Selective Adsorption of Lead in Mixed Metals Wastewater System by Lignin-Carbon-Supported Titanate Nanoflower BC@TNS Adsorbent: Performance and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of BC@Ti(OH)X Composite
2.3. Synthesis of BC@ Titanate Nanosheet Composite (BC@TNS)
2.4. Characterization of BC@TNS
2.5. Kinetic and Isotherm Experiments
2.6. Selectivity Assessment
2.7. Recycling and Regeneration
2.8. Statistical Analysis
3. Results and Discussions
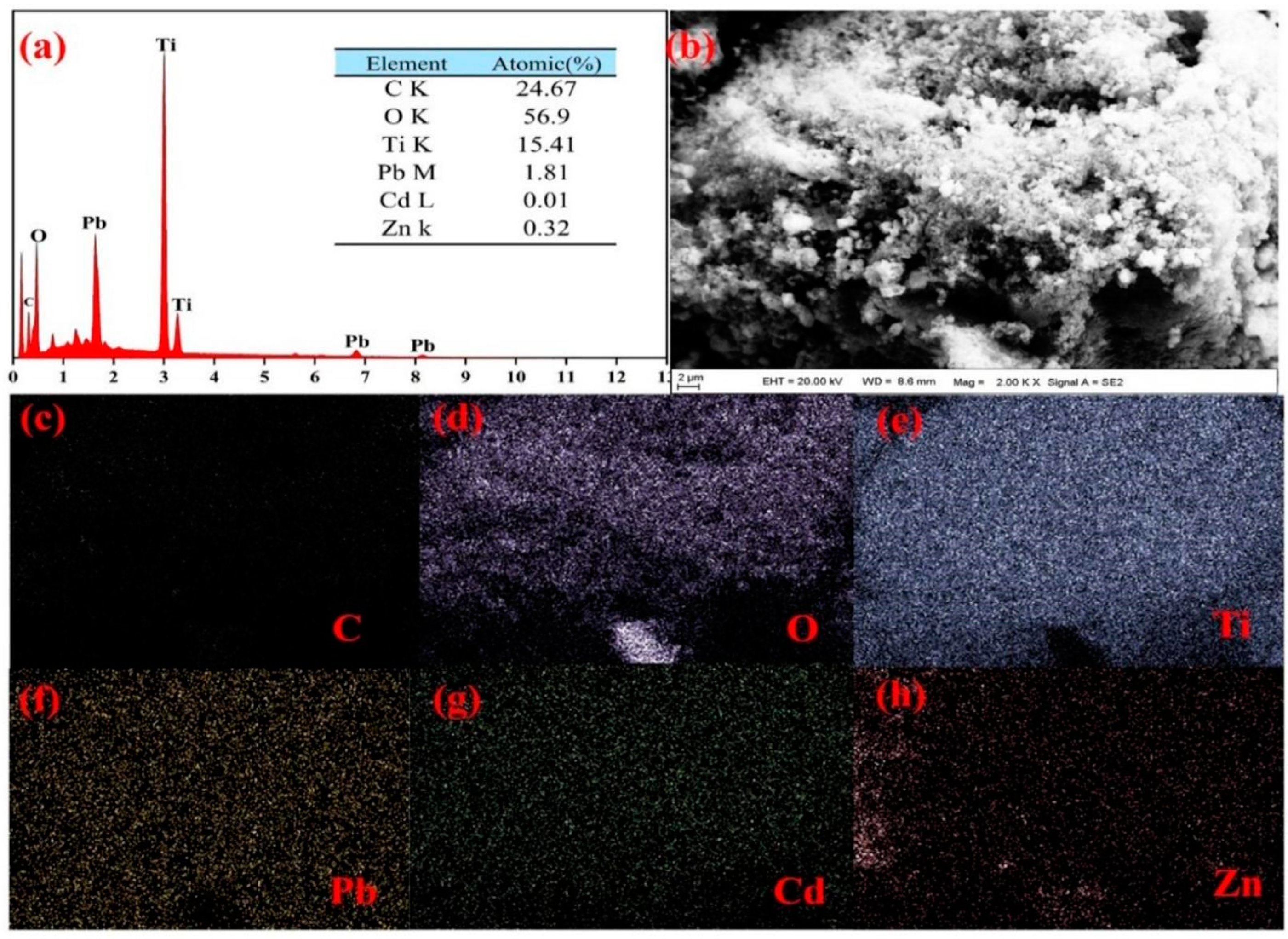
3.1. Morphology and Crystal Phase of BC@TNS
3.2. Adsorption Kinetic of BC@TNS
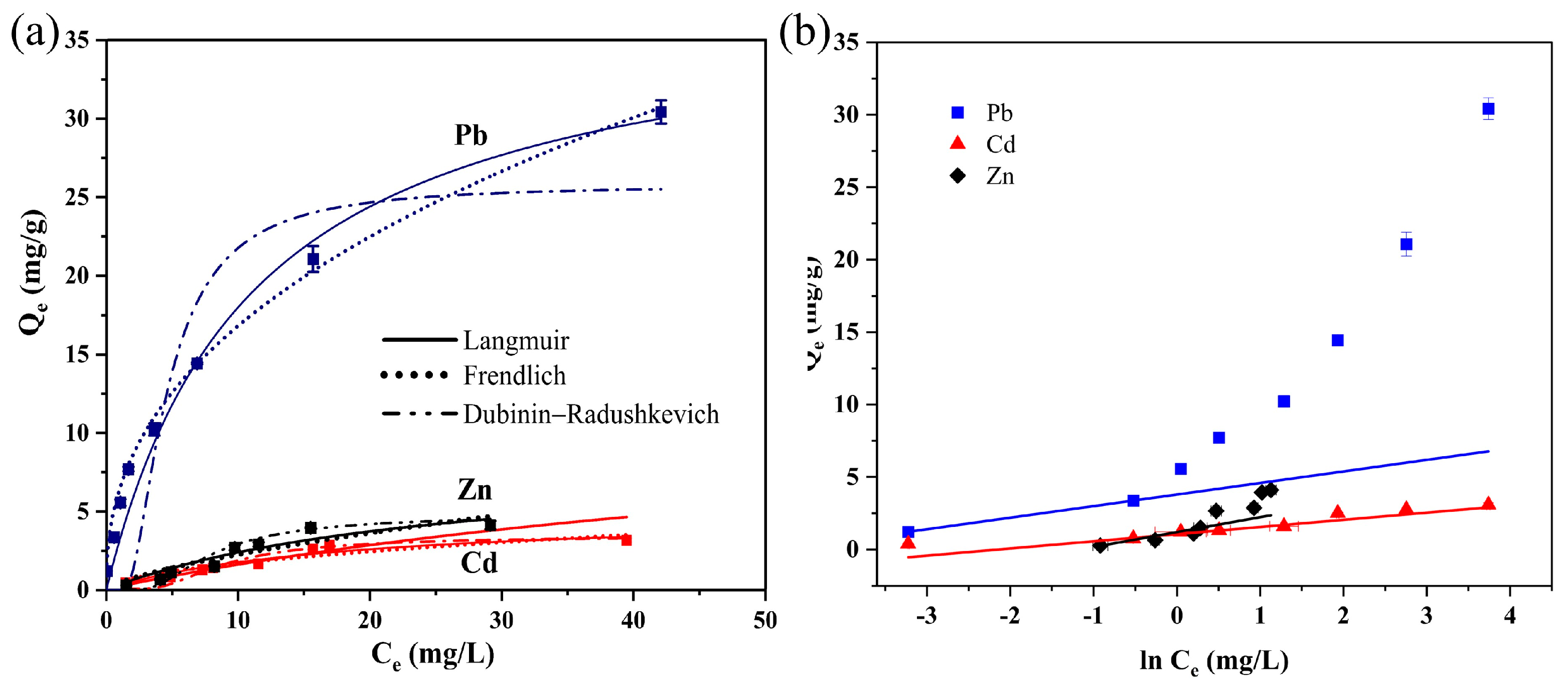
3.3. Adsorption Isotherms of BC@TNS
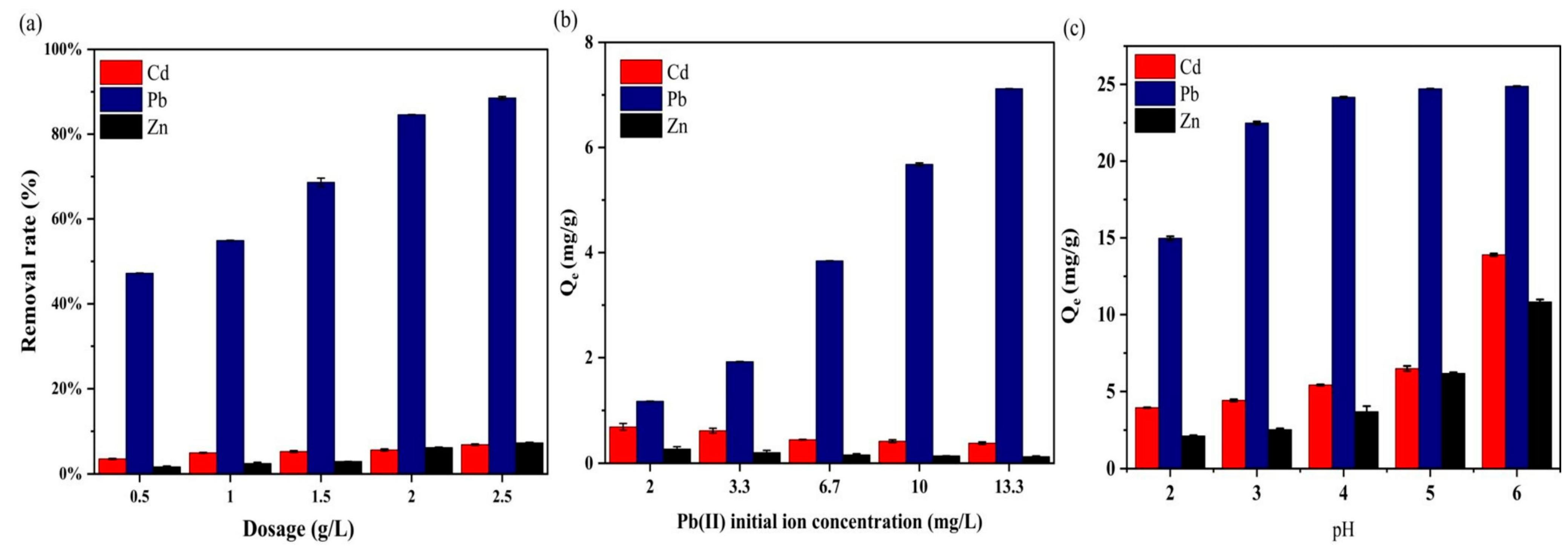
3.4. Adsorption Performance
3.4.1. Effect of BC@TNS Dosage
3.4.2. Effect of Initial Ion Concentration of Pb(II)
3.4.3. Effect of pH Values
3.5. Adsorption Mechanisms
3.6. Selective Adsorption
3.7. Comparison of Selectivity with Other Adsorbents
3.8. Recycling and Regeneration of BC@TNS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dufatanye, I.; Lee, Y.; Kim, H.; Lee, S. Industrial Wastewater Discharge and Compliance Investigation for Environmentally Resilient Rwanda. Water 2022, 14, 3100–3120. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Meng, Y.; Aihemaiti, A.; Xu, Y.; Xiang, H.; Gao, Y.; Chen, X. Preparation, environmental application and prospect of biochar-supported metal nanoparticles: A review. J. Hazard. Mater. 2020, 388, 122026–122048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, Z.; Yan, B.; Zhao, L.; Chen, T.; Yang, X. Effects of active silicon amendment on Pb(II)/Cd(II) adsorption: Performance evaluation and mechanism. J. Hazard. Mater. 2024, 478, 135614–135628. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Jin, C.; Wu, G.; Liu, G.; Kong, Z. Efficient and selective removal of Pb(II) from aqueous solution by a thioether-functionalized lignin-based magnetic adsorbent. RSC Adv. 2022, 12, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shah, H.-u.-R.; Khan, M.S.; Iqbal, A.; Potrich, E.; Amaral, L.S.; Rasheed, S.; Nawaz, H.; Ayub, A.; Naseem, K.; et al. Lead In drinking water: Adsorption method and role of zeolitic imidazolate frameworks for its remediation: A review. J. Clean. Prod. 2022, 368, 133010–133028. [Google Scholar] [CrossRef]
- Chowdhury, I.R.; Chowdhury, S.; Mazumder, M.A.J.; Al-Ahmed, A. Removal of lead ions (Pb2+) from water and wastewater: A review on the low-cost adsorbents. Appl. Water Sci. 2022, 12, 185–208. [Google Scholar] [CrossRef]
- Jolly, Y.N.; Surovi, S.A.; Rahman, S.M.M.; Kabir, J.; Akter, S.; Mamun, K.M.; Rahman, A. A Probabilistic-Deterministic Approach Towards Human Health Risk Assessment and Source Apportionment of Potentially Toxic Elements (PTEs) in Some Contaminated Fish Species. Biol. Trace Elem. Res. 2022, 201, 1996–2010. [Google Scholar] [CrossRef]
- Kaur, H.; Kaleka, A.S.; Rajor, A. Risk assessment of metal contamination in wastewater drains of River Ghaggar in Punjab (India). Sādhanā 2024, 49, 242–255. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, H.-J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers 2022, 14, 2183–2209. [Google Scholar] [CrossRef]
- Liu, X.; Fu, L.; Liu, H.; Zhang, D.; Xiong, C.; Wang, S.; Zhang, L. Design of Zr-MOFs by Introducing Multiple Ligands for Efficient and Selective Capturing of Pb(II) from Aqueous Solutions. ACS Appl. Mater. Interfaces 2023, 15, 5974–5989. [Google Scholar]
- Yang, T.; Liu, Y.; Chen, J.; Liu, J.; Jiang, S.; Zhang, X.; Ji, C. Synthesis of ultrathin hybrid membranes via the co-polymerization of acrylic acid, styrene and molybdenum disulfide and their high adsorption selectivity for lead(II) in the mixture of metal ions. Environ. Pollut. 2024, 350, 124019–124030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Meng, N.; Song, S.; Zhu, Q.; Li, D.; Gong, L.; Ding, Y.; Zhang, R.; Shi, X. Simultaneously-efficient electro-sorption of Pb(II), Cu(II) and Cd(II) by Cu2+ modified superactive carbons. Sep. Purif. Technol. 2024, 338, 126604–126616. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; He, G.; Yilmaz, M.; Yuan, S. KMnO4-activated spinach waste biochar: An efficient adsorbent for adsorption of heavy metal ions in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133174–133187. [Google Scholar] [CrossRef]
- Jia, Y.; Hu, Z.; Mu, J.; Zhang, W.; Xie, Z.; Wang, G. Preparation of biochar as a coating material for biochar-coated urea. Sci. Total Environ. 2020, 731, 139063. [Google Scholar] [CrossRef]
- Lin, M.; Li, F.; Li, X.; Rong, X.; Oh, K. Biochar-clay, biochar-microorganism and biochar-enzyme composites for environmental remediation: A review. Environ. Chem. Lett. 2023, 178, 466–478. [Google Scholar] [CrossRef]
- Yang, S.-D.; Qin, J.-L.; Li, M.-F. Lignin-carbohydrate complex-based polyurethane elastomer with outstanding mechanical strength, photothermal efficiency, and enhanced recyclability. Chem. Eng. J. 2024, 499, 156187. [Google Scholar] [CrossRef]
- Xu, E.; Yu, H.; Wu, W.; Ji, B.; Feng, X.; Xu, H.; Zhong, Y.; Wang, B.; Mao, Z. Preparation of high antioxidant nanolignin and its application in cosmetics. Int. J. Biol. Macromol. 2024, 272, 132635–132647. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Huang, W.; Luo, Y.; Wang, J.; She, D. Silica–magnesium coupling in lignin–based biochar: A promising remediation for composite heavy metal pollution in environment. J. Environ. Manag. 2024, 363, 121392. [Google Scholar] [CrossRef]
- Jin, C.; Liu, G.; Wu, G.; Huo, S.; Liu, Z.; Kong, Z. Facile fabrication of crown ether functionalized lignin-based biosorbent for the selective removal of Pb(II). Ind. Crops Prod. 2020, 155, 112829–112831. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Miao, Y.; Mai, Y.; Li, H.; Chen, X.; Chen, J. A lignin-biochar with high oxygen-containing groups for adsorbing lead ion prepared by simultaneous oxidization and carbonization. Bioresour. Technol. 2020, 307, 123165. [Google Scholar] [CrossRef]
- Wang, B.; Ran, M.; Fang, G.; Wu, T.; Ni, Y. Biochars from Lignin-rich Residue of Furfural Manufacturing Process for Heavy Metal Ions Remediation. Materials 2020, 13, 1037. [Google Scholar] [CrossRef]
- Kopp Alves, A.; Hauschild, T.; Basegio, T.M.; Amorim Berutti, F. Influence of lignin and cellulose from termite-processed biomass on biochar production and evaluation of chromium VI adsorption. Sci. Rep. 2024, 14, 14937. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Gopinath, K.P.; Kumar, P.S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef]
- Castro-Díaz, M.; Uguna, C.N.; Florentino, L.; Díaz-Faes, E.; Stevens, L.A.; Barriocanal, C.; Snape, C.E. Evaluation of hydrochars from lignin hydrous pyrolysis to produce biocokes after carbonization. J. Anal. Appl. Pyrolysis 2017, 124, 742–751. [Google Scholar] [CrossRef]
- Agata, M.-M.; Katarzyna, J.; Tomasz, B.; Wojciech, K.; Tong, H.; Karol, S.; Wojciech, N.; Łukasz, M. Waste-Derived carbon porous materials for enhanced performance in adsorption chillers: A Step toward a circular economy. Appl. Therm. Eng. 2024, 260, 124968. [Google Scholar]
- Xiong, J.; Liang, L.; Shi, W.; Li, Z.; Zhang, Z.; Li, X.; Liu, Y. Application of biochar in modification of fillers in bioretention cells: A review. Ecol. Eng. 2022, 21, 170–186. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Wang, D.; Wang, W.; Jia, S.; Mitsuzakic, N.; Chen, Z. Post-modified biomass derived carbon materials for energy storage supercapacitors: A review. Sustain. Energy Fuels 2023, 7, 3541–3559. [Google Scholar] [CrossRef]
- Cha, G.; Weiß, S.; Thanner, J.; Rosenfeldt, S.; Dudko, V.; Uhlig, F.; Stevenson, M.; Pietsch, I.; Siegel, R.; Friedrich, D.; et al. Gentle, Spontaneous Delamination of Layered Titanate Yielding New Types of Lithium Titanate Nanosheets. Chem. Mater. 2023, 35, 7028–7217. [Google Scholar] [CrossRef]
- Liu, T.; Miao, L.; Yao, F.; Zhang, W.; Zhao, W.; Yang, D.; Feng, Q.; Hu, D. Structure, Properties, Preparation, and Application of Layered Titanates. Inorg. Chem. 2023, 63, 1–26. [Google Scholar] [CrossRef]
- Saker, R.; Shammout, H.; Regdon, G.; Sovány, T. An Overview of Hydrothermally Synthesized Titanate Nanotubes: The Factors Affecting Preparation and Their Promising Pharmaceutical Applications. Pharmaceutics 2024, 16, 635. [Google Scholar] [CrossRef]
- Yang, X.; Guo, N.; Yu, Y.; Li, H.; Xia, H.; Yu, H. Synthesis of magnetic graphene oxide-titanate composites for efficient removal of Pb(II) from wastewater: Performance and mechanism. J. Environ. Manag. 2020, 256, 109943–109951. [Google Scholar] [CrossRef]
- di Bitonto, L.; Volpe, A.; Pagano, M.; Bagnuolo, G.; Mascolo, G.; La Parola, V.; Di Leo, P.; Pastore, C. Amorphous boron-doped sodium titanates hydrates: Efficient and reusable adsorbents for the removal of Pb2+ from water. J. Hazard. Mater. 2017, 324, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, F.; Qian, T.; Liu, H.; Liu, W.; Zhao, D. Natural organic matter resistant powder activated charcoal supported titanate nanotubes for adsorption of Pb(II). Chem. Eng. J. 2017, 315, 191–200. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Zhou, Z.; Shen, L.; Bao, N. Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis. Materials 2019, 12, 157–169. [Google Scholar] [CrossRef]
- Sheng, G.; Hu, J.; Alsaedi, A.; Shammakh, W.; Monaquel, S.; Ye, F.; Li, H.; Huang, Y.; Alshomrani, A.S.; Hayat, T.; et al. Interaction of uranium(VI) with titanate nanotubes by macroscopic and spectroscopic investigation. J. Mol. Liquids 2015, 212, 563–568. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Liu, Z.; Deng, Z.; Tang, F.; Wang, W. Efficient removal of heavy metal ions from water system by titanate nanoflowers. Chem. Eng. J. 2012, 180, 75–80. [Google Scholar] [CrossRef]
- Huang, T.; Pan, B.; Ji, H.; Liu, W. Removal of 17β-Estradiol by Activated Charcoal Supported Titanate Nanotubes (TNTs@AC) through Initial Adsorption and Subsequent Photo-Degradation: Intermediates, DFT calculation, and Mechanisms. Water 2020, 12, 2121–2133. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Chen, G. Adsorption of heavy metal sewage on nano-materials such as titanate/TiO2 added lignin. Results Phys. 2019, 12, 405–411. [Google Scholar] [CrossRef]
- Perez, D.d.S.; Terrones, M.G.H.; Grelier, S.; Nourmamode, A.; Castellan, A.; Ruggiero, R.; Machado, A.E.H. Peroxyformic Acid Pulping of Eucalyptus Grandis Wood Chips and Sugar Cane Bagasse in one Stage and Characterization of the Isolated Lignins. J. Wood Chem. Technol. 1998, 18, 333–365. [Google Scholar] [CrossRef]
- Prakash, D.G.; Gopinath, K.P.; Vinatha, V.; Shreya, S.; Sivaramakrishnan, R.; Lan Chi, N.T. Enhanced production of hydrocarbons from lignin isolated from sugarcane bagasse using formic acid induced supercritical ethanol liquefaction followed by hydrodeoxygenation. Chemosphere 2021, 285, 131491. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Chen, J.; He, X.; Wang, Y.; Song, K.; Xie, Z. Regeneration of commercial selective catalyst reduction catalysts deactivated by Pb and other inorganic elements. J. Environ. Sci. 2016, 47, 100–108. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effects of the Ionic Liquid Structure on Porosity of Lignin-Derived Carbon Materials. ACS Sustain. Chem. Eng. 2023, 11, 15228–15241. [Google Scholar] [CrossRef]
- Bi, J.; Huang, X.; Wang, J.; Tao, Q.; Lu, H.; Luo, L.; Li, G.; Hao, H. Self-assembly of immobilized titanate films with different layers for heavy metal ions removal from wastewater: Synthesis, modeling and mechanism. Chem. Eng. J. 2020, 380, 122564–122576. [Google Scholar] [CrossRef]
- Dang, C.; Sun, F.; Jiang, H.; Huang, T.; Liu, W.; Chen, X.; Ji, H. Pre-accumulation and in-situ destruction of diclofenac by a photo-regenerable activated carbon fiber supported titanate nanotubes composite material: Intermediates, DFT calculation, and ecotoxicity. J. Hazard. Mater. 2020, 400, 123225–123237. [Google Scholar] [CrossRef] [PubMed]
- Kolaei, M.; Tayebi, M.; Masoumi, Z.; Tayyebi, A.; Lee, B.-K. Optimal growth of sodium titanate nanoflower on TiO2 thin film for the fabrication of a novel Ti/TiO2/Na2Ti3O7 photoanode with excellent stability. J. Alloys Compd. 2022, 913, 165337–165350. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Wang, H.; Qin, P.; Yang, D.; Sun, Y.; Kong, F. Application of sodium titanate nanofibers as constructed wetland fillers for efficient removal of heavy metal ions from wastewater. Environ. Pollut. 2019, 248, 938–946. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Teng, H. Structural Features of Nanotubes Synthesized from NaOH Treatment on TiO2 with Different Post-Treatments. Chem. Mater. 2005, 18, 367–373. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Wang, L.; Jiang, H.; Cheng, F.; Hu, X. A simple and effective resin pre-coating treatment on grinded, acid pickled and anodised substrates for stronger adhesive bonding between Ti-6Al-4V titanium alloy and CFRP. Surf. Coat. Technol. 2022, 432, 128072. [Google Scholar] [CrossRef]
- Santander, P.; Butter, B.; Oyarce, E.; Yáñez, M.; Xiao, L.-P.; Sánchez, J. Lignin-based adsorbent materials for metal ion removal from wastewater: A review. Ind. Crops Prod. 2021, 167, 113510. [Google Scholar] [CrossRef]
- Sargın, İ.; Arslan, G.; Kaya, M. Microfungal spores (Ustilago maydis and U. digitariae) immobilised chitosan microcapsules for heavy metal removal. Carbohydr. Polym. 2016, 138, 201–209. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J. Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Waleska, P.; Hess, C. Structural Dynamics of Dispersed Titania During Dehydration and Oxidative Dehydrogenation Studied by In Situ UV Raman Spectroscopy. Catal. Lett. 2018, 148, 2537–2547. [Google Scholar] [CrossRef]
- Backus, E.H.G.; Hosseinpour, S.; Ramanan, C.; Sun, S.; Schlegel, S.J.; Zelenka, M.; Jia, X.; Gebhard, M.; Devi, A.; Wang, H.I.; et al. Ultrafast Surface-Specific Spectroscopy of Water at a Photoexcited TiO2 Model Water-Splitting Photocatalyst. Angew. Chem. Int. Ed. 2024, 63, 5–10. [Google Scholar] [CrossRef]
- Ezzati, R.; Azizi, M.; Ezzati, S. A theoretical approach for evaluating the contributions of pseudo-first-order and pseudo-second-order kinetics models in the Langmuir rate equation. Vacuum 2024, 222, 113018–113024. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Millar, G.J.; Miller, G.L.; Couperthwaite, S.J.; Papworth, S. Factors influencing kinetic and equilibrium behaviour of sodium ion exchange with strong acid cation resin. Sep. Purif. Technol. 2016, 163, 79–91. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Du, P.; Xu, L.; Ke, Z.; Liu, J.; Wang, T.; Chen, S.; Mei, M.; Li, J.; Zhu, S. A highly efficient biomass-based adsorbent fabricated by graft copolymerization: Kinetics, isotherms, mechanism and coadsorption investigations for cationic dye and heavy metal. J. Colloid Interface Sci. 2022, 616, 12–22. [Google Scholar] [CrossRef]
- Singh, S.; Kapoor, D.; Khasnabis, S.; Singh, J.; Ramamurthy, P.C. Mechanism and kinetics of adsorption and removal of heavy metals from wastewater using nanomaterials. Environ. Chem. Lett. 2021, 19, 2351–2381. [Google Scholar] [CrossRef]
- Weintroub, S. Electrons in Action. Nature 1963, 198, 1026. [Google Scholar] [CrossRef]
- Ahmad, S.; Khalid, N.; Daud, M. Adsorption studies of lead on lateritic minerals from aqueous media. Sep. Sci. Technol. 2002, 37, 343–362. [Google Scholar] [CrossRef]
- Tyagi, U. Enhanced adsorption of metal ions onto Vetiveria zizanioides biochar via batch and fixed bed studies. Bioresour. Technol. 2022, 345, 126475. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Ali, I.; Wan, P.; Peng, C.; Tan, X.; Sun, H.; Li, J. Integration of metal organic framework nanoparticles into sodium alginate biopolymer-based three-dimensional membrane capsules for the efficient removal of toxic metal cations from water and real sewage. Int. J. Biol. Macromol. 2024, 266, 131312. [Google Scholar] [CrossRef] [PubMed]
- Lisý, A.; Ház, A.; Nadányi, R.; Jablonský, M.; Šurina, I. About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production. Energies 2022, 15, 6213–6240. [Google Scholar] [CrossRef]
- Dai, W.; Xu, M.; Zhao, Z.; Zheng, J.; Huang, F.; Wang, H.; Liu, C.; Xiao, R. Characteristics and quantification of mechanisms of Cd2+ adsorption by biochars derived from three different plant-based biomass. Arabian J. Chem. 2021, 14, 103119. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Z.; Li, H.; Wu, M.; Zhao, Q.; Pan, B. An electron-scale comparative study on the adsorption of six divalent heavy metal cations on MnFe2O4@CAC hybrid: Experimental and DFT investigations. Chem. Eng. J. 2020, 381, 122656. [Google Scholar] [CrossRef]
- Jawad, A.; Liao, Z.; Zhou, Z.; Khan, A.; Wang, T.; Ifthikar, J.; Shahzad, A.; Chen, Z.; Chen, Z. Fe-MoS4: An Effective and Stable LDH-Based Adsorbent for Selective Removal of Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 28451–28463. [Google Scholar]
- Wojciechowska, A.; Lendzion-Bieluń, Z. Synthesis and Characterization of Magnetic Nanomaterials with Adsorptive Properties of Arsenic Ions. Molecules 2020, 25, 4117. [Google Scholar] [CrossRef]



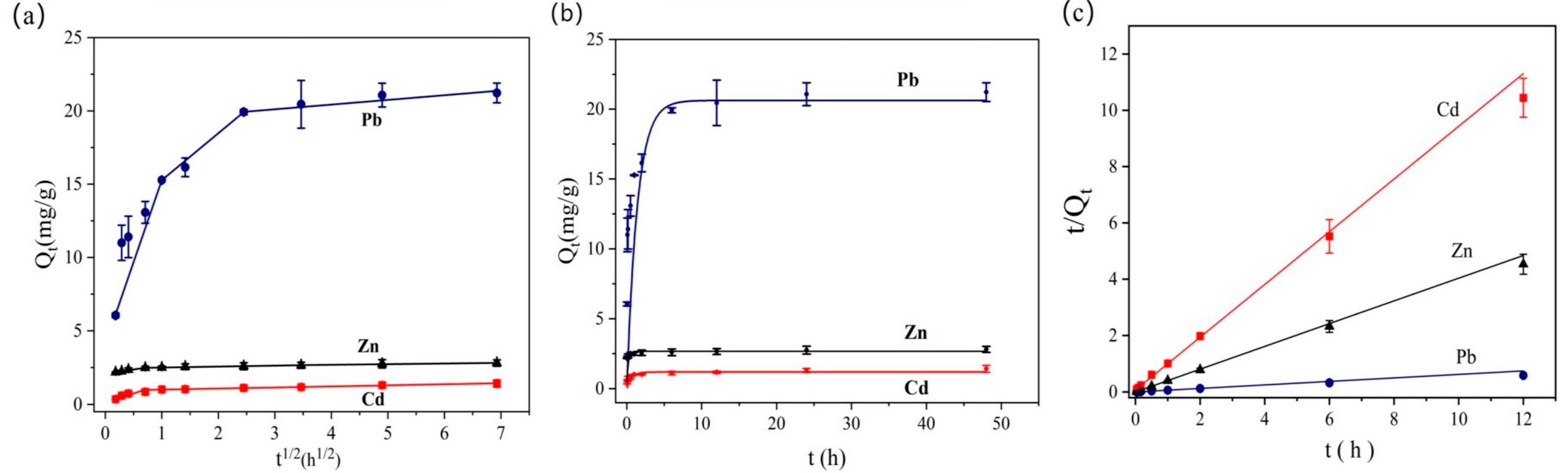

| Kinetic Models | Parameters | Pb(II) | Cd(II) | Zn(II) |
|---|---|---|---|---|
| PFO Model | k1 (h)−1 | 0.708 | 1.563 | 5.476 |
| Qe,Cal (mg/g) | 20.661 | 1.220 | 2.664 | |
| R2 | 0.9989 | 0.9991 | 0.9993 | |
| PSO Model | k2 | 1.101 | 13.432 | 83.820 |
| Qe,Cal (mg/g) | 21.600 | 1.205 | 2.510 | |
| R2 | 0.9998 | 0.9986 | 0.9981 | |
| Qe,exp (mg/g) | 21.225 | 1.412 | 2.825 | |
| Weber Model | K1 | 11.192 | 1.226 | 0.606 |
| C1 | 4.085 | 0.129 | 2.066 | |
| R2 | 0.9961 | 0.8678 | 0.9908 | |
| K2 | 3.202 | 0.017 | 0.0653 | |
| C2 | 12.072 | 0.933 | 2.437 | |
| R2 | 0.9992 | 0.8641 | 0.9543 | |
| K3 | 0.319 | 0.075 | 0.043 | |
| C3 | 19.152 | 0.918 | 2.516 | |
| R2 | 0.9512 | 0.9833 | 0.9571 |
| Isotherm Models | Parameters | Pb(II) | Zn(II) | Cd(II) |
|---|---|---|---|---|
| Langmuir | Qm (mg/g) | 37.890 | 8.470 | 13.381 |
| kL (L/mg) | 0.090 | 0.039 | 0.013 | |
| RL | 0.847 | 0.928 | 0.976 | |
| R2 | 0.998 | 0.997 | 0.9472 | |
| Freundlich | kF (L/mg) | 6.407 | 0.461 | 0.470 |
| 1/n | 0.419 | 0.692 | 0.541 | |
| R2 | 0.9995 | 0.8512 | 0.8696 | |
| Dubinin–Radushkevich | K (mol2/kJ2) | 0.008 | 0.022 | 0.027 |
| E (kJ/mol) | 8.154 | 4.778 | 4.313 | |
| R2 | 0.747 | 0.999 | 0.982 | |
| Temkin | bT (J/mol) | 3107.068 | 2407.555 | 5012.779 |
| KT (L/g) | 116.830 | 3.235 | 8.625 | |
| R2 | 0.9996 | 0.8134 | 0.8384 |
| Material | Element Atomic Percent (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Na | Ti | Pb | Cd | Zn | |
| BC@TNS | 26.88 | 51.76 | 2.95 | 18.40 | 0 | 0 | 0 |
| BC@TNS+ Pb(II), Cd(II), Zn(II) | 34.08 | 45.98 | 2.99 | 15.36 | 0.86 | 0.44 | 0.29 |
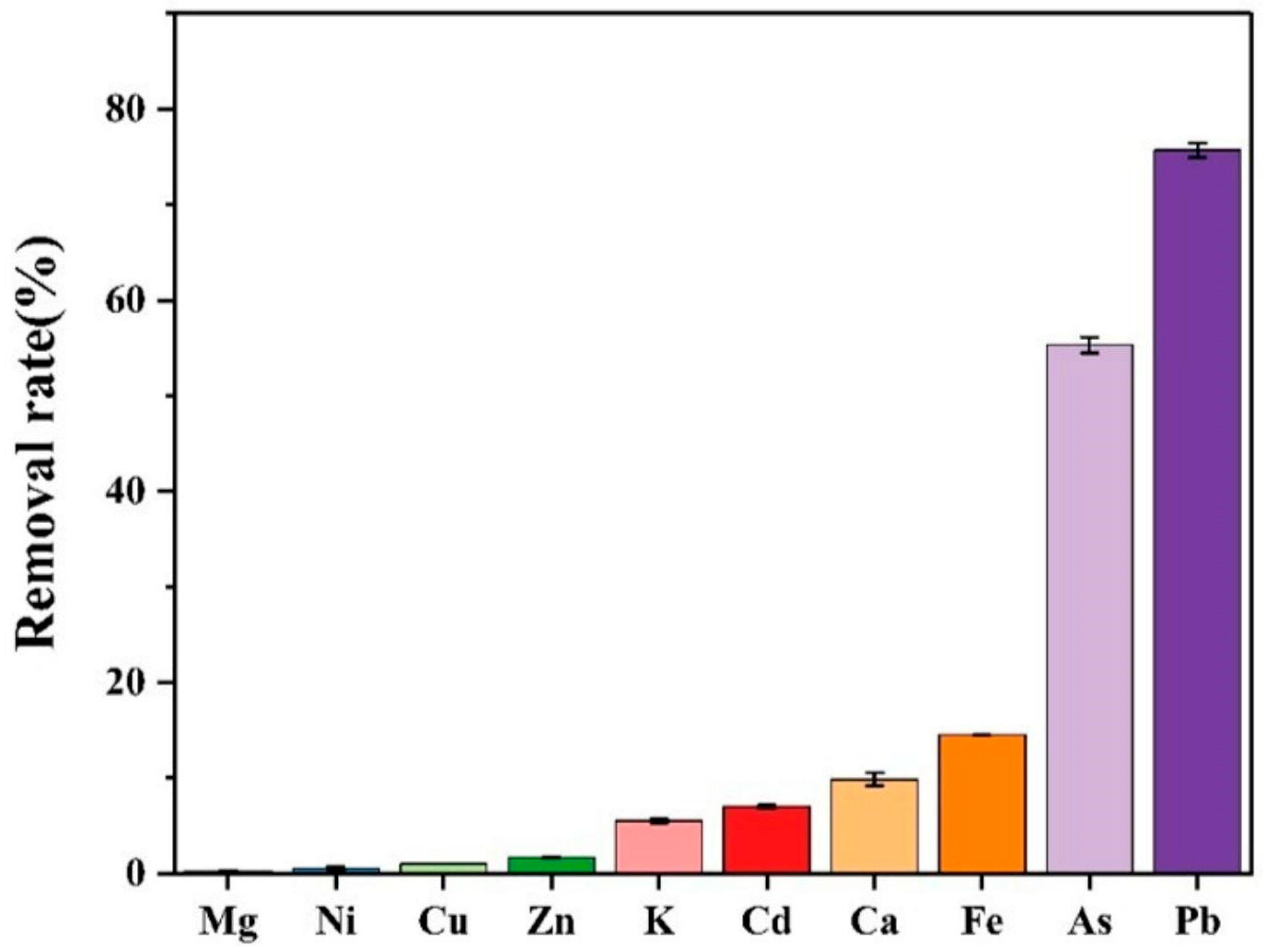
| Mixed Ions | Mn+ Removal (%) | Standard Deviations of Mn+ Removal | kd (mL/g) | k |
|---|---|---|---|---|
| Mg(II) | 0.24 | 0.01549 | 1.51 | 1292.02 |
| Ni(II) | 0.56 | 0.18092 | 3.51 | 555.11 |
| Cu(II) | 1.01 | 0.01457 | 6.38 | 305.29 |
| Zn(II) | 1.64 | 0.07099 | 7.89 | 246.57 |
| K(II) | 5.49 | 0.22627 | 10.44 | 186.40 |
| Cd(II) | 6.97 | 0.17678 | 15.54 | 125.24 |
| Ca(II) | 9.86 | 0.68354 | 68.39 | 28.46 |
| Fe(III) | 14.51 | 0.00731 | 106.10 | 18.35 |
| As(III)/As(V) | 55.31 | 0.84197 | 773.67 | 2.52 |
| Pb(II) | 75.68 | 0.75522 | 1946.60 | 1.00 |
| Adsorbent | Qm (mg/g) | Ref | ||
|---|---|---|---|---|
| Pb(II) | Cd(II) | Zn(II) | ||
| CTS-U. maydis | 49.57 | 27.86 | [49] | |
| CTS/PAM gel | 138.41 | 86.00 | [50] | |
| Cotton derived carbonaceous | 111.10 | 40.20 | [52] | |
| Cu-SAC | 59.92 | 25.12 | [54] | |
| BC -CTS-PMDA | 9.24 | 30.12 | [23] | |
| L@MNP | 49.90 | 12.50 | 7.80 | [4] |
| Fe3O4&g-C3N4 | 137.00 | 102.00 | 45.00 | [67] |
| CB NSs | 14.65 | 8.87 | 10.90 | [68] |
| BC@TNS | 37.89 | 13.38 | 8.47 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Zhong, L.; Yang, Z.; Tang, C.-Y.; Law, W.-C.; Wu, R.; Xie, F. Selective Adsorption of Lead in Mixed Metals Wastewater System by Lignin-Carbon-Supported Titanate Nanoflower BC@TNS Adsorbent: Performance and Mechanism. Coatings 2025, 15, 317. https://doi.org/10.3390/coatings15030317
Feng J, Zhong L, Yang Z, Tang C-Y, Law W-C, Wu R, Xie F. Selective Adsorption of Lead in Mixed Metals Wastewater System by Lignin-Carbon-Supported Titanate Nanoflower BC@TNS Adsorbent: Performance and Mechanism. Coatings. 2025; 15(3):317. https://doi.org/10.3390/coatings15030317
Chicago/Turabian StyleFeng, Jielan, Lei Zhong, Zekun Yang, Chak-Yin Tang, Wing-Cheung Law, Ruchun Wu, and Fengwei Xie. 2025. "Selective Adsorption of Lead in Mixed Metals Wastewater System by Lignin-Carbon-Supported Titanate Nanoflower BC@TNS Adsorbent: Performance and Mechanism" Coatings 15, no. 3: 317. https://doi.org/10.3390/coatings15030317
APA StyleFeng, J., Zhong, L., Yang, Z., Tang, C.-Y., Law, W.-C., Wu, R., & Xie, F. (2025). Selective Adsorption of Lead in Mixed Metals Wastewater System by Lignin-Carbon-Supported Titanate Nanoflower BC@TNS Adsorbent: Performance and Mechanism. Coatings, 15(3), 317. https://doi.org/10.3390/coatings15030317










