Hydrothermal Carbonization Coating on AISI 1018 Steel for Seawater Corrosion Protection
Abstract
1. Introduction
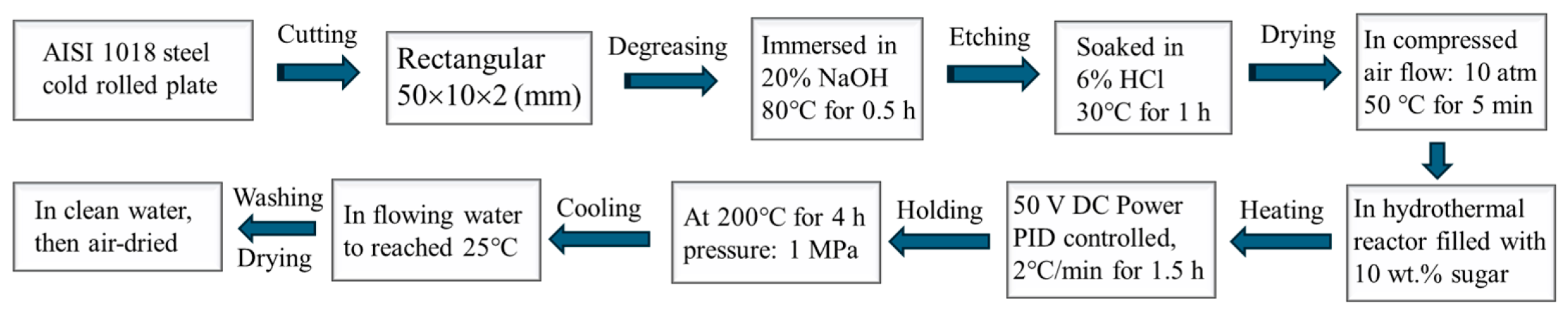
2. Materials and Methods
3. Results and Discussion
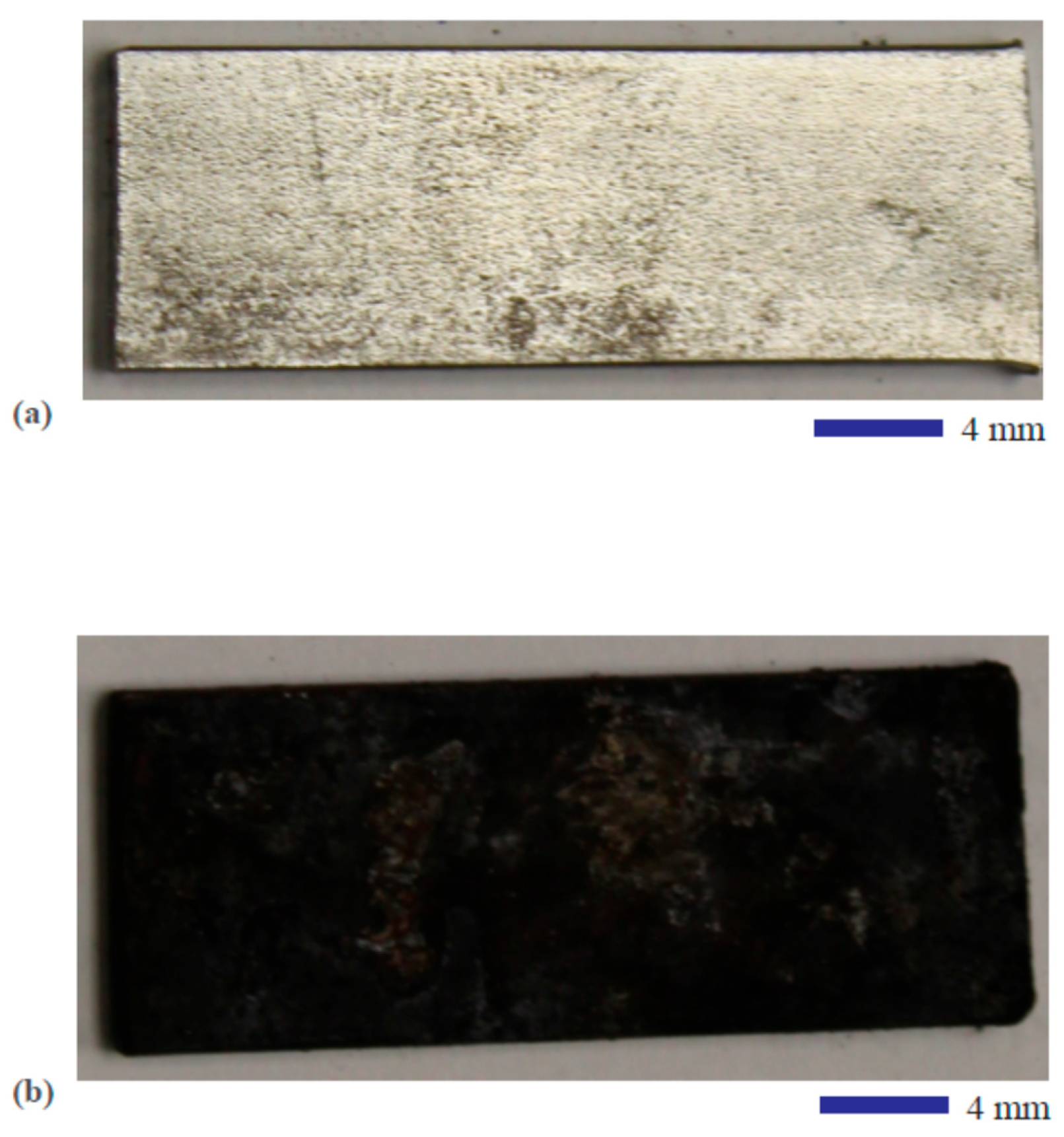
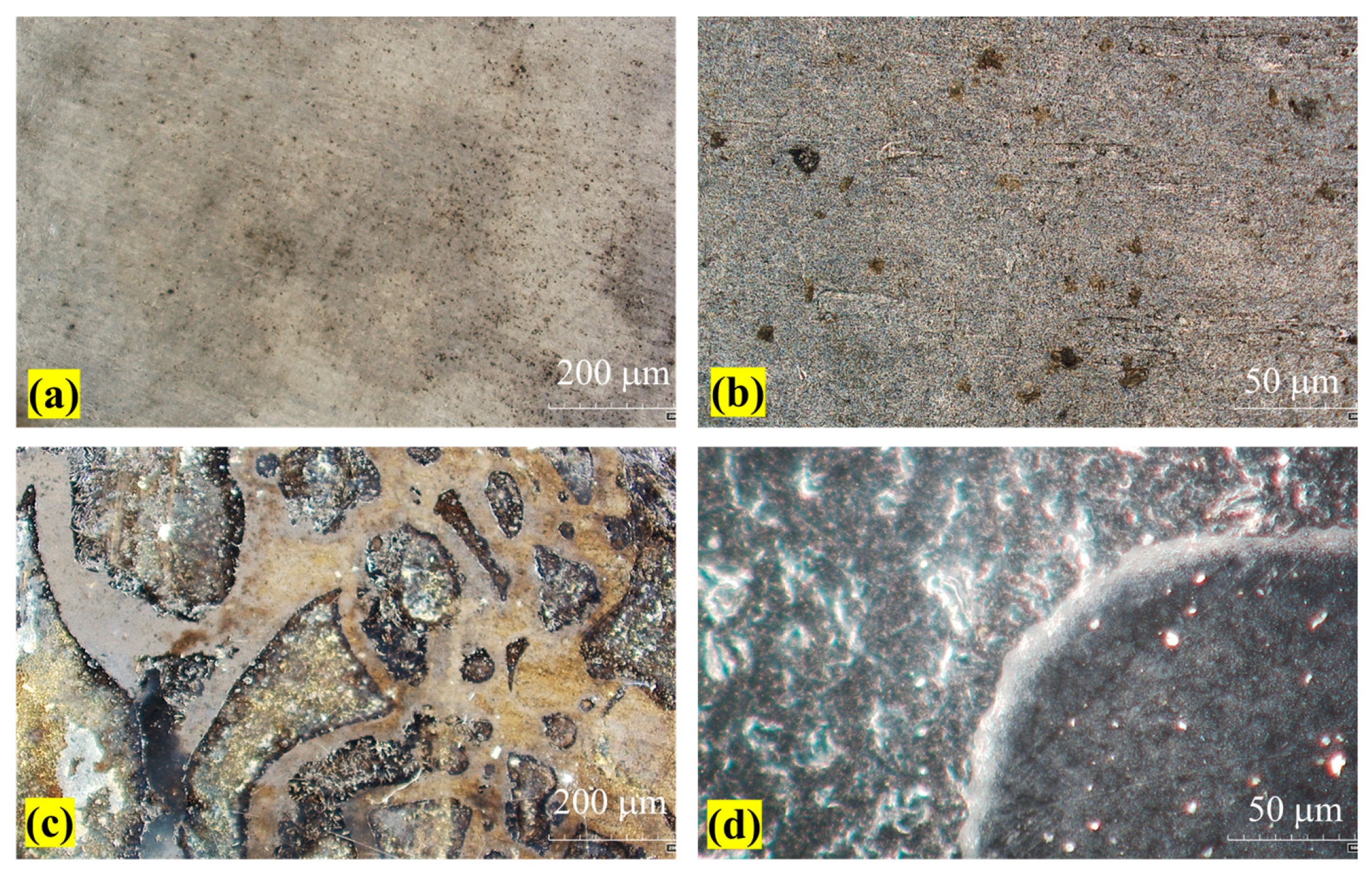
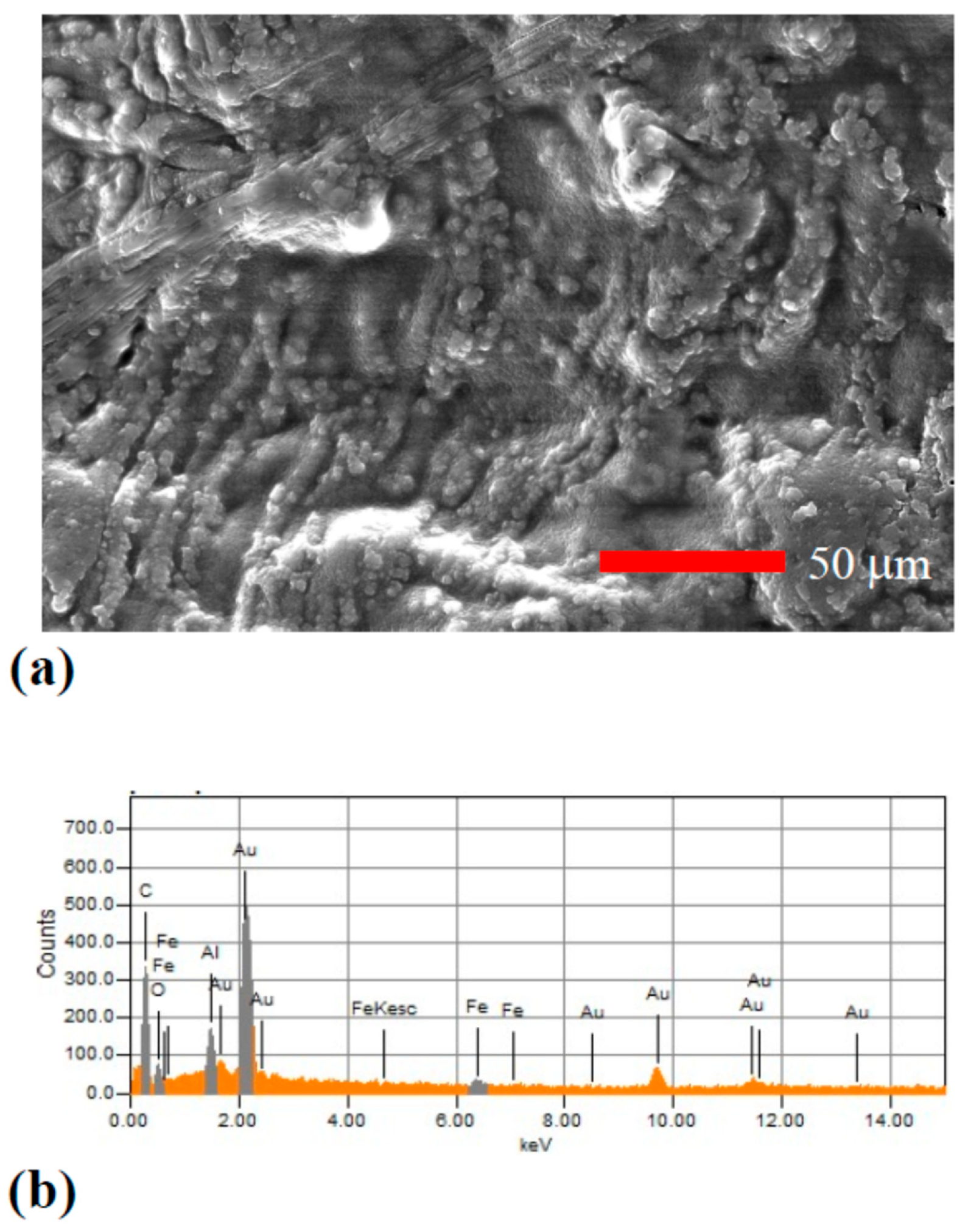
3.1. Microstructure and Composition of the Hydrothermal Carbonization Coating
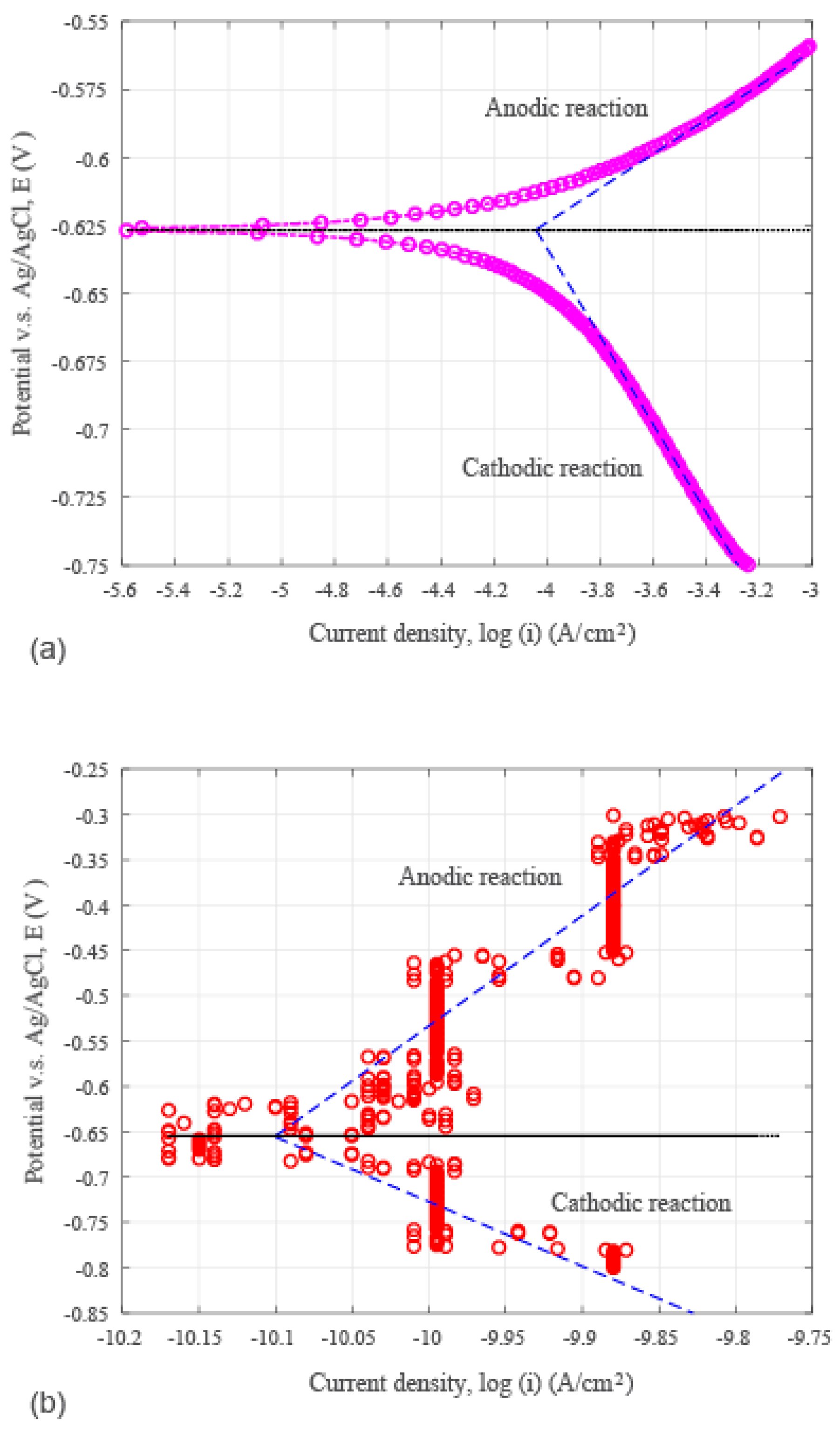
3.2. Seawater Corrosion Behavior of the Hydrothermal Carbonization Coating
3.3. Tafel Constants
- icorr is the corrosion current in amperes per unit area (A/cm2 in this work);
- Ecorr is the corrosion potential in volts vs. the Ag/AgCl reference electrode;
- E is the electrode potential in volts vs. the Ag/AgCl reference electrode;
- βa and βc are the anodic and cathodic β Tafel constants in volts per decade.
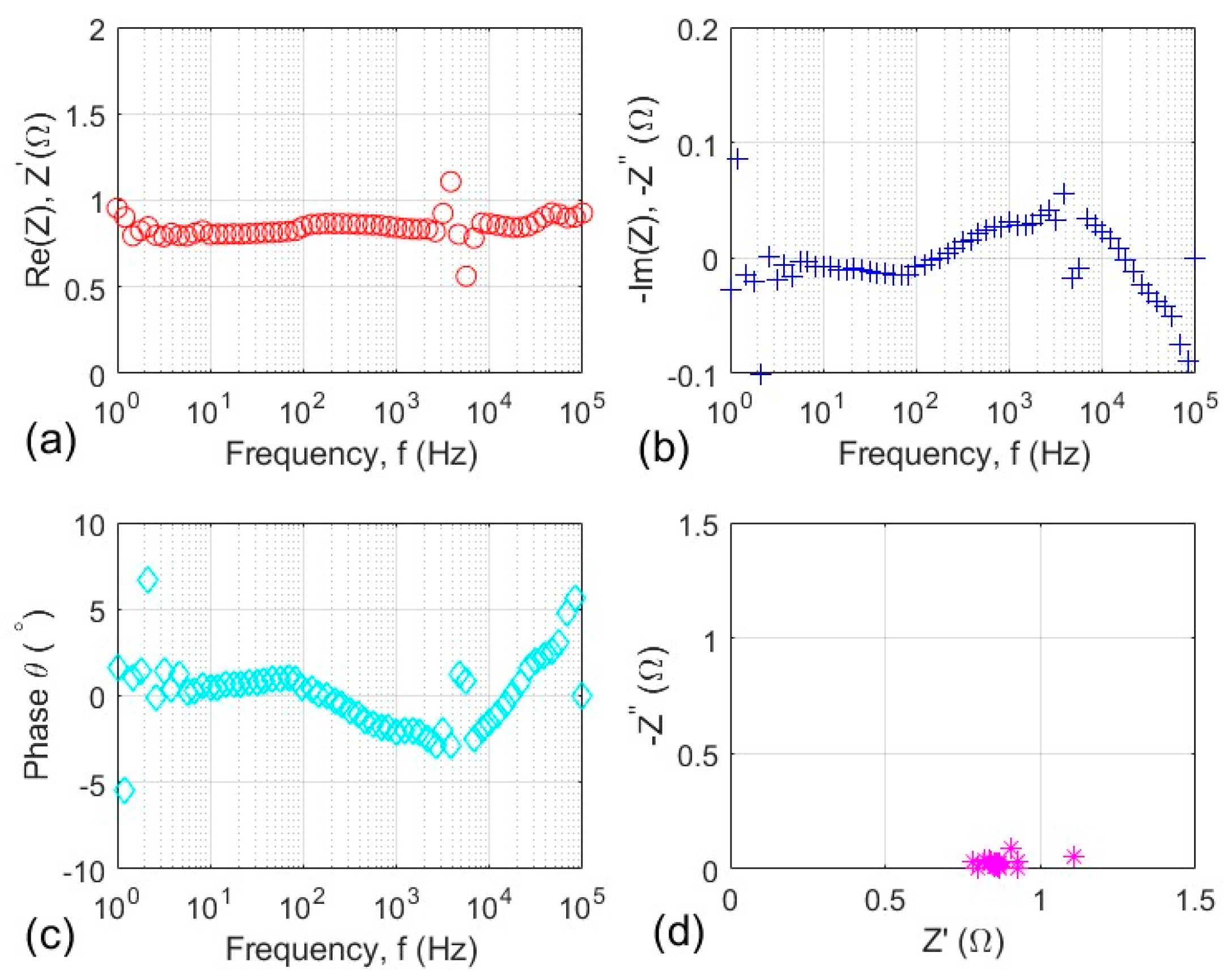
3.4. Electrochemical Impedance
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crespi, P.; Zucca, M.; Valente, M.; Longarini, N. Influence of corrosion effects on the seismic capacity of existing RC bridges. Eng. Fail. Anal. 2022, 140, 106546. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Hu, X.; Zhao, X.L.; Zhang, G.Z.; Shi, C.J. Chloride induced corrosion behavior of carbon steel rebar in seawater mixed Portland cement-SF-MK ternary pastes with low w/b ratio. Cem. Concr. Compos. 2025, 163, 106182. [Google Scholar] [CrossRef]
- Yang, G.H.; Liu, H.Q.; Zhong, H.X.; Bai, Z.P.; Zhi, F.F.; Chen, Q.; Zhu, P.F.; Liu, X.; Chu, H.Q.; Jiang, L.H. Inhibition properties and mechanism of DNA primer inhibitor for Q355 mild steel corrosion in simulated seawater. Surf. Interface 2025, 75, 107759. [Google Scholar] [CrossRef]
- Wang, H.Y.; Liang, Z.P.; Wang, Y.Y.; Jin, H.; Cao, H.Y.; Yang, C.C. Sustainable corrosion inhibition of EH40 steel in seawater using carboxymethyl chitosan/L-lysine composite. J. Environ. Chem. Eng. 2025, 13, 119271. [Google Scholar] [CrossRef]
- Dobo, K.I.; Sarkodie, K.; Agyei, E.; Adenutsi, C.D. Assessing the corrosion inhibition potential of novel green and sustainable plant leaf extracts on mild steel in seawater—Preliminary studies. Next Res. 2025, 2, 100548. [Google Scholar] [CrossRef]
- Yang, G.M.; Huang, T.J.; Sun, B.Z.; Wang, Y.S.; Liu, C.; Huang, F.; Liu, Z.Y.; Du, C.W.; Li, X.G. Effect of trace-Mg treatment on stress corrosion cracking behavior of different heat-affected zone microstructures of X70 steel in simulated seawater. Corros. Sci. 2026, 258, 113437. [Google Scholar] [CrossRef]
- Xin, Z.G.; Guo, L.; Wang, F.J.; Luo, Q.; Wang, L.G.; Chen, H.D.; Liu, Z.Y.; Jiang, J.Y. Insights into the corrosion resistance of Cr-Mo alloy reinforcing steel in simulated pore solution and real seawater sea-sand concrete: A preliminary study. Case Stud. Constr. Mater. 2025, 23, e05002. [Google Scholar] [CrossRef]
- Tavangar, R.; Geyter, N.D.; Verbeken, K.; Depover, T. Elucidating the natural evolution of Mg-Fe-CO3 layered double hydroxide in artificial seawater on construction steel and its corrosion resistance. Eng. Fail. Anal. 2025, 182, 110061. [Google Scholar] [CrossRef]
- Jaume, J.; Mercier, D.; Seyeux, A.; Semetey, V.; Zanna, S.; Marcus, P. Dual role of marine bacteria Pseudoalteromonas NCIMB 2021 in corrosion of mild steel in artificial seawater. Corros. Sci. 2025, 255, 113076. [Google Scholar] [CrossRef]
- Gan, Y.X.; Chang, Y.; Chen, C.-C.; Li, M.; Gan, J.B.; Li, J. Seawater corrosion of copper and its alloy coated with hydrothermal carbon. Coatings 2022, 12, 798. [Google Scholar] [CrossRef]
- Li, L.Y.; Cui, L.Y.; Liu, B.; Zeng, L.C.; Chen, X.B.; Li, S.Q.; Wang, Z.L.; Han, E.H. Corrosion resistance of glucose-induced hydrothermal calcium phosphate coating on pure magnesium. Appl. Surf. Sci. 2019, 465, 1066–1077. [Google Scholar] [CrossRef]
- Lin, Y.; Cai, S.; Jiang, S.; Xie, D.; Ling, R.; Sun, J.; Wei, J.; Shen, K.; Xu, G. Enhanced corrosion resistance and bonding strength of Mg substituted β-tricalcium phosphate/Mg(OH)2 composite coating on magnesium alloys via one-step hydrothermal method. J. Mech. Behav. Biomed. Mater. 2019, 90, 547–555. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Zhu, L.G.; Cai, S.; Shen, S.B. Corrosion-resistant fluoridated Ca-Mg-P composite coating on magnesium alloys prepared via hydrothermal assisted sol-gel process. J. Mater. Res. 2018, 33, 3793–3800. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M.H. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Xie, J.S.; Zhang, J.H.; Liu, S.J.; Li, Z.H.; Zhang, L.; Wu, R.Z.; Hou, L.G.; Zhang, M.L. Hydrothermal synthesis of protective coating on Mg alloy for degradable implant applications. Coatings 2019, 9, 160. [Google Scholar] [CrossRef]
- Song, D.; Guo, G.H.; Jiang, J.H.; Zhang, L.W.; Ma, A.B.; Ma, X.L.; Chen, J.Q.; Cheng, Z.J. Hydrothermal synthesis and corrosion behavior of the protective coating on Mg-2Zn-Mn-Ca-Ce alloy. Prog. Nat. Sci. Mater. Int. 2016, 26, 590–599. [Google Scholar] [CrossRef]
- Song, D.; Li, C.; Zhang, L.W.; Ma, X.L.; Guo, G.H.; Zhang, F.; Jiang, J.H.; Ma, A.B. Decreasing bio-degradation rate of the hydrothermal-synthesizing coated Mg alloy via pre-solid-solution treatment. Materials 2017, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Y.K.; Ryu, M.H.; Bae, T.S.; Lee, M.H. Corrosion resistance and bioactivity enhancement of MAO coated Mg alloy depending on the time of hydrothermal treatment in Ca-EDTA solution. Sci. Rep. 2017, 7, 9061. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.D.; Zhang, K.Y.; Sun, W.; Wu, T.T.; He, H.R.; Liu, G.C. Hydrothermal synthesis of corrosion resistant hydrotalcite conversion coating on AZ91D alloy. Mater. Lett. 2013, 106, 111–114. [Google Scholar] [CrossRef]
- Wang, C.W.; Liu, C.; Lin, D.J.; Yeh, M.L.; Lee, T.M. Hydrothermal treatment and butylphosphonic acid derived self-assembled monolayers for improving the surface chemistry and corrosion resistance of AZ61 magnesium alloy. Sci. Rep. 2017, 7, 16910. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, Y.L.; Liu, C.L. Hydroxyapatite coating on fluorine-treated magnesium alloy by hydrothermal method and its electrochemical corrosion behaviour in Hank’s solution. Prot. Met. Phys. Chem. Surf. 2019, 55, 127–135. [Google Scholar] [CrossRef]
- Ling, L.; Cai, S.; Li, Q.Q.; Sun, J.Y.; Bao, X.G.; Xu, G.H. Recent advances in hydrothermal modification of calcium phosphorus coating on magnesium alloy. J. Magnes. Alloys 2022, 10, 62–81. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, G.M.; Zhao, Q.; Zhang, Y.H.; Xing, G.J.; Li, D.L. Anticorrosive magnesium hydroxide coating on AZ31 magnesium alloy by hydrothermal method. J. Phys. Conf. Ser. 2009, 188, 12044. [Google Scholar] [CrossRef]
- Roldan, L.; Santos, I.; Armenise, S.; Fraile, J.M.; Garcıa-Bordeje, E. The formation of a hydrothermal carbon coating on graphite microfiber felts for using as structured acid catalyst. Carbon 2012, 50, 1363–1372. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Replication and coating of silica templates by hydrothermal carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Luan, D.Y.; Madhavi, S.; Hu, Y.; Lou, X.W. Assembling carbon-coated α-Fe2O3 hollow nanohorns on the CNT backbone for superior lithium storage capability. Energy Environ. Sci. 2012, 5, 5252–5256. [Google Scholar] [CrossRef]
- Wang, Z.F.; Xiao, P.F.; He, N.Y. Synthesis and characteristics of carbon encapsulated magnetic nanoparticles produced by a hydrothermal reaction. Carbon 2006, 44, 3277–3284. [Google Scholar] [CrossRef]
- Ashik, U.P.M.; Asano, S.; Kudo, S.; Minh, D.P.; Appari, S.; Hisahiro, E.; Hayashi, J. The distinctive effects of glucose-derived carbon on the performance of Ni-based catalysts in methane dry reforming. Catalysts 2020, 10, 21. [Google Scholar] [CrossRef]
- Teng, F.; Hu, Z.H.; Ma, X.H.; Zhang, L.C.; Ding, C.X.; Yu, Y.; Chen, C.H. Hydrothermal synthesis of plate-like carbon-coated Li3V2(PO4)3 and its low temperature performance for high power lithium ion batteries. Electrochim. Acta 2013, 91, 43–49. [Google Scholar] [CrossRef]
- Pei, B.; Wang, Q.; Zhang, W.X.; Yang, Z.H.; Chen, M. Enhanced performance of LiFePO4 through hydrothermal synthesis coupled with carbon coating and cupric ion doping. Electrochim. Acta 2011, 56, 5667–5672. [Google Scholar] [CrossRef]
- Marcus, P. Corrosion Mechanisms in Theory and Practices, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 47. [Google Scholar]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Cheng, Y.F.; Huang, W.W.; Zhang, Z.Y.; Lei, Z.F.; Shimizu, K.; Utsumi, M. Fertilizer potential of liquid product from hydrothermal treatment of swine manure. Waste Manag. 2018, 77, 166–171. [Google Scholar] [CrossRef] [PubMed]


| Element Type | Carbon (C) | Manganese (Mn) | Phosphorus (P) | Sulfur (S) |
|---|---|---|---|---|
| Content (wt.%) | 0.15~0.20 | 0.60~0.90 | <0.05 | <0.04 |
| Specimen Type | βc (V/dec) | βa (V/dec) | icorr (A/cm2) | Ecorr (V) |
|---|---|---|---|---|
| With coating | −0.713 | 1.215 | 7.943 × 10−11 | −0.655 |
| Without coating | −0.162 | 0.063 | 8.642 × 10−5 | −0.627 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, Y.X. Hydrothermal Carbonization Coating on AISI 1018 Steel for Seawater Corrosion Protection. Coatings 2025, 15, 1346. https://doi.org/10.3390/coatings15111346
Gan YX. Hydrothermal Carbonization Coating on AISI 1018 Steel for Seawater Corrosion Protection. Coatings. 2025; 15(11):1346. https://doi.org/10.3390/coatings15111346
Chicago/Turabian StyleGan, Yong X. 2025. "Hydrothermal Carbonization Coating on AISI 1018 Steel for Seawater Corrosion Protection" Coatings 15, no. 11: 1346. https://doi.org/10.3390/coatings15111346
APA StyleGan, Y. X. (2025). Hydrothermal Carbonization Coating on AISI 1018 Steel for Seawater Corrosion Protection. Coatings, 15(11), 1346. https://doi.org/10.3390/coatings15111346






