Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GLDA-LDH
2.3. Kinetics
2.4. Factors Affecting Adsorption
2.5. Mixing Dynamics
2.6. Soil Adsorption
2.7. Characterization Methods
3. Results and Discussion
3.1. Characterization of GLDA-LDH
3.2. Adsorption Tests
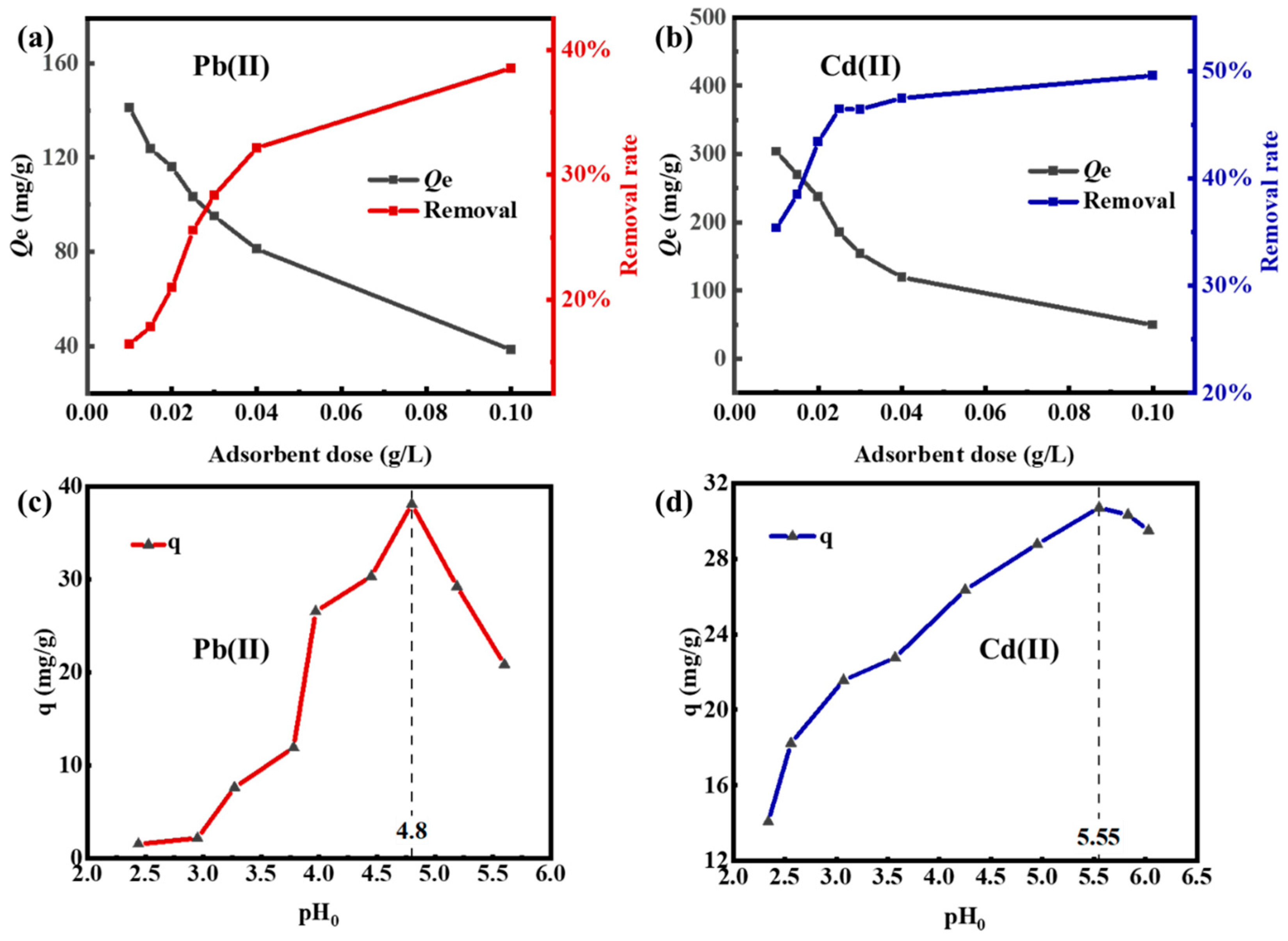
3.2.1. Effect of Adsorbent Dose and Initial pH
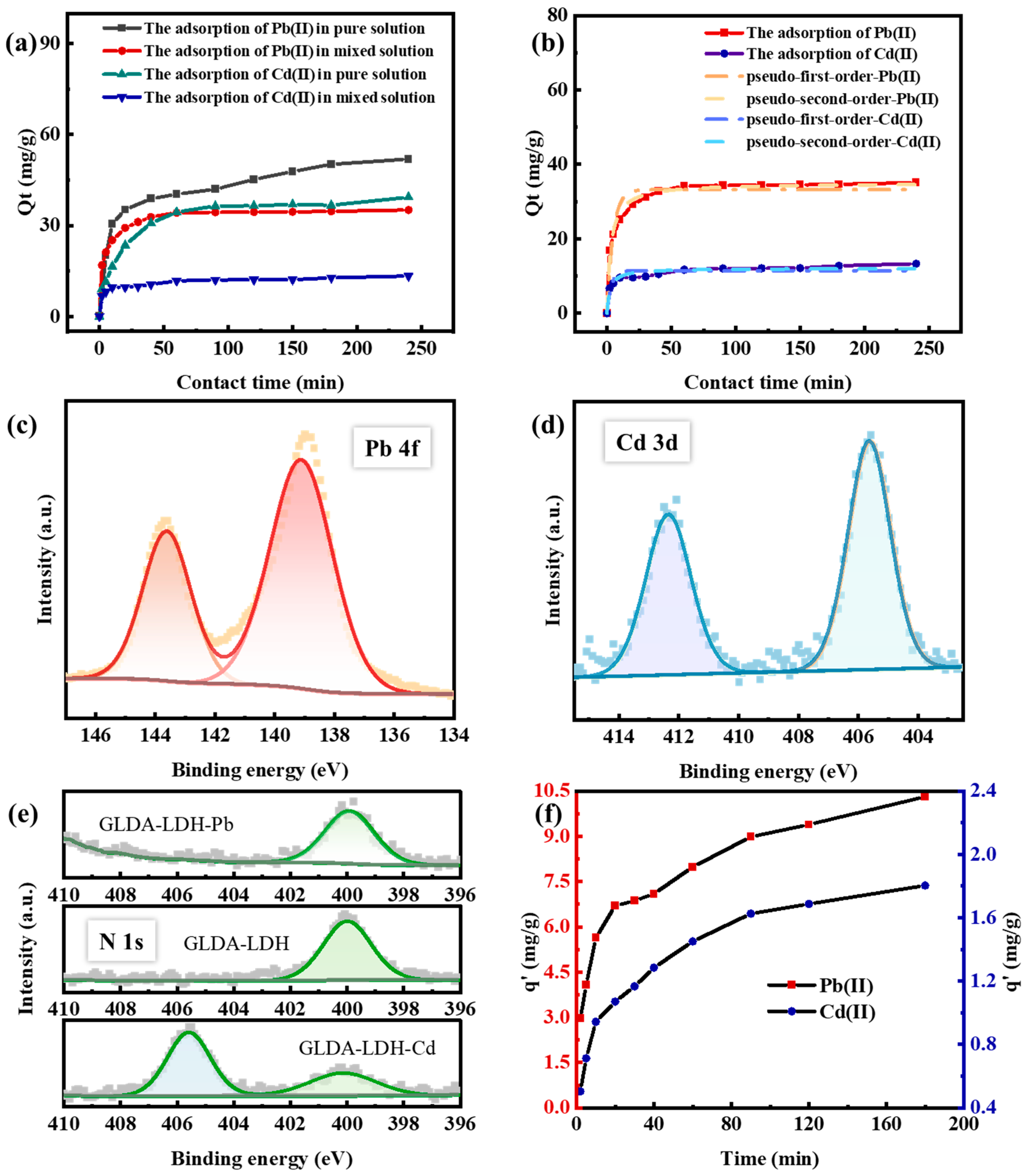
3.2.2. Effect of Contact Time and Kinetic Analysis
3.2.3. Adsorption Isotherm
3.2.4. Competitive Adsorption and Soil Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Hafsteinsdóttir, E.G.; Camenzuli, D.; Rocavert, A.L.; Walworth, J.; Gore, D.B. Chemical immobilization of metals and metalloids by phosphates. Appl. Geochem. 2015, 59, 47–62. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, D. Synthesis and characterization of a new class of stabilized apatite nanoparticles and applying the particles to in situ Pb immobilization in a fire-range soil. Chemosphere 2013, 91, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UIO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Zang, L.; Zhao, Y.; Wei, Q.; Han, J. Removal of Pb from contaminated kaolin by pulsed electrochemical treatment coupled with a permeable reactive barrier: Tuning removal efficiency and energy consumption. Toxics 2023, 11, 961. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Shahrokhi-Shahraki, R.; Benally, C.; El-Din, M.G.; Park, J. High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 2021, 264, 128455. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Abou El-Reash, Y.G.; Youssef, H.M.; Kotp, Y.H.; Hegazey, R.M. Utilization of rice husk and waste aluminum cans for the synthesis of some nanosized zeolite, zeolite/zeolite, and geopolymer/zeolite products for the efficient removal of Co(II), Cu(II), and Zn(II) ions from aqueous media. J. Hazard. Mater. 2021, 401, 123813. [Google Scholar] [CrossRef]
- Xiong, J.; Zhou, M.; Qu, C.; Yu, D.; Chen, C.; Wang, M.; Tan, W. Quantitative analysis of Pb adsorption on sulfhydryl-modified biochar. Biochar 2021, 3, 37–49. [Google Scholar] [CrossRef]
- Zeng, Q.; Huang, Y.; Huang, L.; Hu, L.; Sun, W.; Zhong, H.; He, Z. High adsorption capacity and super selectivity for Pb(II) by a novel adsorbent: Nano humboldtine/almandine composite prepared from natural almandine. Chemosphere 2020, 253, 126650. [Google Scholar] [CrossRef]
- Zhong, J.; Zhou, J.; Xiao, M.; Liu, J.; Shen, J.; Liu, J.; Ren, S. Design and syntheses of functionalized copper-based MOFs and its adsorption behavior for Pb(II). Chin. Chem. Lett. 2022, 33, 973–978. [Google Scholar] [CrossRef]
- Dai, C.; Wu, X.; Wang, Q.; Bai, Y.; Zhao, D.; Fu, J.; Fu, B.; Ding, H. Layered double hydroxides for efficient treatment of heavy metals and organic pollutants: Recent progress and future perspectives. Sep. Purif. Technol. 2025, 352, 128277. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Abdul Aziz, H.; Azmier Ahmad, M.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Lu, Z.; Qian, L.; Tian, Y.; Li, Y.; Sun, X.; Duan, X. Ternary NiFeMn layered double hydroxides as highly-efficient oxygen evolution catalysts. Chem. Commun. 2016, 52, 908–911. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Ouyang, X.F.; Dang, Z.; Yin, H. Acetate anions intercalated Fe/Mg-layered double hydroxides modified biochar for efficient adsorption of anionic and cationic heavy metal ions from polluted water. Chemosphere 2024, 362, 142652. [Google Scholar] [CrossRef]
- Alhammadi, Y.; Hai, A.; Taher, H.; Banat, F.; AlMarzooqi, F. Enhancing heavy metals filtration: Synergistic effects of banana peel-derived activated carbon and layered double hydroxides in ultrafiltration membranes. Sep. Purif. Technol. 2025, 362, 131875. [Google Scholar] [CrossRef]
- Huang, W.-H.; Chang, Y.-J.; Lee, D.-J. Layered double hydroxide loaded pinecone biochar as adsorbent for heavy metals and phosphate ion removal from water. Bioresour. Technol. 2024, 391, 129984. [Google Scholar] [CrossRef]
- Hameed, R.; Abbas, A.; Lou, J.; Khattak, W.A.; Roha, B.; Iqbal, B.; Li, G.; Zhang, Q.; Zhao, X. Synthesis of biochar-ZnAl-layered double hydroxide composite for effective heavy metal adsorption: Exploring mechanisms and structural transformations. J. Environ. Chem. Eng. 2024, 12, 112687. [Google Scholar] [CrossRef]
- Wang, Q.; Peng, Y.; Chen, M.; Xu, M.; Ding, J.; Yao, Q.; Lu, S. Synthesis of layered double hydroxides from municipal solid waste incineration fly ash for heavy metal adsorption. Sci. Total Environ. 2024, 912, 169482. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Wang, X.; Su, M.; Lin, Q. A novel 3D hierarchical NiFe-LDH/graphitic porous carbon composite as multifunctional adsorbent for efficient removal of cationic/anionic dyes and heavy metal ions. J. Mol. Liq. 2024, 411, 125753. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, X.; Ding, J.; Zhang, N.; Yu, H.; Wang, Y.; Zhang, Q.; Zhang, C.; Meng, Z. Mechanochemical synthesis of carbon-loading layered double hydroxides by waste magnesia-carbon bricks for removal heavy metals. J. Environ. Chem. Eng. 2025, 13, 115286. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, X.; Bai, J.; Yang, C.; Zhang, Y.; Xu, Z.; Jin, X.; Jiang, Y. Facile synthesis of magnetic ZnAl layered double hydroxides and efficient adsorption of malachite green and congo red. Sep. Purif. Technol. 2023, 322, 124305. [Google Scholar] [CrossRef]
- Hou, T.; Yan, L.; Li, J.; Yang, Y.; Shan, L.; Meng, X.; Li, X.; Zhao, Y. Adsorption performance and mechanistic study of heavy metals by facile synthesized magnetic layered double oxide/carbon composite from spent adsorbent. Chem. Eng. J. 2020, 384, 123331. [Google Scholar] [CrossRef]
- Su, J.; Zhao, M.; Zhao, Z.; Sun, L.; Meng, Z. Mg-Al layered double hydroxides modified by 2,4,6-trimercapto-s-triazine for effective removal heavy metals from soil. Chem. Eng. Sci. 2024, 300, 120563. [Google Scholar] [CrossRef]
- Bi, R.; Yin, D.; Lei, B.; Chen, F.; Zhang, R.; Li, W. Mercaptocarboxylic acid intercalated MgAl layered double hydroxide adsorbents for removal of heavy metal ions and recycling of spent adsorbents for photocatalytic degradation of organic dyes. Sep. Purif. Technol. 2022, 289, 120741. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, W.; Liu, W.; Shen, Y.; Zhao, Q. An environmentally friendly depressant for magnesite and calcite flotation separation: Selective depression and adsorption mechanism. J. Environ. Chem. Eng. 2024, 12, 114499. [Google Scholar] [CrossRef]
- Qiu, Y.; Serdechnova, M.; Blawert, C.; Höche, D.; Yaremchenko, A.; Strerath, D.; Bi, Y.; Yang, J.; Zheludkevich, M.L. Impact of nitro and amino functional groups on the intercalation of isophthalic acid in ZnAl layered double hydroxides: An experimental and theoretical study. Nano Mater. Sci. 2024, in press. [Google Scholar] [CrossRef]
- Lv, L.; Sun, P.; Gu, Z.; Du, H.; Pang, X.; Tao, X.; Xu, R.; Xu, L. Removal of chloride ion from aqueous solution by ZnAl-NO3 layered double hydroxides as anion-exchanger. J. Hazard. Mater. 2009, 161, 1444–1449. [Google Scholar] [CrossRef]
- Ding, C.; Wu, J.; Liu, Y.; Sheng, X.; Cheng, X.; Xiong, X.; Zhao, W. A waterborne epoxy composite coating with smart corrosion resistance based on 2-phenylbenzimidazole-5-sulfonic acid/layered double hydroxide composite. Molecules 2023, 28, 5199. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Huang, Y.; Li, J.; Hua, G.; Liu, C.; Li, N.; Lai, B. Efficient removal and selective recovery of phosphorus by calcined La-doped layered double hydroxides. J. Water Process Eng. 2022, 47, 102722. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Sillanpää, M.E.T. Adsorption of Cd(II) and Pb(II) by a novel EGTA-modified chitosan material: Kinetics and isotherms. J. Colloid Interface Sci. 2013, 409, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Huang, Y.; Li, Y.; Wang, C.; Yuan, W.; Cheng, D.; Shen, T.; Zhang, J.; Liu, J.; Yang, C.; et al. Construction of BTA-inbuilt ZIF-8 coated with molybdate-intercalated ZnAl-LDH as core-shell structured nanocontainers toward pH-responsive smart corrosion protection of waterborne epoxy coating. Prog. Org. Coat. 2024, 194, 108623. [Google Scholar] [CrossRef]
- Guo, R.; Ding, C.; Liu, Y.; Cheng, X.; Zhang, L.; Zhao, W.; Sheng, X. In situ growth of layered double hydroxides on zirconium phosphate for reinforcing anti-corrosion and wear resistance of waterborne epoxy coatings. Polymer 2025, 319, 128048. [Google Scholar] [CrossRef]
- Burdzy, K.; Jastrząb, R.; Kołodyńska, D. Glda and ion exchangers: Unlocking sustainable solutions for recovery of rare earth elements. Chem. Eng. J. 2024, 479, 147632. [Google Scholar] [CrossRef]
- Hong, X.; Shi, M.; Ding, Z.; Ding, C.; Du, P.; Xia, M.; Wang, F. Unveiling glutamic acid-functionalized ldhs: Understanding the Cr(VI) removal mechanism from microscopic and macroscopic view points. Phys. Chem. Chem. Phys. 2023, 25, 23519–23529. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Greenwell, H.C.; Krzhizhanovskaya, M.G.; Apperley, D.C.; Pekov, I.V.; Yakovenchuk, V.N. Thermal evolution of natural layered double hydroxides: Insight from quintinite, hydrotalcite, stichtite, and iowaite as reference samples for CO3− and Cl-members of the hydrotalcite supergroup. Minerals 2020, 10, 961. [Google Scholar] [CrossRef]
- Mashkoor, F.; Kim, D.; Zahid Ansari, M.; Hakeem Anwer, A.; Shoeb, M.; Jeong, C. Synergistic effects of multifunctional nanostructured WO3-WS2 decorated on polypyrrole (WO3-WS2/PPy) for the removal of toxic heavy metals from wastewaters and high supercapacitor performance. J. Mol. Liq. 2023, 375, 121312. [Google Scholar] [CrossRef]
- Ai, Z.; Liu, C.; Zhang, Q.; Qu, J.; Li, Z.; He, X. Adding ZnO and SiO2 to scatter the agglomeration of mechanochemically prepared Zn-Al LDH precursor and promote its adsorption toward methyl orange. J. Alloys Compd. 2018, 763, 342–348. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Li, N.; Wang, W.; Nan, J.; Zhao, Z.; Cui, F. Highly efficient removal of bivalent heavy metals from aqueous systems by magnetic porous Fe3O4-MnO2: Adsorption behavior and process study. Chem. Eng. J. 2016, 304, 737–746. [Google Scholar] [CrossRef]
- Feng, J.; Zhong, L.; Yang, Z.; Tang, C.-Y.; Law, W.-C.; Wu, R.; Xie, F. Selective adsorption of lead in mixed metals wastewater system by lignin-carbon-supported titanate nanoflower BC@TNS adsorbent: Performance and mechanism. Coatings 2025, 15, 317. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Shen, F.; Wu, C.; Gu, S. Equilibrium, kinetics and thermodynamics of cadmium ions (Cd2+) removal from aqueous solution using earthworm manure-derived carbon materials. J. Mol. Liq. 2017, 241, 612–621. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Y.; Yu, H.; Yan, L.; Du, B.; Pei, Z. Removal of Cu2+, Cd2+ and Pb2+ from aqueous solutions by magnetic alginate microsphere based on Fe3O4/MgAl-layered double hydroxide. J. Colloid Interface Sci. 2018, 532, 474–484. [Google Scholar] [CrossRef]
- Bashir, A.; Manzoor, T.; Malik, L.A.; Qureashi, A.; Pandith, A.H. Enhanced and selective adsorption of Zn(II), Pb(II), Cd(II), and Hg(II) ions by a dumbbell- and flower-shaped potato starch phosphate polymer: A combined experimental and DFT calculation study. ACS Omega 2020, 5, 4853–4867. [Google Scholar] [CrossRef]
- Zhou, R.; Li, H.; Liu, C.; Liu, Y.; Lee, J.-F.; Lin, Y.-J.; Yan, Z.; Xu, Z.; Yi, X.; Feng, C. Magnetic anaerobic granular sludge for sequestration and immobilization of Pb. Water Res. 2023, 239, 120022. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; Zhang, L.; Yu, Z.; Li, X.; Li, Y.; Cui, Y.; Zhao, Y.; Yan, L. Removal of various aqueous heavy metals by polyethylene glycol modified MgAl-LDH: Adsorption mechanisms and vital role of precipitation. J. Mol. Liq. 2023, 375, 121386. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Zhang, H.; Liu, Y.; Xing, B. Adsorption of Pb(II) and Cd(II) by magnetic activated carbon and its mechanism. Sci. Total Environ. 2021, 757, 143910. [Google Scholar] [CrossRef]
- Liao, W.; Bao, D.; Li, H.-Q.; Yang, P. Cu(II) and Cd(II) removal from aqueous solution with LDH@GO-NH2 and LDH@GO-SH: Kinetics and probable mechanism. Environ. Sci. Pollut. Res. 2021, 28, 65848–65861. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Zhao, H.; Zhang, Z.; Su, J.; Ma, X.; Kong, F.; Xie, Y.; Ma, Z.; Zhang, Q.; et al. Effective adsorption of heavy metals on layered double hydroxides modify through dithiocarbamate prepared by CS2 and different molecular weight of polyethyleneimine. Sep. Purif. Technol. 2023, 327, 124886. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, H.; Zhong, Y.; Lu, G.; Dang, Z.; Zhang, L. Co-adsorption behaviors and mechanisms of Cd(II), Pb(II), and Cr(VI) on sodium dodecyl sulfate modified attapulgite clay-supported nano zero-valent iron: Competitive or synergistic effect? Environ. Res. 2025, 271, 121107. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xu, L.; Wu, D.; Li, Q.; Ai, Y.; Liu, W.; Li, D.; Zhou, Y.; Zhang, B.; et al. EDTA functionalized Mg/Al hydroxides modified biochar for Pb(II) and Cd(II) removal: Adsorption performance and mechanism. Sep. Purif. Technol. 2024, 335, 126199. [Google Scholar] [CrossRef]
- Xiao, L.; Yue, C.; Lu, G.; Qiu, G.; Guo, M.; Zhang, M. L-cysteine intercalated ZnAl-LDH: An ultrafast and high-efficiency adsorbent for lead removal from complex wastewater. J. Water Process Eng. 2025, 70, 107051. [Google Scholar] [CrossRef]
- Babu Poudel, M.; Shin, M.; Joo Kim, H. Interface engineering of MIL-88 derived MnFe-LDH and MnFe2O3 on three-dimensional carbon nanofibers for the efficient adsorption of Cr(VI), Pb(II), and As(III) ions. Sep. Purif. Technol. 2022, 287, 120463. [Google Scholar] [CrossRef]





| Samples | (003) | |
|---|---|---|
| 2θ (°) | d (nm) | |
| NO3-LDH | 9.90 | 0.89 |
| GLDA-LDH | 7.46 | 1.18 |
| Sample | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|
| GLDA-LDH | 39 | 0.05603 | 5.571 |
| Ion | Pseudo First Order | Pseudo Second Order | ||
|---|---|---|---|---|
| K1 | R12 | K2 | R22 | |
| Pb(II) | 0.06629 | 0.9613 | 0.0015 | 0.9811 |
| Cd(II) | 0.05524 | 0.9652 | 0.0019 | 0.9839 |
| T (K) | Ion | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm | b | RL2 | Kf | n | RF2 | ||
| 298 | Pb(II) | 219.2 | 0.017 | 0.997 | 10.057 | 1.43 | 0.984 |
| 308 | 167.2 | 0.006 | 0.999 | 4.391 | 1.32 | 0.989 | |
| 318 | 91.4 | −0.00045 | 0.992 | 0.706 | 1.06 | 0.943 | |
| 298 | Cd(II) | 121.9 | 0.0018 | 0.992 | 1.667 | 1.19 | 0.985 |
| 308 | 61.7 | 0.0077 | 0.999 | 1.585 | 1.57 | 0.993 | |
| 318 | 58.9 | 0.0015 | 0.994 | 0.504 | 1.15 | 0.991 | |
| Adsorbent | Adsorption Capacity (mg/g) | Reference | |
|---|---|---|---|
| Pb(II) | Cd(II) | ||
| GLDA-LDH | 219.2 | 121.9 | This work |
| Potato starch phosphate polymer | 106.25 | 91.84 | [43] |
| ZnAl-LDH-BC600 | 128.4 | 99.8 | [18] |
| M-AGS | 200.0 | / | [44] |
| LDH-200 | 125.9 | 69.11 | [45] |
| Magnetized activated carbons | 253.2 | 73.3 | [46] |
| LDH@GO-SH | / | 102.77 | [47] |
| LDH-DTC600 | 181.7 | 125.5 | [48] |
| Ion | Pseudo First Order | Pseudo Second Order | ||
|---|---|---|---|---|
| K1 | R12 | K2 | R22 | |
| Pb(II) | 0.2129 | 0.9357 | 0.0097 | 0.9866 |
| Cd(II) | 0.3017 | 0.8713 | 0.0353 | 0.9421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.; Guang, Z.; Wang, Z.; Liu, Y.; Cheng, X.; Liu, Y. Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications. Coatings 2025, 15, 613. https://doi.org/10.3390/coatings15050613
Zheng K, Guang Z, Wang Z, Liu Y, Cheng X, Liu Y. Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications. Coatings. 2025; 15(5):613. https://doi.org/10.3390/coatings15050613
Chicago/Turabian StyleZheng, Kai, Zhengkai Guang, Zihan Wang, Yangu Liu, Xiaoling Cheng, and Yuan Liu. 2025. "Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications" Coatings 15, no. 5: 613. https://doi.org/10.3390/coatings15050613
APA StyleZheng, K., Guang, Z., Wang, Z., Liu, Y., Cheng, X., & Liu, Y. (2025). Robust Adsorption of Pb(II) and Cd(II) by GLDA-Intercalated ZnAl-LDH: Structural Engineering, Mechanistic Insights, and Environmental Applications. Coatings, 15(5), 613. https://doi.org/10.3390/coatings15050613







