A Review of Anticoagulant Surface Modification Strategies for Blood-Contacting Materials: From Inertness to Bioinspired and Biointegration
Abstract
1. Introduction
2. Thrombogenesis Mechanisms on the Surface of Implantable Devices
3. Anticoagulant Surface Modification Strategies, Characteristics, and Limitations
3.1. Carbon-Based Bio-Inert Coatings
3.1.1. Pyrolytic Carbon Coatings
3.1.2. Diamond-like Carbon (DLC) Films
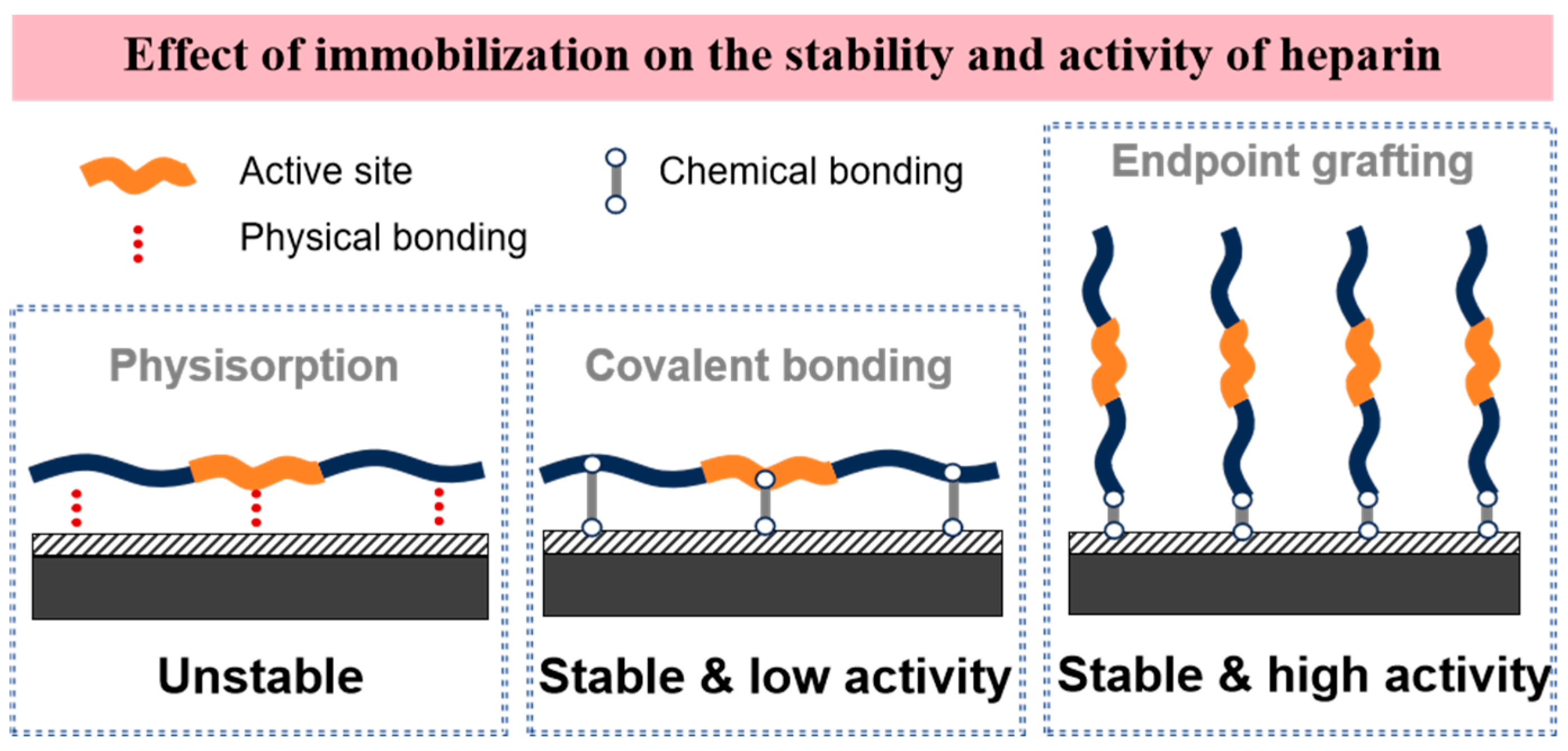
3.2. Heparin-Based Drug Coatings
3.3. Hydrophilic Coatings
3.3.1. Phosphorylcholine (MPC) Polymers
3.3.2. PEG Coatings
3.3.3. Hydrogel Coatings
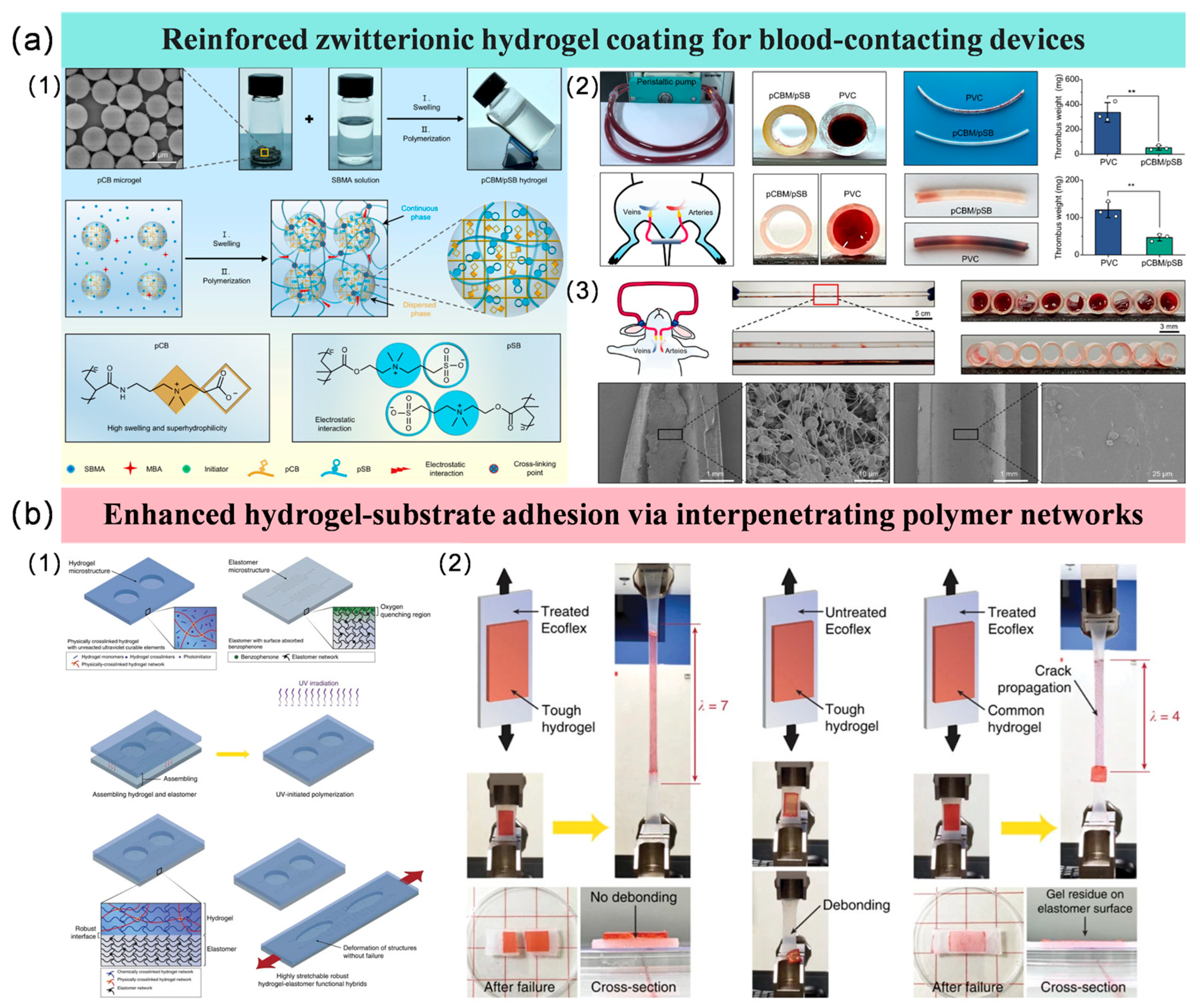
3.4. Hydrophobic Liquid-Infused Surfaces

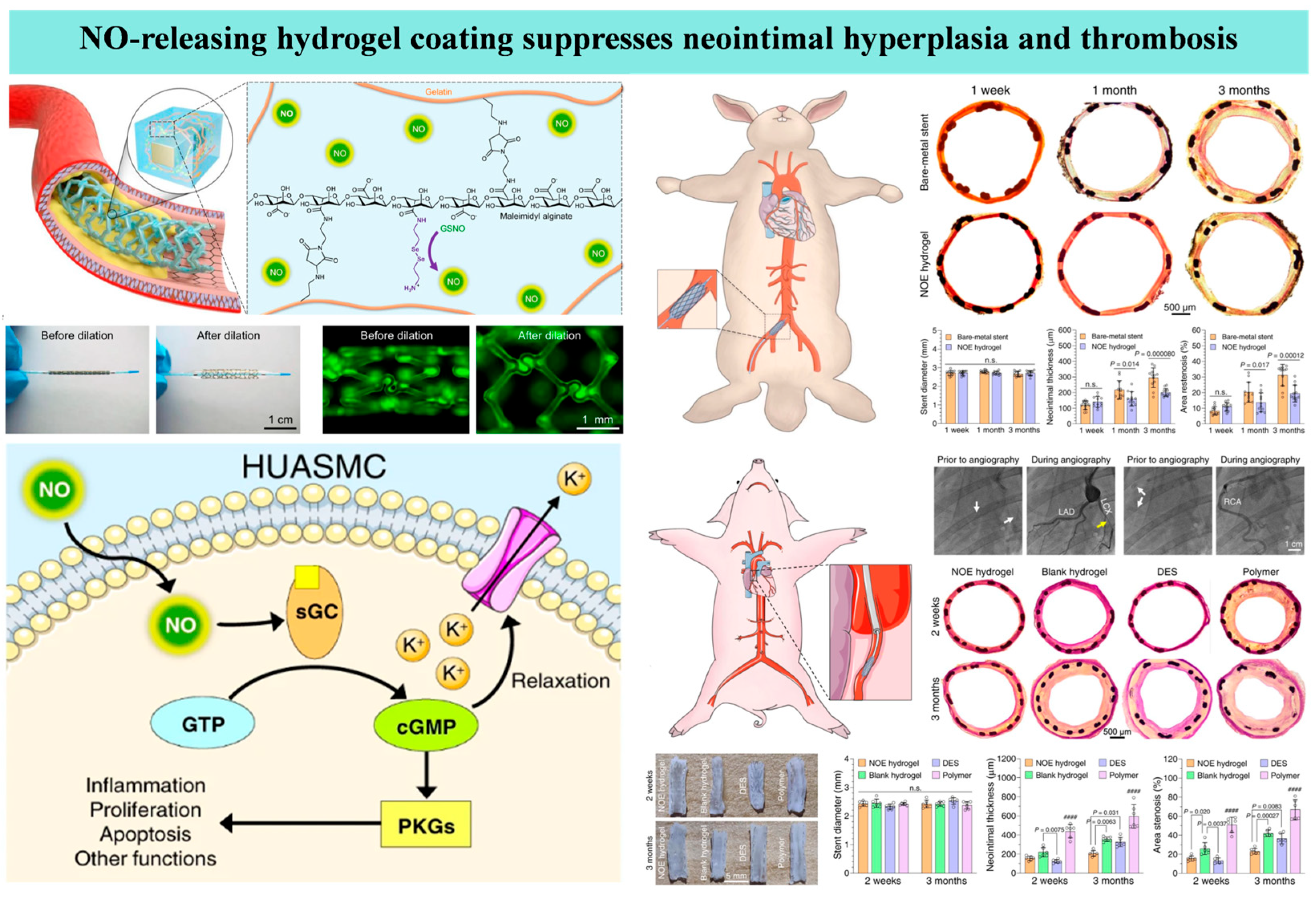
3.5. Endothelium-Mimetic Nitric Oxide-Releasing Coatings
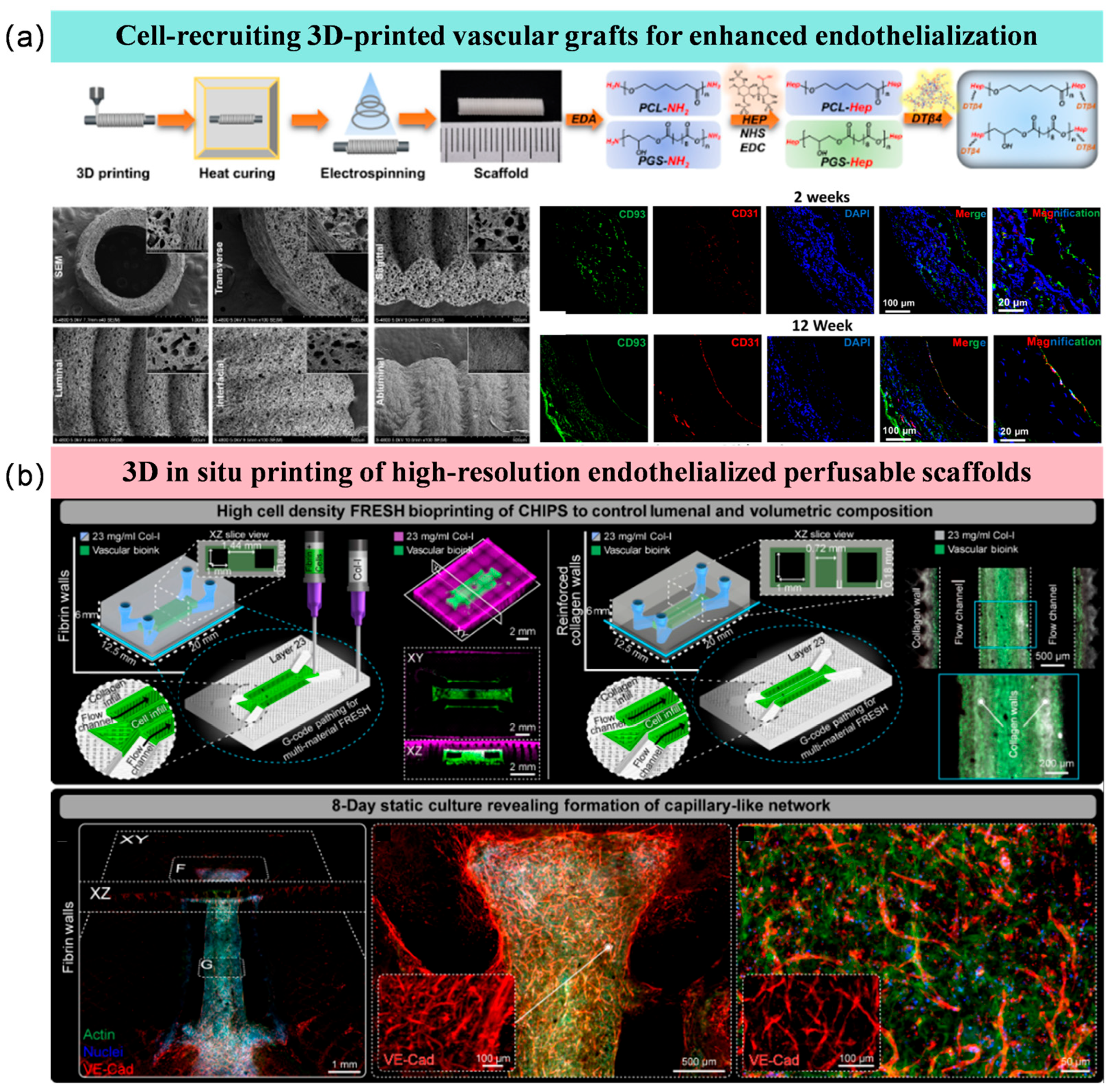
3.6. Endothelialized Surfaces
3.7. Discussion and Comparison of Different Anticoagulation Strategies
3.7.1. Technical Maturity of Anticoagulation Strategies
3.7.2. Analytical Comparison Between Anticoagulation Technologies
3.7.3. Analysis of Integrated Anticoagulation Strategies
3.7.4. Impact of Inter-Individual Biological Variability on the Performance of Anticoagulation Strategies
3.7.5. The Key Molecular and Mechanical Thresholds for Anticoagulant Materials
3.7.6. The Translational Challenges of Anticoagulation Strategies
3.7.7. Analysis of Production Stability and Costs for Anticoagulation Strategies
3.7.8. Experimental Conditions Capable of Simulating the Real Hemodynamic Environment for Anti-Coagulation Strategies
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaffer, I.H.; Weitz, J.I. The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue engineering at the blood-contacting surface: A review of challenges and strategies in vascular graft development. Adv. Healthc. Mater. 2018, 7, 1701461. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D.V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Luu, C.H.; Nguyen, N.T.; Ta, H.T. Unravelling surface modification strategies for preventing medical device-induced thrombosis. Adv. Healthc. Mater. 2024, 13, 2301039. [Google Scholar] [CrossRef]
- Fredenburgh, J.C.; Gross, P.L.; Weitz, J.I. Emerging anticoagulant strategies. Blood 2017, 129, 147–154. [Google Scholar] [CrossRef]
- Ashcraft, M.; Douglass, M.; Chen, Y.; Handa, H. Combination strategies for antithrombotic biomaterials: An emerging trend towards hemocompatibility. Biomater. Sci. 2021, 9, 2413–2423. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Huang, N. The structure, formation, and effect of plasma protein layer on the blood contact materials: A review. Biosurf. Biotribol. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef] [PubMed]
- Gbyli, R.; Mercaldi, A.; Sundaram, H.; Amoako, K.A. Achieving totally local anticoagulation on blood contacting devices. Adv. Mater. Interfaces 2018, 5, 1700954. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, W.; Cheng, S.; Li, J.; Zhang, H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction 2023, 11, 1371–1394. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood compatible materials: State of the art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Yousefi, S.; Borna, H.; Shirvan, A.R.; Wen, C.; Nouri, A. Surface modification of mechanical heart valves: A review. Eur. Polym. J. 2024, 205, 112726. [Google Scholar] [CrossRef]
- Ely, J.L.; Emken, M.R.; Accuntius, J.A.; Wilde, D.S.; Haubold, A.D.; More, R.B.; Bokros, J.C. Pure pyrolytic carbon: Preparation and properties of a new material, On-X carbon for mechanical heart valve prostheses. J. Heart Valve Dis. 1998, 7, 626–632. [Google Scholar] [PubMed]
- Zeng, H.; Yin, W.; Catausan, G.; Moldovan, N.; Carlisle, J. Ultrananocrystalline diamond integration with pyrolytic carbon components of mechanical heart valves. Diam. Relat. Mater. 2016, 61, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Gusmini, M.; Audenino, A.L.; Bergamasco, G.; Ieropoli, O.; Bignardi, C. Multiscale characterization of isotropic pyrolytic carbon used for mechanical heart valve production. Processes 2021, 9, 338. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Cianciulli, T.; Zhang, Z.; Chappard, D.; Lax, J.A.; Saccheri, M.C.; Redruello, H.J.; Jordana, J.L.; Prezioso, H.A.; et al. Clinical device-related article pivoting system fracture in a bileaflet mechanical valve: A case report. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90B, 952–961. [Google Scholar] [CrossRef]
- Zeng, H.J.; Jarvik, R.; Catausan, G.; Moldovan, N.; Carlisle, J. Diamond coated artificial cardiovascular devices. Surf. Coat. Technol. 2016, 302, 420–425. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films-from first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- Krishnan, L.K.; Varghese, N.; Muraleedharan, C.V.; Bhuvaneshwar, G.S.; Derangère, F.; Sampeur, Y.; Suryanarayanan, R. Quantitation of platelet adhesion to Ti and DLC-coated Ti in vitro using I-125-labeled platelets. Biomol. Eng. 2002, 19, 251–253. [Google Scholar] [CrossRef]
- Saito, T.; Hasebe, T.; Yohena, S.; Matsuoka, Y.; Kamijo, A.; Takahashi, K.; Suzuki, T. Antithrombogenicity of fluorinated diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 1116–1119. [Google Scholar] [CrossRef]
- Hasebe, T.; Yohena, S.; Kamijo, A.; Okazaki, Y.; Hotta, A.; Takahashi, K.; Suzuki, T. Fluorine doping into diamond-like carbon coatings inhibits protein adsorption and platelet activation. J. Biomed. Mater. Res. 2007, 83A, 1192–1199. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Ogwu, A.A.; Maguire, P.D.; McLaughlin, J.A.D. Platelet adhesion on silicon modified hydrogenated amorphous carbon films. Biomaterials 2004, 25, 239–245. [Google Scholar] [CrossRef]
- Maguire, P.D.; McLaughlin, J.A.; Okpalugo, T.I.T.; Lemoine, P.; Papakonstantinou, P.; McAdams, E.T.; Needham, M.; Ogwu, A.A.; Ball, M.; Abbas, G.A. Mechanical stability, corrosion performance and bioresponse of amorphous diamond-like carbon for medical stents and guidewires. Diam. Relat. Mater. 2005, 14, 1277–1288. [Google Scholar] [CrossRef]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef]
- Christensen, K.; Larsson, R.; Emanuelsson, H.; Elgue, G.; Larsson, A. Heparin coating of the stent graft-effects on platelets, coagulation and complement activation. Biomaterials 2001, 22, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Willers, A.; Arens, J.; Mariani, S.; Pels, H.; Maessen, J.G.; Hackeng, T.M.; Lorusso, R.; Swol, J. New Trends, Advantages and Disadvantages in Anticoagulation and Coating Methods Used in Extracorporeal Life Support Devices. Membranes 2021, 11, 617. [Google Scholar] [CrossRef]
- Gore, S.; Andersson, J.; Biran, R.; Underwood, C.; Riesenfeld, J. Heparin surfaces: Impact of immobilization chemistry on hemocompatibility and protein adsorption. J. Biomed. Mater. Res. Part B 2014, 102B, 1817–1824. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Bai, Y.; Yi, P.; Sun, C.; Shi, S.; Gong, Y. Achieving superior anticoagulation of endothelial membrane mimetic coating by heparin grafting at zwitterionic biocompatible interfaces. Int. J. Biol. Macromol. 2024, 257, 128574. [Google Scholar] [CrossRef]
- Cherng, W.; Pan, Y.; Wu, T.; Chou, C.; Yeh, C.; Ho, J. Hemocompatibility and adhesion of heparin/dopamine and heparin/collagen self-assembly multilayers coated on a titanium substrate. Appl. Surf. Sci. 2019, 463, 732–740. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Wang, X.; Li, Q. Surface modification with hydrophilic and heparin-loaded coating for endothelialization and anticoagulation promotion of vascular scaffold. Int. J. Biol. Macromol. 2022, 219, 1146–1154. [Google Scholar] [CrossRef]
- Zhang, Y.; Man, J.; Liu, J.; Li, J.; Song, X.; Wang, J.; Li, J.; Chen, Y. Construction of the Mussel-Inspired PDAM/Lysine/Heparin Composite Coating Combining Multiple Anticoagulant Strategies. ACS Appl. Mater. Interfaces 2023, 15, 27719–27731. [Google Scholar] [CrossRef]
- Chen, J.; Huang, N.; Li, Q.; Chu, C.H.; Li, J.; Maitz, M.F. The effect of electrostatic heparin/collagen layer-by-layer coating degradation on the biocompatibility. Appl. Surf. Sci. 2016, 362, 281–289. [Google Scholar] [CrossRef]
- Pan, C.; Pang, L.; Gao, F.; Wang, Y.; Liu, T.; Ye, W.; Hou, Y. Anticoagulation and endothelial cell behaviors of heparin-loaded graphene oxide coating on titanium surface. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 333–340. [Google Scholar] [CrossRef]
- Li, C.; Wang, F.; Ge, P.; Mao, Y.; Wang, L. Anti-acute thrombogenic surface using coaxial electrospraying coating for vascular graft application. Mater. Lett. 2017, 205, 15–19. [Google Scholar] [CrossRef]
- Lee, S.; Jo, H.; Lim, K.; Lim, D.; Lee, S.; Lee, J.; Kim, W.; Jeong, M.; Lim, J.; Kwon, I.; et al. Heparin coating on 3D printed poly (l-lactic acid) biodegradable cardiovascular stent via mild surface modification approach for coronary artery implantation. Chem. Eng. J. Adv. 2019, 378, 122116. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A heparin-functionalized woven stent graft for endovascular exclusion. Colloids Surf. B Biointerfaces 2019, 180, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, H.; Wang, L.; Zhou, D.; Duan, X.; Zhang, X.; Yin, J.; Luan, S.; Shi, H. Heparin-network-mediated long-lasting coatings on intravascular catheters for adaptive antithrombosis and antibacterial infection. Nat. Commun. 2024, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Balikci, E.; Yilmaz, B.; Tahmasebifar, A.; Baran, E.; Kara, E. Surface modification strategies for hemodialysis catheters to prevent catheter-related infections: A review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 314–327. [Google Scholar] [CrossRef]
- Yu, C.; Yang, H.; Wang, L.; Thomson, J.; Turng, L.; Guan, G. Surface modification of polytetrafluoroethylene (PTFE) with a heparin-immobilized extracellular matrix (ECM) coating for small-diameter vascular grafts applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112301. [Google Scholar] [CrossRef]
- Qiu, X.; Lee, B.; Ning, X.; Murthy, N.; Dong, N.; Li, S. End-point immobilization of heparin on plasma-treated surface of electrospun polycarbonate-urethane vascular graft. Acta Biomater. 2017, 51, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chan, C.; Pauls, J.; Semenzin, C.; Ainola, C.; Peng, H.; Fu, C.; Whittaker, A.; Silver, H.; John, F.F. Investigation of heparin-loaded poly(ethylene glycol)-based hydrogels as anti-thrombogenic surface coatings for extracorporeal membrane oxygenation. J. Mater. Chem. B 2022, 10, 4974–4983. [Google Scholar] [CrossRef]
- Li, Q.; Wen, C.; Yang, J.; Zhou, X.; Zhu, Y.; Zheng, J.; Cheng, G.; Bai, J.; Xu, T.; Ji, J.; et al. Zwitterionic Biomaterials. Chem. Rev. 2022, 122, 17073–17154. [Google Scholar] [CrossRef]
- Amoako, K.; Ukita, R.; Cook, K. Antifouling Zwitterionic Polymer Coatings for Blood-Bearing Medical Devices. Langmuir 2025, 41, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, S.; Xia, W.; Lundergan, L.; Yuan, H.; Xu, J. Zwitterionic Polymers for Biomedical Applications: Antimicrobial and Antifouling Strategies toward Implantable Medical Devices and Drug Delivery. Langmuir 2024, 40, 23125–23145. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices Kazuhiko Ishihara. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef]
- Lewis, A.L.; Stratford, P.W. A Review on Phosphorylcholine-Coated Stents. J. Long Term Eff. Med. Implant. 2017, 27, 233–252. [Google Scholar] [CrossRef]
- Du, Y.; Chen, X.; Gong, Y.; Wang, B.; Li, X.; Wang, J.; Shu, Y.; Zhao, Y. Phosphorylcholine-terminated dendritic polyethersulfone membrane with efficient hemocompatibility and antifouling properties for potential hemodialysis application. Mater. Today Chem. 2025, 48, 102993. [Google Scholar] [CrossRef]
- Lv, D.; Li, P.; Zhou, L.; Wang, R.; Chen, H.; Li, X.; Zhao, Y.; Wang, J.; Huang, N. Synthesis, evaluation of phospholipid biomimetic polycarbonate for potential cardiovascular stents coating. React. Funct. Polym. 2021, 163, 104897. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xue, Y.; Chen, T.; Huang, D.; Wang, Y.; Ren, K.; Wang, Y.; Fu, G.; Ji, J. Biodegradable phosphorylcholine copolymer for cardiovascular stent coating. J. Mater. Chem. B 2020, 8, 5361–5368. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, T.; Pan, Q.; Zhang, S.; Zhang, S.; Geng, Z.; Zhu, B. A Universal and Versatile Zwitterionic Coating for Blood-Contacting Catheters with Long Lengths and Complex Geometries. Adv. Sci. 2025, 12, 2502411. [Google Scholar] [CrossRef]
- Asif, S.; Asawa, K.; Inoue, Y.; Ishihara, K.; Lindell, B.; Holmgren, R.; Nilsson, B.; Rydén, A.; Jensen-Waern, M.; Teramura, Y.; et al. Validation of an MPC Polymer Coating to Attenuate Surface-Induced Crosstalk between the Complement and Coagulation Systems in Whole Blood in In Vitro and In Vivo Models. Macromol. Biosci. 2019, 19, 1800485. [Google Scholar] [CrossRef]
- Zhang, M.; Pauls, J.; Bartnikowski, N.; Haymet, A.; Chan, C.; Suen, J.; Schneider, B.; Ki, K.; Whittaker, A.; Dargusch, M.; et al. Anti-thrombogenic Surface Coatings for Extracorporeal Membrane Oxygenation: A Narrative Review. ACS Biomater. Sci. Eng. 2021, 7, 4402–4419. [Google Scholar] [CrossRef]
- Sheng, D.; Zhang, L.; Jia, H.; Guo, B.; Zhang, X.; Li, Y. Phosphorylcholine/Heparin Composite Coatings on Artificial Lung Membrane for Enhanced Hemo-compatibility. Langmuir 2023, 39, 9796–9807. [Google Scholar] [CrossRef]
- Lu, X.; Kang, Y.; Wang, X.; Wang, W.; Liu, C.; Jiang, H.; Yua, Y. An Anticoagulant Coating Based on a Block Phosphocholine Copolymer for Potential Applications in Blood Contact Devices. Adv. Mater. 2025, 12, 2400954. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, X.; Lu, Q.; Li, R.; Bai, Y.; Li, X.; Zhang, S.; Gong, Y. Anchorable phosphorylcholine copolymer synthesis and cell membrane mimetic antifouling coating fabrication for blood compatible applications. J. Mater. Chem. B 2020, 8, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Yang, P.; Li, J.; Li, S.; Li, P.; Zhao, Y.; Huang, N. Immobilization of poly(MPC) brushes onto titanium surface by combining dopamine self-polymerization and ATRP: Preparation, characterization and evaluation of hemocompatibility in vitro. Appl. Surf. Sci. 2015, 349, 445–451. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Xu, C.; Zhuang, X.; Wang, G.; Dong, X.; Qi, M. Preparation of covalently grafted phosphorylcholine coating on amino-rich 35NLT’ cobalt-chromium alloy surface and its biocompatibility evaluation. Surf. Interf. 2025, 58, 105919. [Google Scholar] [CrossRef]
- Wang, J.; Li, X. Enhancing protein resistance of hydrogels based on poly(2-hydroxyethyl methacrylate) and poly(2-methacryloyloxyethyl phosphorylcholine) with interpenetrating network structure. J. Appl. Polym. Sci. 2011, 121, 3347–3352. [Google Scholar] [CrossRef]
- Kobayashi, K.; Ohuchi, K.; Hoshi, H. Segmented polyurethane modified by photopolymerization and cross-linking with 2-methacryloyloxyethyl phosphorylcholine polymer for blood-contacting surfaces of ventricular assist devices. J. Artif. Organs. 2005, 8, 237–244. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Ishihara, K. Phosphorylcholine-containing polymers for biomedical applications. Anal. Bioanal. Chem. 2005, 381, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Yao, L.; Wang, J.; Chen, H. Antifouling Polymer Coatings for Bioactive Surfaces. Langmuir 2025, 41, 6471–6496. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Lv, Q.; Han, Y.; Zhang, L.; Zhang, G.; Chen, Y.; Sun, K.; Li, W.; Chen, Q.; et al. Dual-Functional Antimicrobial and Anticoagulant Coatings: Synergistic Mechanisms, Research Advances, and Translational Challenges. ChemBioChem 2025, 26, e202500425. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hadi, M.; Niu, J.; Zhou, Q.; Ran, F. Anticoagulant Macromolecules. Macromolecules 2023, 56, 4387–4430. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, L.; Muhammad, K.; Ren, X.; Guo, J.; Xia, S.; Zhang, W.; Feng, Y. Construction of Hemocompatible and Histocompatible Surface by Grafting Antithrombotic Peptide ACH11 and Hydrophilic PEG. ACS Biomater. Sci. Eng. 2019, 5, 2846–2857. [Google Scholar] [CrossRef]
- Välimäki, S.; Khakalo, A.; Ora, A.; Johansson, L.; Rojas, O.; Kostiainen, M. Effect of PEG–PDMAEMA Block Copolymer Architecture on Polyelectrolyte Complex Formation with Heparin. Biomacromolecules 2016, 17, 2891–2900. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, S.; Zhang, D.; Zhang, W.; Zhang, H.; Zou, J.; Mao, Z.; Yuan, Y.; Gao, C.; Liu, R. Impact of antifouling PEG layer on the performance of functional peptides in regulating cell behaviors. J. Am. Chem. Soc. 2019, 141, 16772–16780. [Google Scholar] [CrossRef]
- Hansson, K.; Tosatti, S.; Isaksson, J.; Wetterö, J.; Textor, M.; Lindahl, T.; Tengvall, P. Whole blood coagulation on protein adsorption-resistant PEG and peptide functionalised PEG-coated titanium surfaces. Biomaterials 2005, 26, 861–872. [Google Scholar] [CrossRef]
- Amaral, S.; Lozano-Fernández, T.; Sabin, J.; Gallego, A.; Morais, A.; Reis, R.L.; González-Fernández, Á.; Pashkuleva, I.; Novoa-Carballal, R. End-on PEGylation of heparin: Effect on anticoagulant activity and complexation with protamine. Int. J. Biol. Macromol. 2023, 249, 125957. [Google Scholar] [CrossRef]
- Pan, C.; Hou, Y.; Ding, H.; Dong, Y. Enhancing anticoagulation and endothelial cell proliferation of titanium surface by sequential immobilization of poly(ethylene glycol) and collagen. Appl. Surf. Sci. 2013, 287, 443–450. [Google Scholar] [CrossRef]
- Alibeik, S.; Zhu, S.; Yau, J.W.; Weitz, J.I.; Brash, J.L. Dual surface modification with PEG and corn trypsin inhibitor: Effect of PEG: CTI ratio on protein resistance and anticoagulant properties. J. Biomed. Mater. Res. A 2012, 100, 856–862. [Google Scholar] [PubMed]
- Kalaska, B.; Kamiński, K.; Miklosz, J.; Nakai, K.; Yusa, S.; Pawlak, D.; Nowakowska, M.; Mogielnicki, A.; Szczubiałka, K. Anticoagulant Properties of Poly(sodium 2-(acrylamido)-2-methylpropanesulfonate)-Based Di- and Triblock Polymers. Biomacromolecules 2018, 19, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Swieton, J.; Miklosz, J.; Bielicka, N.; Frackiewicz, A.; Depczynski, K.; Stolarek, M.; Bonarek, P.; Kaminski, K.; Rozga, P.; Yusa, S.; et al. Synthesis, biological evaluation and reversal of sulfonated di- and triblock copolymers as novel parenteral anticoagulants. Adv. Healthc. Mater. 2024, 13, 2402191. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, Y.; Gong, Y.; Zhang, Y.; Yu, Y.; Peng, H.; Fu, C. Recent advances in antifouling surface polymer brushes. ACS Appl. Polym. Mater. 2024, 6, 1–27. [Google Scholar] [CrossRef]
- Pasche, S.; De Paul, S.M.; Vörös, J.; Spencer, N.D.; Textor, M. Poly(l-lysine)-graft-poly(ethylene glycol) assembled monolayers on niobium oxide surfaces: A quantitative study of the influence of polymer interfacial architecture on resistance to protein adsorption by tof-sims and in situ owls. Langmuir 2003, 19, 9216–9225. [Google Scholar] [CrossRef]
- Unsworth, L.D.; Sheardown, H.; Brash, J.L. Protein-resistant poly(ethylene oxide)-grafted surfaces: Chain density-dependent multiple mechanisms of action. Langmuir 2008, 24, 1924–1929. [Google Scholar] [CrossRef]
- Faulón Marruecos, D.; Kienle, D.F.; Kaar, J.L.; Schwartz, D.K. Grafting density impacts local nanoscale hydrophobicity in poly(ethylene glycol) brushes. ACS Macro. Lett. 2018, 7, 498–503. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Cao, W.; Wang, J.; Zhang, P.; Ji, J. Stability Study of Anticoagulant Hydrogel Coatings Toward Long-Term Cardiovascular Devices. Langmuir 2025, 41, 2591–2599. [Google Scholar] [CrossRef]
- Yao, M.; Wei, Z.; Li, J.; Guo, Z.; Yan, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Yao, F.; et al. Microgel reinforced zwitterionic hydrogel coating for blood-contacting biomedical devices. Nat. Commun. 2022, 13, 5339. [Google Scholar] [CrossRef]
- Chan, D.; Chien, J.C.; Axpe, E.; Blankemeier, L.; Baker, S.W.; Swaminathan, S.; Piunova, V.; Zubarev, D.; Maikawa, C.; Grosskopf, A.K.; et al. Combinatorial polyacrylamide hydrogels for preventing biofouling on implantable biosensors. Adv. Mater. 2022, 34, 2109764. [Google Scholar] [CrossRef]
- Maitz, M.F.; Zitzmann, J.; Hanke, J.; Renneberg, C.; Tsurkan, M.; Sperling, C.; Freudenberg, U.; Werner, C. Adaptive release of heparin from anticoagulant hydrogels triggered by different blood coagulation factors. Biomaterials 2017, 135, 53–61. [Google Scholar] [CrossRef]
- Pu, H.; Yu, T.; Xiong, Y.; Wang, C.; Zhou, Z.; Li, G.; Wang, Y. Thrombin-regulated multi-functional hydrogel coating for decellularized extracellular matrix materials to enhance the anticoagulant and endothelialization properties. Biomaterials 2026, 326, 123724. [Google Scholar] [CrossRef]
- Balaoing, L.R.; Post, A.D.; Lin, A.Y.; Tseng, H.; Moake, J.L.; Grande-Allen, K.J. Laminin peptide-immobilized hydrogels modulate valve endothelial cell hemostatic regulation. PLoS ONE 2015, 10, e0130749. [Google Scholar] [CrossRef]
- Seeto, W.J.; Tian, Y.; Lipke, E.A. Peptide-grafted poly(ethylene glycol) hydrogels support dynamic adhesion of endothelial progenitor cells. Acta Biomater. 2013, 9, 8279–8289. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, H.; Liu, Y.; Tang, Q.; Wu, P.; Lin, T.; Li, T.; Sun, D. Sodium alginate-hydrogel coatings on extracorporeal membrane oxygenation for anticoagulation. Front. Cardiovasc. Med. 2022, 9, 966649. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, W.; Wu, P.; Liu, J.; Li, S.; Li, S.; Yu, M.; Ning, M.; Bai, R.; Li, T.; et al. A One-pot-synthesized Double-layered Anticoagulant Hydrogel Tube. Chem. Res. Chin. Univ. 2021, 37, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xie, Q.; Cha, R.; Ding, L.; Jia, L.; Mou, L.; Cheng, S.; Wang, N.; Li, Z.; Sun, Y.; et al. Anticoagulant Hydrogel Tubes with Poly (ε-Caprolactone) Sheaths for Small-Diameter Vascular Grafts. Adv. Healthc. Mater. 2021, 10, 2100839. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wang, W.; Zheng, X.; Chen, S.; Wang, S.; Wang, F.; Wang, L.; Hou, Y.; Li, C. Mechanical bionic compression resistant fiber/hydrogel composite artificial heart valve suitable for transcatheter surgery. Compos. B Eng. 2025, 296, 112234. [Google Scholar] [CrossRef]
- Yu, Y.; Yuk, H.; Parada, G.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, 1807101. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Han, X.; Pan, Y.; Wang, P.; Wang, T.; Lu, T. A Universal Strategy for Tough Adhesion of Wet Soft Material. Adv. Funct. Mater. 2020, 30, 2003207. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Ma, S.; Ma, Z.; Yu, B.; Cai, M.; Zhou, F. Universal Strategy for Growing a Tenacious Hydrogel Coating from a Sticky Initiation Layer. Adv. Mater. 2022, 11, 34. [Google Scholar] [CrossRef]
- Hao, D.; Wang, Z.; Liu, M.; Guo, X.; Wang, S.; Jiang, L. Strong Anchoring of Hydrogels through Superwetting-Assisted High-Density Interfacial Grafting. Angew. Chem. Int. Ed. Engl. 2023, 135, e202215034. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Parada, G.; Liu, X.; Zhao, X. Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 12028. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces withpressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Epsteina, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg, J. Liquid-infused structured surfaces with exceptional anti-biofouling performance. Proc. Natl. Acad. Sci. USA 2012, 109, 13182–13187. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Dai, J.; Yan, Y. Preserving exposed hydrophilic bumps on multi-bioinspired slippery surface arrays unlocks high-efficiency fog collection and photocatalytic cleaning. Nat. Commun. 2025, 16, 9793. [Google Scholar] [CrossRef]
- Kim, W.Y.; Yoon, S.M.; Park, S.R.; Kim, M.; Lee, S.; Choi, S.; Shin, S.; Kwon, S.; Kim, C.; Lee, K.; et al. Digitally fabricated 3D slippery architectures for multifunctional liquid manipulation. Nat. Commun. 2025, 16, 9026. [Google Scholar] [CrossRef]
- Manabe, K.; Kyung, K.; Shiratori, S. Biocompatible slippery fluid-Infused films composed of chitosan and alginate via layer-by-layer self-assembly and their antithrombogenicity. ACS Appl. Mater. Interfaces 2015, 7, 4763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, Y.; Meng, K.; Zheng, X.; Li, Y.; Chen, H. Enhanced Anticoagulation of Hierarchy Liquid Infused Surfaces in Blood Flow. ACS Appl. Mater. Interfaces 2023, 15, 55447–55455. [Google Scholar] [CrossRef]
- Leslie, D.C.; Waterhouse, A.; Berthet, J.; Valentin, T.; Watters, A.; Jain, A.; Kim, P.; Hatton, B.; Nedder, A.; Donovan, K.; et al. A bioinspired omniphobic surface coating on medical devices prevents thrombosis and biofouling. Nat. Biotechnol. 2014, 32, 1134–1140. [Google Scholar] [CrossRef]
- Roberts, T.; Choi, J.; Wendorff, D.; Harea, G.; Beely, B.; Sieck, K.; Douglass, M.; Singha, P.; Dean, J.; Handa, H.; et al. Tethered liquid perfluorocarbon coating for 72 hour heparin-free extracorporeal life support. ASAIO J. 2021, 67, 798–808. [Google Scholar] [CrossRef]
- Roberts, T.; Leslie, D.; Cap, A.; Cancio, L.; Batchinsky, A. Tethered liquid omniphobic surface coating reduces surface thrombogenicity, delays clot formation and decrease clot strength ex vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 108, 496–502. [Google Scholar] [CrossRef]
- Roberts, T.; Harea, G.; Singha, P.; Sieck, K.; Batchinsky, A. Heparin-free extracorporeal life support using tethered liquid perfluorocarbon: A feasibility and efficacy study. ASAIO J. 2019, 66, 809–817. [Google Scholar] [CrossRef]
- Howell, C.; Vu, T.; Johnson, C.; Hou, X.; Ahanotu, O.; Alvarenga, J.; Leslie, D.; Uzun, O.; Waterhouse, A.; Kim, P.; et al. Stability of surface-immobilized lubricant interfaces under flow. Chem. Mater. 2015, 27, 1792–1800. [Google Scholar] [CrossRef]
- Howell, C.; Grinthal, A.; Sunny, S.; Aizenberg, M.; Aizenberg, J. Designing liquid-infused surfaces for medical applications: A review. Adv. Mater. 2018, 30, 1802724. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.; McKinley, G.; Varanasi, K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2012, 9, 1772–1780. [Google Scholar] [CrossRef]
- Daniel, D.; Timonen, J.; Li, R.; Velling, S.; Aizenberg, J. Oleoplaning droplets on lubricated surfaces. Nat. Phys. 2017, 13, 1020–1025. [Google Scholar] [CrossRef]
- Yu, H.; Qiu, H.; Ma, W.; Maitz, M.; Tu, Q.; Xiong, K.; Chen, J.; Huang, N.; Yang, Z. Endothelium-Mimicking Surface Combats Thrombosis and Biofouling via Synergistic Long- and Short-Distance Defense Strategy. Small 2021, 17, 2100729. [Google Scholar] [CrossRef]
- Qiu, H.; Qi, P.; Liu, J.; Yang, Y.; Tan, X.; Xiao, Y.; Maitz, M.; Huang, N.; Yang, Z. Biomimetic engineering endothelium-like coating on cardiovascular stent through heparin and nitric oxide-generating compound synergistic modification strategy. Biomaterials 2019, 207, 10–22. [Google Scholar] [CrossRef]
- Handa, H.; Brisbois, E.; Major, T.; Refahiyat, L.; Amoako, K.; Annich, G.M.; Bartlett, R.; Meyerhoff, M. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-doped poly (vinyl chloride) matrix with poly (lactide-co-glycolide) additive. J. Mater. Chem. B 2013, 1, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Devine, R.; Goudie, M.; Singha, P.; Schmiedt, C.; Douglass, M.; Brisbois, E.; Handa, H. Mimicking the Endothelium: Dual Action Heparinized Nitric Oxide Releasing Surface. ACS Appl. Mater. Interfaces 2020, 12, 20158–20171. [Google Scholar] [CrossRef]
- Suchyta, D.; Handa, H.; Meyerhoff, M. A nitric oxide-releasing heparin conjugate for delivery of a combined antiplatelet/anticoagulant agent. Mol. Pharm. 2014, 11, 645–650. [Google Scholar] [CrossRef]
- Simon-Walker, R.; Romero, R.; Staver, J.M.; Zang, Y.; Reynolds, M.M.; Popat, K.C.; Kipper, M. Glycocalyx-inspired nitric oxide-releasing surfaces reduce platelet adhesion and activation on titanium. ACS Biomater. Sci. Eng 2017, 3, 68–77. [Google Scholar]
- Zhang, J.; Ke, X.; Huang, M.; Pei, X.; Gao, S.; Wu, D.; Chen, J.; Weng, Y. NO released via both a Cu-MOF-based donor and surface-catalyzed generation enhances anticoagulation and antibacterial surface effects. Biomater. Sci. 2023, 11, 322–338. [Google Scholar] [CrossRef]
- Douglass, M.; Goudie, M.; Pant, J.; Singha, P.; Hopkins, S.; Devine, R.; Schmiedt, C.; Handa, H. Catalyzed Nitric Oxide Release via Cu Nanoparticles Leads to an Increase in Antimicrobial Effects and Hemocompatibility for Short-Term Extracorporeal Circulation. ACS Appl. Bio. Mater. 2019, 2, 2539–2548. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, J.; Zhuang, W.; Deng, L.; Li, L.; Yu, H.; Wang, J.; Huang, N.; Wang, Y. Multifunctional coatings that mimic the endothelium: Surface bound active heparin nanoparticles within situ generation of nitric oxide from nitrosothiols. J. Mater. Chem. B 2018, 6, 5582–5595. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, T.; Jeakle, M.; Meyerhoff, M.; Bartlett, R.; Major, T. The Effects of the Combined Argatroban/Nitric Oxide-Releasing Polymer on Platelet Microparticle-Induced Thrombogenicity in Coated Extracorporeal Circuits. ASAIO J. 2021, 67, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, P.; Huang, L.; Tan, X.; Zhou, N.; Yang, T.; Qiu, H.; Dai, X.; Michael, S.; Tu, Q.; et al. A tough nitric oxide-eluting hydrogel coating suppresses neointimal hyperplasia on vascular stent. Nat. Commun. 2021, 12, 7079. [Google Scholar] [CrossRef]
- Fallon, B.P.; Lautner-Csorba, O.; Major, T.C.; Lautner, G.; Harvey, S.; Langley, M.; Johnson, M.; Saveski, C.; Matusko, N.; Rabah, R.; et al. Extracorporeal life support without systemic anticoagulation: A nitric oxide-based non-thrombogenic circuit for the artificial placenta in an ovine model. Pediatr. Res. 2024, 95, 93–101. [Google Scholar] [CrossRef]
- Jeakle, M.; Major, T.; Meyerhoff, M.; Bartlett, R. Comparison of Diazeniumdiolated Dialkylhexanediamines as Nitric Oxide Release Agents on Nonthrombogenicity in an Extracorporeal Circulation Model. ACS Appl. Bio Mater. 2020, 3, 466–476. [Google Scholar] [PubMed]
- Rao, J.; Pan, H.; Yang, Y.; Liu, Y.; Lin, H.; Zhao, X. Nitric oxide-producing cardiovascular stent coatings for prevention of thrombosis and restenosis. Front. Bioeng. Biotechnol. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Y.; Xiong, K.; Li, X.; Qi, P.; Tu, Q.; Jing, F.; Weng, Y.; Wang, J.; Huang, N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials 2015, 63, 80–92. [Google Scholar] [CrossRef]
- Brisbois, E.J.; Kim, M.; Wang, X.; Mohammed, A.; Major, T.C.; Wu, J.; Brownstein, J.; Xi, C.; Handa, H.; Bartlett, R.; et al. Improved hemocompatibility of multilumen catheters via nitric oxide (NO) release from S-nitroso-N-acetylpenicillamine (SNAP) composite filled lumen. ACS Appl. Mater. Interfaces 2016, 8, 29270–29279. [Google Scholar] [CrossRef]
- Sánchez, P.F.; Brey, E.M.; Briceño, J.C. Endothelialization mechanisms in vascular grafts. J. Tissue Eng. Regen. Med. 2018, 12, 2164–2178. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, Y. Surface engineering of cardiovascular devices for improved hemocompatibility and rapid endothelialization. Adv. Healthc. Mater. 2020, 9, 2000920. [Google Scholar] [CrossRef]
- Giol, E.D.; Van Vlierberghe, S.; Unger, R.E.; Schaubroeck, D.; Ottevaere, H.; Thienpont, H.; Kirkpatrick, C.; Dubruel, P. Endothelialization and Anticoagulation Potential of Surface-Modified PET Intended for Vascular Applications. Macromol. Biosci. 2018, 18, 1800125. [Google Scholar]
- Zeng, Z.; Hu, C.; Liang, Q.; Tang, L.; Cheng, D.; Ruan, C. Coaxial-printed small-diameter polyelectrolyte-based tubes with an electrostatic self-assembly of heparin and YIGSR peptide for antithrombogenicity and endothelialization. Bioact. Mater. 2021, 6, 1628–1638. [Google Scholar] [CrossRef]
- Tang, D.; Chen, S.; Hou, D.; Gao, J.; Jiang, L.; Shi, J.; Liang, Q.; Kong, D.; Wang, S. Regulation of macrophage polarization and promotion of endothelialization by NO generating and PEG-YIGSR modified vascular graft. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Shan, Y.; Jia, B.; Ye, M.; Shen, H.; Chen, W.; Zhang, H. Application of heparin/collagen-redv selective active interface on ePTFE films to enhance endothelialization and anticoagulation. Artif. Organs 2018, 42, 824–834. [Google Scholar] [PubMed]
- Xue, W.; Nasr, S.; Guan, G.; Gao, L.; Zhao, F.; Gao, J.; Wang, F.; Qian, C.; Wang, L. An Efficient Surface Modification Strategy Improving Endothelialization with Polydopamine Nanoparticles and REDV Peptides for Stent-Grafts. ACS Appl. Bio. Mater. 2019, 2, 3820–3827. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Chen, Y.; Zhang, K.; Maitz, M.; Pan, C.; Chen, J.; Huang, N. Extracellular matrix inspired surface functionalization with heparin, fibronectin and VEGF provides an anticoagulant and endothelialization supporting microenvironment. Appl. Surf. Sci. 2014, 320, 871–882. [Google Scholar] [CrossRef]
- Sgarioto, M.; Vigneron, P.; Patterson, J.; Malherbe, F.; Nagel, M.; Egles, C. Collagen type I together with fibronectin provide a better support for endothelialization. C. R. Biol. 2012, 335, 520–528. [Google Scholar]
- Liu, T.; Liu, S.; Zhang, K.; Chen, J.; Huang, N. Endothelialization of implanted cardiovascular biomaterial surfaces: The development from in vitro to in vivo. J. Biomed. Mater. Res. A 2014, 102, 3754–3772. [Google Scholar] [PubMed]
- Xiao, W.; Chen, W.; Wang, Y.; Zhang, C.; Zhang, X.; Zhang, S.; Wu, W. Recombinant DTβ4-inspired porous 3D vascular graft enhanced antithrombogenicity and recruited circulating CD93+/CD34+ cells for endothelialization. Sci. Adv. 2022, 8, eabn1958. [Google Scholar] [PubMed]
- Zhang, S.; Qi, C.; Zhang, W.; Zhou, H.; Wu, N.; Yang, M.; Meng, S.; Liu, Z.; Kong, T. In Situ Endothelialization of Free-Form 3D Network of Interconnected Tubular Channels via Interfacial Coacervation by Aqueous-in-Aqueous Embedded Bioprinting. Adv. Mater. 2023, 35, 2209263. [Google Scholar] [CrossRef]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Bakirci, E.; Moss, S.; Coffin, B.D.; Feinberg, A.W. 3D bioprinting of collagen-based high-resolution internally perfusable scaffolds for engineering fully biologic tissue systems. Sci. Adv. 2025, 11, eadu5905. [Google Scholar]
- You, S.; Xiang, Y.; Hwang, H.H.; Berry, D.B.; Kiratitanaporn, W.; Guan, J.; Yao, E.; Tang, M.; Zhong, Z.; Ma, X.; et al. High cell density and high-resolution 3D bioprinting for fabricating vascularized tissues. Sci. Adv. 2023, 9, eade7923. [Google Scholar] [CrossRef]
- Ouyang, L.; Armstrong, J.P.; Chen, Q.; Lin, Y.; Stevens, M.M. Void-free 3D bioprinting for in situ endothelialization and microfluidic perfusion. Adv. Funct. Mater. 2020, 30, 1908349. [Google Scholar]
- Du, Y.; Chen, X.; Chen, H.; Wang, B.; Gong, Y.; Li, X.; Wang, J.; Zhao, Y. Metal-polyphenol network coating for cardiovascular implants to prevent thrombosis and promote endothelialization. Mater. Today Commun. 2025, 49, 113974. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Xiang, Z.; Wang, J.; Ren, S.; Zhao, J.; Fu, D.; Wang, Y. Dual-mode nitric oxide releasing vascular grafts for vascular homeostasis and antibacterial defense. Acta Biomater. 2025, 11, S1742–S7061. [Google Scholar] [CrossRef]
- Yuan, S.; Cai, W.; Szakalas-Gratzl, G.; Kottke-Marchant, K.; Tweden, K.; Marchant, R.E. Immobilization of heparin oligosaccharides onto radiofrequency plasma modified pyrolytic carbon-coated graphite. J. Appl. Biomater. 1995, 6, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Arnander, C.; Bagger-Sjöbäck, D.; Frebelius, S.; Larsson, R.; Swedenborg, J. Long-term stability in vivo of a thromboresistant heparinized surface. Biomaterials 1987, 8, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Y.; Sheardown, H.; Brook, M.A. Immobilization of heparin on a silicone surface through a heterobifunctional PEG spacer. Biomaterials 2005, 26, 7418–7424. [Google Scholar] [CrossRef] [PubMed]
- Camasão, D.B.; Mantovani, D. The mechanical characterization of blood vessels and their substitutes in the continuous quest for physiological-relevant performances. A critical review. Mater. Today Bio 2021, 7, 100106. [Google Scholar] [CrossRef]
- Ahmed, A.; Wang, X.; Yang, M. Biocompatible materials of pulsatile and rotary blood pumps: A brief review. Rev. Adv. Mater. Sci. 2020, 59, 322–339. [Google Scholar] [CrossRef]
- Yamazaki, K.; Kihara, S.; Akimoto, T.; Tagusari, O.; Kawai, A.; Umezu, M.; Tomioka, J.; Kormos, R.L.; Griffith, B.P.; Kurosawa, H. EVAHEART™: An implantable centrifugal blood pump for long-term circulatory support. Jpn. J. Thorac. Cardiovasc. Surg. 2002, 50, 461–465. [Google Scholar] [CrossRef]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Griffith, K.; Jenkins, E.; Pagani, F. First American experience with the Terumo DuraHeart™ left ventricular assist system. Perfusion 2009, 24, 83–89. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Ziemer, G.; Wendel, H.P. Induction of EPC homing on biofunctionalized vascular grafts for rapid in vivo self-endothelialization—A review of current strategies. Biotechnol. Adv. 2010, 28, 119–129. [Google Scholar] [CrossRef]
- Du, Z.; Hu, X.; Lin, Y.; Chen, L.; Huang, Y.; Fan, J.; Yang, S. Applications of microfluidic chip technology in microvascular thrombosis research. Mikrochim. Acta 2025, 192, 371. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ma, H.; Xu, F.; Wang, X.; Sun, W. Microfluidics in cardiovascular disease research: State of the art and future outlook. Microsyst. Nanoeng. 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Chen, S.; Zhang, C.; Liao, J.; Chen, Y.; Deng, S.; Mao, Z.; Zhang, T.; Tian, N.; Song, Y.; et al. Recent advances in microfluidic technology of arterial thrombosis investigations. Platelets 2024, 35, 2316743. [Google Scholar] [CrossRef] [PubMed]

| NO Flux (mol·cm−2·min−1) | Characteristics | Implantable and Interventional Application | |
|---|---|---|---|
| Physical doping or embedding of NO donors | >1.0 × 10−10 | high initial NO flux, but short duration (ranging from hours to days) | short-term |
| Chemically bound NO-releasing coatings | 0.5–1.0 × 10−10 | more sustained release (from days to weeks) | medium-term |
| Catalytic NO-generating coatings | 0.5–4.0 × 10−10 | hold potential for “on-demand” release | long-term |
| Representative Strategies | Strategy Type | Protein Adsorption Value (Fibrinogen) | Critical Stable Shear Stress |
|---|---|---|---|
| Hydrogel/Zwitterionic coatings (MPC, PEG) | Passive anti-adsorption | Very low (<5 ng/cm2) | Low to moderate (~10–50 dyn/cm2) |
| Liquid-infused surfaces (LIS) | Passive anti-adsorption | Extremely low (near-complete repellency) | Highly variable (structure-dependent) |
| Heparinized coatings (End-point immobilized) | Bioactive | Moderate | Moderate to high (>50 dyn/cm2) |
| Nitric oxide (NO)-releasing coatings | Bioactive | Not directly related (primarily inhibits platelets) | Moderate (depends on the matrix) |
| Endothelialization surfaces | Biointegrative | Physiological protein adsorption | High (after tissue integration) |
| Hydrophilic Coatings | Liquid Infused Surfaces | Endothelialization Surface | |
|---|---|---|---|
| Core Mechanism | Hydration layer: Creates a physical and thermodynamic barrier to minimize non-specific protein adsorption. | Slippery and dynamic liquid interface: Prevents adhesion of blood components. | Living and functional endothelial layer: Actively secretes anticoagulant factors. |
| Technology Readiness Level | High: Clinical use in short-term devices like catheters and dialysis circuits. | Low-Medium: Preclinical research stage. | Medium: Active preclinical and early clinical exploration. |
| Key Advantages | Good initial hemocompatibility. | Broad-spectrum anti-adhesion properties. | Truly biological, self-healing, and long-term solution. |
| Major Limitations and Challenges | Limited Long-term Stability: Prone to hydrolysis, oxidative degradation, and wear. | Lubricant Loss: Lubricant depletion under shear stress is the primary challenge. | Cell source: Obtaining sufficient functional endothelial cells is difficult. Hyperplasia risk: Leading to restenosis. |
| Long-Term Application Prospect | Moderate: Suitable for medium-term implants pending improvements in material stability against degradation. | Uncertain: High potential is entirely dependent on solving the fundamental challenge of long-term lubricant retention. | High (if successful): Represents the ultimate “gold standard” for permanent implants if functional stability is achieved. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Deng, Z.; Wang, Y.; Zhao, C. A Review of Anticoagulant Surface Modification Strategies for Blood-Contacting Materials: From Inertness to Bioinspired and Biointegration. Coatings 2025, 15, 1486. https://doi.org/10.3390/coatings15121486
Zhang S, Deng Z, Wang Y, Zhao C. A Review of Anticoagulant Surface Modification Strategies for Blood-Contacting Materials: From Inertness to Bioinspired and Biointegration. Coatings. 2025; 15(12):1486. https://doi.org/10.3390/coatings15121486
Chicago/Turabian StyleZhang, Shuguang, Zhixiang Deng, Yuhe Wang, and Chao Zhao. 2025. "A Review of Anticoagulant Surface Modification Strategies for Blood-Contacting Materials: From Inertness to Bioinspired and Biointegration" Coatings 15, no. 12: 1486. https://doi.org/10.3390/coatings15121486
APA StyleZhang, S., Deng, Z., Wang, Y., & Zhao, C. (2025). A Review of Anticoagulant Surface Modification Strategies for Blood-Contacting Materials: From Inertness to Bioinspired and Biointegration. Coatings, 15(12), 1486. https://doi.org/10.3390/coatings15121486





