1. Introduction
Earthen sites serve as invaluable resources for historical research, offering critical insights into human civilization across various periods. While computational technologies play a key role in digitally reconstructing cultural heritage [
1], the preservation of physical artifacts remains essential. Once exposed to the atmosphere, earthen sites quickly become colonized by microorganisms, leading to severe biodeterioration and irreversible damage to artifacts [
2]. Consequently, alongside advancements in digital preservation, the development of novel bioprotective materials to mitigate the biological degradation of physical artifacts is an essential component of conservation. Understanding the microbial community structure on earthen site surfaces is fundamental to identifying and combating microbe-induced deterioration, providing both scientific insights and guiding principles for the long-term preservation of these sites.
The primary factors contributing to biodeterioration in earthen sites include atmospheric conditions conducive to fungal growth, particularly in humid environments such as tropical climates, where the high moisture content promotes the development of biofilms and plant growth on monuments and structures [
3]. Consistently high temperatures and an elevated relative humidity, as observed in Cuba, accelerate fungal proliferation, leading to the biodeterioration of artifacts housed in museums [
4]. Furthermore, the presence of abundant groundwater at archeological sites, coupled with the inherent moisture content of the earthen materials, creates a high-temperature, high-humidity environment, especially in poorly ventilated indoor sites or outdoor sites subject to fluctuating temperatures and humidity. This environment is ideal for the growth of microorganisms like mosses and molds [
5,
6,
7,
8,
9]. The composition and structure of the archeological artifacts themselves also provide suitable substrates for microbial colonization and proliferation [
10]. Elevated levels of soluble salts within the earthen site, coupled with fluctuating temperatures and humidity, can cause salt efflorescence on the surface of the earthen materials [
11], which is a major cause of weathering in earthen structures and artifacts. In addition, the pH and the presence of certain minerals within the earthen site also serve as significant drivers for microbial growth [
12].
Researchers worldwide have investigated numerous archeologically significant sites that are affected by biodeterioration to inhibit microbial growth. The bactericidal mechanisms of microbial inhibitors primarily rely on disrupting cellular metabolic processes, thereby impairing physiological functions and ultimately leading to cell death [
13]. Based on their modes of action, these agents can be broadly classified into oxidizing and non-oxidizing types. Oxidizing agents such as hydrogen peroxide, halogens (e.g., chlorine and bromine), and ozone are represented by their ability to release reactive oxygen species, which damage microbial structures. Although these agents exhibit significant bactericidal effects, their application is limited. In contrast, non-oxidizing agents, including quaternary ammonium compounds, acrolein and its derivatives, and aldehyde amines, which interfere with microbial metabolism or disrupt cell membranes, are more widely employed [
14,
15]. These non-oxidizing compounds can be further categorized by their chemical structure into organic biocides—such as chlorine-containing compounds (e.g., sodium hypochlorite) and iodine-containing compounds (e.g., povidone-iodine)—and inorganic biocides, which include phenols (e.g., phenol), aldehydes (e.g., formaldehyde), and quaternary ammonium salts (e.g., benzalkonium chloride) [
16].
The Sanyangzhuang site, a Han Dynasty settlement discovered in 2003 in Henan Province [
17], exhibits a high-salinity and high-humidity environment due to its location on an ancient Yellow River course. This setting has been led to significant biodeterioration, with mosses and molds extensively colonizing the surface of the site, as evidenced by field investigations (
Figure 1A–C). This microbial growth threatens the site’s preservation by contaminating artifacts, obscuring archeological features, and accelerating corrosion through the production of acidic byproducts [
18]. Furthermore, microbial activity generates pigments, impacting the appearance of the site and diminishing its artistic and historical value [
19,
20].
This research focuses on the conservation of the Sanyangzhuang site, emphasizing the need for future research and public access. The protective materials must be colorless, transparent, strong, chemically inert with the soil, highly resistant to aging, and safe to apply [
21,
22]. To address the site’s high salinity and humidity and to quickly control moss and fungal growth, we developed a FACA tri-action biocide. This formulation is designed to rapidly eliminate fungi, moss, lichens, and ants without causing pollution, thus preventing further damage to the earthen site and preserving the authenticity of its artifacts. This approach effectively mitigates most biological and microbial threats, promoting a harmonious archeological ecosystem.
2. Materials and Methods
2.1. Reagents and Instruments
Reagents: (1) Diluents: ultrapure water, anhydrous ethanol, acetone, propylene glycol, polyethylene glycol, etc. (2) Sodium hydroxide solution: 40 mg·mL−1. (3) Hydrochloric acid solution: 1:11 dilution of concentrated HCl. (4) Algal culture medium: BG-11. (5) FACA: dissolved and diluted in sterile ultrapure water to prepare stock solutions of 5.0 mg·mL−1 and 0.5 mg·mL−1 for immediate use. (6) Mold culture medium: potato dextrose agar (PDA).
Instruments: (1) Spectrophotometer (C9405CC). (2) Cuvette. (3) pH measurement devices: digital pH meter (range: 0–14.00 pH, accuracy: ±0.02 pH) and precision pH test strips (range: 6.4–8.0). (4) Laboratory centrifuge (operational range: 2000–5000 rpm). (5) Test tubes. (6) Hemocytometer (for cell counting). (7) Microporous filter membranes (Material: cellulose acetate or nitrocellulose; diameter: 50 mm; pore sizes: 0.22 × 10−3 mm, 0.45 × 10−3 mm, and 0.65 × 10−3 mm). Preparation: membranes were immersed in distilled water and sterilized by autoclaving at 121 ± 1 °C for 20 min, then cooled and stored aseptically.
2.2. The Bactericidal Mechanism of the FACA
To effectively control fungal contamination at archeological sites, it is crucial to first identify the specific fungal species present and then screen for the most effective antifungal agent through drug sensitivity testing. At the Sanyangzhuang site, we developed FACA, a novel tri-action biocide. This formulation primarily comprises a broad-spectrum, highly effective biocide that combines Phenylcarbamoylthiazoles and isothiaquinolones [
23,
24]. The optimal ratio of the two types of drugs is 2:5. Phenylcarbamoylthiazoles is a kind of biocide that is used abroad and is relatively new in China. Its biocide effect exceeds that of existing similar domestic products. FACA mainly exerts its bactericidal action by breaking the bonds of proteins in bacteria and algae. Once FACA comes into contact with microorganisms, it can rapidly inhibit their growth. This inhibitory effect is irreversible, leading to the death of microbial cells. Before the death of microbial cells, microorganisms treated with FACA can no longer synthesize enzymes or secrete adhesive substances.
The inhibitory effect of succinate dehydrogenase was enhanced by regulating and replacing the heterocyclic electron-absorbing groups of Phenylcarbamoylthiazoles. Nano-preparations suitable for stabilizing and enhancing drug efficacy were prepared using nanotechnology. Meanwhile, they could rapidly penetrate to the soil surface [
25,
26], act on smaller microbial communities, cause rapid depolymerization, and prevent the formation of biofilms [
27]. Active molecules can rapidly penetrate the cell membrane and act on protein groups, thereby terminating the normal redox process of the cell. For systems that have already formed biofilms, their active components do not react with the slime layer in the biofilm but rapidly penetrate deeper into the biofilm, acting on the microbial community at the junction of the biofilm and the soil surface, destroying its viscosity and causing the biofilm to fall off. The sterilization speed is fast, achieving a sterilization rate of 99.8% within three minutes. After completing its bactericidal effect, it can rapidly degrade into carbon dioxide and other biofriendly substances without causing the accumulation of harmful ions in the soil. It is colorless, odorless, and has no impact on the environment.
2.3. Substrates
This study was conducted at the Sanyangzhuang Han Dynasty settlement site, located north of Sanyangzhuang Village, Liangzhuang Town, Neihuang County, Henan Province. The stratigraphic sequence of the area comprises quaternary light yellow and gray clay layers, along with fine sandy silt and gravel layers, representing unconsolidated, loose sediments of varying thicknesses of approximately 7–10 m. Neihuang County lies within the former course of the Yellow River and has been subjected to multiple breaches of the Yellow, Wei, and Zhang rivers. Consequently, the parent materials of the soil were classified into two main categories: alluvial and aeolian deposits.
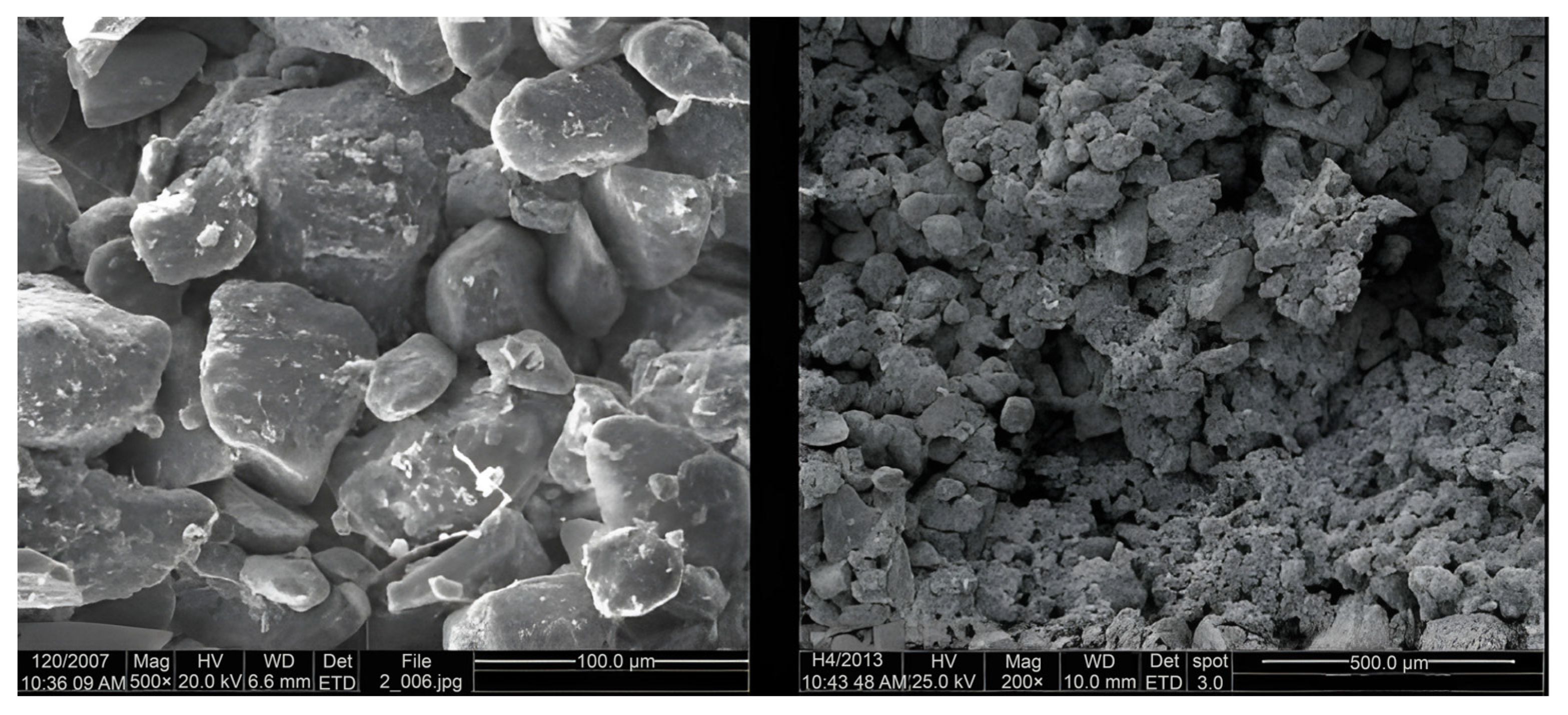
X-ray diffraction analysis was performed on the crystalline structure of the soil samples.
Figure 2 presents electron microscope images of the soil sample from the Sanyangzhuang site, revealing numerous pores within the soil structure and a relatively loose accumulation of mineral grains.
The ionic composition and concentration of salts in the Sanyangzhuang site soil samples were analyzed using ion chromatography.
Table 1 and
Table 2 present the results of the analysis. The analysis indicates that the soil from the archeological site is predominantly anionic, with sulfate being the most abundant element. Calcium and sodium ions are the most prevalent cations. Overall, the soil exhibits a high concentration of soluble salts.
Elevated levels of soluble salts within the earthen matrix, coupled with fluctuating temperature and humidity, can cause salt efflorescence on the surface of the earthen material [
28,
29].
2.4. Microbial Identification and Analysis
The microbial community comprises a complex ecosystem. It encompasses algae, fungi, lichens, bacteria, and protozoa, along with small animals such as mites and lower and higher plants [
18,
30]. The removal of epibionts from river embankments is essentially the elimination of several types of plants and microorganisms, including epiphytic molds, algae, mosses, and lichens.
In order to understand the types of mold in the Sanyangzhuang site, from April 2020 to March 2021, sampling and analyses were conducted in the four different seasons: spring, summer, autumn, and winter. The air in the No. 2 Courtyard protection hall of the Sanyangzhuang site and the soil surfaces in different areas were taken as objects. After multiple transformations and purifications, the morphological characteristic PCR technology was adopted to analyze and study the isolated molds (pollution sources) [
31]. Finally, 10 fungi with uniform morphology, stable genetics, and no pollution were obtained as the strains for the fungicidal experiment. Moreover, in this experiment, the representative eukaryotic Chlorella vulgaris and Anabaena variabilis were used as the experimental organisms for the algal killing experiments (
Table 3).
2.5. Preservation and Cultivation of Strains
- (1)
Preservation and cultivation of algae strains
After obtaining pure algal strains from the Institute of Hydrobiology, Chinese Academy of Sciences, the algal strains were preserved as required for use in the experiments. The algal strains can be preserved on the inclined surface of the solid culture medium in the test tube. Add 0.8% agar to the culture medium, sterilize it, pour it into test tubes, cool it to form an inclined surface, and then inoculate the algae and seal it with cotton plugs. It can be preserved for a relatively long time under lower light and temperature conditions. It should be transferred approximately every two months. If experiments are conducted frequently, the reserve cultures should be stored in liquid culture media. Add approximately 100 mL of the culture medium to a 250 mL conical flask, inoculate the algae, and cultivate under the same temperature and light conditions as required by the experiment. Transfer once a week to ensure the culture grows well and there is always a sufficient quantity available for the experiment. For fast-growing algae, the inoculation amount should be 1% of the concentration of algae cells before transfer. Generally, transfer should be carried out before the algae enter the growth arrest period.
- (2)
Preservation and cultivation of mold strains
After obtaining the pure strains, preserve the strains as required. The preservation of bacteria can adopt the bran preservation method, using bran as a carrier to adsorb the inoculated spores, and then storing them under low-temperature and dry conditions. The production method is to mix bran and water in a certain ratio of 1:1.2 according to the different water requirements of different strains. The filling volume is 2/5 of the test tube volume. After moist heat sterilization, cool it and then inoculate it with freshly cultivated strains. Then, incubate it in a constant temperature box at 30 ± 1 °C for 60–72 h until the spores are fully developed. Place the test tube in a desiccant containing calcium chloride and other desiccants, dry it at room temperature for several days, and then transfer it to a low-temperature storage place for preservation. After drying, the test tubes can also be sealed with a flame and then stored.
3. Evaluation of Bactericidal Effectiveness
3.1. Minimum Algae Killing Concentration Test (Paper Slice Method)
The BG-11 agar plates were prepared under sterility conditions. After cooling, 0.2 mL of the test algal solution was inoculated, evenly spread, and allowed to stand for 10 min. A gradient of FACA (0.5–10 × 10−3 mg·mL−1) was prepared. Filter paper disks were prepared using a 6 mm punch, autoclaved (121 °C, 20 min), and dried. The filter paper disks were immersed in different concentrations of FACA, and after removing excess liquid, they were placed on the surface of the agar plates (four disks per plate, placed at intervals). Filter paper disks treated with sterile water served as the control. Each concentration was tested in triplicate. After 2–5 days of incubation, the algal inhibition zone diameter was measured to assess algal inhibitory activity.
3.2. Minimum Algaecidal Concentration Test (Oscillation Method)
In a 250 mL conical flask, 100 mL of BG-11 medium was added and autoclaved at 121 °C for 30 min after sealing. Inoculation was performed after UV irradiation on an ultra-clean workbench. Based on the results presented in
Section 3.1, a 10,000 × 10
−3 mg·mL
−1 FACA was prepared and sterile-filtered through a microporous membrane. Subsequently, 0.005 mL, 0.01 mL, 0.02 mL, 0.05 mL, and 0.1 mL of the stock solution were added to the medium to create a concentration gradient of 0.5–10 × 10
−3 mg·mL
−1, with a control group lacking the algicide. Each group was set up in triplicate, with 5 mL of algal solution inoculated into each flask and cultured for 2–5 d. The color change in the culture solution was recorded every 24 h to determine the minimum inhibitory concentration (MIC).
3.3. Algaecide Performance Testing
- (1)
Contact Mixing
The prepared algal solution was transferred to a 1000 mL beaker, and the FACA of the appropriate concentration was added. The mixture was stirred according to the following procedure: 50 rpm (2 min), 200 rpm (2 min), and 50 rpm (10 min
−2 h). After 30 min of static incubation (neutralizing agent added, if necessary), a 25 mL sample was taken, vigorously shaken, and the chlorophyll content was determined. Chlorophyll extraction methods are commonly used to determine the quantity of algae in biofilms [
28,
29,
30], and the removal rate was calculated to assess the algicidal effect.
- (2)
Determination of Biomass of Test Algae (Chlorophyll a)
In an ultra-clean workbench, 10 mL of the uniformly shaken algal solution was placed in a 10 mL centrifuge tube and filtered through a 0.45 × 10−3 mm microporous membrane, and the filter paper was returned to the centrifuge tube and frozen for ≥12 h. Ninety-percent ethanol was preheated to 80 °C and rapidly added to the filter paper, and the mixture was sonicated for 10 min (protected from light) in a 2 min water bath, followed by static extraction for 2–6 h. After filtration through a 0.22 × 10−3 mm microporous membrane, the volume was brought to 10 mL with 90% ethanol.
The OD
665 and OD
750 were measured, and after adding 1 mol/L HCl, OD
665, and OD
750, they were measured again. Chlorophyll a content was calculated, and the chlorophyll a concentration was calculated using the following formula [
32,
33]:
In this context, Chla denotes the concentration of chlorophyll a (mg/L), OD665 represents the absorbance at 665 nm, OD750 signifies the absorbance at 750 nm, OD665a indicates the acidified absorbance at 665 nm, OD750a represents the acidified absorbance at 775 nm, Vethanol is the volume of the ethanol extract (mL), and Vsample′ is the volume of the sample taken (mL).
- (3)
Calculation of algaecide concentration
The inhibition rate of the algaecide is given by Equation (2):
where IR indicates the algal inhibition rate. OD
tn represents the absorbance of the treatment group on the nth day and OD
t0 denotes the initial absorbance of the treatment group. OD
cn signifies the absorbance of the control group on the nth day and OD
c0 is the initial absorbance of the control group. The calculated IR values, correlated with the algicide concentrations, were used to assess algicidal performance, thereby informing field applications.
3.4. Minimum Mold Concentration Detection
Potato dextrose agar (PDA) plates were prepared under sterile conditions. After cooling, 0.2 mL of the spore suspension was added and evenly spread. The plates were left to stand for 10 min. A gradient of FACA (0.5–10 × 10−3 mg·mL−1) was prepared and sterilized at 121 °C for 20 min, followed by drying. The filter paper disks were soaked in the FACA, and the excess liquid was removed. The disks were then placed on the plates (four disks per plate), with sterile water used as a control. Three replicates were used for each concentration. After incubation for 2–5 days, the diameters of the inhibition zones were measured to assess the antifungal activity of the FACA.
3.5. Minimum Inhibitory Concentration Test for Mold Growth
Dissolve 15 mL of nutrient agar (45–48 °C) in a water bath and maintain it in a reserve. Inoculate with 1 mL of mixed spores, mix thoroughly, and then add 1 mL of the test solution to a gradient. Pour the mixture into Petri dishes to form a second agar layer and spread evenly by rotation. Incubate it in a 28 °C mold incubator and regularly observe the mold growth. A blank control was set up and the minimum inhibitory concentration (MIC) at which no spore growth occurred was determined. Three replicates were used for each concentration. Mold growth grading was set up as 0 grade (no growth), 1 grade (initial growth), or 2 grade (obvious growth and sporulation).
3.6. Detection of Mold-Killing Performance
A 200 mL suspension of fungal spores was introduced into a 500 mL Erlenmeyer flask, followed by the addition of FACA according to the protocol outlined in
Section 3.4 and
Section 3.5. The mixture was then incubated on a rotary shaker at 150 r/min for a duration ranging from 10 min to 2 h. At regular intervals, 2 mL samples were collected and serially diluted with sterile saline. Subsequently, 0.2 mL aliquots were spread onto PDA plates and incubated at 28 °C. Fungal growth was monitored to assess the efficacy of the FACA in eliminating fungi.
4. Result and Discussions
4.1. Effect on the Algal Suppression Zone of the Two Species of Algae Tested
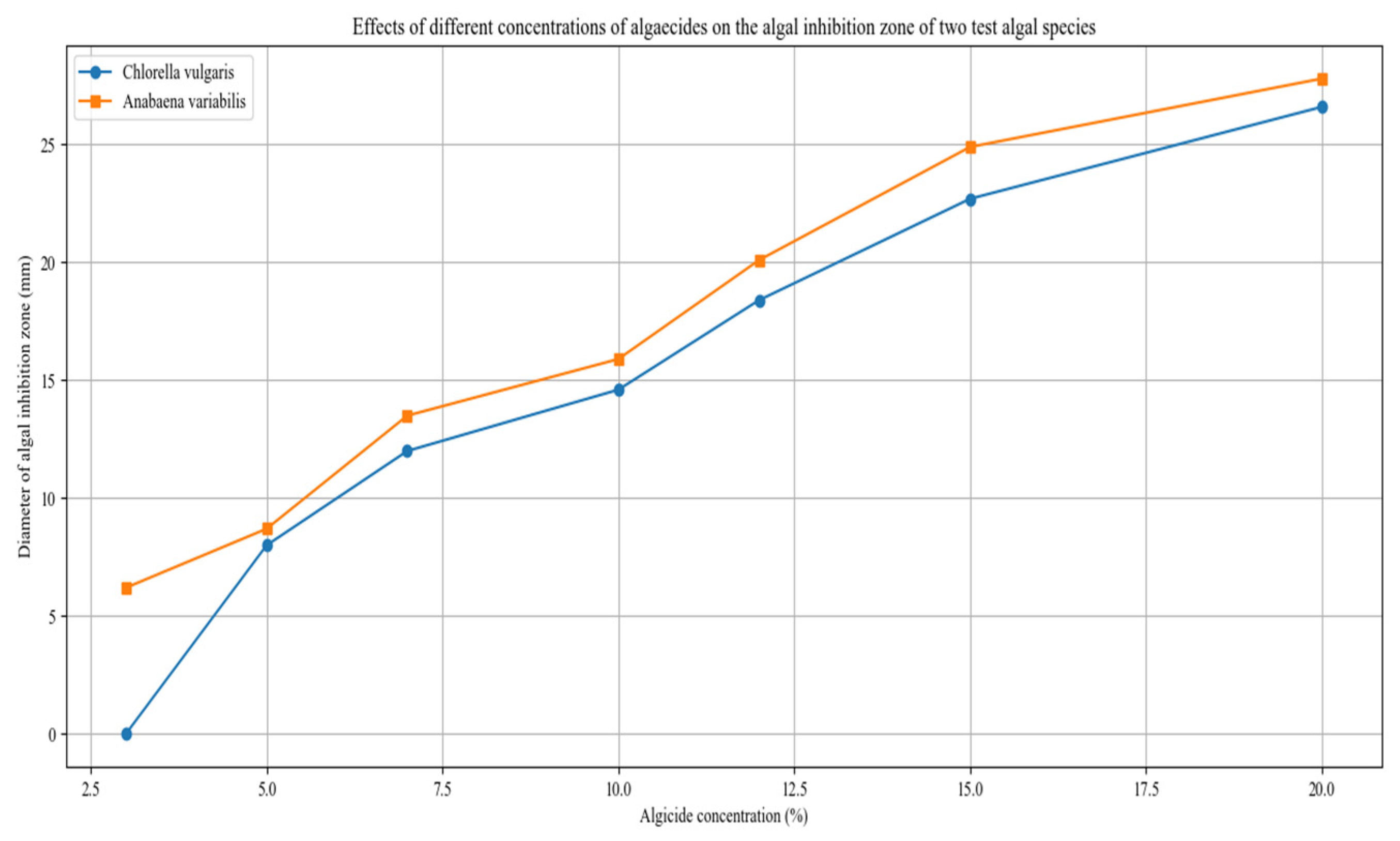
Figure 3 shows that increasing the concentration of the algicide leads to a larger algal inhibition zone. After 5 days of treatment, the maximum inhibition zone for Chlorella vulgaris measured 26.6 mm, while that for Anabaena variabilis reached 27.8 mm. These findings indicate that the algicidal effect becomes significant when the algicide concentration exceeds 15%.
4.2. Effect on the Growth of the Two Kinds of Algae Tested
Table 4 demonstrates that for Chlorella vulgaris, algal growth is uninhibited at algicide concentrations below 5%. Slight growth is observed when concentrations range between 5% and 13%, whereas growth ceases above 13%, accompanied by a yellowing of the culture medium. In contrast, Anabaena variabilis exhibits robust growth at concentrations below 7%, shows limited growth between 7% and 13%, and experiences mortality at concentrations above 15%, also with a noticeable yellowing of the culture medium. Based on the combined data for both algal species, the minimum inhibitory concentration (MIC) was determined to be 15%.
4.3. Results of Algae Killing Test
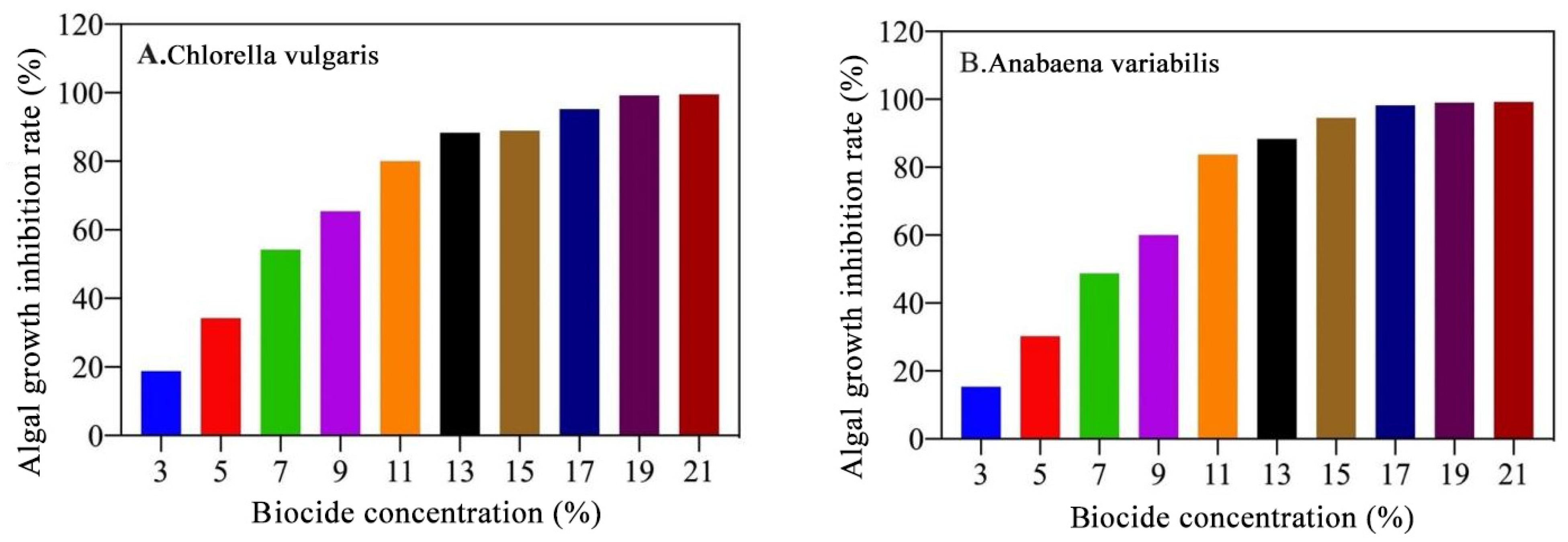
Based on the test results of the algae-inhibiting circle and the minimum algae-inhibiting concentration, the algae-inhibiting rate determination test was carried out. As shown in
Figure 4, when the algicide concentration exceeds 15%, chlorophyll extraction analysis reveals an algae-killing rate of over 90%. This indicates that a concentration above 15% is effective for removing algae in cases of biological fouling.
The rapid death of algae observed through the degradation of chlorophyll a indicates that FACA disrupts the photosynthetic pathway through protein denaturation or membrane destruction. Based on the above algaecidal experimental results and by comparing the bactericidal concentrations of domestic and foreign fungicides, it was found that FACA can directly exert a good inhibitory effect on algae at a concentration of 20% to 30%, which represents a relatively low concentration.
4.4. Effect on the Inhibition Circle of 10 Tested Molds
Using the key strains identified in
Section 2.4 as target organisms, we evaluated the effect of various fungicide concentrations on mold growth by measuring the inhibition zones across ten tested strains. The results show that mold development is significantly inhibited at concentrations ranging from 3% to 20%. Moreover, as the concentration of FACA increases, the diameter of the mold inhibition zones gradually expands, demonstrating enhanced antifungal efficacy. With the increase in culture time, the inhibition zone of the high-concentration biocide continued to grow. In the low-concentration biocide FACA (3%~10%), the fungicidal effect on P.v and P.c was the most significant. In the high-concentration biocide FACA (12%~20%), the fungicidal effect on R.s, A.n, and P.c was significant. In conclusion, as the concentration of FACA increased, it continuously exerted inhibitory effects on the 10 tested molds.
4.5. Effects on the Growth of 10 Kinds of Tested Molds
The impact of varying concentrations of fungicide FACA on the growth of ten tested fungal strains is presented in
Table 5. The results indicate that at fungicide FACA concentrations ranging from 3% to 7%, all ten fungal strains exhibited continued growth, albeit with a reduced growth rate compared to the control group. At a concentration of 12%, no growth was observed in any of the ten fungal strains relative to the control group. A comprehensive analysis suggests that the minimum inhibitory concentration (MIC) of the fungicide against the tested fungal strains is 12%, demonstrating a significant inhibitory effect.
4.6. Effect of Fungicide
The concentration gradient of the fungicidal performance test was determined according to the minimum inhibitory concentration in
Table 6, and the representatives F.c and R.s variabilis were selected as the test molds. As demonstrated in
Table 7, both the concentration and exposure duration of the fungicide significantly influenced the growth inhibition of F.c. The higher the concentration, the longer the action time and the more obvious the inhibitory effect of the fungicide on F.c. When the concentration of the fungicide was 20%, mold growth was completely inhibited even after 10 min.
When the concentration and action time of the fungicides were similar to those of F.c, the higher the concentration, the longer the action time, and the more obvious the inhibitory effect of fungicides on R.s. When the concentration of the fungicide was 15%, the growth of mold was completely inhibited even after 30 min. Considering the two kinds of mold, it is recommended to use a 20% concentration and 10 min treatment time (
Table 8).
Therefore, low concentrations (3%–10%) can slow down the growth of fungi but cannot prevent spore production. A 12% FACA was the lowest fungicidal concentration (MFC), completely inhibiting all the fungi in the subjects. A percentage of 20% of FACA achieved instant sterilization (within 10 min), which is crucial for rapid field applications.
4.7. Comparative Efficacy of FACA Against Algae and Fungi
Algae require a higher concentration (15%) to be completely inhibited, while fungi need a concentration of 12% to be eradicated. This difference may stem from cell wall composition, fungi contain chitin, and the fact that FACA is more easily penetrated. In terms of metabolic vulnerability, algae rely on photosynthesis and may temporarily recover at sublethal doses.
Based on the above conclusions, it can be concluded that FACA can kill a wide variety of algae, fungi, and bacteria in the circulating water system. Its usage concentration is low. Compared with existing biocides, its efficacy exceeds 30% 0.5 × 10−3 mg·mL−1 and has a very good bactericidal and algacidal effect. In addition, it shows a fast speed of sterilization and algae removal, taking effect within 10 min.
4.8. Field Evaluation
4.8.1. Moss Removal Critical Processes
The removal of moss, as documented in the literature, primarily involves two methods: manual abrasion, which is straightforward but often incomplete, leading to moss regrowth and potential damage to the earthen sites, and chemical treatment, which typically employs aqueous solutions of ammonium sulfate or specific fungicides. Inorganic materials, such as copper sulfate, have also been utilized. However, in the context of earthen site preservation, the inherent fragility of materials necessitates careful consideration. Water-based solvents can compromise the granular structure of the soil and hinder the preservation of the site’s original appearance. Consequently, conventional inorganic treatments have been excluded from this project. Ethanol was selected as the solvent due to its rapid volatility, non-toxicity, non-polluting nature, and its minimal impact on the soil’s aggregate structure. The FACA tri-action biocide was chosen as the primary treatment for moss control in the wetland environment of earthen sites. The treatment should proceed systematically, from one side of the archeological site to the other. Initially, a soft brush or sponge should be used to gently remove the moss. A 20% concentration of FACA tri-action biocide, using ethanol as a solvent, should be prepared. This solution should be placed in a 2500 mL spray bottle and applied evenly to the surface of the moss substrate in a mist form. After the solvent evaporates and the remaining moss withers, the surface moss should be carefully removed with a brush to achieve both treatment and preventative goals.
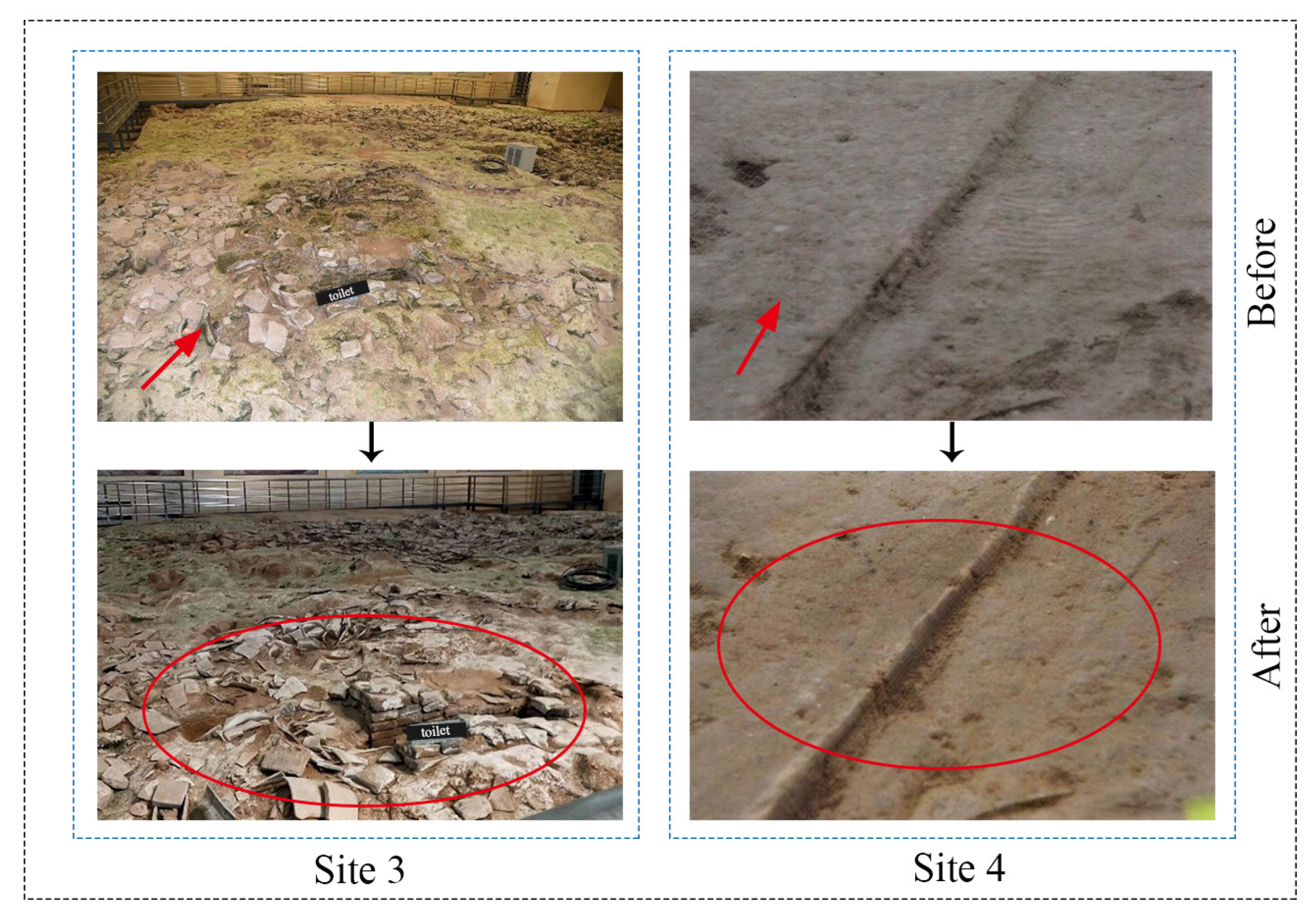
Following the comprehensive remediation of the Sanyangzhuang site’s exterior field area, the selection criteria were as follows: the area was covered with green moss and weeds. After eradication, the biological corrosion phenomena ceased, moss growth and propagation were halted, and the area was clearly revealed, preserving its original appearance during excavation (
Figure 5).
4.8.2. Critical Processes for Mold Removal
The control of biodeterioration is achieved through physical and chemical methodologies. Chemical treatments, whether broad- or narrow-spectrum, are more frequently employed in heritage conservation than physical or mechanical interventions [
32,
34,
35,
36]. Based on the analysis and identification of on-site soil characteristics and fungal species, a comprehensive management strategy combining physical and chemical methods was adopted in this study. Ethanol, characterized by its rapid volatility, non-toxicity, non-polluting nature, and lack of impact on soil aggregate structure, is utilized as a solvent for solution spraying to eliminate fungi. In parallel, fungal proliferation was further inhibited by modifying the site’s temperature and humidity. For instance, ventilation fans were installed within the site’s passageways to disrupt the warm, humid conditions preferred by the fungi, thereby preventing an environment conducive to their propagation.
Based on the deterioration analysis, fungal identification, and causal investigations, a specific treatment protocol was devised. The process involves mixing ethanol with FACA and water in a ratio of 1:2:2. This solution is then loaded into a 2500 mL spray bottle and applied evenly to the affected earthen surfaces in a fine mist. After the solvent evaporates and the fungi desiccate, the residual matter is gently removed with a soft brush. To enhance its preventative measures, a second application, following the same ratio, is scheduled one week later, thereby integrating both eradication and prevention strategies.
Employing the methodologies and materials outlined, the experimental archeological sample block from the Sanyangzhuang site underwent remediation to eliminate the proliferation of mold. This process resulted in the complete removal of the efflorescence present on the surface, effectively inhibiting further mold growth. Consequently, the exposed archeological remains are now clearly visible, preserving the site’s original appearance as it was during excavation (
Figure 6).
4.8.3. Site Construction Effect
Based on the current condition of the Sanyangzhuang site and the results of the previous year’s field trials, we will continue to implement in situ treatment using a rotational application of the FACA tri-action biocide. This involves the large-scale spraying of the surface of the site, which exhibits significant biological deterioration, including mold, insect infestations, and the presence of biofilms. Following a ten-day biocide application, biological damage was successfully mitigated. The accompanying figures illustrate the efficacy of the fungicide, bactericide, and insecticide treatments (
Figure 7).
5. Conclusions
Microbial species isolated from distinct waterlogged archeological sites differ, owing to variations in their geographical context and climatic conditions. Microbial purification techniques enabled the successful extraction and identification of key fungal strains associated with deterioration at the Sanyangzhuang site, including Rhizopus, Mucor, Aspergillus, and Penicillium. Antifungal efficacy was assessed using quantitative inhibition assays. Standardized spore suspensions were prepared, and the performance of the antifungal agents was evaluated using both disk diffusion and agar incorporation methods. Our research indicates that the FACA tri-action biocide exhibits robust inhibitory effects against key fungal agents responsible for the deterioration of archeological sites. The FACA tri-action biocide utilized is a biodegradable compound that does not produce foam and does not affect heat transfer efficiency. The complementary bactericides and algaecides employed in the treatment are odorless, environmentally and artifact-friendly, efficient, broad-spectrum, economical, and practical. For instance, the FACA formulation was applied as a protective material over a 1000 m2 area of the Sanyangzhuang earthen site. After one year of field trials, the treatment outcomes were outstanding, achieving a bactericidal rate of 99.8%, a moss removal rate of 99.8%, and a fungal inhibition rate of 99.8%, with no recurrence observed. Through indoor and on-site tests, it was verified that FACA has a fast sterilization speed, taking effect within 10 min. Moreover, it is used at a low concentration with an efficacy exceeding 30%. Even 0.5 mg/L has a very good sterilization and algaecidal effect.
Future research should explore the combination of FACA with other antimicrobial agents to enhance the bactericidal efficacy of the composite antimicrobial agent and broaden its antimicrobial spectrum. Additionally, expanding the scope of drug screening and optimizing the component ratios of the composite agent will be critical for determining the optimal formulation. These efforts will provide valuable references for the protection and conservation of similar earthen sites in the Central Plain region.