Abstract
Ships and offshore equipment operating in marine environments often face issues such as seawater corrosion and biofouling, leading to significant economic losses. To address the corrosion problems of ships and offshore equipment, heavy-duty anticorrosive coatings are widely used for corrosion protection in marine environments due to their long-term effectiveness, cost-efficiency, and excellent applicability. In this study, silane coupling agent (KH-560) was employed to modify sodium silicate, and the modified sodium silicate was then incorporated as a reinforcing phase into polyurethane to ultimately prepare a modified sodium silicate/polyurethane coating. The feasibility of the modified sodium silicate/polyurethane coating was investigated by characterizing its conventional physicochemical properties, weather resistance, acid and alkali resistance, and salt spray corrosion resistance. Experimental results indicate that the silane coupling agent acts as a bridge between the organic and inorganic interfaces through the hydrolysis and condensation reactions of its bifunctional groups, forming an interfacial layer connected by hydrogen bonds and covalent bonds, thereby improving the compatibility between the organic resin and inorganic sodium silicate. Comprehensive performance analysis revealed that when the content of modified sodium silicate was 60 wt%, the coating hardness reached 4H. Additionally, electrochemical tests demonstrated that the coating exhibited higher impedance (9.62 × 104 Ω/cm2) and lower corrosion current density (5.82 × 10−7 A/cm2). This study provides a theoretical and experimental basis for the development of high-performance anticorrosive coatings for marine applications.
1. Introduction
The marine environment, characterized by high temperature and humidity, is complex and harsh, posing significant challenges to instruments and equipment operating under such conditions. These challenges include seawater corrosion [1,2], marine biofouling [3,4], and mechanical wear [5,6], among others. In particular, seawater corrosion has a profound impact on maritime transportation, coastal equipment, and offshore structures. Figure 1 illustrates examples of corrosion in marine facilities, Figure 1a and Figure 1b,c present the corrosion status of coastal and offshore equipment, respectively. To address the corrosion issues of instruments and equipment in marine environments, extensive research has been conducted, primarily focusing on organic and inorganic coatings. Among these, organic coatings have been widely studied and applied due to their ease of application and excellent anti-corrosion properties, making them one of the most commonly used methods for preventing metal corrosion [7,8].

Figure 1.
Examples of corrosion in offshore equipment. (a) coastal equipment; (b,c) offshore equipment.
Polyurethane (PU) coatings exhibit excellent performance at both high and low temperatures, and the cured film is non-toxic, making them suitable for the production of environmentally friendly coatings with low volatile organic compound (VOC) emissions [9,10]. Jothi et al. [11] prepared a corrosion-resistant PU coating on the surface of AZ31 Mg alloy. Experimental results demonstrated that, compared to the bare substrate, the PU-coated magnesium alloy exhibited higher charge transfer resistance (Rct) values (8.72 × 107 Ω/cm2) and coating resistance (Rf) values (1.88 × 107 Ω/cm2), as well as a lower corrosion current density (Icorr) (2.01 × 10−10 A/cm2), indicating that the coating significantly enhanced the alloy’s corrosion resistance. Despite the numerous superior properties of PU, it still has limitations in the field of heavy-duty anti-corrosion, such as poor wear resistance and susceptibility to damage. To address these issues, researchers have incorporated organic and inorganic fillers into PU coatings to improve their hardness, wear resistance, and corrosion resistance. Kumar et al. [12] fabricated a superhydrophobic PU coating embedded with SiO2 nanoparticles on an aluminum substrate. Electrochemical tests revealed that, compared to the bare aluminum substrate, the coated aluminum exhibited an increased corrosion potential and a reduced corrosion current density, demonstrating the enhanced corrosion resistance of the coating.
Silicates, owing to their excellent properties such as water resistance, high-temperature resistance, and flame retardancy, have been incorporated into coatings to achieve modification. Although PU exhibits outstanding performance, it suffers from poor water resistance and weather resistance. By adding silicates to PU resin for composite modification, the drawbacks of PU resin can be mitigated while enhancing the mechanical properties of the coating [13,14]. Skosana et al. [15] introduced nanoclay into PU coatings using ultrasonic dispersion and high-shear mixing methods. The incorporation of nanoclay improved the adhesion of the PU coating, with the most significant enhancement (34%) observed at a nanoclay content of 3 wt%. Kalendová et al. [16] investigated the flash rust inhibition properties of soluble alkaline silicates, such as sodium silicate, potassium silicate, and lithium silicate, using water-based dispersion binders for primers. The results demonstrated that sodium silicate is an excellent flash rust inhibitor and can be used in styrene-acrylate water-based coatings. Sahoo et al. [17] synthesized a composite material using polybutyl acrylate and sodium silicate through an emulsifier-free emulsion technique. The hydrophobic polybutyl acrylate was intercalated into the hydrophilic sodium silicate through the reaction, enhancing the material’s hydrophilicity and causing significant expansion of the composite. Wang et al. [18] developed a water-based silicate anti-corrosion coating by synthesizing epoxy-modified silicate emulsions with varying epoxy resin contents. They prepared zinc-rich water-based silicate coatings using the modified silicate emulsions and studied the influence of epoxy resin content on the mechanical properties and corrosion resistance of the coatings. The results indicated that as the epoxy resin content increased, the impact resistance of the coating improved, the pencil hardness decreased, the adhesion remained unaffected, and the corrosion resistance was enhanced.
The modified sodium silicate/PU composite material can be applied in high-temperature and high-humidity marine environments. In this study, an organic ester curing agent was selected to cure sodium silicate. As an organic compound, the organic ester can rapidly hydrolyze under alkaline conditions to produce acetic acid, which reacts with sodium silicate to form silica gel, thereby curing the sodium silicate. Simultaneously, the organic ester, being an organic substance, is mutually soluble and compatible with moisture-cured PU. The diols or polyols generated from the hydrolysis of the organic ester can also react with isocyanate, which is beneficial for the bonding performance and uniformity of the cured film. The KH-560 silane coupling agent was used to modify sodium silicate, improving the interfacial bonding between the organic and inorganic phases. By measuring the contact angle between the organic and inorganic phases, the modification effect of different contents of the silane coupling agent on sodium silicate was compared, and the optimal ratio between the two was determined. Fourier-transform infrared spectroscopy (FTIR) was employed to verify the modification effect of the silane coupling agent on sodium silicate. Additionally, the influence of the silane coupling agent on the coating performance was investigated through water contact angle tests, adhesion tests, and other experiments before and after modification.
2. Materials and Methods
2.1. Experimental Materials
Moisture-cured PU (STWD959, viscosity was 250 cps at 20 °C) was supplied by Shunde New Materials Co., Ltd. (Shanghai, China). Liquid sodium silicate was provided by Yatai United Chemical Co., Ltd. (Wuxi, China) (the modulus and Baume degree were 2.31 and 50°). The silane coupling agent was obtained from Nanjing Nengde New Material Technology Co., Ltd. (Nanjing, China) (γ-Glycidoxypropyltrimethoxysilane, KH-560). The organic ester curing agent was supplied by Zhangjiagang Yuanbang Chemical Materials Co., Ltd. (Suzhou, China) (Polycarbonate, HK-12A). Polyether defoamer was used in anti-corrosion coatings (Dow, Midland, MI, USA). The experimental substrate selected was low-carbon steel (Q235), which is commonly used in marine engineering equipment due to its relatively poor corrosion resistance. The specific specifications of the low-carbon steel used are listed in Table 1.

Table 1.
Composition and content of elements in low-carbon steel.
2.2. Preparation of Materials
2.2.1. Preparation of Modified Sodium Silicate
Silane coupling agents, as a type of surfactant, contain two different functional groups that can form covalent bonds at the organic–inorganic interface, thereby enhancing the mechanical properties of the material. In this experiment, a KH-560 silane coupling agent was used to modify sodium silicate. The specific procedure is as follows:
Hydrolysis of the silane coupling agent: A mixed solution was prepared with a ratio of silane coupling agent–deionized water–anhydrous ethanol = 1:3:6. First, deionized water and anhydrous ethanol were mixed at a ratio of 1:2 under stirring at 800 rpm/min. Then, the silane coupling agent was slowly added dropwise into the stirring mixture of deionized water and anhydrous ethanol. The pH of the solution was adjusted to 4–5, and stirring was continued for 4 h to ensure complete hydrolysis of the coupling agent.
Modification of sodium silicate: At room temperature, the hydrolyzed KH-560 silane coupling agent was mixed with sodium silicate solution at different ratios under stirring for 20 min to obtain the modified sodium silicate solution. The ratios of sodium silicate to silane coupling agent are shown in Table 2.

Table 2.
Ratios of silane coupling agent to sodium silicate solution.
2.2.2. Preparation of Modified Sodium Silicate/PU Coatings
The modified sodium silicate/PU coatings were prepared using a one-step method. The specific steps are as follows: Component A: A uniformly mixed solution of sodium silicate modified with the silane coupling agent; Component B: A mixture of PU resin and the organic ester curing agent. Component A and Component B were mixed under stirring at 500 rpm for 20–30 min. Subsequently, additives such as defoamers were added, and the mixture was stirred until uniformly dispersed. The prepared coating was sprayed onto Q235 low-carbon steel plates and phosphated tinplate test panels using a spray gun. The coated panels were then dried in an oven at 60 °C (humidity ≥ 30 wt%) to form a film and cured for seven days before conducting relevant performance tests.
In this experiment, the modified sodium silicate was added at concentrations of 60 wt%, 70 wt%, 80 wt%, and 90 wt%, along with a blank control group. This is because when the content of modified sodium silicate is below 60 wt%, the adhesion of the coating is poor. Therefore, the minimum content was set at 60 wt% in this study. The specific formulations of the coatings are shown in Table 3. The thickness of the coating was controlled at 200 ± 10 μm.

Table 3.
Formulations of modified sodium silicate/PU coatings.
2.3. Characterization
The water contact angle of the coatings was measured using a contact angle goniometer (JYC-2, Shanghai, China). The molecular structure of the modified material was analyzed using a Fourier-transform infrared spectrometer (FTIR-8400S, Shimadzu, Japan) with a scanning range of 400 cm−1 to 4000 cm−1. The viscosity of the coatings was measured using 4 paint cups (Guangzhou, China). The hardness of the coatings was measured with a pencil hardness tester (Guangzhou, China). The bending resistance of the coatings was tested using a conical mandrel bending tester (Guangzhou, China). The impact resistance of the coatings was evaluated using a film impact tester (Guangzhou, China). The friction and wear resistance of the coatings were tested using a coating abrasion tester (Guangzhou, China). Neutral salt spray tests were performed using a neutral salt spray test chamber (Shanghai, China). The morphology of the coating was analyzed using an optical microscope (×400) (Shanghai, China). The corrosion resistance of the coatings was evaluated using an electrochemical workstation (Herisau, Switzerland). The corrosion current density of the samples was obtained by extrapolating and fitting the potentiodynamic polarization curves using the Stern-Geary equation:
where βa and βb are the Tafel slopes for the anodic and cathodic reactions, respectively.
3. Results and Discussion
3.1. Analysis of Sodium Silicate Coatings Modified with Silane Coupling Agent
3.1.1. FTIR Analysis
Figure 2 shows the FTIR analysis results of the coatings before and after modification. The changes in the characteristic peaks of the infrared spectra of sodium silicate/PU and KH560-treated silicate/PU were analyzed to investigate the molecular-level modifications of silicate with silane coupling agent KH560. The results are shown in Figure 2. Among them, the absorption peak near 3412 cm−1 corresponds to the -OH stretching vibration, the peak near 900 cm−1 is attributed to the epoxy group vibration, and the peak near 1100 cm−1 is caused by the Si-O-Si vibration. As shown in the figure, after modification with the silane coupling agent, the absorption peak near 1100 cm−1, resulting from the stretching vibration of Si-O-Si, is enhanced, indicating that the silane coupling agent has formed chemical bonds with the inorganic silicate. The peak near 3412 cm−1, caused by the stretching vibration of hydroxyl groups (-OH), is significantly stronger than that of the unmodified silicate and polyurethane material. This may be due to the hydrolysis of the silane coupling agent, which forms silanols. Some of these silanols did not react with polar groups to form bonds, leading to an increase in the number of -OH groups on the surface. During the modification process, the silane coupling agent undergoes hydrolysis and condensation reactions with the Si-OH bonds on the surface of the silicate, introducing epoxy groups to the silicate surface. Therefore, the peak observed near 880 cm−1 in the infrared spectrum can be attributed to the characteristic absorption peak of the epoxy group.
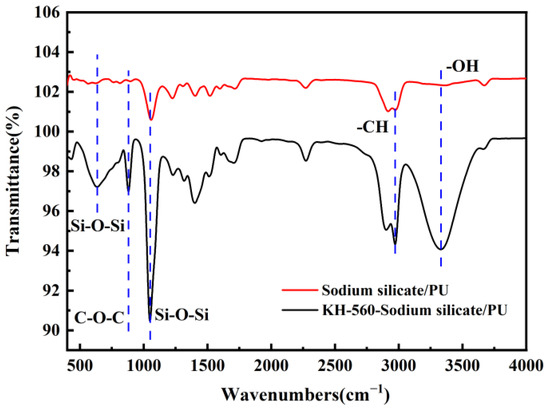
Figure 2.
FTIR spectra of sodium silicate modified with KH-560.
3.1.2. Contact Angle
Figure 3 depicts the contact angle measurement results of sodium silicate solution and PU coatings before and after modification. The success of the modification of sodium silicate by the silane coupling agent is indirectly demonstrated through changes in the contact angle. As illustrated in the figure, the higher the content of the silane coupling agent, the smaller the contact angle between the sodium silicate solution and the organic resin. Figure 4 presents the contact angle test data of sodium silicate with the polyurethane organic phase before and after modification. The results of contact angle measurements initially decrease and then increase with the rising percentage of KH-560. When the content of the modifier exceeds 2.0 wt%, the contact angle between the two begins to stabilize. The silane alkoxy groups of the silane coupling agent can react with the hydroxyl groups on the surface of inorganic materials. Once the number of coupling agents bonded to the surface of sodium silicate reaches saturation, no further reactions occur with the modifier, and the contact angle no longer changes. In summary, when the content of KH-560 reaches 2.0 wt%, the compatibility between the modified sodium silicate and the organic phase is optimal.
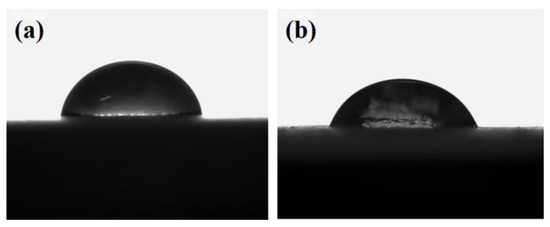
Figure 3.
Morphology of contact angle testing. (a) Contact angle of silicate solution on surface of PU coating before modification. (b) Contact angle of silicate solution on surface of PU coating after modification.
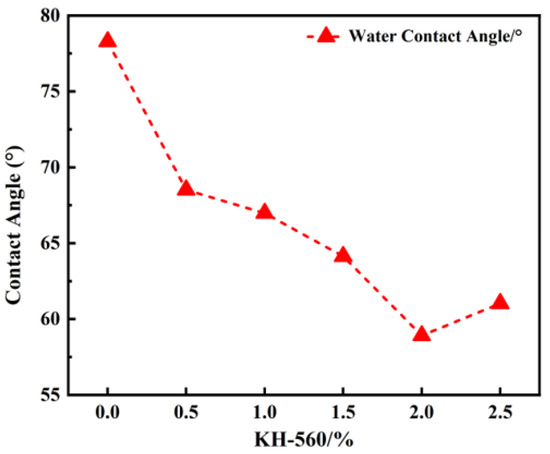
Figure 4.
Contact angle test data.
3.2. Properties of Modified Sodium Silicate/PU Coatings
3.2.1. Microstructure of the Coatings
Figure 5 shows optical micrographs of the modified sodium silicate/PU coatings. As can be seen, the surface of the pure resin coating is relatively smooth, with noticeable reflective phenomena. However, defects such as shrinkage pores are observed on the coating surface, indicating poor density. In contrast, the surface roughness of the coatings with added sodium silicate significantly increases, and the reflective phenomena are reduced. This is primarily due to the uneven distribution of silicon nanoparticles from the sodium silicate on the surface. The uneven coating surface forms a micro-nano structure, enhancing the surface hydrophobicity. As the content of sodium silicate increases, its distribution on the coating surface becomes more uniform, reducing the roughness of the coating and resulting in a smoother surface.
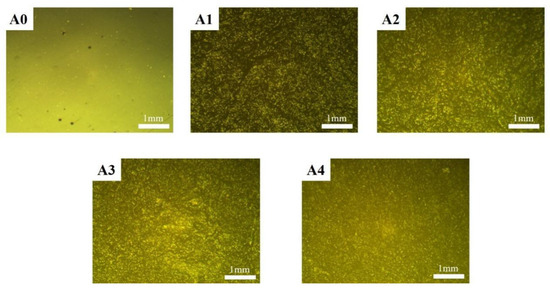
Figure 5.
Optical micrographs of modified sodium silicate/PU coatings.
3.2.2. Conventional Physical Properties
The results of the conventional physicochemical property tests for the coatings are illustrated in Table 4. Compared to the pure resin coating, some properties of the sodium silicate/PU coatings were enhanced. The solid content of the coatings increased from 66 wt% to over 79 wt%, and the content of volatile organic compounds (VOCs) was reduced. As the content of sodium silicate increased, the hardness, impact resistance, bending resistance, and wear resistance of the coatings improved. The increase in the drying time of the coating film is mainly due to the higher proportion of sodium silicate relative to the resin, as sodium silicate also requires time to cure. The addition of sodium silicate enhanced the bending and impact resistance of the coating film, increasing its elasticity. This is primarily because the incorporation of sodium silicate forms a three-dimensional network structure within the coating. The two-phase materials are interpenetrated and entangled throughout the network, creating a unique polymer structure. The interpenetrating polymer network exhibits unique interactions, combining two functionally different materials to achieve complementary properties. Additionally, due to structural characteristics such as interfacial interpenetration and dual-phase connectivity, these materials demonstrate special synergistic effects in performance or functionality, thereby improving the mechanical strength of the material.

Table 4.
Conventional physicochemical and mechanical properties of the coatings.
3.2.3. Water Contact Angle of the Coatings
Figure 6 presents the water contact angle test results of the sodium silicate/PU coatings. As can be seen, the water contact angle of the pure resin coating is 73.52°, while the contact angle increases to 76.54° after adding 60 wt% sodium silicate. As the content of sodium silicate increases, the water contact angle of the coating gradually decreases. The contact angle is 75.15° for the coating with 70 wt% sodium silicate and 74.99° for the coating with 80 wt% sodium silicate. The incorporation of sodium silicate embeds silicon-containing groups into the resin chains, forming a dense and stable three-dimensional network structure, which increases the surface roughness of the coating. During the wetting process of the liquid on the solid surface, the liquid is hindered by the rough structure and cannot overcome the raised features. Upon reaching thermodynamic equilibrium, the liquid no longer wets the surface, resulting in a contact angle hysteresis effect and an increase in the contact angle [19]. However, when the sodium silicate content reaches 90 wt%, the hydrophobicity of the coating decreases, and the water contact angle drops to 73.20°, which is lower than that of the pure resin coating. This is attributed to two factors: first, the reduction in surface roughness leads to a smaller contact angle; second, the increased content of sodium silicate on the coating surface, which is inherently hydrophilic, contributes to the decrease in hydrophobicity.
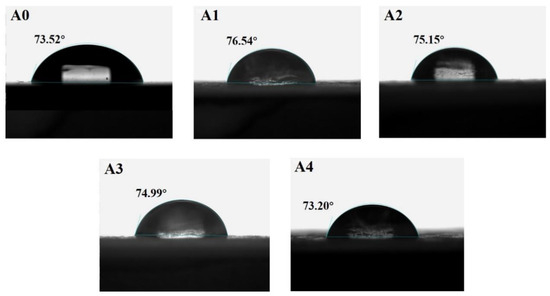
Figure 6.
Water contact angle of modified sodium silicate/PU coatings. A0 (73.52°), A1 (76.54°), A2 (75.15°), A3 (74.99°), A4 (73.20°).
3.2.4. Salt Spray Resistance of the Coatings
Figure 7 displays the macroscopic morphology of the coatings after different durations of neutral salt spray testing. After 192 h of neutral salt spray testing, a large number of blisters appeared on the surfaces of samples A3 and A4, distributed far from the cross-cut areas. The color of the area within 0.5 cm around the cross-cut on sample A1 became lighter. After 336 h of salt spray testing, the number of blisters on the surfaces of samples A3 and A4 increased. The lighter-colored area around the cross-cut on sample A1 continued to expand, and several blisters appeared near the cross-cut. Six blisters appeared on the surface of sample A2, and one blister appeared on the surface of sample A3. After 500 h of neutral salt spray testing, the cross-cut areas on samples A3 and A4 began to show outward corrosion. The edges of the cross-cut on sample A1 started to lift, and the coating began to detach from the substrate. The number of blisters on samples A1 and A2 increased, and corrosion began to spread from the cross-cut areas on sample A1. After 1000 h of neutral salt spray testing, the lighter-colored area around the cross-cut on sample A0 continued to expand, with more blisters appearing nearby. The corrosion at the center of the cross-cut increased. Corrosion also appeared on the surfaces of samples A1 and A2.
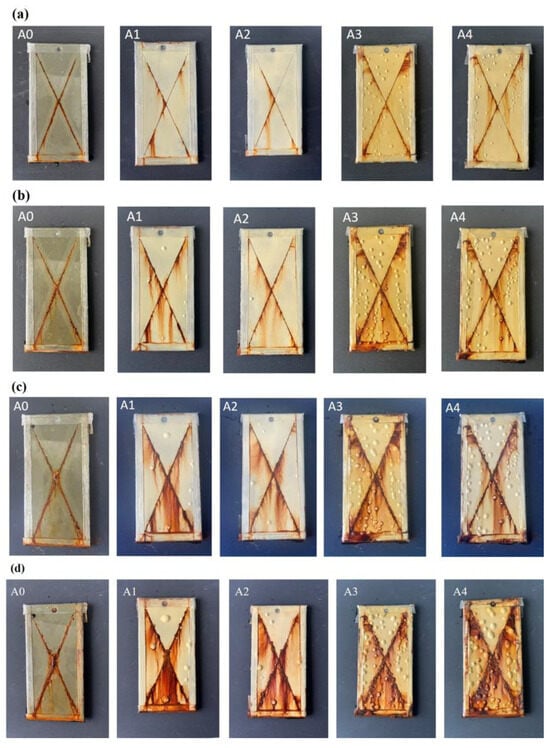
Figure 7.
Macroscopic morphology of coatings after neutral salt spray testing. (a) 192 h, (b) 336 h, (c) 500 h, (d) 1000 h.
3.2.5. Electrochemical Testing
Figure 8 illustrates the potentiodynamic polarization curves of coatings with different sodium silicate contents and bare low-carbon steel after immersion in a 3.5 wt% sodium chloride solution for 24 h. It is generally believed that the corrosion potential is related to the thermodynamic tendency of corrosion, while the corrosion current density, as a kinetic parameter, reflects the actual corrosion rate [20]. Studies have shown that surfaces with higher corrosion potentials and lower corrosion current densities exhibit better corrosion resistance [21]. As seen from the potentiodynamic polarization curves, compared to bare Q235, the samples coated with the coatings exhibit lower corrosion current densities and higher corrosion potentials, indicating that the coatings provide a certain level of protection for the low-carbon steel substrate. Table 5 shows the corrosion potential and corrosion current density of the coatings obtained by fitting the potentiodynamic polarization curves. From Table 5, it can be observed that, compared to bare low-carbon steel, the corrosion current density of the A1 samples decreased from 1.29 × 10−5 A/cm2 to 6.29 × 10−7 A/cm2, a reduction of two orders of magnitude. The corrosion potential shifted positively from −0.93 V to −0.53 V, indicating an improvement in corrosion resistance. The corrosion current densities of the pure resin coatings and the sodium silicate-added coatings are within the same order of magnitude. However, compared to the pure resin coatings, the sodium silicate-added coatings exhibit higher corrosion potentials, suggesting better durability. This is primarily due to the physical and chemical reactions between sodium silicate and the organic resin, which embed silicon-containing groups into the resin chains, forming a dense and stable three-dimensional network structure. This structure acts as a physical barrier, preventing the penetration of corrosive media and achieving corrosion protection. As the proportion of sodium silicate increases, the corrosion potential of the coatings gradually decreases. Among them, the coating with 60 wt% sodium silicate content exhibits the lowest corrosion current and the highest corrosion potential, demonstrating the best corrosion resistance.
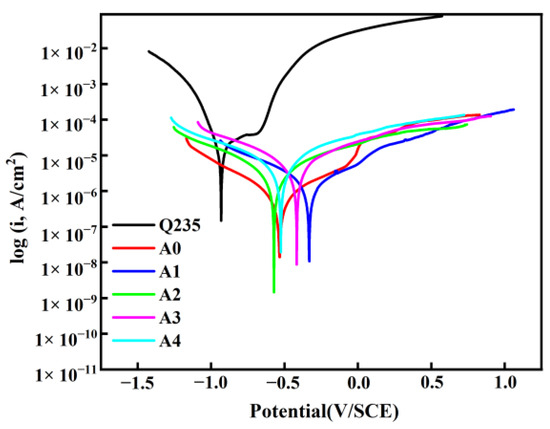
Figure 8.
Potentiodynamic polarization curves of modified sodium silicate/PU coatings.

Table 5.
Corrosion parameters of the coatings.
Figure 9 displays the Nyquist and Bode plots of silicate/isocyanate coatings after 24 h immersion in 3.5 wt% NaCl solution. In the Nyquist plot, a larger semicircle radius indicates the superior corrosion resistance of the coating. As observed in Figure 9a, the Nyquist curve exhibits an incomplete semicircular arc at high frequencies, suggesting either a large capacitive loop diameter or excellent anti-corrosion performance. The coating with 60% silicate additive demonstrates the largest semicircle radius in the Nyquist plot, confirming its optimal corrosion resistance. The Nyquist curves of the coated samples were fitted using an electrochemical equivalent circuit, as shown in Figure 10. Here, Rs represents the resistance generated by the 3.5 wt% NaCl solution during charge transfer, C.P.E denotes the non-ideal double-layer capacitance at the surface of the PU/silicate coating, and Rp is the charge transfer resistance of the electrode reaction at the surface of the PU/silicate coating. The value of the charge transfer resistance is inversely proportional to the corrosion rate of the coating. Table 6 presents the fitting results of the electrochemical equivalent circuit for the EIS of the PU/silicate coatings. From the table, it can be observed that the coating with 90% silicate content has the smallest charge transfer resistance Rp, with a value of 18.0 kΩ/cm2. As the silicate content increases, the Rp value of the coating shows a gradual increasing trend, reaching a maximum of 92.5 kΩ/cm2 at 60 wt% silicate content. Therefore, as the silicate content decreases, the corrosion resistance of the coating gradually improves, with the 60 wt% silicate coating exhibiting the best corrosion resistance. This result is consistent with the findings from the polarization curves.
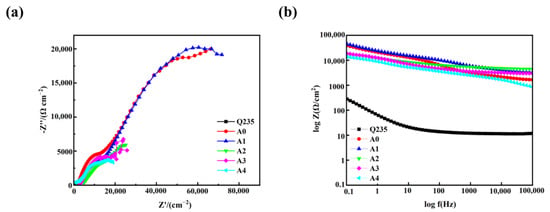
Figure 9.
(a) Nyquist and (b) Bode plots of modified sodium silicate/PU coatings in the 3.5 wt% NaCl solution.
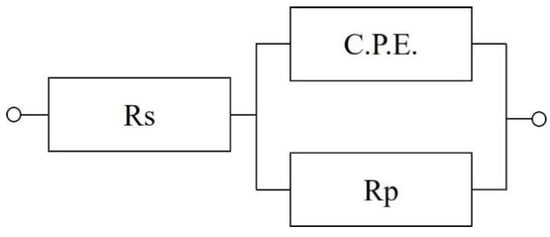
Figure 10.
Electrochemical equivalent circuit.

Table 6.
Fitting results of the equivalent circuit for EIS (electrochemical impedance spectroscopy) of modified sodium silicate/PU coatings.
The Bode plot of the coating is illustrated in Figure 9b. Typically, the corrosion resistance of a sample is evaluated using the |Z| value at a low frequency (0.01 Hz), denoted as |Z|0.01 Hz [22]. Research indicates that surfaces with higher |Z|0.01 Hz values exhibit superior corrosion resistance. As shown in the figure, the specimen coated with the surface layer demonstrates a higher |Z| value in the low-frequency region. Table 7 presents the |Z| values at 0.01 Hz for modified sodium silicate/PU coatings. The specimen with 60 wt% silicate content exhibits the highest impedance value at low frequency, measuring 9.62 × 104 Ω/cm2. The impedance value of the pure resin coating is slightly lower than that of the coating with 60 wt% silicate, at 6.64 × 104 Ω/cm2. The |Z| values for coatings with 70 wt%, 80 wt%, and 90 wt% silicate content fall within an intermediate range, at 2.51 × 104 Ω/cm2, 2.58 × 104 Ω/cm2, and 1.90 × 104 Ω/cm2, respectively. In contrast, the bare Q235 low-carbon steel substrate shows the lowest impedance value at 8.72 × 102 Ω/cm2. The comprehensive results reveal that the coating with 60 wt% silicate content exhibits the optimal corrosion resistance. An appropriate amount of silicate enhances the corrosion resistance of the coating, while excessive silicate content diminishes its protective properties. This phenomenon is primarily attributed to the fact that the presence of silicate reduces the hydrophobicity of the coating, thereby weakening its function as a physical barrier. Consequently, corrosive ions, along with water, can penetrate the coating and corrode the substrate surface.

Table 7.
Impedance (|Z|) values of modified sodium silicate/PU coatings at low frequency (0.01 Hz).
4. Conclusions
This study developed a novel sodium silicate-modified polyurethane heavy-duty anti-corrosion coating system to address the corrosion protection challenges of marine equipment. The coating formulation employed polyurethane resin as the film-forming matrix, with KH-560 silane coupling agent-modified sodium silicate serving as the functional filler, along with HK-12A organic ester curing agent and other auxiliary additives for composition optimization. A systematic investigation was conducted to evaluate the effect of modified silicate content on the coating properties. The experimental results demonstrate that the incorporation of modified sodium silicate significantly increased the coating’s solid content to over 79%. Remarkably, the modified sodium silicate substantially enhanced several key mechanical properties of the coating, including hardness, impact resistance, and bending resistance, while maintaining excellent flexibility. Surface modification with the silane coupling agent increased the water contact angle of sodium silicate, thereby improving the coating’s barrier properties against penetration by liquid corrosive media. Electrochemical impedance spectroscopy tests revealed that the coating containing 60 wt% modified sodium silicate exhibited optimal protective performance, with an impedance value of 9.62 × 104 Ω/cm2 and a low corrosion current density of 5.82 × 10−7 A/cm2. Equivalent circuit modeling further confirmed that the 60 wt% modified sodium silicate coating possessed the best anti-corrosion characteristics, which was consistent with the polarization curve analysis. The comprehensive evaluation indicates that this coating system exhibits promising potential for effective corrosion protection in harsh marine environments.
Author Contributions
Conceptualization, M.L. and Z.T.; methodology, M.L. and Y.Z.; software, M.L.; validation, M.L., Z.T. and S.X.; formal analysis, M.L. and H.W.; investigation, R.M., Z.M. and S.Z.; resources, M.L. and Y.Z.; data curation, M.L. and T.J.; writing—original draft preparation, M.L.; writing—review and editing, Z.T. and Y.Z.; visualization, N.Z. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Zhen Ma was employed by Business development of Edwards in China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Li, N.; Zhang, W.; Yan, X.; Zhang, M.; Han, L.; Cai, Y. Effect of Tropical Marine Atmospheric Environment on Corrosion Behaviour of the 7B04-T74 Aluminium Alloy. Metals 2023, 13, 995. [Google Scholar] [CrossRef]
- Li, C.; Peng, C.; Li, X.; Liu, Y.; Liu, Y.; Wang, Z.; Pai, J.; Wang, C. Initial Corrosion Behavior of EH36 Marine Steel in Simulated Polar Marine Environment. Int. J. Electrochem. Sci. 2022, 17, 22126. [Google Scholar]
- Cao, Z.; Cao, P. Research Progress on Low-Surface-Energy Antifouling Coatings for Ship Hulls: A Review. Biomimetics 2023, 8, 502. [Google Scholar] [CrossRef]
- Leonardi, A.; Zhang, A.; Düzen, N.; Nick, A.; John, A.; Jessica, L.; Anthony, S.; Rachel, A.; Christopher, K. Amphiphilic Nitroxide-Bearing Siloxane-Based Block Copolymer Coatings for Enhanced Marine Fouling Release. ACS Appl. Mater. Interfaces 2021, 13, 28790–28801. [Google Scholar]
- Lu, G.; Zou, Y.; Chen, X.; Shi, R.; Wang, G.; Zhu, L.; Xie, X.; Sun, W.; Yang, J.; Chang, S.; et al. Micro-morphological analysis, lubricating behaviors, and wear failure characteristics and mechanisms of propeller hub bearings in marine environments. Wear 2023, 530, 205047. [Google Scholar]
- Zhou, Y.K.; Kang, J.J.; Yue, W.; Fu, Z.; Zhu, L.; She, D. Wet Sliding Wear of HVOF-Sprayed WC-10Co4Cr Coatings in Simulated Seawater Drilling Fluid. J. Therm. Spray Technol. 2021, 30, 2174–2186. [Google Scholar]
- Rodrigues, R.; Gaboreau, S.; Gance, J.; Ioannis, I.; Stéphanie, B. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2021, 269, 121240. [Google Scholar]
- He, K.; Liu, Y.; He, Y. Organic-Inorganic Interpenetrating Technology-Based Nano Water-Borne Paint for Steel Structures. Nanosci. Nanotechnol. Lett. 2019, 11, 434–440. [Google Scholar] [CrossRef]
- Kiani, V.; Asl, A.; Khosravi, H.; Eivaz, M.; Ahmadi, H. Designing a smart polyurethane anti-corrosion coating loaded with APTES/IMZ modified halloysite nanotubes. Surf. Coat. Technol. 2024, 492, 131179. [Google Scholar]
- Zhang, L.; Chen, Y.; Wu, K.; Sun, G.; Liu, R.; Luo, J. One-pot efficient synthesis of amino-functionalized polyurethane capsules via photopolymerization for self-healing anticorrosion coatings. Prog. Org. Coat. 2024, 189, 108348. [Google Scholar]
- Jothi, V.; Adesina, A.; Rahman, M.; Nirmal, R. Improved Adhesion and Corrosion Resistant Performance of Polyurethane Coatings on Anodized Mg Alloy for Aerospace Applications. J. Mater. Eng. Perform. 2020, 29, 2586–2596. [Google Scholar]
- Kumar, A.; Meena, M. Fabrication of durable corrosion-resistant polyurethane/SiO2 nanoparticle composite coating on aluminium. Colloid Polym. Sci. 2021, 299, 915–924. [Google Scholar]
- Jelić, A.; Sekulić, M.; Travica, M.; Gržetić, J.; Ugrinović, V.; Marinković, A.D.; Božić, A.; Stamenović, M.; Putić, S. Determination of Mechanical Properties of Epoxy Composite Materials Reinforced with Silicate Nanofillers Using Digital Image Correlation (DIC). Polymers 2022, 14, 1255. [Google Scholar] [CrossRef]
- Liu, W.; Song, J.; Zhao, H.; Cui, Z.; Zhang, S.; Qiao, Y.; Cao, C.; Li, W. Mechanically stable and superhydrophobic nano-SiO2@silane/silicate coating for enhanced impermeability of mortar. Constr. Build. Mater. 2025, 470, 140541. [Google Scholar]
- Skosana, S.; Khoathane, C.; Malwela, T. Enhancing the adhesion strength of polyurethane coatings by dispersing layered silicates via sonication and high-shear mixing method. Polym. Bull. 2021, 78, 203–221. [Google Scholar]
- Kalendová, A.; Vesely, D.; Kalenda, R. Nanoparticles of soluble alkaline silicates as flash rusting inhibitors in water-borne paints. Anti-Corros. Methods Mater. 2006, 53, 79–87. [Google Scholar]
- Sahoo, P.K.; Samal, R.; Swain, S.K.; Rana, P.K. Synthesis of poly(butyl acrylate)/sodium silicate nanocomposite fire retardant. Eur. Polym. J. 2008, 44, 3522–3528. [Google Scholar]
- Wang, J.; Qi, Y.; Zhao, X.; Zhang, Z. Electrochemical Investigation of Corrosion Behavior of Epoxy Modified Silicate Zinc-Rich Coatings in 3.5% NaCl Solution. Coatings 2020, 10, 444. [Google Scholar] [CrossRef]
- Mohamed, A.; Abdullah, A.; Younan, N. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar]
- Kong, J.; Hou, T.; Wang, Q.; Yin, L.; Zhou, F.; Zhou, Z.; Li, L. Influence of titanium or aluminum doping on the electrochemical properties of CrN coatings in artificial seawater. Surf. Coat. Technol. 2016, 307, 118–124. [Google Scholar]
- Sattari, M.; Olad, A.; Maryami, F.; Ahadzadeh, I.; Nofouzi, K. Facile fabrication of durable and fluorine-free liquid infused surfaces on aluminum substrates with excellent anti-icing, anticorrosion, and antibiofouling properties. Surf. Interfaces 2023, 38, 102860. [Google Scholar] [CrossRef]
- Sun, H.; Lei, F.; Li, T.; Han, H.; Li, B.; Li, D.; Sun, D. Facile Fabrication of Novel Multifunctional Lubricant-Infused Surfaces with Exceptional Tribological and Anticorrosive Properties. ACS Appl. Mater. Interfaces 2021, 13, 6678–6687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).