Sonochemical Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride for the Development of Films with PLA for the Adsorption of Hexavalent Chromium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride by Sonochemistry
2.3. Preparation of Films PLA/ZrO2
2.4. Characterization
2.5. Adsorption of Hexavalent Chromium (Cr VI)
3. Results and Discussions
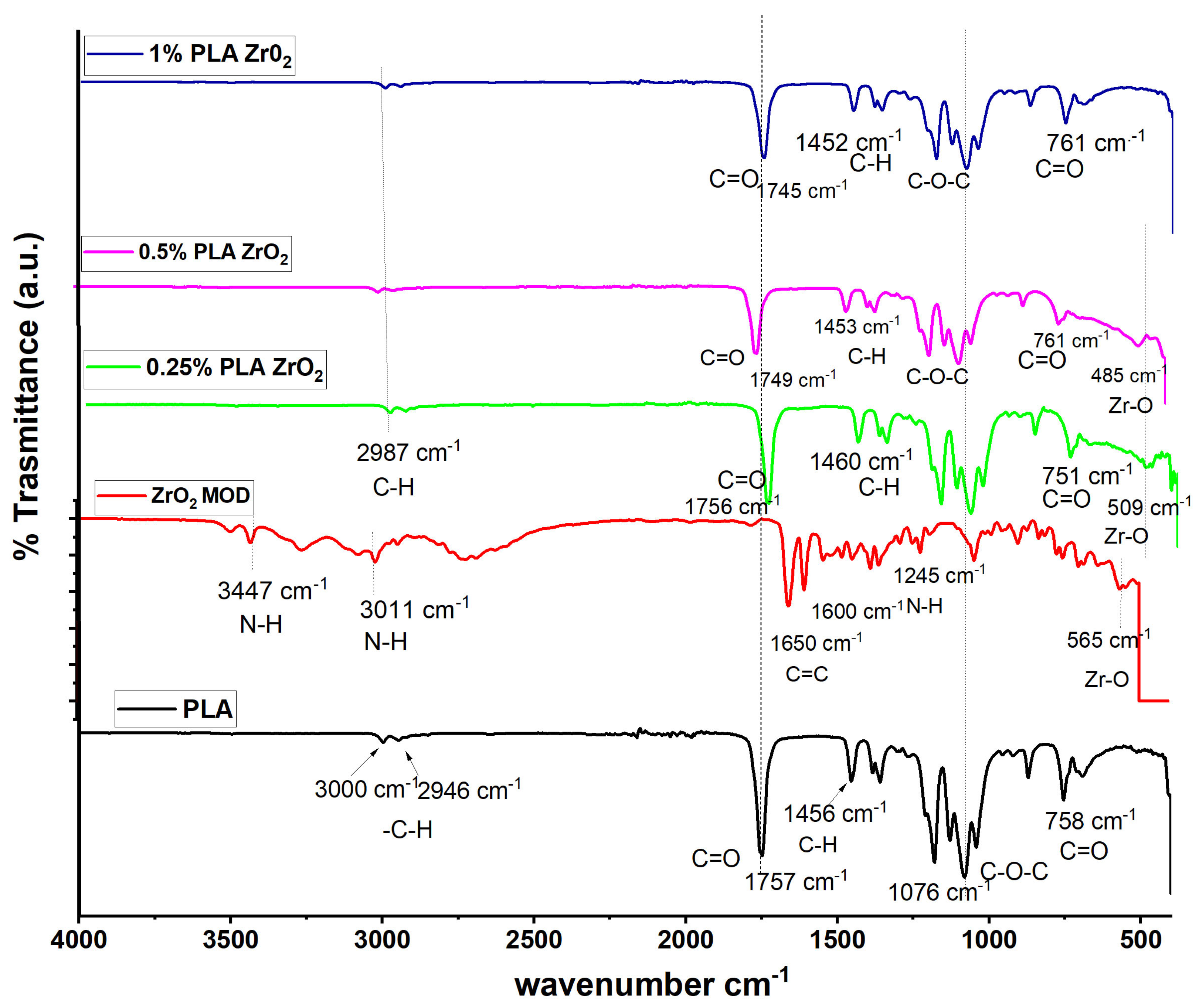
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2. X-Ray Diffraction (XRD)
3.3. Thermogravimetric Analysis (TGA)
3.4. Scanning Electron Microscopy (SEM)
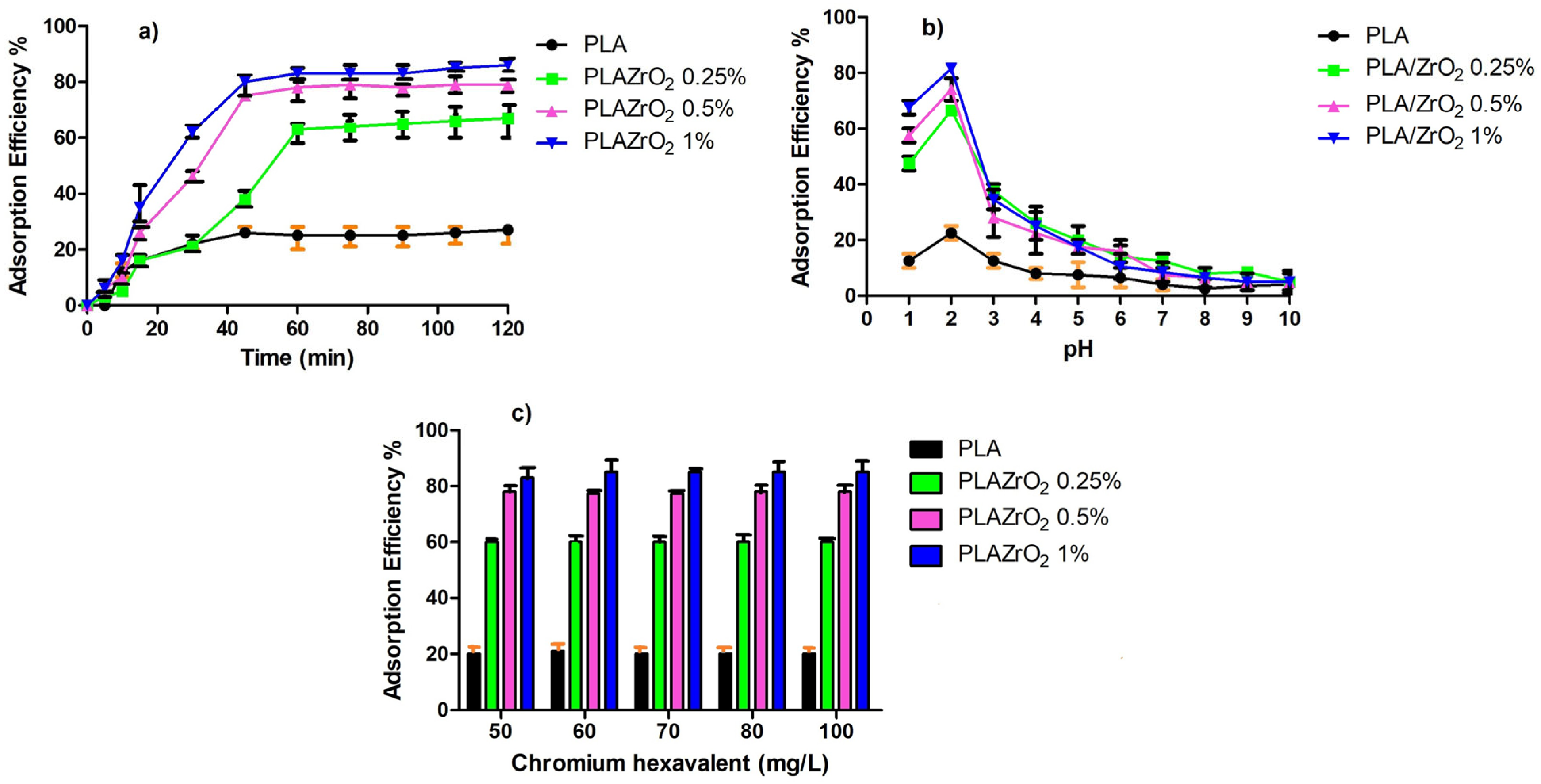
3.5. Adsorption of Hexavalent Chromium Cr VI onto Films
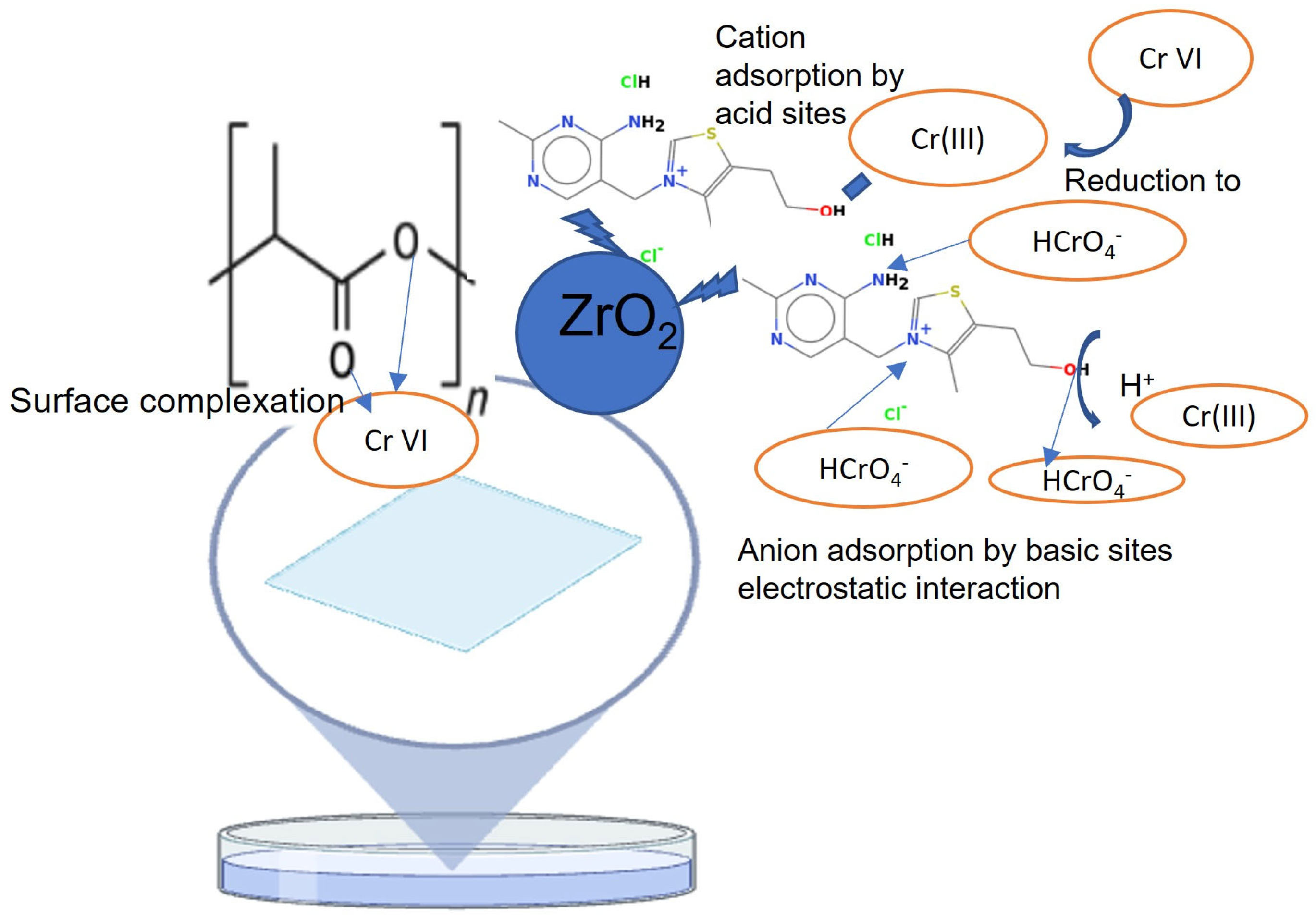
3.6. Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Gore, C.T.; Omwoma, S.; Chen, W.; Song, Y.F. Interweaved LDH/PAN nanocomposite films: Application in the design of effective hexavalent chromium adsorption technology. Chem. Eng. J. 2016, 284, 794–801. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, Y.; Hu, G.; Hong, S.; Su, Z.; Zhang, Q.; Jia, G. Hexavalent chromium and cellular senescence: A comprehensive analysis from chromate-exposed occupational population and chromate-inhaled mouse model. J. Hazard. Mater. 2025, 493, 138387. [Google Scholar] [CrossRef] [PubMed]
- Gopal Reddi, M.R.; Gomathi, T.; Saranya, M.; Sudha, P.N. Adsorption and kinetic studies on the removal of chromium and copper onto chitosan-g-maleic anhydride-g-ethylene dimethacrylate. Int. J. Biol. Macromol. 2017, 104, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zhang, J.; Zuo, W.; Ding, Y. Efficient removal of chromium from water by Mn3O4@ZnO/Mn3O4 composite under simulated sunlight irradiation: Synergy of photocatalytic reduction and adsorption. Appl. Catal. B 2017, 214, 126–136. [Google Scholar] [CrossRef]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Hu, B.; Luo, H. Adsorption of hexavalent chromium onto montmorillonite modified with hydroxyaluminum and cetyltrimethylammonium bromide. Appl. Surf. Sci. 2010, 257, 769–775. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Srinives, S.; Mohapatra, B.C.; Boddu, V.M.; Hao, J.; Meng, X.; Mulchandani, A. Hexavalent chromium removal mechanism using conducting polymers. J. Hazard. Mater. 2013, 252, 99–106. [Google Scholar] [CrossRef]
- Yuan, X.; Li, J.; Luo, L.; Zhong, Z.; Xie, X. Advances in sorptive removal of hexavalent chromium (Cr (VI)) in aqueous solutions using polymeric materials. Polymers 2023, 15, 388. [Google Scholar] [CrossRef]
- González-López, M.E.; Laureano-Anzaldo, C.M.; Pérez-Fonseca, A.A.; Arellano, M.; Robledo-Ortíz, J.R. Chemically modified polysaccharides for hexavalent chromium adsorption. Sep. Purif. Rev. 2021, 50, 333–362. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, C.; Sun, D.; Wang, Q.; Gu, H.; Zhang, X.; Wei, S. Polyaniline coating with various substrates for hexavalent chromium removal. Appl. Surf. Sci. 2015, 334, 7–14. [Google Scholar] [CrossRef]
- Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A.; Oyedotun, K.O.; Ighaloi, J.O. Multiwalled carbon nanotubes versus metal-organic frameworks: A review of their hexavalent chromium adsorption performance. Int. J. Environ. Anal. Chem. 2024, 104, 6027–6049. [Google Scholar] [CrossRef]
- Naimi-Joubani, M.; Shirzad-Siboni, M.; Yang, J.K.; Gholami, M.; Farzadkia, M. Photocatalytic reduction of hexavalent chromium with illuminated ZnO/TiO2 composite. J. Ind. Eng. Chem. 2015, 22, 317–323. [Google Scholar] [CrossRef]
- Mikhaylov, V.I.; Krivoshapkina, E.F.; Trigub, A.L.; Stalugin, V.V.; Krivoshapkin, P.V. Detection and adsorption of Cr (VI) ions by mesoporous Fe–Alumina films. ACS Sustain. Chem. Eng. 2018, 6, 9283–9292. [Google Scholar] [CrossRef]
- Sanchéz-Huerta, R.; Andrade-Guel, M.; Cabello-Alvarado, C.; Ávila-Orta, C.; Cadenas-Pliego, G.; Bartolo-Pérez, P.; Nery-Flores, S. Incorporation of thiamine hydrochloride in carbon black nanoparticles to improve the adsorption of uremic toxins and their hemolytic behavior. J. Indian Chem. Soc. 2025, 102, 102213. [Google Scholar] [CrossRef]
- Kandel, D.R.; Poudel, M.B.; Radoor, S.; Chang, S.; Lee, J. Decoration of dandelion-like manganese-doped iron oxide microflowers on plasma-treated biochar for alleviation of heavy metal pollution in water. Chemosphere 2024, 357, 141757. [Google Scholar] [CrossRef]
- Chang, S.; Kandel, D.R.; Lee, U.; Tran, H.M.; Lee, J. Improving separation efficiency of various micropollutants from water by polymer-enhanced ultrafiltration using oxidized alginate featuring less viscous and lower filtration resistance. J. Environ. Chem. Eng. 2024, 12, 114753. [Google Scholar] [CrossRef]
- Andrade-Guel, M.; Cabello-Alvarado, C.; Romero-Huitzil, R.L.; Rodríguez-Fernández, O.S.; Ávila-Orta, C.A.; Cadenas-Pliego, G.; Medellín-Banda, D.I.; Gallardo-Vega, C.; Cepeda-Garza, J. Nanocomposite PLA/C20A nanoclay by ultrasound-assisted melt extrusion for adsorption of uremic toxins and methylene blue dye. Nanomaterials 2021, 11, 2477. [Google Scholar] [CrossRef]
- Cabello-Alvarado, C.J.; Andrade-Guel, M.L.; Medellín-Banda, D.I.; Melo-Lopez, L.; Avila-Orta, C.A. Polymer Composites: Smart Synthetic Fibers Approach in Energy and Environmental Care. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Bao, C.; Chen, M.; Jin, X.; Hu, D.; Huang, Q. Efficient and stable photocatalytic reduction of aqueous hexavalent chromium ions by polyaniline surface-hybridized ZnO nanosheets. J. Mol. Liq. 2019, 279, 133–145. [Google Scholar] [CrossRef]
- Kumar, R.; Kim, S.J.; Kim, K.H.; Lee, S.H.; Park, H.S.; Jeon, B.H. Removal of hazardous hexavalent chromium from aqueous phase using zirconium oxide-immobilized alginate beads. Appl. Geochem. 2018, 88, 113–121. [Google Scholar] [CrossRef]
- Gilcreas, F.W. Standard methods for the examination of water and waste water. Am. J. Public Health Nations Health 1966, 56, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, N.; Kumar, S.A.; Keerthana, P.; Stanley, S.; Kumar, A. Schiff’s base (SB) modified zirconium dioxide reinforced PLA bio-composite film for industrial packaging applications. Comp. Commun. 2021, 25, 100750. [Google Scholar]
- Andrade-Guel, M.; Cabello-Alvarado, C.J.; Cadenas-Pliego, G.; Ávila-Orta, C.A. PLA-ZnO/TiO2 nanocomposite obtained by ultrasound-assisted melt-extrusion for adsorption of methylene blue. Nanomaterials 2022, 12, 4248. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of polylactic acid films with selenium microparticles and its application for food packaging. Coatings 2020, 10, 280. [Google Scholar] [CrossRef]
- De Azevedo Gonç, R.C.; Da Silva, E.O. Effect of the addiction of metal oxide nanoparticles on the physical, chemical and thermal properties of PVA based nanocomposites. Mater. Sci. Appl. 2018, 9, 473–488. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R. Effect of PLA grades and morphologies on hydrolytic degradation at composting temperature: Assessment of structural modification and kinetic parameters. Polym. Degrad. Stab. 2013, 98, 1006–1014. [Google Scholar] [CrossRef]
- Siriprom, W.; Sangwaranatee, N.; Chantarasunthon, K.; Teanchai, K.; Chamchoi, N. Characterization and analyzation of the poly (L-lactic acid)(PLA) films. Mater. Today Proc. 2018, 5, 14803–14806. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Quintard, G.; Massardier-Nageotte, V. Biobased additive plasticizing Polylactic acid (PLA). Polimeros 2015, 25, 581–590. [Google Scholar] [CrossRef]
- Guel, M.L.A.; Jiménez, L.D.; Hernández, D.A.C. Ultrasound-assisted sol-gel synthesis of ZrO2. Ultrason. Sonochem. 2017, 35, 514–517. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Valapa, R.B.; Nayak, S.K.; Varghese, T.O. Investigation on the influence of EVA content on the mechanical and thermal characteristics of poly (lactic acid) blends. J. Polym. Environ. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Topuz, M.; Dikici, B.; Kasapoglu, A.E.; Zhao, X.; Niinomi, M. Systematic characterization and enhanced corrosion resistance of novel β-type Ti-30Zr-5Mo biomedical alloys with halloysite nanotubes (HNTs) and zirconia (ZrO2)-reinforced polylactic acid (PLA) matrix coatings. Mater. Today Commun. 2024, 40, 110110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, D.; Wang, J.; Ding, Y.; Wang, Y.; Feng, L.; Hu, Y. Aging properties of polyethylene and polylactic acid microplastics and their adsorption behavior of Cd (II) and Cr (VI) in aquatic environments. Chemosphere 2024, 363, 142833. [Google Scholar] [CrossRef]
- Nameni, M.; Alavi Moghadam, M.R.; Arami, M. Adsorption of hexavalent chromium from aqueous solutions by wheat bran. Int. J. Environ. Sci. Technol. 2008, 5, 161–168. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñiz-Valdez, C.R.; Rodríguez-Rosales, N.A.; Ortiz-Cuellar, J.C.; Martínez-Villafañe, J.F.; Gómez-Casas, J.; Cadenas-Pliego, G.; Cabello-Alvarado, C.J.; Andrade-Guel, M.; Galindo-Valdés, J.S. Sonochemical Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride for the Development of Films with PLA for the Adsorption of Hexavalent Chromium. Coatings 2025, 15, 1484. https://doi.org/10.3390/coatings15121484
Muñiz-Valdez CR, Rodríguez-Rosales NA, Ortiz-Cuellar JC, Martínez-Villafañe JF, Gómez-Casas J, Cadenas-Pliego G, Cabello-Alvarado CJ, Andrade-Guel M, Galindo-Valdés JS. Sonochemical Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride for the Development of Films with PLA for the Adsorption of Hexavalent Chromium. Coatings. 2025; 15(12):1484. https://doi.org/10.3390/coatings15121484
Chicago/Turabian StyleMuñiz-Valdez, Carlos Rodrigo, Nelly Abigaíl Rodríguez-Rosales, Juan Carlos Ortiz-Cuellar, Jesús Fernando Martínez-Villafañe, Josué Gómez-Casas, Gregorio Cadenas-Pliego, Christian Javier Cabello-Alvarado, Marlene Andrade-Guel, and Jesús Salvador Galindo-Valdés. 2025. "Sonochemical Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride for the Development of Films with PLA for the Adsorption of Hexavalent Chromium" Coatings 15, no. 12: 1484. https://doi.org/10.3390/coatings15121484
APA StyleMuñiz-Valdez, C. R., Rodríguez-Rosales, N. A., Ortiz-Cuellar, J. C., Martínez-Villafañe, J. F., Gómez-Casas, J., Cadenas-Pliego, G., Cabello-Alvarado, C. J., Andrade-Guel, M., & Galindo-Valdés, J. S. (2025). Sonochemical Modification of ZrO2 Nanoparticles with Thiamine Hydrochloride for the Development of Films with PLA for the Adsorption of Hexavalent Chromium. Coatings, 15(12), 1484. https://doi.org/10.3390/coatings15121484









