Abstract
In recent decades, applications related to sensing have grown increasingly, transforming and expanding their fields into innovative research. Lately, researchers have demonstrated that immobilizing metal nanoparticles on glass-based platforms may render innovative perspectives for sensing applications. As a result, the focus of this study was to develop glass-based platforms functionalized with silver nanoparticles, intending them to be utilized in sensing applications. The purpose of using glass-based platforms is due to their availability and eco-friendly features, which will make them suitable for such applications. The study uses a glass-based platform functionalized/modified with organosilanes (such as mercaptoalkyl trialkoxysilane), which can have a high affinity for Ag NPs. By decorating the glass surface with Ag NPs, it becomes active for the adsorption of the mercapto derivatives and further usage in sensing applications (specific drugs with an antitumoral, anti-hypertensive, antiarthritic role, neurotransmitters, etc.) but also for specific classes of pollutants for environmental applications. Therefore, the desired purpose of this study was to develop glass-based platforms decorated with Ag NPs and their further use in the selective adsorption of thioderivatives (cysteine was selected as a model component) even from a mixture of amino acids (cysteine, alanine, and threonine).
1. Introduction
Mercapto derivatives (also known as thioderivatives) have a wide range of applications in various fields due to their unique chemical properties. The importance of mercapto derivatives is extremely high, with thiolic groups being common in proteins, drugs, pesticides, etc., and, due to their specific reactivity, they can generate specific properties. The thiolic groups can easily form disulfide bonds or can be strongly bonded to specific cations or even metals (such as Ag, Au, Cu) forming hybrid structures [1,2,3,4]. Based on the disulfide bonds, thiolated surfaces can be used to bind specific analytes, driving the design of chemical sensors for specific applications, including medical ones that can be valorized in the detection of certain ions, molecules, or pathogens [5]. This is why there is a great challenge in developing sensing materials for these derivatives, or at least selectively adsorbing them on specific surfaces at a very low level where existing analytical techniques cannot detect and quantify them. After their desorption, they can then be determined by existing techniques. Moreover, Surface-Enhanced Raman Scattering (SERS) is improved by the presence of mercapto derivatives because the presence of the mercapto groups improves the interaction between specific nanoparticles, including silver and gold, increasing its sensitivity [6,7].
Another interesting approach to developing activated surface platforms suitable for promoting selective retention is using organosilanes (like methoxy and sulfhydryl groups) for immobilizing biomolecules [8]. To selectively bind and localize diverse biomolecules, it needs to identify a specific surface; therefore, glass-based platforms were used [8,9]. Furthermore, mercaptoalkyl silanes develop a resistant layer on specific surfaces and are capable of binding/have an affinity for several biomolecules, such as protein/amino acids/enzyme adsorption [10,11,12]. Glass is recommended because of its high stability, which can assure long-term functionality, reusability, and stability, and is a low-cost product [13,14].
An important aspect of the immobilization technique was to decorate the glass-based platforms with Ag NPs through mercapto derivates to generate a binding layer for diverse amino acids. Researchers have stated that compared with various nanostructures, silver nanoparticles are gaining importance as they provide a stable and very large surface area for enzyme immobilization [15]. Upadhyay et al. [16] demonstrated a novel technique for enzyme immobilization on a glass-based platform by integrating a cysteine layer on the glass-silanized substrate, which was able to covalently immobilize alkaline phosphatase. In other research, Halliwell et al. [17] described a modifying method for the immobilization of oligonucleotides on glass substrates. The most used technique for the attachment of biomolecules involves a reaction with organofunctional silanes, like (3-mercaptopropyl) trimethoxysilane-MPTMS, followed by the covalent attachment of the biological molecule to the newly introduced functional group (-SH) on the surface.
In this paper, we are reporting the development of Ag NP-decorated glass surfaces able to selectively bind thioderivatives (the model compound was selected to be cysteine) even from a mixture of amino acids. It is worth mentioning that Ag NPs were previously prepared according to our paper [18] and were anchored onto the glass surface via mercaptopropyl moieties. Firstly, the primary objective of this study is to design thiol-silanized glass platforms with two agents to enhance the affinity of decoration with Ag NPs. Consequently, the novelty of this study consists of the treatment of Ag NP-decorated glass platforms with three amino acids (cysteine, alanine, and threonine, alone or as a mixture) in order to demonstrate the specificity over the cysteine. This study represents a proof of concept that the layer by layer modification approach we have developed here, based on the silanization with agents bearing mercapto moieties, followed by decoration with silver nanoparticles is suitable for further binding of thioderivatives. Up to now, based on our knowledge, there has not been such a study using glass and build up based on an approach using Lego-like suprastructures that are able to selectively adsorb mercapto derivatives. Additional studies will be used in order to find the best technique to quantify the concentration of the thioderivatives (especially for medical applications) with SERS being a desired detection method.
2. Materials and Methods
2.1. Chemicals
The reagents isopropyl alcohol, ammonia (NH3) solution 25%, hydrogen peroxide (H2O2) 35%, hydrochloric acid (HCl) 37%, MPTMS and (3-mercaptopropyl) triethoxysilane–MPTES, cysteine (cys), alanine (ala), and threonine (thr) were produced by Sigma-Aldrich (Darmstadt, Germany). The Ag NPs used were synthesized and characterized according to previous studies [18,19]. The Ag NPs used in this study have a spherical shape, measuring 8–12 nm, with a tendency to agglomerate, and were synthesized by the solvothermal method using polyethylene glycol as a reducing agent at 260 °C and 3 bar pressure for 1 h (HT4 synthesis [18]). The microscope slides (26 × 76 mm, 1.0–1.2 mm thickness) were produced by Labbox (Barcelona, Spain).
2.2. Cleaning Pre-Treatment
All glass samples were washed in an aqueous solution with detergent and rinsed with distilled water (DW), followed by cleaning with isopropyl alcohol. Slides were dipped in a mixture of NH3 25%:H2O2:H2O = 1:1:5 (in volume) for 30 minutes (min) at 30 °C, followed by cleansing with DW, and treated with a mixture of HCl 37%: H2O2:H2O = 1:1:5 (in volume) for 30 min at 85 °C. After these treatments, all samples were washed with DW, DW–isopropyl alcohol = 1:1, and isopropyl alcohol. The glass surface’s functionalization was immediately performed [20,21].
2.3. Glass Surface Modification
The glass-based platforms were performed using MPTMS and MPTES as thioalkyl silanization agents for functionalizing the glass surface. These two silanizing agents were chosen because, following an intensive study of the literature, they show a very high affinity for Ag or Au NPs. All slides were immersed in a 1, 3, and 5% MPTMS/MPTES (v:v) alcoholic solution at 30 °C, 25 rpm for 24 h. The silanization agent excess was washed with isopropyl alcohol, isopropyl alcohol–DW = 1:1, and DW, and further, the silanized glass slides were dried at room temperature. All treatments were performed using tightly closed PVC centrifuge tubes [20,21].
2.4. Decoration of Thiopropylated Glass Surfaces with Ag NPs
In the second step, glass platforms were decorated using Ag NPs. All samples were immersed in a 1.2 ppm Ag NP colloidal solution at room temperature and 20 rpm for 8 days. The adsorption of Ag NPs was monitored by measurements at 405 nm with an UV-Vis Evolution 300 Thermo Fisher Scientific spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Ag NP quantification was performed using a six-point calibration curve covering the 0.08–1.2 ppm range. The Ag NPs deposited (mg/m2) were calculated according to the curve calibration (y = mx + b) and using the following Equation (1):
2.5. Structural Quantification of the Ag NPs’ Deposited Amount
The maximum amount of Ag NPs deposited on the functionalized glass surface specimen was calculated by following two simple approaches, further called A and B.
Approach A works on the hypothesis that the glass surface features a uniform -OH group density, and all -OH groups would become, after silanization, -SH groups; each -SH group will link to a single, spherical Ag nanoparticle, thus resulting in a monolayered Ag NP deposit. In synthesis, by computing the amount of -OH groups on the specimen’s surface and by equivalating -OH groups by -SH groups (supposedly, each -SH will link to an Ag nanoparticle of a given radius), we can compute, theoretically, the mass of a single Ag nanoparticle and, further, the amount of Ag NPs deposited. However, steric effects could influence the number of -SH groups available to interact with Ag, with favor or disfavor nanoparticles having a shape different from spherical (for example, a better packing will result for large particles of an oval shape rather than for a spherical shape when linking to the -SH groups); also, it is probable that instead of a single nanoparticle, some aggregates may link to the functional groups. For these reasons, another approach, called B, was followed, and the results were compared.
Approach B assumes that, in most cases, not all the functional groups will be linked to a single Ag nanoparticle as the nanoparticles increase in size. The principle is that by projecting the areas of the spherical, average-sized, monolayered Ag NPs (AAg NPs) over the area of the specimen and summing them, the result should be less or equal to the area of the specimen (As), according to Equation (2).
where n is the number of Ag NPs. If n is at its highest, one can infer that an important number of -SH connects each with a single Ag nanoparticle (if enough Ag NPs were provided). This may correspond to the case of small-sized Ag NPs. If n has its smallest value, large Ag NPs’ predominance should be presumed; in that case, an Ag nanoparticle may link to-SH groups by a covalent bond.
2.6. Adsorption of Amino Acids to Ag-Glass-Decorated Platforms
Adsorption processes of amino acids are carried out with the aim of expanding the applicability of glass-based platforms silanized with thiolalkyl agents and decorated with Ag NPs, but at the same time, to study and observe the affinity of certain bioactive compounds on the “-SH” groups on the surface of the glass (Figure 1). Considering the well-known affinity of the SH moiety over the Ag and Au surface (even in the case of macroscopic metallic surfaces), cysteine was selected as an amino acid with an SH group, while the other two amino acids, alanine (which is a simple amino acid without any functional group) and threonine (which has a -OH group), were selected as reference amino acids. The silanized glass platforms decorated with Ag NPs were immersed in a 0.2% solution of cys, ala, and thr, respectively, in their volumetric mixture (cys:ala:thr~CAT = 1:1:1) to observe the affinity of the functional groups. The effect of amino acid decoration was observed on conductivity over 21 days and the processes took place in a controlled environment at 37 ± 1 °C and with a slight orbital shaking (25 rpm).
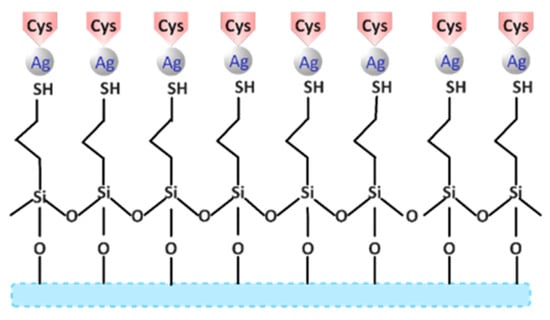
Figure 1.
Schematic representation of cysteine adsorption on a glass surface silanized with MPTMS and decorated with Ag NPs.
2.7. Characterization Methods
The obtained glass-based platforms were characterized by FTIR spectroscopy and microscopy, UV-Vis, and conductivity.
An UV-Vis Evolution 300 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) includes a long-lifetime xenon flash lamp and an extended wavelength ranging up to 900 nm silicon photodiode detector. Moreover, the equipment includes photo-etched slits for greater accuracy and a stepper-motor-driven slit drive with intelligent optimization. The grating features are 1200 lines/mm, 240 nm blazed, and holographic grating for exceptional stray light performance. Additionally, the spectrophotometer presents Vision Pro 4.5. software, which offers advanced scanning, multiple fixed wavelength measurements, and multi-wavelength measurements with customized UVcalc [22].
The Fourier transform infrared spectroscopy (FTIR) spectra were recorded with a Nicolet iS50 FTIR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), using the attenuated total reflection accessory (ATR) (Thermo Fisher Scientific, Waltham, MA, USA). All spectra were obtained as the average of 32 scans in domain 400 and 4000 cm−1 at a resolution of 4 cm−1. FTIR 2D maps were obtained with a Nicolet iN10 MX FTIR microscope equipped with a DTGS detector between 600 and 4000 cm−1. Information about the spatial distribution of the components was extracted from the 2D FTIR maps.
Conductivity measurements were performed at Inolab-WTW Multi 9630 IDS (Intelligent, Digital Sensors) for standard parameters pH, conductivity, and dissolved oxygen. Digital signal transfer eliminates electromagnetic interferences, allocates correct calibration data, and provides sensor data. The intelligent sensor rating QSC visually shows the actual condition of a pH electrode and increases operational reliability [23].
3. Results and Discussions
3.1. Decoration of Glass Platforms with Ag NPs–UV-Vis Spectroscopy
The affinity of glass for metal nanoparticles involved functionalization with thioalkylsilanes as silanizing agents, since “-SH” groups have a high affinity for metal NPs such as Ag [24].
Figure 2 represents the adsorption and affinity of Ag NPs upon the silanizing agent. In the case of glass platforms silanized with MPTMS, a greater amount of deposited Ag is observed compared to those with MPTES. Additionally, the affinity of Ag NPs increased with increasing silanizing agent concentration, and the platforms with 5% MPTMS/MPTES presented the highest amounts of Ag. As a general comment, in the case of MPTMS, the binding of the Ag NPs seems to be more dependent on the concentration of the silanization agent used, and thus, the decoration with Ag NPs for the MPTMS 5% is ~2 times higher expressed as μg Ag NPs retained/m2 of the glass surface. In the case of the MPTES, the concentration plays a lower role, with the content of the Ag NPs deposited onto the surface being ~79, 90, and 101 μg/m2 depending on the concentration of MPTES, 1, 3, and 5%, respectively [25,26]. Otherwise, the Ag NPs presented a high affinity for glass platforms silanized with MPTMS, a fact that is also confirmed for Au NPs [27].
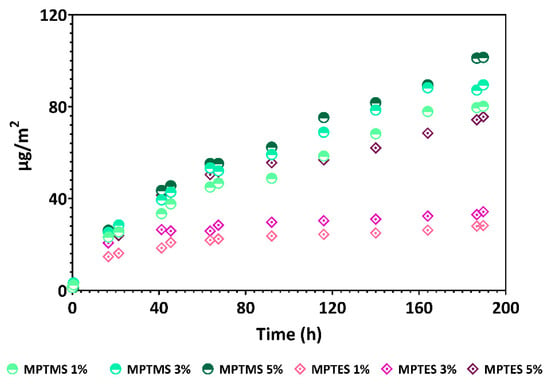
Figure 2.
Decoration of mercaptopropylated glass platforms with Ag NPs.
3.2. Theoretical Decoration Capacity
Within Approach A, calculations were made with various average radii of the Ag NPs (8–12 nm), according to findings in [18]. Moreover, -SH density ranged from 2 to 4 functional groups/nm2 (2.5 was found in the previous study [28]) for microscope glass. The maximum amount of deposited Ag (around 100 µg/m2) was obtained here and taken for comparison. Coefficients were derived by fitting the theoretical results to experimental data, such as: 0.33 for SH density = 4, 0.45 for SH density = 3, and 0.66 for SH density = 2, meaning that, for example, if the OH density is 0.66, there is a maximum 66% chance that these two groups will link single Ag nanoparticles. For convenience, an overall coefficient of 0.42 was adopted (as areas of extreme SH densities have small probabilities to exist) and then applied to the computed Ag amounts. The results are plotted in Figure 3, ranging from 18 to 126 µg/m2. A comparison with the maximum values of the experimental results presented in Figure 3 concludes that both SH concentration and Ag NPs’ radii are taking various, distributed values. Overall, most probably, the highest radius is about 11 nm, being consistent with the value (11.3 nm) given in [18]. A maximum theoretical value of 126 µg/m2 was computed; this case corresponds in an experimental setting to the situation when aggregates are building up.
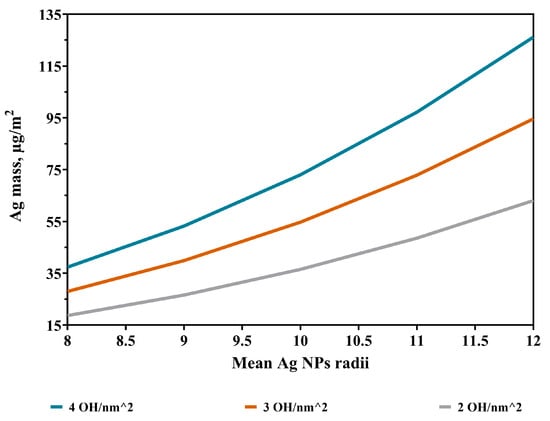
Figure 3.
Deposited Ag NPs for various Ag NP sizes and OH concentrations.
Approach A’s calculations results, as unexpectedly close to the experimental results they are, suggest considering the Ag NPs as material points connected to the SH groups. Therefore, above this value, to avoid overlapping nanoparticles, multilayered Ag structures should develop, which contradicts our monolayered hypothesis; therefore, there is a need for the geometrical Approach B.
Figure 4 shows a nonlinear decrease in the number of Ag NPs with the nanoparticles’ radii. Moreover, there is a significant difference in the order of magnitude if compared to the number of -OH groups/specimen (inset image)—confirming that, in fact, a single Ag nanoparticle covers an area of hundreds of -SH groups—which can be important because these nanoparticles are well anchored onto the surface.
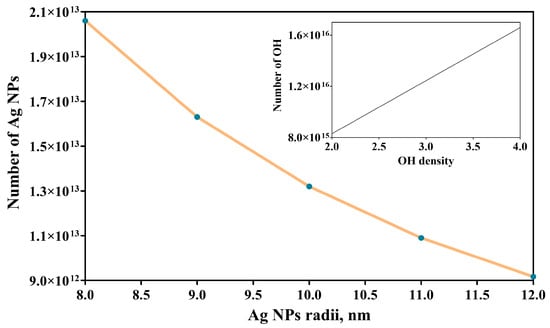
Figure 4.
Variation in the number of Ag NPs with the nanoparticles’ radii and of the number of -OH groups with -OH density (inset image).
3.3. FTIR Microscopy
Adsorption of amino acids (AAs) on silanized surfaces occurs due to the interactions of H bonds that can appear at “-COOH”, and other functional groups of AA (“-OH”, “-SH” etc.), but also with the silanol groups on the surfaces [29]. The following figures display the FTIR maps for silanized and decorated glass platforms with Ag and amino acids, with each figure representing the process and decoration step by step.
Figure 5 presents the maps FTIR for the Ag NP glass-decorated platforms. For the spatial distribution of the samples, the following wavelengths were considered: 2940/2974 cm−1, 2837/2886 cm−1, 2561/2566 cm−1, 1344/1342 cm−1, 1272 cm−1, and 821 cm−1. From the maps above, it can be concluded that the surface is homogeneous (especially based on the very small differences of the absorbance values over the surface, at the specific wavelength), and the mercaptopropyl and Ag NPs are relatively uniformly dispersed on the surface of the glass-decorated platforms [30,31].
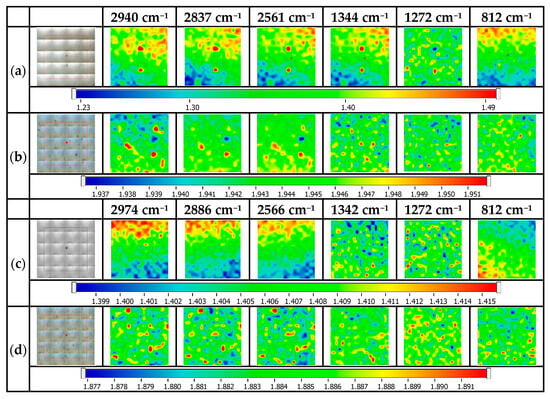
Figure 5.
FTIR maps for Ag NP glass-decorated platforms: (a) MPTMS 1%@glass, (b) Ag NPs@MPTMS 1%@glass, (c) MPTES 1%@glass, (d) Ag NPs@MPTES 1%@glass.
Figure 6 illustrates the maps for cysteine@Ag NP-decorated glass platforms. Therefore, the most representative absorption bands for cysteine are observed at 2984 cm−1 for the C-H functional group, 2550 cm−1 related to the S-H functional group, and N-H at 1586 cm−1, and at 1394 cm−1, the presence of the C=O functional group was observed. The FTIR maps correspond to the cys@Ag NP-decorated glass platforms and registered at ~2837 cm−1, 2565 cm−1, 1586 cm−1, 1344 cm−1, and 812 cm−1 [32]. As a general idea from the maps above, it can be concluded that the surface is quite homogeneous, with the components (mercaptopropyl, Ag NPs, and cysteine) being uniformly distributed on the surface of the glass platforms [30,33]. The best homogeneity was obtained for the cys@Ag NPs@MPTMS 5% sample, which means that the use of 5% MPTMS in the glass surface silanization can assure a homogeneous silanization and, further, induces a homogeneous Ag NPs decoration essential in cysteine capture. In fact, these results correlate well with the experimental data from Figure 2 (but also theoretical simulation data). Moreover, good results are obtained for the samples silanized with 5% MPTES and decorated with Ag NPs.
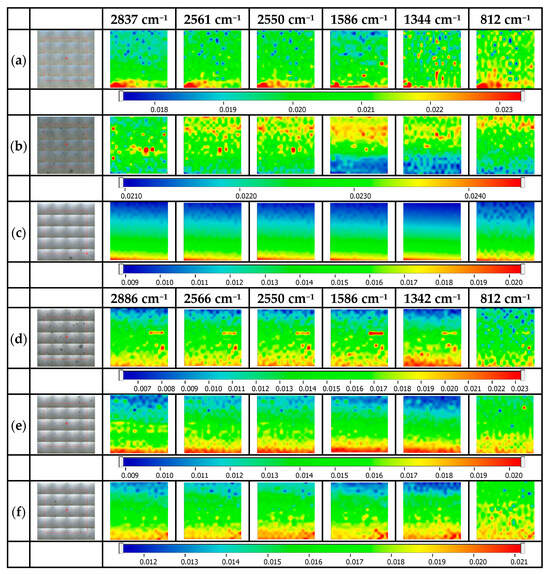
Figure 6.
FTIR maps for cysteine@Ag NP-decorated glass platforms. (a) cys@Ag NPs@MPTMS 1%, (b) cys@Ag NPs@MPTMS 3%, (c) cys@Ag NPs@MPTMS 5%, (d) cys@Ag NPs@MPTES 1%, (e) cys @Ag NPs@MPTES 3%, (f) cys @Ag NPs@MPTES 5%.
Figure 7 illustrates the spatial distribution of the alanine and Ag NPs on the surface of the glass-based platforms. The spectrum of alanine presents some representative absorption bands, such as 2981, 2601, and 1460–1420 cm−1 related to the C-H functional groups, 1620–1527 cm−1 for N-H, and 1354–1390 cm−1, which can be used to evaluate its distribution over the glass surface [34]. Considering the specific bands of glass, mercaptopropyl moiety, and alanine, the following peaks were considered for the surface evaluation: ~2837/2886 cm−1, 2565 cm−1, 1619 cm−1, 1344 cm−1, and 812 cm−1. From the map images, it can be concluded that the alanine distribution on the surface is not as good as for cysteine because alanine is mainly absorbed via some weaker interactions. This behavior is visible at all three MPTMS/ MPTES concentrations (1, 3, or 5%). It is important to mention that even the maps recorded at 812 cm−1 (specific to glass) are different because of the shielding effect of the subsequent layers (aminopropylated layer or alanine) [35].
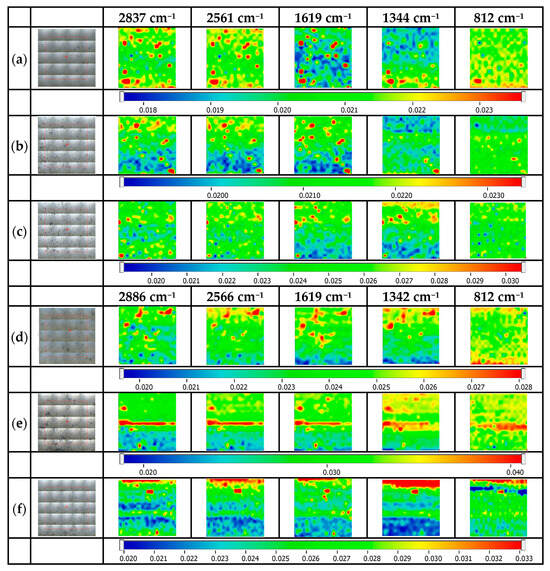
Figure 7.
FTIR maps for alanine@Ag NP-decorated glass platforms. (a) ala@Ag NPs@MPTMS 1%, (b) ala@Ag NPs@MPTMS 3%, (c) ala@Ag NPs@MPTMS 5%, (d) ala@Ag NPs@MPTES 1%, (e) ala@Ag NPs@MPTES 3%, (f) ala@Ag NPs@MPTES 5%.
Figure 8 illustrates the maps for thr@Ag NP-decorated glass platforms. Additionally, in the case of threonine, the discussions are like those for ala@Ag NP-decorated glass platforms but, because of the presence of the additional OH on the structure, some stronger interactions and assembling processes can occur at the surface, and thus, at least in the case of glass surfaces modified with MPTES, the surface homogeneity is better. The specific absorption bands of threonine are 2960–3170 cm−1 for C-H functional groups, 2714–2874 cm−1 for the N-H functional groups, and 1637–1410 cm−1 specific for the C=O functional groups [36]. The FTIR maps were recorded at 2837/2886 cm−1, 2714 cm−1, 2561/2566 cm−1, 1344/1342 cm−1, and 812 cm−1 [36,37].
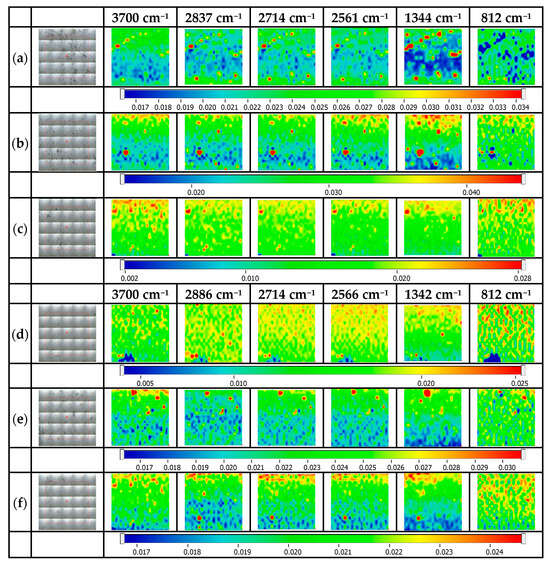
Figure 8.
FTIR maps for thr@Ag NP-decorated glass platforms. (a) thr@Ag NPs@MPTMS 1%, (b) thr@Ag NPs@MPTMS 3%, (c) thr@Ag NPs@MPTMS 5%, (d) thr@Ag NPs@MPTES 1%, (e) thr@Ag NPs@MPTES 3%, (f) thr@Ag NPs@MPTES 5%.
Figure 9 illustrates maps for CAT@Ag NP-decorated glass platforms. The FTIR maps are presented for the specific wavelengths: 3700 cm−1, 2940/2974 cm−1, 2714 cm−1, 2561/2566 cm−1, 2550 cm−1, 1619/1619 cm−1, 1344/1342 cm−1, and 812 cm−1. Considering that cys, ala, and thr are simultaneously present in the mixture, it is expected that cys will be strongly adsorbed onto the Ag NP-decorated glass surfaces, but also the other amino acids can further interact with the as-obtained self-assembled surface. Reasonable surface homogeneity is observed for the CAT@Ag NPs@MPTMS and CAT@Ag NPs@MPTES; the differences that can occur are mostly justified by the assembling process, which appears once the thioderivatives are absorbed onto the silver-decorated glass surface.
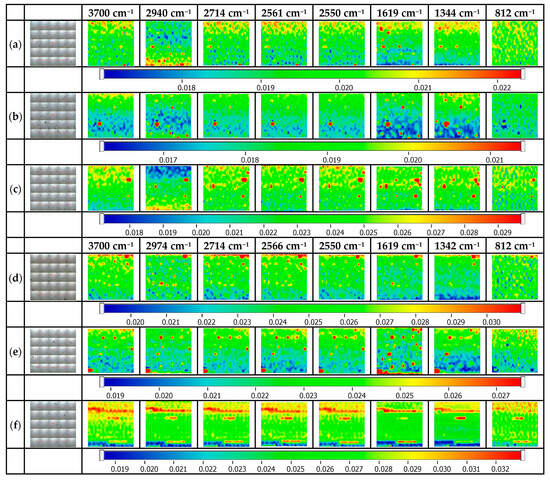
Figure 9.
FTIR maps for CAT@Ag NP-decorated glass platforms. (a) CAT@Ag NPs@MPTMS 1%, (b) CAT @Ag NPs@MPTMS 3%, (c) CAT@Ag NPs@MPTMS 5%, (d) CAT@Ag NPs@MPTES 1%, (e) CAT@Ag NPs@MPTES 3%, (f) CAT@Ag NPs@MPTES 5%.
3.4. Conductivity
Figure 10 illustrates the influence of amino acids on conductivity. In this case, the three amino acids were taken into consideration: cysteine, alanine, and threonine, respectively, with their complex 1:1:1 volumetric mixture (CAT).
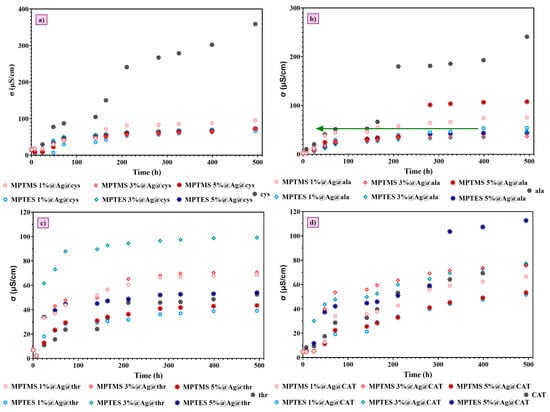
Figure 10.
Evolution of the conductivity in time. Glass-platforms with (a) cysteine; (b) alanine; (c) threonine; (d) CAT.
Figure 10 illustrates the evolution of the conductivity of the amino acids’ solutions over almost 21 days. In all cases, the conductivity of the blank solution (pure amino acid solution) is compared with the amino acid solution, which is in contact with the Ag NP-decorated mercaptopropylated glass surface. As expected, based on the FTIR microscopy data (especially Figure 6), cysteine absorption is the most important, and consequently, the change in conductivity was 250–273 μS/cm. The second most important change in the conductivity was recorded for the alanine, with the conductivity change being 131–202 μS/cm. In the case of threonine and the mixture of the three amino acids, the change in the conductivity can be positive or negative compared to the blank sample, which means that threonine, a polar amino acid with hydroxyl (-OH) and amine (-NH2) functional groups, can interact with glass surfaces through hydrogen bonding and electrostatic interactions, and can induce a different effect, where probably deposition and dissolution processes are concurring [38].
These data show that cysteine is adsorbed more effectively compared to other amino acids onto Ag NP-decorated glass surfaces, opening new perspectives for its selective absorption and quantification even from complex aqueous solutions. Future studies will build upon these preliminary data, and several types of biomolecules containing a -SH group in their structures will be deposited on the surface of silanized glass platforms decorated with Ag NPs, which will also be characterized in terms of their biological properties, stability, and reusability.
4. Conclusions
In conclusion, successful glass surface modification was performed by a layer by layer approach not presented in the literature. In the first step, the glass is modified with mercaptopropyl moieties (by silanization) followed by the decoration with Ag NPs (second step), and preliminary evaluation regarding their adsorption capacity over the mercapto derivatives was conducted using cysteine as a model molecule. It is worth mentioning that according to our mathematical computation results, the decoration with nanoparticles is close to the theoretical value for the modification with MPTMS 5% (80% compared to the theoretical value of 126 μg/m2) and 55%–65% for the other MPTMS-modified surfaces (with 1 and 3%), while in the case of MPTES, the functionalization degree is lower. Based on these results, the adsorption efficiency of these derivatives on the Ag NPs-decorated glass surface was clearly assessed using three independent amino acids: cysteine, alanine, and threonine (alanine and threonine being reference amino acids without SH moiety) as well as their mixture based on the conductometric assays. Further research will be conducted to evaluate the SERS capacity of these Ag NPs-decorated glass surfaces and to extend the study to a wider range of biologically active mercapto derivatives.
Author Contributions
Conceptualization, Z.G., D.F. and A.F.; Methodology, C.-I.I., A.S., L.M. (Ludmila Motelica), L.M. (Liliana Marinescu) and O.-C.O.; Software, Z.G.; Validation, Z.G. and O.-C.O.; Formal analysis, C.-I.I., L.M. (Ludmila Motelica), L.M. (Liliana Marinescu) and D.-R.T.; Investigation, A.S., L.M. (Ludmila Motelica), L.M. (Liliana Marinescu), D.-R.T. and O.-C.O.; Data curation, L.M. (Liliana Marinescu); Writing—original draft, C.-I.I., A.S. and Z.G.; Writing—review & editing, C.-I.I., D.F. and A.F.; Visualization, D.F.; Supervision, A.F.; Project administration, A.F.; Funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
A financial contribution was received from the national project “Functionalization and decoration with nanoparticles of the glass surface. A promising approach to inducing new applications”- PN-III-P1-1.1-TE-2021-1242, Ctr. No. TE95/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to the Romanian Government for providing access to the research infrastructure of the National Center for Micro and Nanomaterials through the National Program titled “Installations and Strategic Objectives of National Interest”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engels, H.-W.; Weidenhaupt, H.-J.; Pieroth, M.; Hofmann, W.; Menting, K.-H.; Mergenhagen, T.; Schmoll, R.; Uhrlandt, S. 4. Chemicals and Additives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; pp. 1–67. [Google Scholar]
- Available online: https://go.drugbank.com/drugs/DB01033 (accessed on 7 December 2023).
- Franchini, C.; Muraglia, M.; Corbo, F.; Florio, M.A.; Di Mola, A.; Rosato, A.; Matucci, R.; Nesi, M.; van Bambeke, F.; Vitali, C. Synthesis and biological evaluation of 2-mercapto-1,3-benzothiazole derivatives with potential antimicrobial activity. Arch. Pharm. 2009, 342, 605–613. [Google Scholar]
- Ishiia, K.; Katayama, M.; Hori, K.; Yodoi, J.; Nakanishi, T. Effects of 2-mercaptoethanol on survival and differentiation of fetal mouse brain neurons cultured in vitro. Neurosci. Lett. 1993, 163, 159–162. [Google Scholar]
- Hock, N.; Racaniello, G.F.; Aspinall, S.; Denora, N.; Khutoryanskiy, V.V.; Bernkop-Schnurch, A. Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body. Adv. Sci. 2022, 9, e2102451. [Google Scholar]
- Ru, E.C.L.; Blackie, E.; Meyer, M.; Etchegoin, P.G. Surface Enhanced Raman Scattering Enhancement Factors: A Comprehensive Study. J. Phys. Chem. C 2007, 111, 13794–13803. [Google Scholar]
- Silvestri, A.; Patzold, J.; Fratzl, P.; Scheffel, A.; Faivre, D. Surface-Enhanced Raman Scattering Microspectroscopy Enables the Direct Characterization of Biomineral-Associated Organic Material on Single Calcareous Microskeletons. J. Phys. Chem. Lett. 2020, 11, 8623–8629. [Google Scholar]
- Kanada, S.; Ono, K.; Fukuba, T.; Yamamoto, T.; Fujii, T. Modification of the Glass Surface Property in PDMS-Glass Hybrid Microfluidic Devices. Anal. Sci. 2012, 28, 39–44. [Google Scholar]
- Tsai, M.-Y.; Hsu, C.-C.; Chen, P.-H.; Lin, C.-S.; Chen, A. Surface modification on a glass surface with a combination technique of sol–gel and air brushing processes. Appl. Surf. Sci. 2011, 257, 8640–8646. [Google Scholar]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.K. From protein engineering to immobilization: Promising strategies for the upgrade of industrial enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef]
- Englade-Franklin, L.E.; Saner, C.K.; Garno, J.C. Spatially selective surface platforms for binding fibrinogen prepared by particle lithography with organosilanes. Interface Focus 2013, 3, 20120102. [Google Scholar]
- Highland, Z.L.; Garno, J.C. Spatially selective binding of green fluorescent protein on designed organosilane nanopatterns prepared with particle lithography. Biointerphases 2017, 12, 02C402. [Google Scholar]
- Vistas, C.R.; Águas, A.C.P.; Ferreira, G.N.M. Silanization of glass chips—A factorial approach for optimization. Appl. Surf. Sci. 2013, 286, 314–318. [Google Scholar] [CrossRef]
- Shakeri, A.; Jarad, N.A.; Leung, A.; Soleymani, L.; Didar, T.F. Biofunctionalization of Glass- and Paper-Based Microfluidic Devices: A Review. Adv. Mater. Interfaces 2019, 6, 1900940. [Google Scholar]
- Petkova, G.A.; Záruba, K.; Žvátora, P.; Král, V. Gold and silver nanoparticles for biomolecule immobilization and enzymatic catalysis. Nanoscale Res. Lett. 2012, 7, 287. [Google Scholar] [PubMed]
- Upadhyay, L.S.B.; Verma, N. Dual immobilization of biomolecule on the glass surface using cysteine as a bifunctional linker. Process Biochem. 2014, 49, 1139–1143. [Google Scholar] [CrossRef]
- Halliwell, C.M.; Cass, A.E.G. A Factorial Analysis of Silanization Conditions for the Immobilization of Oligonucleotides on Glass Surfaces. Anal. Chem. 2001, 73, 2476–2483. [Google Scholar]
- Marinescu, L.; Ficai, D.; Ficai, A.; Oprea, O.; Nicoara, A.I.; Vasile, B.S.; Boanta, L.; Marin, A.; Andronescu, E.; Holban, A.M. Comparative Antimicrobial Activity of Silver Nanoparticles Obtained by Wet Chemical Reduction and Solvothermal Methods. Int. J. Mol. Sci. 2022, 23, 5982. [Google Scholar] [CrossRef]
- Gheorghe-Barbu, I.; Corbu, V.M.; Vrancianu, C.O.; Marinas, I.C.; Popa, M.; Dumbrava, A.S.; Nita-Lazar, M.; Pecete, I.; Muntean, A.A.; Popa, M.I.; et al. Phenotypic and Genotypic Characterization of Recently Isolated Multidrug-Resistant Acinetobacter baumannii Clinical and Aquatic Strains and Demonstration of Silver Nanoparticle Potency. Microorganisms 2023, 11, 2439. [Google Scholar] [CrossRef]
- Pallavicini, P.; Dacarro, G.; Galli, M.; Patrini, M. Spectroscopic evaluation of surface functionalization efficiency in the preparation of mercaptopropyltrimethoxysilane self-assembled monolayers on glass. J. Colloid Interface Sci. 2009, 332, 432–438. [Google Scholar]
- Ilie, C.-I.; Spoială, A.; Motelica, L.; Marinescu, L.; Dolete, G.; Trușcă, D.-R.; Oprea, O.-C.; Ficai, D.; Ficai, A. Decoration of a Glass Surface with AgNPs Using Thio-Derivates for Environmental Applications. Coatings 2024, 14, 96. [Google Scholar] [CrossRef]
- Scientific, T. Thermo Scientific Evolution 300 UV-Vis Spectrophotometer; Thermo Electron Scientific Instruments LLC: Madison, WI, USA, 2013; pp. 1–16. [Google Scholar]
- WTW. inoLab® Multi 9630 IDS; WTW GmbH: London, UK, 2014; pp. 1–8. [Google Scholar]
- Chen, W.-S.; Chen, H.-R.; Lee, C.-H. The Photocatalytic Performance of Ag-Decorated SiO2 Nanoparticles (NPs) and Binding Ability Between Ag NPs and Modifiers. Coatings 2022, 12, 146. [Google Scholar] [CrossRef]
- Gao, C.; Guo, T.; Ye, X.S.; Zhang, H.F.; Liu, H.N.; Wu, Z.J. Adsorption of Silver Nanoparticles on Modified Surfaces. Key Eng. Mater. 2015, 645–646, 75–79. [Google Scholar]
- Polowczyk, I.; Koźlecki, T.; Bastrzyk, A. Adsorption of Silver Nanoparticles on Glass Beads Surface. J Adsorpt. Sci. Technol. 2015, 33, 731–738. [Google Scholar]
- Wang, Y.; Tang, L. Chemisorption assembly of Au nanorods on mercaptosilanized glass substrate for label-free nanoplasmon biochip. Anal. Chim. Acta 2013, 796, 122–129. [Google Scholar] [PubMed]
- Liu, X.M.; Thomason, J.L.; Jones, F.R. The Concentration of Hydroxyl Groups on Glass Surfaces and Their Effect on the Structure of Silane Deposits. In Silanes Other Coupling Agents Volume 5; CRC Press: Boca Raton, FL, USA, 2009; pp. 25–38. [Google Scholar]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Affinity Scale for the Interaction of Amino Acids with Silica Surfaces. J. Phys. Chem. C 2009, 113, 5741–5750. [Google Scholar]
- Spoiala, A.; Ilie, C.I.; Dolete, G.; Petrisor, G.; Trusca, R.D.; Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Ditu, M.L. The Development of Alginate/Ag NPs/Caffeic Acid Composite Membranes as Adsorbents for Water Purification. Membranes 2023, 13, 591. [Google Scholar] [CrossRef]
- Gothe, P.K.; Gaur, D.; Achanta, V.G. MPTMS self-assembled monolayer deposition for ultra-thin gold films for plasmonics. J. Phys. Commun. 2018, 2, 035005. [Google Scholar]
- Shen, L.M.; Chen, Q.; Sun, Z.Y.; Chen, X.W.; Wang, J.H. Assay of biothiols by regulating the growth of silver nanoparticles with C-dots as reducing agent. Anal. Chem. 2014, 86, 5002–5008. [Google Scholar]
- Kogelheide, F.; Kartaschew, K.; Strack, M.; Baldus, S.; Metzler-Nolte, N.; Havenith, M.; Awakowicz, P.; Stapelmann, K.; Lackmann, J.-W. FTIR spectroscopy of cysteine as a ready-to-use method for the investigation of plasma-induced chemical modifications of macromolecules. J. Phys. D Appl. Phys. 2016, 49, 084004. [Google Scholar]
- Bowles, J.; Jähnigen, S.; Vuilleumier, R.; Calvo, F.; Clavaguéra, C.; Agostini, F. Influence of the environment on the infrared spectrum of alanine: An effective mode analysis. J. Chem. Phys. 2023, 158, 094305. [Google Scholar]
- Liu, J.; Zhou, F.-S.; Guo, R.; Jiang, Y.; Fan, X.; He, A.; Zhai, Y.; Weng, S.; Yang, Z.; Xu, Y.; et al. Analysis of an Alanine/Arginine Mixture by Using TLC/FTIR Technique. J. Spectrosc. 2014, 2014, 925705. [Google Scholar]
- Gaillard, T.; Trivella, A.; Stote, R.H.; Hellwig, P. Far infrared spectra of solid state L-serine, L-threonine, L-cysteine, and L-methionine in different protonation states. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 301–307. [Google Scholar] [PubMed]
- Ramesh Kumar, G.; Gokul Raj, S. Growth and PhysioChemical Properties of Second-Order Nonlinear Optical L-Threonine Single Crystals. Adv. Mater. Sci. Eng. 2009, 2009, 704294. [Google Scholar]
- Razvag, Y.; Gutkin, V.; Reches, M. Probing the interaction of individual amino acids with inorganic surfaces using atomic force spectroscopy. Langmuir 2013, 29, 10102–10109. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).