Effect of Gun Geometry on MCrAlX Coating Microstructure and In-Flight Oxidation Deposited by Low-Temperature High-Velocity Air Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Selection
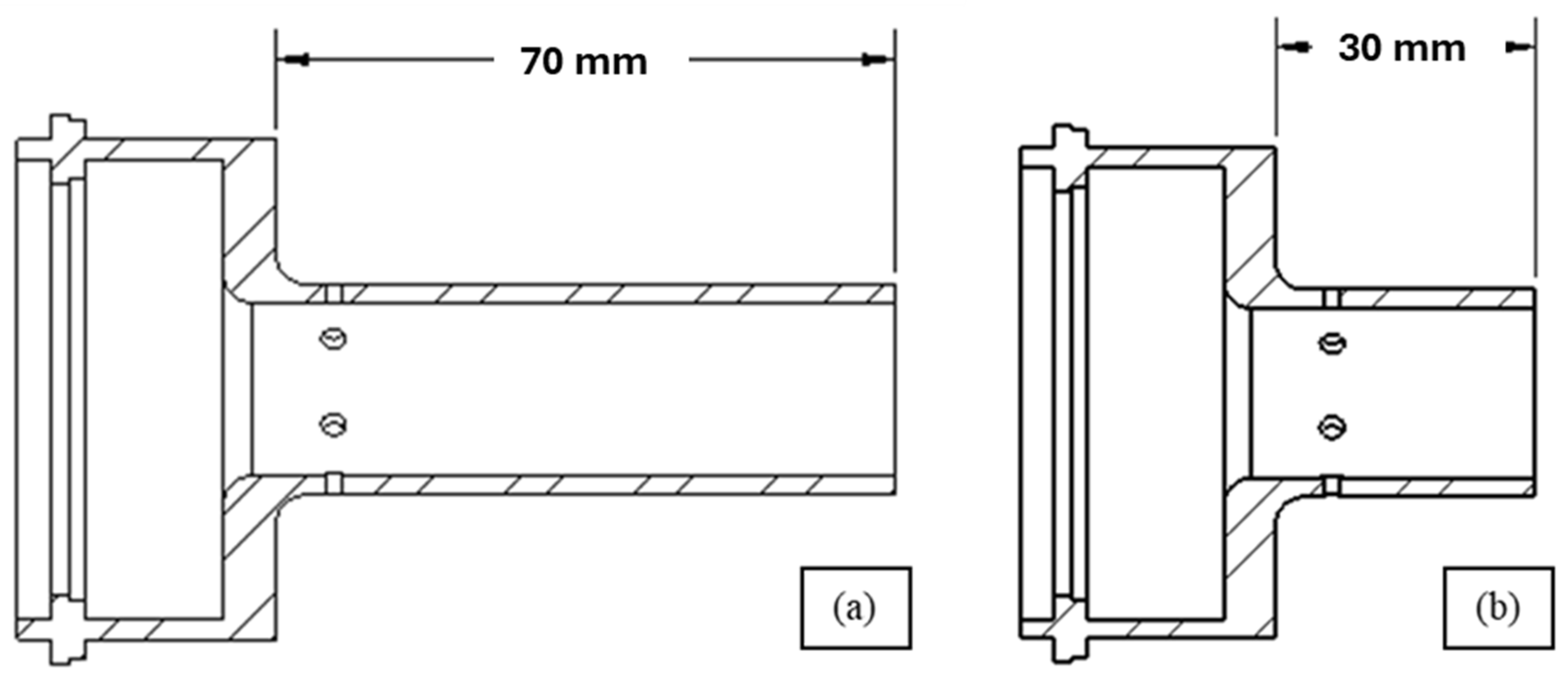
2.2. High-Velocity Air Fuel Deposition
2.3. Particle Diagnosis
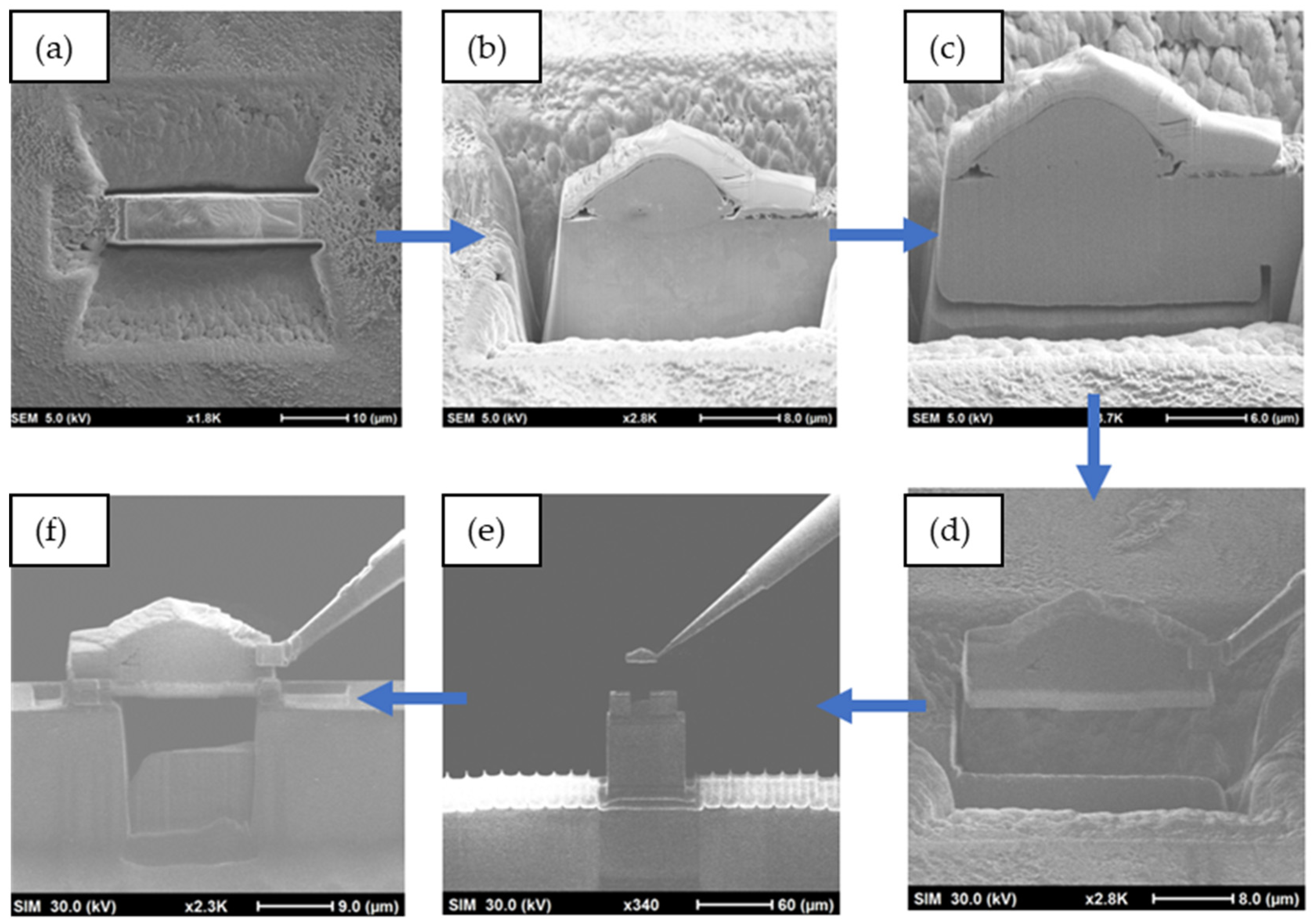
2.4. Characterization Techniques
3. Results and Discussion
3.1. Powder Characterization
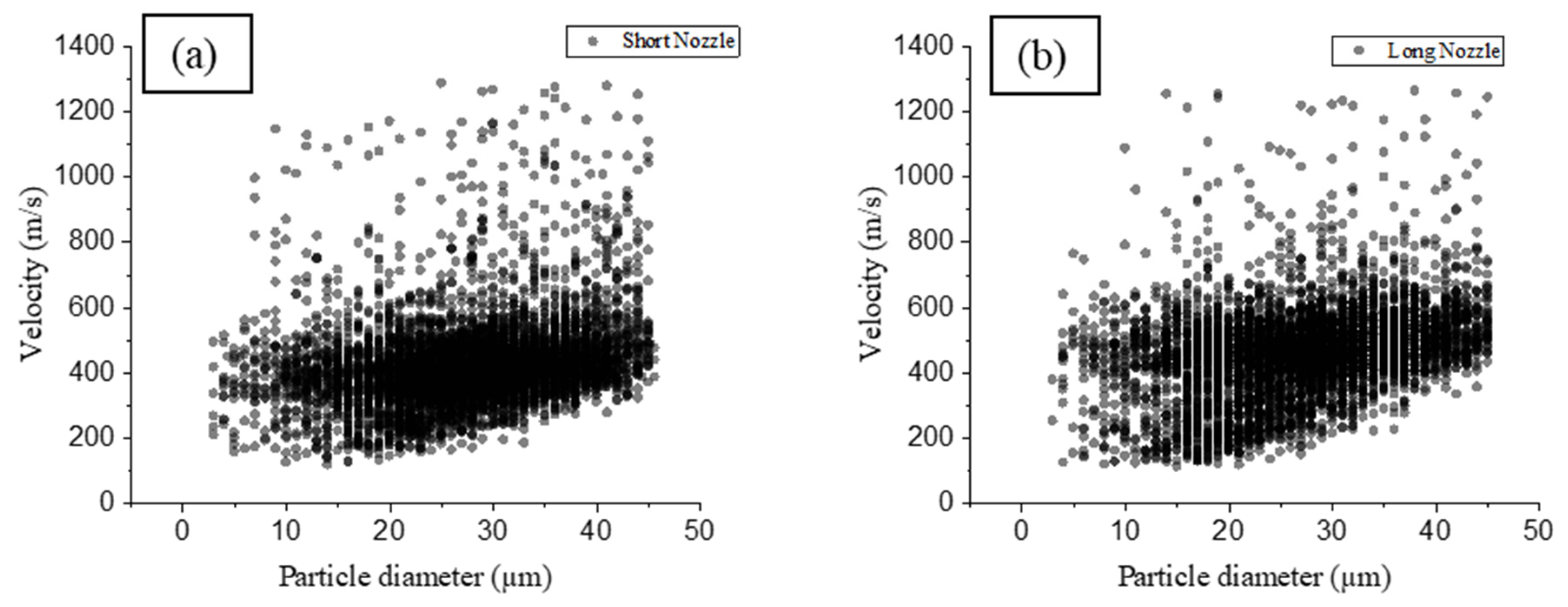
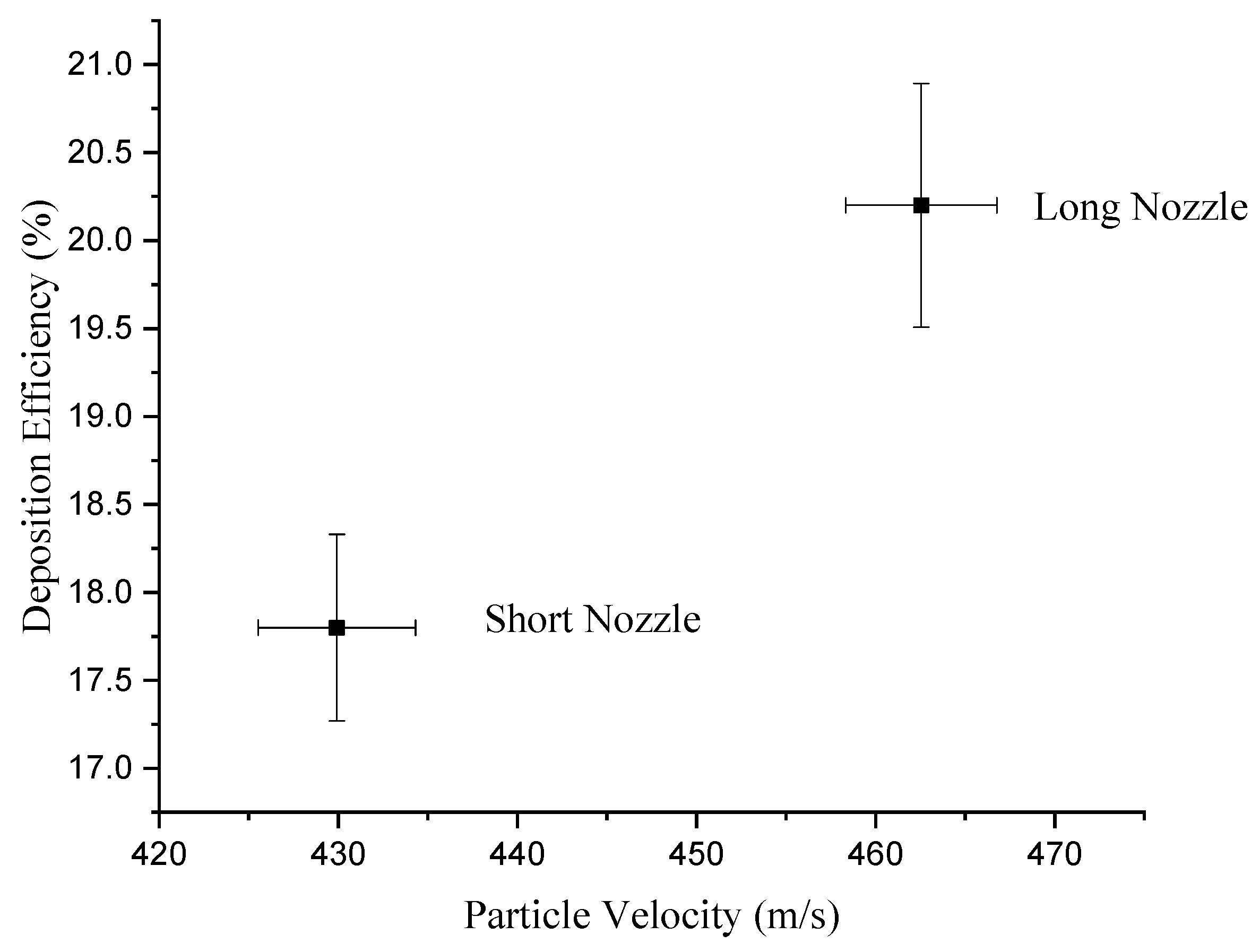
3.2. Particle Diagnostics
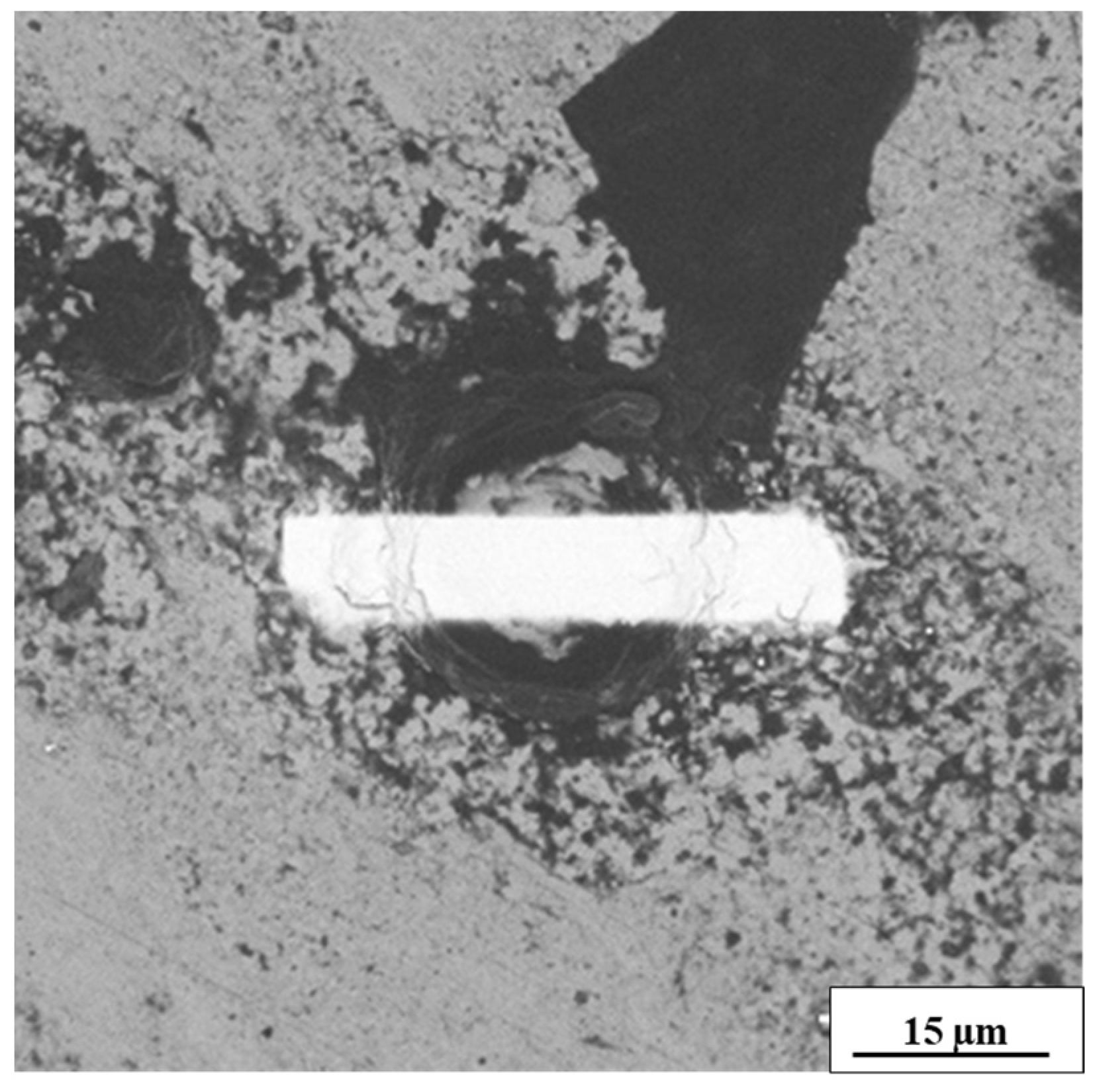
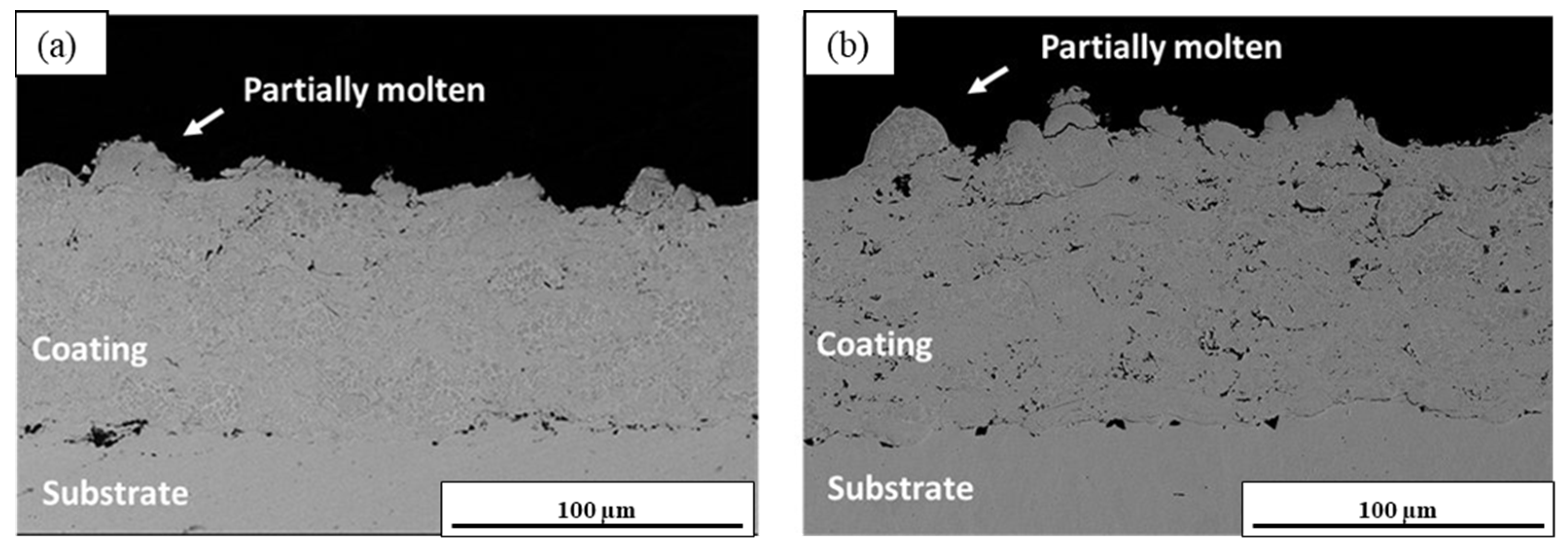
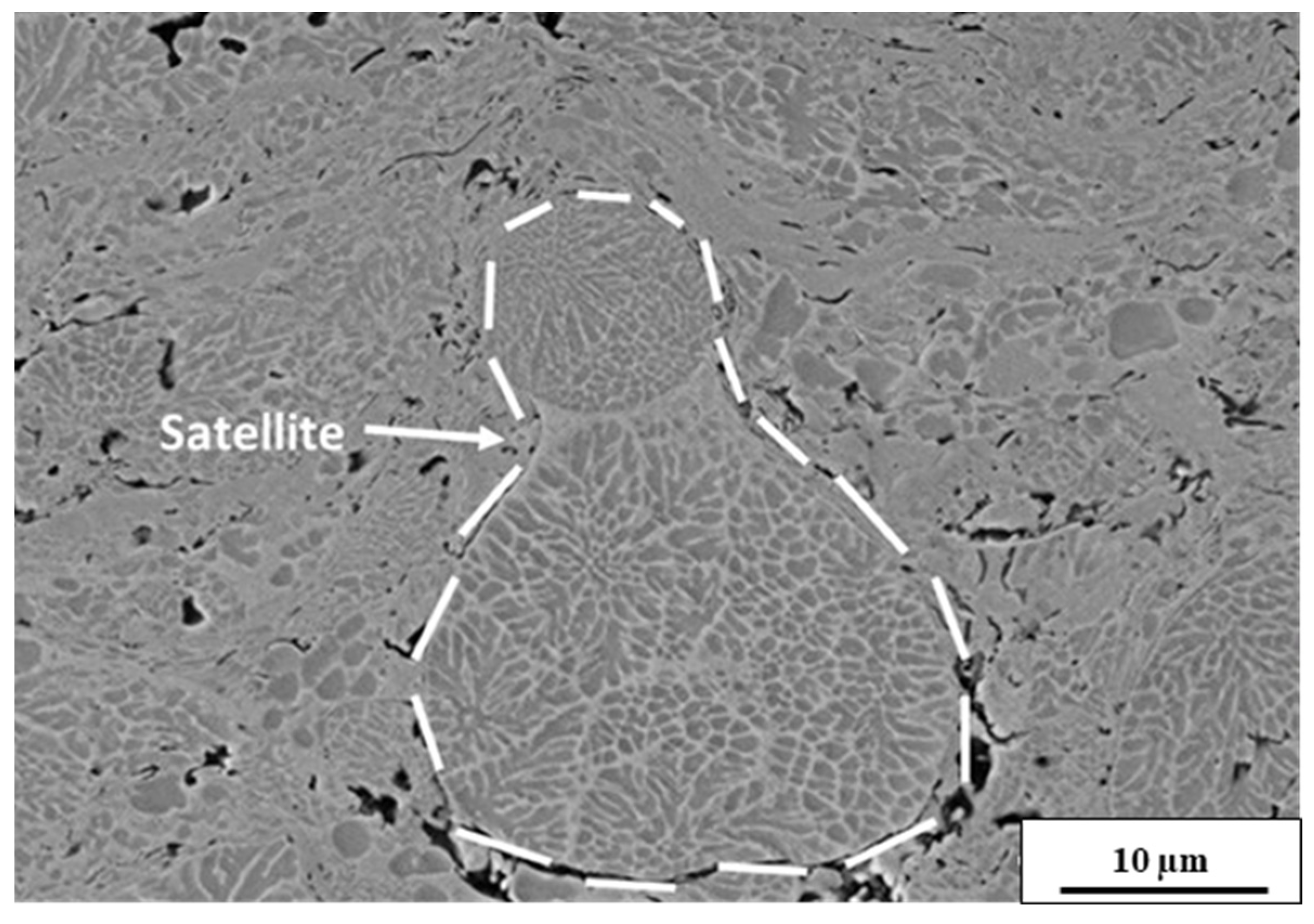
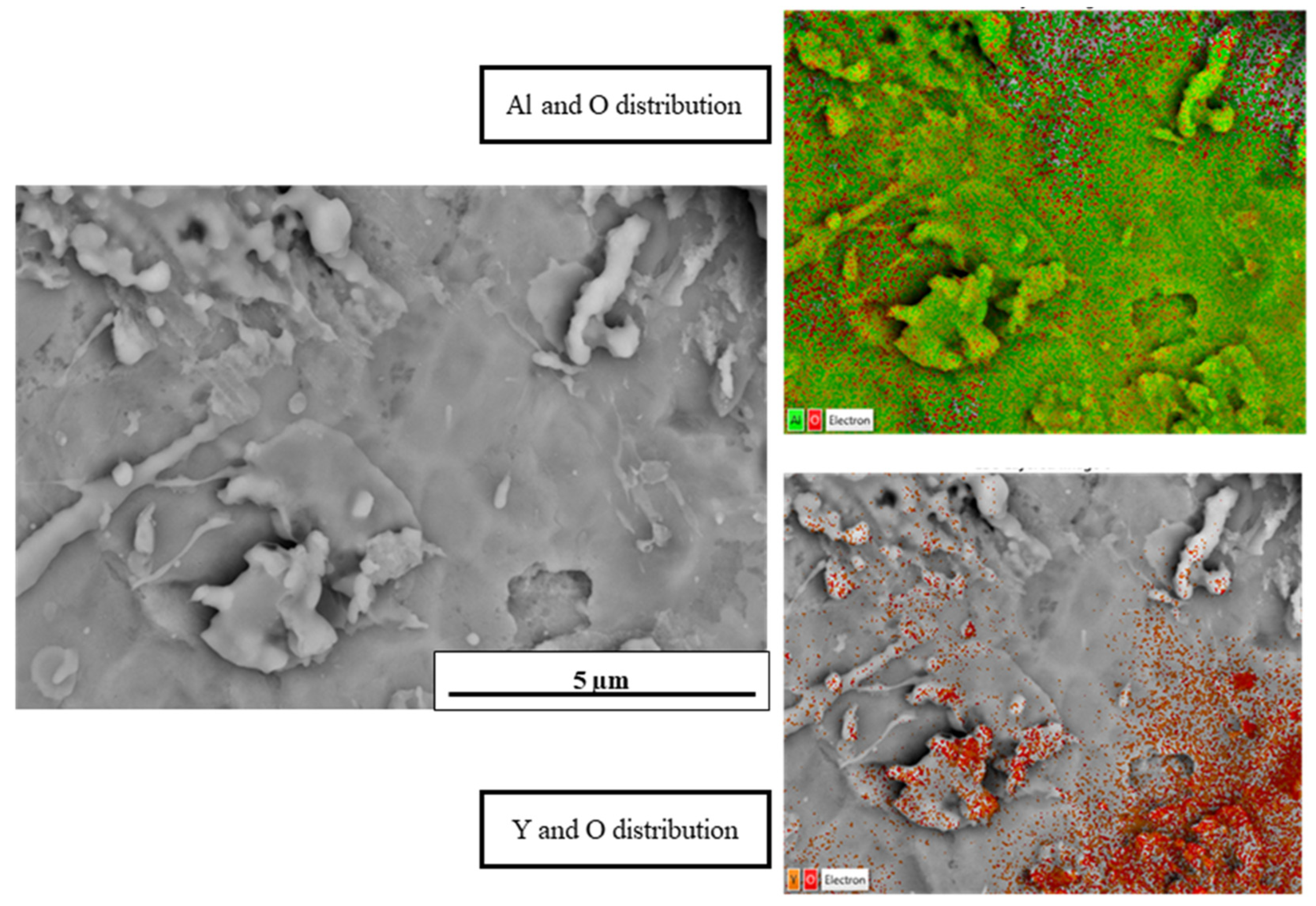
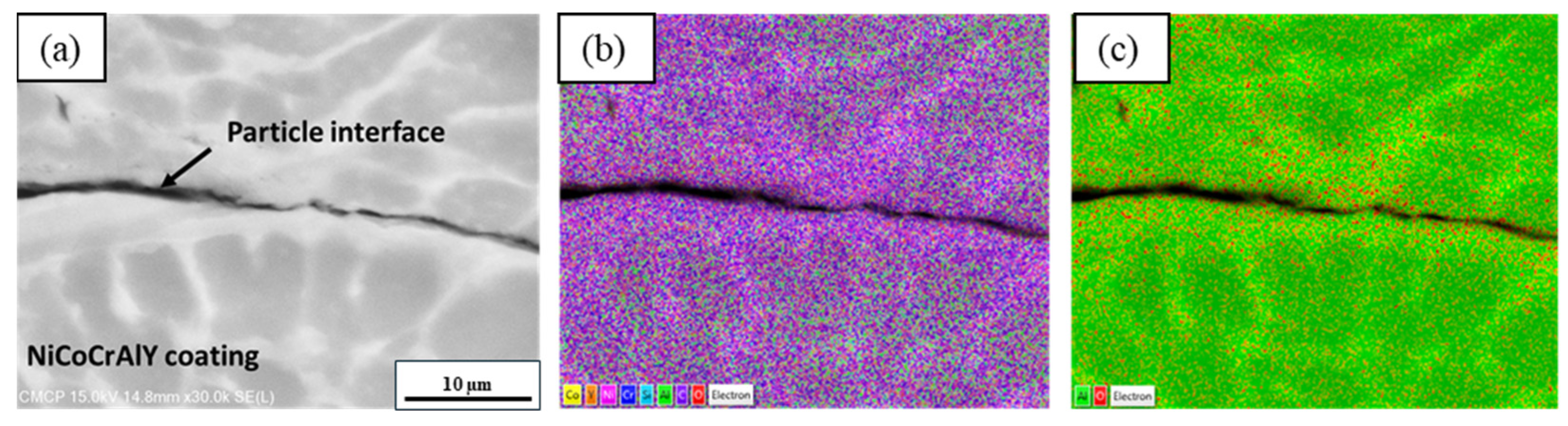
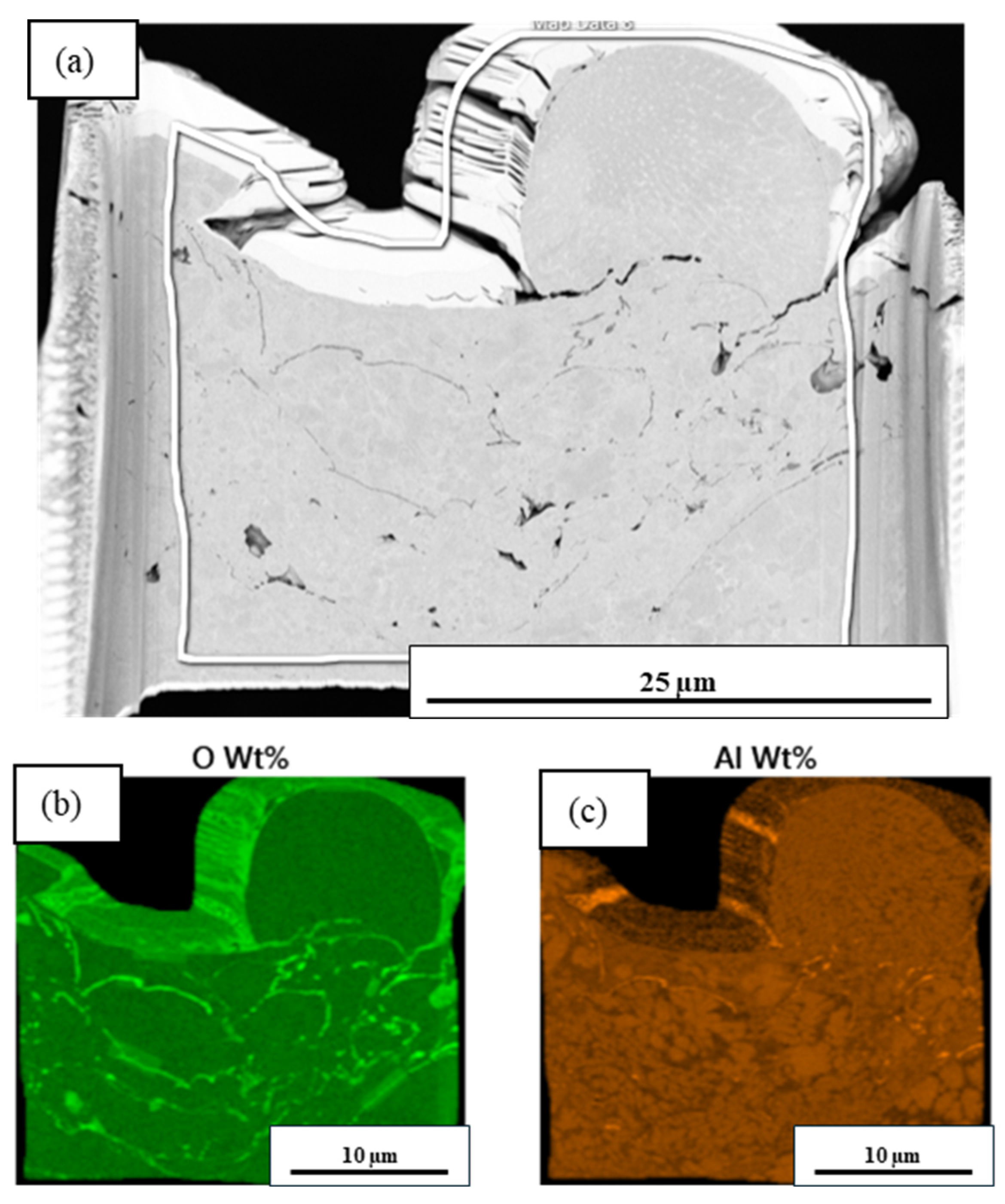
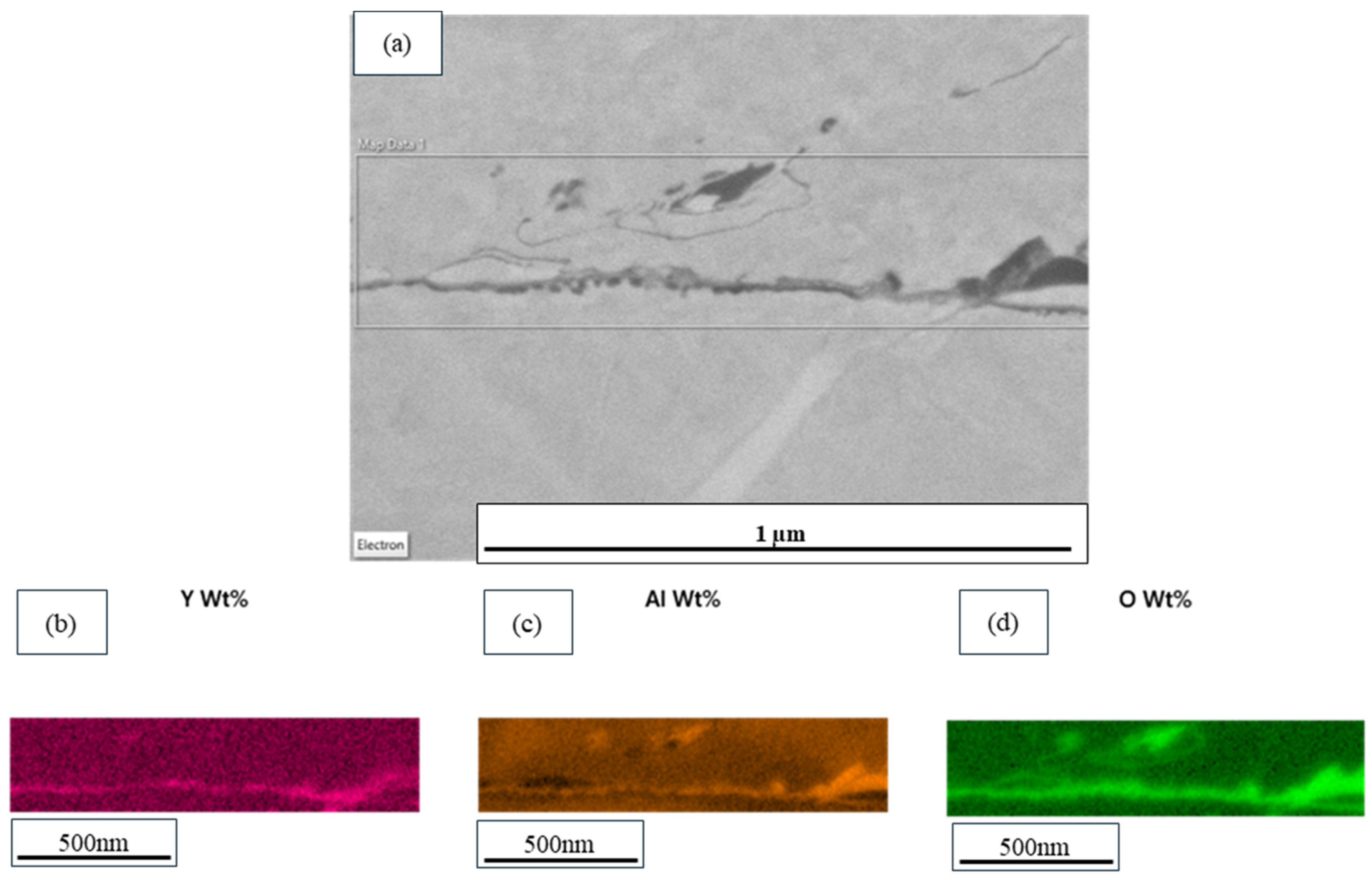
3.3. Microstructural Analysis
3.4. Compositional Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HVAF | High-Velocity Air Fuel |
| HVOF | High-Velocity Oxygen-Fuel |
| TBC | Thermal Barrier Coatings |
| SEM | Scanning Electron Microscopy |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| FIB | Focused Ion Beam |
| EDS | Energy Dispersive Spectroscopy |
| BC | Bond Coat |
| TC | Topcoat |
| YSZ | Yttria-Stabilized Zirconia |
| TGO | Thermally Grown Oxide |
| AC-HVAF | Activated Combustion High-Velocity Air Fuel |
| ID-HVAF | Internal Diameter High-Velocity Air Fuel |
| GIS | Gas Injection System |
| BSE | Backscattered Electron |
| SE | Secondary Electron |
| GA | Gas Atomized |
References
- Osyka, A.S.; Rybnikov, A.I.; Leontiev, S.A.; Nikitin, N.V.; Malashenko, I.S. Experience with metal/ceramic coating in stationary gas turbines. Surf. Coat. Technol. 1995, 76–77, 86–94. [Google Scholar] [CrossRef]
- Gildersleeve, V.V.E.J.; Viswanathan, V.; Lance, M.J.; Haynes, J.A.; Pint, B.A.; Sampath, S. Role of bond coat processing methods on the durability of plasma sprayed thermal barrier systems. Surf. Coat. Technol. 2019, 375, 782–792. [Google Scholar] [CrossRef]
- Boyce, M.P. (Ed.) Gas Turbine Engineering Handbook; Butterworth-Heinemann: Oxford, UK, 2012; pp. i–ii. Available online: https://www.sciencedirect.com/science/article/pii/B978012383842100024X (accessed on 28 March 2022).
- Fauchais, P.L.; Heberlein, J.V.R.; Boulos, M.I. Thermal Spray Fundamentals: From Powder to Part; Springer Science & Business Media: New York, NY, USA, 2014. [Google Scholar]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Moskal, G. Thermal barrier coatings: Characteristics of microstructure and properties, generation and directions of development of bond. J. Achiev. Mater. Manuf. Eng. 2009, 37, 323–331. [Google Scholar]
- International Workshop on Surface Engineering and Coatings; Rajagopal, I.; National Aerospace Laboratories. International Workshop on Surface Engineering and Coatings: June 25–30, 1998; Allied Publishers: New Delhi, India, 1999. [Google Scholar]
- Karaoglanli, A.C.; Ozgurluk, Y.; Doleker, K.M. Comparison of microstructure and oxidation behavior of CoNiCrAlY coatings produced by APS, SSAPS, D-gun, HVOF and CGDS techniques. Vacuum 2020, 180, 109609. [Google Scholar] [CrossRef]
- Szymanski, K.; Goral, M.; Kubaszek, T.; Monteiro, P. Microstructure of TBC coatings deposited by HVAF and PS-PVD methods. Solid State Phenom. 2015, 227, 373–376. [Google Scholar] [CrossRef]
- Joshi, S.; Nylen, P. Advanced Coatings by Thermal Spray Processes. Technologies 2019, 7, 79. [Google Scholar] [CrossRef]
- Kuroda, S.; Watanabe, M.; Kim, K.; Katanoda, H. Current Status and Future Prospects of Warm Spray Technology. J. Therm. Spray Technol. 2011, 20, 653–676. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Mauer, G.; Vaßen, R. Enhanced characteristics of HVOF-sprayed MCrAlY bond coats for TBC applications. J. Therm. Spray Technol. 2011, 20, 1209–1216. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; Xu, Y.; Chen, X.; Han, X. Effects of Fuel Types and Process Parameters on the Performance of an Activated Combustion High Velocity Air-Fuel (AC-HVAF) Thermal Spray System. J. Therm. Spray Technol. 2021, 30, 1875–1890. [Google Scholar] [CrossRef]
- Lyphout, C.; Bjorklund, S. Internal Diameter HVAF Spraying for Wear and Corrosion Applications. J. Therm. Spray Technol. 2015, 24, 235–243. [Google Scholar] [CrossRef]
- Mauer, G.; Vaßen, R.; Stöver, D. Plasma and Particle Temperature Measurements in Thermal Spray: Approaches and Applications. J. Therm. Spray Technol. 2010, 20, 391–406. [Google Scholar] [CrossRef]
- Giannuzzi, L.A.; Drown, J.L.; Brown, S.R.; Irwin, R.B.; Stevie, F.A. Focused Ion Beam Milling and Micromanipulation Lift-Out for Site Specific Cross-Section Tem Specimen Preparation. MRS Proc. 1997, 480, 19–27. [Google Scholar] [CrossRef]
- Chandrakar, R.; Bessette, S.; Brodush, N.; Gauvin, R. High-Resolution Imaging and X-Ray Microanalysis of Oxide at Low Energy using Scanning Electron Microscope and Triple Beam FIB Microscope. Microsc. Microanal. 2024, 30 (Suppl. S1), ozae044.332. [Google Scholar] [CrossRef]
- Chen, W.R.; Irissou, E.; Wu, X.; Legoux, J.-G.; Marple, B.R. The Oxidation Behavior of TBC with Cold Spray CoNiCrAlY Bond Coat. J. Therm. Spray Technol. 2011, 20, 132–138. [Google Scholar] [CrossRef]
- Zakeri, A.; Ghadami, F.; Rouhaghdam, A.S.; Saeedi, B. Study on production of modified MCrAlY powder with nano oxide dispersoids as HVOF thermal spray feedstock using mechanical milling. Mater. Res. Express 2020, 7, 015030. [Google Scholar] [CrossRef]
- Strondl, A.; Lyckfeldt, O.; Brodin, H.; Ackelid, U. Characterization and Control of Powder Properties for Additive Manufacturing. JOM 2015, 67, 549–554. [Google Scholar] [CrossRef]
- Giese, S.; Neumeier, S.; Amberger-Matschkal, D.; Bergholz, J.; Vaßen, R.; Göken, M. Microtensile creep testing of freestanding MCrAlY bond coats. J. Mater. Res. 2019, 34, 2643–2652. [Google Scholar] [CrossRef]
- Kim, D.J.; Seo, D.Y.; Huang, X.; Yang, Q.; Kim, Y.-W. Cyclic oxidation behavior of a beta gamma powder metallurgy TiAl–4Nb–3Mn alloy coated with a NiCrAlY coating. Surf. Coat. Technol. 2012, 206, 3048–3054. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Chen, Y.; Zhao, X.; Guo, F.; Xiao, P. Effect of microstructure of a NiCoCrAlY coating fabricated by high-velocity air fuel on the isothermal oxidation. Corros. Sci. 2019, 159, 108126. [Google Scholar] [CrossRef]
- Xiong, L.; Chuang, A.C.; Thomas, J.; Prost, T.; White, E.; Anderson, I.; Singh, D. Defect and satellite characteristics of additive manufacturing metal powders. Adv. Powder Technol. 2022, 33, 103486. [Google Scholar] [CrossRef]
- Chen, W.R.; Wu, X.; Marple, B.R.; Nagy, D.R.; Patnaik, P.C. TGO growth behaviour in TBCs with APS and HVOF bond coats. Surf. Coat. Technol. 2008, 202, 2677–2683. [Google Scholar] [CrossRef]
- Gao, X.; Li, C.; Zhang, D.; Gao, H.; Han, X. Numerical analysis of the activated combustion high-velocity air-fuel (AC-HVAF) thermal spray process: A survey on the parameters of operation and nozzle geometry. Surf. Coat. Technol. 2021, 405, 126588. [Google Scholar] [CrossRef]
- Jenkins, R.; Yin, S.; Aldwell, B.; Meyer, M.; Lupoi, R. New insights into the in-process densification mechanism of cold spray Al coatings: Low deposition efficiency induced densification. J. Mater. Sci. Technol. 2019, 35, 427–431. [Google Scholar] [CrossRef]
- Mauer, G.; Rauwald, K.-H.; Sohn, Y.J.; Vaßen, R. The Potential of High-Velocity Air-Fuel Spraying (HVAF) to Manufacture Bond Coats for Thermal Barrier Coating Systems. J. Therm. Spray Technol. 2024, 33, 746–755. [Google Scholar] [CrossRef]
- Hejrani, E.; Sebold, D.; Nowak, W.J.; Mauer, G.; Naumenko, D.; Vaßen, R.; Quadakkers, W.J. Isothermal and cyclic oxidation behavior of free standing MCrAlY coatings manufactured by high-velocity atmospheric plasma spraying. Surf. Coat. Technol. 2017, 313, 191–201. [Google Scholar] [CrossRef]
- Mauer, G.; Sebold, D.; Vaßen, R.; Hejrani, E.; Naumenko, D.; Quadakkers, W.J. Impact of processing conditions and feedstock characteristics on thermally sprayed MCrAlY bondcoat properties. Surf. Coat. Technol. 2017, 318, 114–121. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Zhang, H.; Zhao, C.; Zhao, X.; Guo, F.; Xiao, P. Superior oxidation and spallation resistant NiCoCrAlY bond coat via homogenizing the yttrium distribution. Corros. Sci. 2019, 159, 108145. [Google Scholar] [CrossRef]
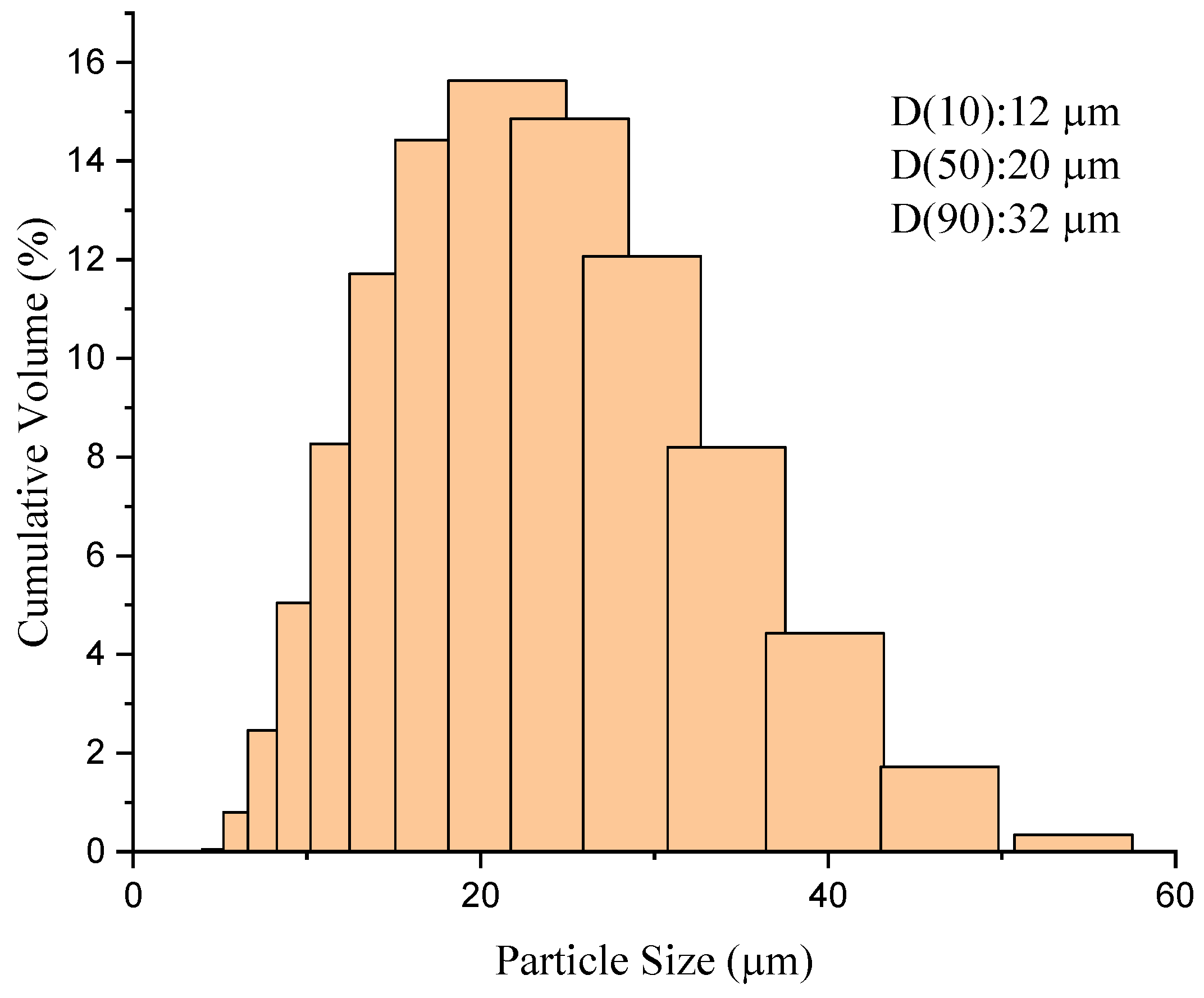


| Amdry 386 | |||||||
|---|---|---|---|---|---|---|---|
| Powder cut size (µm) | −63 + 5 | ||||||
| Material | Ni | Co | Cr | Al | Hf | Y | Si |
| wt.% | 47.6 | 22 | 17 | 12 | 0.5 | 0.5 | 0.4 |
| Parameters | |
|---|---|
| Substrate | Stainless Steel |
| Fuel | Propylene |
| Powder feed rate (g/min) | 100 |
| Carrier gas flow (L/min) | 18 |
| Air flow rate (L/min) | 3300 |
| Fuel flow rate (g/min) | 200 |
| Stand-off distance (mm) | 75 |
| Robot speed (mm/s) | 1000 |
| Nozzle length (mm) | 30 and 70 |
| Coating | Average Oxygen Content (wt.%) | Oxide Layer Thickness (nm) |
|---|---|---|
| Short Nozzle | 17.5 ± 1.7 | 27.0 ± 3.8 |
| Long Nozzle | 14.1 ± 1.6 | 22.5 ± 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamana, M.S.; Thoutam, A.K.; de Castilho, B.C.N.M.; Ben Ettouil, F.; Chandrakar, R.; Bessette, S.; Brodusch, N.; Gauvin, R.; Dolatabadi, A.; Moreau, C. Effect of Gun Geometry on MCrAlX Coating Microstructure and In-Flight Oxidation Deposited by Low-Temperature High-Velocity Air Fuel. Coatings 2025, 15, 357. https://doi.org/10.3390/coatings15030357
Lamana MS, Thoutam AK, de Castilho BCNM, Ben Ettouil F, Chandrakar R, Bessette S, Brodusch N, Gauvin R, Dolatabadi A, Moreau C. Effect of Gun Geometry on MCrAlX Coating Microstructure and In-Flight Oxidation Deposited by Low-Temperature High-Velocity Air Fuel. Coatings. 2025; 15(3):357. https://doi.org/10.3390/coatings15030357
Chicago/Turabian StyleLamana, Murilo Sergio, Aravind Kumar Thoutam, Bruno C. N. M. de Castilho, Fadhel Ben Ettouil, Ritvij Chandrakar, Stephanie Bessette, Nicolas Brodusch, Raynald Gauvin, Ali Dolatabadi, and Christian Moreau. 2025. "Effect of Gun Geometry on MCrAlX Coating Microstructure and In-Flight Oxidation Deposited by Low-Temperature High-Velocity Air Fuel" Coatings 15, no. 3: 357. https://doi.org/10.3390/coatings15030357
APA StyleLamana, M. S., Thoutam, A. K., de Castilho, B. C. N. M., Ben Ettouil, F., Chandrakar, R., Bessette, S., Brodusch, N., Gauvin, R., Dolatabadi, A., & Moreau, C. (2025). Effect of Gun Geometry on MCrAlX Coating Microstructure and In-Flight Oxidation Deposited by Low-Temperature High-Velocity Air Fuel. Coatings, 15(3), 357. https://doi.org/10.3390/coatings15030357







