A Preparation Method for Improving the Thermal Conductivity of Graphene Film
Abstract
1. Introduction
2. Experiment
2.1. Experimental Material
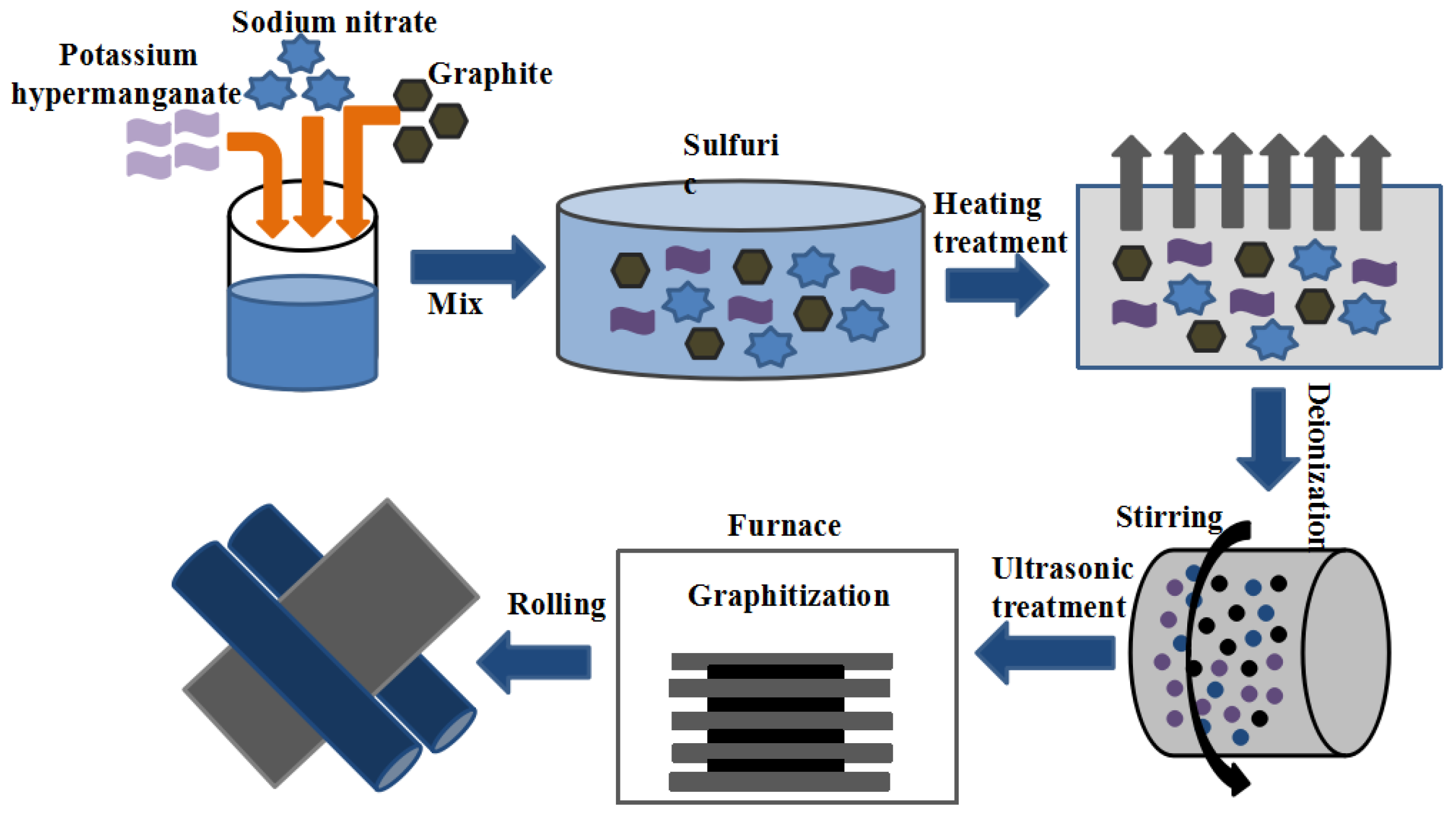
2.2. Preparation of Carbon-Rich Molecules
2.3. Preparation of Graphene Films
2.4. Characterization
3. Results
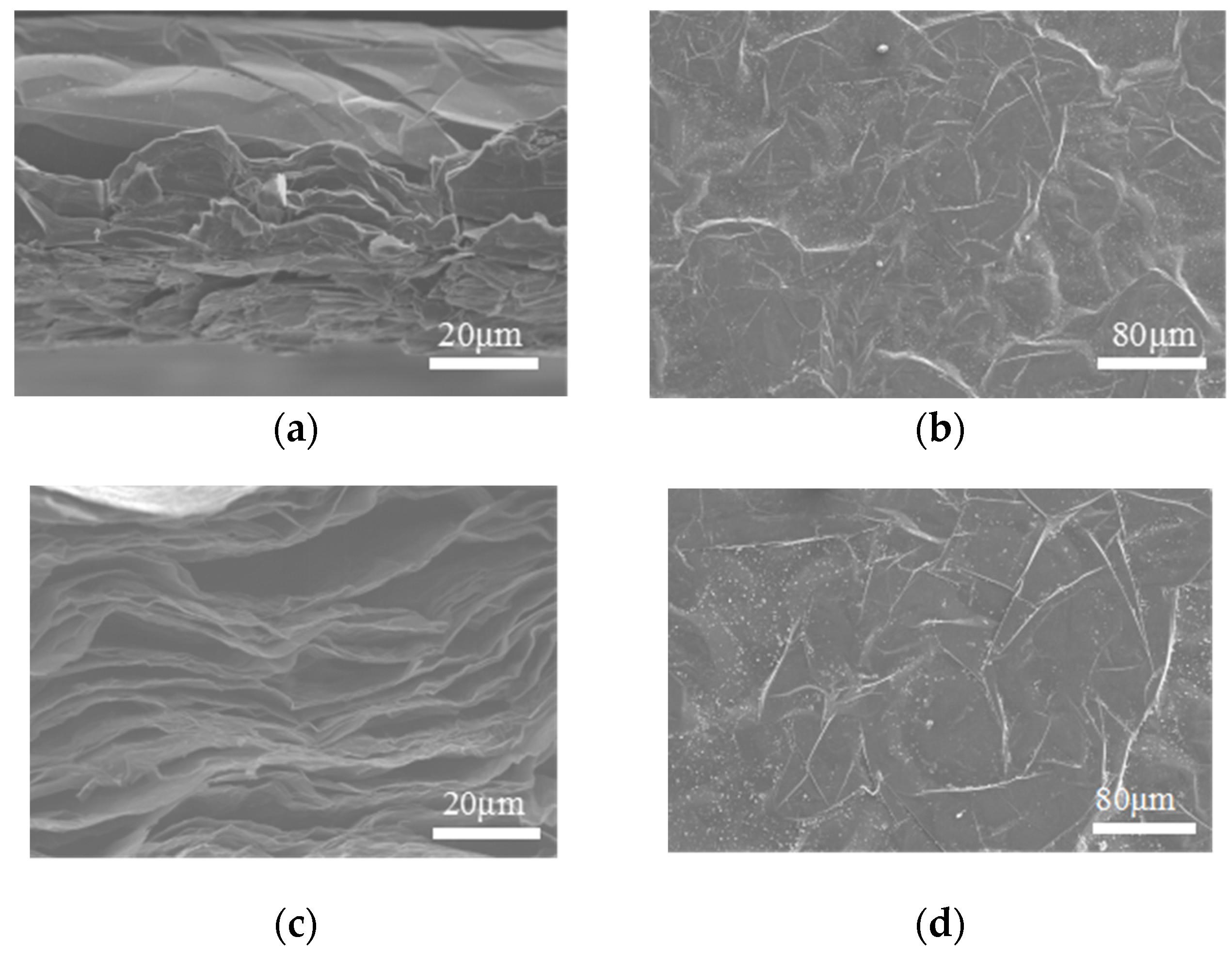
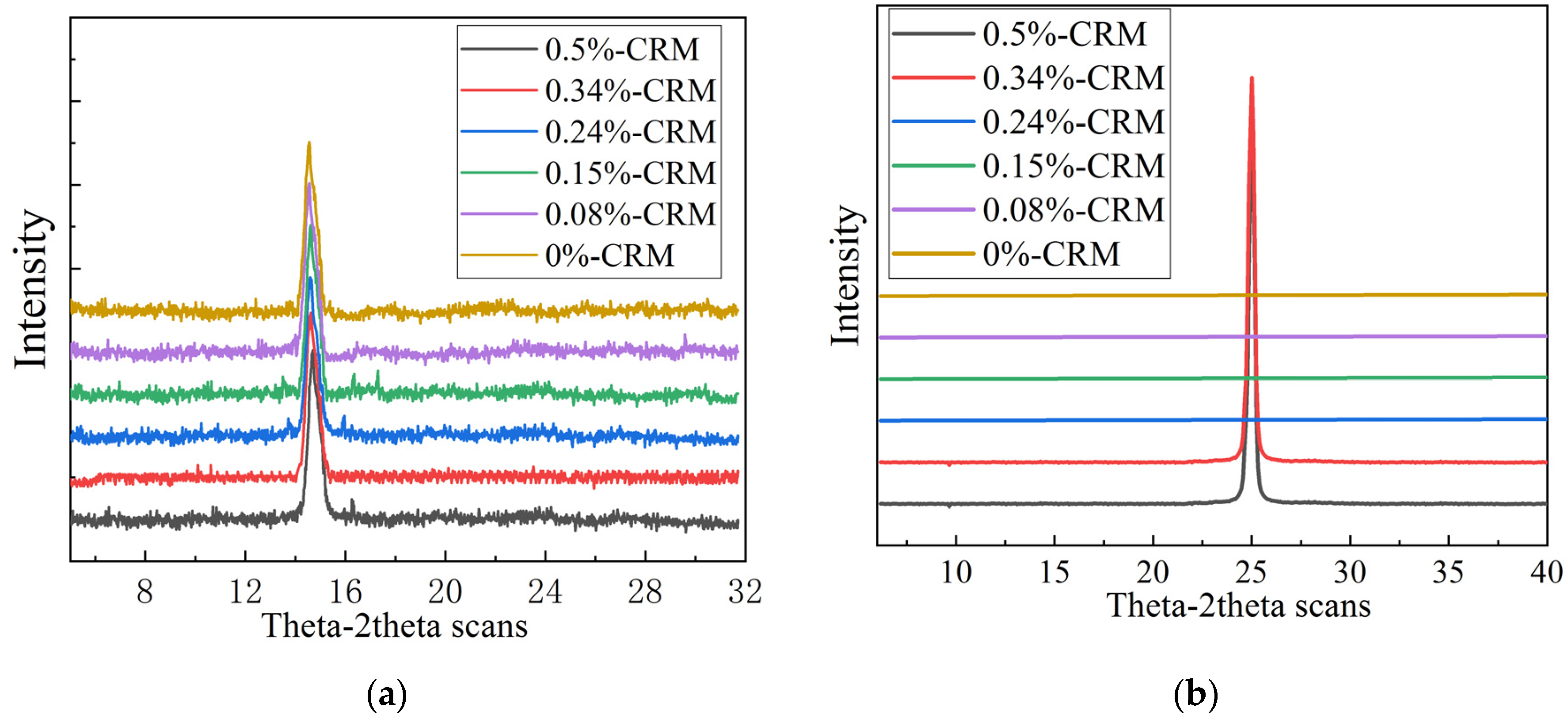
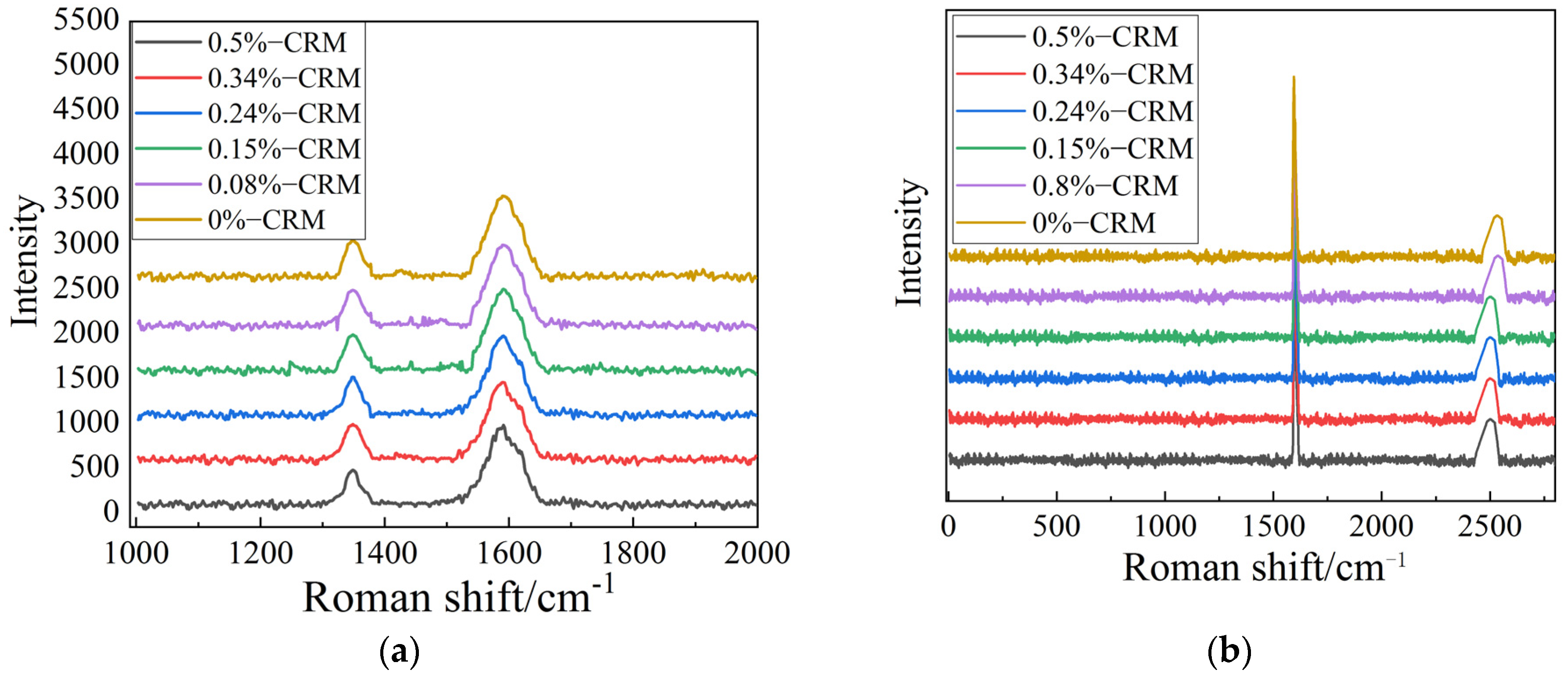
3.1. Effect of Adding Carbon-Rich Molecules to Graphene Films
3.2. Effect of Carbon-Rich Molecules on Thermal Conductivity of Graphene Film
3.3. Effect of Carbon-Rich Molecules on Mechanical Properties of Graphene Film
3.4. The Heat Dissipation Capacity of Graphene Films
3.5. Evaluating the Heat Dissipation Effect of Graphene Film on the New Energy Car Seats
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, K.X.; Gao, L.Y.; Li, Z.; Sun, R.; Liu, Z.Q. Research Progress of Electroplated Nanotwinned Copper in Microelectronic Packaging. Materials 2023, 16, 4614. [Google Scholar] [CrossRef] [PubMed]
- Xin, G.; Sun, H.; Hu, T.; Fard, H.R.; Sun, X.; Koratkar, N.; Borca-Tasciuc, T.; Lian, J. Large-area freestanding graphene paper for superior thermal management. Adv. Mater. 2014, 26, 4521–4526. [Google Scholar] [CrossRef] [PubMed]
- Wertz, J.T.; Kuczynski, J.P.; Boday, D.J. Thermally conductive-silicone composites with thermally reversible cross-links. ACS Appl. Mater. Interfaces 2016, 8, 56–64. [Google Scholar] [CrossRef]
- Raab, S.J.; Guschlbauer, R.; Lodes, M.A.; Körner, C. Thermal and electrical conductivity of 99.9% pure copper processed via selective electron beam melting. Adv. Eng. Mater. 2016, 18, 1661–1666. [Google Scholar] [CrossRef]
- Hu, Y.; Du, G.; Chen, N. A novel approach for Al2O3/epoxy composites with high strength and thermal conductivity. Compos. Sci. Technol. 2016, 124, 25–32. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Qian, W.; Zhang, Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, posttreatment, and bulk applications for composites and energy storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Balandina, A.A.; Nika, D.L. Phononics in low-dimensions: Engineering phonons in nanostructures and graphene. Mater. Today 2012, 15, 266. [Google Scholar]
- Song, S.H.; Xu, Z.; Liu, Z. Enhanced Thermal Conductivity of Graphene-Based Polymer Composites for Heat Sink Applications. Carbon 2016, 100, 628–635. [Google Scholar]
- Xu, X.; Li, X.; Fang, M.; Wang, W. Graphene Films for Next-Generation Thermal Management in Electronics. Adv. Funct. Mater. 2020, 30, 7–15. [Google Scholar]
- Baiocco, G.; Genna, S. Electrophoretic deposition of graphene coating on copper for improved thermoelectric performance of wire rods. Int. J. Adv. Manuf. Technol. 2024, 133, 5761–5776. [Google Scholar] [CrossRef]
- Seol, J.H.; Jo, I.; Moore, A.L.; Lindsay, L.; Aitken, Z.H.; Pettes, M.T.; Li, X.; Yao, Z.; Huang, R.; Broido, D.; et al. Two-dimensional phonon trans port in supported graphene. Science 2010, 328, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhai, W.T.; Zheng, W.G. Ultrathin flexible graphene film: An excelle nt thermal conducting material with efficient EMI shielding. Adv. Funct. Al Mater. 2014, 24, 4542–4548. [Google Scholar] [CrossRef]
- Kumar, P.; Shahzad, F.; Yu, S.; Hong, S.M.; Kim, Y.-H.; Koo, C.M. Large-area reduced graphene oxide thin film with excellent thermal conductivity and electromagnetic interference shielding effectiveness. Carbon 2015, 94, 494–500. [Google Scholar] [CrossRef]
- Meng, X.; Pan, H.; Zhu, C.; Chen, Z.; Lu, T.; Xu, D.; Li, Y.; Zhu, S. Coupled chiral structure in graphene-based film for ultrahigh thermal condu ctivity in both in-plane and through-plane directions. ACS Appl. Mater. Interfaces 2018, 10, 22611–22622. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Xiong, X. Free-standing paper-like heat spreading films based on graphene oxide-aromatic molecule c omposites. J. Mater. Sci. Mater. Ls Electron. 2018, 29, 3050–3055. [Google Scholar] [CrossRef]
- Dai, B.; Fu, L.; Liao, L.; Liu, N.; Yan, K.; Chen, Y.; Liu, Z. High-quality single-layer graphene via repar ative reduction of graphene oxide. Nano Res. 2011, 4, 434–439. [Google Scholar] [CrossRef]
- Song, N.; Cui, S.Q. Layered nanofibrillated cellulose hybrid films as flexible lateral heat spreaders: The effect of graphe ne defect. Carbon 2017, 115, 338–346. [Google Scholar] [CrossRef]
- Haskins, J.; Kınacı, A.; Sevik, C.; Sevinçli, H.; Cuniberti, G.; Çağın, T. Control of thermal and electronic transport in defect-engineered graphene nanoribbons. ACS Nano 2011, 5, 3779–3787. [Google Scholar] [CrossRef]
- Zhang, H.J.; Lee, G.; Cho, K. Thermal transport in graphene and effects of vacancy defects. Phys. Rev. B 2011, 84, 115460. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Two-dimensional phonon transport in graphene. J. Phys. Condens. Matter 2012, 24, 233203. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wu, Z.; Wang, W.; Bi, K.; Liang, Z.; Yang, J.; Chen, Y.; Xu, Z.; Ni, Z. Defect-engineered heat transport in graphene: A route to highefficient thermal rectification. Sci. Rep. 2015, 5, 11962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Pu, H.; Lv, P.; Zhu, Z.; Yang, C.; Zheng, R.; Wang, Z.; Liu, C.; Hu, E.; Zheng, J.; et al. Healing of reduced graphene oxide with methane hydrogen plasma. Carbon 2017, 120, 274–280. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Miao, J.; Wu, X.; Chandrasekharan, N.; Qiu, H.; Yang, J. Enhanced thermal conductivity of graphene/ polyimide hybrid film via a novel “molecular welding” strategy. Carbon 2018, 126, 319–327. [Google Scholar] [CrossRef]
- Ren, S.; Liu, B.; Wang, M.; Han, G.; Zhao, H.; Zhang, Y. Highly Bright Carbon Quantum Dots for Flexible Anti-counterfeiting. J. Mater. Chem. C 2022, 1039, 23–31. [Google Scholar] [CrossRef]
- Wu, P.; Gao, L.; Lv, Z.; Zhang, D. Research progress of carbon quantum dots in metal corrosion protection. Corros. Prot. 2022, 43, 83–87. [Google Scholar]
- Wang, Y.; Zhang, L.; Liu, J. Bioinspired Carbon Quantum Dots Nanofluid Fabricated by a Facile Two-Step Synthesis toward High Performance Lubrication. ACS Appl. Mater. Interfaces 2023, 15, 4567–4578. [Google Scholar]
- Qin, T.; Deng, L.; Zhang, N.; Zhang, P.; Liu, Y.; Qin, L.; Huang, S.; Qiu, L.; Peng, S. Flexible polyaniline/carbon quantum dots films for enhanced electrochromic performance. Ceram. Int. 2024, 50, 53254–53263. [Google Scholar] [CrossRef]
- Niu, R.-H.; Li, Y.-W.; Ma, S.-L.; Yi, Z.-L.; Xie, L.-J.; Su, F.-Y.; Jia, H.; Chen, C.-M. Asymmetric structure endows thermal radiation and heat conduction in graphene films for enhancing dual-mode heat dissipation. J. Mater. Chem. A Mater. Energy Sustain. 2025, 11, 7890–7902. [Google Scholar] [CrossRef]
- Gang, Z.; Miao, Z.; Liu, Y.; Huang, J.; Chen, F.; Fu, Q. High thermal conductivity and increased thickness graphene nanosheet films prepared through metal ion-free route. Ceram. Int. 2022, 48, 3711–3719. [Google Scholar] [CrossRef]
- Bai, T.; Huang, K.; Liu, F.; Shi, R.; Ren, W.; Pei, S.; Gao, P.; Liu, Z. Nanoscale Mechanism of Microstructure-Dependent Thermal Diffusivity in Thick Graphene Sheets. Acta Phys. Chim. Sin. 2025, 41, 25–32. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh thermal conductive yet super flexible graphene films. Adv. Mater. 2017, 29, 270–289. [Google Scholar] [CrossRef]
- Xiong, K.; Yang, T.; Sun, Z.; Ma, C.; Wang, J.; Ge, X.; Qiao, W.; Ling, L. Modified graphene film powder scraps for repreparation of highly thermally conductive flexible graphite heat spreaders. Carbon 2024, 11, 8–27. [Google Scholar]
- Miao, J.; Li, H.; Qiu, H.; Wu, X.; Yang, J. Graphene/PANI hybrid film with enhanced thermal conductivity by in situ polymerization. J. Mater. Sci. 2018, 53, 8855–8865. [Google Scholar] [CrossRef]

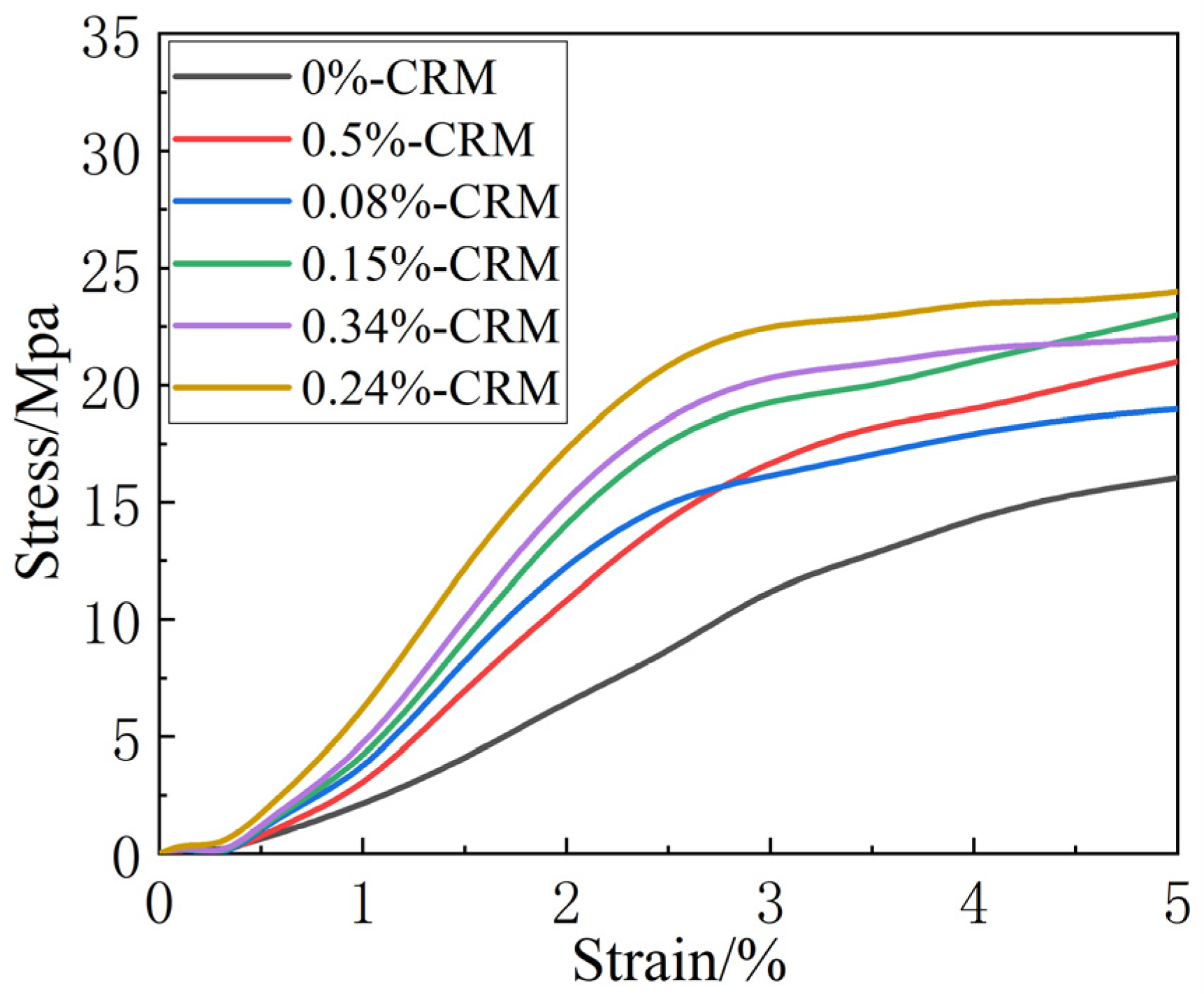

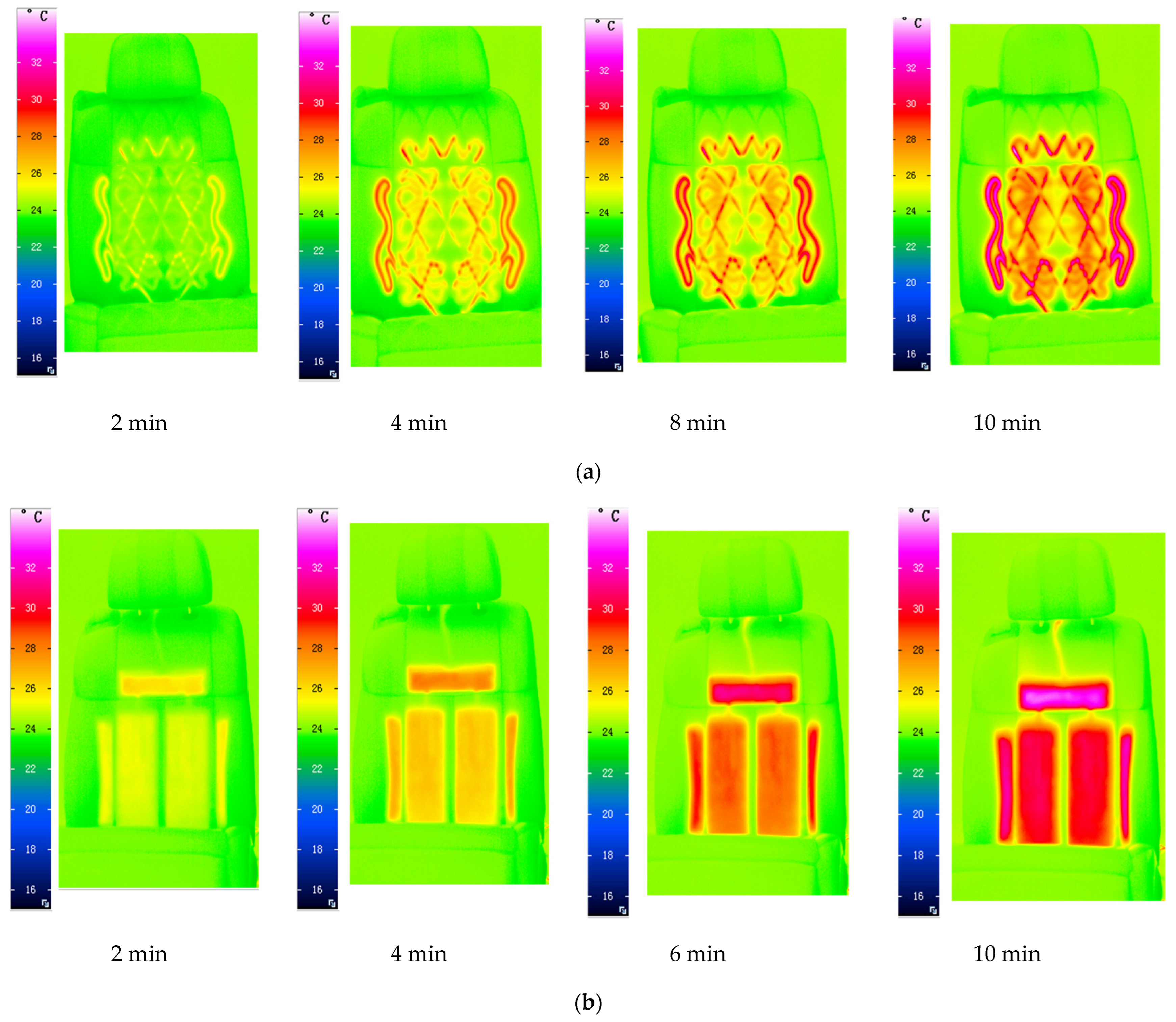
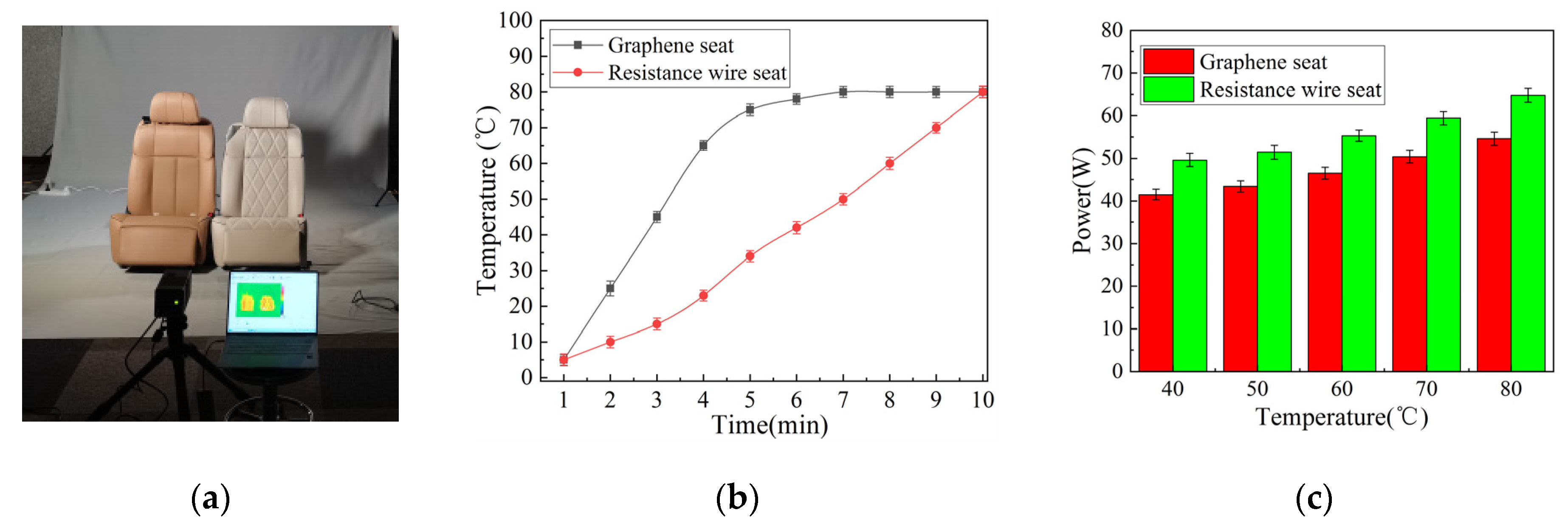
| Sample Number | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| CRM quality score% | 0.00 | 0.08 | 0.15 | 0.24 | 0.34 | 0.5 |
| Ambient Temperature/°C | Mass Fraction of CRMs/% | Modulus of Elasticity/Mpa | Maximum Tensile Force/N |
|---|---|---|---|
| 0 | 0 | 949.44 | 18.32 |
| 0.08 | 1015.82 | 21.13 | |
| 0.15 | 1198.43 | 23.14 | |
| 0.24 | 1267.52 | 25.67 | |
| 0.34 | 1199.32 | 23.02 | |
| 0.5 | 1023.35 | 21.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Jia, X. A Preparation Method for Improving the Thermal Conductivity of Graphene Film. Coatings 2025, 15, 560. https://doi.org/10.3390/coatings15050560
Zhao X, Jia X. A Preparation Method for Improving the Thermal Conductivity of Graphene Film. Coatings. 2025; 15(5):560. https://doi.org/10.3390/coatings15050560
Chicago/Turabian StyleZhao, Xia, and Xin Jia. 2025. "A Preparation Method for Improving the Thermal Conductivity of Graphene Film" Coatings 15, no. 5: 560. https://doi.org/10.3390/coatings15050560
APA StyleZhao, X., & Jia, X. (2025). A Preparation Method for Improving the Thermal Conductivity of Graphene Film. Coatings, 15(5), 560. https://doi.org/10.3390/coatings15050560






