Concentration-Optimized Minocycline-Modified Antimicrobial Coatings on Polyetheretherketone for the Prevention of Implant-Associated Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Surface Characterization
2.4. Release Behavior of Mino
2.5. In Vitro Antibacterial Assays
2.5.1. Bacterial Culture
2.5.2. Zone of Inhibition (ZOI) Assay
2.5.3. Turbidity Assay
2.5.4. Colony Counting Assay
2.6. In Vitro Biocompatibility Assays
2.6.1. Cell Culture
2.6.2. Cell Adhesion
2.6.3. Cell Proliferation
2.7. Statistical Analysis
3. Results
3.1. Surface Characterization
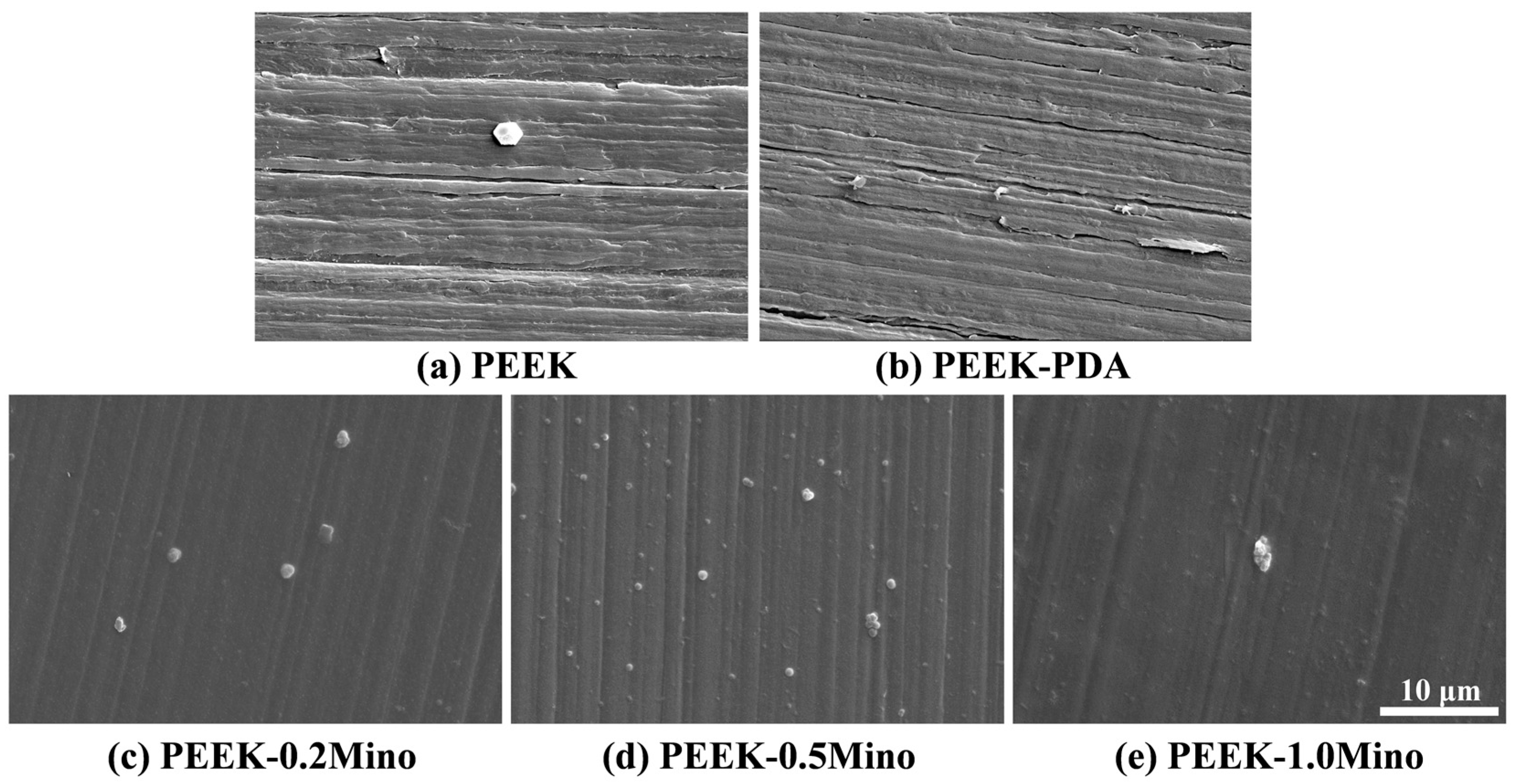
3.1.1. Surface Morphology Characterization by SEM
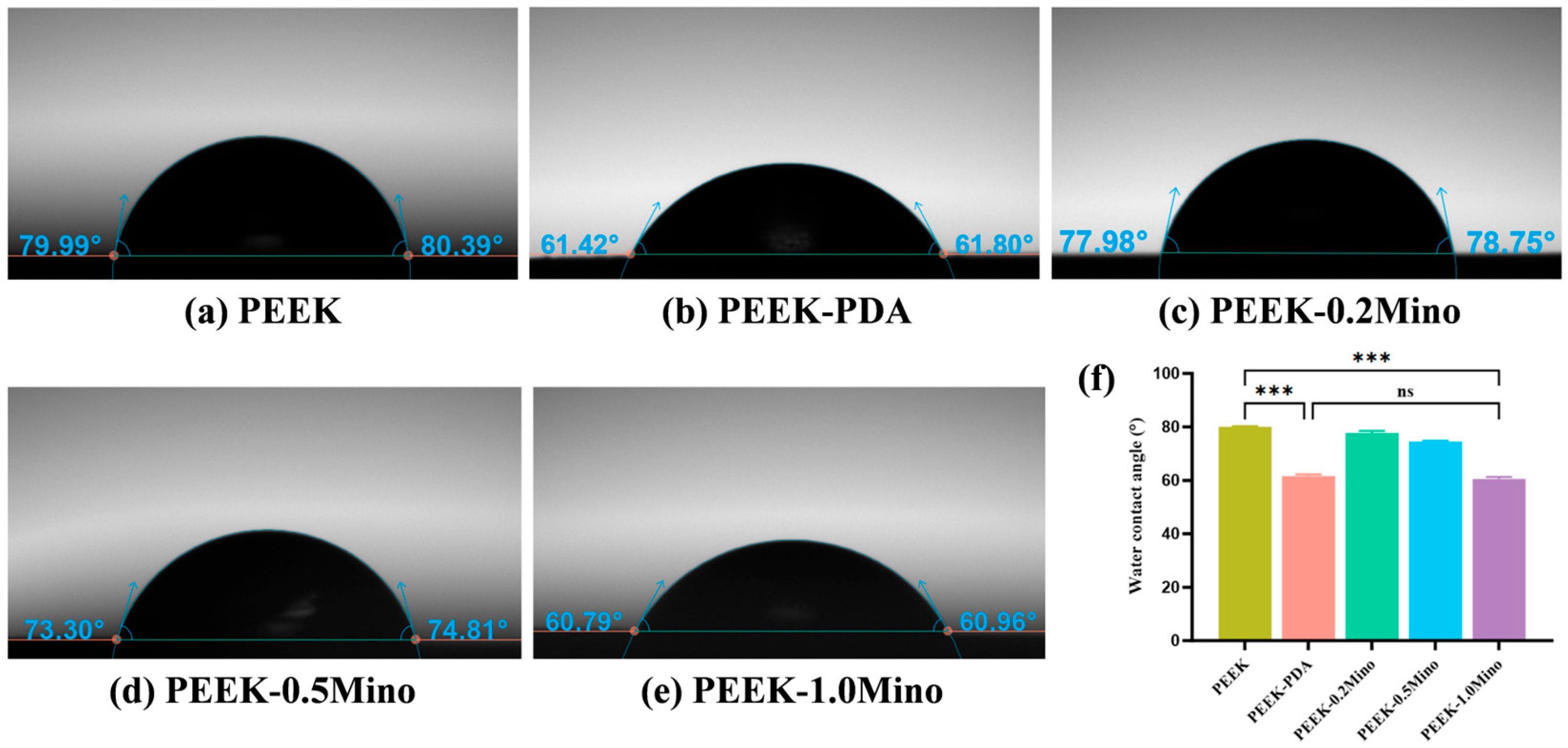
3.1.2. Water Contact Angle and Wettability Analysis
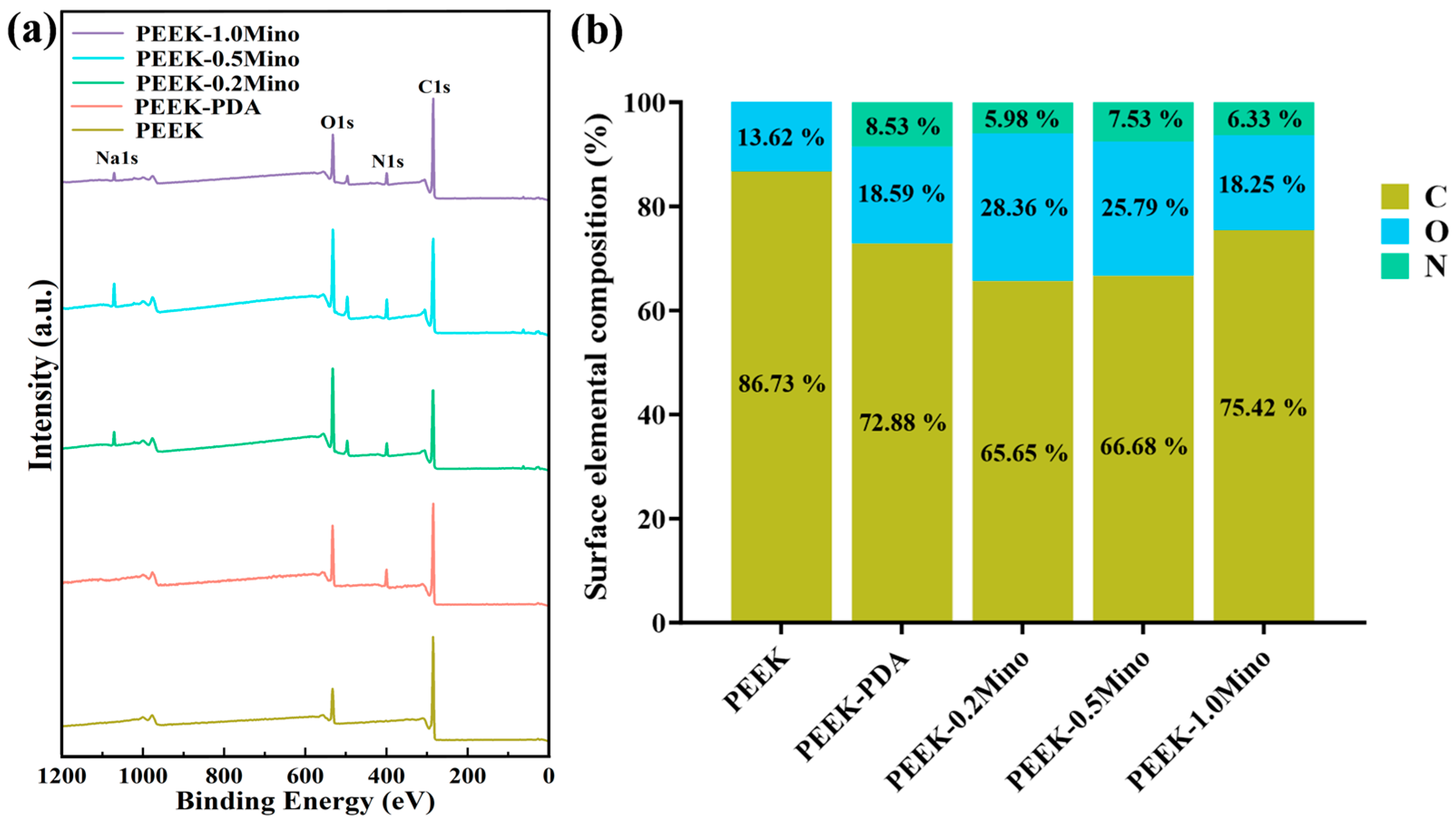
3.1.3. Surface Chemical Composition Analysis by XPS
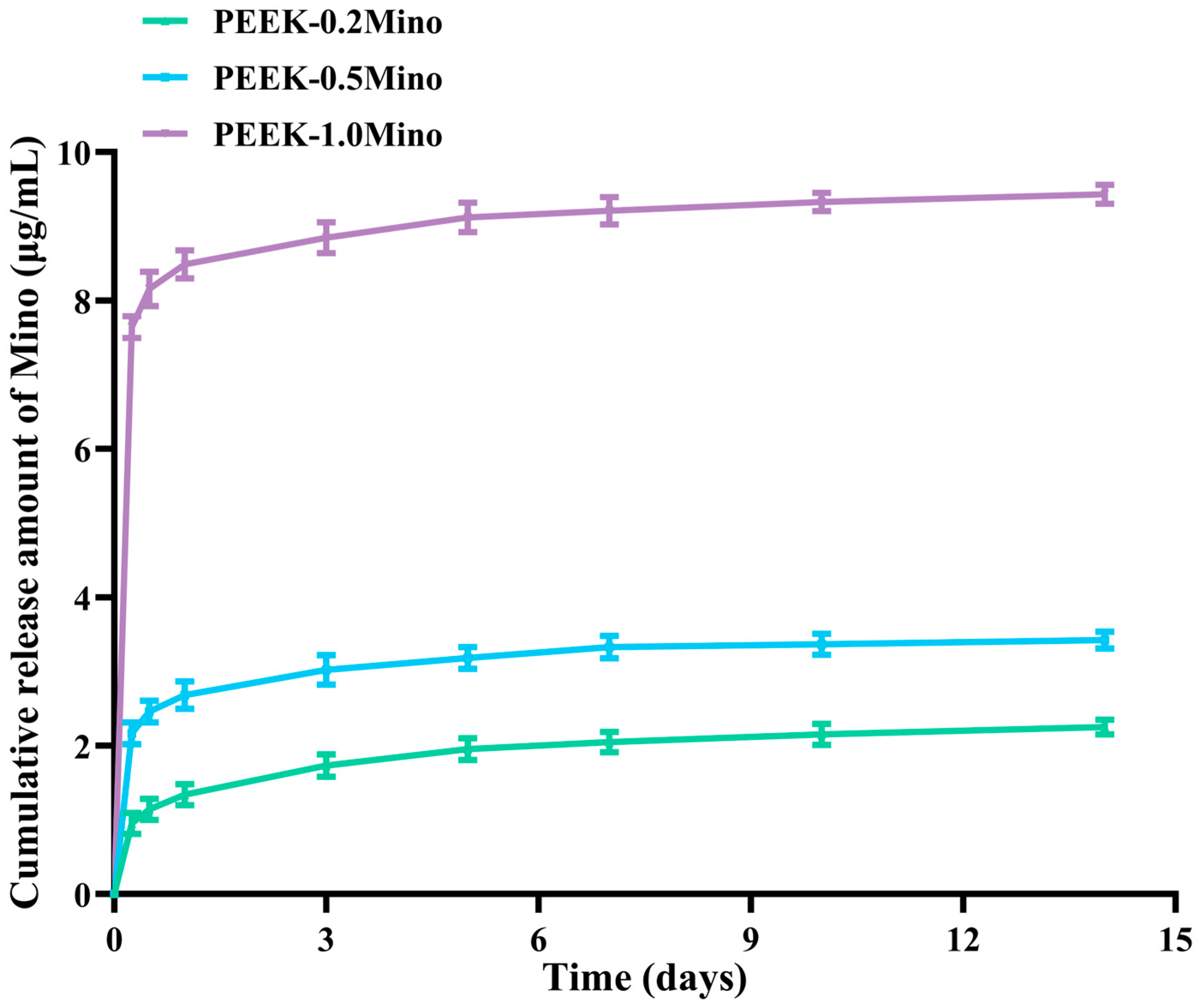
3.2. Release Behavior of Mino
3.3. Antibacterial Performance Evaluation
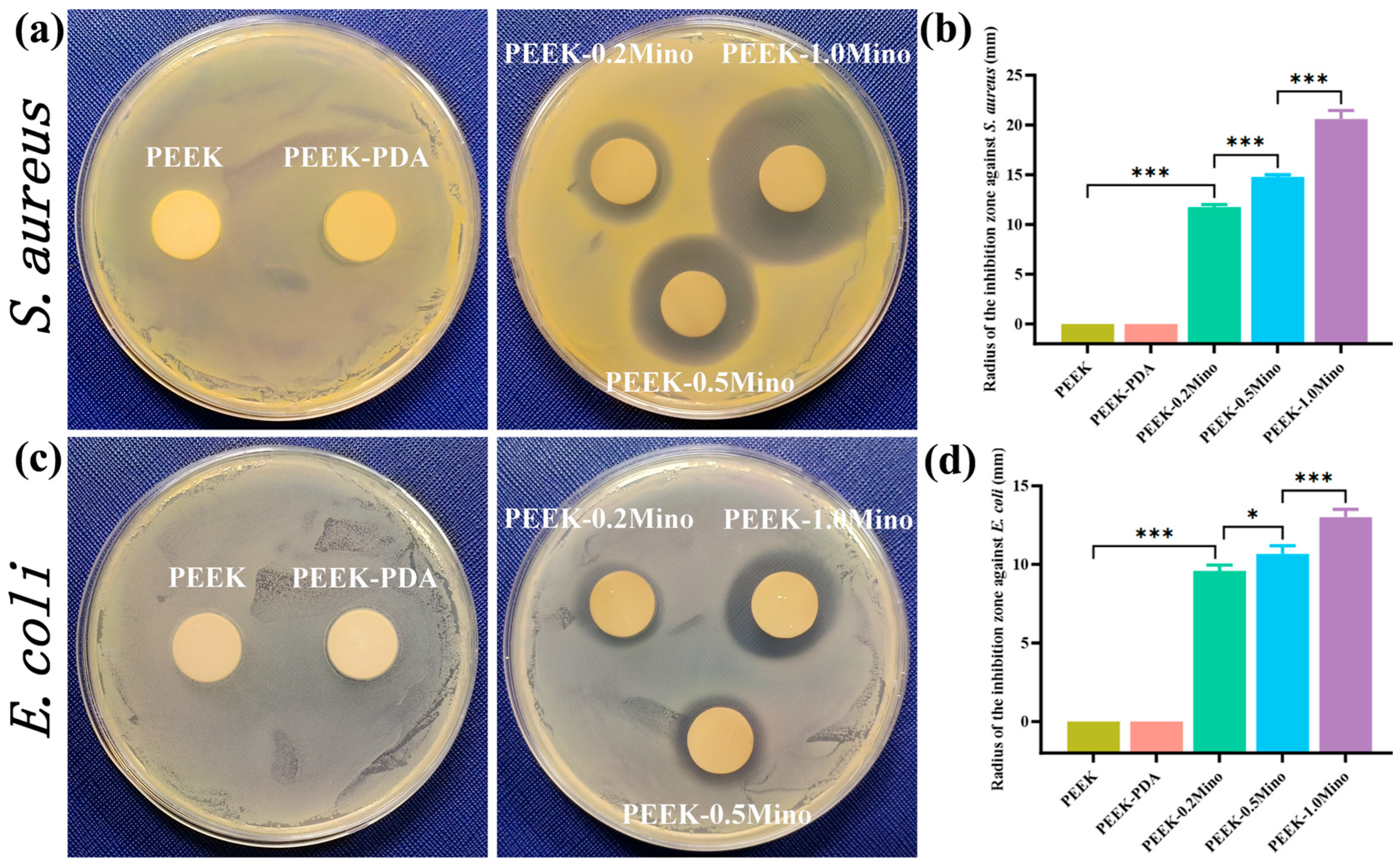
3.3.1. ZOI Assay
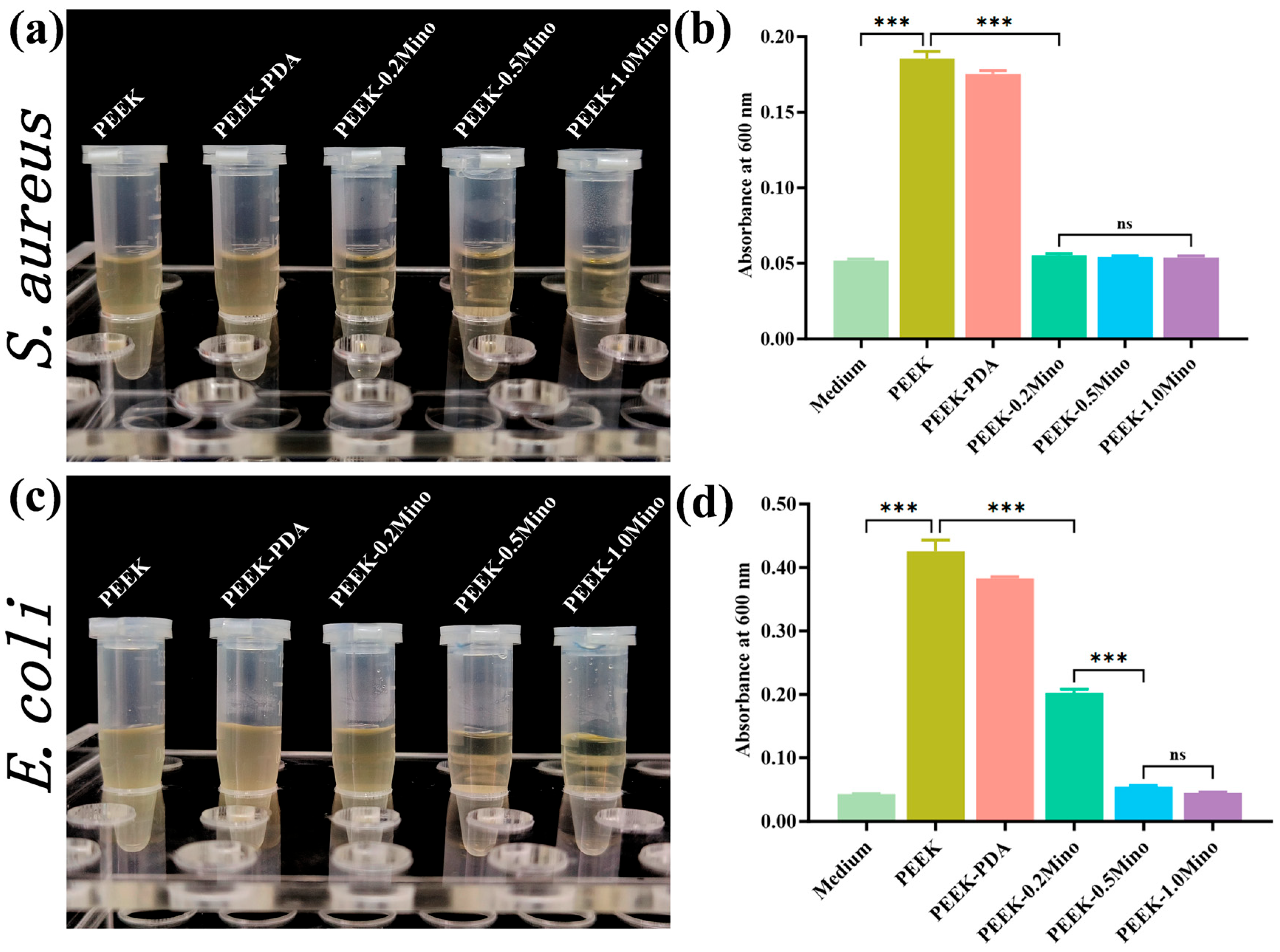
3.3.2. Turbidity Assay
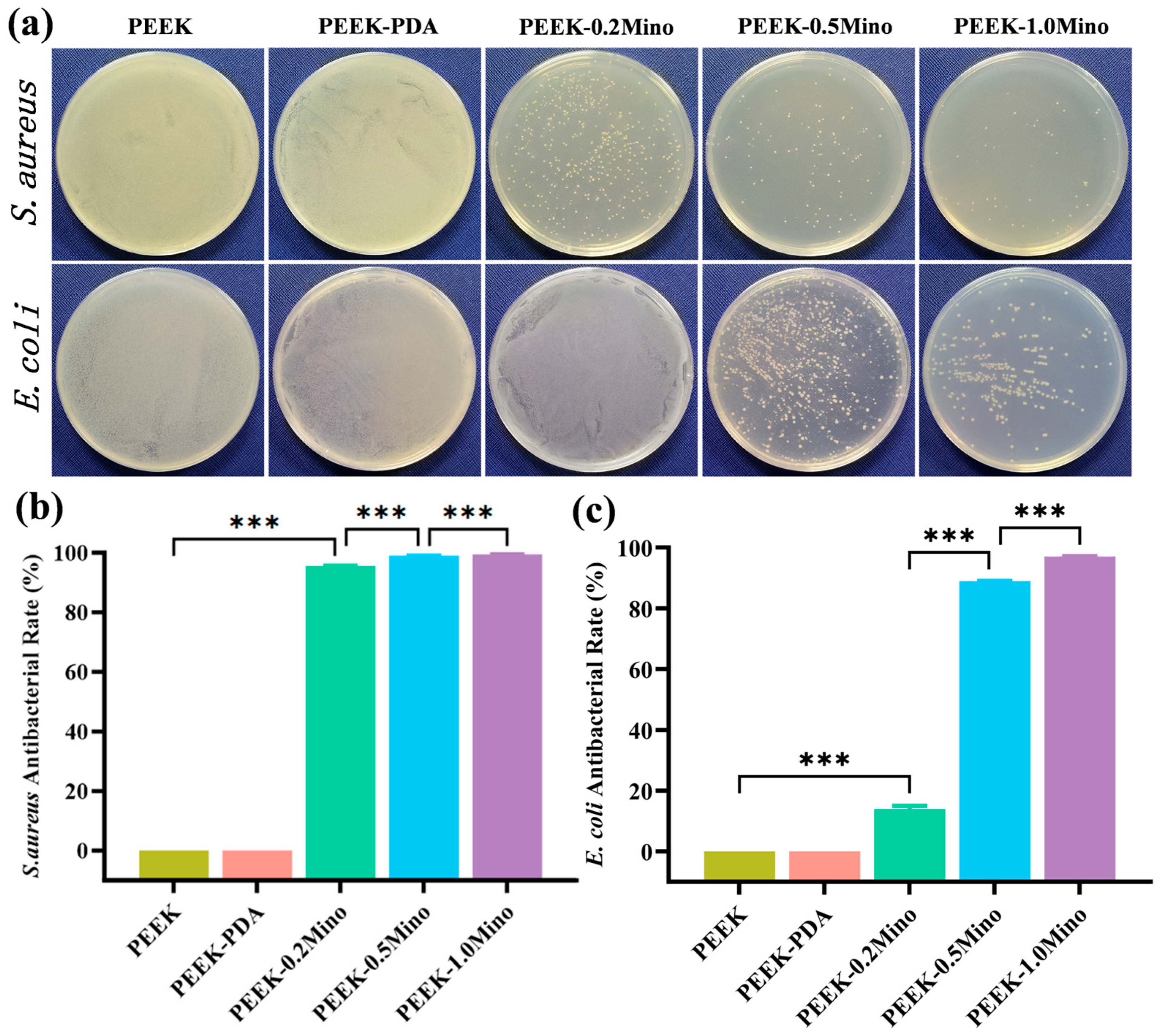
3.3.3. Colony Counting Assay
3.4. Biocompatibility Evaluation
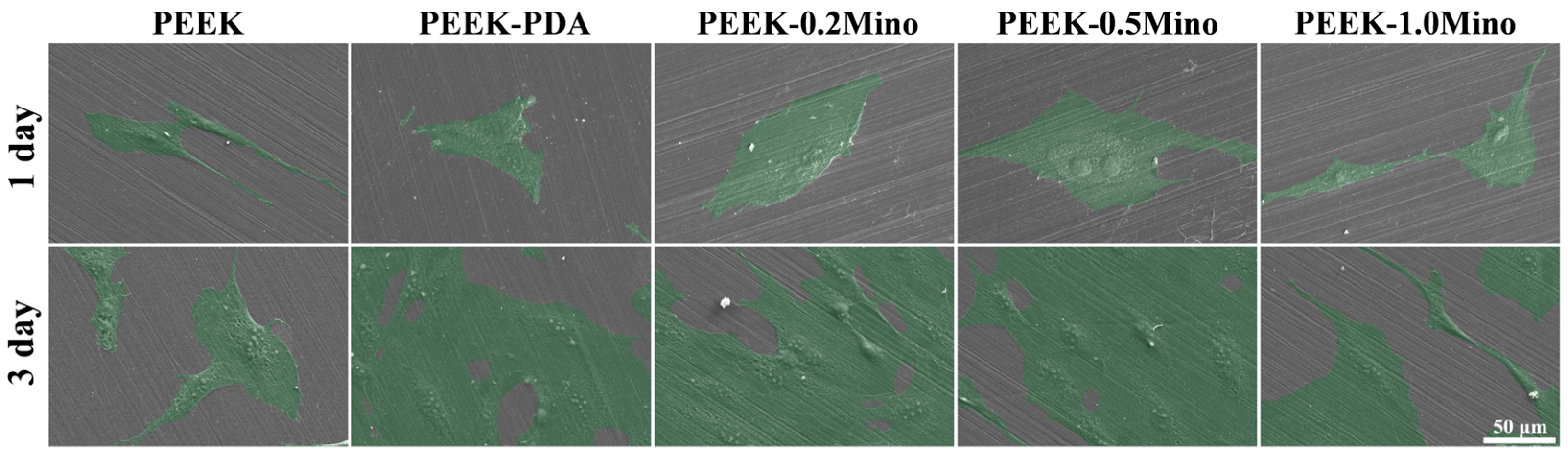
3.4.1. Cell Adhesion
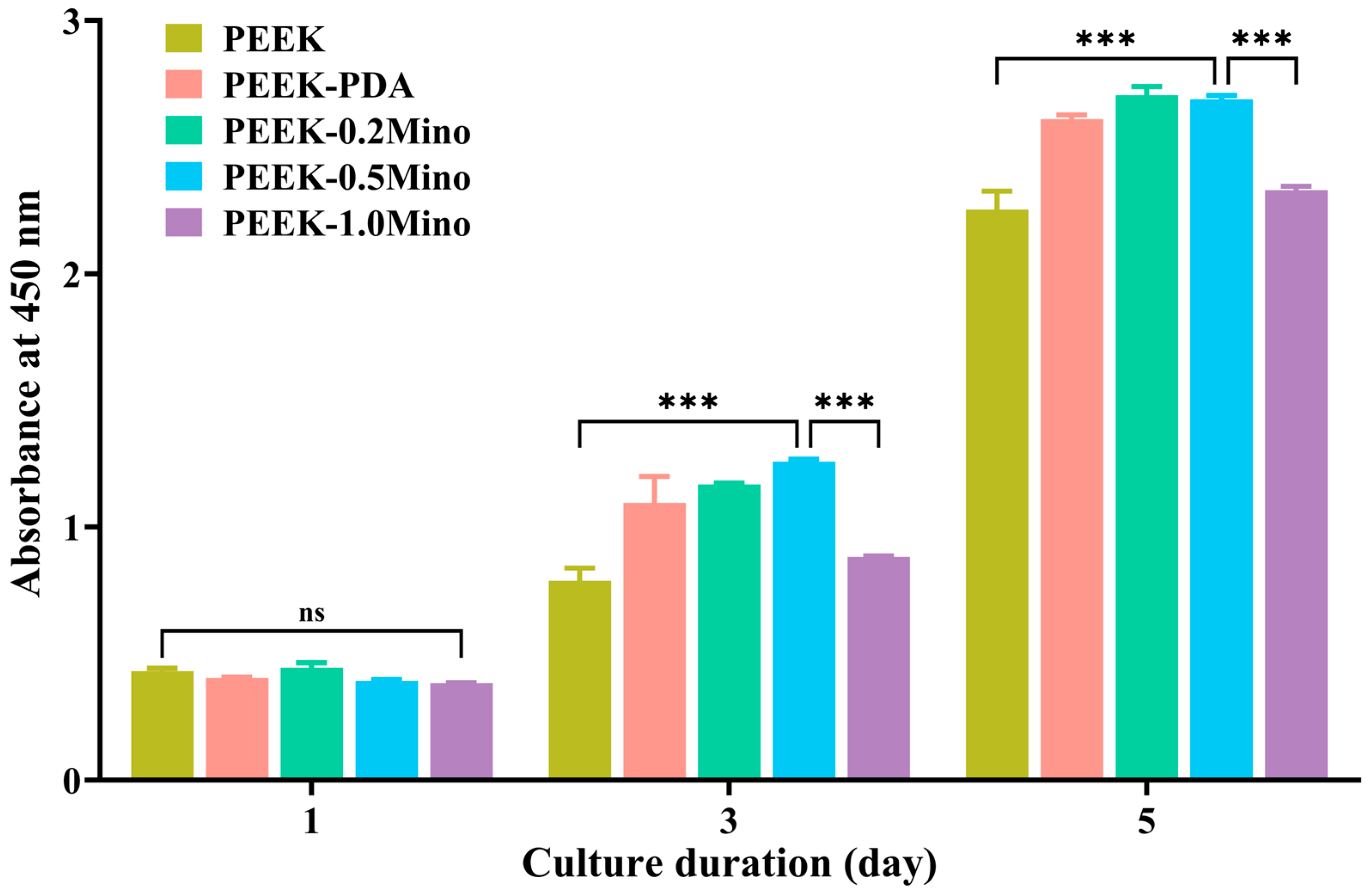
3.4.2. Cell Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory biomaterials for implant-associated infections: From conventional to advanced therapeutic strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Patel, R. Practical Guidance for Clinical Microbiology Laboratories: Microbiologic diagnosis of implant-associated infections. Clin. Microbiol. Rev. 2024, 37, e0010423. [Google Scholar] [CrossRef]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef]
- Chu, G.; Guan, M.; Jin, J.; Luo, Y.; Luo, Z.; Shi, T.; Liu, T.; Zhang, C.; Wang, Y. Mechanochemically Reprogrammed Interface Orchestrates Neutrophil Bactericidal Activity and Apoptosis for Preventing Implant-Associated Infection. Adv. Mater. 2024, 36, e2311855. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Clinical presentation and treatment of orthopaedic implant-associated infection. J. Intern. Med. 2014, 276, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Y.; Yin, C.; Dai, S.; Fan, X.; Jiang, Y.; Liu, X.; Fang, J.; Yi, B.; Zhou, Q.; et al. Recent Progress in antibacterial hydrogel coatings for targeting biofilm to prevent orthopedic implant-associated infections. Front. Microbiol. 2023, 14, 1343202. [Google Scholar] [CrossRef]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the balance: Antibiotic effects on host-microbiota mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface modification approaches for prevention of implant associated infections. Colloids Surf. B Biointerfaces 2020, 193, 111116. [Google Scholar] [CrossRef]
- Li, S.; Yue, Y.; Wang, W.; Han, M.; Wan, X.; Li, Q.; Chen, X.; Cao, J.; Zhang, Y.; Li, J.; et al. Ultrasound-Activated Probiotics Vesicles Coating for Titanium Implant Infections Through Bacterial Cuproptosis-Like Death and Immunoregulation. Adv. Mater. 2024, 36, e2405953. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yang, M.; Li, M.; Zhou, L.; Liu, Y.; Liu, L.; Zheng, Y. Comprehensive review of polyetheretherketone use in dentistry. J. Prosthodont. Res. 2025, 69, 215–232. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, A.; Bai, J.; Liao, Q.; Wu, Y.; Zhang, W.; Guan, M.; Tong, L.; Geng, D.; Zhao, X.; et al. A programmed surface on polyetheretherketone for sequentially dictating osteoimmunomodulation and bone regeneration to achieve ameliorative osseointegration under osteoporotic conditions. Bioact. Mater. 2022, 14, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, H.; Li, M.; Fu, J.; Dong, J.; Liu, Y.; Liu, L. Polyetheretherketone surface engineered with a degradable hybrid coating for accelerating osteogenesis. Mater. Lett. 2023, 331, 133515. [Google Scholar] [CrossRef]
- Liu, L.H.; Zhang, W.F.; Yuan, L.; Liu, Y.; Zheng, Y.Y. Ameliorative antibacterial, anti-inflammatory, and osteogenic activity of sulfonate-bearing polyetheretherketone toward orthopedic and dental implants. Mater. Lett. 2021, 305, 130774. [Google Scholar] [CrossRef]
- Chen, Y.; Ni, H.; Park, J.-J.; Lv, S. A Review of the Preparation, Modification, and Applications of Polyetheretherketone Coating. Coatings 2024, 14, 1451. [Google Scholar] [CrossRef]
- Yee, A.; Xin, X.; Liu, H.; Ma, L.; Cheng, K. Fabrication of PVTF Electroactive Coatings on PEEK Implant to Provide Surface Potential for Enhancing Osteogenesis. Coatings 2025, 15, 261. [Google Scholar] [CrossRef]
- Xiao, T.; Fan, L.; Liu, R.; Huang, X.; Wang, S.; Xiao, L.; Pang, Y.; Li, D.; Liu, J.; Min, Y. Fabrication of Dexamethasone-Loaded Dual-Metal-Organic Frameworks on Polyetheretherketone Implants with Bacteriostasis and Angiogenesis Properties for Promoting Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 50836–50850. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tan, X.; Zheng, L.; Tang, H.; Hu, S.; Zhai, Q.; Jing, X.; Liang, P.; Zhang, Y.; He, Q.; et al. A Dual-Antioxidative Coating on Transmucosal Component of Implant to Repair Connective Tissue Barrier for Treatment of Peri-Implantitis. Adv. Healthc. Mater. 2023, 12, e2301733. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, H.; Wang, S.; Zong, Y.; Dai, Q.; Wu, H.; Liang, K.; Yang, J. Mussel-inspired self-assembly platform for staged implant osseointegration: Combining early anti-infection and late osteoinduction. Mater. Design. 2023, 228, 111857. [Google Scholar] [CrossRef]
- Garner, S.J.; Dalby, M.J.; Nobbs, A.H.; Barbour, M.E. A novel chlorhexidine-hexametaphosphate coating for titanium with antibiofilm efficacy and stem cell cytocompatibility. J. Mater. Sci. Mater. Med. 2021, 32, 139. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Q.; Yang, Z.; Yang, W.; Wang, L.; Ma, J.; Fu, Y. An efficient approach to endow TiNbTaZr implant with osteogenic differentiation and antibacterial activity in vitro. Mater. Design. 2022, 221, 110987. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Zhang, Y.; He, H.; Xiong, S.; Chen, P.; Li, C.; Wang, L.; Lu, G.; Xu, Y. A dual-functional PEEK implant coating for anti-bacterial and accelerated osseointegration. Colloids Surf. B Biointerfaces 2023, 224, 113196. [Google Scholar] [CrossRef] [PubMed]
- Bozic Cvijan, B.; Korac Jacic, J.; Bajcetic, M. The Impact of Copper Ions on the Activity of Antibiotic Drugs. Molecules 2023, 28, 5133. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, L.; Zhou, J.; Chen, J.; Ma, Y.; Han, Y. Low-dose Cu ions assisted by mild thermal stimulus inducing bacterial cuproptosis-like death for antibiosis and biointegration. Adv. Funct. Mater. 2024, 34, 2308197. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Y.; Jin, M.; Yuan, Z.; Bond, P.; Guo, J. Both silver ions and silver nanoparticles facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes. Water Res. 2020, 169, 115229. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef]
- Jennings, J.A.; Carpenter, D.P.; Troxel, K.S.; Beenken, K.E.; Smeltzer, M.S.; Courtney, H.S.; Haggard, W.O. Novel antibiotic-loaded point-of-care implant coating inhibits biofilm. Clin. Orthop. Relat. Res. 2015, 473, 2270–2282. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lai, Y.; Guo, Y.; Cai, Z.; Mao, C.; Lu, M.; Ren, C.; Ong, J.L.; Chen, W. Aspirin/amoxicillin loaded chitosan microparticles and polydopamine modified titanium implants to combat infections and promote osteogenesis. Sci. Rep. 2024, 14, 7624. [Google Scholar] [CrossRef]
- Brogden, R.N.; Speight, T.M.; Avery, G.S. Minocycline: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 1975, 9, 251–291. [Google Scholar] [CrossRef]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef]
- Geng, Y.; Xue, H.; Zhang, Z.; Panayi, A.C.; Knoedler, S.; Zhou, W.; Mi, B.; Liu, G. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 2023, 305, 120555. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Zhao, L.; Mehwish, H.M.; Wu, Y.; Mahmood, S. Chitosan and its derivatives: Synthesis, biotechnological applications, and future challenges. Appl. Microbiol. Biotechnol. 2019, 103, 1557–1571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Li, M.; Wang, S.; Fu, J.; Yang, M.; Yan, C.; Liu, Y.; Zheng, Y. Dose-dependent enhancement of in vitro osteogenic activity on strontium-decorated polyetheretherketone. Sci. Rep. 2025, 15, 3063. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Shrestha, A.; Wang, S.; Wu, Q.; Li, L.; Guan, C.; Wang, C.; Fu, T.; Liu, W.; et al. Effects of Programmed Local Delivery from a Micro/Nano-Hierarchical Surface on Titanium Implant on Infection Clearance and Osteogenic Induction in an Infected Bone Defect. Adv. Healthc. Mater. 2019, 8, e1900002. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Zhou, H.; He, C.; Yang, X.; Fu, J.; Wang, H.; Liu, Y.; Zheng, Y. Surface bisphosphonation of polyetheretherketone to manipulate immune response for advanced osseointegration. Mater. Design. 2023, 232, 112151. [Google Scholar] [CrossRef]
- Li, X.; Yuan, S.; Chen, S.; Luo, R.; Xiong, K.; Yang, Z.; Wang, J.; Huang, N. Proliferation and functionality of human umbilical vein endothelial cells on angiopoietin-1 immobilized 316L stainless steel. J. Mater. Chem. B 2015, 3, 8717–8728. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Adsorption characteristics and mechanisms of O-Carboxymethyl chitosan on chalcopyrite and molybdenite. J. Colloid Interface Sci. 2019, 552, 659–670. [Google Scholar] [CrossRef]
- Nobles, K.; Janorkar, A.V.; Roach, M.D.; Walker, L.; Williamson, R.S. In Vitro Evaluation of Optimized PEEK Surfaces for Enhanced Osseointegration. Coatings 2024, 14, 518. [Google Scholar] [CrossRef]
- Liu, L.; Dong, J.; Zhang, W.; He, C.; Liu, Y.; Zheng, Y. The single-step fabrication of a poly (sodium vinylsulfonate)-grafted polyetheretherketone surface to ameliorate its osteogenic activity. Coatings 2022, 12, 868. [Google Scholar] [CrossRef]
- Ramsay, K.A.; McTavish, S.M.; Wardell, S.J.; Lamont, I.L. The effects of sub-inhibitory antibiotic concentrations on Pseudomonas aeruginosa: Reduced susceptibility due to mutations. Front Microbiol. 2021, 12, 789550. [Google Scholar] [CrossRef] [PubMed]
- Ching, C.; Zaman, M.H. Development and selection of low-level multi-drug resistance over an extended range of sub-inhibitory ciprofloxacin concentrations in Escherichia coli. Sci. Rep. 2020, 10, 8754. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57. [Google Scholar] [CrossRef]
- Qian, H.; Lei, T.; Hua, L.; Zhang, Y.; Wang, D.; Nan, J.; Liu, W.; Sun, Y.; Hu, Y.; Lei, P. Fabrication, bacteriostasis and osteointegration properties researches of the additively-manufactured porous tantalum scaffolds loading vancomycin. Bioact. Mater. 2023, 24, 450–462. [Google Scholar] [CrossRef]
- Shin, H.-J.; Yang, S.; Lim, Y. Antibiotic susceptibility of Staphylococcus aureus with different degrees of biofilm formation. J. Anal. Sci. Technol. 2021, 12, 41. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of Antibiotic Resistance Mechanisms in Gram-Negative Bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A review of the mechanisms that confer antibiotic resistance in pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Fernandez, L.; Hancock, R.E. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef]

| B.E. (eV) | Attribution | Relative Composition of Carbon Species (%) | ||
|---|---|---|---|---|
| PEEK-0.2Mino | PEEK-0.5Mino | PEEK-1.0Mino | ||
| 284.8 | C-C/C-H | 45.03 | 59.76 | 75.84 |
| 286.2 | C-O-C, C-OH, C-N | 42.72 | 24.48 | 13.85 |
| 287.8 | C=O, COOH, O=C-NH | 10.14 | 14.25 | 10.31 |
| 291.3 | π→π* | 2.11 | 1.51 | 0.00 |
| B.E. (eV) | Attribution | Relative Composition of Oxygen Species (%) | ||
|---|---|---|---|---|
| PEEK-0.2Mino | PEEK-0.5Mino | PEEK-1.0Mino | ||
| 531.1 | COOH, C=O, O=C-NH | 31.84 | 44.26 | 46.92 |
| 532.7 | C-O-C, C-O-H, bound H2O | 65.62 | 48.67 | 46.05 |
| 535.7 | gas phase H2O | 2.54 | 7.07 | 7.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, L.; Zhang, Y.; Yu, P.; Hu, Q.; Liu, Y.; Zheng, Y. Concentration-Optimized Minocycline-Modified Antimicrobial Coatings on Polyetheretherketone for the Prevention of Implant-Associated Infections. Coatings 2025, 15, 622. https://doi.org/10.3390/coatings15060622
Zhang Y, Zhang L, Zhang Y, Yu P, Hu Q, Liu Y, Zheng Y. Concentration-Optimized Minocycline-Modified Antimicrobial Coatings on Polyetheretherketone for the Prevention of Implant-Associated Infections. Coatings. 2025; 15(6):622. https://doi.org/10.3390/coatings15060622
Chicago/Turabian StyleZhang, Yongheng, Longyu Zhang, Yuehong Zhang, Pingping Yu, Qisheng Hu, Ying Liu, and Yanyan Zheng. 2025. "Concentration-Optimized Minocycline-Modified Antimicrobial Coatings on Polyetheretherketone for the Prevention of Implant-Associated Infections" Coatings 15, no. 6: 622. https://doi.org/10.3390/coatings15060622
APA StyleZhang, Y., Zhang, L., Zhang, Y., Yu, P., Hu, Q., Liu, Y., & Zheng, Y. (2025). Concentration-Optimized Minocycline-Modified Antimicrobial Coatings on Polyetheretherketone for the Prevention of Implant-Associated Infections. Coatings, 15(6), 622. https://doi.org/10.3390/coatings15060622







