Research Status and Progress on the Current-Carrying Friction and Wear Performance of Conductive Slip Rings in Harsh Environments
Abstract
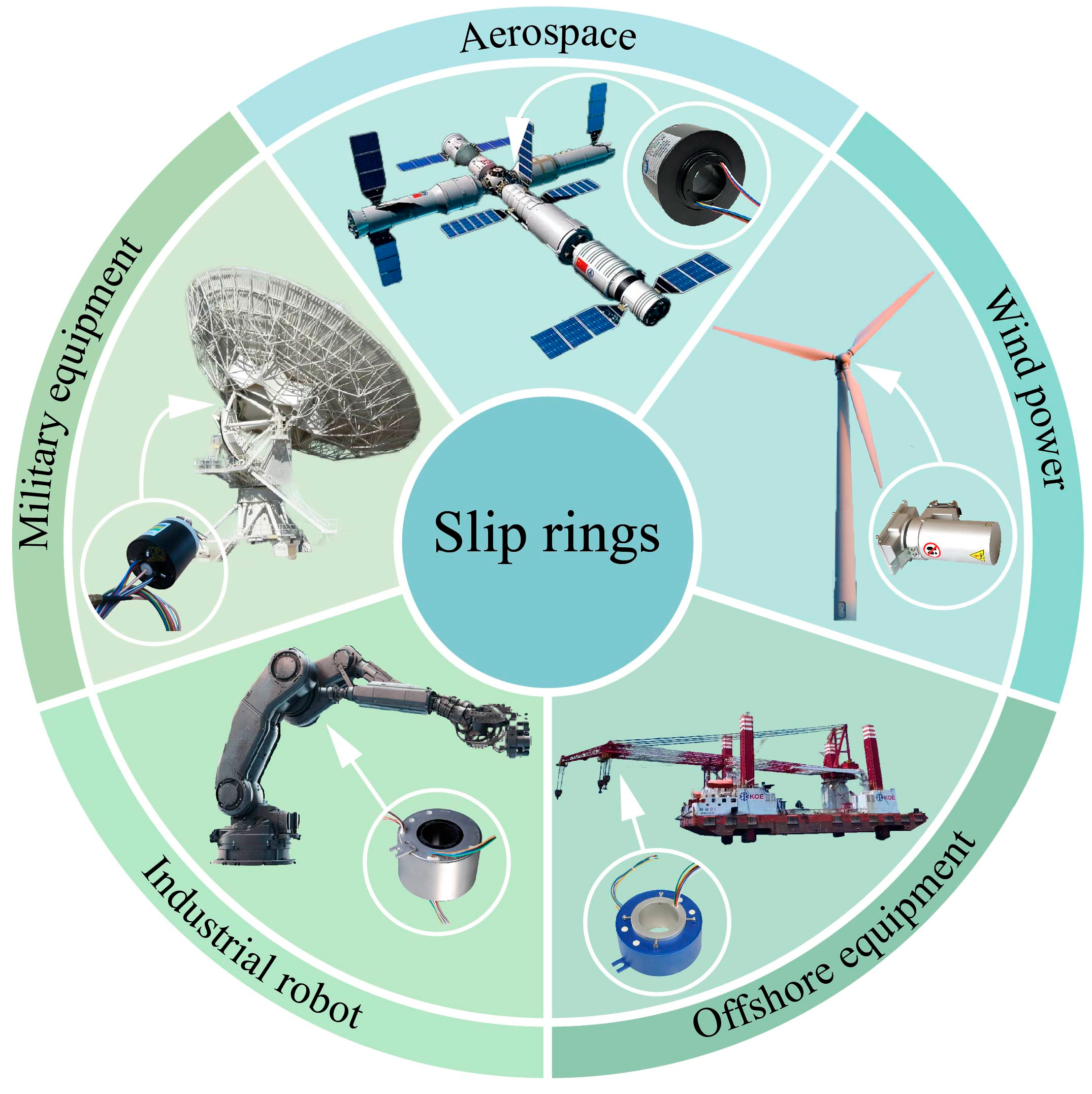
1. Introduction
2. Effects of Harsh Environments on the Current-Carrying Friction and Wear Behavior of Slip Rings
2.1. Effects of High Temperature
2.1.1. Effects of High Temperature on the Physical Properties of Materials
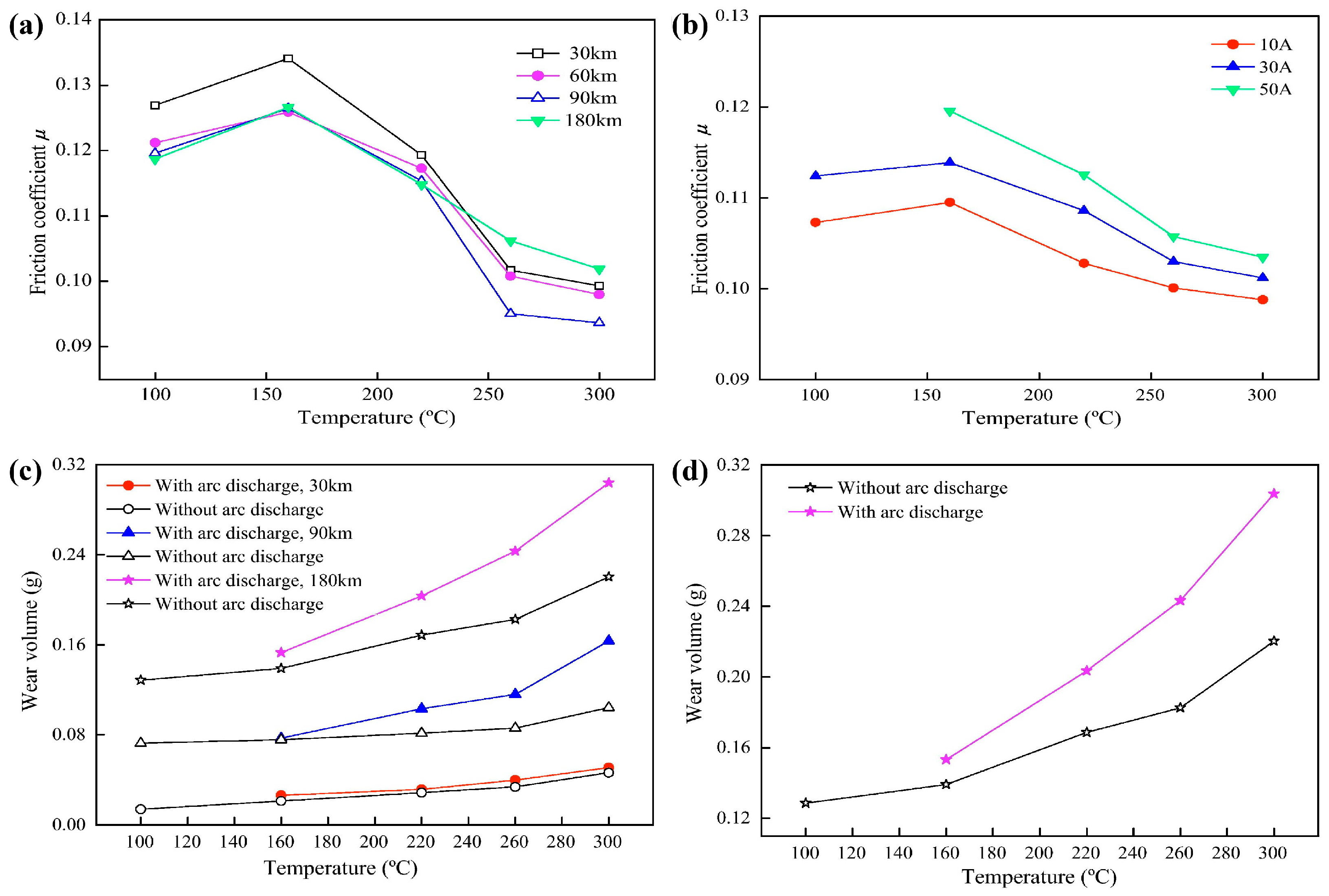
2.1.2. Effects of High Temperature on Tribological Behavior
2.1.3. Oxidative Wear Mechanism Under High Temperature
2.1.4. Adhesive Wear and Arc Erosion at High Temperatures
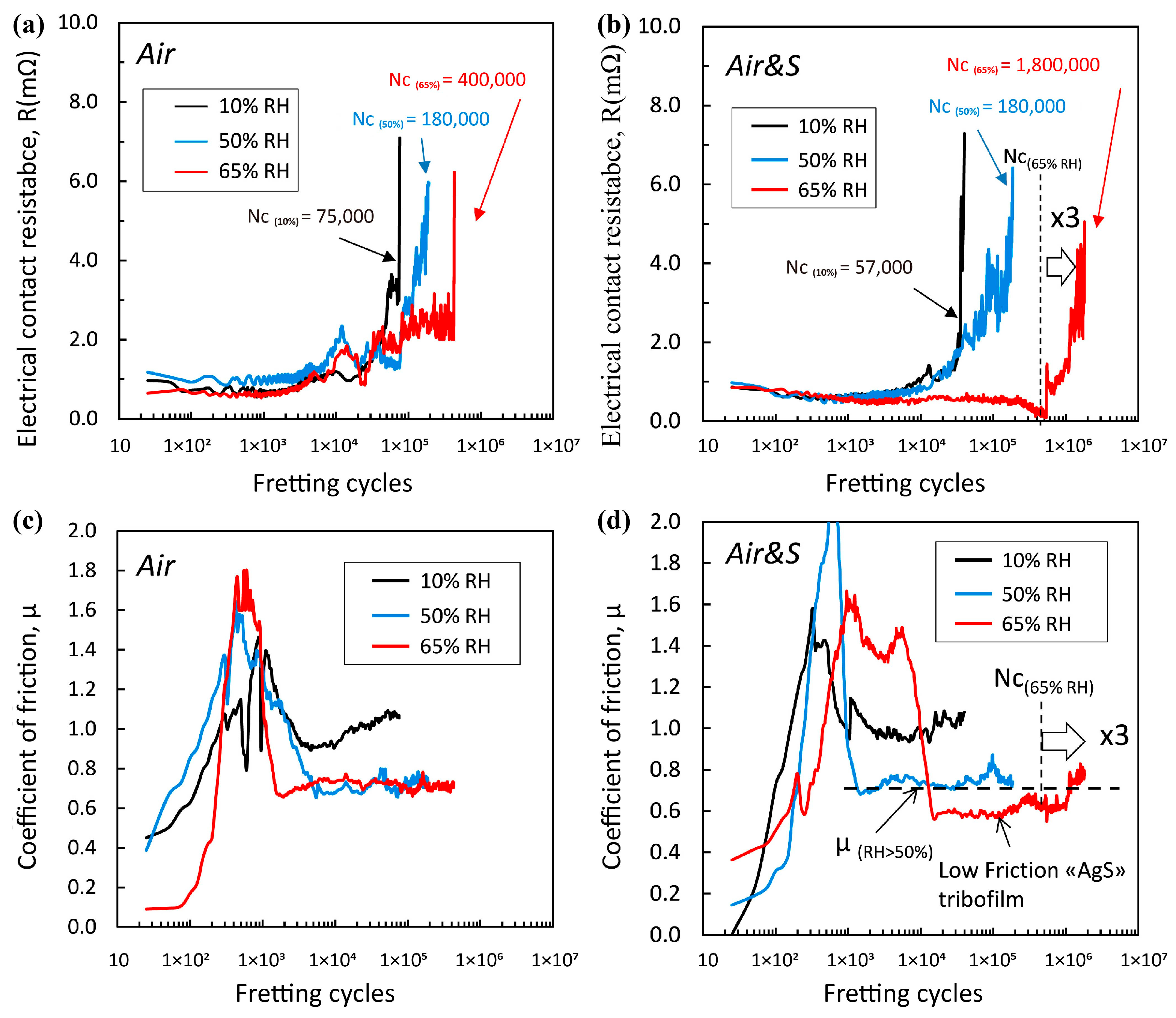
2.2. Effect of Humidity
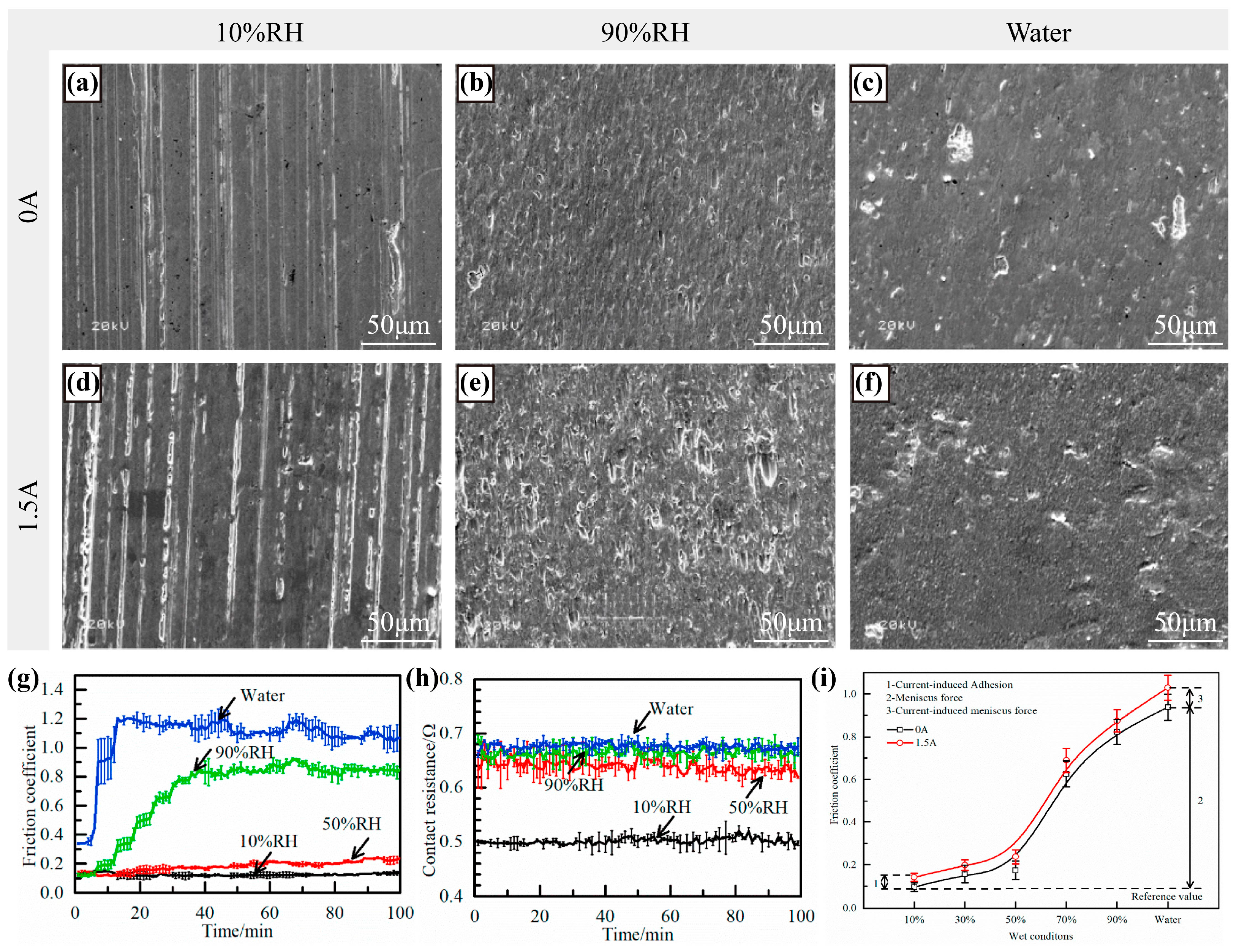
2.2.1. Effects of Humidity on the Formation of the Lubricating Film
2.2.2. Effects of Humidity on Electrochemical Corrosion
2.2.3. Effects of Humidity on Wear Modes
2.3. Effects of Corrosive Gases
2.3.1. Formation and Characteristics of Corrosion Products
2.3.2. Synergistic Mechanism of Corrosion and Wear
2.3.3. Corrosion-Resistant Materials and Protection Strategies
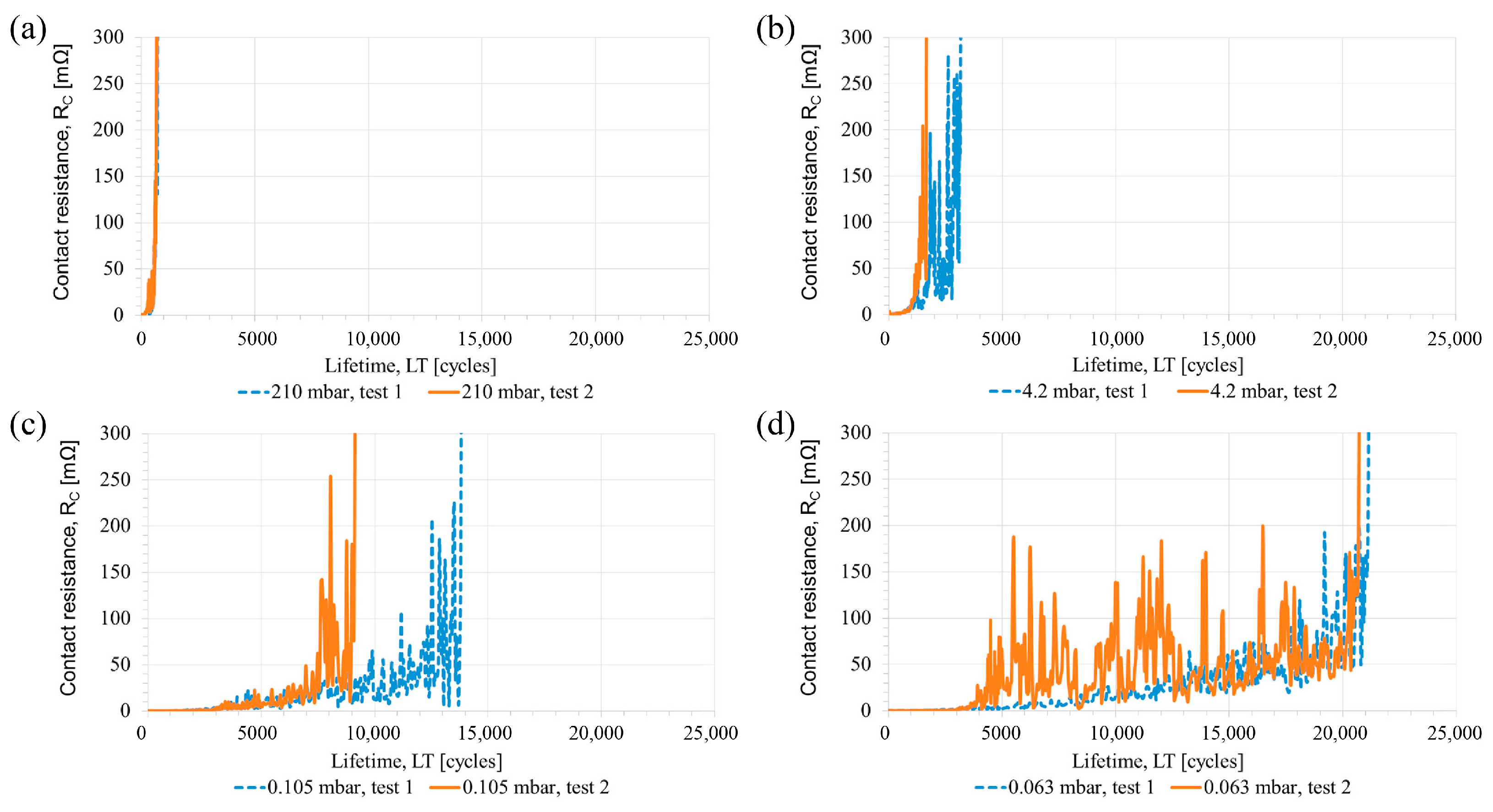
2.4. Effects of Vacuum Environment
2.4.1. Adhesive Wear Caused by the Absence of Oxide Film
2.4.2. Performance of Solid Lubricants in Vacuum
2.4.3. Arc Discharge Effects in Vacuum
2.5. Summary
3. Simulation and Modeling Research
3.1. Multiphysics Modeling
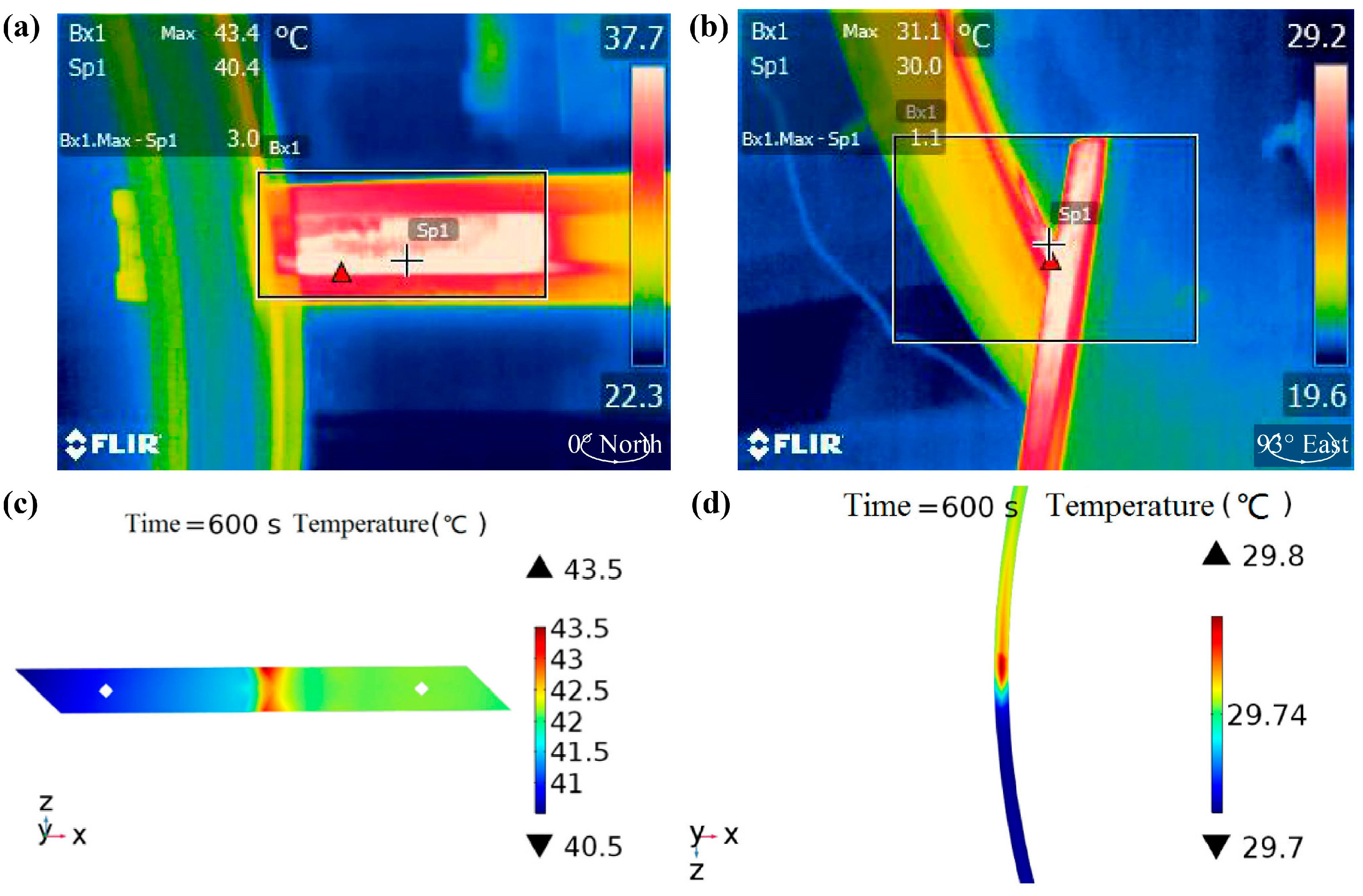
3.1.1. Temperature Field Modeling
3.1.2. Electromagnetic–Fluid Coupled Modeling
3.1.3. Rough Surface Contact Modeling
3.2. Wear Models and Prediction
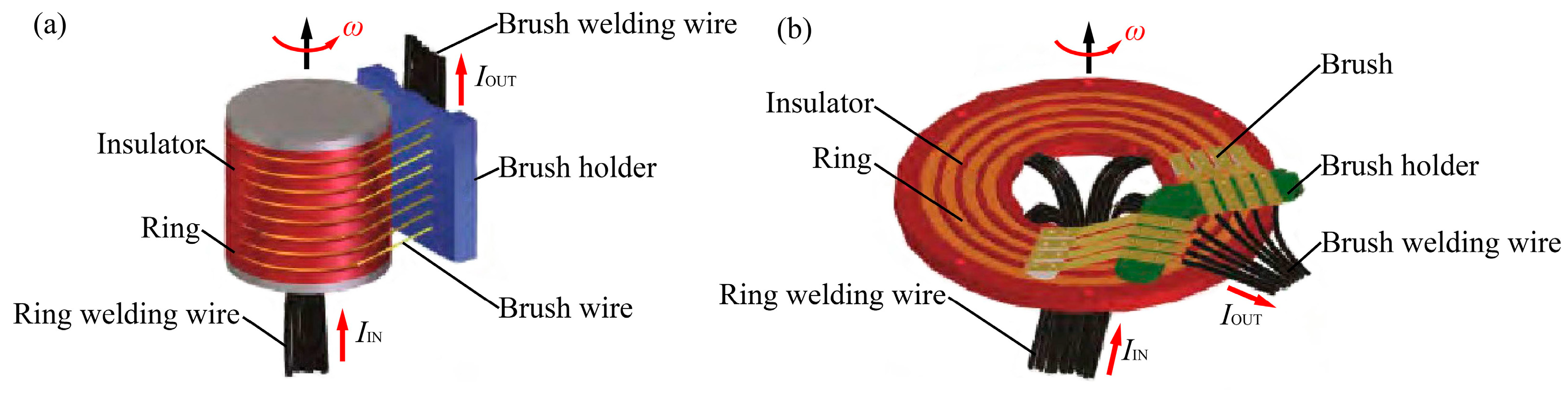
3.2.1. Modeling of Brush–Slip Ring Systems
3.2.2. Contact Resistance Modeling
3.2.3. Development of Wear Prediction Models
3.2.4. Digital Twin and Life Prediction
3.2.5. Molecular Dynamics Simulation
3.3. Summary
4. Research on Improving Current-Carrying Friction and Wear Performance of Slip Rings Under Harsh Environments
4.1. Material Modification Methods
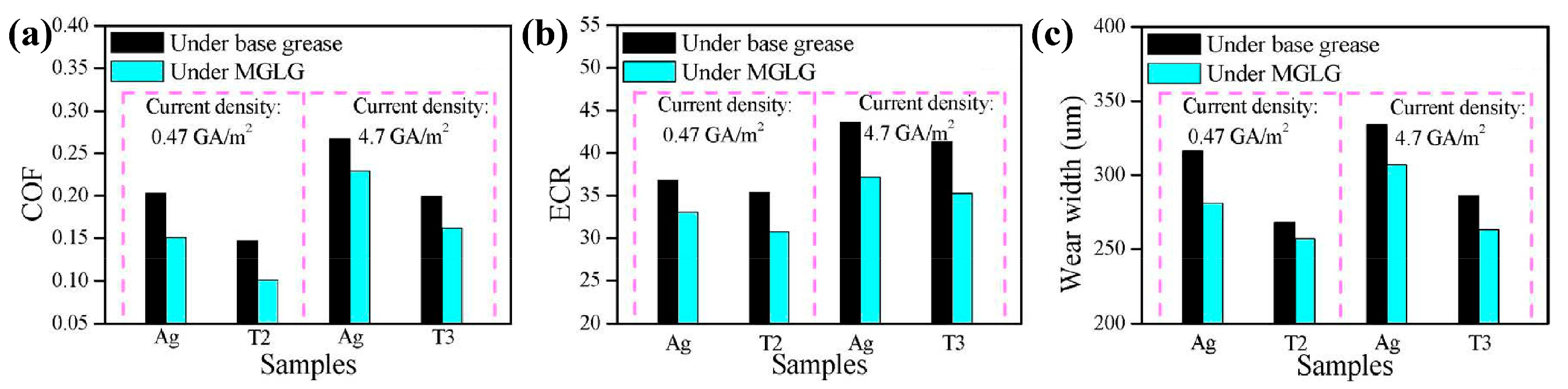
4.2. Surface Coating Technology
4.3. Summary
5. Conclusions
- The extent to which environmental factors exert influence varies significantly. High temperature and humidity are the most critical environmental factors. Elevated temperature (>150 °C) can increase the wear rate by 200%–500% through the synergistic action of multiple mechanisms, including material softening, accelerated oxidation, and arc intensification. Humidity exhibits a distinctive non-monotonic effect: 40%–60% relative humidity is the optimal operating window, and deviations from this range lead the wear mechanism to shift from adhesive wear to fatigue spalling. The corrosion–wear synergistic effect of corrosive gases is irreversible and is markedly intensified under high humidity. Vacuum conditions increase the coefficient of friction by a factor of 2–3, yet enhance the stability of contact resistance, necessitating entirely different material solutions.
- The synergistic effects of multiple factors far exceed the simple superposition of single factors, yet quantitative descriptions remain severely inadequate. The coupling of high temperature and humidity accelerates electrochemical corrosion, while the coupling of high temperature and current may induce thermal runaway. However, current research mainly relies on single-factor experiments, lacking systematic studies on multi-factor coupling, making it difficult to accurately predict material behavior under real service conditions.
- The depth of mechanistic understanding is uneven, and cross-scale linkages are lacking. The macroscopic phenomena influenced by high temperature and humidity are relatively well documented, but the elucidation of microscopic mechanisms is weak. Fundamental questions—such as the nature of the “low-friction–high-wear” paradox, the dual role of oxide films, and the dynamic cycling of lubricant films—still lack clear answers. A cross-scale theoretical framework linking atomic bonding to macroscopic wear has yet to be established.
- The practicality of simulation technologies is limited. Multiphysics models have successfully captured electro-thermal-mechanical coupling effects, but their descriptions of key processes such as chemical reactions, phase transitions, and arc discharges are overly simplified. Most wear prediction models are based on empirical correlations, and their applicability during mechanism transitions remains questionable. More critically, parameter acquisition is difficult and validation is insufficient, leaving extrapolation capability and long-term predictive reliability unverified. Simulation studies addressing environmental factors are far fewer than those focusing on operational parameters, creating a gap between research and practical needs.
- Material optimization strategies are diverse but lack systematic design principles. Copper-, silver-, and gold-based materials, as well as novel composite materials, each possess their own advantages and disadvantages. Although numerous surface coating technologies exist, there is still a lack of systematic design criteria and long-term stability validation for the rational selection of materials and coating systems according to specific environments.
- Cross-scale mechanistic linkage and dynamic process tracking. Establish a multiscale integrated framework spanning from first-principles calculations to macroscopic finite-element analysis; develop in situ multiphysics characterization techniques to track in real time the dynamic processes of lubricant-film formation–rupture, oxide-film growth–spallation, and arc initiation–extinction, thereby elucidating the quantitative links between microscopic mechanisms and macroscopic performance.
- Quantitative theory of multi-factor coupling effects. Systematically conduct studies on multi-factor interactions and establish physical models for quantitatively describing coupling effects. Develop phenomenological models grounded in mechanistic understanding, integrate machine learning to extract patterns from big data, and advance failure criteria and lifetime prediction models under multi-factor conditions.
- Extreme-environment parameter databases and high-fidelity modeling. Systematically measure material parameters under high temperature–high current, extreme humidity, and corrosive atmospheres, and establish an open, shared database. Develop comprehensive physical models encompassing chemical reactions, phase transformations, and arc discharge to improve the accuracy and reliability of simulation predictions. Prioritize breakthroughs in modeling methods for environmental factors.
- Materials genome engineering and intelligent response systems. Integrate computational materials science, high-throughput experiments, and machine learning to establish a rapid iterative process of “computational screening–experimental validation–performance optimization.” Develop performance-driven inverse design methods for materials, and explore intelligent material systems such as temperature-responsive self-lubricating materials and humidity-adaptive coatings, achieving a transition from passive adaptation to active control.
- Digital twins and intelligent health management. Integrate physics-based models with data-driven approaches to establish real-time condition monitoring and remaining useful life (RUL) prediction systems for slip ring systems. Develop adaptive algorithms that dynamically update model parameters based on real-time data, and construct intelligent decision-making systems to optimize operating conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Zhang, K.; Liu, J.; Qu, Q.; Jiang, J.; Li, R. Research and development review of space conductive slip ring technology. J. Mech. Eng. 2022, 58, 334–348. [Google Scholar]
- Yu, X.; Li, H.; Gao, X.; Chhattal, M.; Zheng, Q.; Wu, W.; Gong, Z.; Wang, N.; Liu, J. Effect of Heavy Ion Irradiation on Friction of GNSs: Implications for Aerospace Lubrication. ACS Appl. Nano Mater. 2024, 7, 12035–12042. [Google Scholar] [CrossRef]
- Yan, S.; Wang, A.; Liu, R.; Ma, X.; Qi, M.; Zhao, Z.; Zhao, T.; You, C. Study of Ordered Phase Precipitation Behaviour and Hardening Mechanism of Au-20Ag-10Cu Alloy during Deformation-Aging Treatment. J. Alloys Compd. 2024, 976, 173007. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Kang, Y.; Li, Z.; Li, H. Progress on Current-Carry Friction and Wear: An Overview from Measurements to Mechanism. Coatings 2022, 12, 1345. [Google Scholar] [CrossRef]
- Papastergiou, K.D.; Macpherson, D.E. An Airborne Radar Power Supply with Contactless Transfer of Energy—Part II: Converter Design. IEEE Trans. Ind. Electron. 2007, 54, 2885–2893. [Google Scholar] [CrossRef]
- Ayaz, E.; Altun, O.; Keysan, O. Carrier Phase Shift Method of SPWM for Concurrent Wired and Wireless Power Transfer Systems. IEEE Trans. Ind. Electron. 2023, 70, 8645–8654. [Google Scholar] [CrossRef]
- Ludois, D.C.; Reed, J.K.; Hanson, K. Capacitive Power Transfer for Rotor Field Current in Synchronous Machines. IEEE Trans. Power Electron. 2012, 27, 4638–4645. [Google Scholar] [CrossRef]
- Zhou, X.; Dai, C.; Liu, G.; Tan, X.; Zhang, Z.; Pan, Y.; Pan, H.; Ahmed, A. A Hybrid Wind Energy Harvester Based on a Double-Rotor Reverse Synergy Mechanism for High-Speed Railway. Sustain. Cities Soc. 2023, 90, 104389. [Google Scholar] [CrossRef]
- Jabr, H.M.; Lu, D.; Kar, N.C. Design and Implementation of Neuro-Fuzzy Vector Control for Wind-Driven Doubly-Fed Induction Generator. IEEE Trans. Sustain. Energy 2011, 2, 404–413. [Google Scholar] [CrossRef]
- Zhao, Q.; Lai, J.; Hu, X.; Chu, H.K. Dual-Segment Continuum Robot with Continuous Rotational Motion along the Deformable Backbone. IEEE/ASME Trans. Mechatron. 2022, 27, 4994–5004. [Google Scholar] [CrossRef]
- Edwards, E.C.; Holcombe, A.; Brown, S.; Ransley, E.; Hann, M.; Greaves, D. Evolution of Floating Offshore Wind Platforms: A Review of at-Sea Devices. Renew. Sustain. Energy Rev. 2023, 183, 113416. [Google Scholar] [CrossRef]
- Hod, O.; Meyer, E.; Zheng, Q.; Urbakh, M. Structural Superlubricity and Ultralow Friction across the Length Scales. Nature 2018, 563, 485–492. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, T.; Erdemir, A.; Li, Q. Tribology of Two-Dimensional Materials: From Mechanisms to Modulating Strategies. Mater. Today 2019, 26, 67–86. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, J.; Jin, Z.; Prakash, B.; Hu, Y. A Review of Recent Advances in Tribology. Friction 2020, 8, 221–300. [Google Scholar] [CrossRef]
- Murua, J.; Ibañez, I.; Dianova, A.; Domínguez-Meister, S.; Larrañaga, O.; Larrañaga, A.; Braceras, I. Tribological and Electric Contact Resistance Properties of Pulsed Plasma Duplex Treatments on a Low Alloy Steel. Surf. Coat. Technol. 2023, 454, 129155. [Google Scholar] [CrossRef]
- Shen, M.; Ji, D.; Hu, Q.; Xiao, L.; Li, Q. Current-Carrying Tribological Behavior of C/Cu Contact Pairs in Extreme Temperature and Humidity Environments for Railway Catenary Systems. Sci. China Technol. Sci. 2024, 67, 2537–2548. [Google Scholar] [CrossRef]
- Shin, W.-G.; Lee, S.-H. Determination of Accelerated Condition for Brush Wear of Small Brush-Type DC Motor in Using Design of Experiment (DOE) Based on the Taguchi Method. J. Mech. Sci. Technol. 2011, 25, 317–322. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Hu, M.; Zhou, C.; Xiao, Q.; Yang, W.; Chen, D. Research on the Wear Properties of Carbon Strips and Contact Wires at Frigid Temperatures. Wear 2021, 486–487, 204122. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Milone, C.; Mastronardo, E.; Piperopoulos, E.; Iemmo, L.; Di Bartolomeo, A. Effect of Temperature and Morphology on the Electrical Properties of PET/Conductive Nanofillers Composites. Compos. Part B Eng. 2018, 135, 149–154. [Google Scholar] [CrossRef]
- Jedrzejczyk, P.; Fouvry, S.; Chalandon, P. A Fast Methodology to Quantify Electrical-Contact Behaviour under Fretting Loading Conditions. Wear 2009, 267, 1731–1740. [Google Scholar] [CrossRef]
- Ogar, P.; Ugryumova, E.; Koryakyn, I. The Influence of the Mechanical Properties of Copper at Elevated Temperatures on the Tightness of the Sealing Joint. Mater. Today Proc. 2021, 38, 1764–1768. [Google Scholar] [CrossRef]
- Ji, D.-H.; Xiao, L.; Hu, Q.; Chen, S.; Li, Q.; Shen, M. The Effect of Temperature on the Current-Carrying Tribological Behaviour of C/Cu Contact Pairs in High Humidity Environments. Tribol. Lett. 2024, 72, 63. [Google Scholar] [CrossRef]
- Wang, H.; Gao, G.; Lei, D.; Wang, Q.; Xiao, S.; Xie, Y.; Xu, Z.; Ma, Y.; Dong, K.; Chen, Q.; et al. Influence of Interface Temperature on the Electric Contact Characteristics of a C-Cu Sliding System. Coatings 2022, 12, 1713. [Google Scholar] [CrossRef]
- Lyu, Y.; Bergseth, E.; Wahlström, J.; Olofsson, U. A Pin-on-Disc Study on the Tribology of Cast Iron, Sinter and Composite Railway Brake Blocks at Low Temperatures. Wear 2019, 424–425, 48–52. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Liu, D.; Gu, Y.; Zheng, R.; Ma, R.; Li, S.; Wang, Y.; Shi, Y. The Tribological Performance of Metal-/Resin-Impregnated Graphite under Harsh Condition. Lubricants 2022, 10, 2. [Google Scholar] [CrossRef]
- Ding, T.; Wang, X.; Chen, G.; Zhu, M. Effect of temperature on the friction and wear properties of a carbon/copper friction pair with and without electric current. Chin. Mech. Eng. 2010, 21, 843–847. [Google Scholar]
- Turel, A.; Slavič, J.; Boltežar, M. Wear Rate vs. Dynamic and Material Properties at Elevated Temperatures for a Copper-Graphite Brush. J. Mech. Eng. 2018, 3, 169–175. [Google Scholar]
- Yu, Y.; Feng, S.; Liu, Y.; Yan, Z.; Chen, G.; Yang, J.; Zhang, W. Effect of Temperature on the Mechanical Properties, Conductivity, and Microstructure of Multi-Layer Graphene/Copper Composites Fabricated by Extrusion. J. Mater. Eng. Perform. 2024, 34, 7773–7785. [Google Scholar] [CrossRef]
- Mei, G.; Fu, W.; Chen, G.; Zhang, W. Effect of High-Density Current on the Wear of Carbon Sliders against Cu–Ag Wires. Wear 2020, 452–453, 203275. [Google Scholar] [CrossRef]
- Wang, H.; Gao, G.; Wei, W.; Yang, Z.; Yin, G.; Xie, W.; He, Z.; Ni, Z.; Yang, Y.; Wu, G. Influence of the Interface Temperature on the Damage Morphology and Material Transfer of C–Cu Sliding Contact under Different Current Amplitudes. J. Mater. Sci. 2022, 57, 5006–5021. [Google Scholar] [CrossRef]
- Ding, T.; Chen, G.X.; Bu, J.; Zhang, W.H. Effect of Temperature and Arc Discharge on Friction and Wear Behaviours of Carbon Strip/Copper Contact Wire in Pantograph–Catenary Systems. Wear 2011, 271, 1629–1636. [Google Scholar] [CrossRef]
- Liu, X.; Gao, M.; Hu, M.; Cao, Y.; Guan, X.; Zhang, W.; Zhang, S.; Xiao, Q.; Zheng, Y.; Chen, D.; et al. Study the Influence of Power-Current Impact with Different Conditions on the Current-Carrying Wear Property of Carbon Brush/Friction Disc Contact. Tribol. Int. 2023, 189, 108985. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, J.-K.; Xiao, S.-X.; Xu, G.-M.; Chen, J.; Zhang, C. Effect of Electrical Current on the Tribological Property of Cu-Graphite Brush. Tribol. Lett. 2024, 72, 29. [Google Scholar] [CrossRef]
- Kalin, M.; Poljanec, D. Influence of the Contact Parameters and Several Graphite Materials on the Tribological Behaviour of Graphite/Copper Two-Disc Electrical Contacts. Tribol. Int. 2018, 126, 192–205. [Google Scholar] [CrossRef]
- Azevedo, C.R.F.; Sinatora, A. Failure Analysis of a Railway Copper Contact Strip. Eng. Fail. Anal. 2004, 11, 829–841. [Google Scholar] [CrossRef]
- Wang, Y.A.; Li, J.X.; Yan, Y.; Qiao, L.J. Effect of Pv Factor on Sliding Friction and Wear of Copper-Impregnated Metallized Carbon. Wear 2012, 289, 119–123. [Google Scholar] [CrossRef]
- Liu, X.; Hu, M.; Li, Z.; Zhou, C.; Xiao, Q.; Yang, W.; Chen, D. Effect of Copper Contents on the Current-Carrying Wear Properties of Carbon Brush under Different Temperatures Conditions. J. Mater. Res. Technol. 2021, 15, 3110–3121. [Google Scholar] [CrossRef]
- Ren, W.; Chen, G.; Dong, B.; Song, Q.; Feng, X.; Li, L.; Yang, H.; He, Q. Experimental Study of the Effect of Contact Line Profiles on the Wear Mechanism of a Skateboard. Tribol. Int. 2024, 192, 109317. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Wang, C.; Xiao, S.-X.; Chen, J.; Zhang, C. Sliding Electrical Contact Properties of Highly Oriented Copper Fiber Brush. Wear 2023, 512–513, 204541. [Google Scholar] [CrossRef]
- Wang, H.B.; Liu, X.T.; Che, J.J.; Zhao, H.W.; Bhushan, B.; Tong, J.; Zhuang, J.; Ma, Y.H. Analysis of Abnormal Wear on a Certain Type of CRH380 High-Speed Railway Grounding Brush. Mater. Werkst. 2020, 51, 452–460. [Google Scholar] [CrossRef]
- Dong, B.; Hu, Y.; Chen, G.; Huang, H.; Wu, Y.; Cui, X.; Gao, G.; Wu, G. Experimental study on arc discharge and temperature rise of carbon slide under current-carrying pantograph–catenary friction. Lubr. Eng. 2016, 41, 41–45. [Google Scholar]
- Cao, Z.; Xia, Y.; Liu, L.; Feng, X. Study on the Conductive and Tribological Properties of Copper Sliding Electrical Contacts Lubricated by Ionic Liquids. Tribol. Int. 2019, 130, 27–35. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Liu, Z.; Li, J.; Sun, Y.; Shangguan, B.; Zhang, Y. Effect of Relative Humidity on the Tribological/Conductive Properties of Cu/Cu Rolling Contact Pairs. Wear 2019, 436–437, 203023. [Google Scholar] [CrossRef]
- Hu, Z.L.; Chen, Z.H.; Xia, J.T. Study on Surface Film in the Wear of Electrographite Brushes against Copper Commutators for Variable Current and Humidity. Wear 2008, 264, 11–17. [Google Scholar] [CrossRef]
- Zhi, X.; Zhou, N.; Cheng, Y.; Wang, X.; Wei, H.; Chen, G.; Zhang, W. Effect and Behaviors of Ambient Humidity on the Wear of Metal-Impregnated Carbon Strip in Pantograph-Catenary System. Tribol. Int. 2023, 188, 108864. [Google Scholar] [CrossRef]
- Lyu, Y.; Bergseth, E.; Tu, M.; Olofsson, U. Effect of Humidity on the Tribological Behaviour and Airborne Particle Emissions of Railway Brake Block Materials. Tribol. Int. 2018, 118, 360–367. [Google Scholar] [CrossRef]
- Wu, R.; Song, C.; Wu, H.; Lv, B.; Zhang, Y.; Zhang, Y. Effect of Relative Humidity on the Current-Carrying Tribological Properties of Cu–C Sliding Contact Pairs. Wear 2022, 492–493, 204219. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, Y.; Zhou, Z.; Qian, G.; Zhang, J.; Huang, X.; Zhang, X. Effect of Electrical Current Density, Apparent Contact Pressure, and Sliding Velocity on the Electrical Sliding Wear Behavior of Cu–Ti3AlC2 Composites. Wear 2020, 444–445, 203156. [Google Scholar] [CrossRef]
- Grandin, M.; Wiklund, U. Wear Phenomena and Tribofilm Formation of Copper/Copper-Graphite Sliding Electrical Contact Materials. Wear 2018, 398–399, 227–235. [Google Scholar] [CrossRef]
- Abdo, J.; Shamseldeen, E.; Lafdee, K. Humidity Effects on Carbon–Carbon Composites (Fiber Pre-Form + CVI). Mater. Sci. Eng. A 2008, 472, 2–14. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Liu, Z.; Li, J.; Wang, L.; Sun, C.; Zhang, Y. Tribological and Conductive Behavior of Cu/Cu Rolling Current-Carrying Pairs in a Water Environment. Tribol. Int. 2020, 143, 106055. [Google Scholar] [CrossRef]
- Pompanon, F.; Fouvry, S.; Alquier, O. Influence of Humidity on the Endurance of Silver-Plated Electrical Contacts Subjected to Fretting Wear. Surf. Coat. Technol. 2018, 354, 246–256. [Google Scholar] [CrossRef]
- Qin, H.; Wang, J.; Li, H.; Zhao, X.; Yi, J.; Zhao, Y.; Ren, Z. Effect of operating conditions on surface film formation of carbon brush/slip ring friction pairs. Surf. Technol. 2023, 52, 178–188. [Google Scholar] [CrossRef]
- Sung, I.H.; Kim, J.W.; Noh, H.J.; Jang, H. Effect of Displacement and Humidity on Contact Resistance of Copper Electrical Contacts. Tribol. Int. 2016, 95, 256–261. [Google Scholar] [CrossRef]
- Song, C.; Sun, Y.; Zhang, Y.; Pang, X.; Chen, T.; Sun, C.; Zhang, Y. Current-Carrying Property and Damage Mechanism of Rolling Contact Pairs in Different Water Conditions: From Vapor to Liquid. Wear 2021, 477, 203857. [Google Scholar] [CrossRef]
- Pompanon, F.; Fouvry, S.; Alquier, O. Influence of the Relative Humidity and H2S—SO2 Polluted Air on the Fretting Wear Behavior of Silver-Plated Electrical Contacts. Wear 2022, 508–509, 204455. [Google Scholar] [CrossRef]
- Walgern, J.; Fischer, K.; Hentschel, P.; Kolios, A. Reliability of Electrical and Hydraulic Pitch Systems in Wind Turbines Based on Field-Data Analysis. Energy Rep. 2023, 9, 3273–3281. [Google Scholar] [CrossRef]
- Smith, F.; Brownlie, F.; Hodgkiess, T.; Toumpis, A.; Pearson, A.; Galloway, A.M. Effect of Salinity on the Corrosive Wear Behaviour of Engineering Steels in Aqueous Solutions. Wear 2020, 462–463, 203515. [Google Scholar] [CrossRef]
- Jiang, J.; Stack, M.M.; Neville, A. Modelling the Tribo-Corrosion Interaction in Aqueous Sliding Conditions. Tribol. Int. 2002, 35, 669–679. [Google Scholar] [CrossRef]
- Hoque, M.A.; Yao, C.-W.; Khanal, M.; Lian, I. Tribocorrosion Behavior of Micro/Nanoscale Surface Coatings. Sensors 2022, 22, 9974. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Islam, M.A.; Xie, Y.; Stack, M.M. Some Thoughts on Modeling Abrasion-Corrosion: Wear by Hard Particles in Corrosive Environments. J. Bio Tribo Corros. 2024, 10, 12. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, D.; Wang, Y.; Zuo, X.; Zhu, R.; Mia, M.F. Tribo-Electrical Properties of Copper Matrix Composites in Salt-Fog Environment. Tribol. Int. 2024, 192, 109299. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhang, Y.Z.; Du, S.M.; Song, C.F.; Yang, Z.H.; Shangguan, B. Tribological Properties of Pure Carbon Strip Affected by Dynamic Contact Force during Current-Carrying Sliding. Tribol. Int. 2018, 123, 256–265. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, L.; Zhang, H.; Feng, K.; Ju, P.; Duan, W.; Ji, L.; Li, H.; Liu, X.; Zhou, H.; et al. Magnetron Sputtering NbSe2 Film as Lubricant for Space Current-Carrying Sliding Contact. Friction 2023, 11, 383–394. [Google Scholar] [CrossRef]
- Kang, X.; Xie, X.; Zhang, L. Tribological behavior and self-lubricating mechanism of Ag–C fiber brushes under air and vacuum conditions. Wear 2020, 460–461, 203458. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Liu, L.-M.; Zhang, C.; Zhang, L.; Zhou, K.-C. Sliding Electrical Contact Behavior of Brass Fiber Brush against Coin-Silver and Au Plating. Wear 2016, 368–369, 461–469. [Google Scholar] [CrossRef]
- Pei, L.-L.; Ju, P.-F.; Ji, L.; Li, H.-X.; Liu, X.-H.; Zhou, H.-D.; Chen, J.-M. Vacuum Current-Carrying Tribological Behavior of MoS2-Ti Films with Different Conductivities. Chin. Phys. B 2022, 31, 66201. [Google Scholar] [CrossRef]
- Pei, L.; Ji, L.; Li, H.; Cai, H.; Xue, Y. Current-Carrying Tribological Behavior of Textured Au/MoS2 Coatings in Vacuum. Surf. Topogr. Metrol. Prop. 2024, 12, 25006. [Google Scholar] [CrossRef]
- Qian, G.; Feng, Y.; Chen, F.; Liu, W.; Zhang, X.; Liu, Y. Effect of Current Polarity on Electrical Sliding Wear Behavior of Cu-WS2-Graphite-WS2 Nanotube Composites in Air and Vacuum Conditions. Sci. China Technol. Sci. 2013, 56, 2839–2846. [Google Scholar] [CrossRef]
- Song, J.; Yuan, H.; Schinow, V. Fretting Corrosion Behavior of Electrical Contacts with Tin Coating in Atmosphere and Vacuum. Wear 2019, 426–427, 1439–1445. [Google Scholar] [CrossRef]
- Rau, J.S.; Schmidt, O.; Schneider, R.; Debastiani, R.; Greiner, C. Three Regimes in the Tribo-Oxidation of High Purity Copper at Temperatures of up to 150 °C. Adv. Eng. Mater. 2022, 24, 2200518. [Google Scholar] [CrossRef]
- Wang, H.; Gao, G.; Deng, L.; Li, X.; Wang, X.; Wang, Q.; Wu, G. Study on Current-Carrying Tribological Characteristics of C-Cu Sliding Electric Contacts under Different Water Content. Coatings 2022, 13, 42. [Google Scholar] [CrossRef]
- Kharin, S.N. Mathematical Models of Heat and Mass Transfer in Electrical Contacts. In Proceedings of the 2015 IEEE 61st Holm Conference on Electrical Contacts (Holm), San Diego, CA USA, 11–14 October 2015; IEEE: San Diego, CA, USA, 2015; pp. 1–21. [Google Scholar]
- Li, C.; Zhu, N.; Wu, G.; Gao, G.; Wu, J. Study on the mathematical model of dynamic contact resistance in pantograph–catenary systems. High Volt. Eng. 2015, 41, 3554–3560. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Q.; Guo, F.; Tang, A.; Wang, X.; Chen, X. Mathematical Model of Contact Resistance in Pantograph-Catenary System Considering Rough Surface Characteristics. IEEE Trans. Transp. Electrif. 2022, 8, 455–465. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Mu, X.; Huang, K.; Wang, H.; Gao, S. An Extended Habedank’s Equation-Based EMTP Model of Pantograph Arcing Considering Pantograph-Catenary Interactions and Train Speeds. IEEE Trans. Power Deliv. 2016, 31, 1186–1194. [Google Scholar] [CrossRef]
- Derosa, S.; Nåvik, P.; Collina, A.; Bucca, G.; Rønnquist, A. A Heuristic Wear Model for the Contact Strip and Contact Wire in Pantograph—Catenary Interaction for Railway Operations under 15 kV 16.67 Hz AC Systems. Wear 2020, 456–457, 203401. [Google Scholar] [CrossRef]
- Zhou, N.; Zhi, X.; Cheng, Y.; Sun, Y.; Wang, J.; Gu, Z.; Li, Z.; Zhang, W. Contact Strip of Pantograph Heuristic Wear Model and Its Application. Tribol. Int. 2024, 194, 109546. [Google Scholar] [CrossRef]
- Nie, L.; Wang, X.; Zhang, J.; Ma, Z.; Zhang, L.; Guo, W. Dynamic Characteristics Analysis and Vibration Control of Coupled Bending-Torsional System for Axial Flow Hydraulic Generating Set. Chaos Solitons Fractals 2024, 187, 115401. [Google Scholar] [CrossRef]
- Shen, F.; Ke, L.-L. Numerical Study of Coupled Electrical-Thermal-Mechanical-Wear Behavior in Electrical Contacts. Metals 2021, 11, 955. [Google Scholar] [CrossRef]
- Blanchet, T.A. Modeling Temperature Rise in Multi-Track Reciprocating Frictional Sliding. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 2155–2168. [Google Scholar] [CrossRef]
- Yu, X.; Song, M.; Wang, Z. Simulation Study on Surface Temperature Distribution of Collector Strip Material under Pantograph-Catenary Arc of Urban Rail. IEEE Access 2023, 11, 68358–68365. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Xiao, S.; Duan, X.; Gao, G.; Wei, W.; Wu, G.; Rotaru, M.; Sykulski, J.K. Multi-physics Analysis and Optimisation of High-speed Train Pantograph–Catenary Systems Allowing for Velocity Skin Effect. High Volt. 2020, 5, 654–661. [Google Scholar] [CrossRef]
- Liao, G.; Wang, W.; Wang, B.; Chen, Q.; Liu, X. Transient Mixed-Lubrication and Contact Behavior Analysis of Metal Liquid Film under Magneto-Thermal Effect. Int. J. Mech. Sci. 2024, 271, 109142. [Google Scholar] [CrossRef]
- Morris, S.A.; Leighton, M.; Morris, N.J. Electrical Field Strength in Rough Infinite Line Contact Elastohydrodynamic Conjunctions. Lubricants 2022, 10, 87. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, K.; Kou, Y.; Zheng, X.; Li, Y. Modelling of Magneto-Electro-Thermo-Mechanical Coupled Behavior of the Lubricating Liquid Film for the Electromagnetic Launch. Int. J. Heat Mass Transf. 2022, 196, 123267. [Google Scholar] [CrossRef]
- Guo, F.; Gu, X.; Li, L.; Wang, Z.; Wang, T.; Jia, S. Effect of Surface Microparameters on Contact Temperature of Sliding Electrical Contact. IEEE Trans. Ind. Inform. 2022, 18, 5972–5981. [Google Scholar] [CrossRef]
- Suchan, A.; Ponick, B. Brush Dynamics in Electrically Excited Synchronous Machines and Their Influence on the Field Winding. In Proceedings of the IECON 2020 The 46th Annual Conference of the IEEE Industrial Electronics Society, Singapore, 18–21 October 2020; pp. 889–894. [Google Scholar]
- Hermann Houenouvo, A.T.; Hofmann, W. Numerical Analysis by Current Flow of Mechanical Components in Double-Fed Asynchronous Generators in Wind Turbines. In Proceedings of the 2013 IEEE International Conference on Industrial Technology (ICIT), Cape Town, South Africa, 25–28 February 2013; pp. 368–373. [Google Scholar]
- Zuo, X.; Du, M.; Zhou, Y. Influence of Contact Parameters on the Coupling Temperature of Copper-Brass Electrical Contacts. Eng. Fail. Anal. 2022, 136, 106205. [Google Scholar] [CrossRef]
- Deeva, V.; Slobodyan, S. Influence of Gravity and Thermodynamics on the Sliding Electrical Contact. Tribol. Int. 2017, 105, 299–303. [Google Scholar] [CrossRef]
- Sincero, G.C.R.; Cros, J.; Viarouge, P. Arc Models for Simulation of Brush Motor Commutations. IEEE Trans. Magn. 2008, 44, 1518–1521. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Zhu, J.; Xu, W. Research on Electrical Contact Characteristics of Brush-Rail Device with Transient Large Current. IEEE Access 2023, 11, 37560–37569. [Google Scholar] [CrossRef]
- Zhang, M.; Zuo, X.; Zhou, Y. Fractal Contact Resistance Model of Wind Pitch Slip Ring Considering Wear and Self-Excited Vibration. Ind. Lubr. Tribol. 2024, 76, 214–230. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Yi, P.; Lai, X. A Micro Contact Model for Electrical Contact Resistance Prediction between Roughness Surface and Carbon Fiber Paper. Int. J. Mech. Sci. 2017, 124–125, 37–47. [Google Scholar] [CrossRef]
- Bucca, G.; Collina, A. A Procedure for the Wear Prediction of Collector Strip and Contact Wire in Pantograph–Catenary System. Wear 2009, 266, 46–59. [Google Scholar] [CrossRef]
- Bucca, G.; Collina, A. Electromechanical Interaction between Carbon-Based Pantograph Strip and Copper Contact Wire: A Heuristic Wear Model. Tribol. Int. 2015, 92, 47–56. [Google Scholar] [CrossRef]
- Derosa, S.; Nåvik, P.; Collina, A.; Bucca, G.; Rønnquist, A. Contact Point Lateral Speed Effects on Contact Strip Wear in Pantograph—Catenary Interaction for Railway Operations under 15 kV 16.67 Hz AC Systems. Wear 2021, 486–487, 204103. [Google Scholar] [CrossRef]
- Mei, G.; Fan, J.; Liu, D. Long-Sliding Distance Experiment and Heuristic Model Prediction of the Electrical Sliding Abrasion of an Overhead Wire/Current Collector. Tribol. Int. 2023, 180, 108212. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Y.; Zhang, X.; Zhi, X.; Zhang, W. Wear Rate and Profile Prediction of Cu-Impregnated Carbon Strip for High-Speed Pantograph. Wear 2023, 530–531, 205056. [Google Scholar] [CrossRef]
- Balakrishna, G.V.; Gnanamoorthy, R. Prediction of Temperature Rise in Ball-on-Disk Contacts under Electromechanical Loading. Tribol. Int. 2024, 194, 109476. [Google Scholar] [CrossRef]
- Hansen, E.; Vaitkunaite, G.; Schneider, J.; Gumbsch, P.; Frohnapfel, B. Establishment and Calibration of a Digital Twin to Replicate the Friction Behaviour of a Pin-on-Disk Tribometer. Lubricants 2023, 11, 75. [Google Scholar] [CrossRef]
- Zhao, S.; Ma, H.; Jiang, M.; Li, C.; Liu, Y.; Zhao, X.; Chen, X. Study of Carbon Brush and Slip-Ring System Abrasion from Electric Contact Friction under Special Environments. IEEE Access 2021, 9, 9308–9317. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Z.; Zhang, J. Molecular dynamics simulation of the friction and wear behavior of Au coating on conductive slip rings. Tribology 2018, 38, 108–114. [Google Scholar] [CrossRef]
- Xie, B.; Ju, P.; Ji, L.; Li, H.; Zhou, H.; Chen, J. Research progress in tribology of electrical contact materials. Tribology 2019, 39, 656–668. [Google Scholar] [CrossRef]
- Huang, W.; Yu, H.; Wang, L.; Wu, X.; Ouyang, C.; Zhang, Y.; He, J. State of the Art and Prospects in Sliver- and Copper-Matrix Composite Electrical Contact Materials. Mater. Today Commun. 2023, 37, 107256. [Google Scholar] [CrossRef]
- Liu, X.; Guan, X.; Zhang, T.-M.; Zhong, Y.; Zhang, W.; Zhang, S.; Xiao, Q.; Zheng, Y.; Gao, M.; Chen, D.; et al. A Comparative Investigation into the Material Matching Performance of Different Friction Pairs under Current-Carrying Friction. Tribol. Int. 2023, 185, 108543. [Google Scholar] [CrossRef]
- Qian, G.; Feng, Y.; Li, B.; Huang, S.; Liu, H.; Ding, K. Effect of Electrical Current on the Tribological Behavior of the Cu-WS2-G Composites in Air and Vacuum. Chin. J. Mech. Eng. 2013, 26, 384–392. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Qin, L.; Qiu, W.; Li, S.; Jiang, Y.; Jia, Y.; Li, Z. Electrical Sliding Friction Wear Behaviors and Mechanisms of Cu–Sn Matrix Composites Containing MoS2/Graphite. Wear 2024, 548–549, 205388. [Google Scholar] [CrossRef]
- Ray, N.; Kempf, B.; Mützel, T.; Heringhaus, F.; Froyen, L.; Vanmeensel, K.; Vleugels, J. Effect of Ni Addition on the Contact Resistance of Ag-WC Electrical Contacts. J. Alloys Compd. 2016, 670, 188–197. [Google Scholar] [CrossRef]
- Wu, C.; Yi, D.; Weng, W.; Li, S.; Zhou, J.; Zheng, F. Arc Erosion Behavior of Ag/Ni Electrical Contact Materials. Mater. Des. 2015, 85, 511–519. [Google Scholar] [CrossRef]
- Wang, H.; Cai, Q.; Wang, J.; Zhang, Y.; Hu, D.; Wang, Y. First-Principles and Experimental Investigations on Physical Properties and Arc Erosion Behavior of Metal-Doped AgSnO2 Electrical Contact Materials. Ceram. Int. 2023, 49, 26033–26048. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.B.; Zhang, P.; Zhang, M.; Zhang, Y.M.; Sun, Z.M. Arc Erosion Behavior of Ag/Ti3AlC2 Electrical Contact Materials. J. Alloys Compd. 2018, 740, 669–676. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, J.; Cai, Q.; Hu, D. Enhancement of Arc Erosion Resistance in AgCuO Electrical Contact Materials through Rare Earth Element Doping: First-Principles and Experimental Studies. Int. J. Mol. Sci. 2023, 24, 12627. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Y.; Ge, J.; Li, L.; Li, Z.; Ding, M. Arc Erosion Mechanism of Ag-Ti3SiC2 Material. J. Alloys Compd. 2020, 817, 152741. [Google Scholar] [CrossRef]
- Gopal, V.; Chandran, M.; Rao, M.S.R.; Mischler, S.; Cao, S.; Manivasagam, G. Tribocorrosion and Electrochemical Behaviour of Nanocrystalline Diamond Coated Ti Based Alloys for Orthopaedic Application. Tribol. Int. 2017, 106, 88–100. [Google Scholar] [CrossRef]
- Braceras, I.; Ibáñez, I.; Taher, M.; Mao, F.; del Barrio, A.; De Urturi, S.S.; Berastegui, P.; Andersson, A.M.; Jansson, U. On the Electro-Tribological Properties and Degradation Resistance of Silver-Aluminum Coatings. Wear 2018, 414–415, 202–211. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Shen, F.; Ke, L.-L. A Comparative Study on the Electrical Contact Behavior of CuZn40 and AgCu10 Alloys under Fretting Wear: Effect of Current Load. Tribol. Int. 2024, 194, 109523. [Google Scholar] [CrossRef]
- Shu, S.; Yuan, Q.; Dai, W.; Wu, M.; Dai, D.; Yang, K.; Wang, B.; Lin, C.-T.; Wuebben, T.; Degenhardt, J.; et al. In-Situ Synthesis of Graphene-like Carbon Encapsulated Copper Particles for Reinforcing Copper Matrix Composites. Mater. Des. 2021, 203, 109586. [Google Scholar] [CrossRef]
- Braceras, I.; Ibáñez, I.; Dominguez-Meister, S.; Velasco, X.; Brizuela, M.; Garmendia, I. Electro-Tribological Properties of Diamond like Carbon Coatings. Friction 2020, 8, 451–461. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.Q.; Chen, X.; Xiang, Z.Y.; Zhao, F. Probing the Effect of the Electric Current on the Tribological Performances of the Electrical Contact Surfaces with Graphene Coating. Tribol. Int. 2023, 178, 108121. [Google Scholar] [CrossRef]
- Hu, M.; Liu, X.; Zhou, C.; Wang, D.; Xiao, Q.; Guan, X.; Zhang, S.; Xu, Z. Comparative Study of the Current-Carrying Tribological Properties of Carbon Graphite Composites with Different Hardnesses. Int. J. Mech. Sci. 2023, 245, 108133. [Google Scholar] [CrossRef]
- Wang, P.; Yue, W.; Lu, Z.; Zhang, G.; Zhu, L. Friction and Wear Properties of MoS2-Based Coatings Sliding against Cu and Al under Electric Current. Tribol. Int. 2018, 127, 379–388. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, R.; Zuo, X.; Xie, W. Tribo-Electrical Behaviors of CNTs-MoS2/Cu Composites under Sliding Electrical Contact with Brass. Tribol. Int. 2023, 180, 108207. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, N.; Zhi, Q.; Zhou, X.; Wang, B.; Yang, J. Role of Cr-Carbide Coating on Graphite Particles in the Mechanical and Wear Behavior of Graphite/Copper Composites. Tribol. Lett. 2024, 72, 8. [Google Scholar] [CrossRef]
- Deng, C.; Yin, J.; Zhang, H.; Xiong, X.; Wang, P.; Sun, M. The Tribological Properties of Cf/Cu/C Composites under Applied Electric Current. Tribol. Int. 2017, 116, 84–94. [Google Scholar] [CrossRef]
- Kang, K.; Yu, B.; Deng, G.; Wang, P.; Yin, J.; Zhang, H.; Luo, G.; Song, H. Effect of Counterpart Materials on Tribological Behaviors of Copper Impregnated Carbon/Carbon Composite under Electric Current Condition. Ceram. Int. 2023, 49, 30008–30018. [Google Scholar] [CrossRef]
- Grandin, M.; Wiklund, U. Wear and Electrical Performance of a Slip-Ring System with Silver–Graphite in Continuous Sliding against PVD Coated Wires. Wear 2016, 348–349, 138–147. [Google Scholar] [CrossRef]
- Honda, Y.; Mikami, Y.; Inoue, M.; Shinagawa, K.; Abe, T. Low-Temperature SiO2 Film Coatings onto Cu Particles Using the Polygonal Barrel-Plasma Chemical Vapor Deposition Method. Appl. Surf. Sci. 2022, 588, 152646. [Google Scholar] [CrossRef]
- Miranzo, P.; Belmonte, M.; Osendi, M.I. From Bulk to Cellular Structures: A Review on Ceramic/Graphene Filler Composites. J. Eur. Ceram. Soc. 2017, 37, 3649–3672. [Google Scholar] [CrossRef]
- Fukuda, N.; Sawa, K.; Ueno, T. Contact Voltage Drop and Brush Wear Characteristics for Various Silver Content of the Silver Graphite Brush in Slip Ring System. In Proceedings of the 2018 IEEE Holm Conference on Electrical Contacts, Albuquerque, New Mexico, 14–18 October 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 521–525. [Google Scholar]
- Wang, J.; Wang, C.; Kang, Y. The Effects of Annealing Treatment on Microstructure and Contact Resistance Properties of Cold Sprayed Ag-SnO2 Coating. J. Alloys Compd. 2017, 714, 698–703. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, L.; Xiao, J.; Qian, Z.; Zhang, T.; Zhou, K. Sliding Electrical Contact Behavior of AuAgCu Brush on Au Plating. Trans. Nonferrous Met. Soc. China 2015, 25, 3029–3036. [Google Scholar] [CrossRef]
- Wang, X.; Yao, P.; Li, Y.; Zhou, H.; Xiao, Y.; Deng, M.; Kang, L.; Zhou, P. Effects of Material Transfer Evolution on Tribological Behavior in CuCrZr Alloy Paired with 7075 Al Alloy under Current-Carrying. Tribol. Int. 2023, 179, 107960. [Google Scholar] [CrossRef]
- André, B.; Lewin, E.; Jansson, U.; Wiklund, U. Friction and Contact Resistance of Nanocomposite Ti–Ni–C Coatings. Wear 2011, 270, 555–566. [Google Scholar] [CrossRef]
- Yin, L.; Gao, W.; Jones, M.I. Wear Behaviour and Electrical Conductivity of β-Sialon-ZrN Composites Fabricated by Reaction Bonding and Gas Pressure Sintering Process. Ceram. Int. 2019, 45, 2266–2274. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, L.; Sprouster, D.J.; Trelewicz, J.R.; Zhong, W.; Yang, Y.; Zinkle, S.J.; Snead, L.L. Tailoring Microstructure in Sintered Cu-Cr-Nb-Zr Alloys for Fusion Components. J. Nucl. Mater. 2021, 551, 152956. [Google Scholar] [CrossRef]
- Bachani, S.K.; Wang, C.-J.; Lou, B.-S.; Chang, L.-C.; Lee, J.-W. Fabrication of TiZrNbTaFeN High-Entropy Alloys Coatings by HiPIMS: Effect of Nitrogen Flow Rate on the Microstructural Development, Mechanical and Tribological Performance, Electrical Properties and Corrosion Characteristics. J. Alloys Compd. 2021, 873, 159605. [Google Scholar] [CrossRef]
- Vasić, B.; Zurutuza, A.; Gajić, R. Spatial Variation of Wear and Electrical Properties across Wrinkles in Chemical Vapour Deposition Graphene. Carbon 2016, 102, 304–310. [Google Scholar] [CrossRef]
- Ayyappadas, C.; Muthuchamy, A.; Raja Annamalai, A.; Agrawal, D.K. An Investigation on the Effect of Sintering Mode on Various Properties of Copper-Graphene Metal Matrix Composite. Adv. Powder Technol. 2017, 28, 1760–1768. [Google Scholar] [CrossRef]
- Aguilar-Rosas, O.A.; Alvis-Sánchez, J.A.; Tormos, B.; Marín-Santibáñez, B.M.; Pérez-González, J.; Farfan-Cabrera, L.I. Enhancement of Low-Viscosity Synthetic Oil Using Graphene Nanoparticles as Additives for Enduring Electrified Tribological Environments. Tribol. Int. 2023, 188, 108848. [Google Scholar] [CrossRef]
- Shiri, S.G.; Abachi, P.; Pourazarang, K.; Rahvard, M.M. Preparation of In-Situ Cu/NbC Nanocomposite and Its Functionally Graded Behavior for Electrical Contact Applications. Trans. Nonferrous Met. Soc. China 2015, 25, 863–872. [Google Scholar] [CrossRef]
- Song, J.; Schinow, V. Correlation between Friction and Wear Properties and Electrical Performance of Silver Coated Electrical Connectors. Wear 2015, 330–331, 400–405. [Google Scholar] [CrossRef]
- Du, J.; Lu, M.; Fang, J.; Li, W.; Chen, D. Current-Carrying Friction of Ag Coatings by Additive Manufacturing: Uncovering the Role of Electric Current. Mater. Res. Lett. 2024, 12, 459–466. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Wang, W. Modeling Contact of Au-Coated Sphere with Rigid Flat: Electrical Contact Resistance, Adhesive Wear and Friction. Int. J. Mech. Sci. 2023, 246, 108152. [Google Scholar] [CrossRef]
- Xu, H.; Fu, T.; Wang, P.; Zhou, Y.; Guo, W.; Su, F.; Li, G.; Xing, Z.; Ma, G. Microstructure and Properties of Plasma Sprayed Copper-Matrix Composite Coatings with Ti3SiC2 Addition. Surf. Coat. Technol. 2023, 460, 129434. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Tan, N.; Zhou, L.; Ma, G.; Hu, D.; Wang, J.; Zhang, G.; Wang, H. Preparation Process and Mechanical Properties of Laser Cladding Gradient Molybdenum Coating on Copper Alloy. Surf. Coat. Technol. 2023, 470, 129888. [Google Scholar] [CrossRef]
- Larsson, E.; Andersson, A.M.; Kassman Rudolphi, Å. Grease Lubricated Fretting of Silver Coated Copper Electrical Contacts. Wear 2017, 376–377, 634–642. [Google Scholar] [CrossRef]
- Du, J.; Lu, M.; Fang, J.; Li, W.; Chen, D. Current-Carrying Friction Behavior and Wear Mechanism of Ag Coatings by Rotary Spray Deposition. Wear 2024, 546–547, 205367. [Google Scholar] [CrossRef]
- Noël, S.; Alamarguy, D.; Brézard-Oudot, A.; Gendre, P. An Investigation of Fretting Wear Behaviour of Nickel Coatings for Electrical Contacts Application in Dry and Lubricated Conditions. Wear 2013, 301, 551–561. [Google Scholar] [CrossRef]
- Cui, R.; Han, Y.; Zhu, Z.; Chen, B.; Ding, Y.; Zhang, Q.; Wang, Q.; Ma, G.; Pei, F.; Ye, Z. Investigation of the Structure and Properties of Electrodeposited Cu/Graphene Composite Coatings for the Electrical Contact Materials of an Ultrahigh Voltage Circuit Breaker. J. Alloys Compd. 2019, 777, 1159–1167. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Hu, Y.; Hou, B. Tribological Performance and Conductive Capacity of Ag Coating under Boundary Lubrication. Tribol. Int. 2017, 110, 161–172. [Google Scholar] [CrossRef]
- Argibay, N.; Prasad, S.V.; Goeke, R.S.; Dugger, M.T.; Michael, J.R. Wear Resistant Electrically Conductive Au–ZnO Nanocomposite Coatings Synthesized by e-Beam Evaporation. Wear 2013, 302, 955–962. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Fu, Y.; Chen, R.; Li, T.; Hou, X.; Li, H. Research Progress in Chemical Vapor Deposition for High-Temperature Anti-Oxidation/Ablation Coatings on Thermal Structural Composites. Compos. Part B Eng. 2025, 291, 112015. [Google Scholar] [CrossRef]
- Antunes, J.; Matos, K.; Carvalho, I.; Carvalho, S.; Ferreira, F.; Cruz, S.M.A. Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles. Coatings 2022, 12, 1399. [Google Scholar] [CrossRef]
- Zellele, D.M.; Yar-Mukhamedova, G.S.; Rutkowska-Gorczyca, M. A Review on Properties of Electrodeposited Nickel Composite Coatings: Ni-Al2O3, Ni-SiC, Ni-ZrO2, Ni-TiO2 and Ni-WC. Materials 2024, 17, 5715. [Google Scholar] [CrossRef] [PubMed]
- Calli, C.; Tazegul, O.; Kayali, E.S. Wear and Corrosion Characteristics of Copper-Based Composite Coatings. Ind. Lubr. Tribol. 2017, 69, 300–305. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, Z.; Gao, L.; Liu, Y.; Wang, F. Influence of Microstructure on the Optical Property of Plasma-Sprayed Al, Cu, and Ag Coatings. Mater. Des. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, L.; Lu, Z.; Xing, Z. Current-Carrying Tribological Performance of CrB2/Cu Composite Coating Prepared by Laser Cladding Technology. J. Mater. Sci. Mater. Electron. 2024, 35, 1558. [Google Scholar] [CrossRef]
- Tebianian, M.; Aghaie, S.; Razavi Jafari, N.S.; Elmi Hosseini, S.R.; Pereira, A.B.; Fernandes, F.A.O.; Farbakhti, M.; Chen, C.; Huo, Y. A Review of the Metal Additive Manufacturing Processes. Materials 2023, 16, 7514. [Google Scholar] [CrossRef]
- Yadav, A.; Srivastava, M.; Jain, P.K. Design and Fabrication of Wire Arc Additive Manufacturing Setup and Enhanced Tailored Properties of Dissimilar Steel Additively Deposited by WAAM Process. Structures 2025, 72, 108228. [Google Scholar] [CrossRef]
- Wang, P.; Ma, G.; Su, F.; Guo, W.; Chen, S.; Zhao, H.; Liu, M.; Wang, H. Effect of Annealing Treatment on the Microstructure and Current Carrying Tribological Properties of In-Situ Formed TiO2−x Coating Prepared by Plasma Spraying. Tribol. Int. 2022, 175, 107872. [Google Scholar] [CrossRef]
- Singer, I.L.; Veracka, M.J.; Boyer, C.N.; Neri, J.M. Wear Behavior of Lubricant-Conditioned Copper Rails and Armatures in a Railgun. IEEE Trans. Plasma Sci. 2011, 39, 138–143. [Google Scholar] [CrossRef]
- Li, X.; Ji, L.; Ju, P.; Li, H.; Zhou, H.; Chen, J.; Gou, X.; Duan, W. Preparation of Au thin films by magnetron sputtering and study on their vacuum current-carrying tribological behavior. Tribology 2022, 42, 283–293. [Google Scholar] [CrossRef]
- Ren, W.; Wang, P.; Fu, Y.; Pan, C.; Song, J. Effects of Temperature on Fretting Corrosion Behaviors of Gold-Plated Copper Alloy Electrical Contacts. Tribol. Int. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, M.; Cheng, G.; Tang, M.; Sun, L.; Chen, Y.; Luo, C. Multilayer Coatings of Periodically Co-Deposited Graphene and Ag Substrate: Improving the Electrified Friction Interface by Modifying the Strength-Ductility Combination. Surf. Coat. Technol. 2024, 482, 130667. [Google Scholar] [CrossRef]
- Cao, Z.; Xia, Y.; Chen, C.; Zheng, K.; Zhang, Y. A Synergetic Strategy Based on Laser Surface Texturing and Lubricating Grease for Improving the Tribological and Electrical Properties of Ag Coating under Current-Carrying Friction. Friction 2021, 9, 978–989. [Google Scholar] [CrossRef]


| Environmental Factor | Reference | Material System | Effect on Friction Coefficient | Effect on Wear Rate | Effect on Contact Resistance | Main Wear Mechanism |
|---|---|---|---|---|---|---|
| High temperature (>150 °C) | [71] | Cu/Cu | Moderate (↓) | Strong (↑) | Moderate (↑) | Thermo-oxidative coupled wear (tribo-oxidation) |
| High humidity (>70% RH) | [72] | C/Cu | Strong (↓) | Moderate (↑) | Strong (unstable) | Electrochemical–mechanical coupling (tribocorrosion) |
| Corrosive gases (SO2, Cl2) | [56] | Silver coating/copper alloy | Moderate (↓) | Moderate (↑) | Strong (↑) | Chemical film–induced brittle spalling |
| Vacuum (<10−3 Pa) | [65] | Ag–C | Strong (↑) | Strong (↑) | Low (↓) | Adhesive wear/mechanical wear |
| Materials | Reference Number | Advantages | Disadvantages | |
|---|---|---|---|---|
| Copper-based electrical contact materials | Copper-base alloys | [106] | Excellent physical and mechanical properties | Strength and conductivity are difficult to reconcile |
| Fiber reinforced (carbon fiber, boron fiber…) | [107] | Self-lubricating, wear-resistant, high strength and temperature-resistance, etc. | Large brittleness, uneven microstructure, anisotropy, higher cost | |
| Ceramic reinforced (SiC/WC/TiN) | [72] | Good wear-resistant and temperature mechanical properties, low coefficient of thermal expansion, lower cost | Weak dispersion and interfacial bonding | |
| New types (Cu-WS2, Cu-G-MoS2…) | [108,109] | Excellent self-lubricating, wear- resistant and environmental adaptability | Reduced mechanical strength | |
| Silver-based electrical contact materials | Silver -base alloys (Ag-Cu, Ag-Cu-Ni…) | [106] | high mechanical strength and wear-resistant, stable contact characteristics under low contact pressure | Poor oxidation resistance and corrosion resistance, lower conductivity than silver |
| Ag/C series | [65] | High weld-resistant, low contact resistance | Low hardness, poor anti-arc erosion capability | |
| Ag/WC series | [110] | Anti-melt welding, heat and wear resistance | Producing WO3 resulting in contact resistance | |
| Ag/Ni series | [111] | Wear-resistant, saving silver | Low weld resistance at high current | |
| Ag/MeO series (Ag/CdO, Ag/SnO2, Ag/ZnO…) | [112] | Better weld and arc resistance, conductivity at high current | Ag/CdO pollutes the environment | |
| New types (Ag-MoS2, Ag-MoS2-G-CNTs…) | [113] | Excellent wear-resistant, anti-vulcanization and environmental adaptability | Reduced mechanical strength | |
| Gold-based electrical contact materials | Gold-base alloys | [106] | Excellent conductivity and mechanical properties | Expensive |
| Lanthanon reinforced | [114] | High melting point, hardness, anti-arc and chemical stability | Expensive | |
| New electrical contact materials (TiNiC, Graphene…) | [115] | Integrating electrical conductivity and lubrication | - | |
| Coating Technology | Reference Number | Combination Method | Material Type | Substrate Heating | Single Layer Thickness/mm | Advantage | Disadvantage | |
|---|---|---|---|---|---|---|---|---|
| Plating technology | Chemical vapor deposition | [154] | Physical bonding | Metallic, ceramic materials | Large | <0.01 | High-quality coating, uniform and controllable thickness, high heat and chemical resistance, high deposition efficiency | High temperature process, toxic reaction gas, slow deposition rate |
| Physical vapor deposition | [155] | Metallic, ceramic materials | Lesser | <0.01 | Low temperature process, high bonding strength, high hardness and wear resistance, no pollution | Slow deposition rates, limited coating thickness, and limited material selection | ||
| Electrodeposit | [156] | Metallic | Nothing | <0.1 | Easy operation, fast deposition speed, no limitation of substrate shape and size, low internal stress and controllable thickness of the plated layer. | Low dimensional accuracy and environmental pollution | ||
| Surface spraying technology | Cold spray technology | [157] | Mechanical bonding | Alloys, ceramic powders | Little | 0.1–9 | Low heat input, high material utilization, high bond strength, coating density, low porosity, and high deposition rate | Difficulty in ensuring coating uniformity, coating thickness limitations, material limitations, need for post-processing |
| Thermal spray technology | [158] | Metallurgical bonding | Alloys, ceramic powders | Large | 0.1–1 | Small heat-affected zone, high deposition efficiency, good coating performance, thickness control | Limited coating bonding, high porosity, high surface roughness, low material utilization | |
| Laser cladding technology | [159] | Metallurgical bonding | Alloy powders, wires, plates | Little | 0.02–2 | High deposition rate, small heat affected zone, high coating quality | Complex process debugging, limited material selection, high cost | |
| Electron beam additive manufacturing technology | [160] | Metallurgical bonding | Alloy powders | Large | 0.001–9 | High precision and complexity, high material utilization, superior mechanical properties, high reparability power | Slower production speeds, heat-affected zones, material selection limitations, high vacuum requirements | |
| Arc Additive Manufacturing Technology | [161] | Metallurgical bonding | Alloy powders, wires, plates | Large | 2–4 | High deposition rate, material adaptability, economy, superior mechanical properties, uniform structure, high production efficiency | Large heat affected zone, poor surface quality, difficult to control, lack of refinement | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhao, X.; Li, W.; Li, Y.; Pan, T.; Yang, W.; Li, X. Research Status and Progress on the Current-Carrying Friction and Wear Performance of Conductive Slip Rings in Harsh Environments. Coatings 2025, 15, 1347. https://doi.org/10.3390/coatings15111347
Wu H, Zhao X, Li W, Li Y, Pan T, Yang W, Li X. Research Status and Progress on the Current-Carrying Friction and Wear Performance of Conductive Slip Rings in Harsh Environments. Coatings. 2025; 15(11):1347. https://doi.org/10.3390/coatings15111347
Chicago/Turabian StyleWu, Hailin, Xinze Zhao, Wanting Li, Yang Li, Tengda Pan, Wei Yang, and Xuetao Li. 2025. "Research Status and Progress on the Current-Carrying Friction and Wear Performance of Conductive Slip Rings in Harsh Environments" Coatings 15, no. 11: 1347. https://doi.org/10.3390/coatings15111347
APA StyleWu, H., Zhao, X., Li, W., Li, Y., Pan, T., Yang, W., & Li, X. (2025). Research Status and Progress on the Current-Carrying Friction and Wear Performance of Conductive Slip Rings in Harsh Environments. Coatings, 15(11), 1347. https://doi.org/10.3390/coatings15111347






