Thin Films of PNDI(2HD)2T and PCPDTBT Polymers Deposited Using the Spin Coater Technique for Use in Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czerwiński, W.; Kaczmarek, H.; Kędziera, D.; Kowalonek, J.; Nowaczyk, J.; Olewnik, E. Przewodzące i Fotoczułe Materiały Polimerowe; Faculty of Chemistry at Nicolaus Copernicus University (UMK): Toruń, Poland, 2012; pp. 2–4. [Google Scholar]
- Tapan, K.D.; Prusty, S. Review on Conducting Polymers and Their Applications. Polym. Technol. Eng. 2012, 14, 1487–1500. [Google Scholar]
- Lelek-Borkowska, U. Podstawy Chemii Polimerów; Akademia Górniczo-Hutnicza im. St. Staszica w Krakowie: Kraków, Poland, 2021; p. 1. [Google Scholar]
- Bokwa, M.; Wierzbicka, B.; Tański, T.; Wiśniowski, M. Polimery Przewodzące i ich Zastosowanie. PSKN 2015, 42, 1–8. [Google Scholar]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Oskay, W.; Barrett, R. Make: The Annotated Build-It-Yourself Science Laboratory; O’Reilly: Sebastopol, CA, USA, 2016; pp. 4–7. [Google Scholar]
- Kaltenhauser, V.; Rath, T.; Edler, M.; Reichmann, A.; Trimmel, G. Exploring polymer/nanoparticle hybrid solar cells in tandem architecture. RSC Adv. 2013, 40, 18643–18650. [Google Scholar] [CrossRef][Green Version]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; McGehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, J.; Liu, S.; Qi, M.; Jia, T.; Zhao, J.; Qingduan, L.; Ying, L.; Cai, Y.-P.; Lu, X.; et al. Understanding of Imine Substitution in Wide-Bandgap Polymer Donor-Induced Efficiency Enhancement in All-Polymer Solar Cells. Chem. Mater. 2019, 31, 8533–8542. [Google Scholar] [CrossRef]
- Xue, C.; Tang, Y.; Liu, S.; Feng, H.; Li, S.; Xia, D. Achieving efficient polymer solar cells based on benzodithiophene-thiazolecontaining wide band gap polymer donors by changing the linkage patterns of two thiazoles. New J. Chem. 2020, 44, 13100–13107. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Liu, S.; Cao, Z.; Jiao, X.; Cai, Y.; Huang, F. Bithieno[3,4-c]pyrrole-4,6-dione-Mediated Crystallinity in Large Bandgap Polymer Donors Directs Charge Transportation and Recombination in Efficient Nonfullerene Polymer Solar Cells. ACS Energy Lett. 2020, 5, 367–375. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, J.; Trang, B.T.T.; Lee, C.; Cho, C.; Na, K.; Jung, J.; Song, C.E.; Ma, B.; Lee, J.-Y.; et al. Rationally Designed Donor-Acceptor Random Copolymers with Optimized Complementary Light Absorption for Highly Efficient All-Polymer Solar Cells. Adv. Funct. Mater. 2017, 38, 1–8. [Google Scholar] [CrossRef]
- Dalila, K.; Kamel, A. PTB7-Th /Non-fullerene acceptors for organic solar cells. Synth. Met. 2022, 291, 1124–1132. [Google Scholar]
- Kim, H.; Nam, S.; Jeong, J.; Lee, S.; Seo, J.; Han, H.; Kim, Y. Organic solar cells based on conjugated polymers: History and recent advances. Korean J. Chem. Eng. 2014, 31, 1095–1104. [Google Scholar] [CrossRef]
- Soci, C.; Hwang, I.W.; Moses, D.; Zhu, Z.; Waller, D.; Gaudiana, R.; Brabec, C.J.; Heeger, A.J. Photoconductivity of a low-bandgap conjugated polymer. Adv. Funct. Mater. 2007, 17, 632–636. [Google Scholar] [CrossRef]
- Fischer, U.; Trefz, D.; Back, J.; Kayunkid, N.; Tornow, B.; Albrecht, S.; Yager, K.G.; Singh, G.; Karim, A.; Neher, D.; Brinkmann, M.; et al. Highly Crystalline Films of PCPDTBT with Branched Side Chains by Solvent Vapor Crystallization: Influence on Opto-Electronic Properties. Adv. Mater. 2014, 7, 1223–1228. [Google Scholar] [CrossRef]
- Kowalski, S.; Allard, S.; Scherf, U. Synthesis of Poly(4,4-dialkyl-cyclopenta[2,1-b:3,4-b′]dithiophene-alt-2,1,3-benzothiadiazole) (PCPDTBT) in a Direct Arylation Scheme. ACS Macro Lett. 2012, 4, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, C.; Liu, F.; Chen, J.; Dyck, O.E.; Duscher, G.; Russell, T.P. Guided crystallization of P3HT in ternary blend solar cell based on P3HT:PCPDTBT:PCBM. Energy Environ. Sci. 2014, 7, 3782–3790. [Google Scholar] [CrossRef]
- Lee, W.; Lee, C.; Yu, H.; Kim, D.-J.; Wang, C.; Woo, H.Y.; Oh, J.H.; Kim, B.J. Side Chain Optimization of Naphthalenediimide-Bithiophene-Based Polymers to Enhance the Electron Mobility and the Performance in All-Polymer Solar Cells. Adv. Funct. Mater. 2016, 10, 1543–1553. [Google Scholar] [CrossRef]
- Jung, J.; Lee, W.; Lee, C.; Ahn, H.; Kim, B.J. Controlling Molecural Orientation of Naphthalenediimide-Based Polymer Acceptors for High Performance All-Polymers Solar Cells. Adv. Energy Mater. 2016, 15, 1–10. [Google Scholar]
- Zhang, Y.; Wang, L.; Chen, X.; Liu, H.; Li, Q. High-Performance Flexible Organic Solar Cells with Enhanced Stability via Novel Interface Engineering. Adv. Funct. Mater. 2024, 34, 2402128. [Google Scholar]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photon. 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 18003. [Google Scholar] [CrossRef]
- Han, X.Y.; Choi, J.Y.; Lee, Y.J.; Ko, E.J.; Choi, M.H.; Suh, I.S.; Moon, D.K. Vertical Phase Separation for Highly Efficient Organic Solar Cells Incorporating Conjugated-Polyelectrolytes. Adv. Mater. Interfaces 2019, 6, 1801396. [Google Scholar] [CrossRef]
- Dettinger, U.; Egelhaaf, H.-J.; Brabec, C.; Latteyer, F.; Peisert, H.; Chassé, T. FTIR Study of the Impact of PC[60]BM on the Photodegradation of the Low Band Gap Polymer PCPDTBT under O2 Environment. Chem. Mater. 2015, 27, 2299–2308. [Google Scholar] [CrossRef]
- von Hauff, E.; da Como, E.; Ludwigs, S. Optoelectronic Properties of PCPDTBT for Photovoltaics: Morphology Control and Molecular Doping. In Elementary Processes in Organic Photovoltaics; Advances in Polymer Science; Leo, K., Ed.; Springer: Cham, Switzerland, 2017; Volume 272, pp. 109–138. [Google Scholar] [CrossRef]
- Ossila. PNDI(2HD)2T|Copolymer of Naphthalene & Bithiophene. Available online: https://www.ossila.com/products/pndi2hd2t (accessed on 12 May 2025).
- Gross, Y.M.; Ludwigs, S. P(NDI2OD-T2) Revisited—Aggregation Control as Key for High Performance n-Type Applications. Synth. Met. 2019, 253, 73–87. [Google Scholar] [CrossRef]

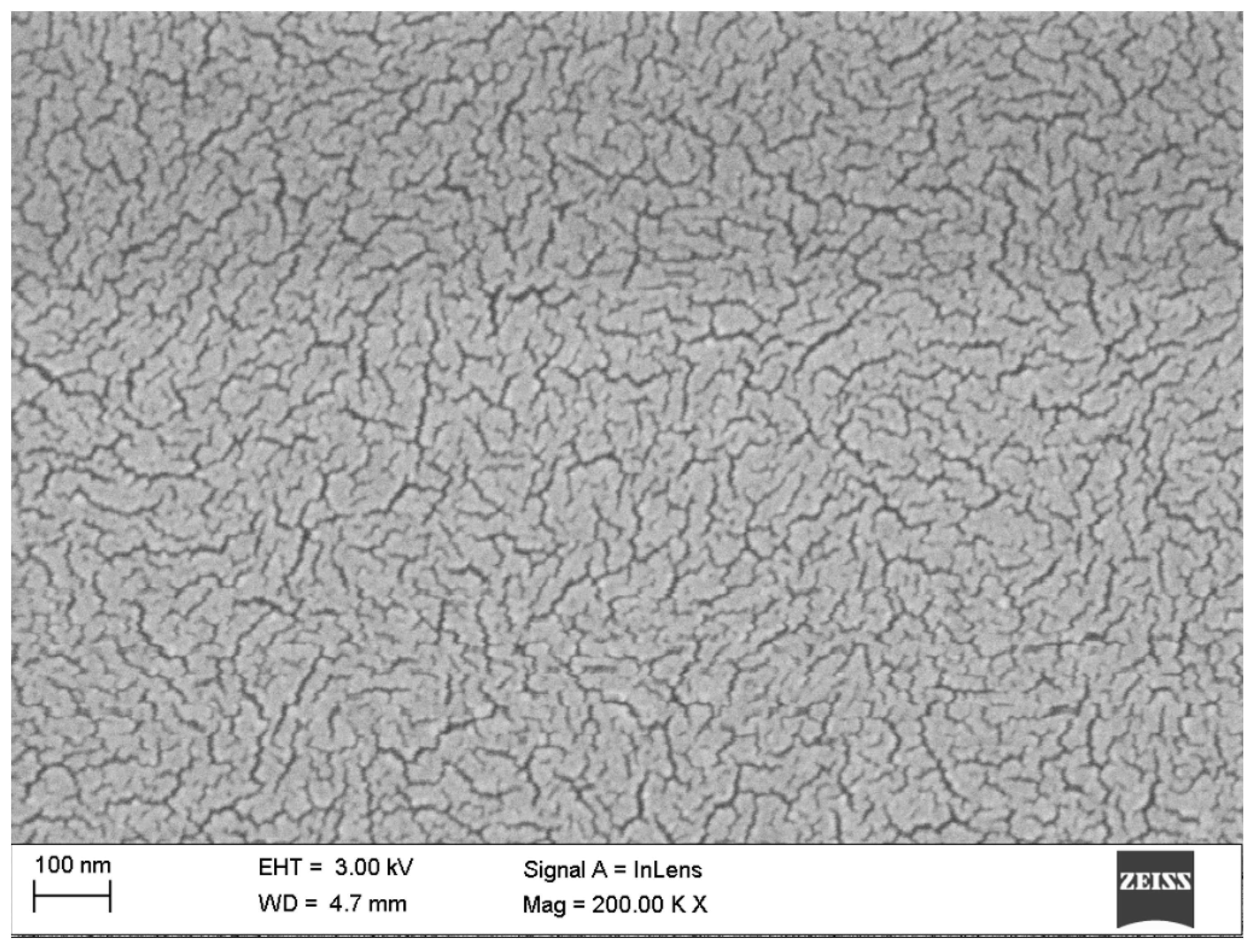

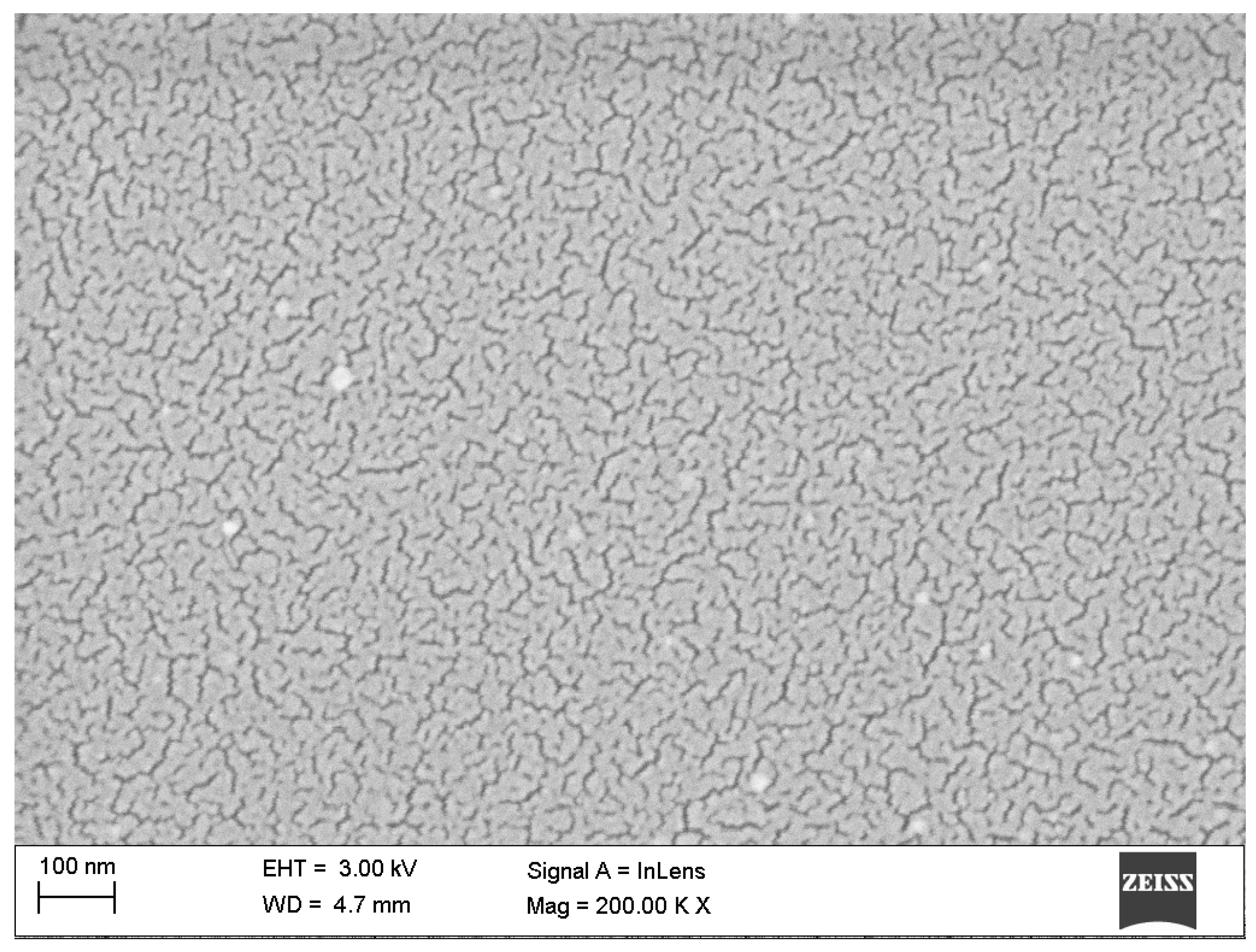
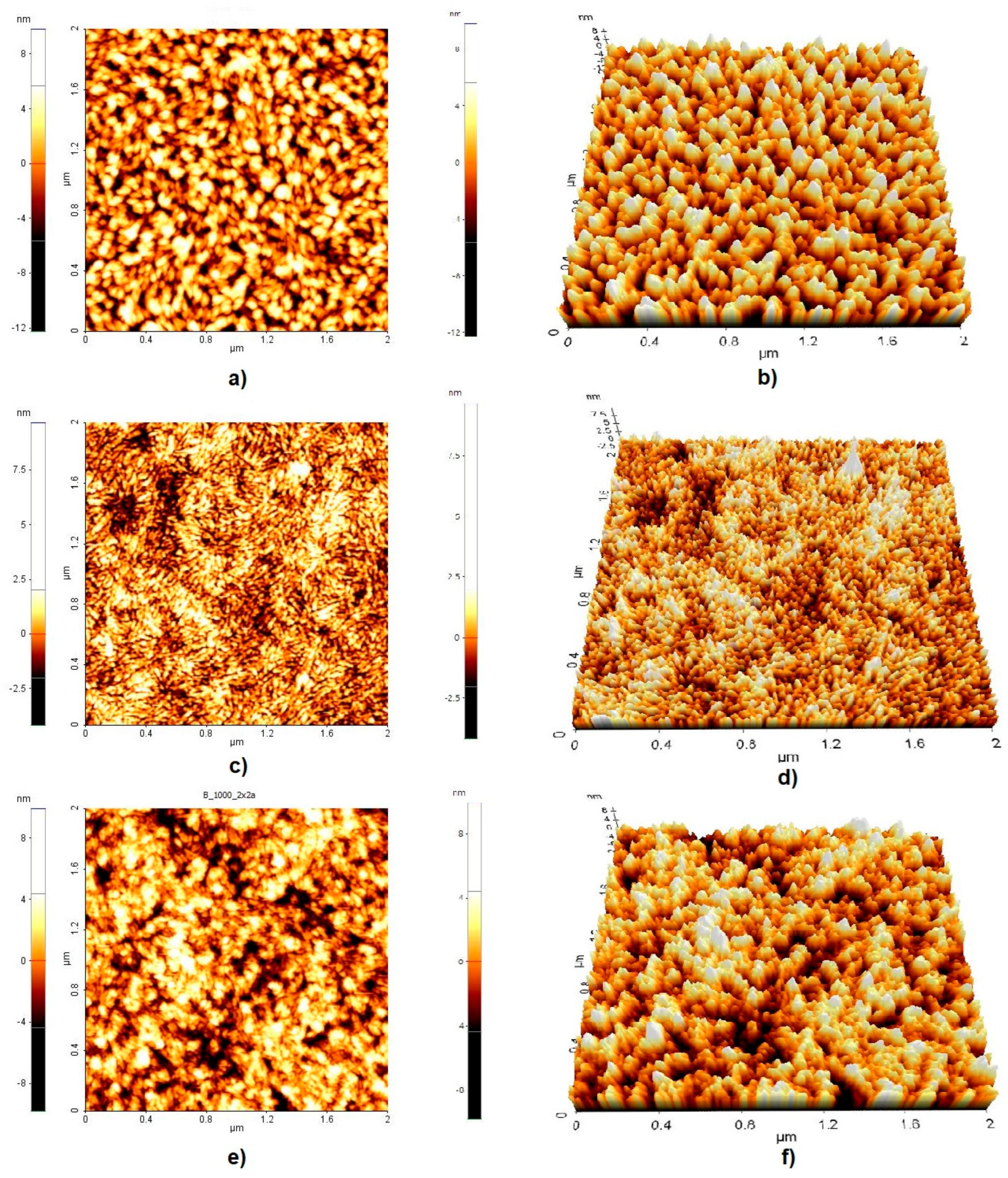
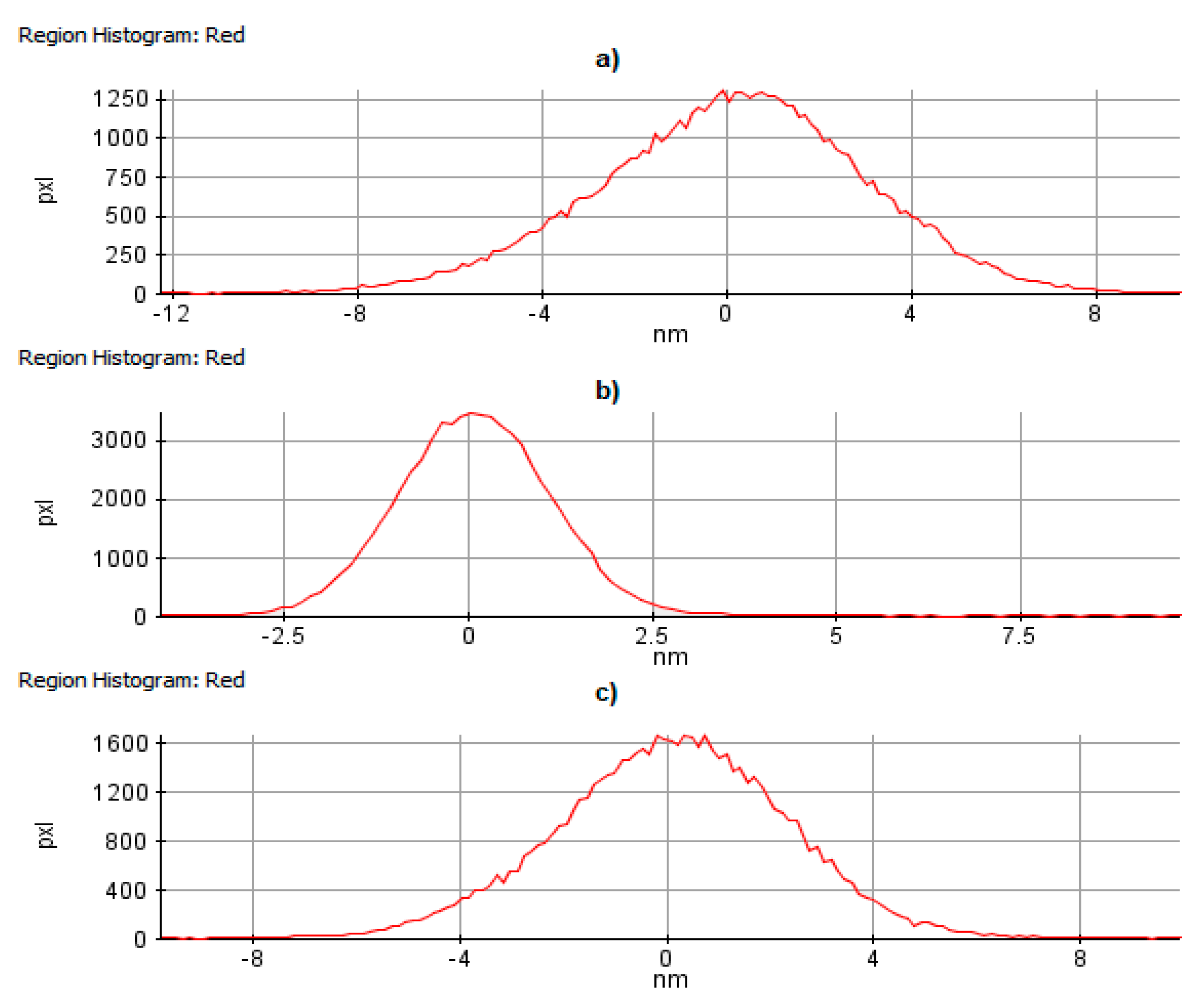

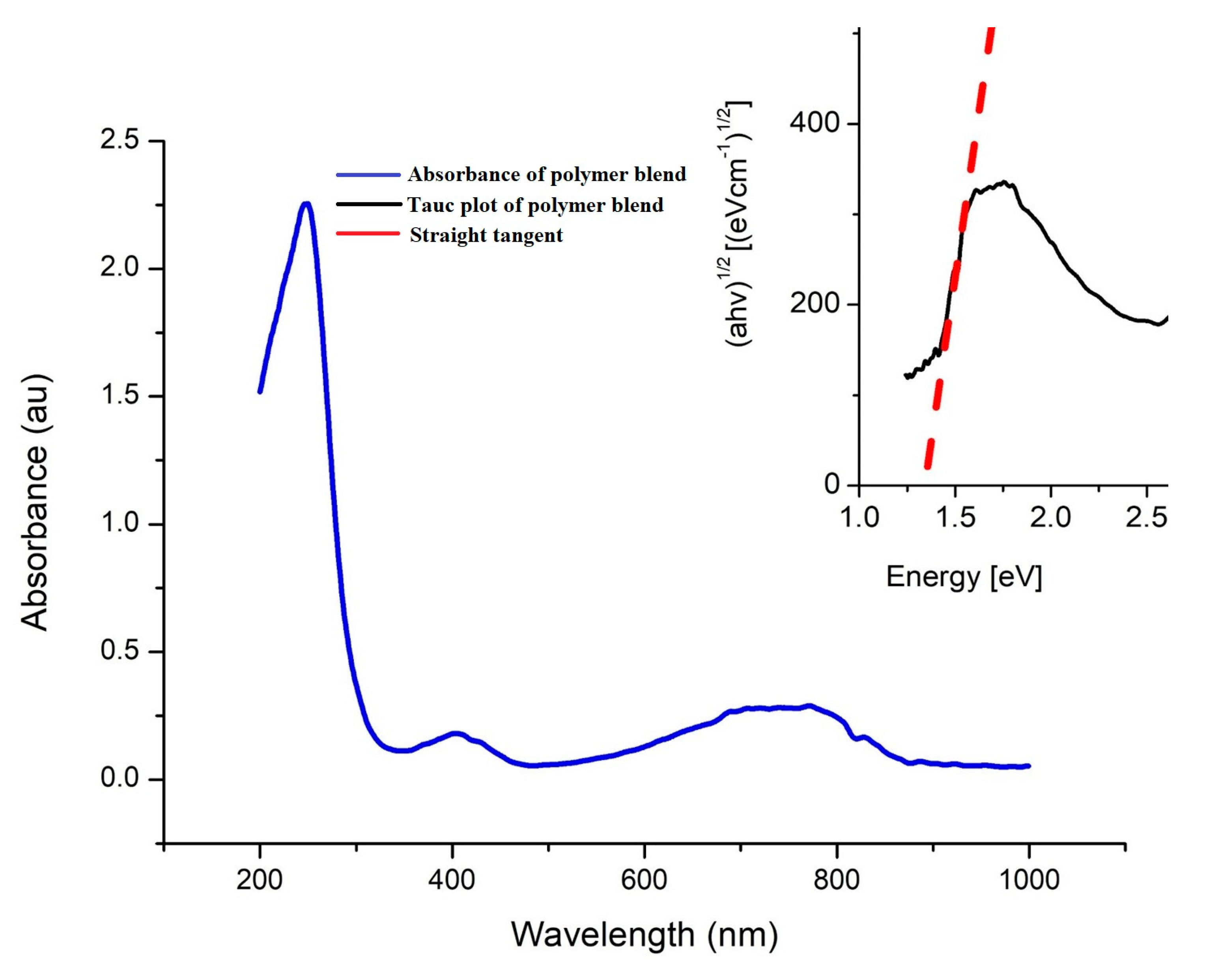
| Sample | Rq [nm] | Ra [nm] | Max. Irregularity [nm] |
|---|---|---|---|
| PCPDTBT | 2.88 | 2.28 | 9.80 |
| PNDI(2HD)2T | 1.04 | 0.82 | 9.66 |
| Blend | 2.22 | 1.76 | 9.91 |
| No | Sheet Resistance [Ω/☐] | Resistivity [kΩ·m] | Conductivity [µS/cm] | Egopt [eV] |
|---|---|---|---|---|
| PCPDTBT | 2.93 × 108 | 0.29 | 34.3 | 1.32 |
| PNDI(2HD)2T | 1.59 × 1010 | 15.87 | 0.63 | 1.37 |
| Blend | 1.1 × 109 | 1.1 | 9.1 | 1.34 |
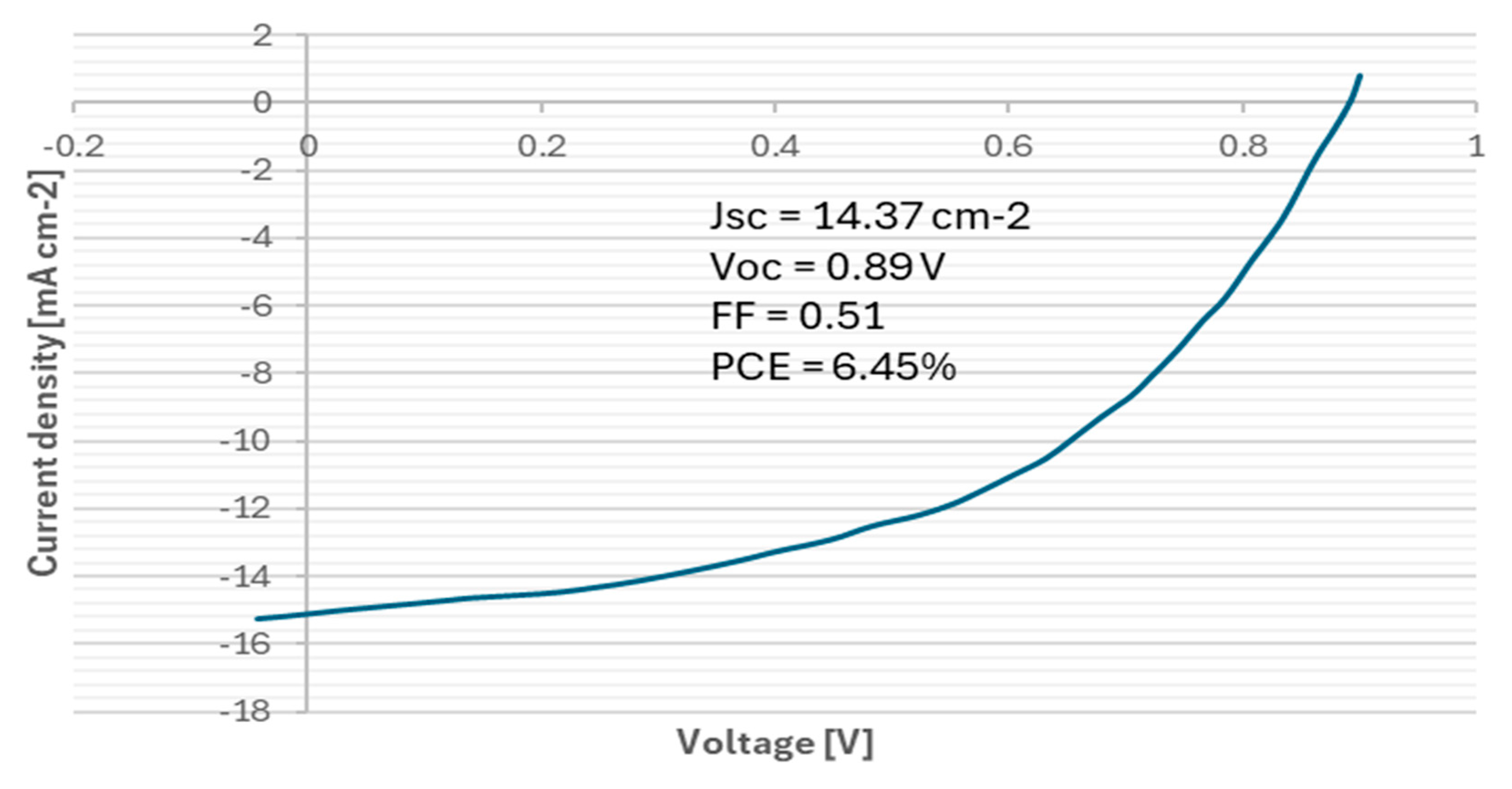
| Parameter | Average Value ± SD | Unit |
| Short-circuit current density (JSC) | 14.37 ± 0.52 | mA·cm⁻2 |
| Open-circuit voltage (VOC) | 0.89 ± 0.03 | V |
| Fill factor (FF) | 0.51 ± 0.02 | – |
| Power conversion efficiency (PCE) | 6.45 ± 0.21 | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sładek, M.; Radek, P.; Szindler, M.M.; Szindler, M. Thin Films of PNDI(2HD)2T and PCPDTBT Polymers Deposited Using the Spin Coater Technique for Use in Solar Cells. Coatings 2025, 15, 603. https://doi.org/10.3390/coatings15050603
Sładek M, Radek P, Szindler MM, Szindler M. Thin Films of PNDI(2HD)2T and PCPDTBT Polymers Deposited Using the Spin Coater Technique for Use in Solar Cells. Coatings. 2025; 15(5):603. https://doi.org/10.3390/coatings15050603
Chicago/Turabian StyleSładek, Michał, Patryk Radek, Magdalena Monika Szindler, and Marek Szindler. 2025. "Thin Films of PNDI(2HD)2T and PCPDTBT Polymers Deposited Using the Spin Coater Technique for Use in Solar Cells" Coatings 15, no. 5: 603. https://doi.org/10.3390/coatings15050603
APA StyleSładek, M., Radek, P., Szindler, M. M., & Szindler, M. (2025). Thin Films of PNDI(2HD)2T and PCPDTBT Polymers Deposited Using the Spin Coater Technique for Use in Solar Cells. Coatings, 15(5), 603. https://doi.org/10.3390/coatings15050603








