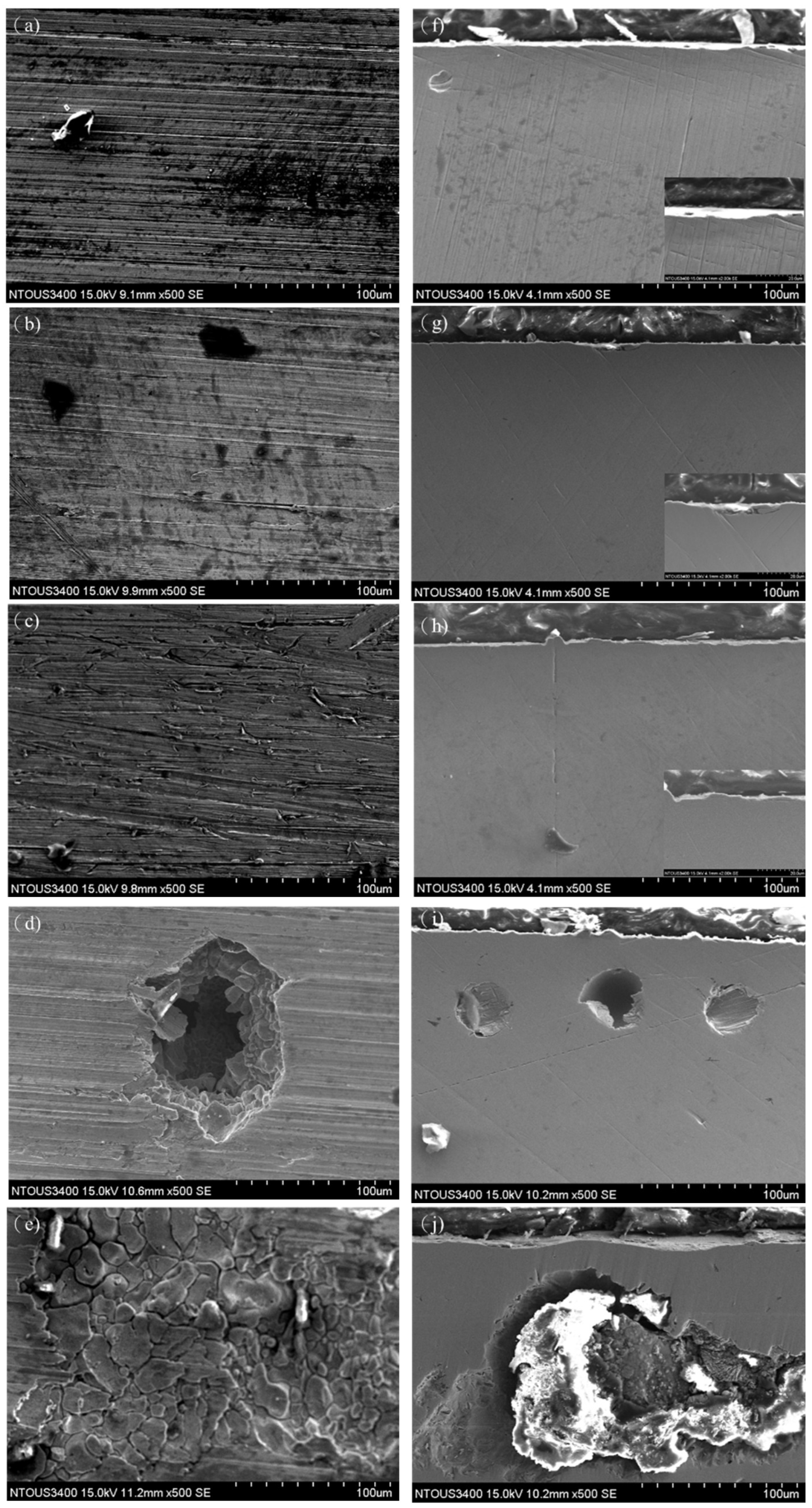
3.4. Corrosion Morphology Analysis Under Various Polarization Potentials
3.5. Friction Coefficient Results Under Various Potentials
3.6. SEM Analysis Under Various Polarization Potentials
4. Discussion
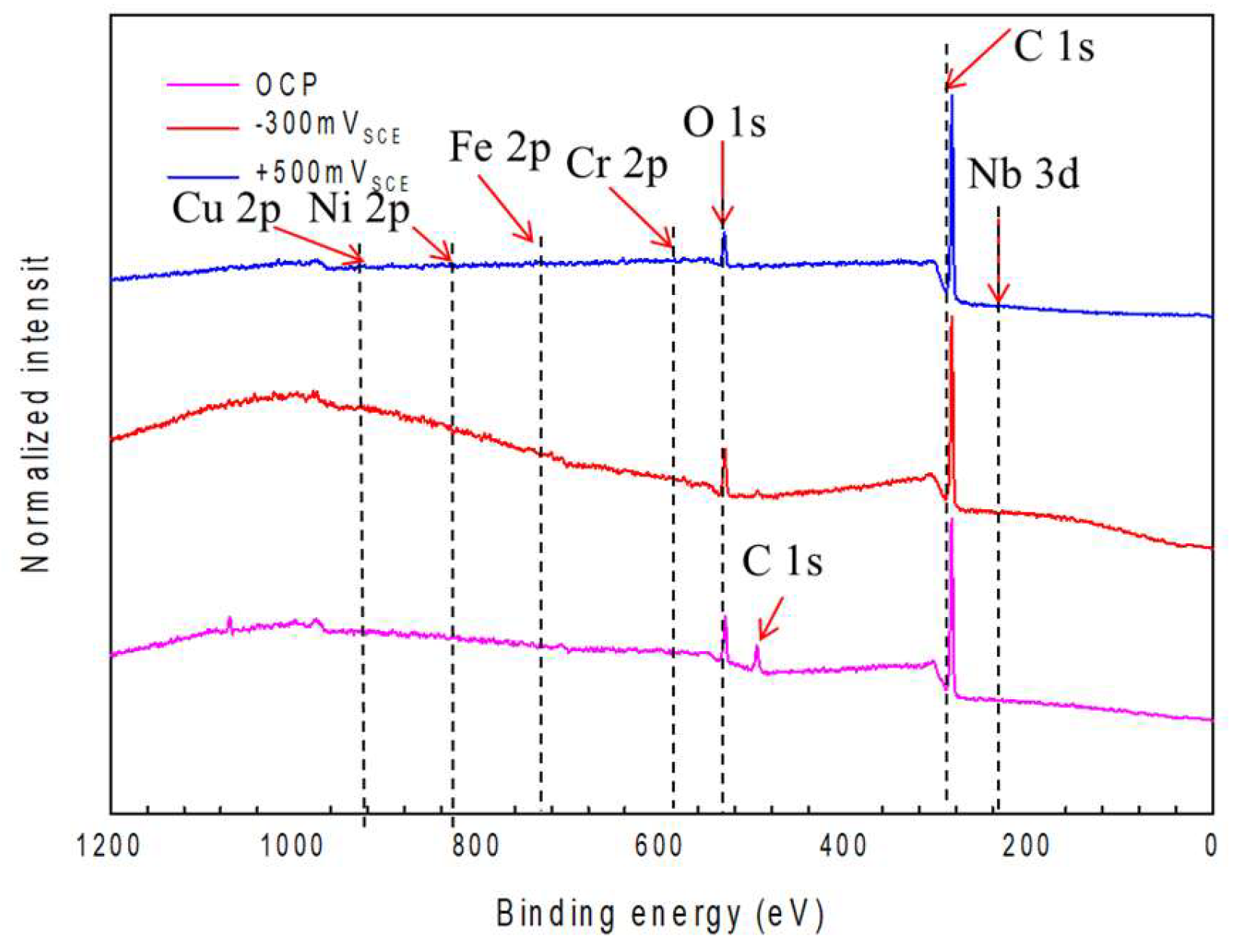
4.1. XPS Peak Analysis of Elements in the Passive Film
4.2. Quantitative Analysis Results
4.3. Tribocorrosion Mechanism
- Initial corrosion stage: due to their relatively higher anodic potential, NbC carbides undergo preferential corrosion, acting as sacrificial anodes that temporarily protect the surrounding matrix.
- Intermediate stage: progressive dissolution of NbC leads to the formation of corrosion channels, which, combined with galvanic coupling to the adjacent matrix, results in localized chromium depletion zones.
- Final stage: after complete dissolution of NbC, the voids left behind serve as initiation sites for pitting corrosion, promoting localized attack.
- During tribocorrosion, generated debris detaches from the contact surface and is expelled outward due to mechanical interaction.
- Once the underlying metallic substrate is exposed due to breakdown of the passive film, further fragmentation occurs, and the fractured particles are dislodged from the surface.
- Some debris is retained within the natural pores of the material and, through repeated contact stress, is mechanically ground into finer particles, which are eventually expelled from the pores during sliding motion.
5. Conclusions
- The corrosion polarization results demonstrated that the SLM specimens exhibited superior corrosion resistance under pure corrosion conditions. This enhancement is attributed to the more uniform distribution of alloying elements and the reduction in inherent porosity after solution treatment, which collectively improve the passivation behavior of the material.
- In contrast, the tribocorrosion polarization curves revealed that SLM specimens showed inferior corrosion resistance under simultaneous mechanical wear and electrochemical attack. The degradation was primarily caused by the expansion of pores under shear stress, which disrupted the integrity of the passive film.
- Microstructural analysis indicated that solution treatment at 1040 °C resulted in a uniform distribution of martensite, reduced porosity, and a more homogeneous microstructure, which significantly improved corrosion resistance.
- SEM observations confirmed that SLM specimens suffered more severe surface degradation under tribocorrosion conditions due to the preferential corrosion of pre-existing pores within the matrix, leading to localized brittle delamination.
- Quantitative analysis further confirmed that both bulk and SLM specimens possessed excellent corrosion resistance. However, wear played a more dominant role than electrochemical corrosion in the overall material loss during tribocorrosion.
- XPS analysis revealed that Cr2O3 formed the most compact and stable passive layer among the corrosion products. This oxide effectively prevented chloride ion penetration, thereby enhancing the long-term stability and protective capability of the passive film.
- SLM technology offers advantages such as the ability to fabricate complex geometries and high material utilization; however, it also has drawbacks, including high equipment costs and relatively slow manufacturing speeds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, P.H.; Xiao, A.C.; Li, J.B.; Jang, J.S.C.; Chu, J.P.; Huang, J.C. Prominent Fe-based bulk amorphous steel alloy with large supercooled liquid region and superior corrosion resistance. J. Alloys Compd. 2014, 586, 94–98. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Xue, Y.; Onggowarsito, C.; Kong, C.; Li, S. Tribological properties and corrosion behaviour of additively manufactured 17-4PH stainless steel: Effects of scanning pattern, build orientation, and single vs. double scan. Mater. Today Commun. 2020, 25, 101535. [Google Scholar] [CrossRef]
- Yang, K.-T.; Kim, M.-K.; Kim, D.; Suhr, J. Investigation of laser powder bed fusion manufacturing and post-processing for surface quality of as-built 17-4PH stainless steel. Surf. Coat. Technol. 2021, 422, 127492. [Google Scholar] [CrossRef]
- Sabooni, S.; Chabok, A.; Feng, S.C.; Blaauw, H.; Pijper, T.C.; Yang, H.J.; Pei, Y. Laser powder bed fusion of 17–4 PH stainless steel: A comparative study on the effect of heat treatment on the microstructure evolution and mechanical properties. Addit. Manuf. 2021, 46, 102176. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Kong, C.; Adabifiroozjaei, E.; Li, S. Microstructure, post thermal treatment response, and tribological properties of 3D printed 17-4 PH stainless steel. Wear 2020, 203367, 456–457. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Haq, M.I.U. 3D printing—A review of processes, materials and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Ali, U.; Esmaeilizadeh, R.; Ahmed, F.; Sarker, D.; Muhammad, W.; Keshavarzkermani, A.; Mahmoodkhani, Y.; Marzbanrad, E.; Toyserkani, E. Identification and characterization of spatter particles and their effect on surface roughness, density and mechanical response of 17-4 PH stainless steel laser powder-bed fusion parts. Mater. Sci. Eng. R 2019, 756, 98–107. [Google Scholar] [CrossRef]
- Ponnusamy, P.; Sharma, B.; Masood, S.H.; Rashid, R.A.R.; Rashid, R.; Palanisamy, S.; Ruan, D. A study of tensile behavior of SLM processed 17-4 PH stainless steel. Mater. Today Proc. 2021, 45, 4531–4534. [Google Scholar] [CrossRef]
- Pasebani, S.; Ghayoor, M.; Badwe, S.; Irrinki, H.; Atre, S.V. Effect of atomizing media and post processing on mechanical properties of 17-4 PH stainless steel manufactured via selective laser melting. Addit. Manuf. 2018, 22, 127–137. [Google Scholar] [CrossRef]
- Kudzal, A.; McWilliams, B.; Hofmeister, C.; Kellogg, F.; Yu, J.; Taggart-Scarff, J.; Liang, J. Effect of scan pattern on the microstructure and mechanical properties of Powder Bed Fusion additive manufactured 17-4 stainless steel. Mater. Des. 2017, 133, 205–215. [Google Scholar] [CrossRef]
- Barroux, A.; Ducommun, N.; Nivet, E.; Laffont, L.; Blanc, C.; Blanca, C.E. Pitting corrosion of 17-4PH stainless steel manufactured by laser beam melting. Corros. Sci. 2020, 169, 108594. [Google Scholar] [CrossRef]
- Alnajjar, M.; Christien, F.; Bosch, C.; Wolski, K. A comparative study of microstructure and hydrogen embrittlement of selective laser melted and wrought 17–4 PH stainless steel. Mater. Sci. Eng. A 2020, 785, 139363. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Wang, H.N.; Huo, P.G.; Bai, P.G.; Du, W.; Li, X.; Li, J.; Zhang, W. Effect of Solution Temperature on the Microstructure and Properties of 17-4PH High-Strength Steel Samples Formed by Selective Laser Melting. Metals 2022, 12, 425. [Google Scholar] [CrossRef]
- Liu, G.L.; Huang, C.Z.; Zou, B.; Liu, H.L.; Liu, Z.Q.; Liu, Y.; Li, C.W. The modification of corrosion resistance of 17-4PH stainless steel by cutting process. J. Manuf. Process. 2020, 49, 447–455. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Machado, C.L.C.; Oliveira, I.G.; Martins, T.R.B.; Masoumi, M. Damage associated with the interaction between hydrogen and microstructure in a high sulfur 17-4PH steel for studs. Eng. Fail. Anal. 2017, 82, 642–647. [Google Scholar] [CrossRef]
- An, S.; Eo, D.-R.; Sohn, I.; Choi, K. Homogenization on solution treatment and its effects on the precipitation-hardening of selective laser melted 17-4PH stainless steel. J. Mater. Sci. Technol. 2023, 166, 47–57. [Google Scholar] [CrossRef]
- Nezhadfar, P.D.; Shrestha, R.; Phan, N.; Shamsaei, N. Fatigue behavior of additively manufactured 17-4 PH stainless steel: Synergistic effects of surface roughness and heat treatment. Int. J. Fatigue 2019, 124, 188–204. [Google Scholar] [CrossRef]
- Lou, X.; Andresen, P.L.; Rebak, R.B. Oxide inclusions in laser additive manufactured stainless steel and their effects on impact toughness and stress corrosion cracking behavior. J. Nucl. Mater. 2018, 499, 182–190. [Google Scholar] [CrossRef]
- Wang, T.; Wang, M.S.; Xu, T.Z.; Wu, C.L.; Zhang, C.H.; Zhang, S.; Chen, H.T.; Chen, J. Enhancing Wear Resistance and Erosion Wear Performance of Laser Additive Manufactured 17-4PHss through Solution Aging Treatment. J. Therm. Spray Technol. 2024, 33, 2350–2366. [Google Scholar] [CrossRef]
- Shen, S.; Li, X.; Zhang, P.; Nan, Y.; Yang, G.; Song, X. Effect of solution-treated temperature on hydrogen embrittlement of 17-4 PH stainless steel. Mater. Sci. Eng. A 2017, 703, 413–421. [Google Scholar] [CrossRef]
- Wang, Z.; Grimm, M.; Lindner, T.; Schubert, F.; Winkler, K.; Berger, R.; Lampke, T. Wear and corrosion properties of low-temperature nitrocarburized 17-4PH SLM components. Surf. Coat. Technol. 2024, 494, 131399. [Google Scholar] [CrossRef]
- García-Hernández, C.; Naranjo, J.A.; Castro-Sastre, M.Á.; Berges, C.; Fernandez-Abia, A.I.; Martín-Pedrosa, F.; Herranz, G.; García-Cabezón, C. Enhancing wear performance: A comparative study of traditional vs. additive manufacturing techniques for 17–4pH SS. Wear 2024, 205258, 540–541. [Google Scholar] [CrossRef]
- Schaller, R.F.; Taylor, J.M.; Rodelas, J.; Schindelholz, E.J. Corrosion Properties of Powder Bed Fusion Additively Manufactured 17-4 PH Stainless Steel. Corrosion 2017, 73, 796–807. [Google Scholar] [CrossRef]
- García-Cabezón, C.; García-Hernández, C.; Castro-Sastre, M.A.; Mendez, M.Z.; Pedrosa, F.N. Heat treatments of 17-4 PH SS processed by SLM to improve its strength and biocompatibility in biomedical applications. J. Mater. Res. Technol. 2023, 26, 3524–3543. [Google Scholar] [CrossRef]
- Cheruvathur, S.; Lass, E.A.; Campbell, C.E. Additive manufacturing of 17-4 PH stainless steel: Post-processing heat treatment to achieve uniform reproducible microstructure. JOM 2016, 68, 930–942. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Pardal, J.M.; da Silva, M.R. Influence of Sulfur Content on the Corrosion Resistance of 17-4PH Stainless Steel. J. Mater. Eng. Perform. 2017, 26, 2512–2519. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.; Chan, K.C.; Chen, Q.; Tang, C.Y. Wear behavior of HVOF-sprayed Fe-based amorphous coatings. Intermet 2012, 29, 80–85. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Castro-Sastre, M.A.; Fernandez-Abia, A.I.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. Microstructure–hardness–corrosion performance of 17–4 precipitation hardening stainless steels processed by selective laser melting in comparison with commercial alloy. Met. Mater. Int. 2022, 28, 2652–2667. [Google Scholar] [CrossRef]
- Lin, T.J.; Sheu, H.H.; Lee, C.Y.; Lee, H.B. The study of mechanical properties and corrosion behavior of the Fe-based amorphous alloy coatings using high velocity oxygen fuel spraying. J. Alloys Compd. 2021, 867, 159132. [Google Scholar] [CrossRef]
- Esfandiari, M.; Dong, H. The corrosion and corrosion–wear behaviour of plasma nitrided 17-4PH. Surf. Coat. Technol. 2007, 202, 466–478. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lin, T.; Sheu, H.H.; Lee, H.B. A study on corrosion and corrosion–wear behavior of Fe-based amorphous alloy coating prepared by high velocity oxygen fuel method. J. Mater. Res. Technol. 2021, 15, 4880–4895. [Google Scholar] [CrossRef]
- Rowolt, C.; Milkereit, B.; Springer, A.; Kreyenschulte, C.; Kessler, O. Dissolution and precipitation of copper-rich phases during heating and cooling of precipitation-hardening steel X5CrNiCuNb16-4 (17-4 PH). Met. Corros. 2020, 55, 13244–13257. [Google Scholar] [CrossRef]
- Shih, T.-S.; Huang, Y.-S.; Chen, C.-F. Constituted oxides/nitrides on nitriding 304, 430 and 17-4 PH stainless steel in salt baths over the temperature range 723 to 923K. Appl. Surf. Sci. 2011, 258, 81–88. [Google Scholar] [CrossRef]
- Barroux, A.; Duguet, T.; Ducommun, N.; Nivet, E.; Delgado, J.; Laffont, L.; Blanc, C. Combined XPS/TEM study of the chemical composition and structure of the passive film formed on additive manufactured 17-4PH stainless steel. Surf. Interface 2021, 22, 100874. [Google Scholar] [CrossRef]
- Biesinger, M.K.; Payne, B.P.; Hart, B.R.; Grosvenor, A.P.; McIntryre, N.T.; Lau, L.; Smart, R.S. Quantitative Chemical State XPS Analysis of First Row Transition Metals, Oxides and Hydroxides. Conf. Ser. 2008, 100, 012025. [Google Scholar] [CrossRef]
- Cao, S.; Mischler, S. Modeling tribocorrosion of passive metals—A review. Curr. Opin. Solid State Mater. Sci. 2018, 22, 127–141. [Google Scholar] [CrossRef]












| Elements | Fe | Cr | Ni | Cu | Mn | Si | Nb | Mo | C | S | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atom% | ||||||||||||
| Bulk Specimen | 75.51 | 15.29 | 4.55 | 3.24 | 0.81 | 0.32 | 0.19 | 0.03 | 0.02 | 0.01 | 0.03 | |
| SLM Specimen | 75.68 | 15.12 | 4.63 | 3.16 | 0.79 | 0.34 | 0.17 | 0.05 | 0.03 | 0.02 | 0.01 | |
| Metal Powder | 75.57 | 15.23 | 4.75 | 3.04 | 0.75 | 0.38 | 0.16 | 0.06 | 0.03 | 0.01 | 0.02 | |
| Bulk Specimen | Ecorr | Icorr | Friction Coefficient |
|---|---|---|---|
| Corrosion | −510 mV | 431.03 μA | - |
| Corrosion wear | −609 mV | 614.61 μA | 0.12 |
| SLM specimen | Ecorr | Icorr | Friction Coefficient |
| Corrosion | −527 mV | 317.82 μA | - |
| Corrosion wear | −612 mV | 653.19 μA | 0.50 |
| Bulk Specimen | Potential | Weight Loss | Friction Coefficient |
|---|---|---|---|
| Corrosion | −524 mV | 5 mg | - |
| Corrosion wear | −619 mV | 17 mg | 0.11 |
| SLM specimen | Potential | Weight loss | Friction Coefficient |
| Corrosion | −553 mV | 2 mg | - |
| Corrosion wear | −625 mV | 29 mg | 0.51 |
| 17-4PH Stainless Steel | Bulk Specimen | SLM Specimen | ||
|---|---|---|---|---|
| Dry Wear | Wet Wear | Dry Wear | Wet Wear | |
| Friction coefficient | 0.56 | 0.14 | 0.69 | 0.53 |
| Weight loss (mg) | 19 | 12 | 56 | 14 |
| mA/cm2 | −500 mVSCE | −300 mVSCE | +100 mVSCE | +500 mVSCE |
|---|---|---|---|---|
| Bulk specimen Corrosion | 0.06 | 0.10 | 0.52 | 1.82 |
| SLM specimen Corrosion | 0.003 | 0.004 | 0.09 | 0.07 |
| Bulk specimen Corrosion wear | 0.08 | 0.18 | 2.82 | 4.77 |
| SLM specimen Corrosion wear | 0.12 | 0.34 | 3.06 | 5.50 |
| Friction Coefficient | OCP | −500 mVSCE | −300 mVSCE | +100 mVSCE | +500 mVSCE |
|---|---|---|---|---|---|
| Bulk specimen | 0.11 | 0.12 | 0.09 | 0.10 | 0.11 |
| SLM specimen | 0.51 | 0.55 | 0.56 | 0.58 | 0.59 |
| Element | Spectral Line | Formula | Binding Energy (eV) | ||
|---|---|---|---|---|---|
| NIST | Reference | Experimental on the Surface | |||
| Fe | 2p1/2, 2p3/2 | Fe | 720.0, 706.7 | 706.6 [34] | 720.0, 706.7 |
| Fe | 2p1/2, 2p3/2, 2p3/2 | FeO | 723.3, 709.6 707.2 | 707.5 [34] | 723.3, 709.6 707.2 |
| Fe | 2p3/2 | Fe2O3 | 711.4 | 711.8 [34] | 711.4 |
| Cr | 2p1/2, 2p3/2 | Cr | 583.5, 574.1 | 573.9 [34] | 583.5, 574.1 |
| Cr | 2p3/2, 2p3/2 | Cr2O3 | 576.2, 575.4 | 575.8 [34] | 576.2, 575.4 |
| Ni | 2p3/2, 2p3/2 | Ni | 853.0, 852.9 | 852.7 [34] | 853.0, 852.9 |
| Ni | 2p3/2 | NiO | 853.4 | 852.8 [34] | 853.4 |
| Cu | 2p3/2, 2p3/2 | Cu | 932.8, 932.6 | 932.4 [34] | 932.8, 932.6 |
| Cu | 2p3/2 | CuO | 934.6 | 932.5 [34] | 934.6 |
| NbC | 3d5/2, 3d5/2 | NbC | 203.7, 202.7 | 203.6 [34] | 203.7, 202.7 |
| NbC | 3d5/2 | NbCO | 205.8 | 206.3 [34] | 205.8 |
| Nb | 3d5/2, 3d5/2 3d5/2 | NbO | 204.7, 203.8 202.8 | 206.9 [34] | 204.7, 203.8 202.8 |
| Nb | 3d5/2, 3d5/2 | NbO2 | 206.1, 205.8 | 207.5 [34] | 206.1, 205.8 |
| Nb | 3d5/2, 3d5/2 | Nb2O5 | 207.1, 206.9 | 209.6 [34] | 207.1, 206.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, B.-X.; Sheu, H.-H.; Lin, M.-Y.; Lee, C.-Y.; Lee, H.-B. Corrosion and Wear Behavior of 17-4PH Stainless Steel Manufactured by Selective Laser Melting and Bulk Material After Solution Treatment. Coatings 2025, 15, 649. https://doi.org/10.3390/coatings15060649
Hou B-X, Sheu H-H, Lin M-Y, Lee C-Y, Lee H-B. Corrosion and Wear Behavior of 17-4PH Stainless Steel Manufactured by Selective Laser Melting and Bulk Material After Solution Treatment. Coatings. 2025; 15(6):649. https://doi.org/10.3390/coatings15060649
Chicago/Turabian StyleHou, Bo-Xun, Hung-Hua Sheu, Ming-Yuan Lin, Chun-Ying Lee, and Hung-Bin Lee. 2025. "Corrosion and Wear Behavior of 17-4PH Stainless Steel Manufactured by Selective Laser Melting and Bulk Material After Solution Treatment" Coatings 15, no. 6: 649. https://doi.org/10.3390/coatings15060649
APA StyleHou, B.-X., Sheu, H.-H., Lin, M.-Y., Lee, C.-Y., & Lee, H.-B. (2025). Corrosion and Wear Behavior of 17-4PH Stainless Steel Manufactured by Selective Laser Melting and Bulk Material After Solution Treatment. Coatings, 15(6), 649. https://doi.org/10.3390/coatings15060649








