Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications
Abstract
1. Introduction
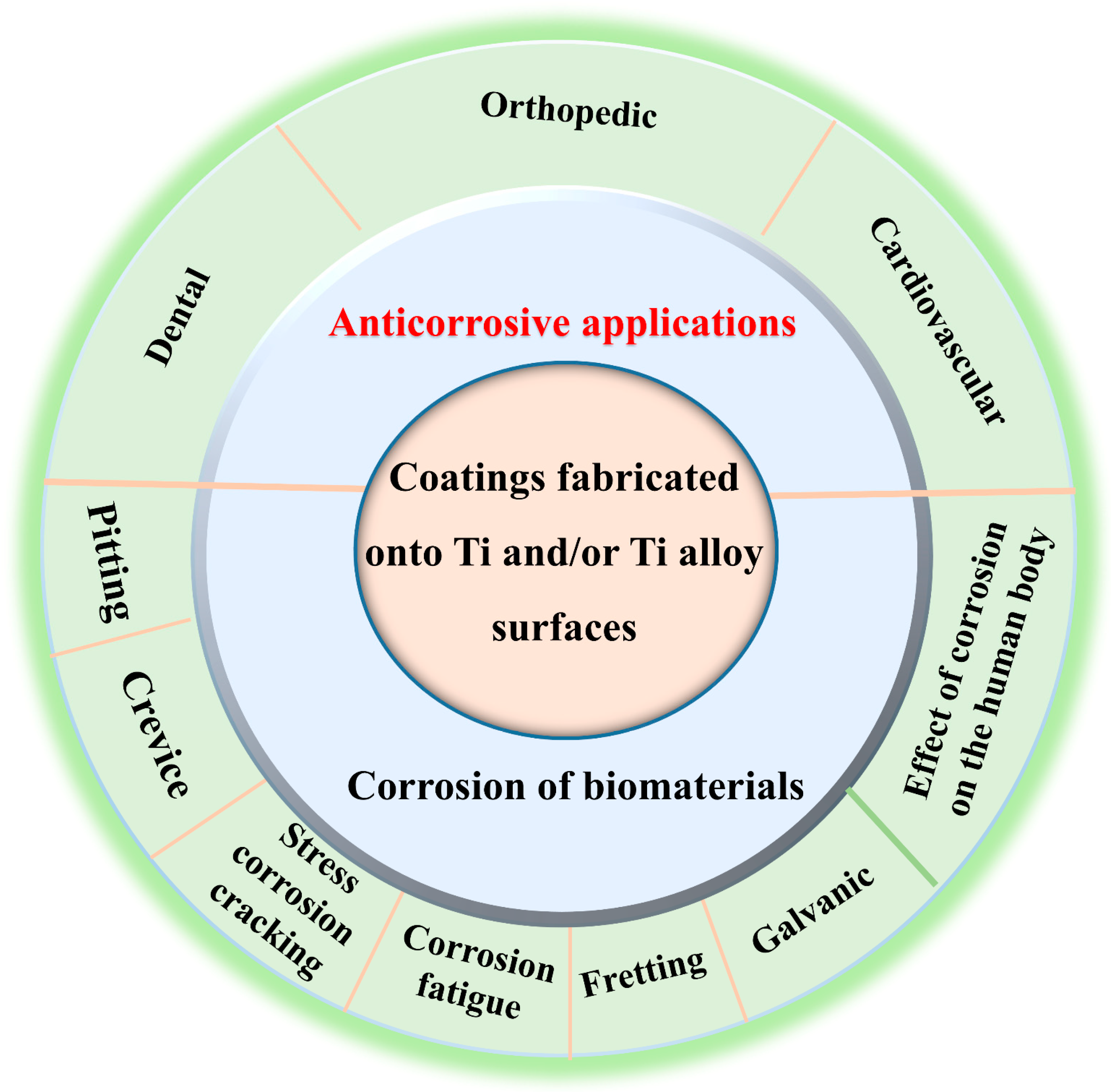
2. Corrosion of Biomaterials
2.1. Corrosion Types
2.1.1. Pitting Corrosion
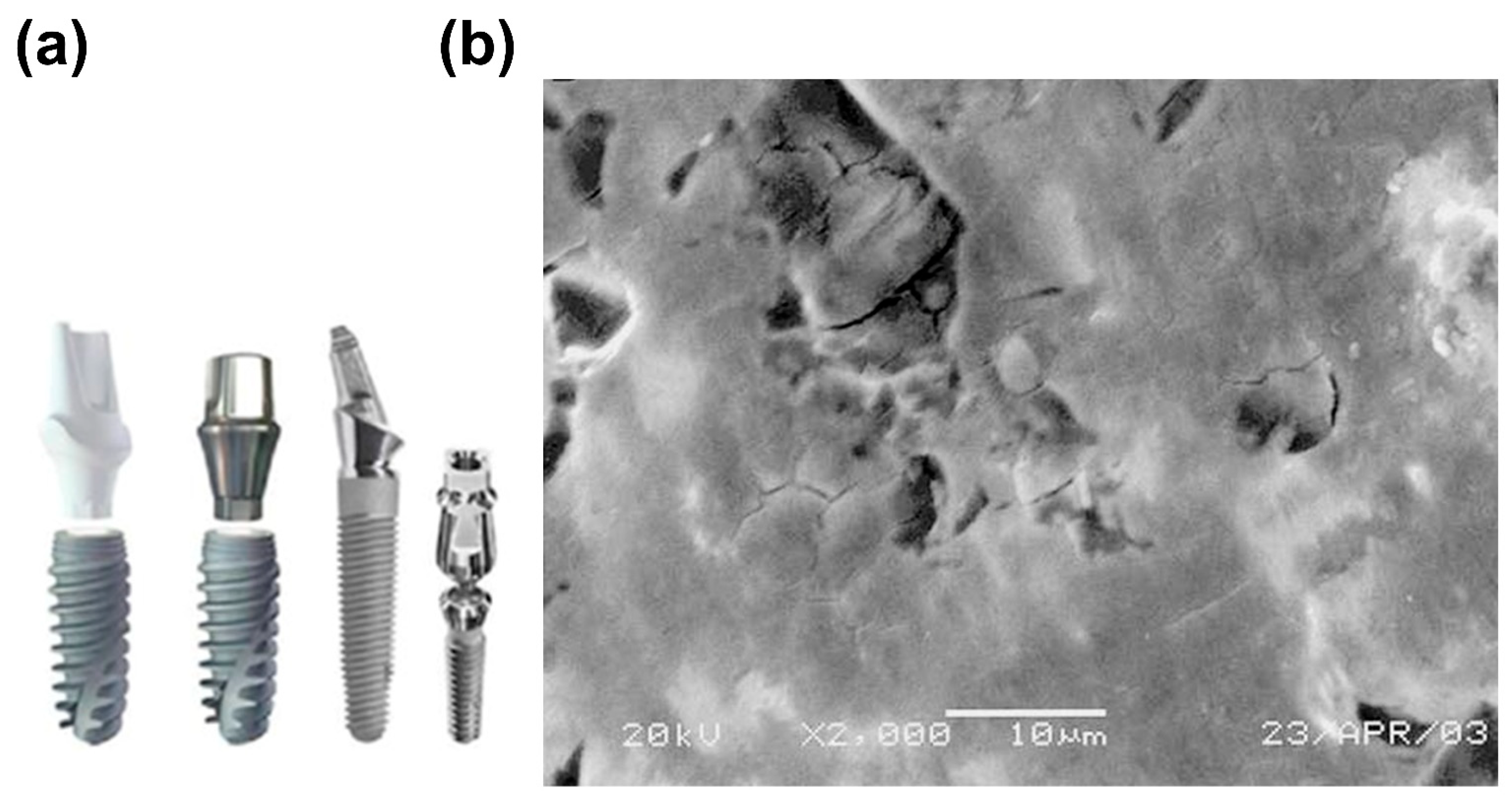
2.1.2. Crevice Corrosion
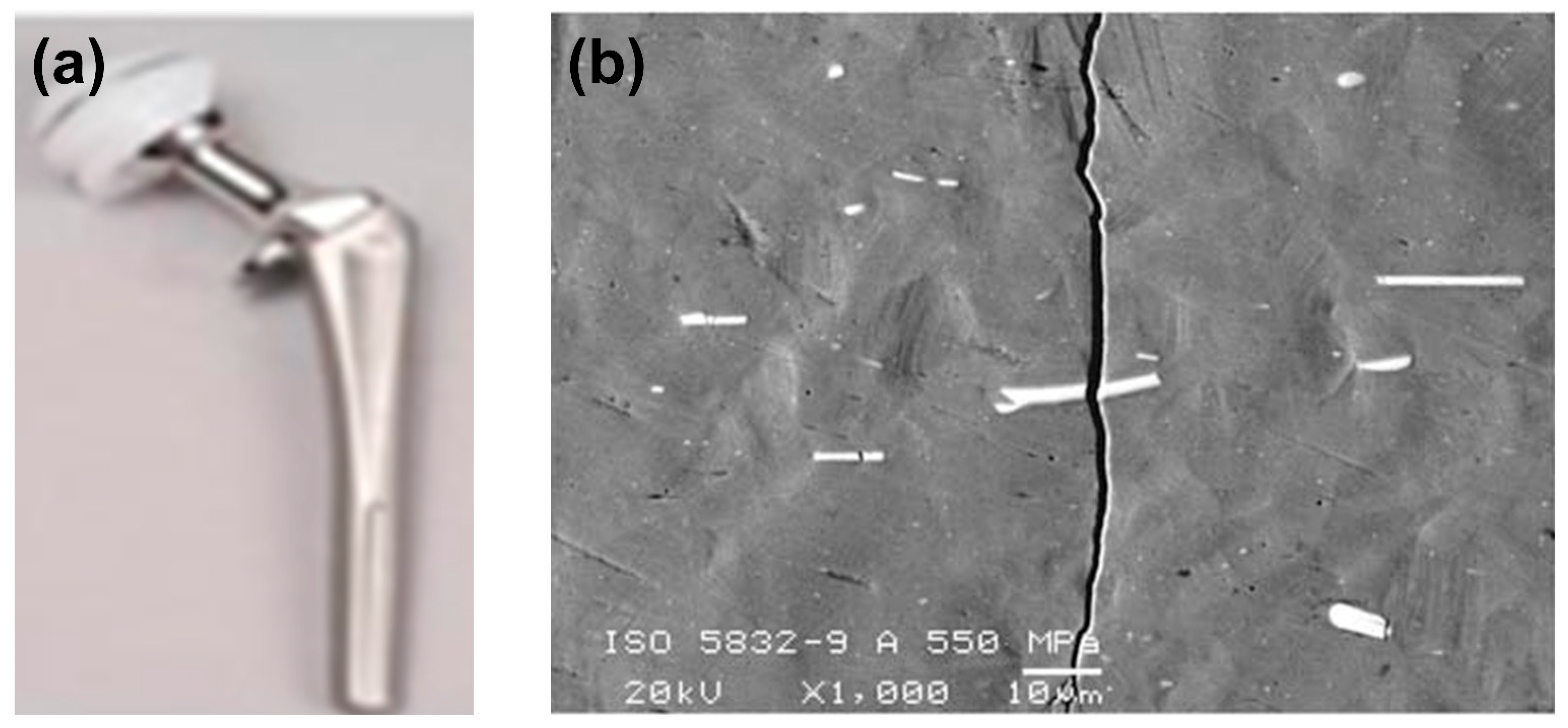
2.1.3. Stress Corrosion Cracking (SCC)
2.1.4. Corrosion Fatigue (CF)
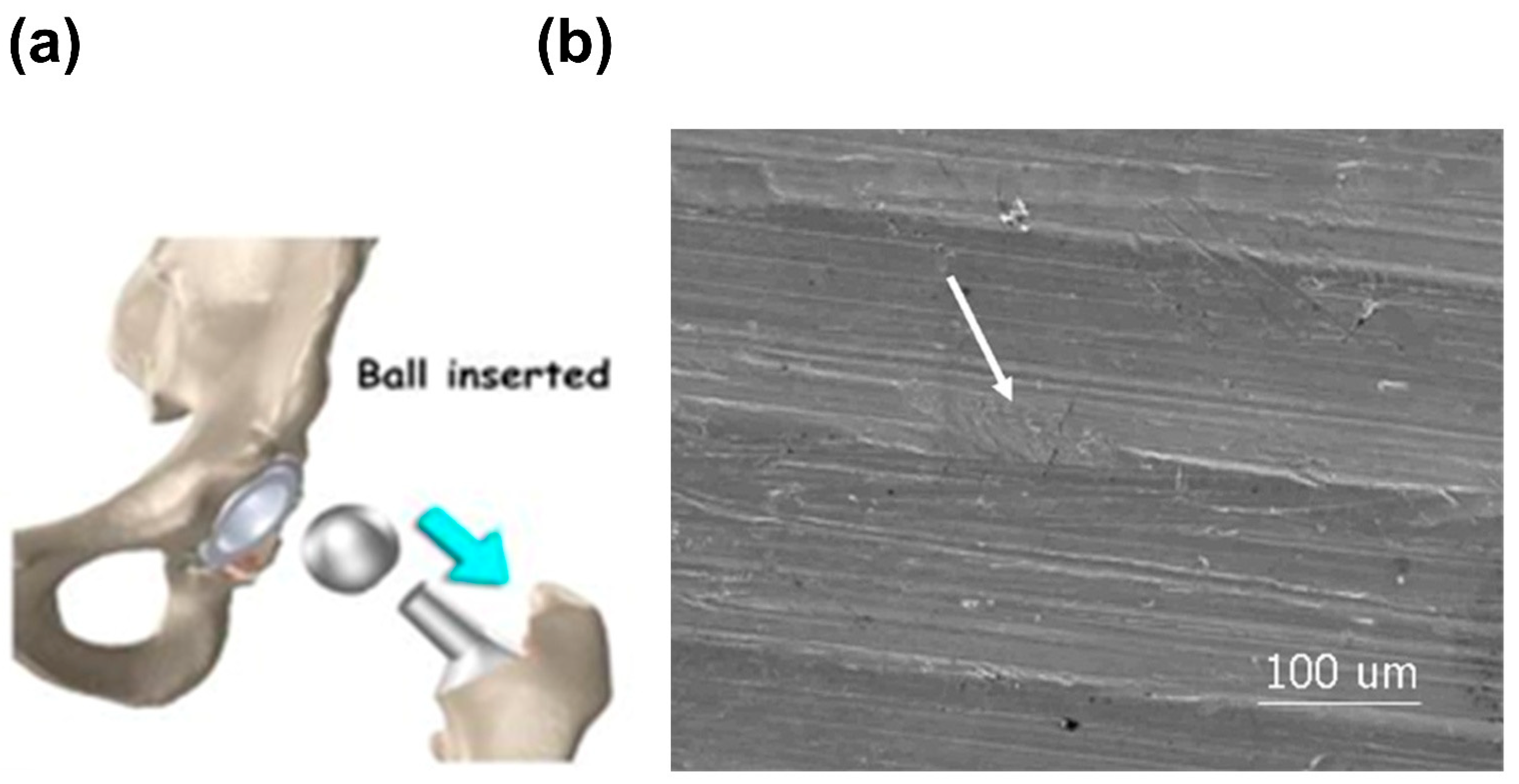
2.1.5. Fretting Corrosion
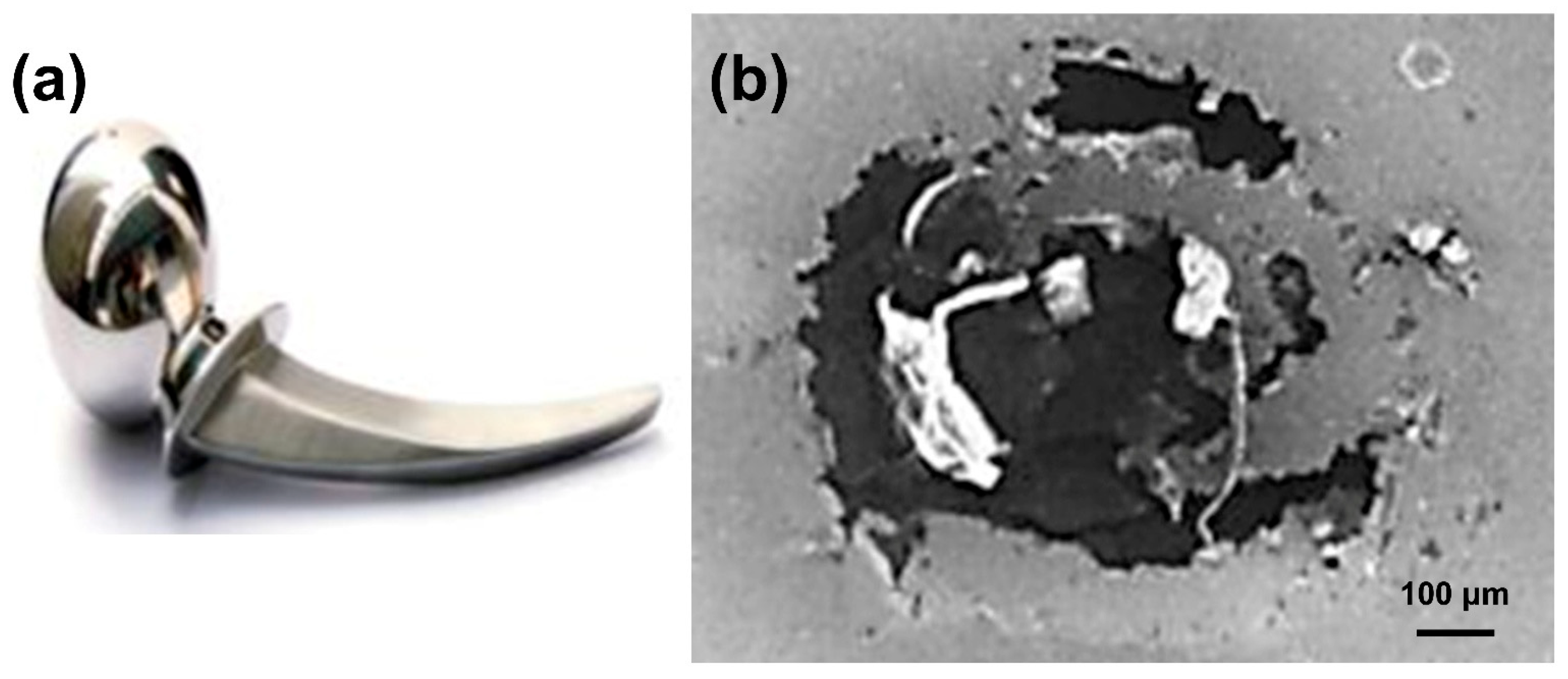
2.1.6. Galvanic Corrosion
2.2. Effect of Corrosion on the Human Body
3. Review of Literature
4. Anticorrosive Applications in Biomaterials for Medical Applications
4.1. Dental Applications
4.1.1. Orthodontic Wires and Brackets
EPD
Ion Plating
4.1.2. Dental Implants
Chemical Vapor Deposition
Sol–Gel Method
Thermal Oxidation
Acid Etching
4.2. Orthopedic Applications
4.2.1. Sol–Gel Method and EPD
4.2.2. Magnetron Sputtering and MAO
4.2.3. Hydrothermal Process
4.2.4. Laser Treatment
4.2.5. Chemical Self-Assembly
4.2.6. Laser Shock Peening (LSP)
4.2.7. EPD and Magnetron Sputtering
4.2.8. Pre-Anodization (PA) and MAO Techniques
4.2.9. Plasma Immersion Ion Implantation and Deposition (PIII&D)
4.2.10. Powder Immersion Reaction Assisted Coating (PIRAC) Technique
4.2.11. AIP
4.3. Cardiovascular Applications
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Guo, W.; Xu, P.; Zhang, S.; Zhang, Y. Progress in corrosion-resistant coatings on surface of low alloy steel. J. Iron Steel Res. Int. 2023, 30, 193–215. [Google Scholar] [CrossRef]
- Ananth, K.P.; Suganya, S.; Mangalaraj, D.; Ferreira, J.M.; Balamurugan, A. Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: An in vitro investigation. Mater. Sci. Eng. C 2013, 33, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.T.; Tang, C.Y.; Chen, L.; Wong, C.T.; Tsui, C.P. In vitro and in vivo performance of bioactive Ti6Al4V/TiC/HA implants fabricated by a rapid microwave sintering technique. Mater. Sci. Eng. C 2014, 42, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, M.; Felix, M.S.; Bernard, C.; Linares, J.M.; Chaves-Jacob, J.; Decherchi, P.; Dousset, E. Biocompatibility of four common orthopedic biomaterials following neuroelectromyostimulation: An in-vivo study. J. Biomed. Mater. Res. Part B 2018, 106, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Tu, J.; Jin, G.; Li, L.; Wang, K.; Wang, H. In vitro and in vivo investigations of a-C/a-C:Ti nanomultilayer coated Ti6Al4V alloy as artificial femoral head. Mater. Sci. Eng. C 2019, 99, 816–826. [Google Scholar] [CrossRef]
- Moon, B.S.; Kim, S.; Kim, H.E.; Jang, T.S. Hierarchical micro-nano structured Ti6Al4V surface topography via two-step etching process for enhanced hydrophilicity and osteoblastic responses. Mater. Sci. Eng. C 2017, 73, 90–98. [Google Scholar] [CrossRef]
- Salaie, R.N.; Besinis, A.; Le, H.; Tredwin, C.; Handy, R.D. The biocompatibility of silver and nanohydroxyapatite coatings on titanium dental implants with human primary osteoblast cells. Mater. Sci. Eng. C 2020, 107, 110210. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, W.; Gao, R.; Zhang, H.; Dong, L.; Qin, J.; Wang, B.; Jia, W.; Li, X. Fatigue behavior and osseointegration of porous Ti-6Al-4V scaffolds with dense core for dental application. Mater. Des. 2020, 195, 108994. [Google Scholar] [CrossRef]
- Rafiee, K.; Naffakh-Moosavy, H.; Tamjid, E. The effect of laser frequency on roughness, microstructure, cell viability and attachment of Ti6Al4V alloy. Mater. Sci. Eng. C 2020, 109, 110637. [Google Scholar] [CrossRef]
- Sidambe, A.T.; Todd, I.; Hatton, P.V. Effects of build orientation induced surface modifications on the in vitro biocompatibility of electron beam melted Ti6Al4V. Powder Metall. 2016, 59, 57–65. [Google Scholar] [CrossRef]
- Amirnejad, M.; Rajabi, M.; Jamaati, R. The effect of crystallographic texture as a distinct effective parameter on the biocorrosion performance of Ti6Al4V alloy in PBS solution. Corros. Sci. 2021, 179, 109100. [Google Scholar] [CrossRef]
- Rao, Q.; Weng, L.; Zhang, J.; Liu, D.; Zhang, W.; Chen, S.; Chen, J.; Li, X.; Qiu, H.; Cao, Y.; et al. Research progress in superhydrophobic titanium-based implants for antibacterial applications. Coatings 2023, 13, 419. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants—A review. Colloids Surf. B 2019, 176, 494–506. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Bushroa, A.R.; Zalnezhad, E.; Sarraf, M.; Basirun, W.J.; Baradaran, S.; Nasiri-Tabrizi, B. Microstructural development and corrosion behavior of self-organized TiO2 nanotubes coated on Ti–6Al–7Nb. Ceram. Int. 2015, 41, 10844–10855. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, G.; Li, Z.; Zeng, X.; Xu, Y.; Zhao, S.; Hu, H.; Zhang, Y.; Ren, T. Tribological behavior of Ti-6Al-4V against cortical bone in different biolubricants. J. Mech. Behav. Biomed. Mater. 2019, 90, 460–471. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Zhang, Y.; Yao, Y.; Mo, Z.; Wang, L.; Fan, Y. Diagonal-symmetrical and midline-symmetrical unit cells with same porosity for bone implant: Mechanical properties evaluation. J. Bionic Eng. 2019, 16, 468–479. [Google Scholar] [CrossRef]
- Khalili, V.; Naji, H. Developing a mechanochemical surface pretreatment to increase the adhesion strength of hydroxyapatite electrophoretic coating on the NiTi alloy as a bone implant. Surf. Coat. Technol. 2020, 397, 125985. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Safavi, M.S.; Khalil-Allafi, J.; Visai, L. Improved osteogenic activity of NiTi orthopedic implant by HAp-Nb2O5 composite coatings: Materials and biological points of view. Biomater. Adv. 2023, 150, 213435. [Google Scholar] [CrossRef]
- Mozetič, M. Surface Modification to Improve Properties of Materials. Materials 2019, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V.; Rokaya, D.; Thunyakitpisal, P.; Qin, J.; Saengkiettiyut, K. Corrosion Resistance of Graphene oxide/Silver Coatings on Ni-Ti alloy and Expression of IL-6 and IL-8 in Human Oral Fibroblasts. Sci. Rep. 2020, 10, 3247. [Google Scholar] [CrossRef] [PubMed]
- Mahlooji, E.; Atapour, M.; Labbaf, S. Electrophoretic deposition of bioactive glass—Chitosan nanocomposite coatings on Ti-6Al-4V for orthopedic applications. Carbohydr. Polym. 2019, 226, 115299. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Abd El-Rahman, A.M. Synthesis and annealing effects on the properties of nanostructured Ti–Al–V–N coatings deposited by plasma enhanced magnetron sputtering. Mater. Chem. Phys. 2015, 149–150, 179–187. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Bandyopadhyay, A.; Bose, S. Plasma sprayed fluoride and zinc doped hydroxyapatite coated titanium for load-bearing implants. Surf. Coat. Technol. 2022, 440, 128464. [Google Scholar] [CrossRef]
- Cisternas, M.; Bhuyan, H.; Retamal, M.J.; Casanova-Morales, N.; Favre, M.; Volkmann, U.G.; Saikia, P.; Diaz-Droguett, D.E.; Mändl, S.; Manova, D.; et al. Study of nitrogen implantation in Ti surface using plasma immersion ion implantation & deposition technique as biocompatible substrate for artificial membranes. Mater. Sci. Eng. C 2020, 113, 111002. [Google Scholar]
- Tian, X.; Zhang, P.; Xu, J. Incorporating zinc ion into titanium surface promotes osteogenesis and osteointegration in implantation early phase. J. Mater. Sci. Mater. Med. 2023, 34, 55. [Google Scholar] [CrossRef] [PubMed]
- Kylychbekov, S.; Allamyradov, Y.; Khuzhakulov, Z.; Majidov, I.; Banga, S.; ben Yosef, J.; Duta, L.; Er, A.O. Bioactivity and mechanical properties of hydroxyapatite on Ti6Al4V and Si(100) surfaces by pulsed laser deposition. Coatings 2023, 13, 1681. [Google Scholar] [CrossRef]
- Xiao, G.; Zeng, H.; Xu, S.; Chen, C.; Zhao, Q.; Liu, X. Preparation of Ti species coating hydrotalcite by chemical vapor deposition for photodegradation of azo dye. J. Environ. Sci. 2017, 60, 14–23. [Google Scholar] [CrossRef]
- Zong, M.; Bai, L.; Liu, Y.; Wang, X.; Zhang, X.; Huang, X.; Hang, R.; Tang, B. Antibacterial ability and angiogenic activity of Cu-Ti-O nanotube arrays. Mater. Sci. Eng. C 2017, 71, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Fu, Q.; Xiang, T.; Hu, W.; Wang, J.; Ding, S.; Liu, P.; Chen, Z. Fabrication of a micro-nanostructured superhydrophobic aluminum surface with excellent corrosion resistance and anti-icing performance. Rsc Adv. 2016, 6, 79389–79400. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Geng, Z.; Huang, X.; Hang, R.; Ma, Y.; Yao, X.; Tang, B. Microstructure and cytotoxicity evaluation of duplex-treated silver-containing antibacterial TiO2 coatings. Mater. Sci. Eng. C 2014, 45, 402–410. [Google Scholar] [CrossRef]
- Yan, Q.; Xue, T.; Liu, S.; Wang, W.; Wang, Y.; Song, X.; Yang, X.; Shang, W. A comparative study of surface characterization and corrosion behavior of micro-arc oxidation treated Ti-6Al-4V alloy prepared by SEBM and SLM. J. Iron Steel Res. Int. 2023, 30, 165–175. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Tian, B.; Xie, D.B.; Wang, F.H. Corrosion behavior of TiN and TiN/Ti composite films on Ti6Al4V alloy in Hank’s solution. J. Appl. Electrochem. 2008, 39, 447–453. [Google Scholar] [CrossRef]
- Zaveri, N.; McEwen, G.D.; Karpagavalli, R.; Zhou, A. Biocorrosion studies of TiO2 nanoparticle-coated Ti-6Al-4V implant in simulated biofluids. J. Nanopart. Res. 2009, 12, 1609–1623. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, X.; Bu, Y.; Zhang, J.; Zhang, J.; Wu, L. Photochemical grafting of fluorinate alkenes on DLC coated Ti6Al4V to improve in vitro cytocompatibility, friction and corrosion resistance. Surf. Coat. Technol. 2012, 208, 51–56. [Google Scholar] [CrossRef]
- Cui, W.F.; Jin, L.; Zhou, L. Surface characteristics and electrochemical corrosion behavior of a pre-anodized microarc oxidation coating on titanium alloy. Mater. Sci. Eng. C 2013, 33, 3775–3779. [Google Scholar] [CrossRef] [PubMed]
- Flamini, D.O.; Saidman, S.B. Corrosion behaviour of Nitinol alloy coated with alkylsilanes and polypyrrole. Mater. Sci. Eng. C 2014, 44, 317–325. [Google Scholar] [CrossRef]
- Chellappa, M.; Vijayalakshmi, U. Electrophoretic deposition of silica and its composite coatings on Ti-6Al-4V, and its in vitro corrosion behaviour for biomedical applications. Mater. Sci. Eng. C 2017, 71, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; He, X.; Gao, Y.; Zhang, X.; Yao, X.; Tang, B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C 2017, 75, 7–15. [Google Scholar] [CrossRef]
- Mthisi, A.; Popoola, A.P.I. Influence of Al2O3 addition on the hardness and in vitro corrosion behavior of laser synthesized Ti-Al2O3 coatings on Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2018, 100, 917–927. [Google Scholar] [CrossRef]
- Yigit, O.; Dikici, B.; Senocak, T.C.; Ozdemir, N. One-step synthesis of nano-hydroxyapatite/graphene nanosheet hybrid coatings on Ti6Al4V alloys by hydrothermal method and their in-vitro corrosion responses. Surf. Coat. Technol. 2020, 394, 125858. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Zhao, H.; Zhang, G.; Ren, T.; Zhang, Y. Enhanced anticorrosion and antiwear properties of Ti-6Al-4V alloys with laser texture and graphene oxide coatings. Tribol. Int. 2020, 152, 106475. [Google Scholar] [CrossRef]
- Yang, C.; Cao, W.; Yang, Z.; Wang, M.; Jing, X.; Tian, Y. The study on the anti-corrosion performance of NiTi alloy in human body solution with the fabricating processes of laser irradiation and PDMS modification. J. Bionic Eng. 2021, 18, 77–91. [Google Scholar] [CrossRef]
- Tian, P.; Zhao, X.; Sun, B.; Cao, H.; Zhao, Y.; Yan, J.; Xue, Y.; Lin, H.; Han, S.; Ren, T.; et al. Enhanced anticorrosion and tribological properties of Ti6Al4V alloys with Fe3O4/HA coatings. Surf. Coat. Technol. 2022, 433, 128118. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. Electrodeposited hydroxyapatite-based biocoatings: Recent progress and future challenges. Coatings 2021, 11, 110. [Google Scholar] [CrossRef]
- Safavi, M.S.; Surmeneva, M.A.; Surmenev, R.A.; Khalil-Allafi, J. RF-magnetron sputter deposited hydroxyapatite-based composite & multilayer coatings: A systematic review from mechanical, corrosion, and biological points of view. Ceram. Int. 2021, 47, 3031–3053. [Google Scholar]
- Safavi, M.S.; Walsh, F.C.; Visai, L.; Khalil-Allafi, J. Progress in niobium oxide-containing coatings for biomedical applications: A critical review. ACS Omega 2022, 7, 9088–9107. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T. In vivo metallic biomaterials and surface modification. Mater. Sci. Eng. A 1999, 267, 260–266. [Google Scholar] [CrossRef]
- Gilbert, J.L. Corrosion in the human body: Metallic implants in the complex body environment. Corrosion 2017, 73, 1478–1495. [Google Scholar] [CrossRef]
- Manivasagam, G.; Dhinasekaran, D.; Rajamanickam, A. Biomedical implants corrosion and its prevention—A review. Recent Pat. Corros. Sci. 2010, 2, 40–54. [Google Scholar] [CrossRef]
- Liens, A.; Ter-Ovanessian, B.; Courtois, N.; Fabregue, D.; Wada, T.; Kato, H.; Chevalier, J. Effect of alloying elements on the microstructure and corrosion behavior of TiZr-based bulk metallic glasses. Corros. Sci. 2020, 177, 108854. [Google Scholar] [CrossRef]
- Shtefan, V.; Navas, N.F.; Kaban, I.; Hantusch, M.; Gebert, A. Pitting corrosion mechanisms of Ti-Cu-(Pd-) based metallic glasses in simulated physiological solution. Corros. Sci. 2025, 251, 112913. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, D.; Wang, S.; Ma, Y.; Chen, J.; Wang, Y.; Zhou, H. Effect of hydrogen charging on microstructural evolution and corrosion behavior of Ti-4Al-2V-1Mo-1Fe alloy. J. Mater. Sci. Technol. 2021, 60, 168–176. [Google Scholar] [CrossRef]
- Virtanen, S.; Milosev, I.; Gomez-Barrena, E.; Trebse, R.; Salo, J.; Konttinen, Y.T. Special modes of corrosion under physiological and simulated physiological conditions. Acta Biomater. 2008, 4, 468–476. [Google Scholar] [CrossRef]
- Liu, J.; Alfantazi, A.; Asselin, E. Effects of temperature and sulfate on the pitting corrosion of titanium in high-temperature chloride solutions. J. Electrochem. Soc. 2015, 162, C189–C196. [Google Scholar] [CrossRef]
- Jirarungsatian, C.; Prateepasen, A. Pitting and uniform corrosion source recognition using acoustic emission parameters. Corros. Sci. 2010, 52, 187–197. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science, 3rd ed.; Springer: New York, NY, USA, 2010; pp. 263–313. [Google Scholar]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M. Corrosion of titanium: Part 1: Aggressive environments and main forms of degradation. J. Appl. Biomater. Funct. Mater. 2017, 15, e291–e302. [Google Scholar] [CrossRef] [PubMed]
- Hollis, A.C.; Scully, J.C. The stress corrosion cracking and hydrogen embrittlement of titanium in methanolhydrochloric acid solutions. Corros. Sci. 1993, 34, 821–835. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Singh Raman, R.K. A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. Jom 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Sudarshan, T.S.; Srivatsan, T.S.; Harvey, D.P., II. Fatigue processes in metals—Role of aqueous environments. Eng. Fract. Mech. 1990, 36, 827–852. [Google Scholar] [CrossRef]
- Larrosa, N.O.; Akid, R.; Ainsworth, R.A. Corrosion-fatigue: A review of damage tolerance models. Int. Mater. Rev. 2017, 63, 283–308. [Google Scholar] [CrossRef]
- Giordani, E.J.; Guimarães, V.A.; Pinto, T.B.; Ferreira, I. Effect of precipitates on the corrosion-fatigue crack initiation of ISO 5832-9 stainless steel biomaterial. Int. J. Fatigue 2004, 26, 1129–1136. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M.; Barril, S. Third body effects and material fluxes in tribocorrosion systems involving a sliding contact. Wear 2004, 256, 517–524. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Zheng, Y.; Yuan, G. Fretting properties of biodegradable Mg-Nd-Zn-Zr alloy in air and in Hank’s solution. Sci. Rep. 2016, 6, 35803. [Google Scholar] [CrossRef]
- Ali, M.M.; Raman, S.G.S.; Pathak, S.D.; Gnanamoorthy, R. Influence of plasma nitriding on fretting wear behaviour of Ti-6Al-4V. Tribol. Int. 2010, 43, 152–160. [Google Scholar]
- Warlimont, H. Springer Handbook of Materials Data, 3rd ed.; Springer: Cham, Switzerland, 2018; pp. 271–278. [Google Scholar]
- Lucas, L.C.; Buchanan, R.A.; Lemons, J.E. Investigations on the galvanic corrosion of multialloy total hip prostheses. J. Biomed. Mater. Res. 1981, 15, 731–747. [Google Scholar] [CrossRef]
- Rostoker, W.; Pretzel, C.W.; Galante, J.O. Couple corrosion among alloys for skeletal prostheses. J. Biomed. Mater. Res. 1974, 8, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H. Is galvanic corrosion between titanium alloy and stainless steel spinal implants a clinical concern? Spine J. 2004, 4, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.S.; Serra, G.G.; Muller, C.A.; Andrade, L.R.; Palermo, E.F.; Elias, C.N.; Meyers, M. Titanium alloy mini-implants for orthodontic anchorage: Immediate loading and metal ion release. Acta Biomater. 2007, 3, 331–339. [Google Scholar] [CrossRef]
- Latysh, V.; Krallics, G.; Alexandrov, I.; Fodor, A. Application of bulk nanostructured materials in medicine. Curr. Appl. Phys. 2006, 6, 262–266. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles-A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef]
- Hussain, H.D.; Ajith, S.D.; Goel, P. Nickel release from stainless steel and nickel titanium archwires—An in vitro study. J. Oral Biol. Craniofac. Res. 2016, 6, 213–218. [Google Scholar] [CrossRef][Green Version]
- Berglund, F.; Carlmark, B. Titanium, sinusitis, and the yellow nail syndrome. Biol. Trace Elem. Res. 2011, 143, 1–7. [Google Scholar] [CrossRef]
- Ataya, A.; Kline, K.P.; Cope, J.; Alnuaimat, H. Titanium exposure and yellow nail syndrome. Respir. Med. Case Rep. 2015, 16, 146–147. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Ostlere, S.J.; McLardy-Smith, P.; Athanasou, N.A.; Gill, H.S.; Murray, D.W. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: Prevalence and metal ion study. J. Arthroplast. 2011, 26, 511–518. [Google Scholar] [CrossRef]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; De Smet, K.; Amstutz, H.C. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin. Orthop. Relat. Res. 2010, 468, 2321–2327. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Della Valle, C.J.; Berger, R.A.; Tetreault, M.; Paprosky, W.G.; Sporer, S.M.; Jacobs, J.J. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J. Bone Jt. Surg. 2012, 94, 1655–1661. [Google Scholar] [CrossRef]
- Cooper, H.J.; Urban, R.M.; Wixson, R.L.; Meneghini, R.M.; Jacobs, J.J. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J. Bone Jt. Surg. 2013, 95, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; O’Dell, N.L.; Singh, B.B.; Ghazi, M.; Whitford, G.M.; Lockwood, P.E. Relating nickel-induced tissue inflammation to nickel release in vivo. J. Biomed. Mater. Res. 2001, 58, 537–544. [Google Scholar] [CrossRef]
- Fage, S.W.; Muris, J.; Jakobsen, S.S.; Thyssen, J.P. Titanium: A review on exposure, release, penetration, allergy, epidemiology, and clinical reactivity. Contact Dermat. 2016, 74, 323–345. [Google Scholar] [CrossRef]
- Lee, T.H.; Wang, C.C.; Huang, T.K.; Chen, L.K.; Chou, M.Y.; Huang, H.H. Corrosion resistance of titanium-containing dental orthodontic wires in fluoride-containing artificial saliva. J. Alloys Compd. 2009, 488, 482–489. [Google Scholar] [CrossRef]
- Stancheva, M.; Bojinov, M. Influence of fluoride content on the barrier layer formation and titanium dissolution in ethylene glycol–water electrolytes. Electrochim. Acta 2012, 78, 65–74. [Google Scholar] [CrossRef]
- Barao, V.A.R.; Mathew, M.T.; Assuncao, W.G.; Yuan, J.C.; Wimmer, M.A.; Sukotjo, C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH—An electrochemical study. Clin. Oral Implant. Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef]
- Vieira, A.C.; Ribeiro, A.R.; Rocha, L.A.; Celis, J.P. Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear 2006, 261, 994–1001. [Google Scholar] [CrossRef]
- Topolska, J.M.; Jagielska, A.; Motyl, S.; Kozub-Budzyń, G.A.; Kępa, L.; Wagner, B.; Wątor, K. Metal leakage from orthodontic appliances chemically alters enamel surface during experimental in vitro simulated treatment. Sci. Rep. 2024, 14, 5412. [Google Scholar] [CrossRef]
- Arora, V.; Pawar, V.R.; Sukumaran, K.; Varthini, N.P.; Kumar, A.V.; Seema, S.; Krishnan, M. Corrosion resistance of surface modified nickel titanium archwires. Angle Orthod. 2014, 84, 358–367. [Google Scholar]
- Li, G.; Zhang, L.; Cai, F.; Yang, Y.; Wang, Q.; Zhang, S. Characterization and corrosion behaviors of TiN/TiAlN multilayer coatings by ion source enhanced hybrid arc ion plating. Surf. Coat. Technol. 2019, 366, 355–365. [Google Scholar] [CrossRef]
- Sugisawa, H.; Kitaura, H.; Ueda, K.; Kimura, K.; Ishida, M.; Ochi, Y.; Kishikawa, A.; Ogawa, S.; Takano-Yamamoto, T. Corrosion resistance and mechanical properties of titanium nitride plating on orthodontic wires. Dent. Mater. J. 2018, 37, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, P.K.; Lin, G.; Yang, D. Effects of Ti/TiN multilayer on corrosion resistance of nickel–titanium orthodontic brackets in artificial saliva. Corros. Sci. 2007, 49, 3783–3796. [Google Scholar] [CrossRef]
- Malhotra, R.; Han, Y.M.; Morin, J.L.P.; Luong-Van, E.K.; Chew, R.J.J.; Castro Neto, A.H.; Nijhuis, C.A.; Rosa, V. Inhibiting corrosion of biomedical-grade Ti-6Al-4V alloys with graphene nanocoating. J. Dent. Res. 2020, 99, 285–292. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of hydroxyapatite-iron oxide-chitosan composite coatings on Ti–13Nb–13Zr alloy for biomedical applications. Thin Solid Film. 2020, 697, 137801. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, X.; Tang, H.; Zhan, Y. Enhancement of wear and corrosion resistance of low modulus beta-type Zr-20Nb-xTi (x = 0, 3) dental alloys through thermal oxidation treatment. Mater. Sci. Eng. C 2017, 76, 260–268. [Google Scholar] [CrossRef]
- Sun, Y.-S.; Huang, H.-H.; Tsai, Y.-H.; Kuo, Y.-L.; Lee, J.-W.; Lee, Y.-J.; Linn, T.Y.; Chen, P. Creating an extracellular matrix-like three-dimension structure to enhance the corrosion resistance and biological responses of titanium implants. J. Res. Sci. 2024, 19, S70–S80. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Souza, G.B.; Lepienski, C.M.; Kuromoto, N.K. Mechanical properties of anodic titanium films containing ions of Ca and P submitted to heat and hydrothermal treatment. J. Mech. Behav. Biomed. Mater. 2016, 64, 18–30. [Google Scholar] [CrossRef]
- Prodana, M.; Duta, M.; Ionita, D.; Bojin, D.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. A new complex ceramic coating with carbon nanotubes, hydroxyapatite and TiO2 nanotubes on Ti surface for biomedical applications. Ceram. Int. 2015, 41, 6318–6325. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-Ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Li, J.; He, X.; Hang, R.; Huang, X.; Tian, L.; Tang, B. Corrosion behavior of Zn-incorporated antibacterial TiO2 porous coating on titanium. Ceram. Int. 2016, 42, 17095–17100. [Google Scholar] [CrossRef]
- de Lima Almeida, L.; da Silva Ferreira, D.W.F.; de Andrade Santana, J.; Huck-Iriart, C.; Kunst, S.R.; Ferreira, J.Z.; Oliveira, C.T.; Sarmento, V.H.V. Effect of the addition of calcium salts on the structure and anticorrosion properties of siloxane-poly(hydroxyethyl methacrylate) hybrid coating applied on Ti-6Al-4V alloy. J. Sol-Gel Sci. Technol. 2020, 96, 690–701. [Google Scholar] [CrossRef]
- Hameed, P.; Gopal, V.; Bjorklund, S.; Ganvir, A.; Sen, D.; Markocsan, N.; Manivasagam, G. Axial suspension plasma spraying: An ultimate technique to tailor Ti6Al4V surface with HAp for orthopaedic applications. Colloids Surf. B 2019, 173, 806–815. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Peon, E.; Garcia-Galvan, F.R.; Barranco, V.; Parra, J.; Jimenez-Morales, A.; Galvan, J.C. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, D.; Qi, F.; Liu, W.; Chen, Y. Heat and hydrothermal treatments on the microstructure evolution and mechanical properties of plasma sprayed hydroxyapatite coatings reinforced with graphene nanoplatelets. J. Mech. Behav. Biomed. Mater. 2020, 101, 103418. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, K.; Basu, B. On the toughness enhancement in hydroxyapatite-based composites. Acta Biomater. 2013, 61, 5198–5215. [Google Scholar] [CrossRef]
- Parau, A.C.; Büyüksungur, S.; Li, G.; Liu, Q.; Badillo, E.; Blum, L.; Schmidt, J.; Pana, I.; Vitelaru, C.; Marinescu, I.M.; et al. Zn-doped CaP coating equips Ti implants with corrosion resistance, biomineralization, antibacterial and immunotolerant activities. J. Adv. Res. 2025; in press. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Komarova, E.G.; Kazantseva, E.A.; Luginin, N.A.; Kashin, A.D.; Uvarkin, P.V.; Sharkeev, Y.P. Enhanced Corrosion Resistance and Mechanical Durability of the Composite PLGA/CaP/Ti Scaffolds for Orthopedic Implants. Polymers 2024, 16, 826. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, G.; Li, Z.; Xu, Y.; Zeng, X.; Zhao, S.; Deng, J.; Hu, H.; Zhang, Y.; Ren, T. Microtribological properties of Ti 6Al 4V alloy treated with self-assembled dopamine and graphene oxide coatings. Tribol. Int. 2019, 137, 46–58. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Hu, J. Performances investigation of Ti6Al4V alloy modified by plasma nitriding + plasma enhanced chemical vapor deposition and laser remelting process in simulated body fluid. Met. Mater. Int. 2020, 27, 4757–4767. [Google Scholar] [CrossRef]
- Hussein, M.A.; Kumar, A.M.; Yilbas, B.S.; Al-Aqeeli, N. Laser nitriding of the newly developed Ti-20Nb-13Zr at.% biomaterial alloy to enhance its mechanical and corrosion properties in simulated body fluid. J. Mater. Eng. Perform. 2017, 26, 5553–5562. [Google Scholar] [CrossRef]
- Zhang, Y.; Addison, O.; Yu, F.; Troconis, B.C.R.; Scully, J.R.; Davenport, A.J. Time-dependent Enhanced Corrosion of Ti6Al4V in the Presence of H2O2 and Albumin. Sci. Rep. 2018, 8, 3185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, Y.; Shi, W.; Hou, B.; Qiao, H.; Qi, J.; Zhang, E.; Qin, G. Improvement of biocorrosion resistance and antibacterial performance of Ti-3Cu alloy subjected to laser shock peening. Opt. Laser Technol. 2023, 158, 108794. [Google Scholar] [CrossRef]
- Poon, R.W.Y.; Yeung, K.W.K.; Liu, X.Y.; Chu, P.K.; Chung, C.Y.; Lu, W.W.; Cheung, K.M.C.; Chan, D. Carbon plasma immersion ion implantation of nickel-titanium shape memory alloys. Biomaterials 2005, 26, 2265–2272. [Google Scholar] [CrossRef]
- Hendry, J.A.; Pilliar, R.M. The fretting corrosion resistance of PVD surface-modified orthopedic implant alloys. J. Biomed. Mater. Res. 2001, 58, 156–166. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Gotman, I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy. Biomaterials 2001, 22, 1853–1859. [Google Scholar] [CrossRef]
- Pohrelyuk, I.M.; Fedirko, V.M.; Tkachuk, O.V.; Proskurnyak, R.V. Corrosion resistance of Ti-6Al-4V alloy with nitride coatings in Ringer’s solution. Corros. Sci. 2013, 66, 392–398. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Tamborim, S.M.; dos Santos, C.B.; Müller, I.L. Tribological, electrochemical and tribo-electrochemical characterization of bare and nitrided Ti6Al4V in simulated body fluid solution. Corros. Sci. 2011, 53, 1786–1793. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Smith, A.G.C.; Sable, C.A.; Echko, M.M.; Wilner, L.B.; Olsen, H.E.; Atalay, H.T.; Awasthi, A.; Bhutta, Z.A.; Boucher, J.L. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Yachia, D.; Beyar, M. New treatment modality for penile urethral strictures using a self-expanding and self-retaining coil stent: Urocoil®. Eur. Urol. 1993, 24, 500–504. [Google Scholar] [CrossRef]
- Kong, H.F.; Wilkinson, J.L.; Coe, J.Y.; Gu, X.P.; Urness, M.; Kim, T.H.; Bass, J.L. Corrosive behaviour of Amplatzer® devices in experimental and biological environments. Cardiol. Young 2002, 12, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.J.L.; Madamba, D.; Sivan, S.; Miyashiro, K.; Dreher, M.L.; Trepanier, C.; Nagaraja, S. The effects of surface processing on in-vivo corrosion of Nitinol stents in a porcine model. Acta Biomater. 2017, 62, 385–396. [Google Scholar] [CrossRef]





| Fabrication Technique | Coating | Substrate | Improved Properties | Application | Year of Publication | Reference |
|---|---|---|---|---|---|---|
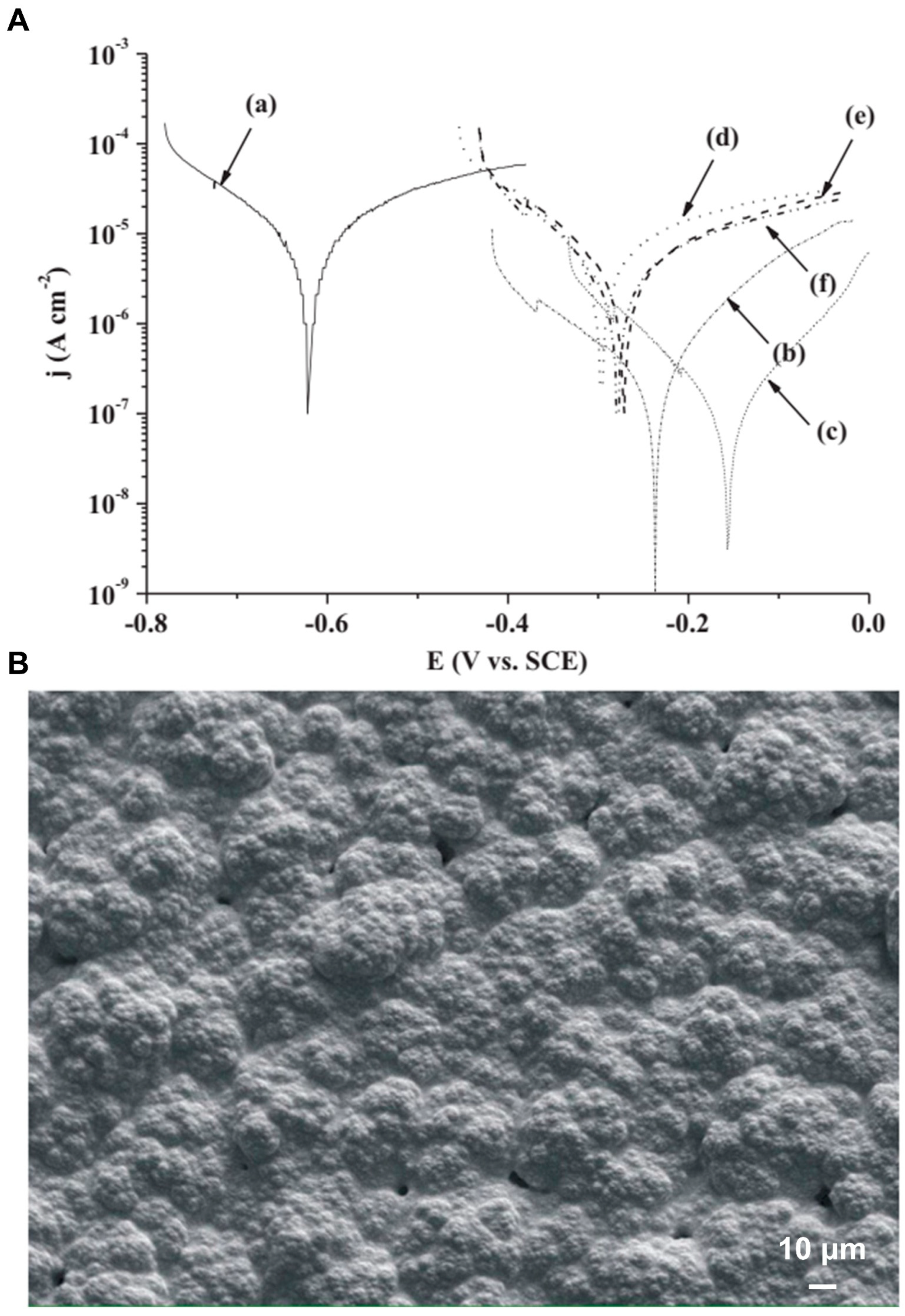
| Arc ion plating (AIP) | TiN and TiN/Ti coating | Ti-6Al-4V alloy | Corrosion resistance | Orthopedics | 2008 | [38] |
| Spin technique | TiO2 Nano-particles coating | Ti-6Al-4V alloy | Corrosion resistance | Orthopedics | 2009 | [39] |
| Magnetron sputtering and photochemical functionalization | F-DLC/ Ti coating | Ti-6Al-4V alloy | Anti-corrosion and friction, biocompatibility, and functional properties | Various applications | 2012 | [40] |
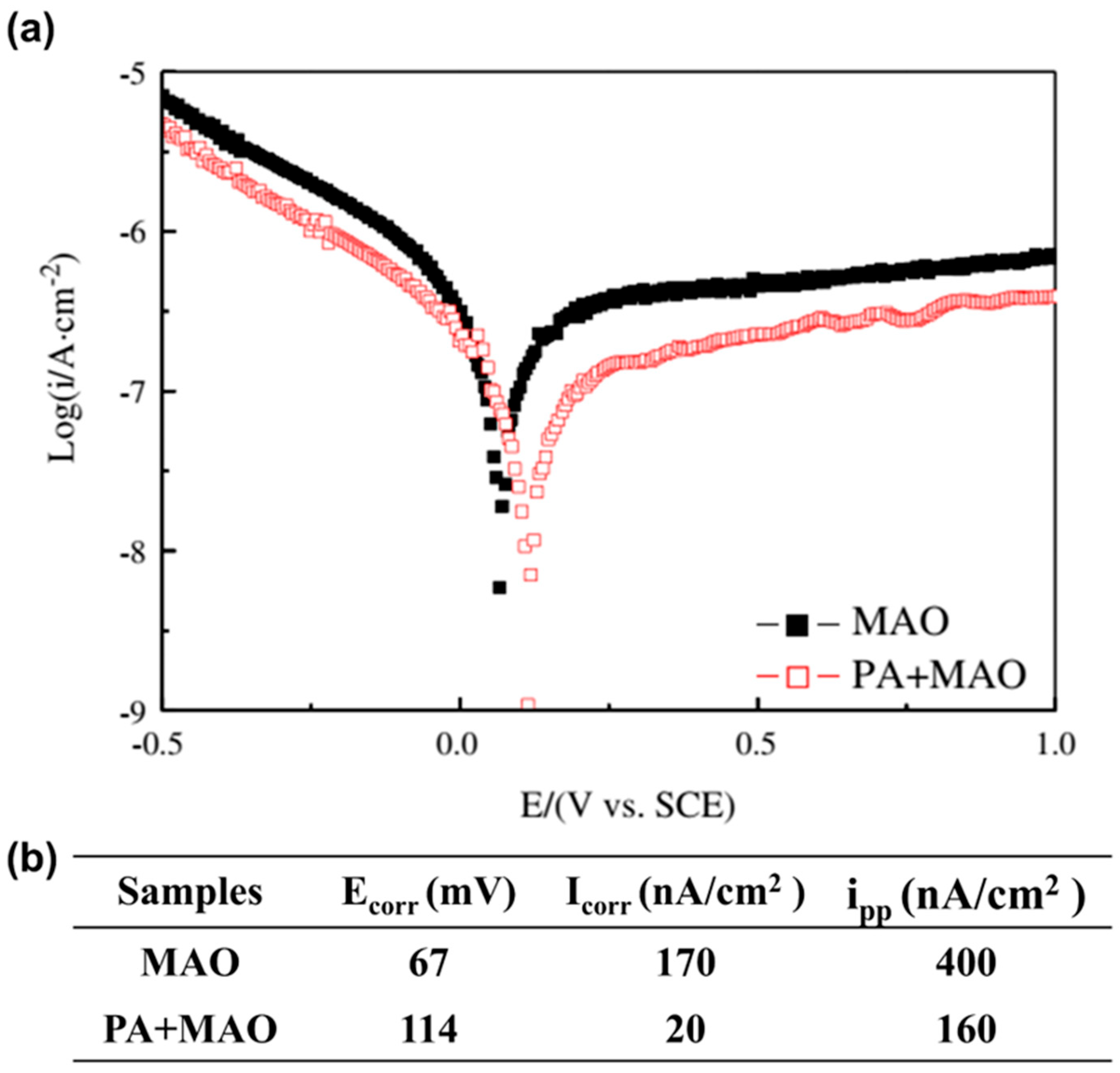
| Pre-anodization (PA) and micro-arc oxidation (MAO) techniques | Porous TiO2 coating | biomedical β Ti alloy | Corrosion resistance | Various applications | 2013 | [41] |
| Polydimethylsiloxane (PDMS) modification | Self-assembled film | NiTi alloy (NiTi) | Corrosion resistance | Biomedical applications | 2014 | [42] |
| Low thermal volatilization sol–gel method | SiO2 and ZnO composite coating | Ti-6Al-4V alloy | Corrosion resistance, mechanical properties | Dental and knee joint implants | 2017 | [43] |
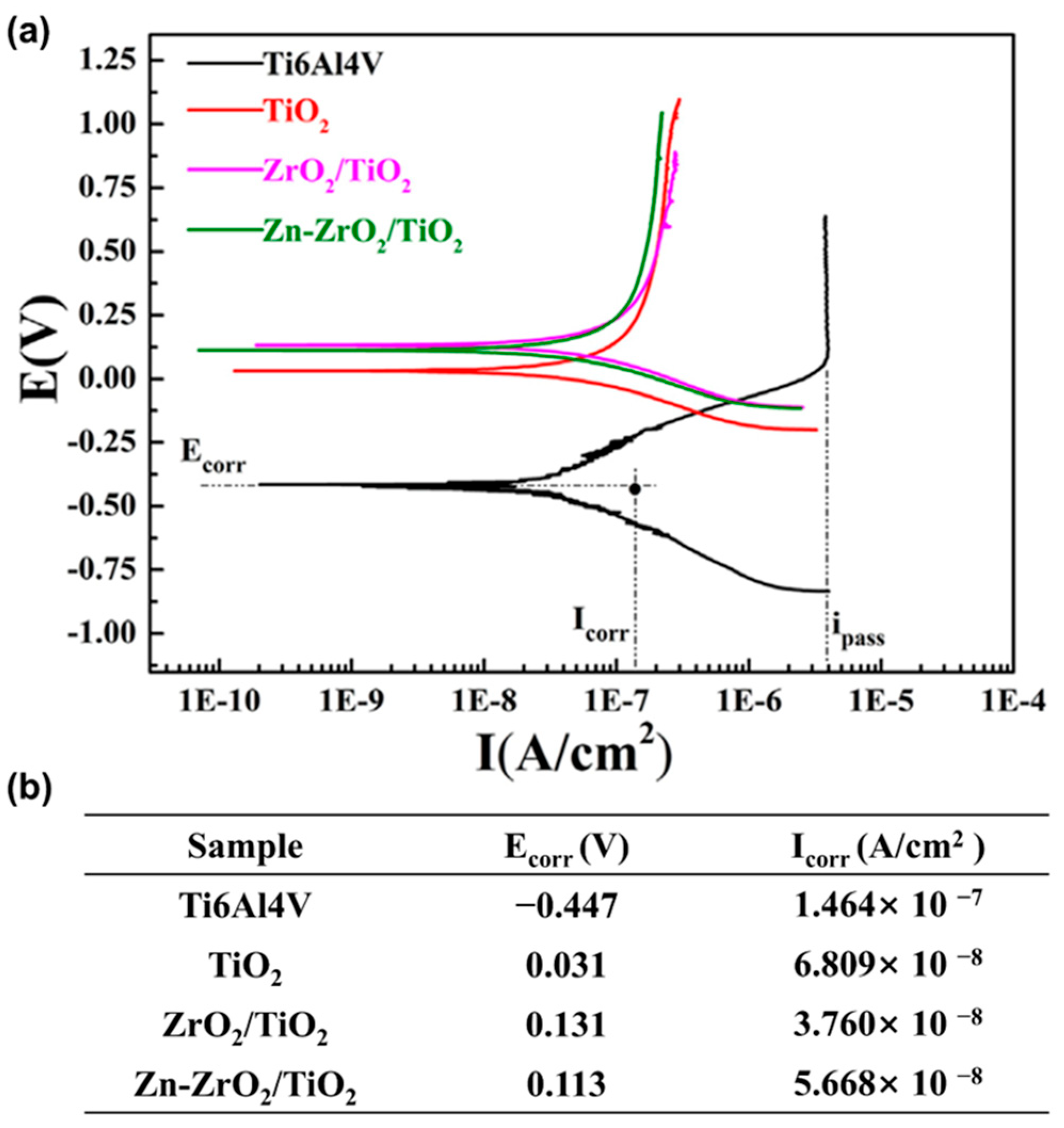
| Magnetron sputtering in combination with MAO | Zn-doped ZrO2/TiO2 coating | Ti-6Al-4V alloy | Anti-corrosive, antibacterial properties, and biocompatibility | orthopedics and dentistry | 2017 | [44] |
| Electrophoretic deposition technique and magnetron sputtering | Ti-Al2O3 coating | Ti-6Al-4V alloy | Anti-corrosive property and hardness | Orthopedics | 2018 | [45] |
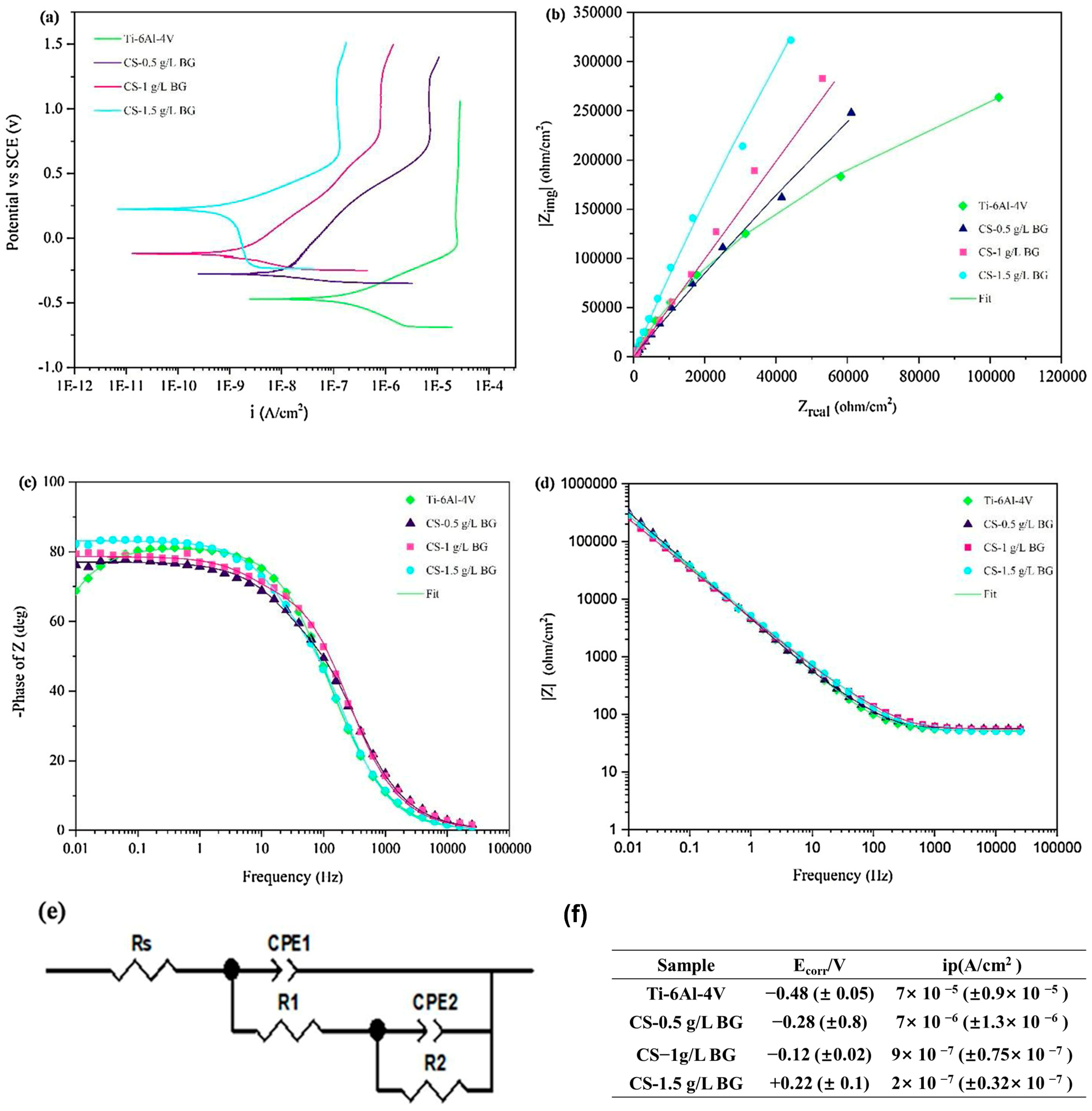
| Sol–gel technique and cathodic electrophoretic deposition (EPD) | Chitosan–Bioactive glass (CS-1.5 g/L BG) nanocomposite coating | Ti-6Al-4V alloy | Bio-corrosion, biological activity, and wetting behavior | Orthopedics | 2019 | [23] |
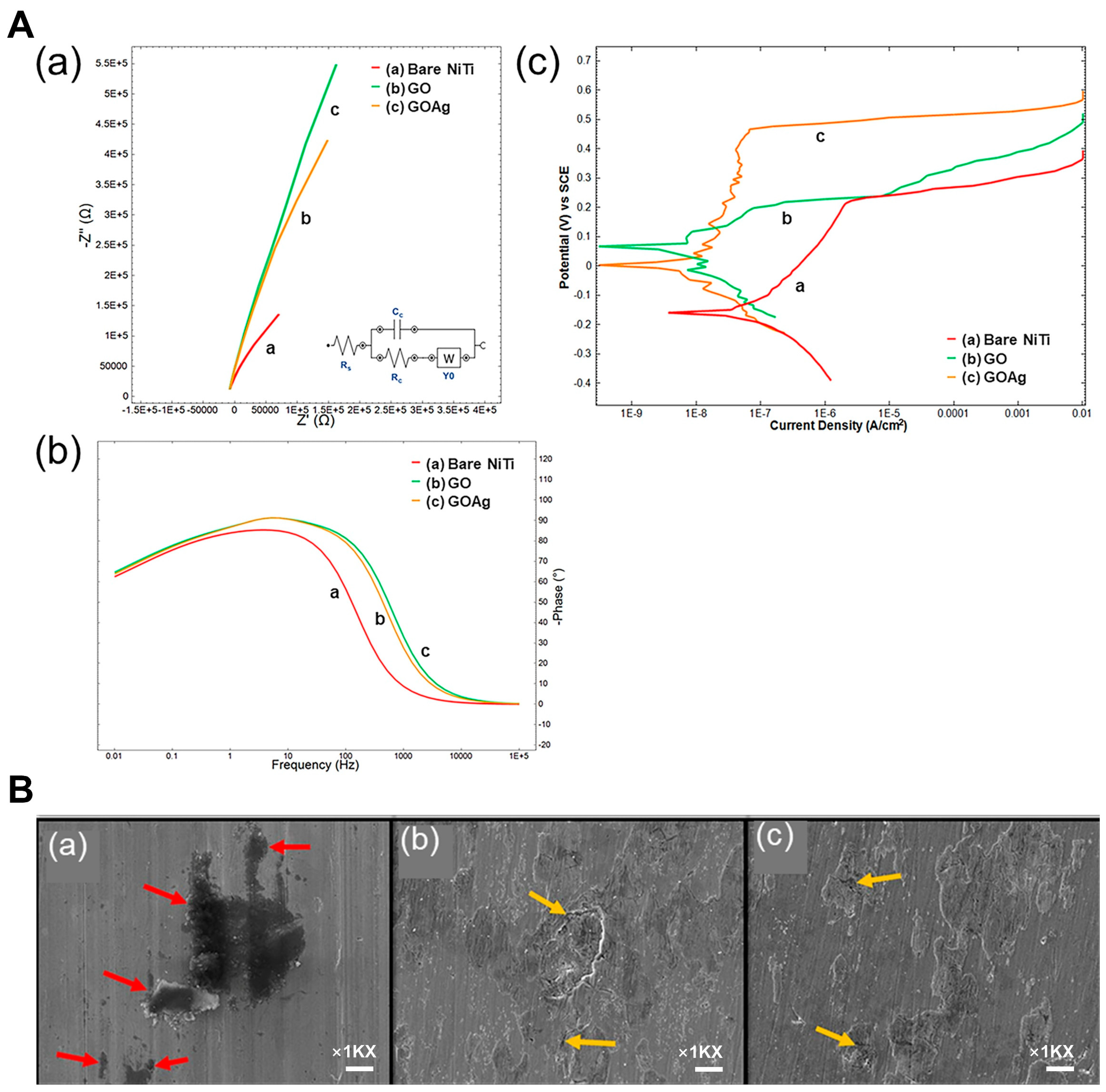
| EPD | Graphene oxide (GO) and GO/silver (GO/Ag) nanocomposite coatings | NiTi alloy | Corrosion resistance, biocompatibility | Orthodontic wires and brackets | 2020 | [22] |
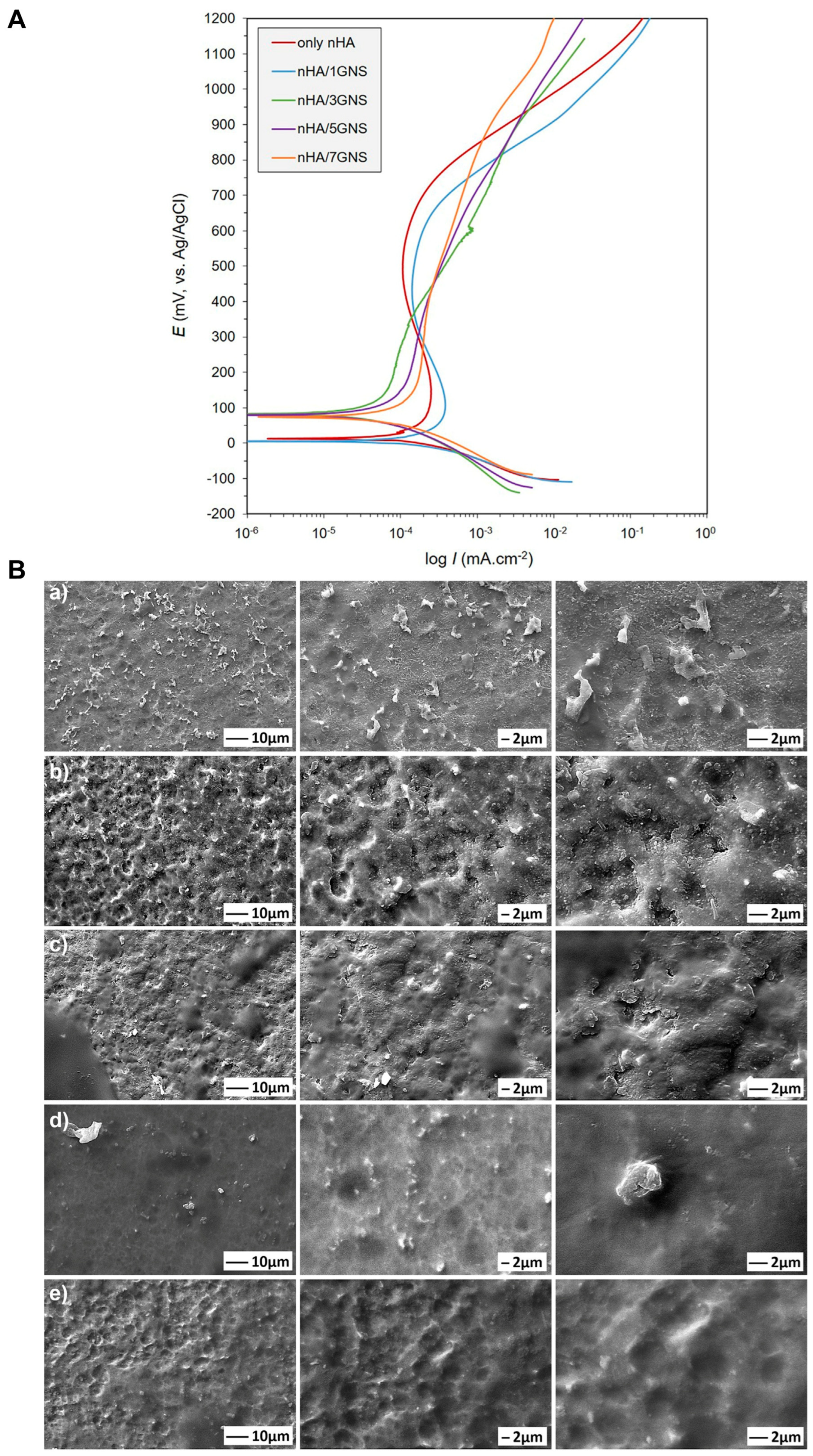
| Hydrothermal process | NanoHA/graphene nanosheet (nHA/GNS) composite coating | Ti-6Al-4V alloy | Corrosion resistance | Orthopedics | 2020 | [46] |
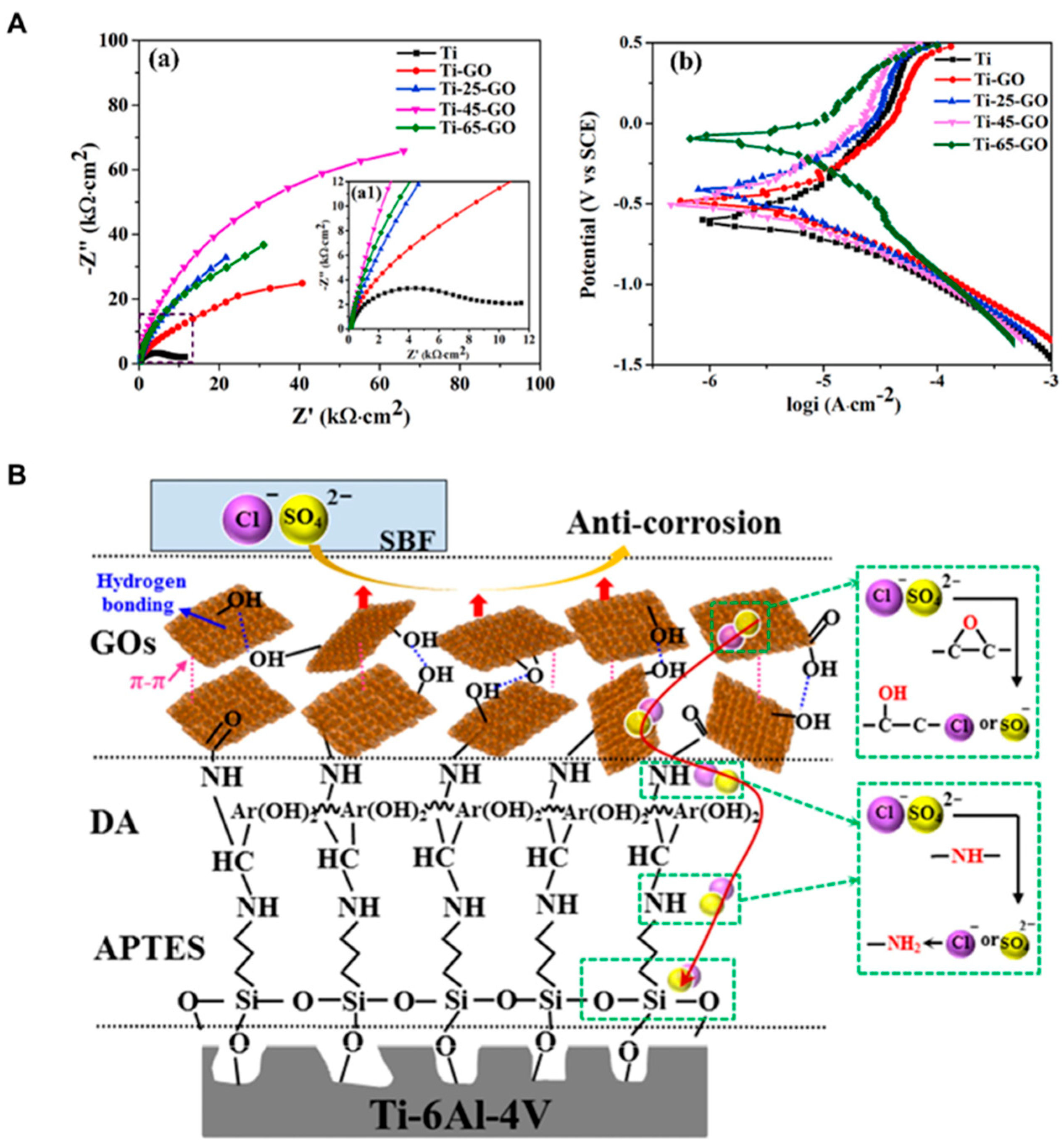
| Laser treatment and chemical assembly | Graphene oxide (GO) coating | Ti-6Al-4V alloy | Corrosion resistance | Biomedical application | 2020 | [47] |
| Laser treatment and polydimethylsiloxane (PDMS) modification | Superhydrophobic surface | NiTi alloy | Corrosion resistance | Medical device | 2021 | [48] |
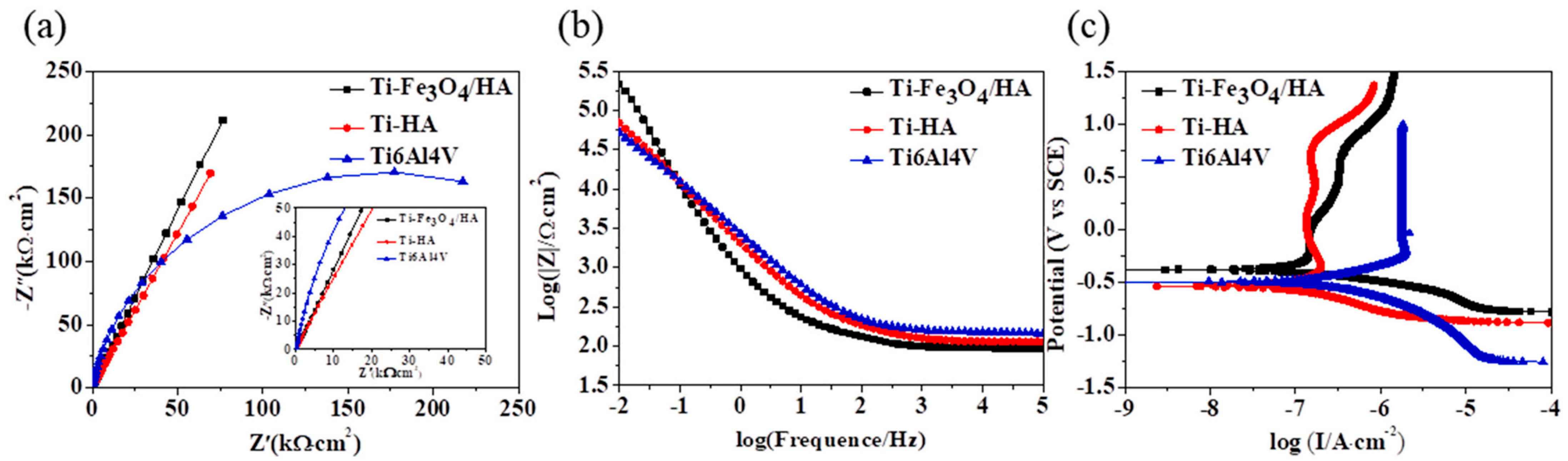
| Chemical self-assembly | Fe3O4-coated HA coating | Ti-6Al-4V alloy | Corrosion resistance | Biomedical application | 2022 | [49] |
| Specimens | Ecorr (V vs. SCE) | Icorr (µA/cm2) | η (%) |
|---|---|---|---|
| Bare NiTi | −0.170 | 0.158 | — |
| GO | 0.031 | 0.017 | 89.24 |
| GOAg | 0.008 | 0.002 | 98.73 |
| Specimen | Rs (Ω) | Rct (kΩ) | Cdl (µF) | Yo (µMho) |
|---|---|---|---|---|
| Bare NiTi | 16.20 | 16.80 | 36.90 | 8.29 |
| GO | 16.50 | −6.12 | 26.60 | 15.60 |
| GOAg | 16.50 | −5.04 | 47.20 | 23.20 |
| Sample | Ecorr (V) | Icorr (μA/cm2) |
|---|---|---|
| Uncoated | −0.602 | 1.1.5 × 10−8 |
| HA | −0.482 | 1.15 × 10−10 |
| Fe3O4 | −0.516 | 2.68 × 10−10 |
| HA@1Fe3O4 | −0.309 | 5.90 × 10−11 |
| HA@3Fe3O4 | −0.364 | 8.20 × 10−11 |
| HA@5Fe3O4 | −0.393 | 1.02 × 10−10 |
| Sample | Rs (Ω × cm2) | R1 (Ω × cm2) | CPE1-T (×10−5, Fsn−1cm−2) | CPE1-P | R2 (Ω × cm2) | CPE2-T (×10−5, Fsn−1cm−2) | CPE2-P | Chi-Squared (X2) |
|---|---|---|---|---|---|---|---|---|
| Ti-6Al-4V | 52.3 | 179.1 | 3.23 | 0.91 | 1.25 × 106 | 6.91 | 0.94 | 3 × 10−4 |
| CS-0.5 g/L BG | 55 | 779.7 | 3.21 | 0.89 | 107 | 1.11 | 0.93 | 7 × 10−4 |
| CS-1 g/L BG | 56.95 | 595.4 | 2.88 | 0.93 | 1.83 × 107 | 1.08 | 0.92 | 1 × 10−3 |
| CS-1.5 g/L BG | 51.38 | 3583 | 1.45 | 0.87 | 1.25 × 1010 | 1.03 | 0.89 | 1 × 10−3 |
| Coating | Ecorr (mV) | Icorr (×10−9, A·cm−2) | Corr. Rate (mpy) | Rp (Ω·cm2) |
|---|---|---|---|---|
| Only nHA | 12.87 | 115 | 0.052 | 75,303 |
| nHA/1GNS | 5.87 | 125 | 1090 | 120,429 |
| nHA/3GNS | 83.11 | 50 | 0.037 | 597,065 |
| nHA/5GNS | 79.89 | 55 | 0.065 | 776,167 |
| nHA/7GNS | 74.90 | 85 | 0.077 | 385,321 |
| Samples | Corrosion Potential (V) | Corrosion Current Density (A/cm2) |
|---|---|---|
| Ti | −0.572 | 1.894 × 10−6 |
| Ti-GO | −0.467 | 1.112 × 10−6 |
| Ti-25-GO | −0.425 | 4.031 × 10−7 |
| Ti-45-GO | −0.442 | 1.425 × 10−7 |
| Ti-65-GO | −0.102 | 3.894 × 10−7 |
| Samples | Corrosion Potential (mV) | Corrosion Current Density (nA/cm2) | Inhibition Efficiency (η) |
|---|---|---|---|
| Ti6Al4V | −489.53 | 1123.03 | - |
| Ti-HA | −532.59 | 157.99 | 85.93% |
| Ti-Fe3O4/HA | −324.60 | 153.33 | 86.33% |
| Samples | Rs (Ω⋅cm2) | CPE (μF/cm2) | n | Rp (107 Ω⋅cm2) |
|---|---|---|---|---|
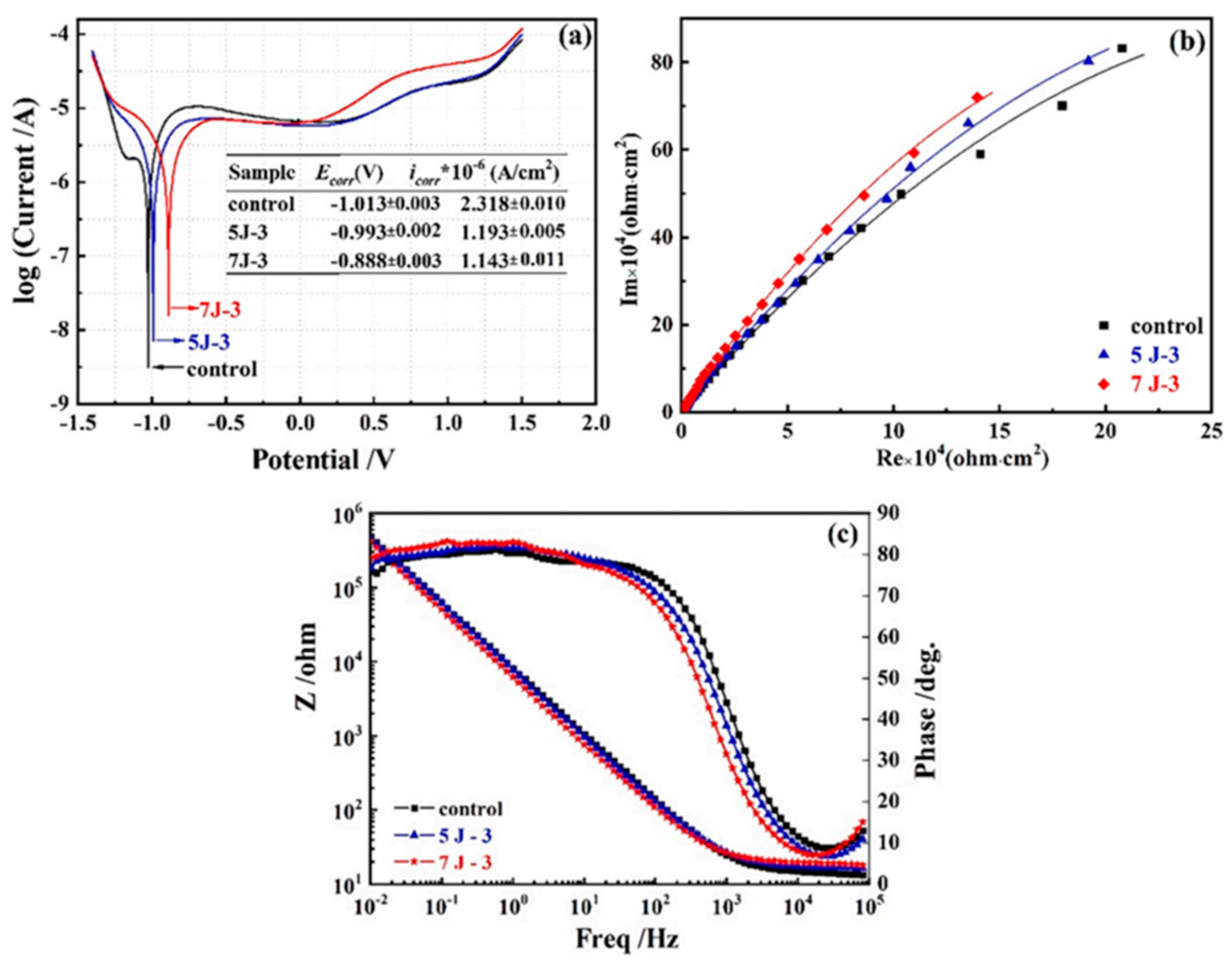
| control | 35.8 ± 1.10 | 9.55 ± 0.84 | 0.886 ± 0.20 | 2.36 ± 0.54 |
| 5 J-3 | 43.7 ± 1.05 | 10.01 ± 0.84 | 0.891 ± 0.21 | 3.58 ± 0.85 |
| 7 J-3 | 44.1 ± 1.26 | 11.92 ± 1.03 | 0.893 ± 0.26 | 5.05 ± 1.74 |
| Samples | Ecorr (V) | Icorr (nA·cm−2) |
|---|---|---|
| Ti6Al4V | −0.2380 | 837.96 |
| DLC/Ti | −0.1010 | 164.42 |
| F-DLC/Ti | 0.0399 | 82.025 |
| T, °C | Corrosion Parameters | Surface Condition | |||
|---|---|---|---|---|---|
| Untreated | Coating I | Coating II | Coating III | ||
| 36 | Ecor, V vs. Ag/AgCl | −0.425 | −0.225 | −0.24 | −0.11 |
| icor × 10−4, A/cm2 | 0.041 | 0.0055 | 0.0027 | 0.0037 | |
| 40 | Ecor, V vs. Ag/AgCl | −0.430 | −0.375 | −0.34 | −0.125 |
| icor × 10−4, A/cm2 | 0.019 | 0.0050 | 0.0070 | 0.02 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Q.; Zhang, J.; Chen, Y.; Yang, Y.; Chen, X.; Liu, D.; Zhu, R.; Li, A.; Lv, Y.; Zheng, S. Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications. Coatings 2025, 15, 599. https://doi.org/10.3390/coatings15050599
Rao Q, Zhang J, Chen Y, Yang Y, Chen X, Liu D, Zhu R, Li A, Lv Y, Zheng S. Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications. Coatings. 2025; 15(5):599. https://doi.org/10.3390/coatings15050599
Chicago/Turabian StyleRao, Qin, Jinshuang Zhang, Yaqing Chen, Yujin Yang, Xu Chen, Donghao Liu, Ruilu Zhu, Ang Li, Yanping Lv, and Shunli Zheng. 2025. "Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications" Coatings 15, no. 5: 599. https://doi.org/10.3390/coatings15050599
APA StyleRao, Q., Zhang, J., Chen, Y., Yang, Y., Chen, X., Liu, D., Zhu, R., Li, A., Lv, Y., & Zheng, S. (2025). Research Progress of the Coatings Fabricated onto Titanium and/or Titanium Alloy Surfaces in Biomaterials for Medical Applications for Anticorrosive Applications. Coatings, 15(5), 599. https://doi.org/10.3390/coatings15050599