Fluoro-Silicon-Modified Polythiourethane Copolymer for Marine Antifouling Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Si-PTU Marine Antifouling Coatings
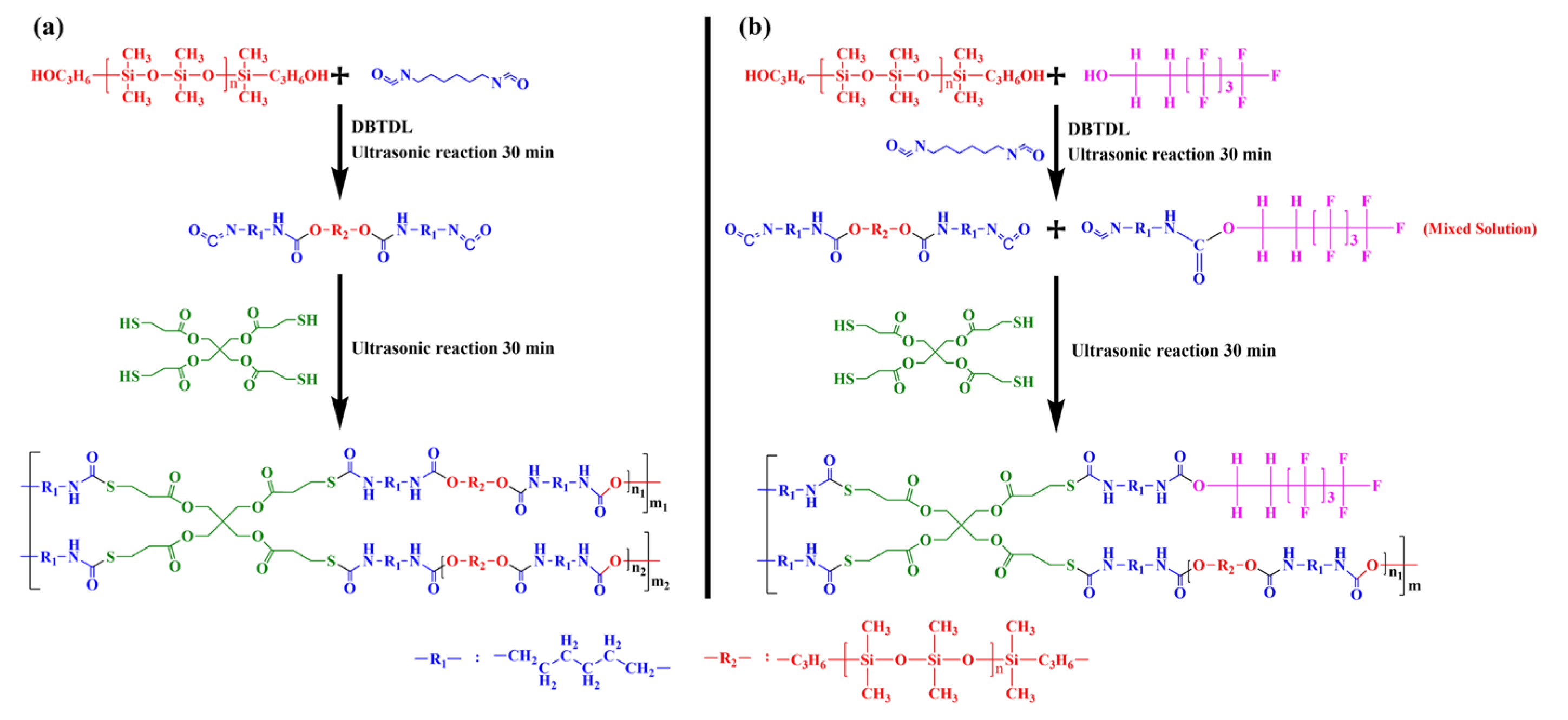
2.3. Preparation of FSi-PTU-x Marine Antifouling Coatings
2.4. Characterization
2.5. Adhesion Tests
2.6. Anti-Protein Adsorption Experiment
2.7. Navicula and Chlorella Vulgaris Colonization Assays
2.8. Actual Marine Test
3. Results and Discussion
3.1. FT-IR Characterization
3.2. Elemental Characterization
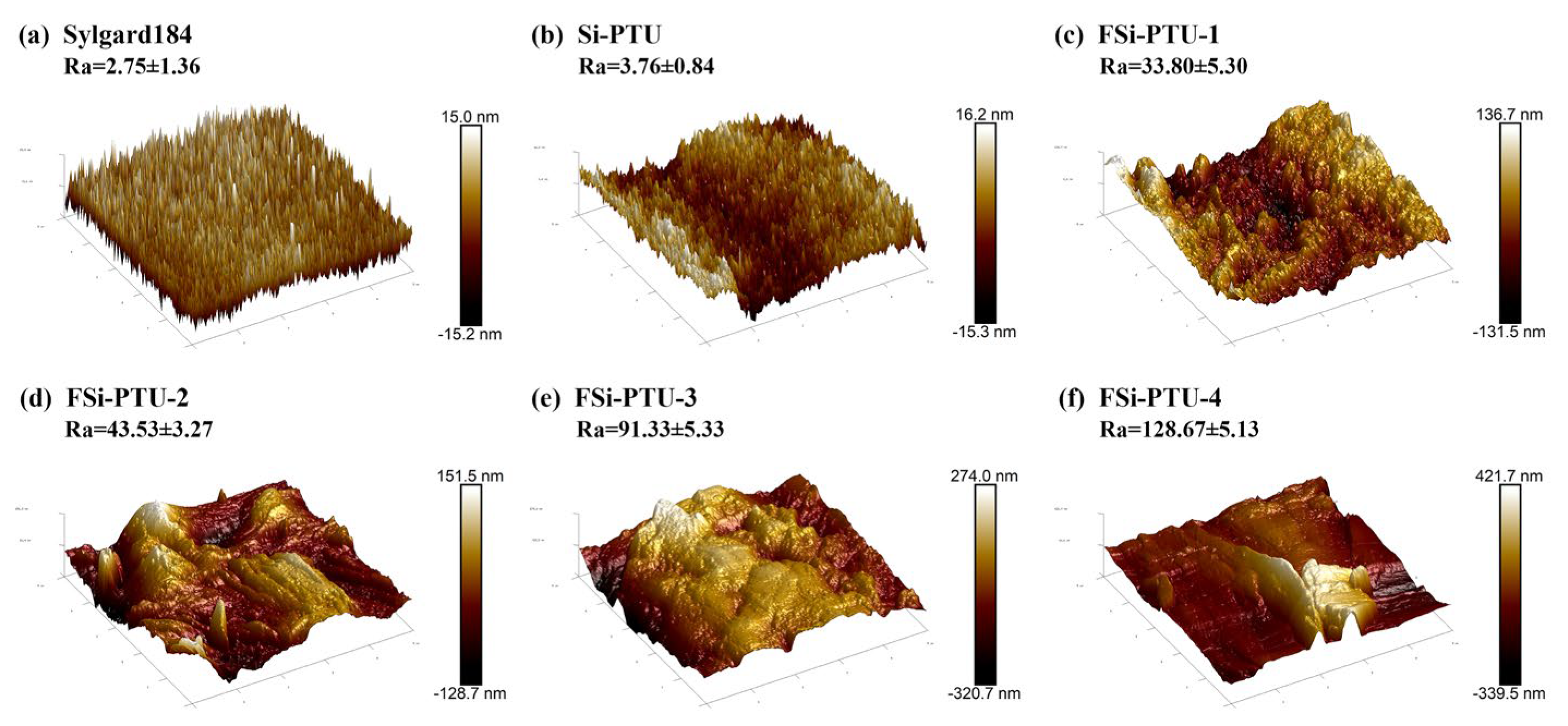
3.3. Topography and Surface Roughness
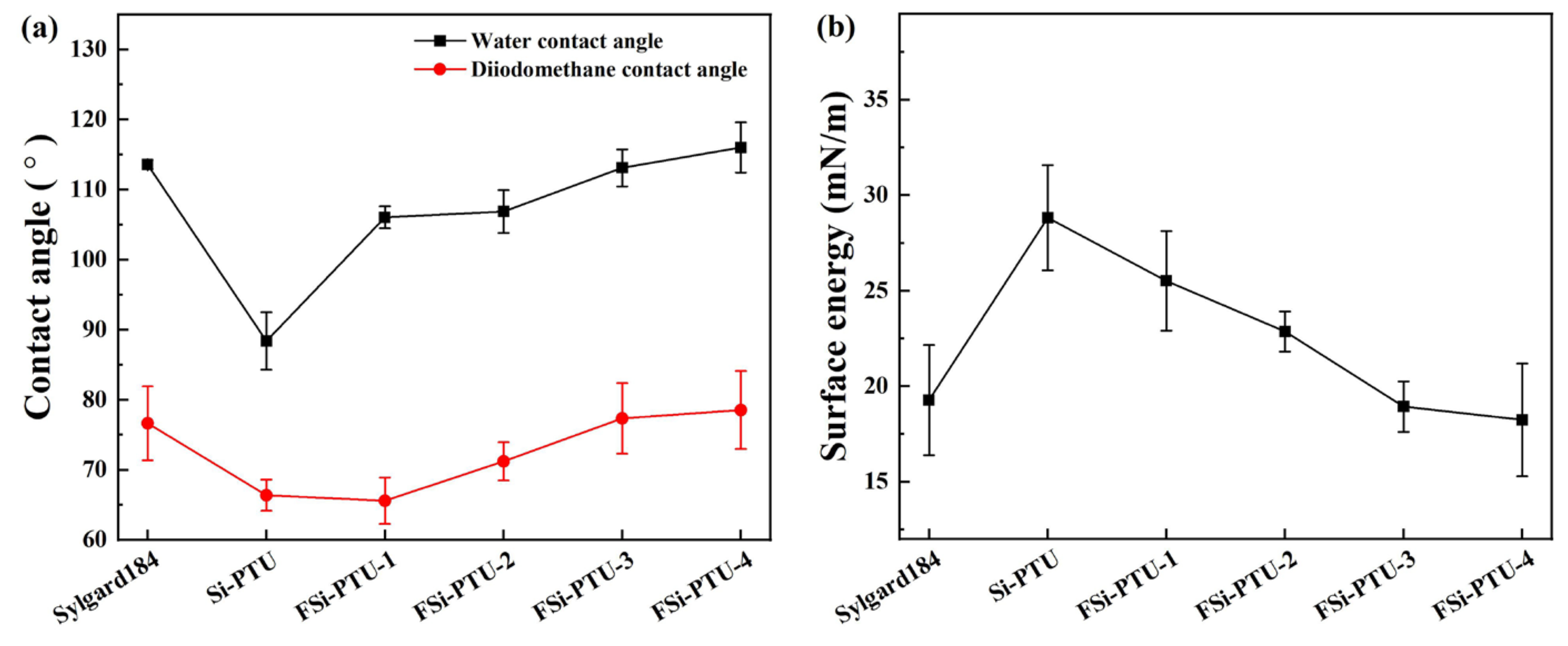
3.4. Water Contact Angle and Surface Energy
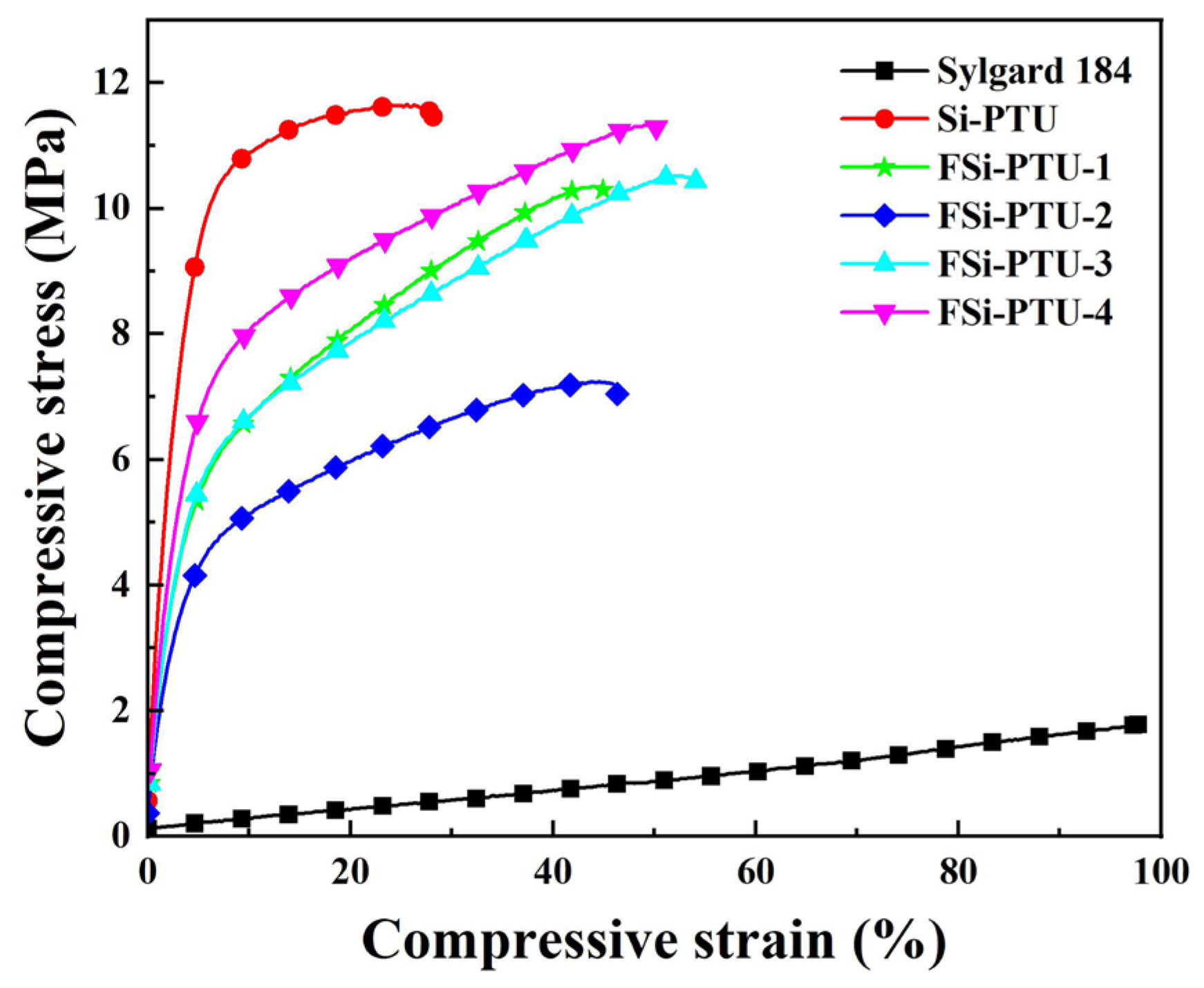
3.5. Tensile Test
3.6. Adhesion Test
3.7. Anti-Protein Adsorption
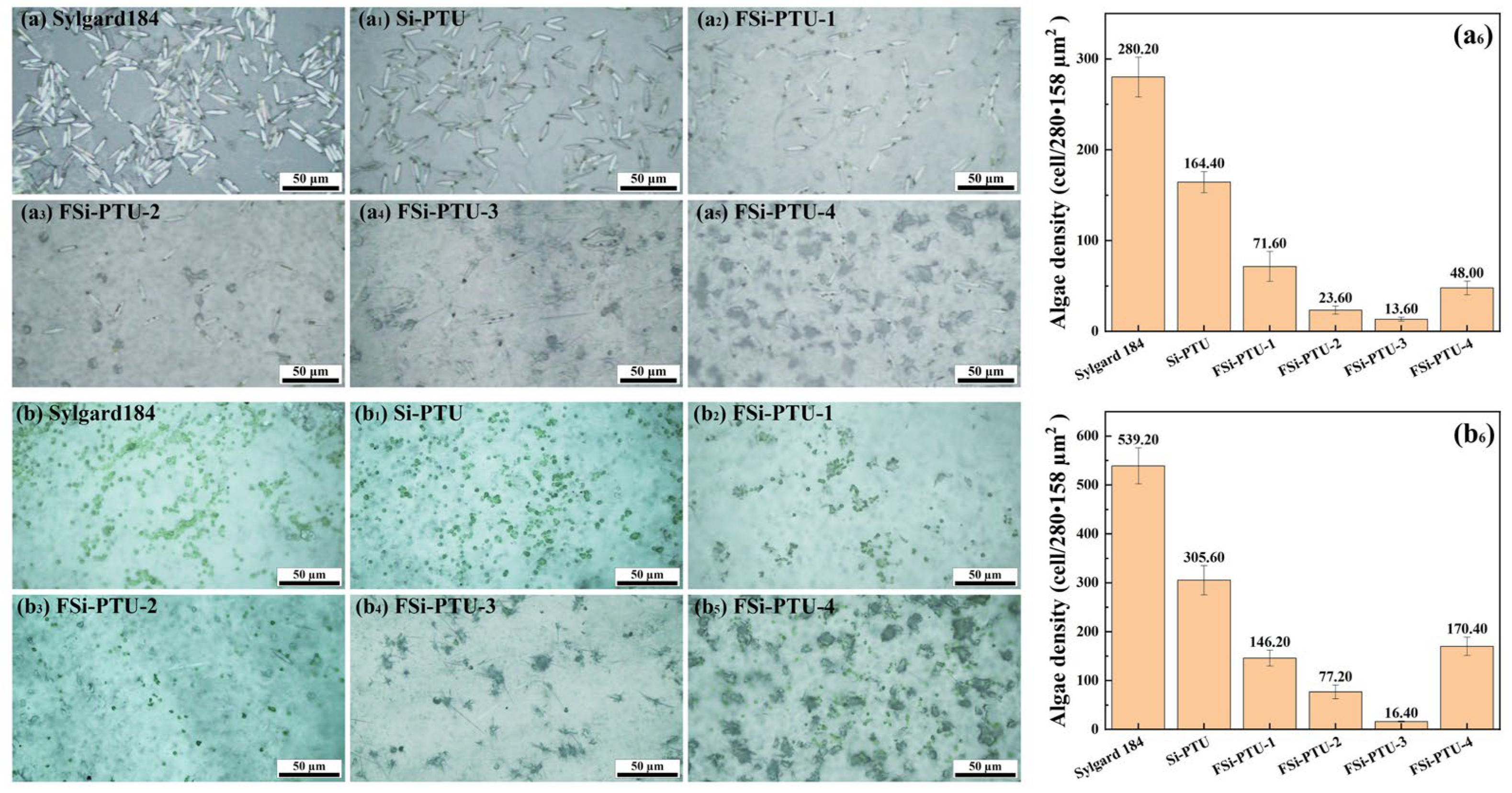
3.8. Navicula and Chlorella Vulgaris Colonization Assays
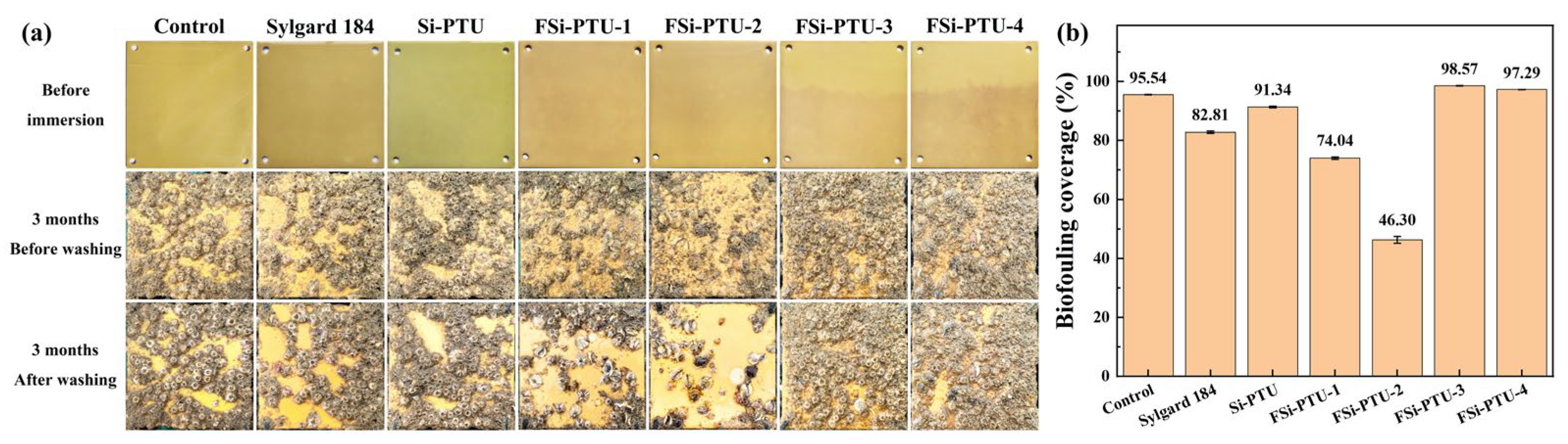
3.9. Actual Marine Test
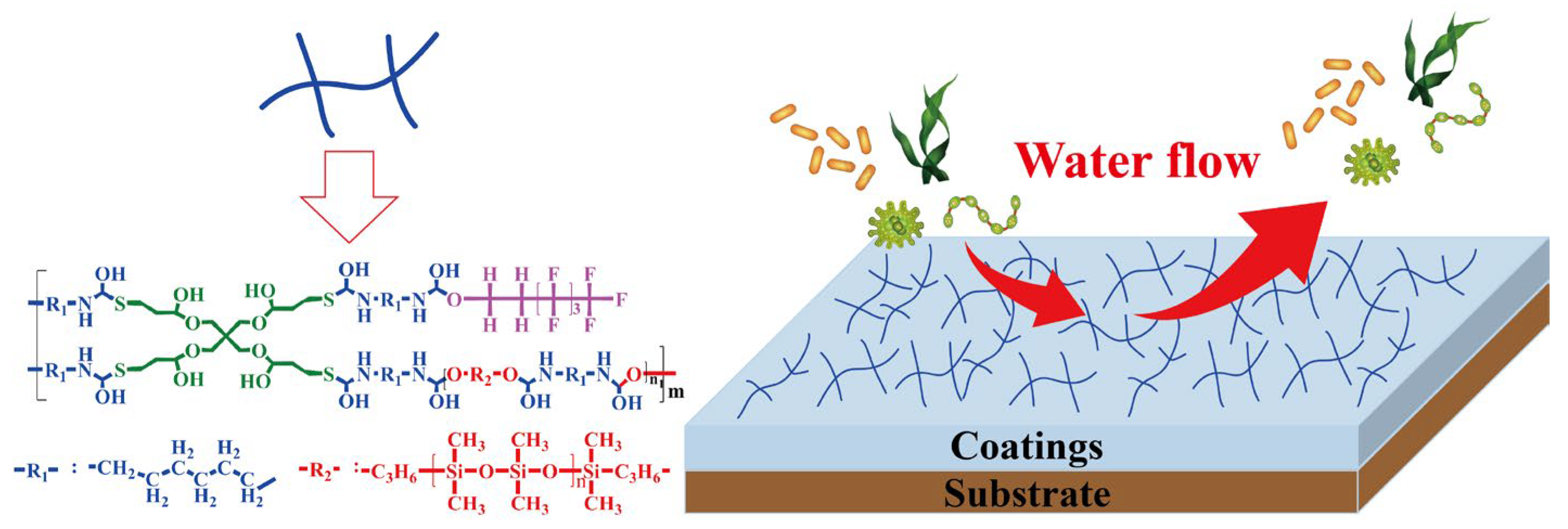
3.10. Antifouling Mechanism of FSi-PTU
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koschitzki, F.; Özcan, O.; Wanka, R.; Krisam, M.; Rosenhahn, A. Marine fouling release performance of amphiphilic carboxybetaine perfluoropolyether methacrylate polymer coatings. Adv. Mater. Interfaces 2024, 11, 2400370–2400383. [Google Scholar] [CrossRef]
- Chen, J.; Bai, W.; Jian, R.; Lin, Y.; Zheng, X.; Wei, F.; Lin, Q.; Lin, F.; Xu, Y. Molecular structure design of polybenzoxazines with low surface energy and low modulus for marine antifouling application. Prog. Org. Coat. 2024, 187, 108165–108176. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, L.; Wang, Y.; Jiang, Q.; Shi, Y.; Wang, G. Tyrosine-based branched polybenzoxazines with antibacterial and surface fouling released performances for marine antifouling coatings. Prog. Org. Coat. 2025, 200, 108978–108992. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.A.; El-Safty, S.A.; Higazy, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Kio, M.; Klauda, J. Advances in emerging hydrogel fouling-release coatings for marine applications. J. Coat. Technol. Res. 2024, 21, 827–856. [Google Scholar] [CrossRef]
- Zhou, A.; Li, S.; Sand, W.; Wang, X.; Geng, Y.; Ban, X.; Jin, Z.; Zhang, R. Self-growing composite photocatalytic materials for surface self-renewal of marine antifouling coatings. Sep. Purif. Technol. 2025, 354, 129387. [Google Scholar] [CrossRef]
- Zheng, N.; Jia, B.; Liu, J.; Wang, X.; Zhang, D.; Zhang, H.; Wang, G. Multi-strategy combined bionic coating for long-term robust protection against marine biofouling. J. Mater. Sci. Technol. 2025, 210, 265–277. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, S.; Janczewski, D.; Velandia, F.J.; Teo, S.L.; Vancso, G.J. Multilayers of fluorinated amphiphilic polyions for marine fouling prevention. Langmuir 2014, 30, 288–296. [Google Scholar] [CrossRef]
- Beyer, J.; Song, Y.; Tollefsen, K.E.; Berge, J.A.; Tveiten, L.; Helland, A.; Oxnevad, S.; Schoyen, M. The ecotoxicology of marine tributyltin (TBT) hotspots: A review. Mar. Environ. Res. 2022, 179, 105689–105703. [Google Scholar] [CrossRef]
- Tang, C.; Qin, X.; Huang, W.; Debi, S.; Zhang, Z.; Guo, J.; Liu, W.; Mo, J. Environmental sources, fate, toxicological effects, and health risks of copper pyrithione: An overview. Front. Env. Sci. Eng. 2024, 18, 132–152. [Google Scholar] [CrossRef]
- Thomas, K.V.; Brooks, S. The environmental fate and effects of antifouling paint biocides. Biofouling 2010, 26, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.C.; Joana, F.; Fernando, P.; Denis, M.S.A.; Susana, L.; Roberto, M. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? Crit. Rev. Env. Sci. Tec. 2021, 52, 3179–3210. [Google Scholar]
- Charlotte, B.; Patrice, C.; Pierre-Yves, G.; Christelle, C.; Patrice, G.; Jerome, C. Comparison of the accumulation and effects of copper pyrithione and copper sulphate on rainbow trout larvae. Environ. Toxicol. Phar. 2023, 104, 104308–104316. [Google Scholar]
- Zheng, L.; Lin, Y.; Wang, D.; Chen, J.; Yang, K.; Zheng, B.; Bai, W.; Jian, R.; Xu, Y. Facile one-pot synthesis of silver nanoparticles encapsulated in natural polymeric urushiol for marine antifouling. RSC Adv. 2020, 10, 13936–13943. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zheng, X.; Lin, Q.; Zheng, G.; Xu, Y.; Lin, F. Urushiol-based benzoxazine containing sulfobetaine groups for sustainable marine antifouling applications. Polymers 2023, 15, 2383. [Google Scholar] [CrossRef]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-based benzoxazine copper polymer with low surface energy, strong substrate adhesion and antibacterial for marine antifouling application. J. Clean. Prod. 2021, 318, 128527–128528. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, Y.; Guo, Y.; Zhang, S.; Xiong, G.; Wang, K.; Zhang, Z. Anti-marine biofouling adhesion performance and mechanism of PDMS fouling-release coating containing PS-PEG hydrogel. Mar. Pollut. Bull. 2023, 194, 115345–115360. [Google Scholar] [CrossRef]
- Arsenie, L.V.; Bangoura, M.A.; Ramonda, M.; Merindol, R.; Hespel, L.; Blanquer, S.; Azemar, F.; Lapinte, V. Photo-switchable polyoxazoline additive for marine fouling release silicone coatings. Prog. Org. Coat. 2025, 200, 109096–109110. [Google Scholar] [CrossRef]
- Qiu, H.; Gapeeva, A.; Hölken, I.; Kaps, S.; Adelung, R.; Baum, M.J. Preventing algae adhesion using lubricant-modified polydimethylsiloxane/polythiourethane nanocomposite. Mater. Design 2022, 214, 110389–110399. [Google Scholar] [CrossRef]
- Xie, R.; Ai, X.; Xie, Q.; Ma, C.; Zhang, G. Non-silicone elastic coating with fouling resistance and fouling release abilities based on degradable hyperbranched polymer. Prog. Org. Coat. 2023, 175, 107350–107357. [Google Scholar] [CrossRef]
- Zhu, P.; Feng, D.; Muhammad, Y.; Song, W.; Muhammad, A.H.; Zhang, C.; Liu, L. Enhanced antifouling capability of PDMS/Cu2O-anchored Fe-based amorphous coatings. Surf. Coat. Tech. 2023, 475, 130192–130203. [Google Scholar] [CrossRef]
- Wang, H.; Chen, R.; Song, D.; Sun, G.; Yu, J.; Liu, Q.; Liu, J.; Zhu, J.; Liu, P.; Wang, J. Silicone-modified polyurea-interpenetrating polymer network fouling release coatings with excellent wear resistance property tailored to regulations. J. Colloid Interf. Sci. 2024, 653, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, S.; Rong, Z.; Zhang, K.; Gao, C.; Wu, Y.; Liu, Y. Silicone low surface energy antifouling coating modified by zwitterionic side chains with strong substrate adhesion. Eur. Polym. J. 2022, 179, 111529–111539. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, Y.; Chen, F.; Gong, S.; Yang, W.; Liang, X.; Lian, Y.; Chen, J.; Wei, F.; Bai, W.; et al. Bioinspired self-stratification fouling release silicone coating with strong adhesion to substrate. Chem. Eng. J. 2022, 446, 137043–137052. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, C.; Lin, X.; Ma, C.; Zhang, G. Nanodiamond reinforced poly(dimethylsiloxane)-based polyurea with self-healing ability for fouling release coating. ACS Appl. Polym. Mater. 2020, 2, 3181–3188. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Q.; Ma, C.; Zhang, G. Fouling release property of polydimethylsiloxane-based polyurea with improved adhesion to substrate. Ind. Eng. Chem. Res. 2016, 55, 6671–6676. [Google Scholar] [CrossRef]
- Soleimani, S.; Jannesari, A.; Yousefzadi, M.; Ghaderi, A.; Shahdadi, A. Eco-friendly foul release coatings based on a novel reduced graphene oxide/Ag nanocomposite prepared by a green synthesis approach. Prog. Org. Coat. 2021, 151, 106107–106120. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, R.; Sun, G.; Liu, Q.; Liu, J.; Yu, J.; Zhu, J.; Wang, J. Ficus religiosa-inspired microstructure-controlled low surface energy coatings with long-term antifouling effect. Colloids Surf. A 2023, 670, 131482–131493. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Pizzi, E.; Puopolo, M.; Vesco, S. Design, manufacturing and testing of anti-fouling/foul-release (AF/FR) amphiphilic coatings. Prog. Org. Coat. 2018, 123, 267–281. [Google Scholar] [CrossRef]
- Zhu, P.; Meng, W.; Huang, Y. Synthesis and antibiofouling properties of crosslinkable copolymers grafted with fluorinated aromatic side chains. RSC Adv. 2017, 7, 3179–3189. [Google Scholar] [CrossRef]
- Wei, C.; Tang, Y.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Facile fabrication of highly omniphobic and self-cleaning surfaces based on water mediated fluorinated nanosilica aggregation. RSC Adv. 2016, 6, 74340–74348. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Zhang, Z. Effects of 3,3,3-trifluoropropyl content in poly (dimethyl-methylphenyl-methyltrifluoropropyl) siloxane coating on its antifouling performance. Prog. Org. Coat. 2024, 197, 108777–108791. [Google Scholar] [CrossRef]
- Xiang, Y.; Xue, H.; Zheng, Z.; Deng, J. Research in fluorinated block copolymer/polystyrene blends with durable antifouling properties based on chain-entanglement. Polymer 2023, 270, 125780–125790. [Google Scholar] [CrossRef]
- Andruzzi, L.; Chiellini, E.; Galli, G.; Li, X.; Kang, S.H.; Ober, C.K. Engineering low surface energy polymers through molecular design: Synthetic routes to fluorinated polystyrene-based block copolymers. J. Mater. Chem. 2002, 12, 1684–1692. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, N.; Li, Z.; Liu, Z.; Wang, G.; Gui, L.; Lin, J. Fast self-healing and antifouling polyurethane/fluorinated polysiloxane-microcapsules-silica composite material. Adv. Compos. Hybrid Mater. 2022, 5, 1899–1909. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Cui, M.; Su, R.; Huang, R. The preparation of antifouling and superhydrophobic polyurethane sponge by grafting Econea to diisocyanate monomer for durable continuous oil/water separation and cleanup of oil spills. Sep. Purif. Technol. 2023, 316, 123826–123837. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, R.; Guo, S.; Sun, G.; Liu, Q.; Liu, J.; Yu, J.; Liu, P.; Zhu, J.; et al. Multiple synergistic effects of indole derivative modified fluorinated acrylate polymer and z-scheme heterojunction over hollow spherical Mo2C/Bi2WO6 for efficient antifouling. Appl. Surf. Sci. 2023, 619, 156527–156537. [Google Scholar] [CrossRef]
- Szmechtyk, T.; Sienkiewicz, N.; Strzelec, K. Polythiourethane microcapsules as novel self-healing systems for epoxy coatings. Polym. Bull. 2017, 75, 149–165. [Google Scholar] [CrossRef]
- Xie, C.; Guo, H.; Zhao, W.; Zhang, L. Environmentally friendly marine antifouling coating based on a synergistic strategy. Langmuir 2020, 36, 2396–2402. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Gui, T.; Guo, H.; Zhang, K.; Meng, F.; Zhan, X.; Liu, Q.; Zhang, Q. Carp-inspired self-regulating marine antifouling coating: Featuring robust controlled release of eugenol and high-efficiency self-healing performance. Chem. Eng. J 2024, 486, 149929–149943. [Google Scholar] [CrossRef]

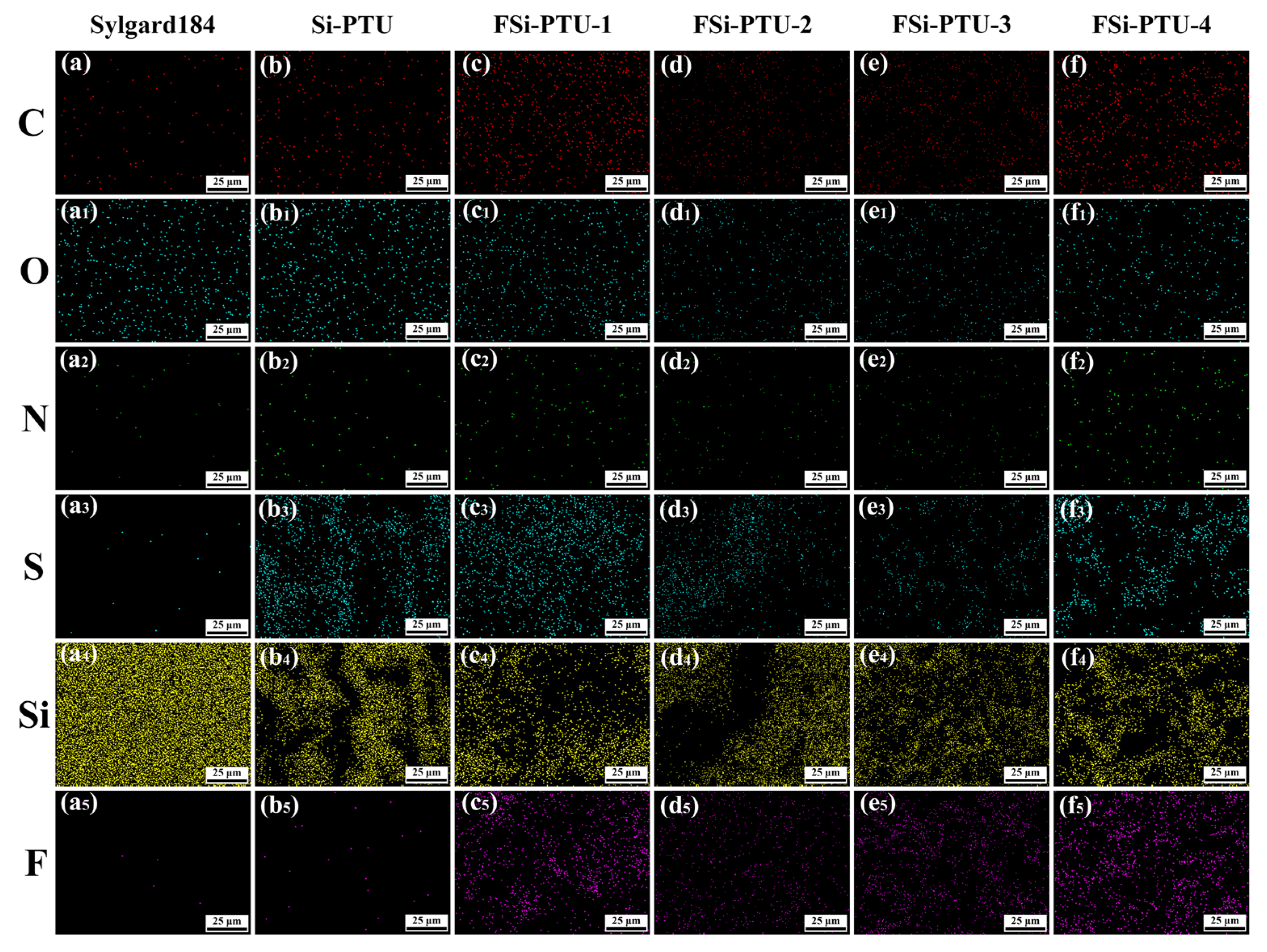


| Elt. | Sylgard 184 | Si-PTU | FSi-PTU-1 | FSi-PTU-2 | FSi-PTU-3 | FSi-PTU-4 |
|---|---|---|---|---|---|---|
| C | 68.89 | 135.90 | 210.38 | 186.16 | 196.74 | 190.07 |
| O | 237.26 | 194.65 | 177.38 | 162.57 | 147.14 | 135.28 |
| N | 8.15 | 13.67 | 17.02 | 16.09 | 9.79 | 13.97 |
| S | 0.00 | 345.64 | 289.84 | 243.06 | 158.62 | 251.14 |
| Si | 2221.81 | 736.22 | 510.21 | 692.30 | 608.57 | 426.28 |
| F | 1.64 | 0.10 | 268.12 | 229.76 | 314.65 | 305.28 |
| Test | Sylgard 184 | Si-PTU | FSi-PTU-1 | FSi-PTU-2 | FSi-PTU-3 | FSi-PTU-4 |
|---|---|---|---|---|---|---|
| Tensile stress (MPa) | 1.74 ± 0.06 | 10.69 ± 0.41 | 10.16 ± 0.18 | 7.19 ± 0.16 | 10.44 ± 0.33 | 11.14 ± 0.16 |
| Elongation at break (%) | 97.84 ± 3.51 | 26.88 ± 2.48 | 44.45 ± 3.68 | 47.47 ± 3.27 | 53.64 ± 2.40 | 50.73 ± 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Liao, X.; Fan, Y.; Li, J.; Jiang, Q.; Zheng, Y.; Huang, Z.; Li, S. Fluoro-Silicon-Modified Polythiourethane Copolymer for Marine Antifouling Coatings. Coatings 2025, 15, 588. https://doi.org/10.3390/coatings15050588
Xie S, Liao X, Fan Y, Li J, Jiang Q, Zheng Y, Huang Z, Li S. Fluoro-Silicon-Modified Polythiourethane Copolymer for Marine Antifouling Coatings. Coatings. 2025; 15(5):588. https://doi.org/10.3390/coatings15050588
Chicago/Turabian StyleXie, Songbo, Xiufen Liao, Yanye Fan, Jiacheng Li, Qiumei Jiang, Yihua Zheng, Zhimin Huang, and Shella Li. 2025. "Fluoro-Silicon-Modified Polythiourethane Copolymer for Marine Antifouling Coatings" Coatings 15, no. 5: 588. https://doi.org/10.3390/coatings15050588
APA StyleXie, S., Liao, X., Fan, Y., Li, J., Jiang, Q., Zheng, Y., Huang, Z., & Li, S. (2025). Fluoro-Silicon-Modified Polythiourethane Copolymer for Marine Antifouling Coatings. Coatings, 15(5), 588. https://doi.org/10.3390/coatings15050588






