Development of Zn-Reinforced Mg Matrix Composites via High Energy Ball Milling Duration: Impact on Mechanical Properties and Biodegradability
Abstract
1. Introduction
2. Materials and Method
Production and Characterization of Powders
3. Results and Discussion
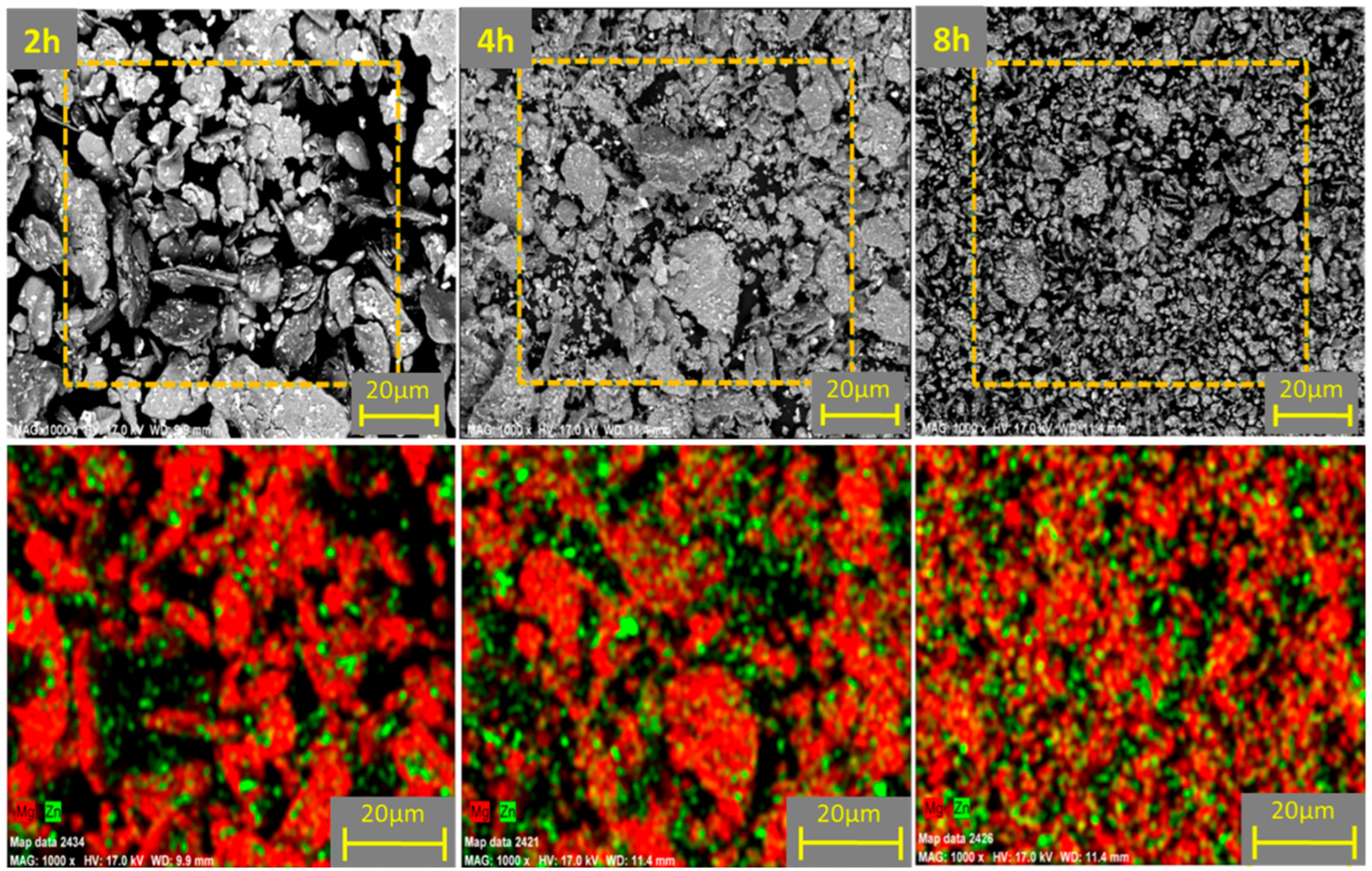
3.1. Powder Morphology and Distribution of the Reinforcement Element in Different Ball Milling Durations
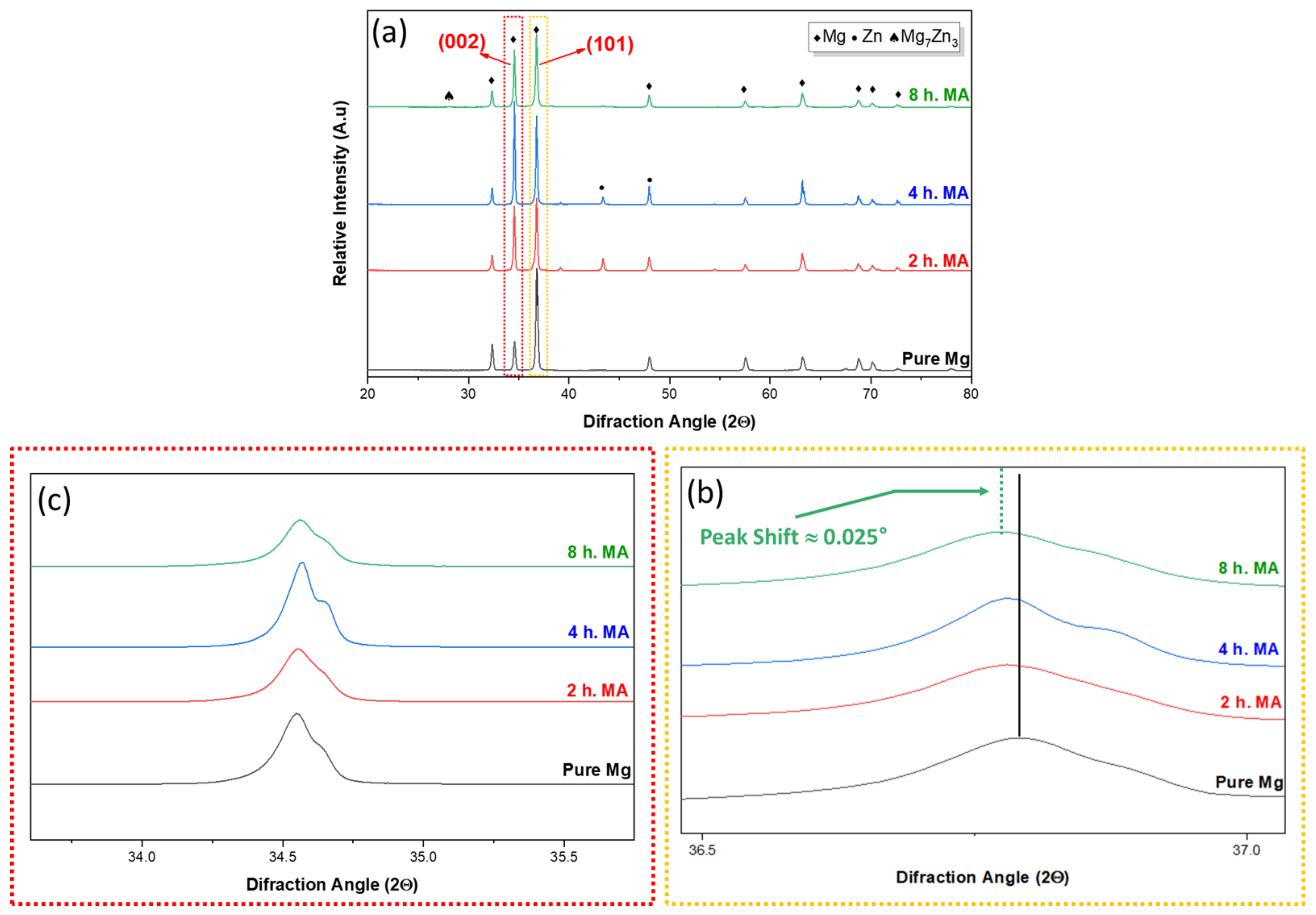
3.2. XRD Results Depending on the Various Milling Time in Mg–6ZN Powder System
3.3. Effect of Variation Milling Time on Sintered Mg–6Zn Alloy System
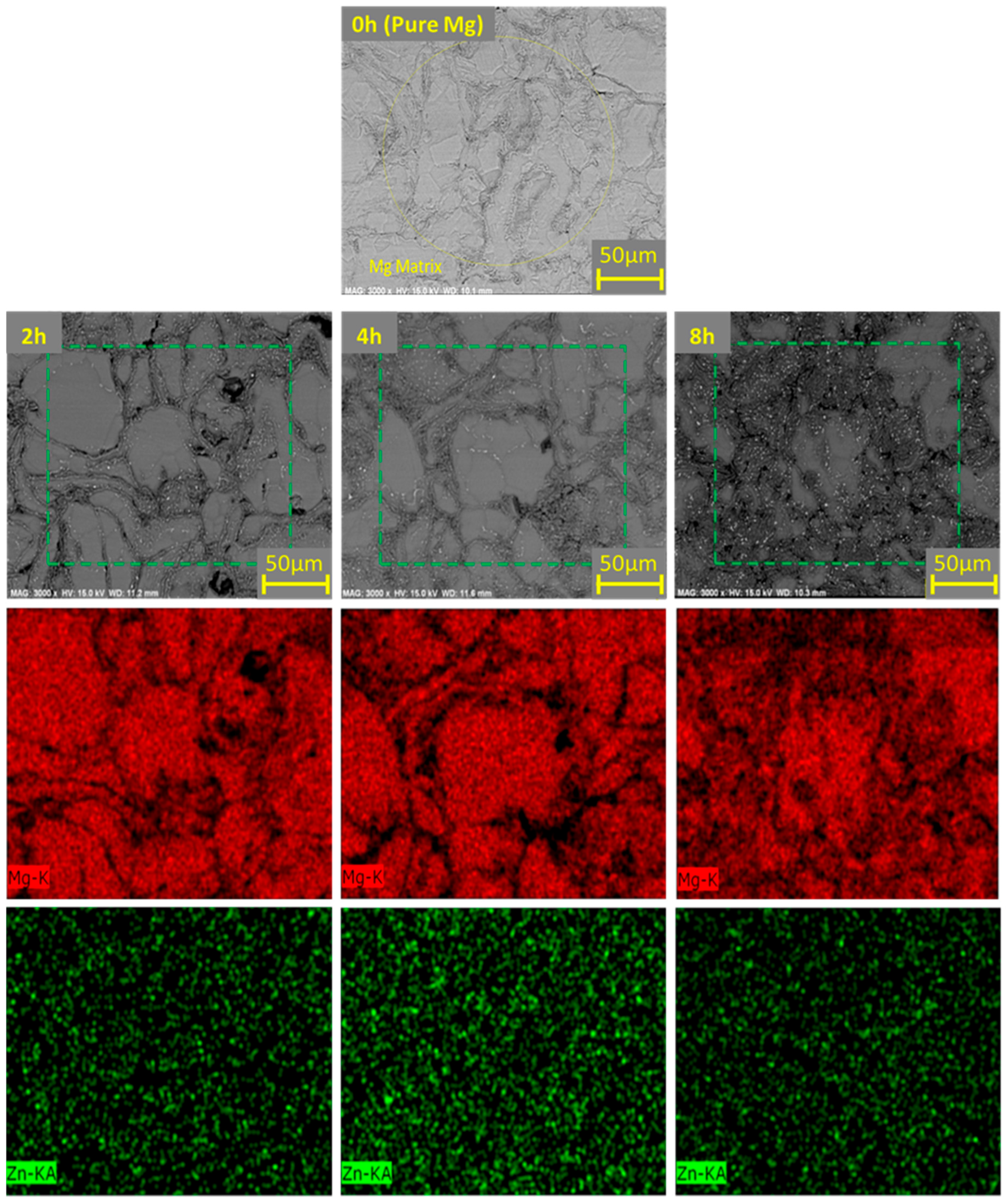
3.3.1. Microstructure and Intermetallic Development in Sintered Materials
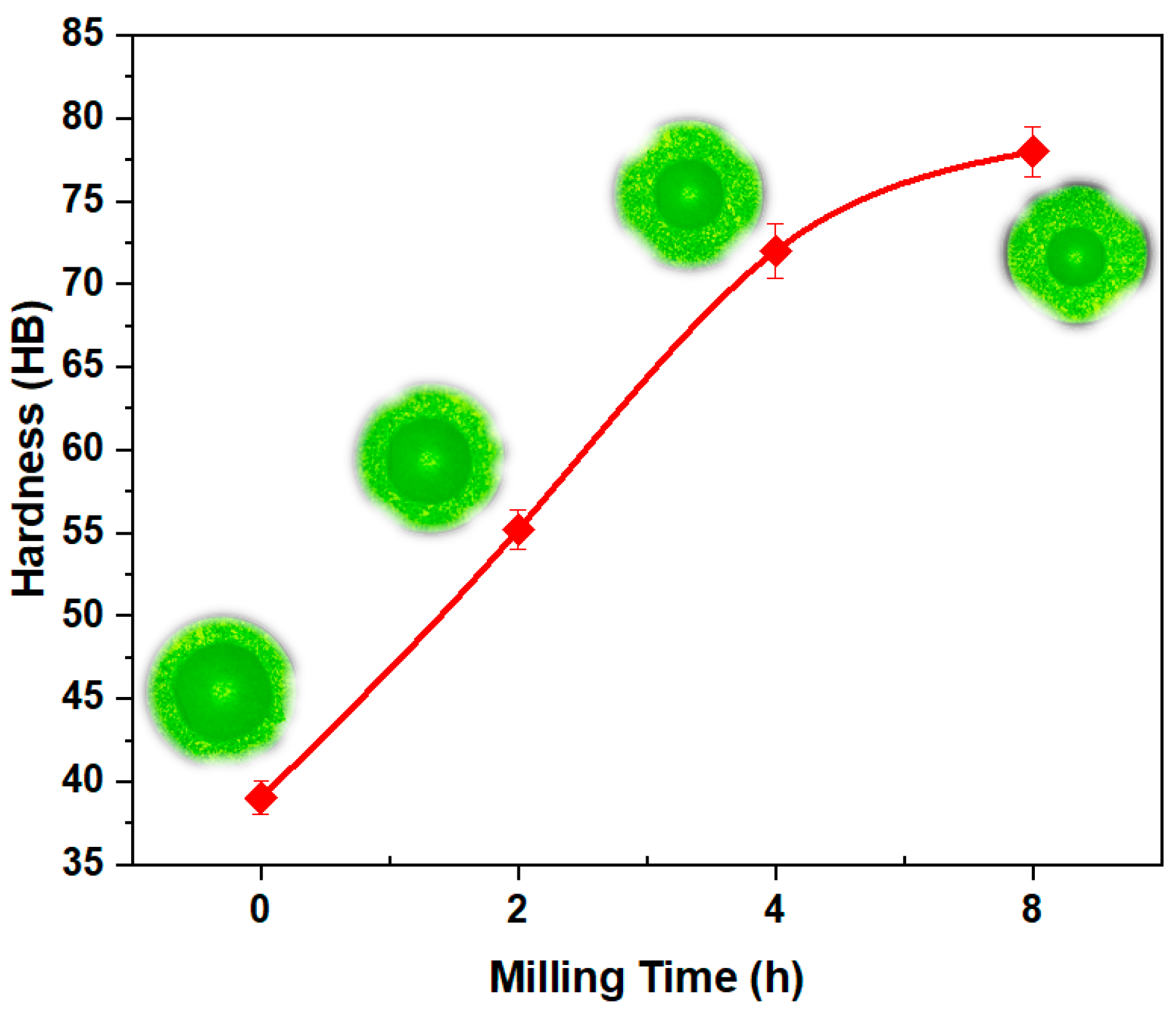
3.3.2. Effect of Milling Time on Hardness and Density in Bulk Materials
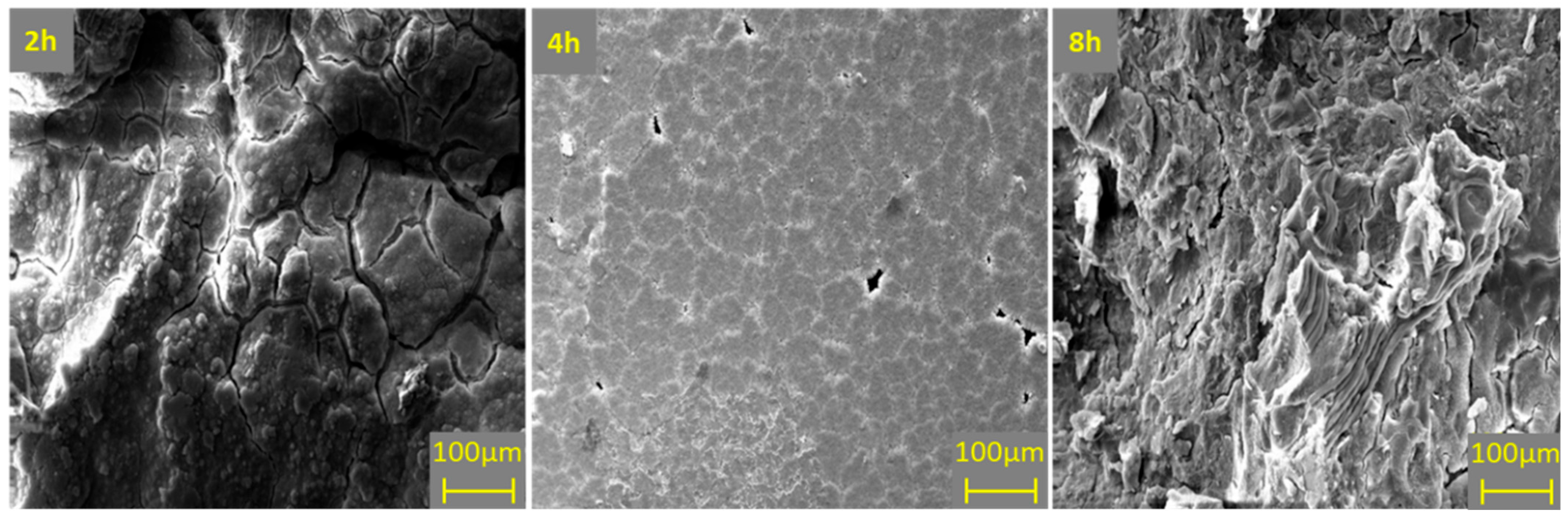
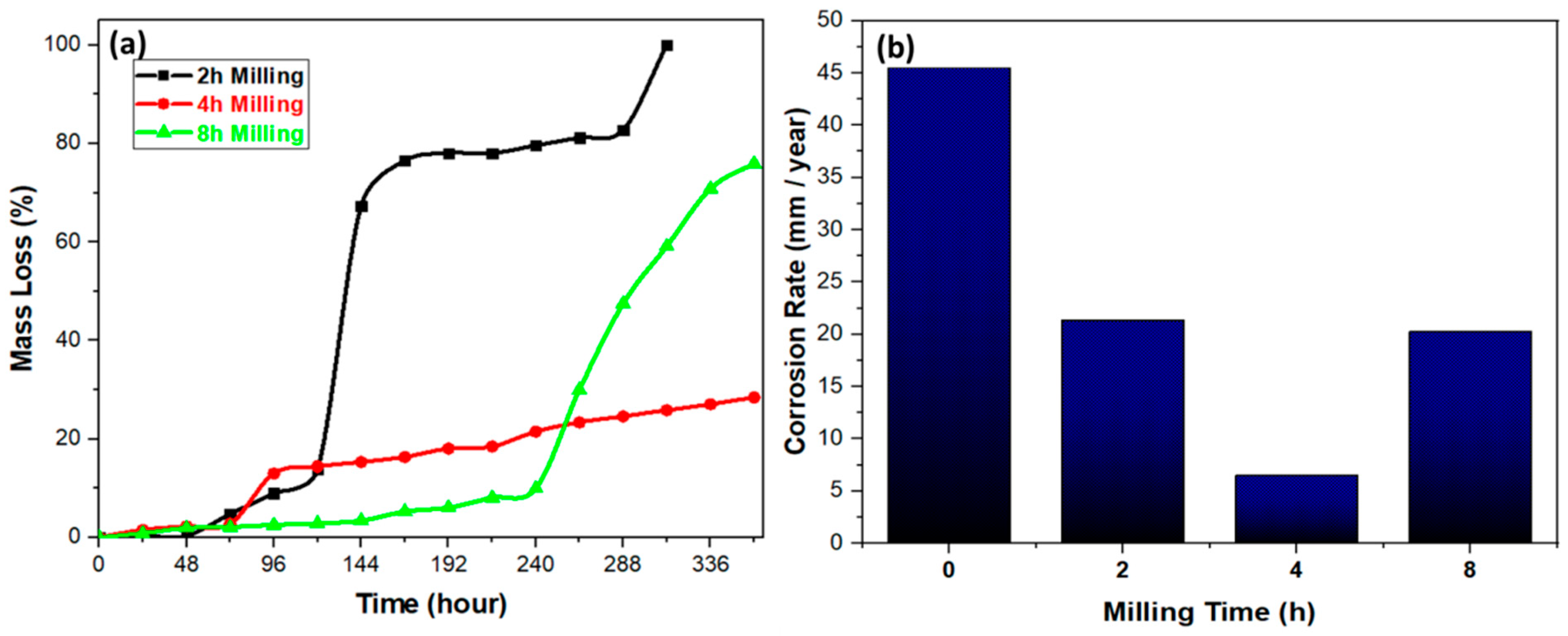
3.3.3. Effect of Ball Milling Time on Degradation Behavior of Mg–6ZN Material System
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaya, A.; Pekgüleryüz, M.; Türkoğlu, S.; Özdoğru, E.F. Ağırlık Tasarrufu Amacıyla Otomobil Motor Bölmesinde Magnezyum Alaşımlarının Kullanımı. OTEKON 2004, 4, 21–23. [Google Scholar]
- Mordike, B.; Ebert, T. Magnesium: Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Friedrich, H.; Schumann, S. Research for a “new age of magnesium” in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Chaffin, G.N.; Jacoby, J.E.; Brusethaug, S. Guidelines for Aluminum Sow Casting and Charging; Aluminum Association: Arlington, VA, USA, 1992. [Google Scholar]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Kaya, A.A.; Kaya, R.A.; Witte, F.; Duygulu, O. Useful corrosion-potential of magnesium alloys as implants. Corros. Sci. Technol. 2008, 7, 162–167. [Google Scholar]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5 Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, mechanical and cytocompatibility of degradable Mg–Zn based orthopedic biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Z.M.; Yu, K.; Chen, L.J. Biodegradable Behaviors of Mg-6%Zn-5%Hydroxyapatite Biomaterial. Adv. Mater. Res. 2011, 239-242, 1287–1291. [Google Scholar] [CrossRef]
- Erçetin, A.; Özgün, Ö.; Aslantaş, K. Toz metal Al2O3 takviyeli Mg5Sn matrisli kompozitler: Üretim ve karakterizasyon. Gazi Üniversitesi Mühendis. Mimar. Fakültesi Derg. 2022, 38, 1003–1012. [Google Scholar] [CrossRef]
- Frieden, E. The evolution of metals as essential elements [with special reference to iron and copper]. In Protein-Metal Interactions; Friedman, M., Ed.; Springer: Boston, MA, USA, 1974; pp. 1–31. [Google Scholar]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Nandy, T.; Jones, J.; Allison, J.; Pollock, T. Microstructural characterization of a die-cast magnesium-rare earth alloy. Scr. Mater. 2001, 45, 1423–1429. [Google Scholar] [CrossRef]
- Özarslan, S.; Şevik, H.; Sorar, İ. Microstructure, mechanical and corrosion properties of novel Mg-Sn-Ce alloys produced by high pressure die casting. Mater. Sci. Eng. C 2019, 105, 110064. [Google Scholar] [CrossRef]
- Gökçe, A. Toz metalurjisi yöntemiyle Mg-Sn alaşımı üretimi ve karakterizasyonu. Acad. Platf.-J. Eng. Sci. 2020, 8, 112–119. [Google Scholar] [CrossRef]
- Özgün, Ö.; Erçetin, A. Microstructural and mechanical properties of Cr-C reinforced Cu matrix composites produced through powder metallurgy method. Tr. J. Nature Sci. 2017, 6, 1–6. [Google Scholar]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Toozandehjani, M.; Matori, K.A.; Ostovan, F.; Aziz, S.A.; Mamat, M.S. Effect of milling time on the microstructure, physical and mechanical properties of Al-Al2O3 nanocomposite synthesized by ball milling and powder metallurgy. Materials 2017, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, H.; Senthilkumar, V. Consolidation behavior of mechanically alloyed aluminum based nanocomposites reinforced with nanoscale Y2O3/Al2O3 particles. Mater. Charact. 2011, 62, 1235–1249. [Google Scholar] [CrossRef]
- Beaulieu, L.; Larcher, D.; Dunlap, R.A.; Dahn, J.R. Nanocomposites in the Sn–Mn–C system produced by mechanical alloying. J. Alloys Compd. 2000, 297, 122–128. [Google Scholar] [CrossRef]
- Benjamin, J.; Volin, T. The mechanism of mechanical alloying. Metall. Trans. 1974, 5, 1929–1934. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Enayati, M.; Tavoosi, M. Synthesis and characterization of Zn/Al2O3 nanocomposite by mechanical alloying. Mater. Sci. Eng. A 2008, 486, 45–48. [Google Scholar] [CrossRef]
- Salur, E.; Acarer, M.; Şavkliyildiz, İ. Improving mechanical properties of nano-sized TiC particle reinforced AA7075 Al alloy composites produced by ball milling and hot pressing. Mater. Today Commun. 2021, 27, 102202. [Google Scholar] [CrossRef]
- Charkhi, A.; Kazemian, H.; Kazemeini, M. Optimized experimental design for natural clinoptilolite zeolite ball milling to produce nano powders. Powder Technol. 2010, 203, 389–396. [Google Scholar] [CrossRef]
- Das, B.K.; Das, T.; Parashar, K.; Thirumurugan, A.; Parashar, S.K.S. Structural, bandgap tuning and electrical properties of Cu doped ZnO nanoparticles synthesized by mechanical alloying. J. Mater. Sci. Mater. Electron. 2017, 28, 15127–15134. [Google Scholar] [CrossRef]
- Sheibani, S.; Heshmati-Manesh, S.; Ataie, A. Influence of Al2O3 nanoparticles on solubility extension of Cr in Cu by mechanical alloying. Acta Mater. 2010, 58, 6828–6834. [Google Scholar] [CrossRef]
- Soni, P. Mechanical Alloying: Fundamentals and Applications; Cambridge International Science Publishing: Cambridge, UK, 2000. [Google Scholar]
- Zhao, Q.; Yu, L.; Liu, Y.; Li, H. Morphology and structure evolution of Y2O3 nanoparticles in ODS steel powders during mechanical alloying and annealing. Adv. Powder Technol. 2015, 26, 1578–1582. [Google Scholar] [CrossRef]
- Gültekin, G.G.; Çanakçı, A.; Canpolat, Ö. Effect of Milling Time and Process Control Agent on Microstructure and Mechanical Properties of AA2024-2 wt.% Cu Metal–Metal Composites Produced by Mechanical Alloying. Metallogr. Microstruct. Anal. 2023, 12, 444–454. [Google Scholar] [CrossRef]
- Wu, W.; Tor, S.B.; Chua, C.K.; Leong, K.F.; Merchant, A. Investigation on processing of ASTM A131 Eh36 high tensile strength steel using selective laser melting. Virtual Phys. Prototyp. 2015, 10, 187–193. [Google Scholar] [CrossRef]
- Forchelet, J.; Zamni, L.; El Maudni El Alami, S.; Hu, J.; Balli, M.; Sari, O. Corrosion behavior of gadolinium and La–Fe–Co–Si compounds in various heat conducting fluids. Int. J. Refrig. 2014, 37, 307–313. [Google Scholar] [CrossRef]
- ASTM International. Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- Torres-Huerta, A.; Nacional, P.; Domínguez-Crespo, M.; Palma-Ramírez, D.; Flores-Vela, A.; Castellanos-Alvarez, E.; Del-Angel-López, D. Preparation and degradation study of HDPE/PLA polymer blends for packaging applications. Rev. Mex. Ing. Quimica 2018, 19, 251–271. [Google Scholar] [CrossRef]
- Sübütay, H.; Şavklıyıldız, İ. The relationship between structural evolution and high energy ball milling duration in tin reinforced Mg alloys. Mater. Today Commun. 2023, 35, 105868. [Google Scholar] [CrossRef]
- Sahu, P.; Samal, S.; Kumar, V. An assessment of the mechanically alloyed equiatomic FeCoNiMnSi high entropy amorphous alloy for non-isothermal crystallization kinetics and magnetocaloric refrigeration application. Mater. Charact. 2024, 216, 114269. [Google Scholar] [CrossRef]
- Wu, Z.; Song, G. Microstructure and properties of brazing joints of magnesium alloy AZ31B. Mater. Res. Innov. 2010, 14, 160–164. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Çuvalcı, O.; Varol, T.; Akçay, S.B.; Güler, O.; Çanakçı, A. Effect of ball mill time and wet pre-milling on the fabrication of Ti powders by recycling Ti machining chips by planetary milling. Powder Technol. 2023, 426, 118637. [Google Scholar] [CrossRef]
- Akdoğan, E.K.; Şavklιyιldιz, I.; Berke, B.; Zhong, Z.; Wang, L.; Weidner, D.; Croft, M.C.; Tsakalakos, T. Pressure effects on phase equilibria and solid solubility in MgO-Y2O3 nanocomposites. J. Appl. Phys. 2012, 111, 053506. [Google Scholar] [CrossRef]
- Akdoğan, E.K.; Şavklıyıldız, I.; Berke, B.; Zhong, Z.; Wang, L.; Vaughan, M.; Tsakalakos, T. High-pressure phase transformations in MgO-Y2O3 nanocomposites. Appl. Phys. Lett. 2011, 99, 141915. [Google Scholar] [CrossRef]
- Şavklıyıldız, I.; Akdoğan, E.K.; Zhong, Z.; Wang, L.; Weidner, D.; Vaughan, M.; Croft, M.C.; Tsakalakos, T. Phase transformations in hypereutectic MgO-Y2O3 nanocomposites at 5.5 GPa. J. Appl. Phys. 2013, 113, 203520. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, W.; Li, S.; Zhang, D.; Zhao, Y.; Lavernia, E.J.; Zhu, Y. Contribution of van der Waals forces to the plasticity of magnesium. Acta Mater. 2016, 107, 127–132. [Google Scholar] [CrossRef]
- Gunes, I.; Uygunoglu, T.; Erdogan, M. Effect of sintering duration on some properties of pure magnesium. Powder Metall. Met. Ceram. 2015, 54, 156–165. [Google Scholar] [CrossRef]
- Avraham, S.; Katsman, A.; Bamberger, M. Optimization of composition and heat treatment design of Mg–Sn–Zn alloys via the CALPHAD method. J. Mater. Sci. 2011, 46, 6941–6951. [Google Scholar] [CrossRef]
- Williams, D.B.; Butler, E. Grain boundary discontinuous precipitation reactions. Int. Met. Rev. 1981, 26, 153–183. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Plichta, M.R.; Franti, G.W.; Russell, K.C. Precipitation at interphase boundaries. Met. Trans. A 1978, 9, 363–371. [Google Scholar] [CrossRef]
- Balluffi, R.W. Grain Boundary Diffusion Mechanisms in Metals; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Villalobos, J.C.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Çelebi, M.; Çanakçi, A.; Güler, O.; Akgül, B.; Karabacak, A.H. Study on effect of milling time on the mechanical and wear properties of the ZA40-B4C-graphene hybrid nanocomposites fabricated by vacuum hot-pressing. JOM 2023, 75, 3935–3950. [Google Scholar] [CrossRef]
- Du, J.; Zhang, A.; Guo, Z.; Yang, M.; Li, M.; Xiong, S. Atomic cluster structures, phase stability and physicochemical properties of binary Mg-X (X= Ag, Al, Ba, Ca, Gd, Sn, Y and Zn) alloys from ab-initio calculations. Intermetallics 2018, 95, 119–129. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying: A critical review. Mater. Res. Lett. 2022, 10, 619–647. [Google Scholar] [CrossRef]
- Keerthipalli, T.; Aepuru, R.; Biswas, A. Review on precipitation, intermetallic and strengthening of aluminum alloys. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2023, 237, 833–850. [Google Scholar] [CrossRef]
- Ercetin, A.; Ercetin, A.; Özgün, Ö.; Aslantas, K.; Der, O.; Yalçın, B.; Simsir, E.; Aamir, M. Microstructural and mechanical behavior investigations of Nb-reinforced Mg–Sn–Al–Zn–Mn matrix magnesium composites. Metals 2023, 13, 1097. [Google Scholar] [CrossRef]
- Esteban-Manzanares, G.; Alizadeh, R.; Papadimitriou, I.; Dickel, D.; Barrett, C.D.; LLorca, J. Atomistic simulations of the interaction of basal dislocations with MgZn2 precipitates in Mg alloys. Mater. Sci. Eng. A 2020, 788, 139555. [Google Scholar] [CrossRef]
- Alizadeh, R.; LLorca, J. Interactions between basal dislocations and β1′ precipitates in Mg–4Zn alloy: Mechanisms and strengthening. Acta Mater. 2020, 186, 475–486. [Google Scholar] [CrossRef]
- Sübütay, H.; Şavklıyıldız, İ. Effect of high-energy ball milling in ternary material system of (Mg-Sn-Na). Crystals 2023, 13, 1230. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Z.; Li, J.; Fang, Q.; Wei, Y. Beyond Orowan hardening: Mapping the four distinct mechanisms associated with dislocation-precipitate interaction. Int. J. Plast. 2023, 169, 103710. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Knight, B.E.; Schuh, C.A. Six decades of the Hall–Petch effect–a survey of grain-size strengthening studies on pure metals. Int. Mater. Rev. 2016, 61, 495–512. [Google Scholar] [CrossRef]
- Dehaghani, M.T.; Ahmadian, M.; Fathi, M. Effect of ball milling on the physical and mechanical properties of the nanostructured Co–Cr–Mo powders. Adv. Powder Technol. 2014, 25, 1793–1799. [Google Scholar] [CrossRef]
- Li, D.; Hou, X.; Zhao, D.; Wang, C.; Xie, X.; Ye, X.; Yang, G.; Hu, P.; Xu, G. Waste Mg alloys hydrogen production from seawater: An integrative overview of medium optimization, hydrogen-producing materials, underlying mechanisms, innovative technologies, and device development. J. Magnes. Alloys 2024, 12, 3491–3515. [Google Scholar] [CrossRef]
- Prokopiev, O.; Sevostianov, I. Dependence of the mechanical properties of sintered hydroxyapatite on the sintering temperature. Mater. Sci. Eng. A 2006, 431, 218–227. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef]
- Doğan, K.; Özgün, M.I.; Sübütay, H.; Salur, E.; Eker, Y.; Kuntoğlu, M.; Aslan, A.; Gupta, M.K.; Acarer, M. Dispersion mechanism-induced variations in microstructural and mechanical behavior of CNT-reinforced aluminum nanocomposites. Arch. Civ. Mech. Eng. 2022, 22, 55. [Google Scholar] [CrossRef]
- Li, K.; Mao, X.; Khanlari, K.; Song, K.; Shi, Q.; Liu, X. Effects of powder size distribution on the microstructural and mechanical properties of a Co–Cr–W–Si alloy fabricated by selective laser melting. J. Alloys Compd. 2020, 825, 153973. [Google Scholar] [CrossRef]
- Farkash, A.; Mittelman, B.; Hayun, S.; Priel, E. Aluminum Matrix Composites with Weak Particle Matrix Interfaces: Effective Elastic Properties Investigated Using Micromechanical Modeling. Materials 2021, 14, 6083. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zou, N.; Li, Q. Effect of ball milling time on microstructure and hardness of porous magnesium/carbon nanofiber composites. JOM 2017, 69, 1236–1243. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.; Wu, S.; Zheng, Y.; Guan, Z.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2021, 7, 192–216. [Google Scholar] [CrossRef]
- Ye, L.; Huang, H.; Sun, C.; Zhuo, X.; Dong, Q.; Liu, H.; Ju, J.; Xue, F.; Bai, J.; Jiang, J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn–Mg binary alloys. J. Mater. Res. Technol. 2022, 16, 1673–1685. [Google Scholar] [CrossRef]
- Li, C.; Huang, T.; Liu, Z. Effects of thermomechanical processing on microstructures, mechanical properties, and biodegradation behaviour of dilute Zn–Mg alloys. J. Mater. Res. Technol. 2023, 23, 2940–2955. [Google Scholar] [CrossRef]
- Ji, C.; Ma, A.; Jiang, J.; Wu, H.; Liu, H.; Guo, S.; Yuan, Y. Regulating mechanical properties and degradation behavior of biodegradable Zn–0.6 Mg alloy via ECAP plus cold rolling. J. Alloys Compd. 2023, 937, 168487. [Google Scholar]
- Ji, C.; Ma, A.; Jiang, J.; Song, D.; Liu, H.; Guo, S. Research status and future prospects of biodegradable Zn-Mg alloys. J. Alloys Compd. 2024, 993, 174669. [Google Scholar] [CrossRef]
- Kuwahara, H.; Al-Abdullat, Y.; Mazaki, N.; Tsutsumi, S.; Aizawa, T. Precipitation of Magnesium Apatite on Pure Magnesium Surface during Immersing in Hank’s Solution. Mater. Trans. 2001, 42, 1317–1321. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Song, Y.; Zhao, C.; Zhang, X.; Xie, C.; Zhang, Y.; Tao, H.; He, Y.; Jiang, Y.; et al. In vitro degradation, hemolysis and MC3T3-E1 cell adhesion of biodegradable Mg–Zn alloy. Mater. Sci. Eng. C 2009, 29, 1907–1912. [Google Scholar] [CrossRef]
- Waizy, H.; Weizbauer, A.; Modrejewski, C.; Witte, F.; Windhagen, H.; Lucas, A.; Kieke, M.; Denkena, B.; Behrens, P.; Meyer-Lindenberg, A.; et al. In vitro corrosion of ZEK100 plates in Hank’s Balanced Salt Solution. Biomed. Eng. Online 2012, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Bouanis, F.; Bentiss, F.; Traisnel, M.; Jama, C. Enhanced corrosion resistance properties of radiofrequency cold plasma nitrided carbon steel: Gravimetric and electrochemical results. Electrochim. Acta 2009, 54, 2371–2378. [Google Scholar] [CrossRef]
- Wen, C.; Tian, Y.; Mai, Z.; Hu, J.; Wang, G. Effect of macropores at the steel-concrete interface on localized corrosion behaviour of steel reinforcement. Cem. Concr. Compos. 2022, 129, 104510. [Google Scholar] [CrossRef]
- Ghali, E.; Dietzel, W.; Kainer, K.-U. General and localized corrosion of magnesium alloys: A critical review. J. Mater. Eng. Perform. 2004, 13, 7–23. [Google Scholar] [CrossRef]
- Op’t Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of pure Mg as a function of grain size and processing route. Adv. Eng. Mater. 2008, 10, 579–582. [Google Scholar] [CrossRef]
- Zeng, R.-C.; Zhang, J.; Huang, W.-J.; Dietzel, W.; Kainer, K.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferr. Met. Soc. China 2006, 16, s763–s771. [Google Scholar] [CrossRef]
- Song, G.-L.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef]
- Jingyuan, Y.; Jianzhong, W.; Qiang, L.; Jian, S.; Jianming, C.; Xudong, S. Effect of Zn on Microstructures and Properties of Mg-Zn Alloys Prepared by Powder Metallurgy Method. Rare Met. Mater. Eng. 2016, 45, 2757–2762. [Google Scholar] [CrossRef]
- Yan, Y.; Chu, X.; Luo, X.; Xu, X.; Zhang, Y.; Dai, Y.; Li, D.; Chen, L.; Xiao, T.; Yu, K. A homogenous microstructural Mg-based matrix model for orthopedic application with generating uniform and smooth corrosion product layer in Ringer’s solution: Study on biodegradable behavior of Mg-Zn alloys prepared by powder metallurgy as a case. J. Magnes. Alloys 2021, 9, 225–240. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]



| Ball Milling Parameters | |
|---|---|
| Milling Speed | 300 rpm |
| Ball/Powder ratio | 10:1 |
| Chamber Fill | 10 g |
| PCA | 2 wt.% Stearic Acid |
| Ball Size | 10 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çetinkal, S.B.; Salur, E.; Arıcı, G.; Degnah, A.; Sarkar, S.; Sübütay, H. Development of Zn-Reinforced Mg Matrix Composites via High Energy Ball Milling Duration: Impact on Mechanical Properties and Biodegradability. Coatings 2025, 15, 561. https://doi.org/10.3390/coatings15050561
Çetinkal SB, Salur E, Arıcı G, Degnah A, Sarkar S, Sübütay H. Development of Zn-Reinforced Mg Matrix Composites via High Energy Ball Milling Duration: Impact on Mechanical Properties and Biodegradability. Coatings. 2025; 15(5):561. https://doi.org/10.3390/coatings15050561
Chicago/Turabian StyleÇetinkal, S. Bilal, Emin Salur, Gökhan Arıcı, Ahmed Degnah, Sayan Sarkar, and Halit Sübütay. 2025. "Development of Zn-Reinforced Mg Matrix Composites via High Energy Ball Milling Duration: Impact on Mechanical Properties and Biodegradability" Coatings 15, no. 5: 561. https://doi.org/10.3390/coatings15050561
APA StyleÇetinkal, S. B., Salur, E., Arıcı, G., Degnah, A., Sarkar, S., & Sübütay, H. (2025). Development of Zn-Reinforced Mg Matrix Composites via High Energy Ball Milling Duration: Impact on Mechanical Properties and Biodegradability. Coatings, 15(5), 561. https://doi.org/10.3390/coatings15050561







