Performance Evolution of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Thermal Barrier Coatings After Thermal Cycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder and Coating Deposition
2.2. Preparation of GYYZO Thermal Barrier Coatings
2.3. Thermal Cycling of TBCs
2.4. Performance Testing and Microstructure Observation
3. Results
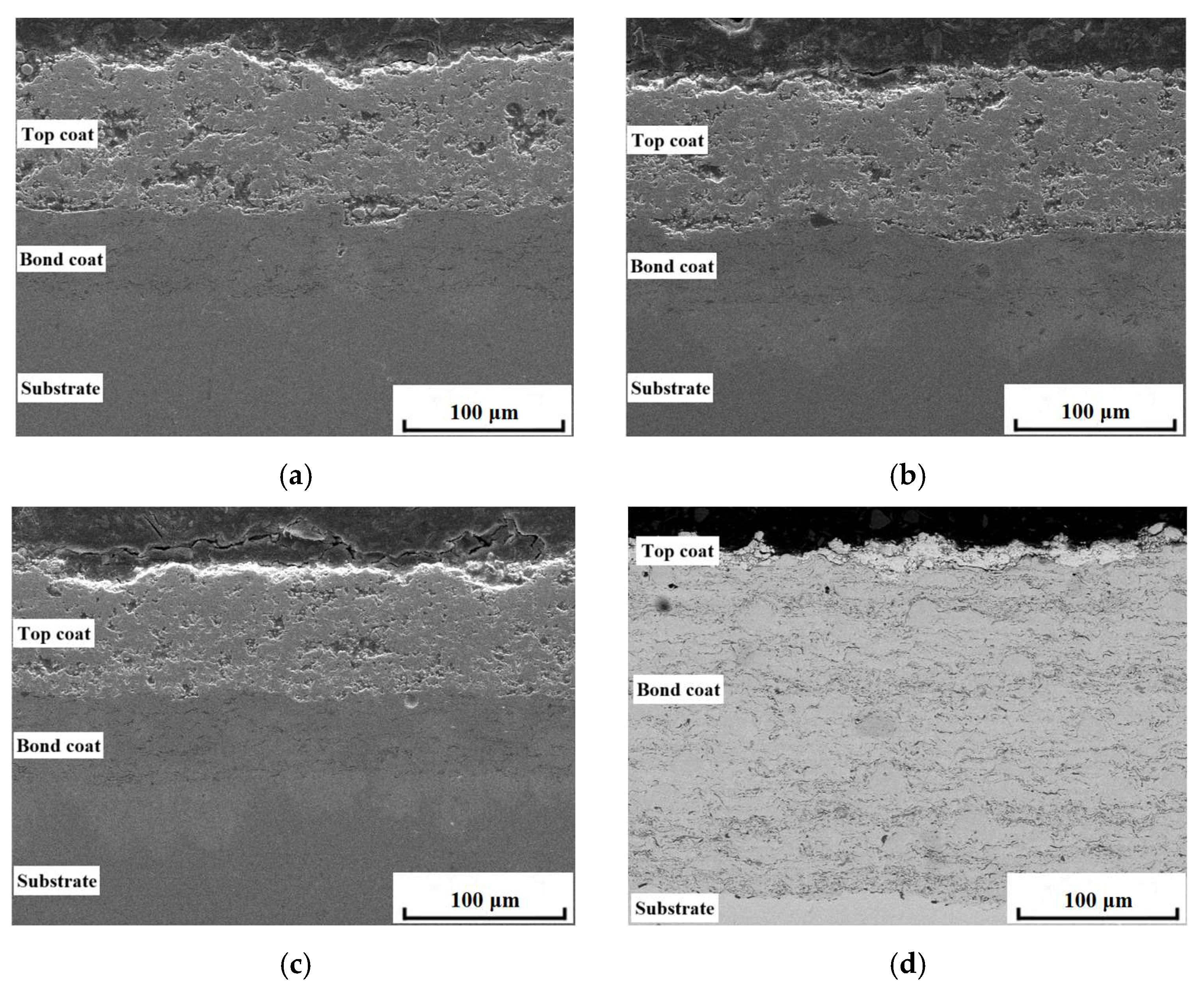
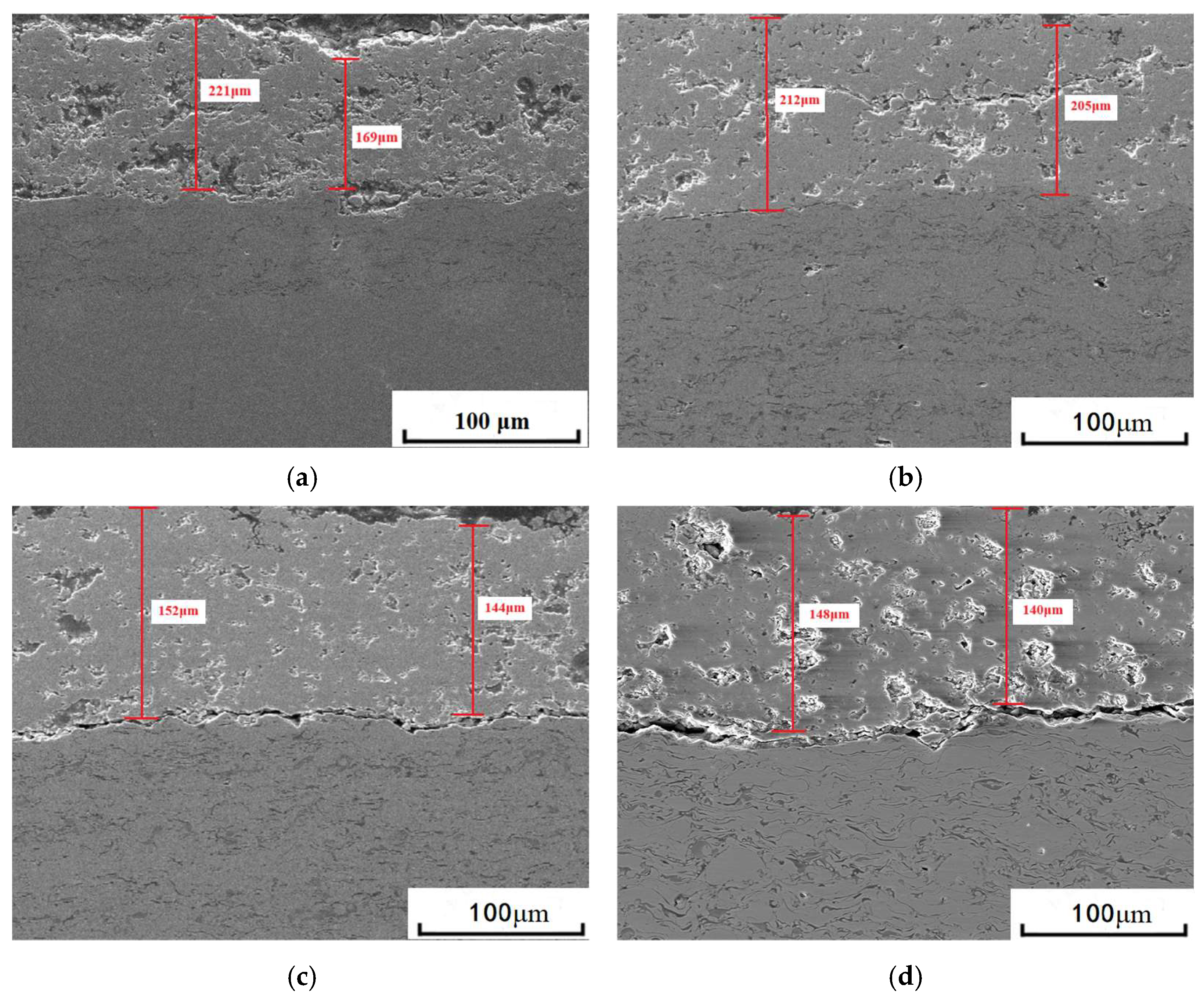
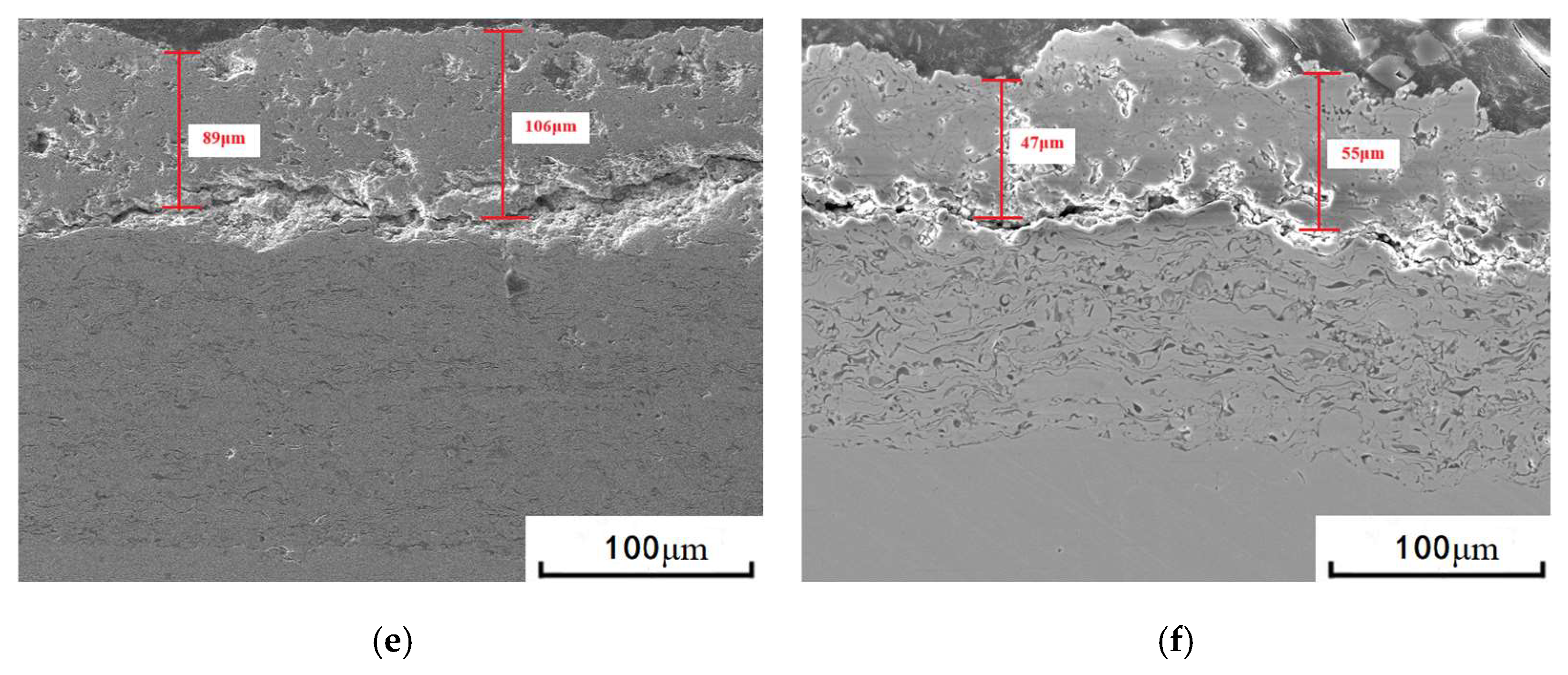
3.1. Microstructure of Coatings Before and After Thermal Cycling
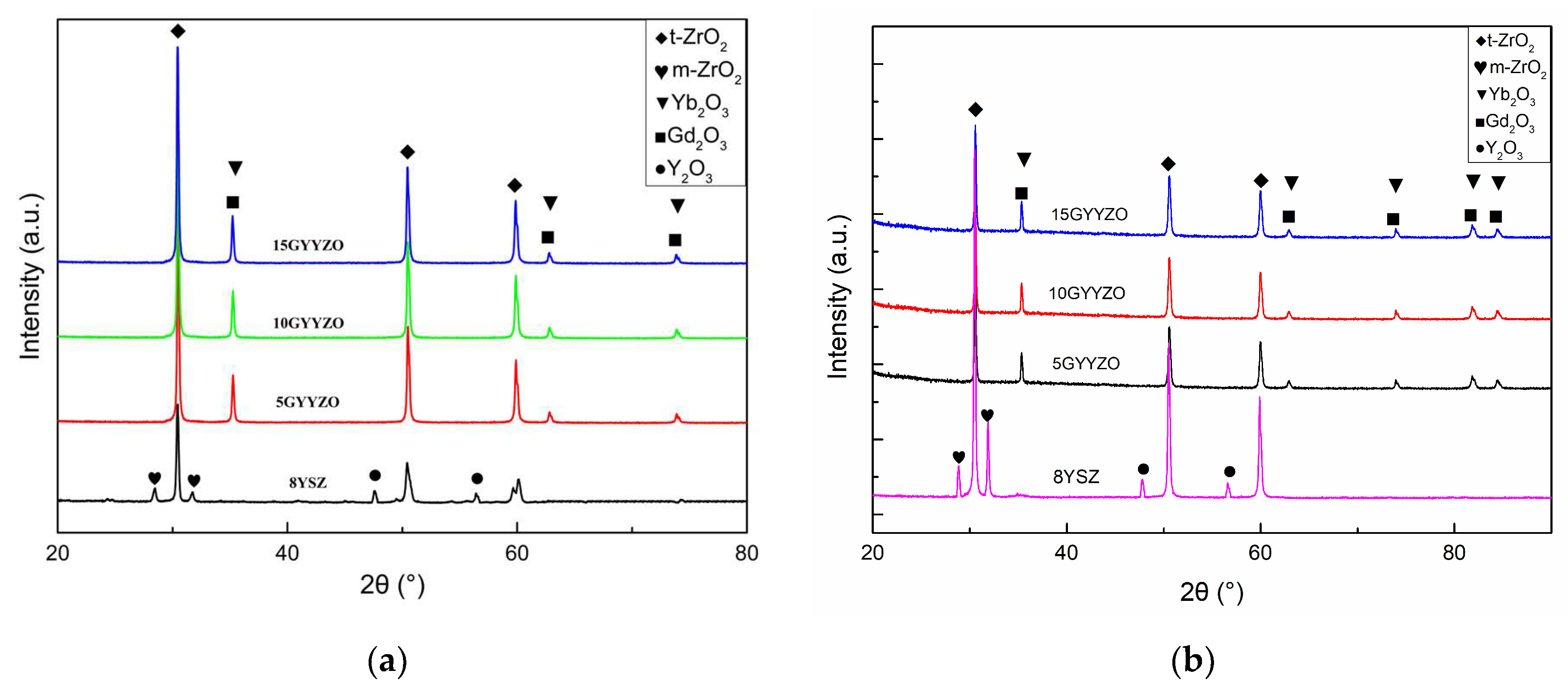
3.2. Phase Analysis
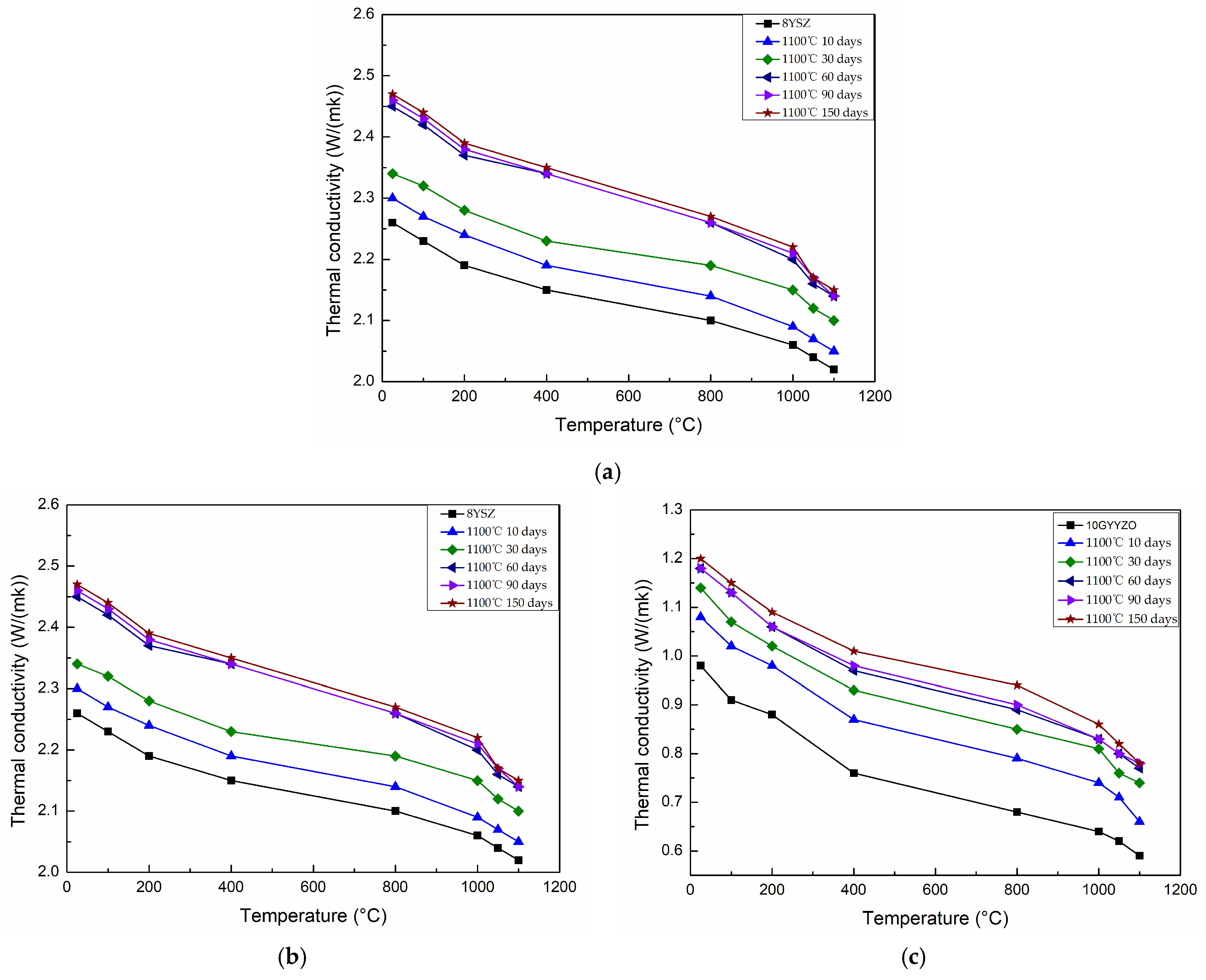
3.3. Evolution Mechanism of Thermal Conductivity Before and After Thermal Cycling
4. Discussion
4.1. Mechanism of ZrO2 Stabilization by Gd2O3, Yb2O3, and Y2O3
4.2. Microstructure and Material Properties
5. Conclusions
- (1)
- Co-doping Gd2O3, Yb2O3, and Y2O3 enables ZrO2 to form a stable pure t-phase. By substituting Zr4+ and generating oxygen vacancy defects/complex aggregates, this system completely suppresses t → m transformation, outperforming single Y3+ doping and dual Yb-Y doping in phase stability.
- (2)
- 10GYYZO balances porosity (15.36%) and defect density, achieving 0.59 W/(m·K) thermal conductivity and 150-cycle life. Its thermal cycling life is 2.5 times that of 8YSZ. This “composition–microstructure–performance” tuning strategy advances beyond structural optimization approaches.
- (3)
- 10GYYZO coatings exhibit superior insulation, long life, and APS compatibility, making them a promising 8YSZ replacement. Avoiding excessive Gd2O3 and verifying CMAS resistance will accelerate industrial adoption.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, D.R.; Oechsner, M.; Padture, N.P. Thermal-barrier coatings for more efficient gas-turbine engines. MRS Bull. 2012, 37, 891–898. [Google Scholar] [CrossRef]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Clarke, D.R.; Levi, C.G. Materials Design for the Next Generation Thermal Barrier Coatings. Annu. Rev. Mater. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Rakoczy, Ł.; Grudzień-Rakoczy, M.; Cygan, R.; Kargul, T.; Maj, Ł.; Zielińska-Lipiec, A. Analysis of the As-Cast Microstructure and Properties of the Ni-Based Superalloy MAR-M247 Produced Via Directional Solidification. Metall. Mater. Trans. A 2023, 54, 3630–3652. [Google Scholar] [CrossRef]
- Arunkumar, T.; Navneet, S.; Premnath, S.; Alan, S.; Ajith, J. A study of mechanical and corrosion behaviour of 8YSZ coating on AZ91D alloy. Mater. Today Proc. 2021, 47, 4322–4325. [Google Scholar]
- Pia, G.; Casnedi, L.; Sanna, U. Porosity and pore size distribution influence on thermal conductivity of yttria-stabilized zirconia: Experimental findings and model predictions. Ceram. Int. 2016, 42, 5802–5809. [Google Scholar] [CrossRef]
- Sharma, A.; Witz, G.; Howell, P.C.; Hitchman, N. Interplay of the phase and the chemical composition of the powder feedstock on the properties of porous 8YSZ thermal barrier coatings. J. Eur. Ceram. Soc. 2021, 41, 3706–3716. [Google Scholar] [CrossRef]
- Gao, P.H.; Jia, H.; Wang, W.; Chen, B.Y.; Guo, Y.C.; Yang, Z.; Wang, J.D. Research progress in characterization of pore structure of thermal spray coatings. Rare. Metal. Mat. Eng. 2019, 48, 3055–3070. [Google Scholar]
- Li, X.; Deng, C.M.; Niu, S.P.; Wang, C.; Sun, Y.N.; Su, W.M.; Liu, M.; Deng, Z.Q.; Zhang, X.F. Effect of calcination temperature on the microstructure, composition and properties of nanometer agglomerated 8YSZ powders for plasma spray-physical vapor deposition (PS-PVD) and coatings thereof. Ceram. Int. 2021, 47, 16632–16640. [Google Scholar] [CrossRef]
- Liu, K.; Cao, Y.; Lv, D.; Du, Y.; Ye, G.; Wang, Y.; Fan, W.; Zhang, L.; Cao, Y. Study on multimodal crack growth of FG-TBCs and its influence on interface cracking. J. Mater. Res. Technol. 2025, 36, 8920–8934. [Google Scholar] [CrossRef]
- Jiang, C.; Hao, W.; Liu, C.; Shi, D.; Song, W. Thermal cycling performance of GYbZ/YSZ thermal barrier coatings with different microstructures based on finite element simulation. J. Alloys Compd. 2025, 1010, 177185. [Google Scholar] [CrossRef]
- Gao, P.-H.; Yang, G.-J.; Cao, S.-T.; Li, J.-P.; Yang, Z.; Guo, Y.-C. Heredity and variation of hollow structure from powders to coatings through atmospheric plasma spraying. Surf. Coat. Technol. 2016, 305, 76–82. [Google Scholar] [CrossRef]
- Gao, P.H.; Zeng, S.C.; Jin, C.; Zhang, B.; Chen, B.Y.; Yang, Z.; Guo, Y.C.; Liang, M.X.; Li, J.P.; Li, Q.P.; et al. Thermal conductivity of multi-sized porous thermal barrier coatings at micro and nano scales after long-term service at high temperature. Coatings 2021, 11, 1183. [Google Scholar] [CrossRef]
- Huo, K.; Xu, C.; Huang, Z.; Xia, J.; Zhang, L.; Zhang, X.; Li, T. Effects of Oxygen Gas Flow During Deposition on the Thermal Shock Life of YSZ Thermal Barrier Coatings Prepared by Electron Beam Physical Vapor Deposition. Coatings 2025, 15, 928. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; Wang, Q.; Ma, Z.; Liu, L. Oxidation behavior of rare earth doped zirconia thermal barrier coating at 1100 °C. Ceram. Int. 2025, 51, 18723–18730. [Google Scholar] [CrossRef]
- Gan, M.; Lai, L.; Chong, X.; Jin, Q.; Yu, W.; Wang, J.; Wang, J.; Chen, L.; He, J.; Feng, J. Enhancing high-temperature phase stability and thermal properties of 5RE(TaxNb1-x)O4 high-entropy ceramics through chemical bonding strength optimization. Chem. Eng. J. 2025, 519, 164894. [Google Scholar] [CrossRef]
- Chen, W.R.; Li, C.; Cheng, Y.; Li, H.; Zhang, X.; Wang, L. Degradation of HVOF-MCrAlY + APS-Nanostructured YSZ Thermal Barrier Coatings. Coatings 2025, 15, 871. [Google Scholar] [CrossRef]
- Kozlovskiy, A.L.; Konuhova, M.; Borgekov, D.B.; Anatoli, I.P. Study of irradiation temperature effect on radiation-induced polymorphic transformation mechanisms in ZrO2ceramics. Opt. Mater. 2024, 156, 115994. [Google Scholar] [CrossRef]
- Gao, P.-H.; Jin, C.; Zeng, S.-C.; Xie, R.-G.; Zhang, B.; Chen, B.-Y.; Yang, Z.; Guo, Y.-C.; Liang, M.-X.; Li, J.-P.; et al. Microstructure and Properties of Densified Gd2O3 Bulk. Materials 2022, 15, 7793. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.H.; Zeng, S.C.; Jin, C.; Zhang, B.; Chen, B.Y.; Yang, Z.; Guo, Y.C.; Liang, M.X.; Li, J.P.; Wang, W.; et al. Mechanical Properties of Multi-Sized Porous Thermal Barrier Coatings at Micro and Nano Scales after Long-Term Service at High Temperature. Coatings 2021, 12, 165. [Google Scholar] [CrossRef]
- Guo, L.; Guo, H.; Peng, H. Thermophysical properties of Yb2O3 doped Gd2Zr2O7 and thermal cycling durability of (Gd0.9Yb0.1)2Zr2O7/YSZ thermal barrier coatings. J. Eur. Ceram. Soc. 2014, 34, 1255–1263. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiang, H.; Feng, Z. Theoretical Investigation on Mechanical and Thermal Properties of a Promising Thermal Barrier Material: Yb3Al5O12. J. Mater. Sci. Technol. 2014, 30, 631–638. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.; Ma, Y.; Peng, H.; Guo, H.; Gong, S. Thermal cycling behavior of (Gd0.9Yb0.1)2Zr2O7/8YSZ gradient thermal barrier coatings deposited on Hf-doped NiAl bond coat by EB-PVD. Surf. Coat. Technol. 2014, 258, 950–955. [Google Scholar] [CrossRef]
- Gao, P.H.; Zeng, S.C.; Jin, C.; Zhang, B.; Chen, B.Y.; Yang, Z.; Guo, Y.C.; Liang, M.X.; Li, J.P.; Li, Q.P.; et al. Effect of Gd2O3 Addition on the Microstructure and Properties of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Ceramics. Materials 2021, 14, 7470. [Google Scholar] [CrossRef]
- Zhang, J.X.; Bai, Y.; Li, E.B.; Dong, H.; Ma, W. Yb2O3-Gd2O3 codoped strontium zirconate composite ceramics for potential thermal barrier coating applications. Int. J. Appl. Ceram. Technol. 2020, 17, 1608–1618. [Google Scholar] [CrossRef]
- Taylor, R.; Brandon, J.R.; Morrell, P. Microstructure, composition and property relationships of plasma-sprayed thermal barrier coatings. Surf. Coat. Technol. 1992, 50, 141–149. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal Barrier Coatings for Gas-Turbine Engine Applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Essmeister, J.; Altun, A.A.; Schwentenwein, M.; Staudacher, M.; Konegger, T. Stereolithography-based additive manufacturing of polymer-derived SiOC/SiC ceramic composites. J. Eur. Ceram. Soc. 2002, 42, 5343–5354. [Google Scholar] [CrossRef]
- Cao, Z.; An, S.; Song, X. Effect of thermal treatment at high temperature on phase stability and transformation of Yb2O3 and Y2O3 co-doped ZrO2 ceramics. Nature 2022, 12, 9955. [Google Scholar] [CrossRef] [PubMed]
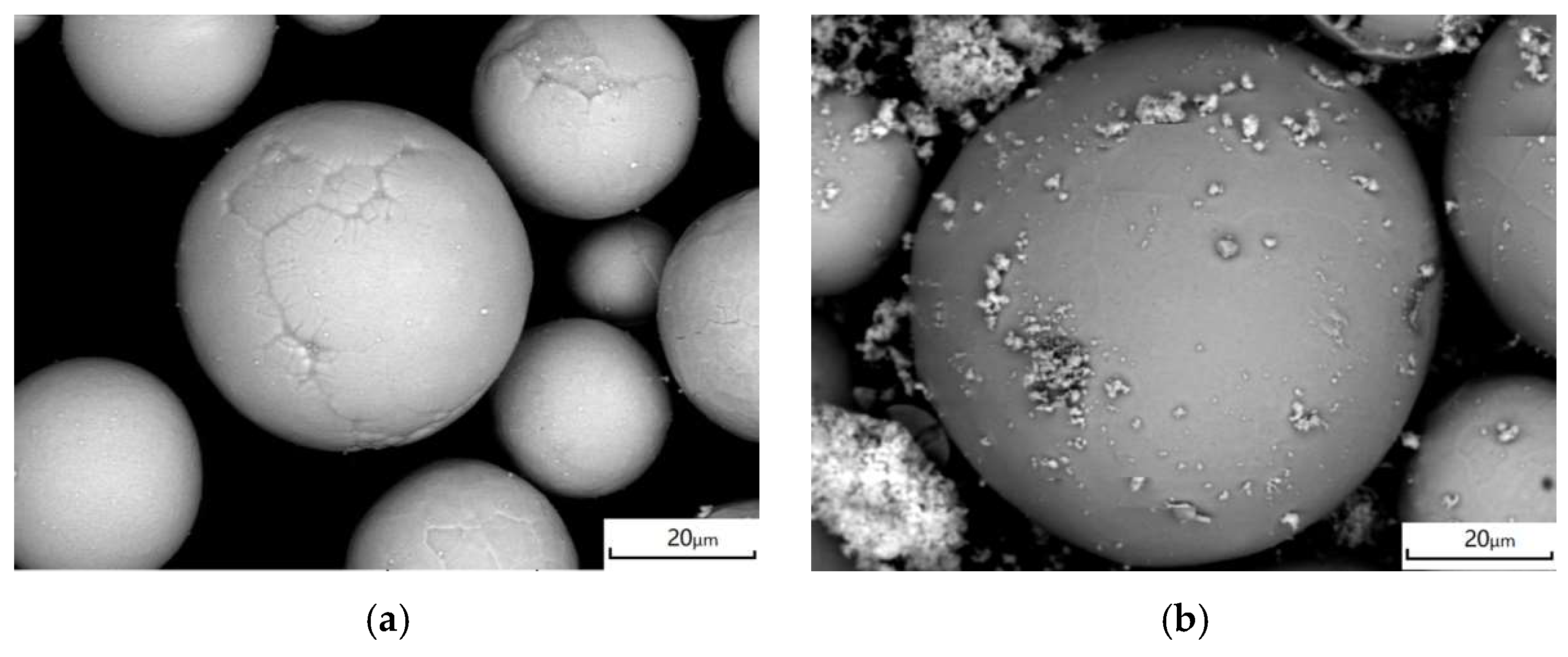

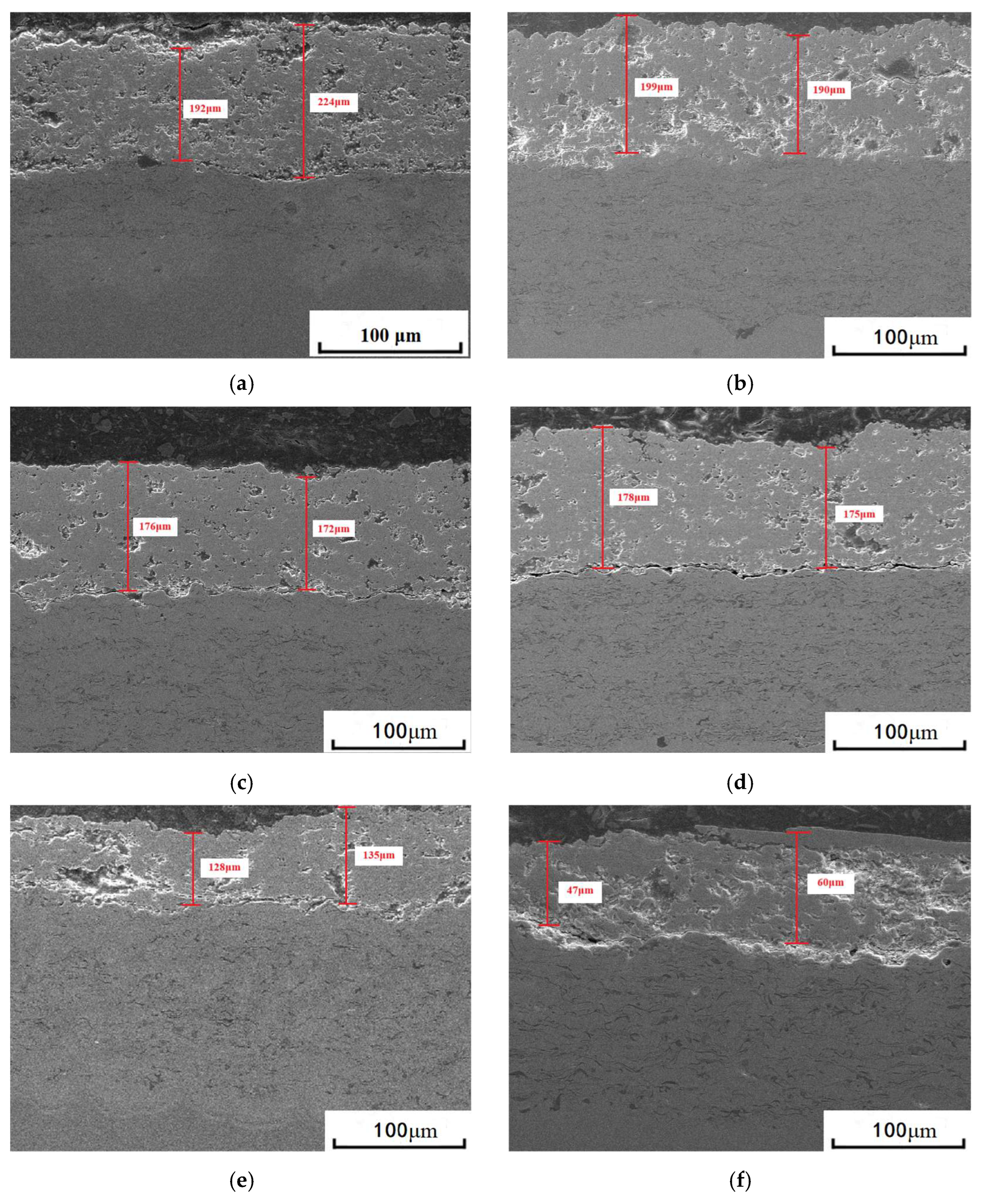
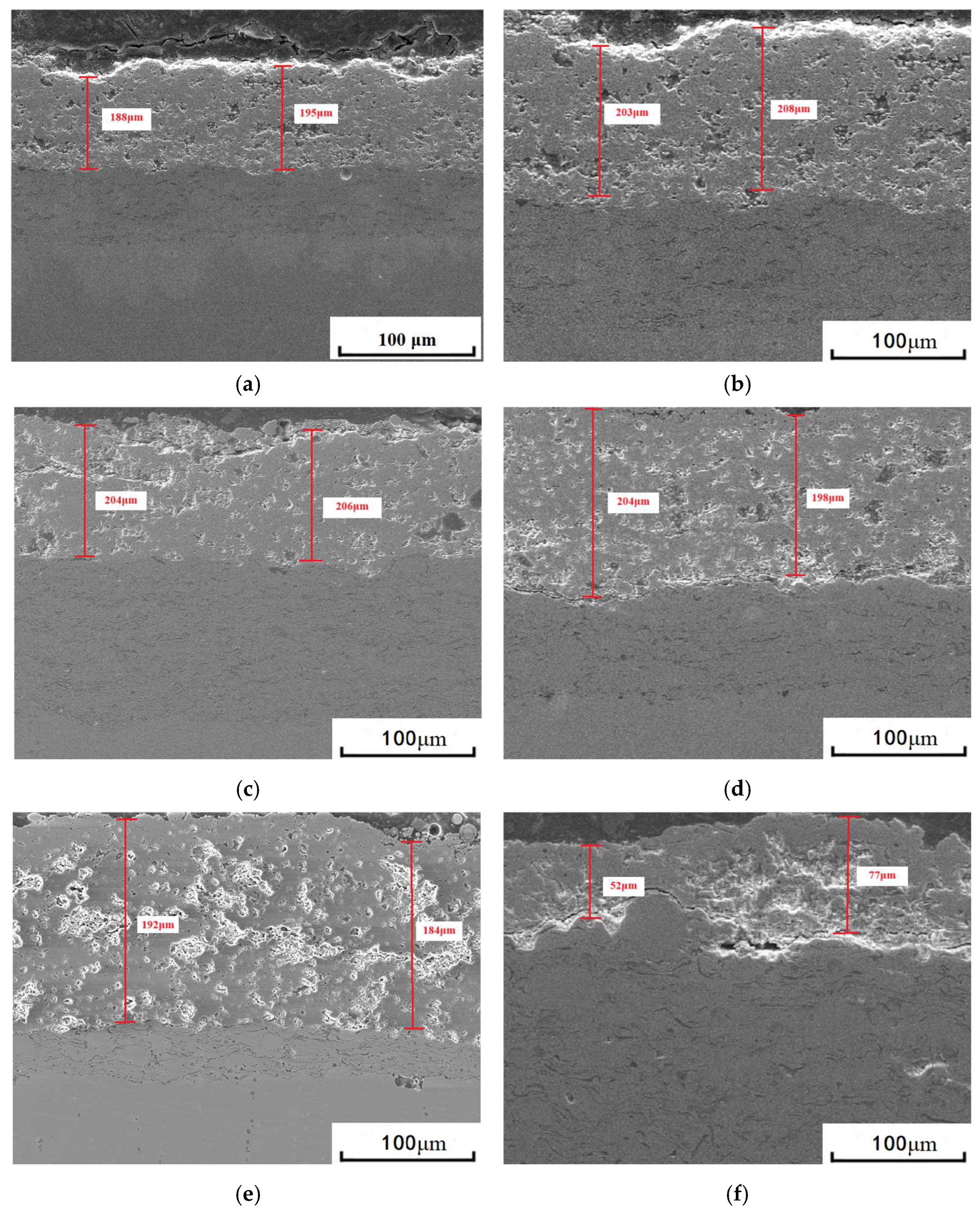
| Item | Experimental Parameters |
|---|---|
| Raw materials | The powders of 5GYYZO, 10GYYZO, 15GYYZO |
| Milling balls | Zirconia balls, with diameters of 10 mm, 20 mm, and 40 mm, each accounting for 1/3 |
| Solvent | Anhydrous ethanol |
| Powder/ball/solvent Ratio | 1:2:2 (Powder/ball/solvent) |
| Milling rotational speed | 260 r/min |
| Milling time | 8 h |
| Coating | Experimental Parameters | |
|---|---|---|
| Bond Coat | Arc Voltage (V) | 67 |
| Arc Current (A) | 540 | |
| H2 Pressure (MPa) and Flow Rate (SLPM) | 0.2 and 2.7 | |
| Ar Pressure (MPa) and Flow Rate (SLPM) | 0.9 and 38.1 | |
| Powder Feed Rate (rad/min) | 3.5 | |
| The standoff distance (mm) | 150 | |
| The substrate preheat temperature (°C) | 100 | |
| The traverse speed (mm/s) | 50 | |
| Top Coat | Arc Voltage (V) | 72 |
| Arc Current (A) | 630 | |
| H2 Pressure (MPa) and Flow Rate (SLPM) | 0.3 and 3.4 | |
| Ar Pressure (MPa) and Flow Rate (SLPM) | 0.9 and 36.5 | |
| Powder Feed Rate (rad/min) | 3.7 | |
| The standoff distance (mm) | 150 | |
| The substrate preheat temperature (°C) | 100 | |
| The traverse speed (mm/s) | 200 | |
| Item | Experimental Parameters |
|---|---|
| Heating rate | 5 °C/min |
| Holding temperature | 1100 °C |
| Holding time | 24 h |
| Cooling rate | 3 °C/min |
| Number of cycles | 10, 30, 60, 90 and 150 times |
| Sample Type | Sample | m-Phase Content (%) | t-Phase Content (%) |
|---|---|---|---|
| Powders | 8YSZ | 27.9 | 72.1 |
| 5GYYZO | 4.3 | 95.7 | |
| 10GYYZO | 1.5 | 98.5 | |
| Coatings | 8YSZ | 39.2 | 60.8 |
| 5GYYZO | 4.4 | 95.6 | |
| 10GYYZO | 1.7 | 98.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, S.; Gao, S.; Wang, Z.; Huang, Y.; He, Q.; Jiang, C. Performance Evolution of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Thermal Barrier Coatings After Thermal Cycling. Coatings 2025, 15, 1380. https://doi.org/10.3390/coatings15121380
Zeng S, Gao S, Wang Z, Huang Y, He Q, Jiang C. Performance Evolution of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Thermal Barrier Coatings After Thermal Cycling. Coatings. 2025; 15(12):1380. https://doi.org/10.3390/coatings15121380
Chicago/Turabian StyleZeng, Shengcong, Shanping Gao, Zhongda Wang, Yisong Huang, Qiwei He, and Chongran Jiang. 2025. "Performance Evolution of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Thermal Barrier Coatings After Thermal Cycling" Coatings 15, no. 12: 1380. https://doi.org/10.3390/coatings15121380
APA StyleZeng, S., Gao, S., Wang, Z., Huang, Y., He, Q., & Jiang, C. (2025). Performance Evolution of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Thermal Barrier Coatings After Thermal Cycling. Coatings, 15(12), 1380. https://doi.org/10.3390/coatings15121380





