Study on Synergistic Enhancement of Surface Properties of Ti-6Al-4V Alloy for Dental Applications by Magnetic Abrasive Finishing
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Morphology
2.3. Phase Analysis
2.4. Surface Micro Vickers Hardness
2.5. Surface Residual Stress
2.6. Electrochemical Corrosion
| NaCl | KCl | NaH2PO4·2H2O | CaCl2·2H2O | Na2S·2H2O | Urea | Distilled Water |
|---|---|---|---|---|---|---|
| 0.4 g | 0.4 g | 0.78 g | 0.795 g | 0.005 g | 1 g | 1000 mL |
2.7. Friction and Wear Performance
2.8. Statistical Analysis
3. Result
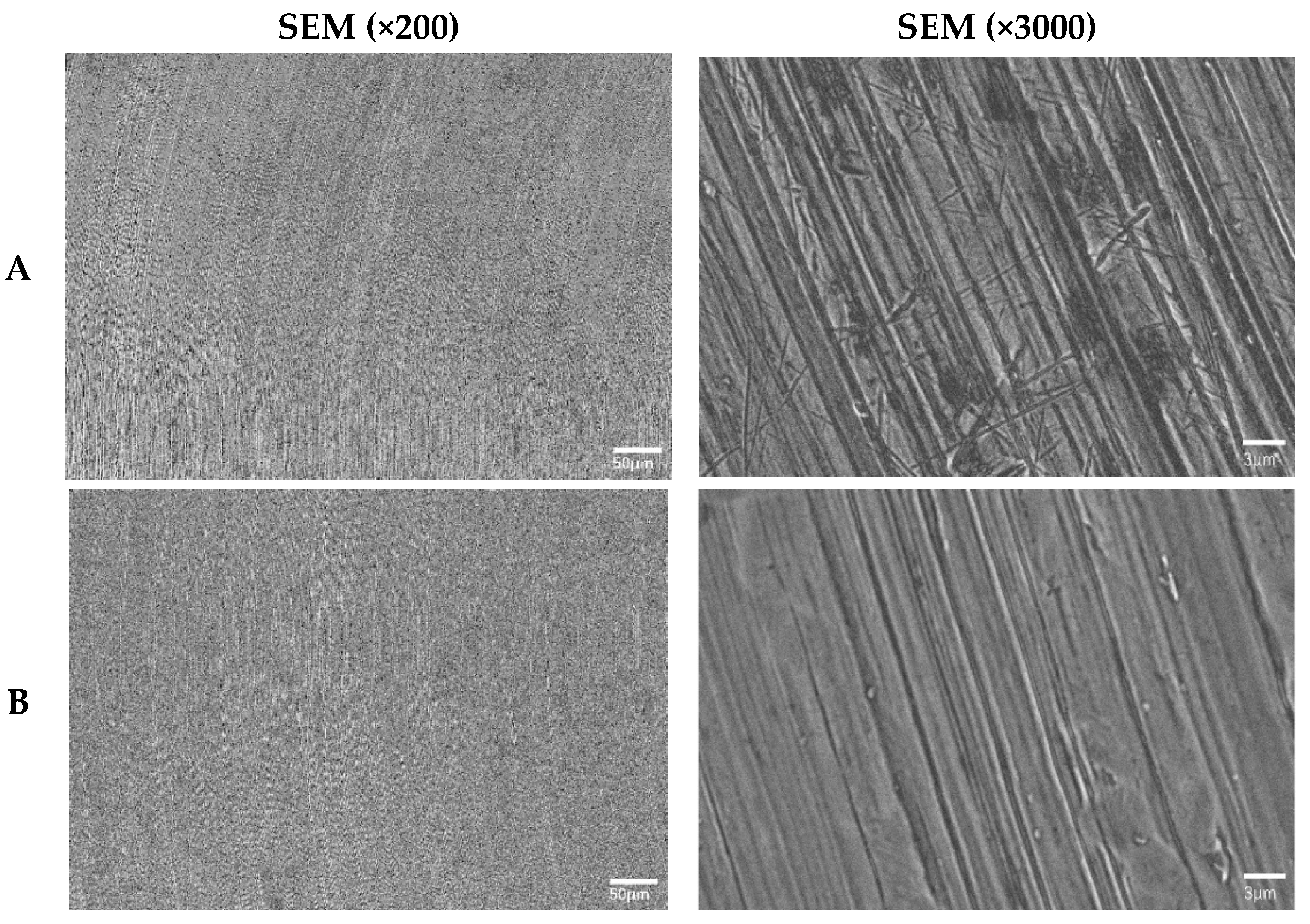
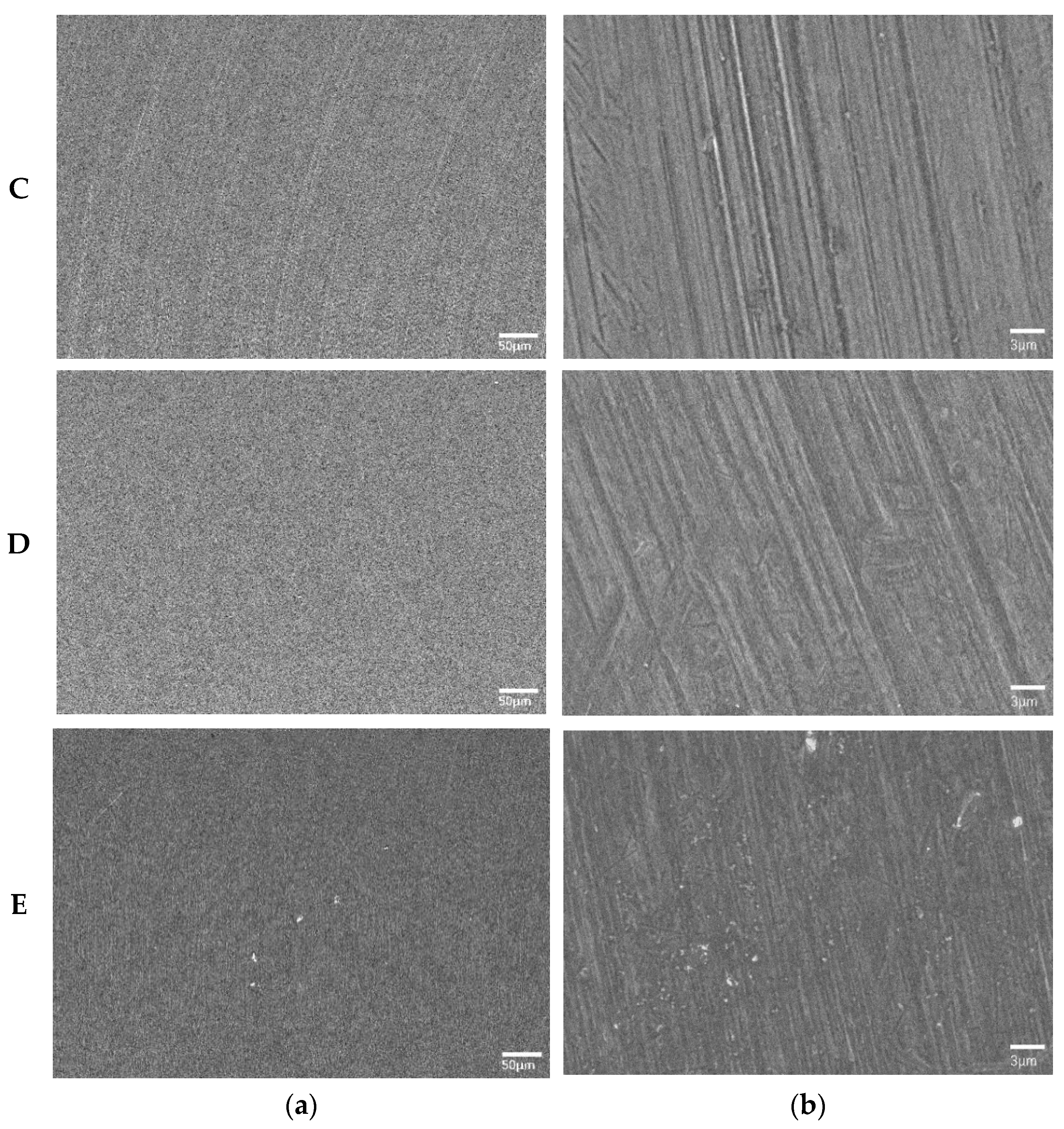
3.1. Surface Morphology
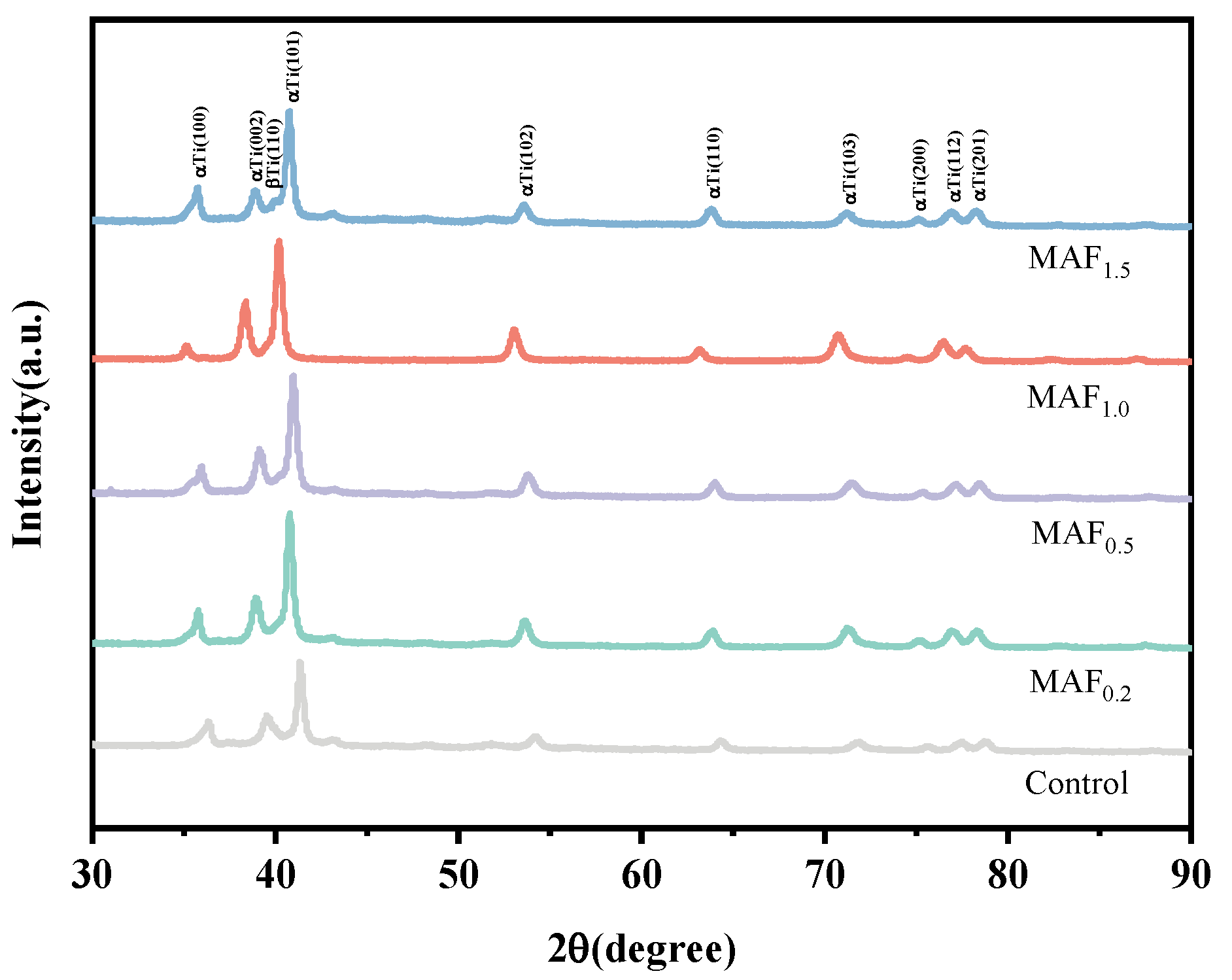
3.2. Phase Analysis
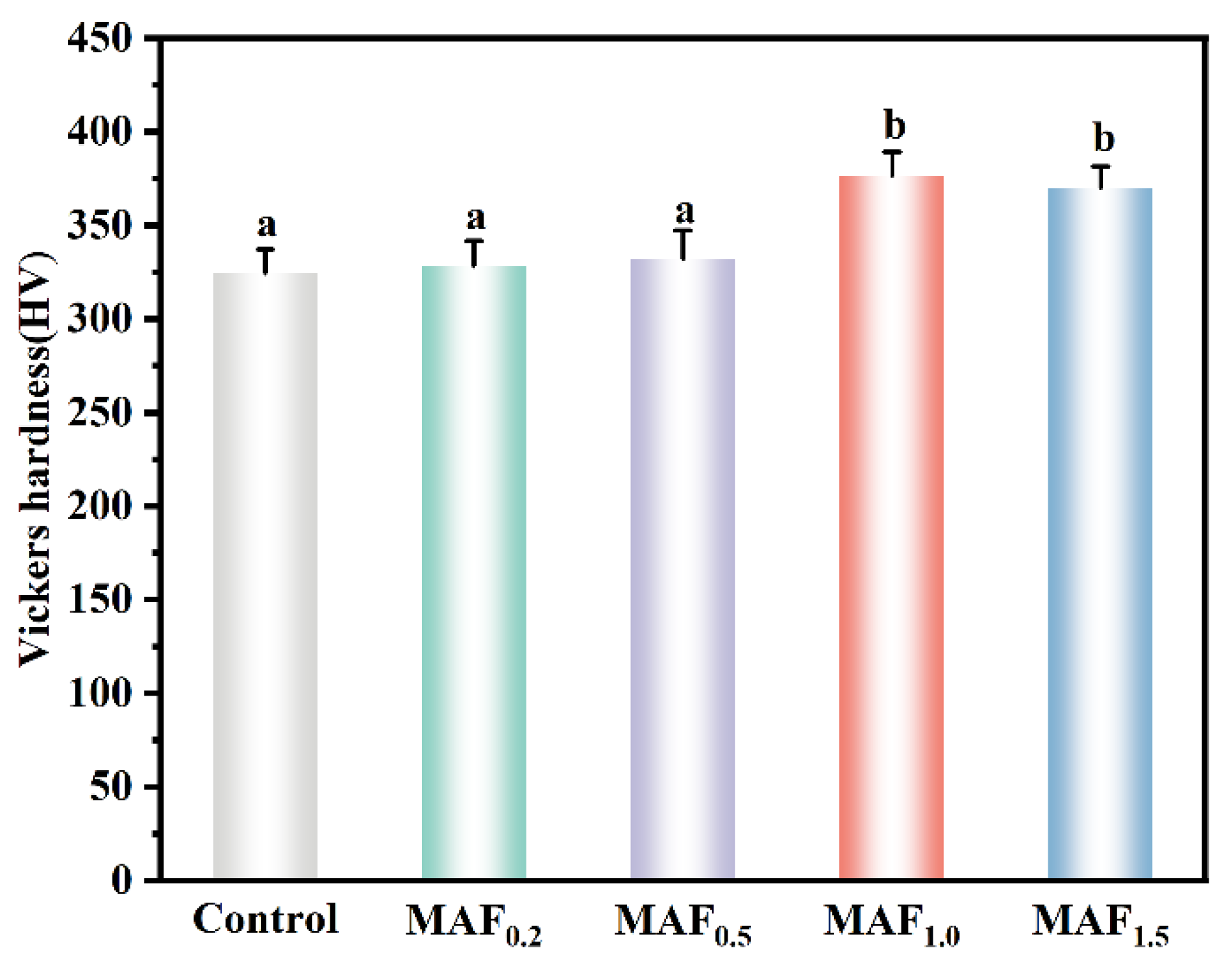
3.3. Surface Micro-Vickers Hardness
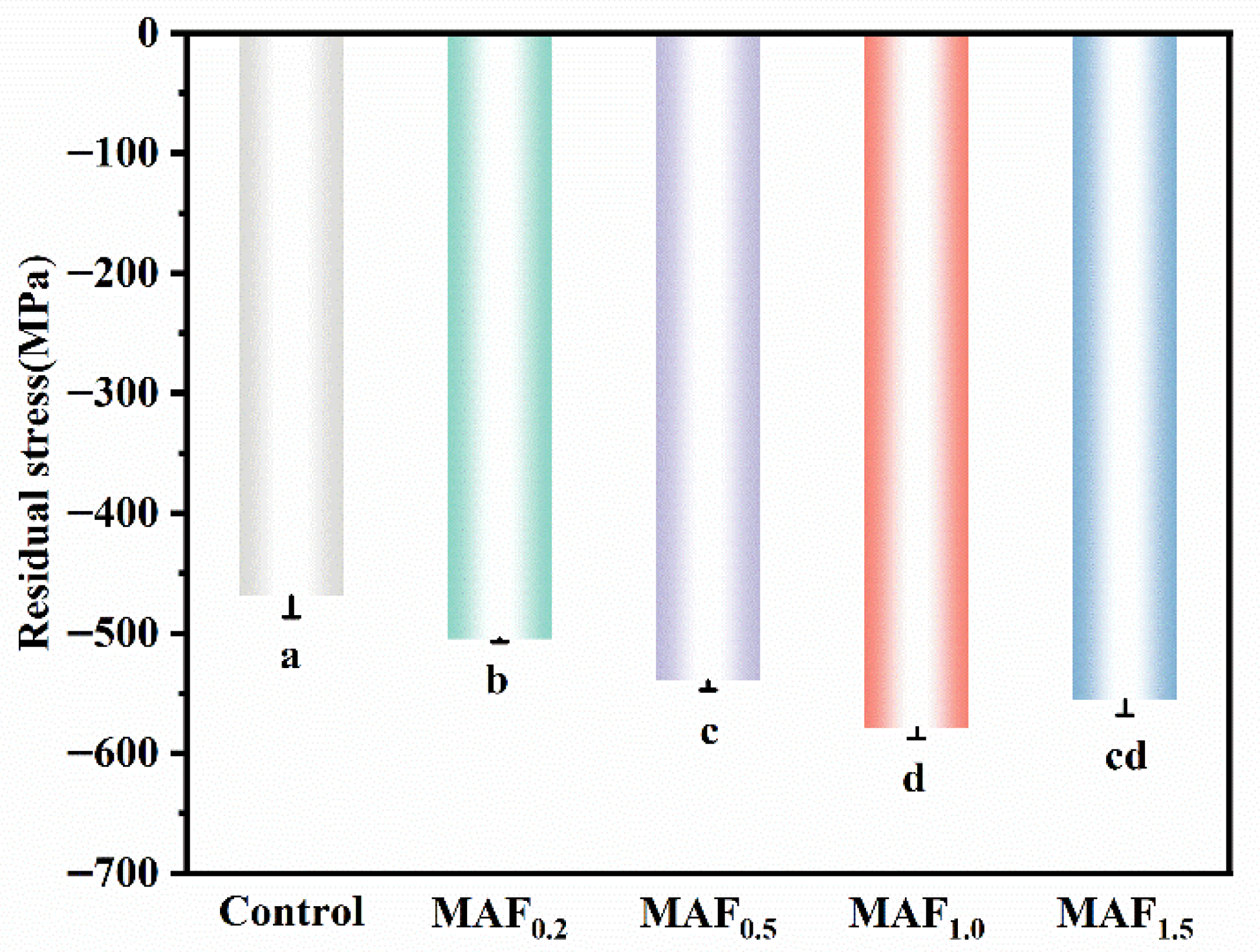
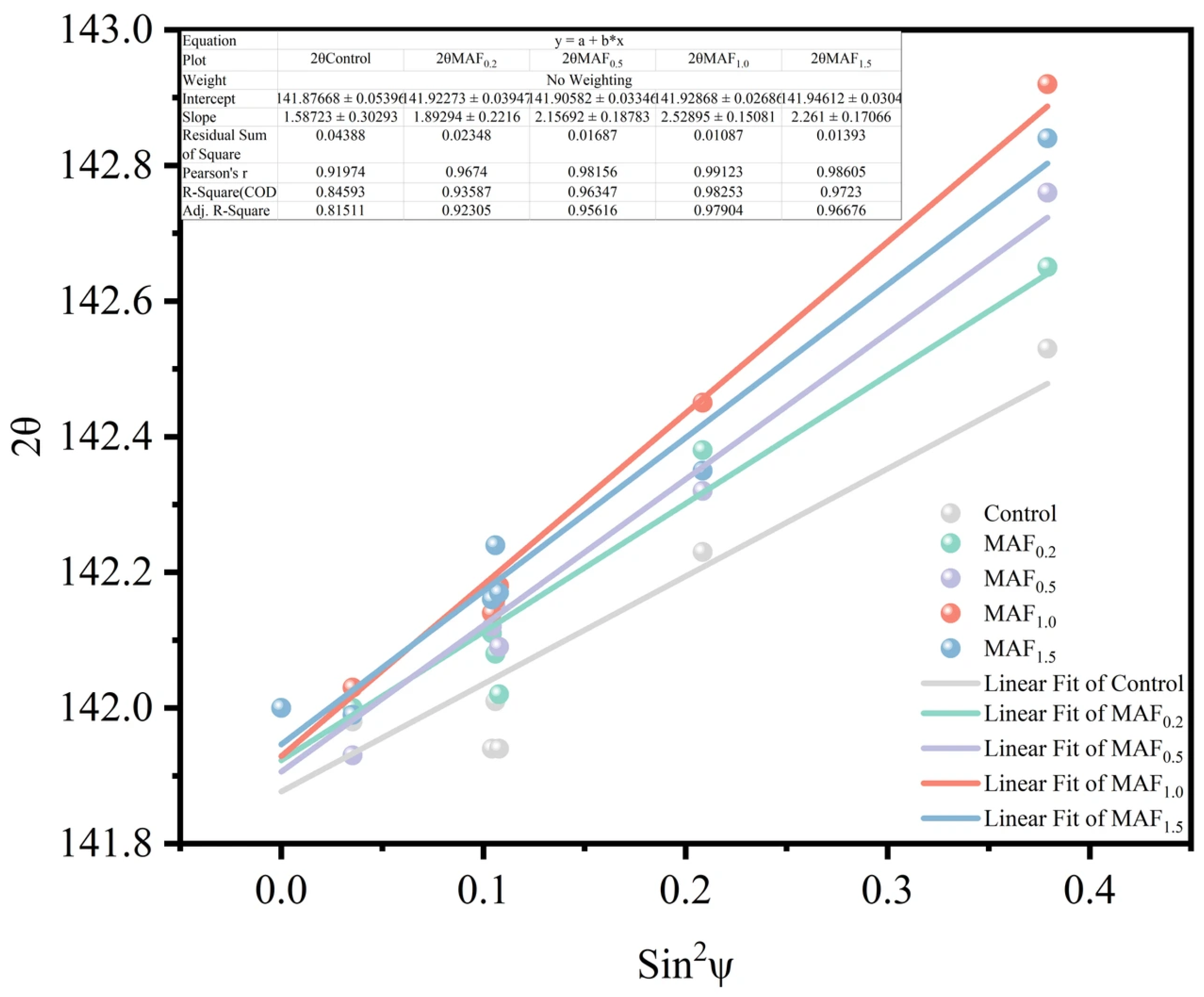
3.4. Surface Residual Stress
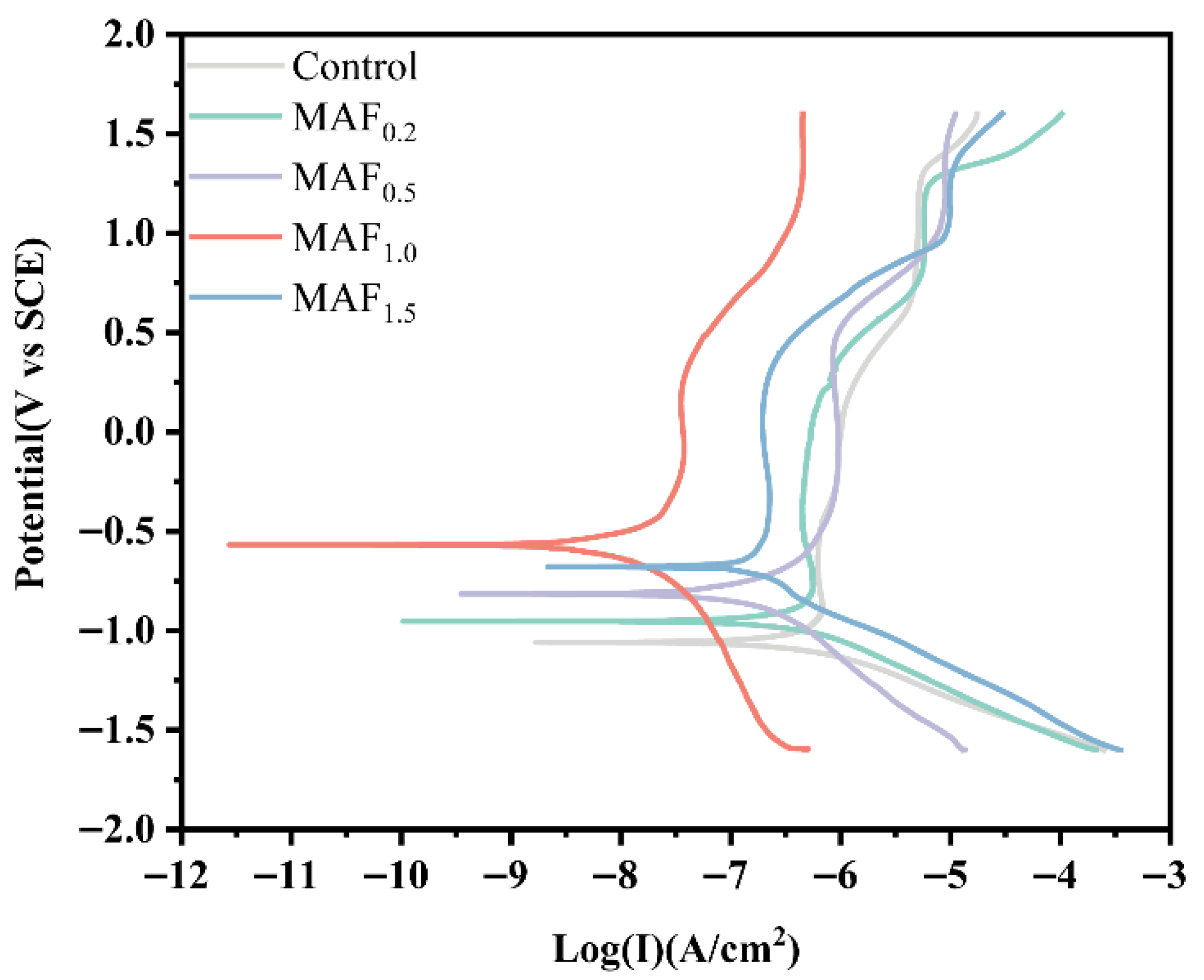
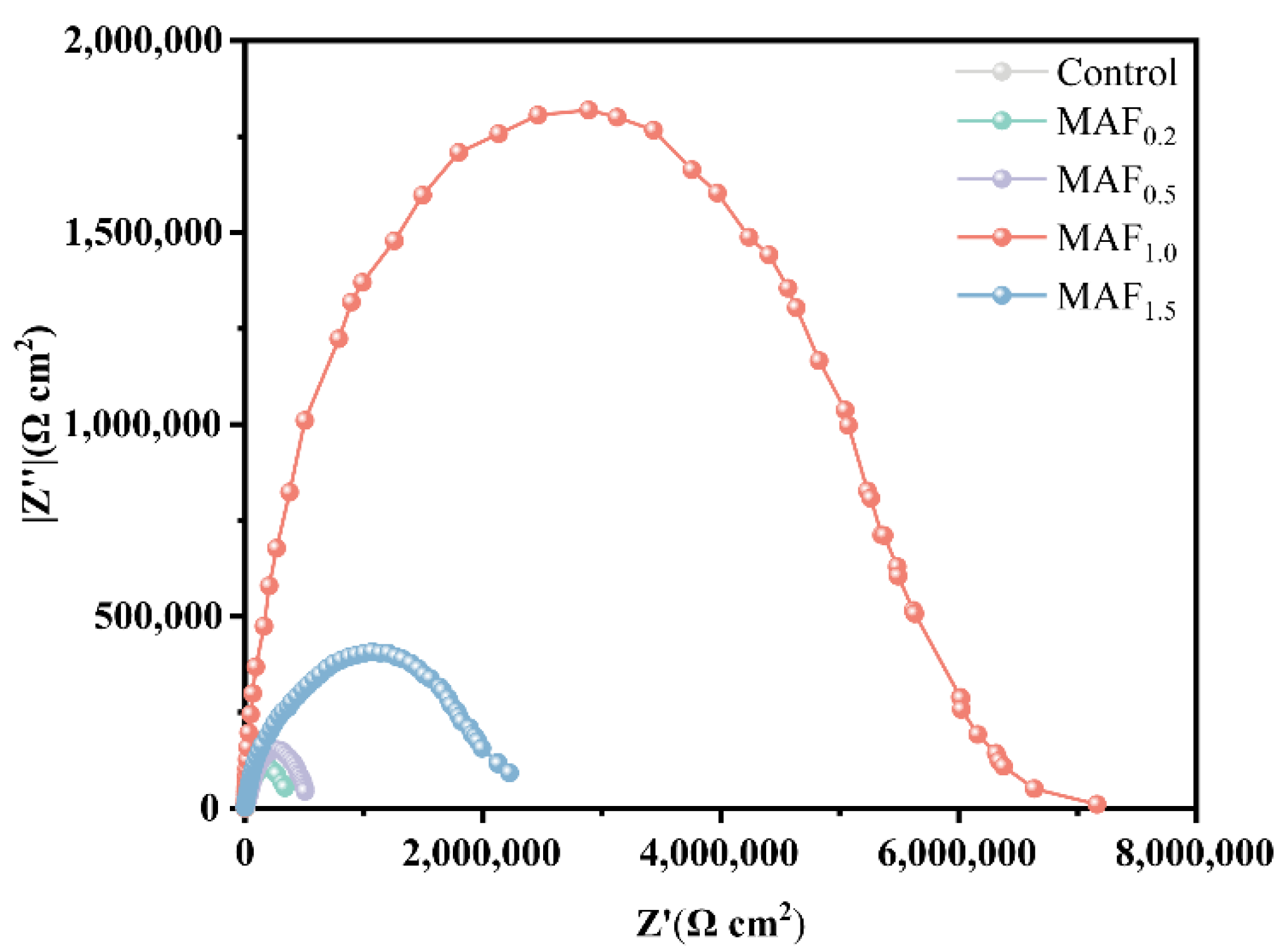
3.5. Electrochemical Corrosion
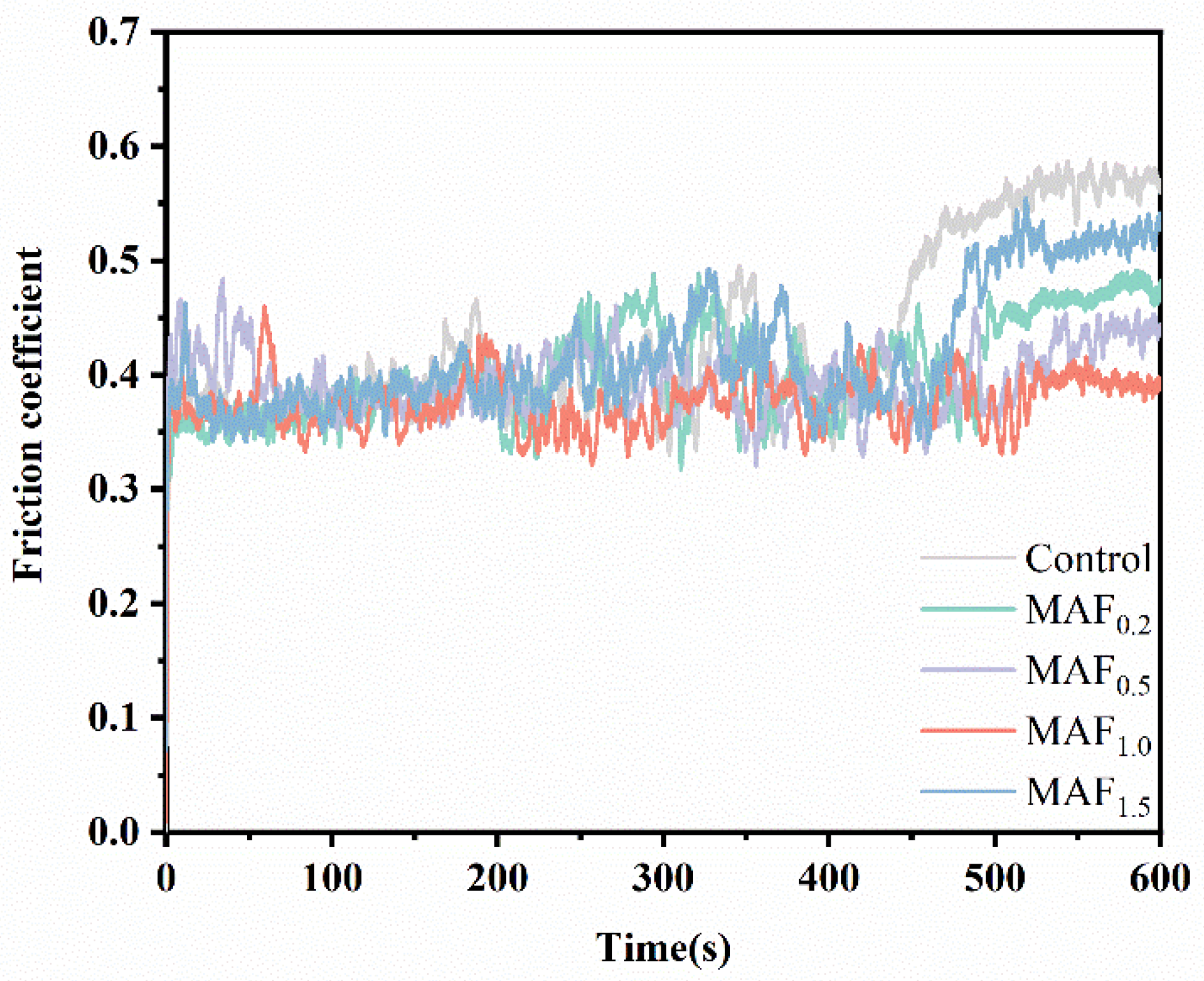
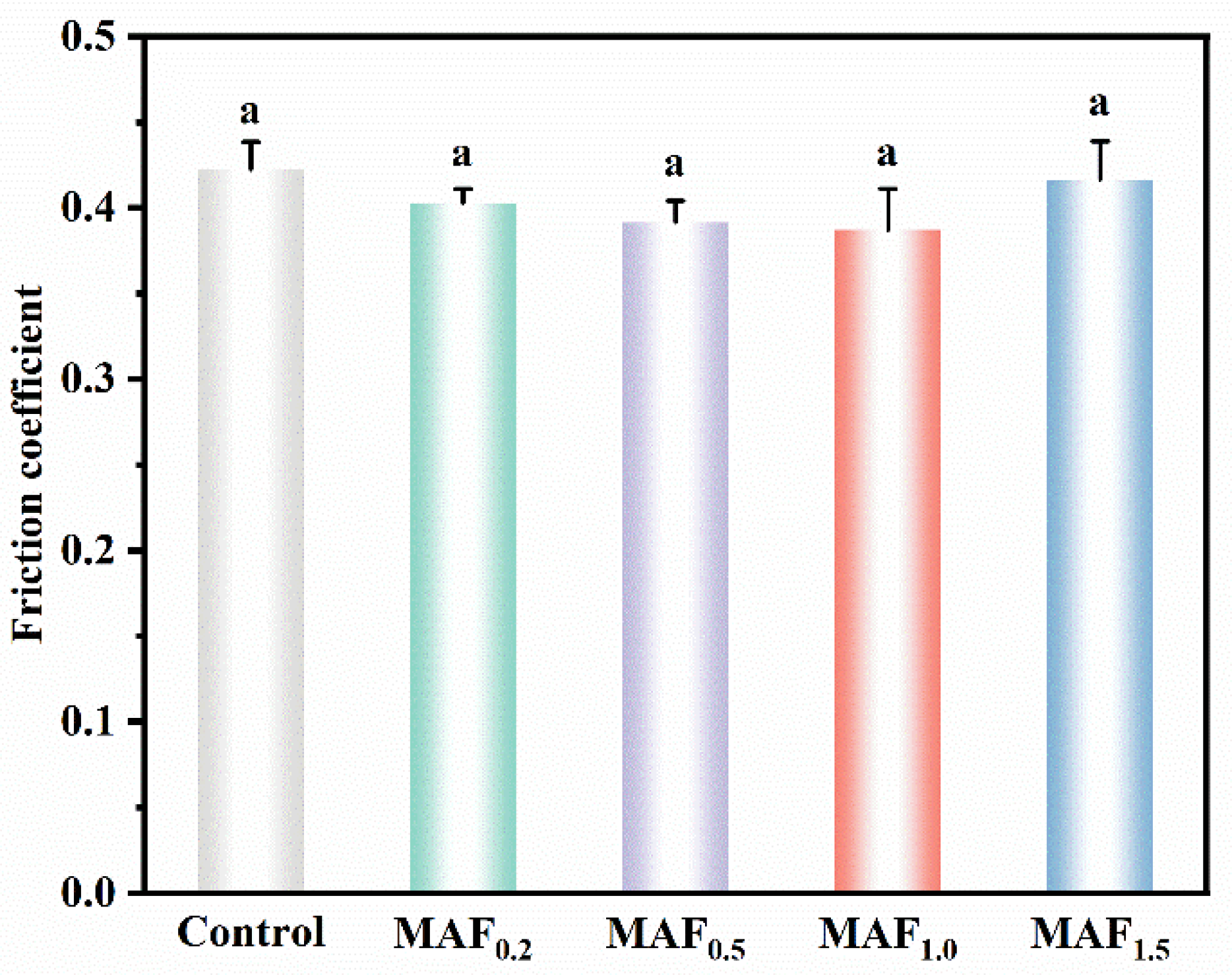
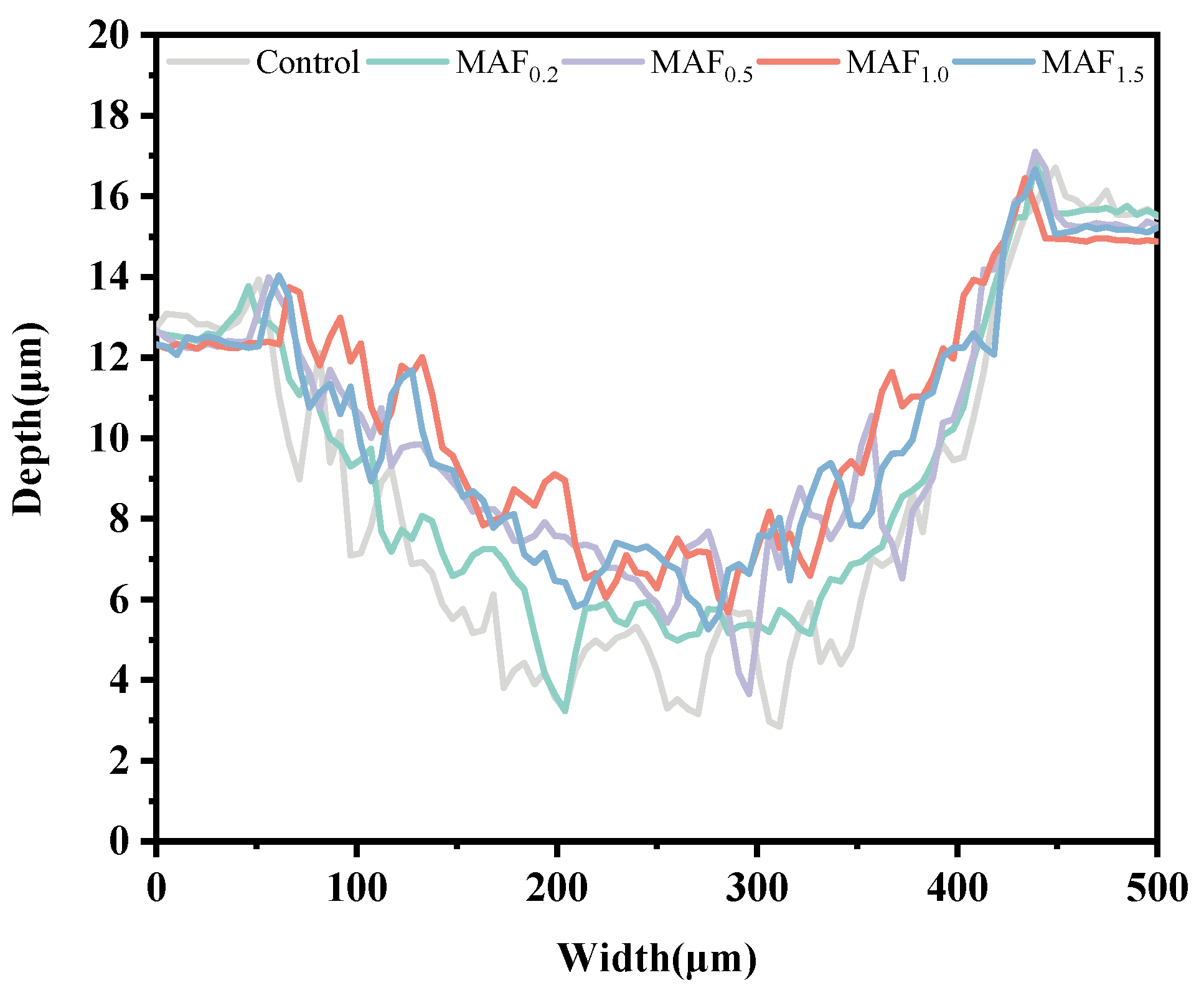
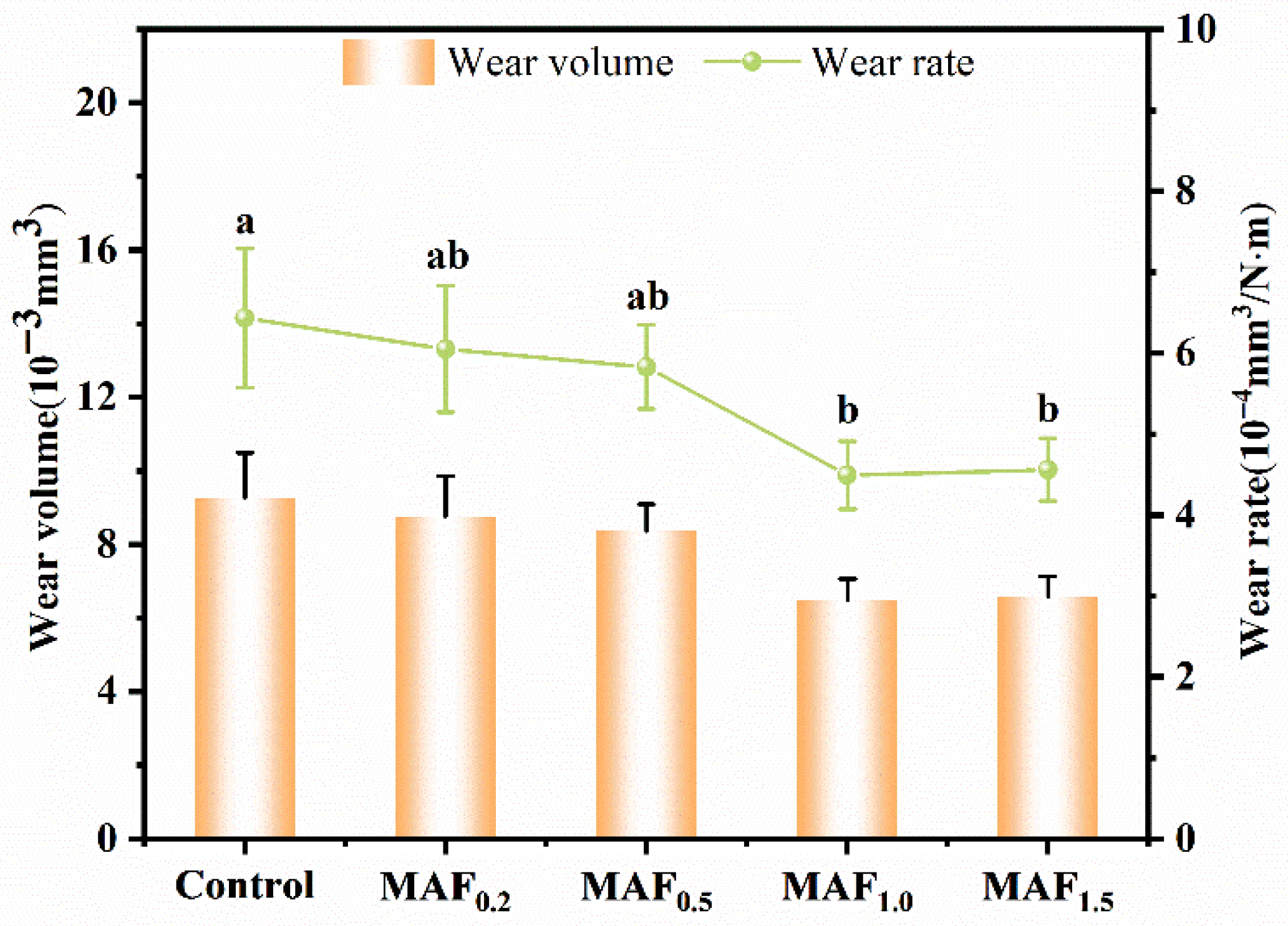
3.6. Friction Wear
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- Lin, C.P.; Shyu, Y.T.; Wu, Y.L.; Tsai, M.H.; Chen, H.S.; Wu, A.Y. Effects of Marginal Bone Loss Progression on Stress Distribution in Different Implant-Abutment Connections and Abutment Materials: A 3D Finite Element Analysis Study. Materials 2022, 15, 5866. [Google Scholar] [CrossRef]
- Muhl, A.; Szabo, P.; Krafcsik, O.; Aigner, Z.; Kopniczky, J.; Akos, N.; Marada, G.; Turzo, K. Comparison of surface aspects of turned and anodized titanium dental implant, or abutment material for an optimal soft tissue integration. Heliyon 2022, 8, e10263. [Google Scholar] [CrossRef]
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: A scoping review. J. Periodontal Res. 2017, 52, 946–954. [Google Scholar] [CrossRef]
- Alhamad, M.; Barao, V.A.R.; Sukotjo, C.; Cooper, L.F.; Mathew, M.T. Ti-Ions and/or Particles in Saliva Potentially Aggravate Dental Implant Corrosion. Materials 2021, 14, 5733. [Google Scholar] [CrossRef]
- Barros, C.D.d.R.; Rocha, J.C.; Bastos, I.N.; Ponciano Gomes, J.A.d.C. Tribocorrosion Resistance of Dental Implant Alloys—Assessment of cp-Ti, Ti6Al4V, and NiCr in Neutral and Acidified Saliva. J. Bio- Tribo-Corros. 2021, 7, 73. [Google Scholar] [CrossRef]
- Hubenova, E.; Mitov, M.; Hubenova, Y. Microbiologically influenced corrosion can cause a dental implant rejection. Electrochim. Acta 2024, 485, 144087. [Google Scholar] [CrossRef]
- Mellado-Valero, A.; Munoz, A.I.; Pina, V.G.; Sola-Ruiz, M.F. Electrochemical Behaviour and Galvanic Effects of Titanium Implants Coupled to Metallic Suprastructures in Artificial Saliva. Materials 2018, 11, 171. [Google Scholar] [CrossRef]
- Yilmaz, E.Ç.; Sadeler, R. A Literature Review on Chewing Simulation and Wear Mechanisms of Dental Biomaterials. J. Bio- Tribo-Corros. 2021, 7, 91. [Google Scholar] [CrossRef]
- Ali, B.; Meddah, H.M.; Merdji, A. Effects of overloading in mastication on the mechanical behaviour of dental implants. Mater. Des. 2013, 47, 210–217. [Google Scholar] [CrossRef]
- Raj, D.; Maity, S.R.; Das, B. State-of-the-art review on laser cladding process as an in-situ repair technique. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2021, 236, 1194–1215. [Google Scholar] [CrossRef]
- Dowling, M.; Al-Hamaoy, A.R.; Obeidi, M.A. Laser surface cladding of metal parts. Results Surf. Interfaces 2023, 12, 100142. [Google Scholar] [CrossRef]
- Heimann, R.B. The nature of plasma spraying. Coatings 2023, 13, 622. [Google Scholar] [CrossRef]
- İzmir, M.; Ercan, B. Anodization of titanium alloys for orthopedic applications. Front. Chem. Sci. Eng. 2018, 13, 28–45. [Google Scholar] [CrossRef]
- Ahmad, S.; Tian, Y.; Arora, K. Magnetic abrasive finishing: Innovations and possibilities. J. Manuf. Process. 2025, 134, 299–336. [Google Scholar] [CrossRef]
- Houshi, M.N. A comprehensive review on magnetic abrasive finishing process. In Advanced Engineering Forum; Trans Tech Publications: Baech, Switzerland, 2016; pp. 1–20. [Google Scholar]
- Maraboina, R.; Pasam, V.K. Enhancing Surface Integrity of Selective Laser Melted Al-Si Alloys through Ultrasonic Assisted Magnetic Abrasive Finishing utilizing SiC Abrasives. Silicon 2023, 16, 1183–1196. [Google Scholar] [CrossRef]
- Wei, D.; Chen, Y.Y.; Li, H.; Yang, J.C. Residual stress evolution and tailoring of cold pilgered Ti-3Al-2.5V tube. Int. J. Mech. Sci. 2022, 225, 107366. [Google Scholar] [CrossRef]
- Zhou, K.; Chen, Y.; Du, Z.W.; Niu, F.L. Surface integrity of titanium part by ultrasonic magnetic abrasive finishing. Int. J. Adv. Manuf. Technol. 2015, 80, 997–1005. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kwak, J.-S. Behavior characteristics of abrasives for improving surface integrity in magnetic pin polishing. J. Mech. Sci. Technol. 2020, 34, 4069–4075. [Google Scholar] [CrossRef]
- Noyan, I.C.; Cohen, J.B. Residual Stress: Measurement by Diffraction and Interpretation; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wang, P.-J.; Ma, L.-W.; Cheng, X.-Q.; Li, X.-G. Influence of grain refinement on the corrosion behavior of metallic materials: A review. Int. J. Miner. Metall. Mater. 2021, 28, 1112–1126. [Google Scholar] [CrossRef]
- Jos, B.; Babu, C.R.; Shaji, S.; Anila, E.I. Study of the structural, optical, electrical and electrochemical properties of copper oxide thin films synthesized by spray pyrolysis. J. Mater. Sci. Mater. Electron. 2024, 35, 233. [Google Scholar] [CrossRef]
- Golgovici, F.; Tudose, A.-E.; Mosinoiu, L.F.; Demetrescu, I. Characterization of NiCrAlY Layers Deposited on 310H Alloy Using the EB-PVD Method After Oxidation in Water at High Temperature and Pressure. Appl. Sci. 2025, 15, 2361. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Du, X.; Yang, G.; Shi, H.; Wang, J. Regulation of trace aluminum addition on the corrosion resistance of Cu-12.5Ni-5Sn-xAl alloy. Mater. Today Commun. 2025, 43, 111616. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, C.; Liu, X.; Liu, R.; Xiao, Z. Improved surface integrity of Ti6Al4V fabricated by selective electron beam melting using ultrasonic surface rolling processing. J. Mater. Process. Technol. 2021, 297, 117264. [Google Scholar] [CrossRef]
- Fujian, M.; Shiyu, L.; Qichao, L.; Yu, L.; Zhihua, S.; Shengfang, Z. Effects of Ultrasonic Assisted Magnetic Abrasive Finishing on Surface Integrity of Titanium Alloy. China Surf. Eng. 2019, 32, 128–136. [Google Scholar]
- Liu, B.; Bruni, S.; Lewis, R. Numerical calculation of wear in rolling contact based on the Archard equation: Effect of contact parameters and consideration of uncertainties. Wear 2022, 490–491, 204188. [Google Scholar] [CrossRef]
- Qiu, Y.; Peng, Y.; Zuo, Y. Ultrasonic impact surface strengthening treatment and fatigue behaviors of titanium alloy thin-walled open hole components. Eng. Fract. Mech. 2024, 307, 110292. [Google Scholar] [CrossRef]
- Zha, X.; Yuan, Z.; Qin, H.; Xi, L.; Guo, Y.; Xu, Z.; Dai, X.; Jiang, F. Investigating the Dynamic Mechanical Properties and Strengthening Mechanisms of Ti-6Al-4V Alloy by Using the Ultrasonic Surface Rolling Process. Materials 2024, 17, 1382. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Zhou, S.; Wang, Y.; Zhang, L.; Zhang, Z. Study on surface properties of Ti-6Al-4V titanium alloy by ultrasonic rolling. Simul. Model. Pract. Theory 2022, 121, 102643. [Google Scholar] [CrossRef]
- Wei, D.; Yang, H.; Yang, J.; Xue, J.; Zhang, P.; Li, H. Synchronously tailoring of residual stress and surface quality of high-strength titanium alloy tube using magnetic field-assisted finishing. J. Manuf. Process. 2025, 141, 638–649. [Google Scholar] [CrossRef]
- Su, K.; Zhang, J.; Lu, L.; Li, H.; Ji, D. Corrosion-fatigue property of anodic oxidation coated 6082-T6 aluminium alloy: Effect of substrate residual stress and microstructure beneath coating-substrate interface. Int. J. Fatigue 2023, 175, 107803. [Google Scholar] [CrossRef]
- Dai, W.; Hao, J.; Li, C.; He, D.; Jia, D.; Zhang, Y.; Tan, Z. Residual stress relaxation and duty cycle on high cycle fatigue life of micro-arc oxidation coated AA7075-T6 alloy. Int. J. Fatigue 2020, 130, 105283. [Google Scholar] [CrossRef]
- Chen, W. An Overview of Near-Neutral pH Stress Corrosion Cracking in Pipelines and Mitigation Strategies for Its Initiation and Growth. Corrosion 2016, 72, 962–977. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, K.; Hu, Y.; Wang, S.; Wang, X. Effect of machining-induced surface residual stress on initiation of stress corrosion cracking in 316 austenitic stainless steel. Corros. Sci. 2016, 108, 173–184. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, G.; Yan, J.; Cheng, X.; Liu, H.; Yang, X. Effect of TiAlSiN coating residual stress on its sliding wear and cutting wear performance. Int. J. Adv. Manuf. Technol. 2022, 123, 3885–3900. [Google Scholar] [CrossRef]
- Lhiabani, A.; Nasri, M.; Shajari, Y.; Seyedraoufi, Z.-S. Effect of compressive residual stress on wear resistance of IGT25+ gas turbine compressor blades made of 1.4923 steel. Modares Mech. Eng. 2022, 22, 167–177. [Google Scholar] [CrossRef]
- Sun, Y.; Shukla, A.; Ramachandran Remya, A.; Kanniyappan, H.; Yang, B.; Harlow, R.; Campbell, S.D.; Thalji, G.; Mathew, M. Fretting-corrosion at the Implant–Abutment Interface Simulating Clinically Relevant Conditions. Dent. Mater. 2024, 40, 1823–1831. [Google Scholar] [CrossRef]
- Revathi, A.; Borras, A.D.; Munoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1354–1368. [Google Scholar] [CrossRef]
- Corne, P.; De March, P.; Cleymand, F.; Geringer, J. Fretting-corrosion behavior on dental implant connection in human saliva. J. Mech. Behav. Biomed. Mater. 2019, 94, 86–92. [Google Scholar] [CrossRef]
- Hu, N.; Xie, L.; Liao, Q.; Gao, A.; Zheng, Y.; Pan, H.; Tong, L.; Yang, D.; Gao, N.; Starink, M.J.; et al. A more defective substrate leads to a less defective passive layer: Enhancing the mechanical strength, corrosion resistance and anti-inflammatory response of the low-modulus Ti-45Nb alloy by grain refinement. Acta Biomater. 2021, 126, 524–536. [Google Scholar] [CrossRef]
- Pathote, D.; Jaiswal, D.; Singh, V.; Gautam, R.K.; Behera, C.K. Wear behavior and microhardness studies of tantalum (Ta)-coated 316L stainless steel by DC magnetron sputtering for the orthopedic applications. J. Mater. Sci. 2022, 57, 21039–21056. [Google Scholar] [CrossRef]
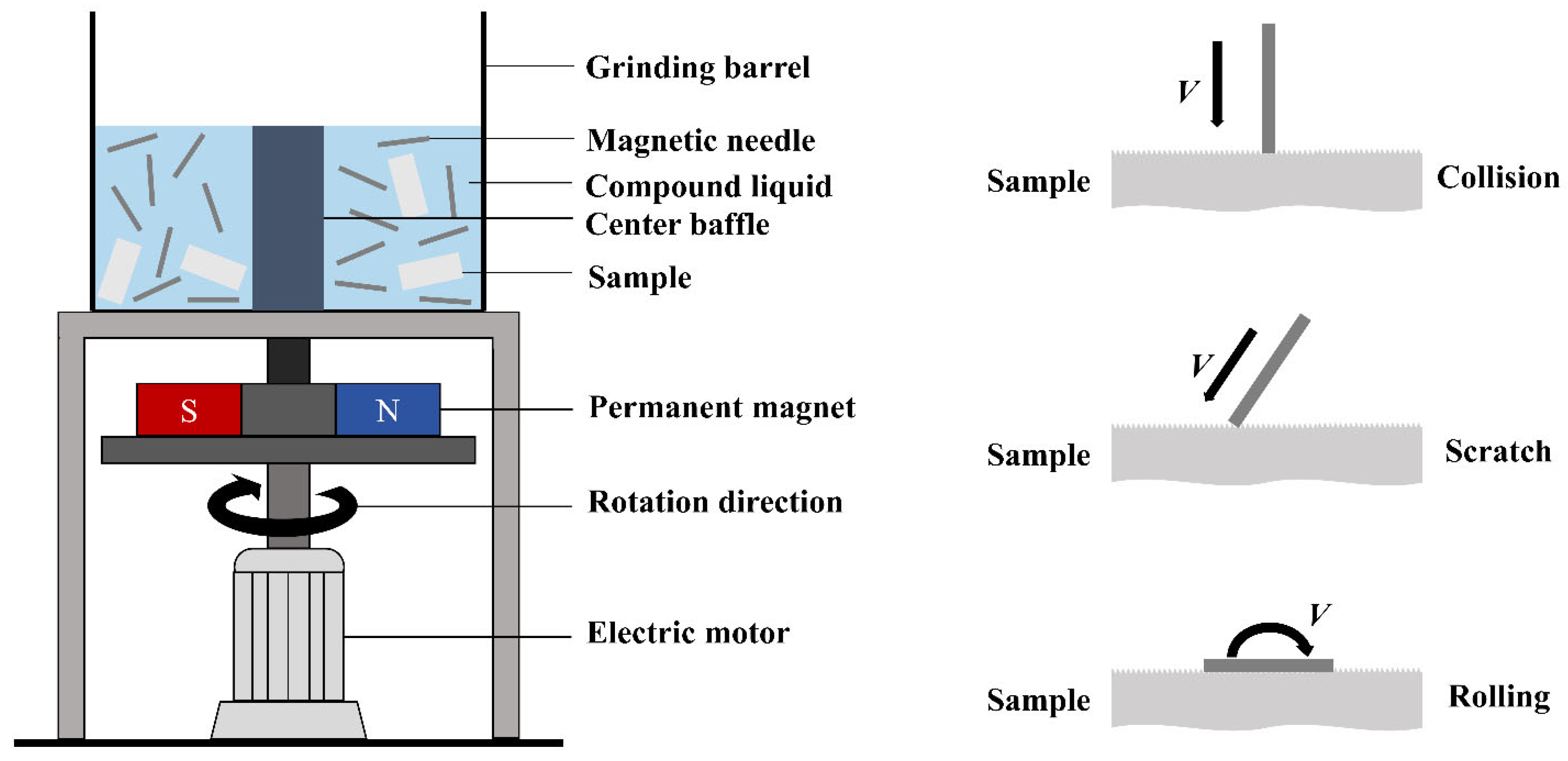
| Group | Magnetic Needle Diameter/mm | Grinding Fluid Ratio | Magnetic Needle Quality/kg | Magnetic Needle Length/mm | Magnetic Pole Rotation Speed/Hz | Grinding Time/min |
|---|---|---|---|---|---|---|
| MAF0.2 | 0.2 | 1/50 | 1.0 | 5.0 | 40 | 60 |
| MAF0.5 | 0.5 | 1/50 | 1.0 | 5.0 | 40 | 60 |
| MAF1.0 | 1.0 | 1/50 | 1.0 | 5.0 | 40 | 60 |
| MAF1.5 | 1.5 | 1/50 | 1.0 | 5.0 | 40 | 60 |
| Group | FWHW (°) | Grain Size (nm) |
|---|---|---|
| Control | 0.338 | 18.5 |
| MAF0.2 | 0.362 | 17.7 |
| MAF0.5 | 0.385 | 17.1 |
| MAF1.0 | 0.400 | 15.4 |
| MAF1.5 | 0.390 | 16.8 |
| Group | Ecorr (V vs. SCE) | Icorr (μA·cm−2) | βa (mv/dec) | |βc| (mv/dec) | Rp ( ·cm2) |
|---|---|---|---|---|---|
| Control | −1.0647 | 0.2869 | 362 | 127 | 1.43 × 105 |
| MAF0.2 | −0.9553 | 0.1349 | 161 | 86 | 1.80 × 105 |
| MAF0.5 | −0.8329 | 0.1164 | 345 | 211 | 4.88 × 105 |
| MAF1.0 | −0.5661 | 0.0114 | 489 | 454 | 8.97 × 106 |
| MAF1.5 | −0.6798 | 0.1069 | 294 | 204 | 4.89 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, L.; Su, H.; Hao, J.; Su, Y. Study on Synergistic Enhancement of Surface Properties of Ti-6Al-4V Alloy for Dental Applications by Magnetic Abrasive Finishing. Coatings 2025, 15, 1364. https://doi.org/10.3390/coatings15121364
Xiong L, Su H, Hao J, Su Y. Study on Synergistic Enhancement of Surface Properties of Ti-6Al-4V Alloy for Dental Applications by Magnetic Abrasive Finishing. Coatings. 2025; 15(12):1364. https://doi.org/10.3390/coatings15121364
Chicago/Turabian StyleXiong, Lang, Hanqi Su, Junjiang Hao, and Yucheng Su. 2025. "Study on Synergistic Enhancement of Surface Properties of Ti-6Al-4V Alloy for Dental Applications by Magnetic Abrasive Finishing" Coatings 15, no. 12: 1364. https://doi.org/10.3390/coatings15121364
APA StyleXiong, L., Su, H., Hao, J., & Su, Y. (2025). Study on Synergistic Enhancement of Surface Properties of Ti-6Al-4V Alloy for Dental Applications by Magnetic Abrasive Finishing. Coatings, 15(12), 1364. https://doi.org/10.3390/coatings15121364







