Surface Modification of AZ31 Mg Alloy Based on PLA or PLGA with Caffeic Acid for Bioengineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Surface Modification
2.2. Surface Analysis
2.3. Mechanical and Tribological Tests
2.4. Corrosion Test
2.5. Cumulative Release Profiles
2.6. Biocompatibility Study
2.6.1. Cell Culture
2.6.2. Cell Viability Assay
2.6.3. Visualization of Cell Attachment and Growth
2.6.4. Statistical Analysis
3. Results and Discussion
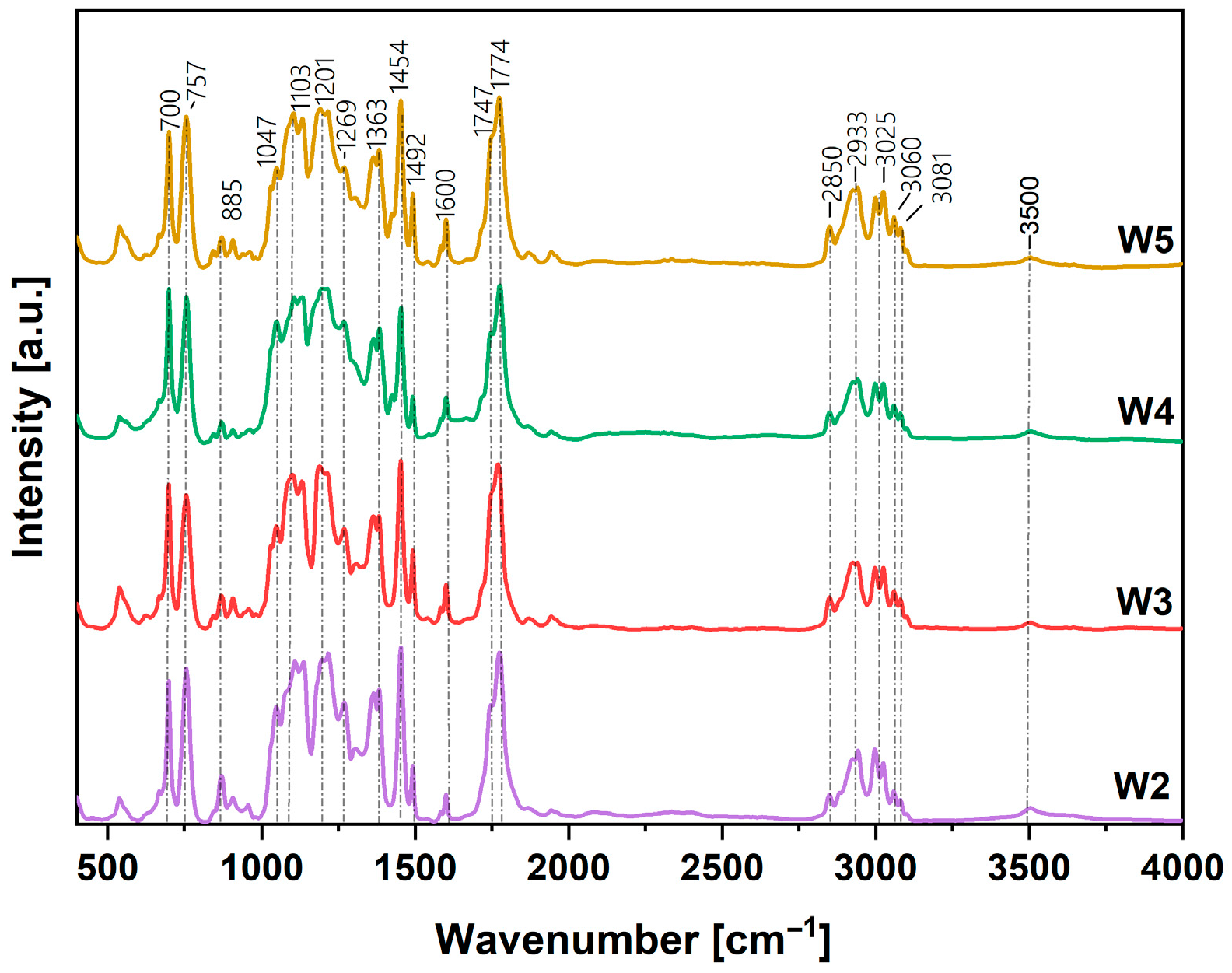
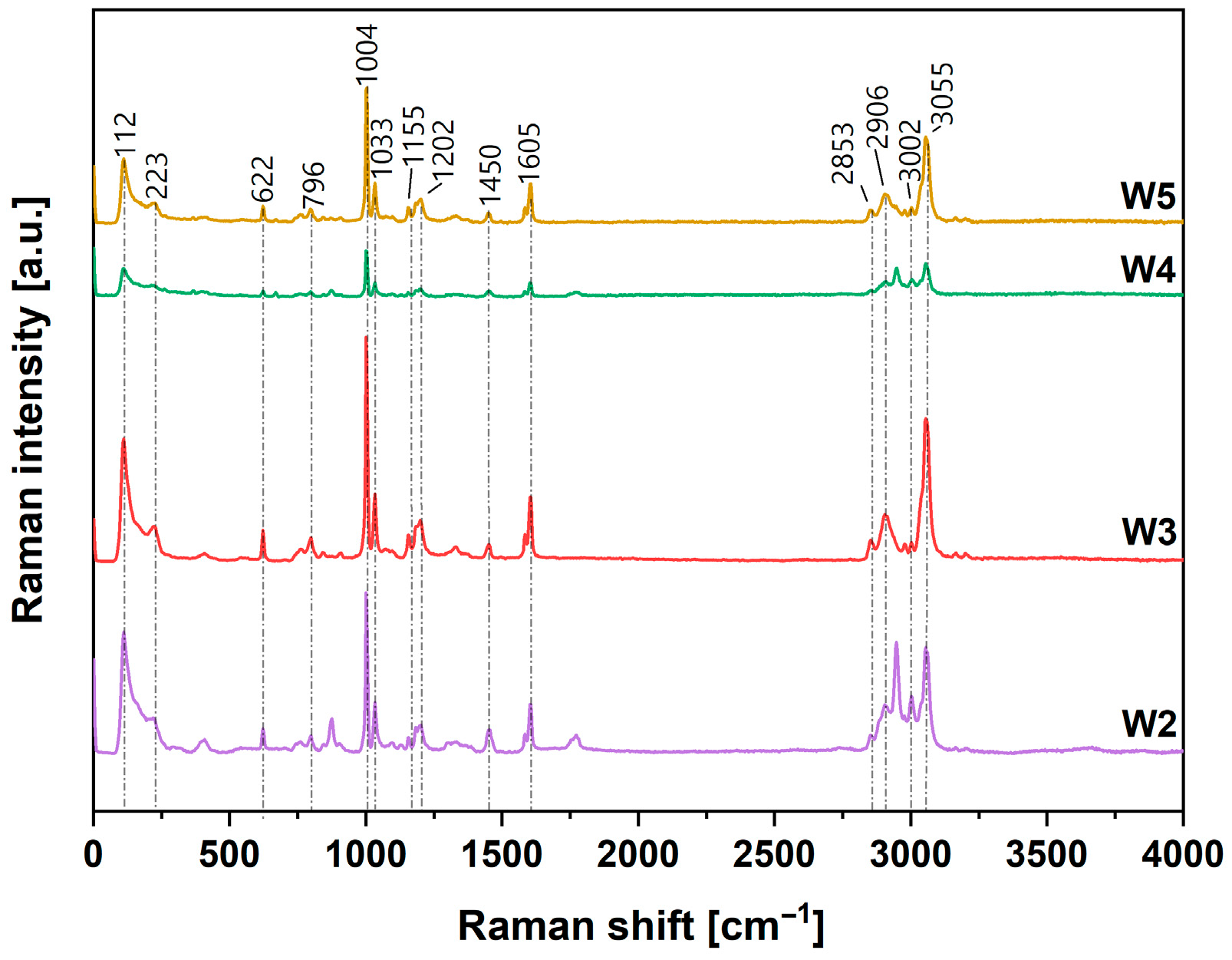
3.1. Chemical Structure of Obtained Coatings
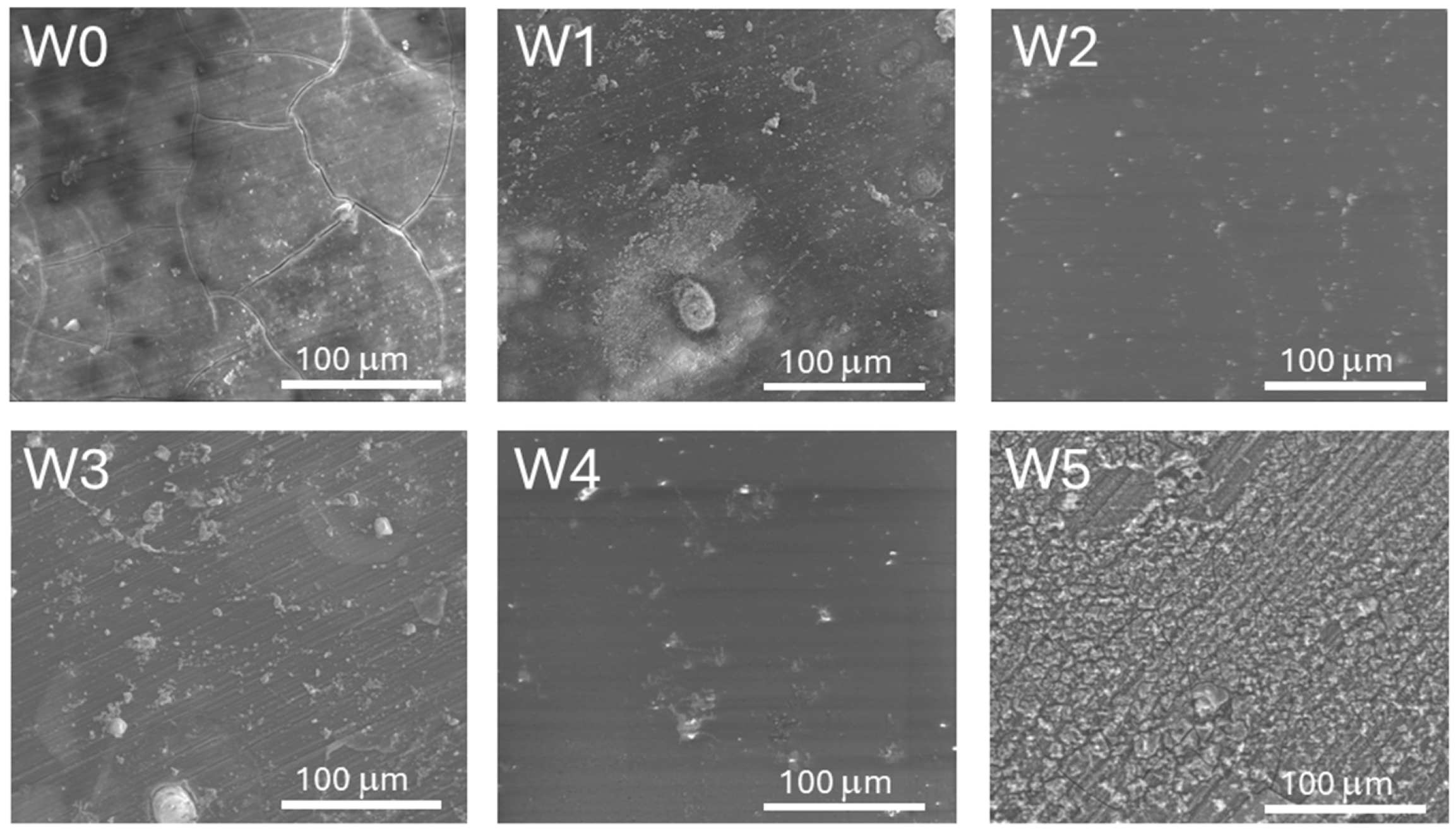
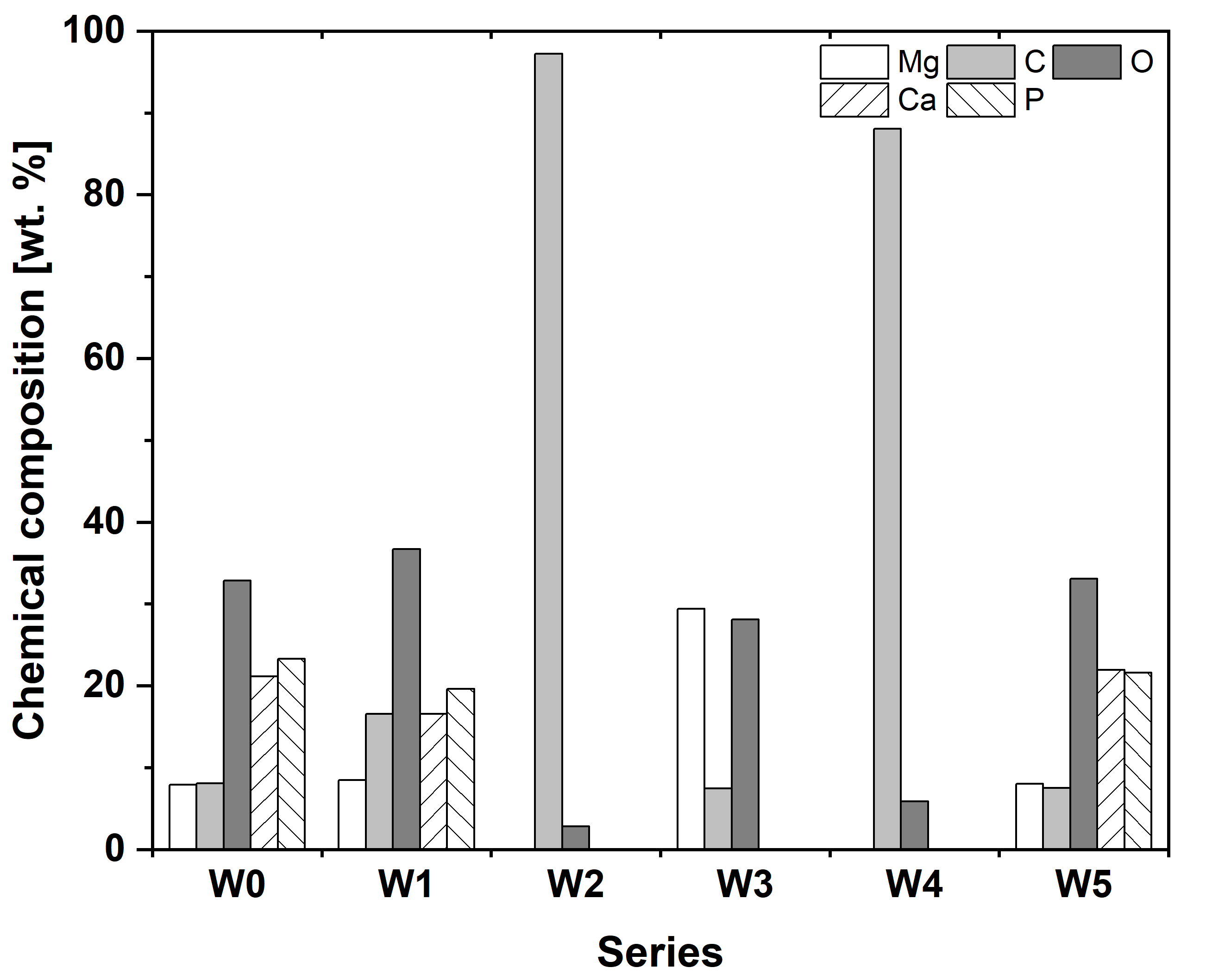
3.2. Microstructure and Chemical Composition
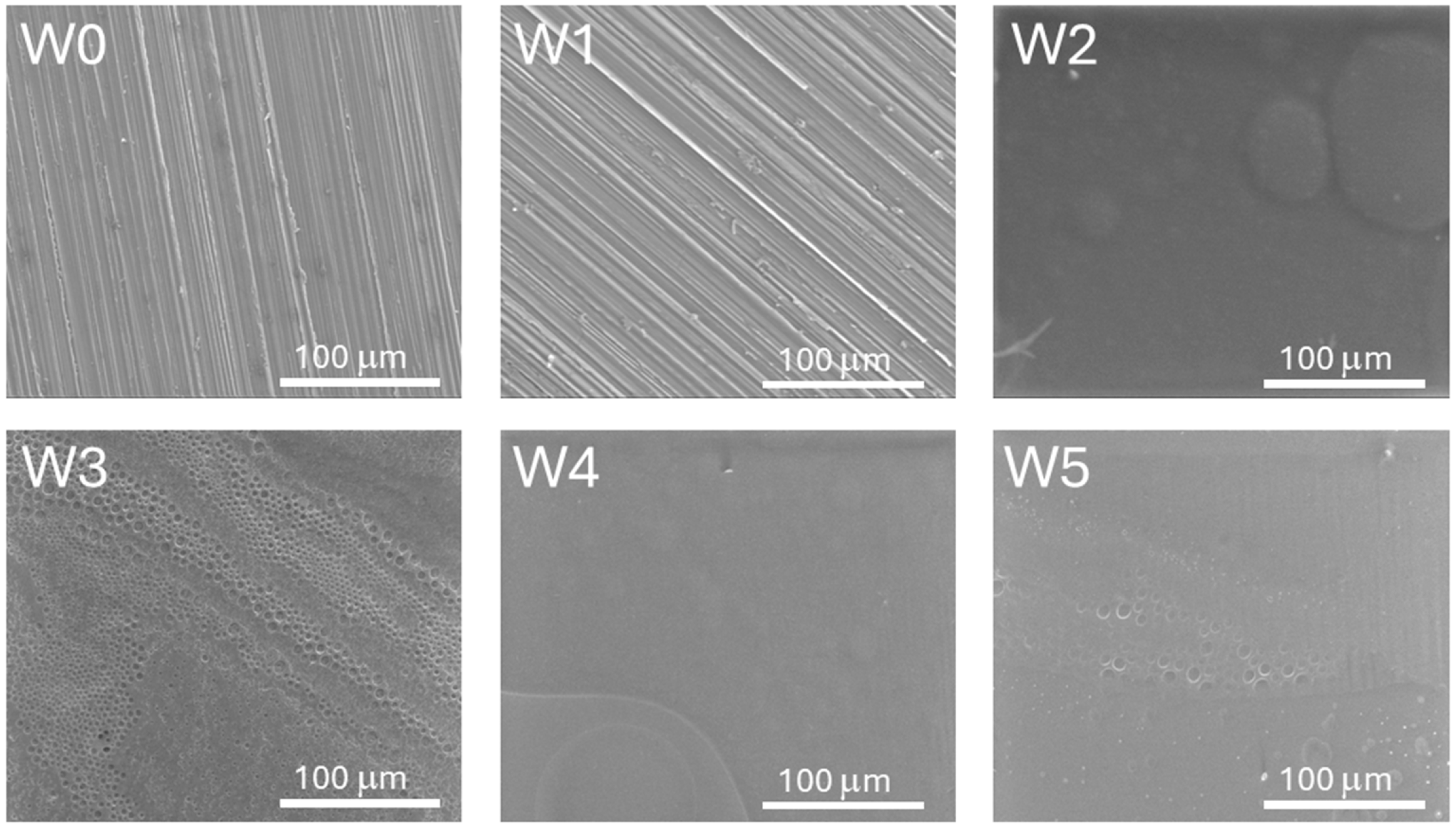
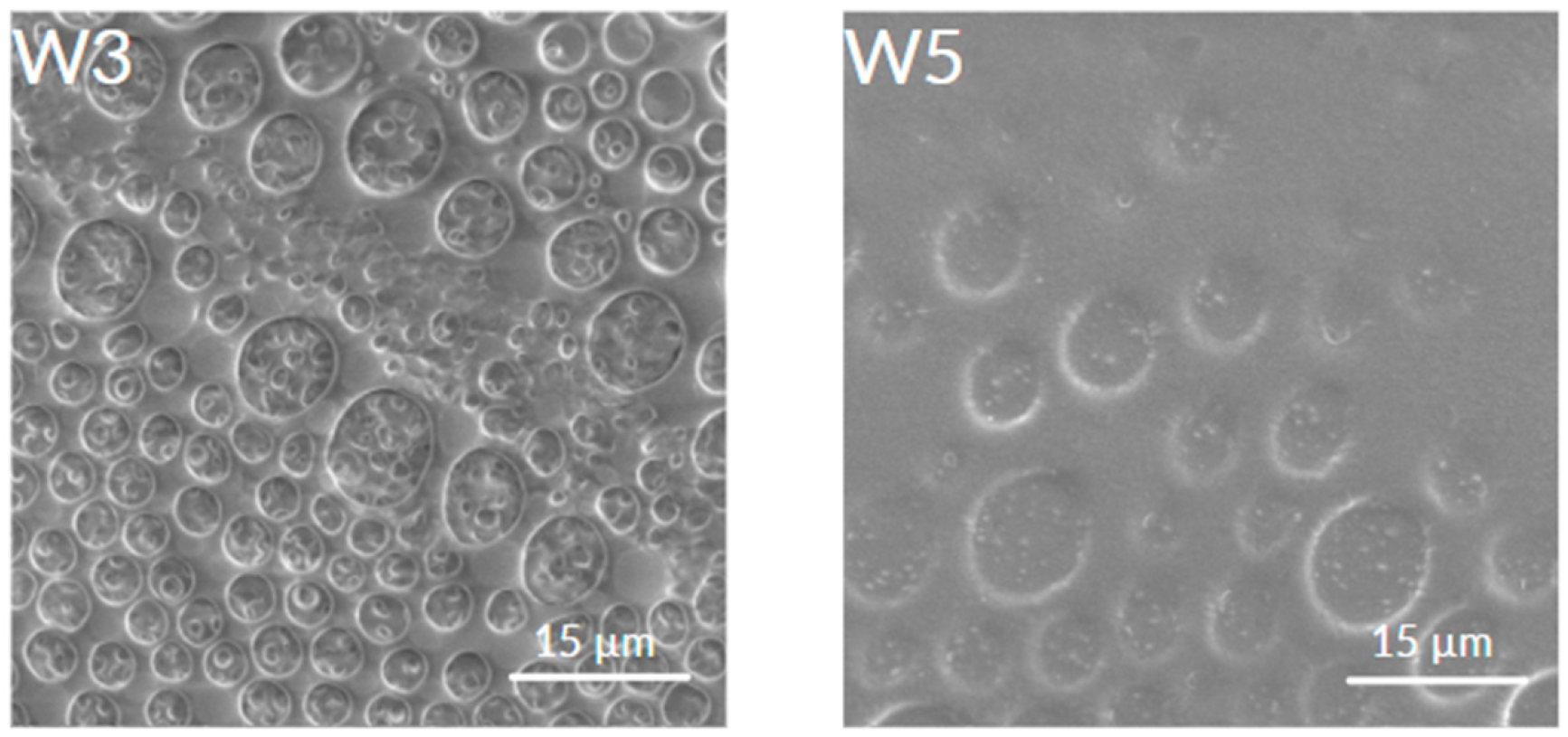
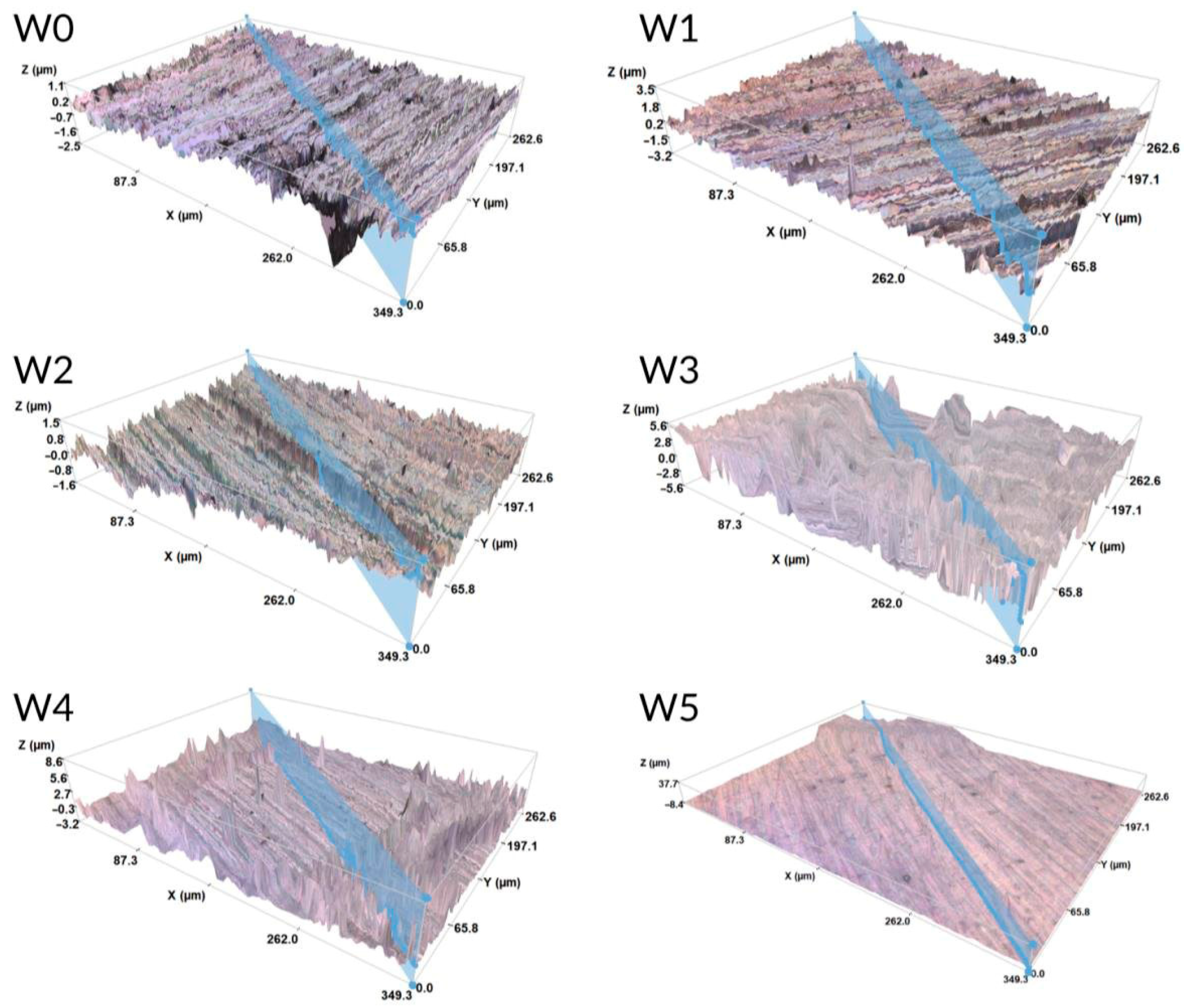
3.3. Surface Topography
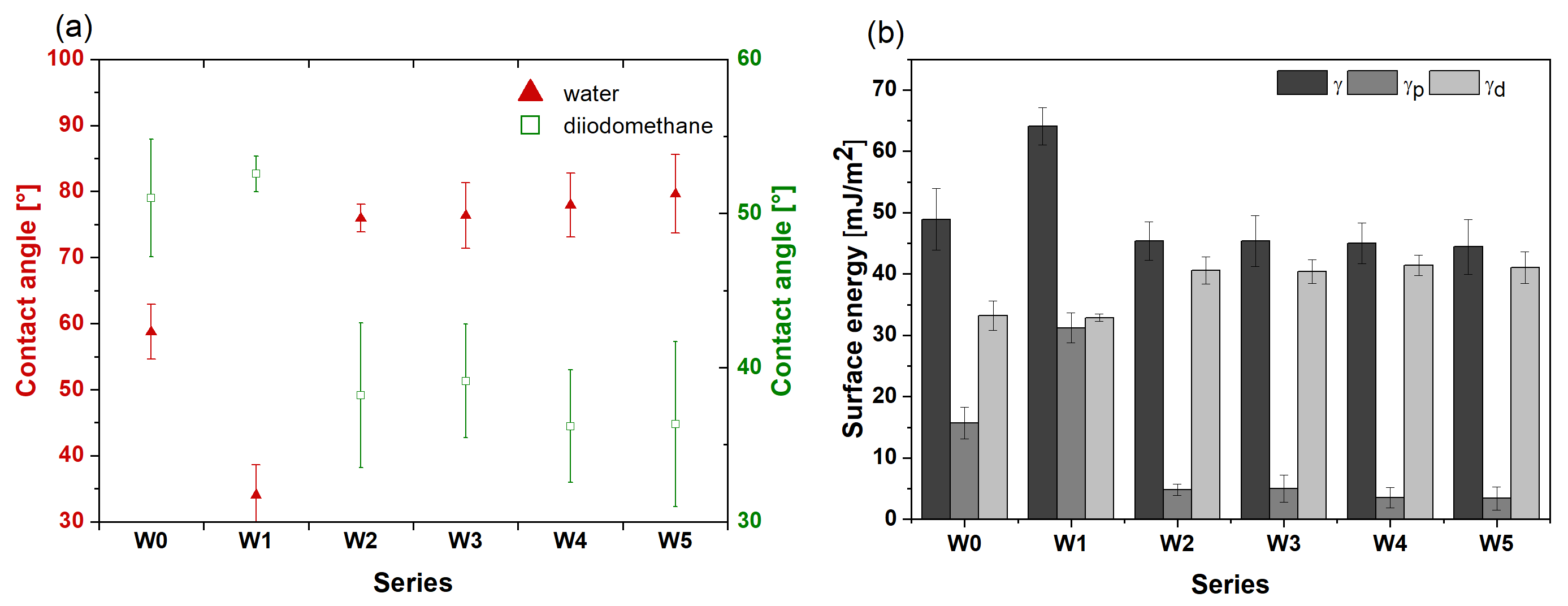
3.4. Surface Wettability
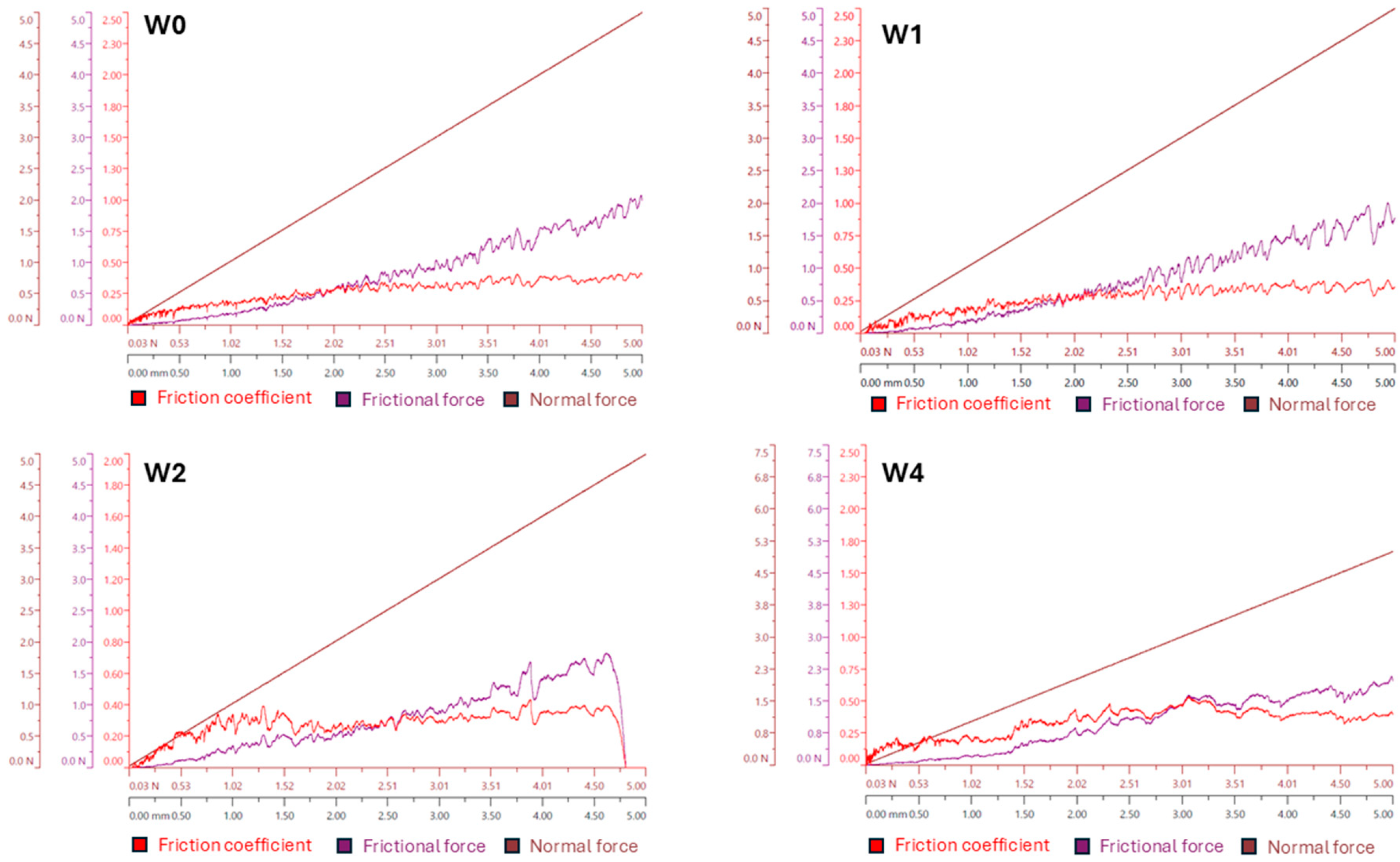
3.5. Tribological Test
3.6. Corrosion Analysis
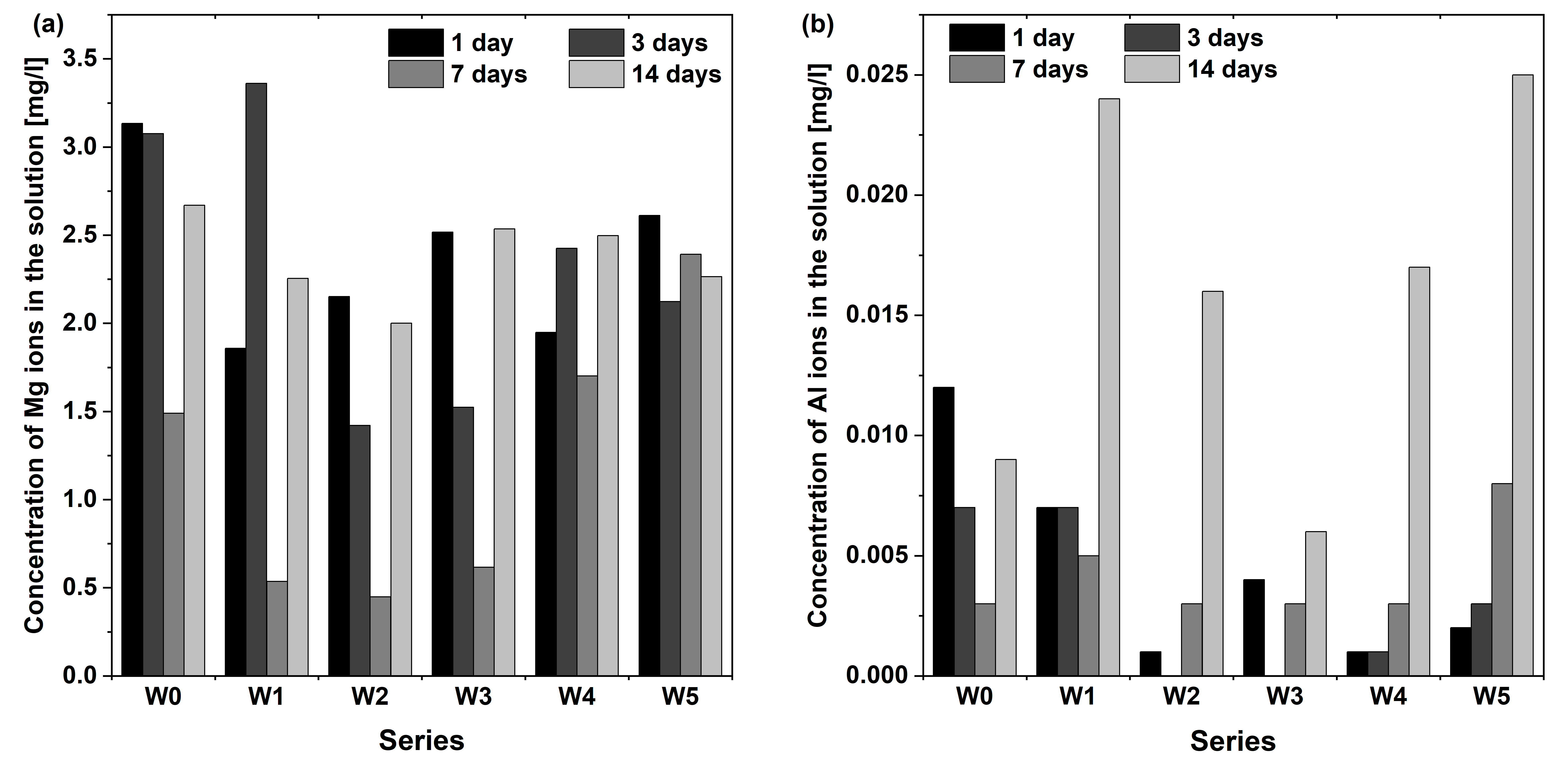
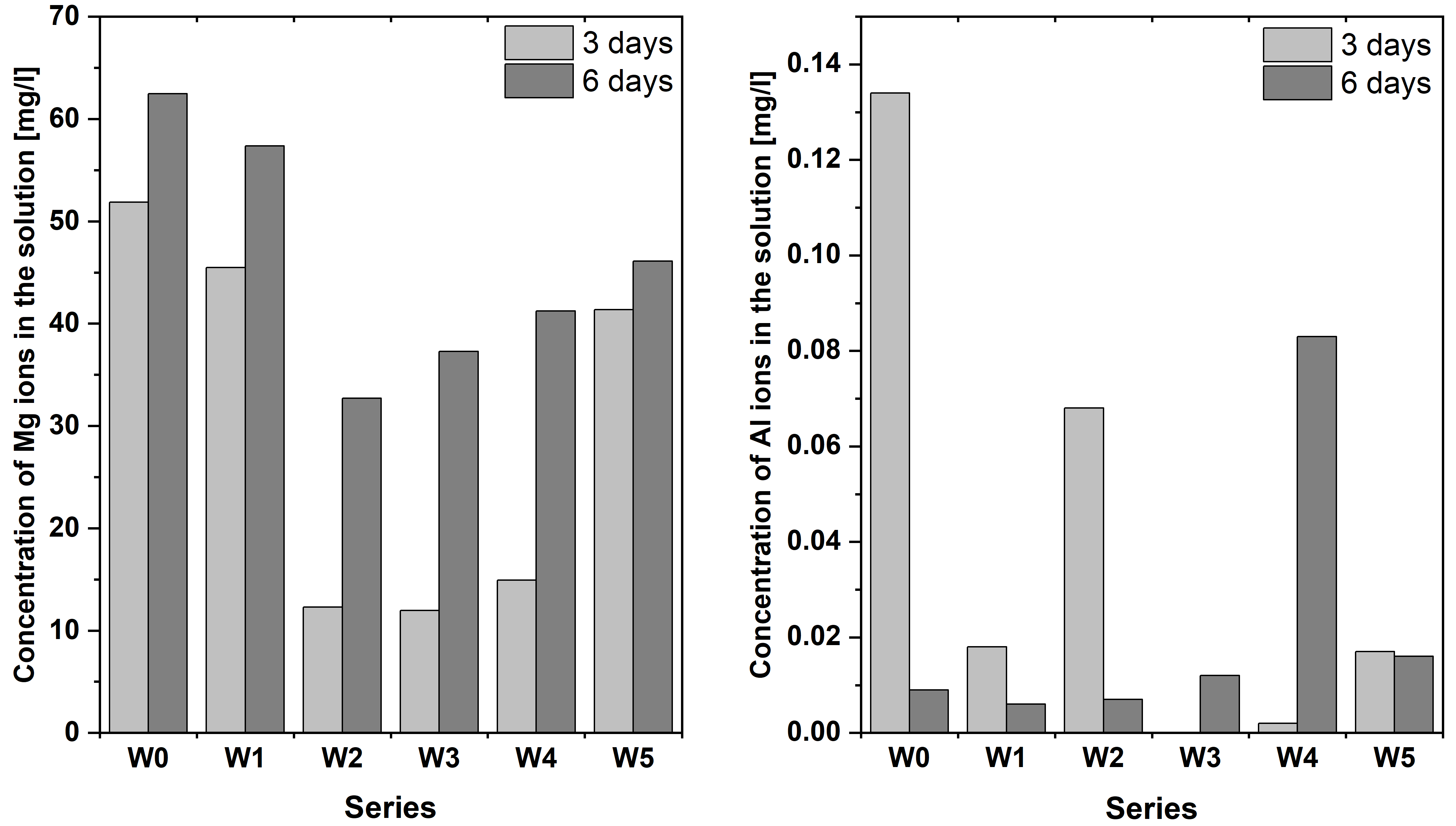
3.7. ICP MS Analysis
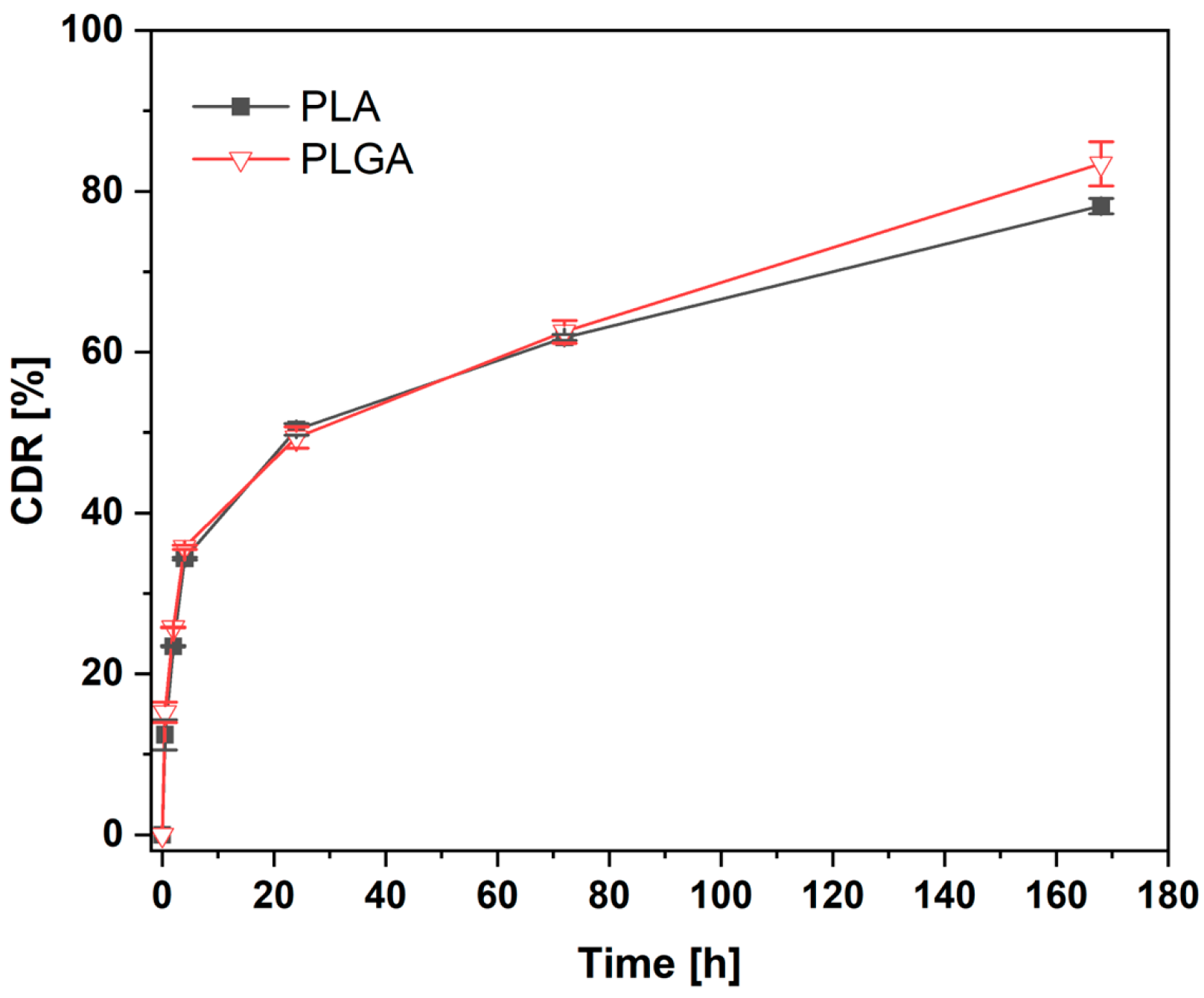
3.8. Cumulative Caffeic Acid Release
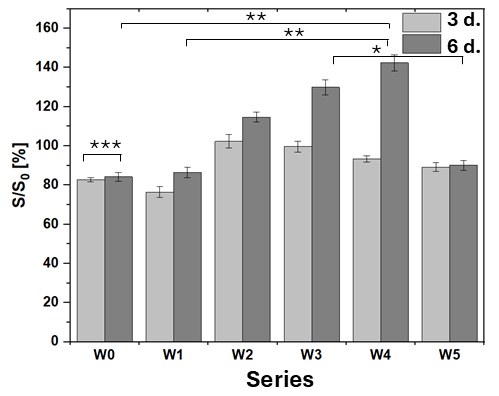
3.9. Evaluation of Biocompatibility
4. Conclusions
- (1)
- Preliminary surface treatment allowed the successful removal of impurities and made a positive impact on the surface finish, increasing microscopic unevenness on the alloy surface of the W1 sample series. This effect allowed for obtaining continuous and well-adherent to the metallic core biopolymer coatings after deposition and also durability tests in SBF solution (except for the samples of W5 series).
- (2)
- Surfaces of PLA, PLGA, and PLGA + CA layers showed higher roughness in comparison to the metallic core and were hydrophilic, which can foster the osseointegration.
- (3)
- The addition of caffeic acid to PLA and PLGA changed the structure of the coating surfaces in comparison to those obtained from both pure biopolymers leading to the formation of porous bubbles and increasing the coefficient of friction values and friction force. The induced porosity for W3 and W5 resulted in a temporary loss of barrier integrity after 7 days, but the W3 coating recovered continuity at 14 days. This porous structure, while initially compromising corrosion protection, is beneficial for the necessary initial burst release of CA and, particularly for W3, supports the best cell proliferation.
- (4)
- PLA and PLGA biopolymer layers on the AZ31 alloy ensured desirable biofunctionality and no obvious cytotoxicity, enabling osseointegration of the implant in contact with the MG-63 osteoblast cell line, as confirmed by the progressive proliferation of the cells on the investigated surfaces of modified AZ31 alloy substrates for all specimens after 6 days. The relative proliferation rate exceeding 75% and the presence of intercellular junctions characteristic of osteoblasts prove the biocompatibility of proposed modifications, proving the potential of such surface modifications of the studied Mg alloy.
- (5)
- Efficient CA release with a possible transport mechanism based on the Korsmeyer–Peppas model and with the characteristic initial “burst release” ensures effective antimicrobial agent release at the initial step of curation after implantation, followed by slower release governed by the diffusion and further erosion of the polymeric coating.
- (6)
- The proposed surface modifications, by depositing PLA and PLGA layers on AZ31 alloy, improved its resistance to corrosion in the applied simulated body fluid (SBF) solution. Layers of the W2 and W4 series preserved their continuity and adhesion after 14-day contact with this solution, and, additionally, the W4 series showed the presence of globular structures typical of apatite. This formation on the surface of the obtained biopolymer layers is beneficial for the application of the material in bone implantology. Barrier protection against Al3+ ions release into the SBF solution for initial periods, less than 14 days, was also confirmed. A major and novel finding is that the CA-containing PLA coating (W3) exhibits superior performance in suppressing the long-term release of potentially toxic Al3+ ions into the SBF solution, which is a critical concern for AZ31-based implants.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Esen, Z.; Dikici, B.; Duygulu, O.; Dericioglu, A.F. Titanium-Magnesium Based Composites: Mechanical Properties and in-Vitro Corrosion Response in Ringer’s Solution. Mater. Sci. Eng. A 2013, 573, 119–126. [Google Scholar] [CrossRef]
- Mahapatro, A. Bio-Functional Nano-Coatings on Metallic Biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Murchio, S.; Emanuelli, L.; De Biasi, R.; Branca Vergano, L.; Giuliani, R.; Tian, S.; Wille, M.L.; Berto, F.; Pellizzari, M.; et al. Metal Additive Manufacturing of Lattice-Based Orthopedic Implants: A Comprehensive Review of Requirements and Design Strategies. Mater. Sci. Eng. R Rep. 2025, 166, 101075. [Google Scholar] [CrossRef]
- Rahman, M.; Dutta, N.K.; Roy Choudhury, N. Magnesium Alloys With Tunable Interfaces as Bone Implant Materials. Front. Bioeng. Biotechnol. 2020, 8, 564. [Google Scholar] [CrossRef]
- Morsada, Z.; Hossain, M.M.; Islam, M.T.; Mobin, M.A.; Saha, S. Recent Progress in Biodegradable and Bioresorbable Materials: From Passive Implants to Active Electronics. Appl. Mater. Today 2021, 25, 101257. [Google Scholar] [CrossRef]
- Khan, A.R.; Grewal, N.S.; Zhou, C.; Yuan, K.; Zhang, H.J.; Jun, Z. Recent Advances in Biodegradable Metals for Implant Applications: Exploring in Vivo and in Vitro Responses. Results Eng. 2023, 20, 101526. [Google Scholar] [CrossRef]
- Mohd Salaha, Z.F.; Abdullah, N.N.A.A.; Chan, K.F.; Gan, H.S.; Mohd Yusop, M.Z.; Ramlee, M.H. Biodegradable Orthopaedic Implants: A Systematic Review of in Vitro and in Vivo Evaluations of Magnesium, Iron, and Zinc Alloys. Results Eng. 2025, 27, 105746. [Google Scholar] [CrossRef]
- Xing, F.; Li, S.; Yin, D.; Xie, J.; Rommens, P.M.; Xiang, Z.; Liu, M.; Ritz, U. Recent Progress in Mg-Based Alloys as a Novel Bioabsorbable Biomaterials for Orthopedic Applications. J. Magnes. Alloy 2022, 10, 1428–1456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Chen, C.; Gu, Y. Advances in Microarc Oxidation Coated AZ31 Mg Alloys for Biomedical Applications. Corros. Sci. 2015, 91, 7–28. [Google Scholar] [CrossRef]
- Ma, D.; Sun, Z.; Zhao, Q.; Zhang, Y.; Li, W.; Wang, J.; Chen, Y.; Zhao, M.; Wang, J.; Huang, J.; et al. In Vitro and in Vivo Degradation Behavior of an Assembled Magnesium Alloy Suture Anchor for Ligament-Bone Reconstruction. Acta Biomater. 2025, 205, 723–736. [Google Scholar] [CrossRef]
- Zhou, K.; Lu, Q.; Qin, J.; Shi, H.; Zhang, P.; Yan, H.; Shi, H.; Wang, X. A View of Magnesium Alloy Modification and Its Application in Orthopedic Implants. J. Mater. Res. Technol. 2025, 36, 1536–1561. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, J.; Wang, J.; Li, W.; Zhao, Q.; Ma, D.; Li, W.; Zhang, Y.; Huang, J.; Zhao, M.; et al. Metabolic Behavior of the Degradation Products of Magnesium Alloys in Bone Tissue. J. Magnes. Alloy 2025. [Google Scholar] [CrossRef]
- Dong, J.; Lin, T.; Shao, H.; Wang, H.; Wang, X.; Song, K.; Li, Q. Advances in Degradation Behavior of Biomedical Magnesium Alloys: A Review. J. Alloys Compd. 2022, 908, 164600. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-Based Biomaterials as Emerging Agents for Bone Repair and Regeneration: From Mechanism to Application. J. Magnes. Alloy 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Cundy, T.; Grey, A.; Reid, I.R. CHAPTER 6—Calcium, Phosphate and Magnesium. In Clinical Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A.P., Ayling, R.M., Eds.; Churchill Livingstone: London, UK, 2014; pp. 93–123. ISBN 978-0-7020-5140-1. [Google Scholar]
- Harris, E.D. Cofactors: Inorganic. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 357–365. ISBN 978-0-12-384885-7. [Google Scholar]
- Yu, T.; Jiang, J.; Yu, Q.; Li, X.; Zeng, F. Structural Insights into the Distortion of the Ribosomal Small Subunit at Different Magnesium Concentrations. Biomolecules 2023, 13, 566. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. A Narrative Review on the Role of Magnesium in Immune Regulation, Inflammation, Infectious Diseases, and Cancer. J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef] [PubMed]
- Yavuzyegit, B.; Karali, A.; De Mori, A.; Smith, N.; Usov, S.; Shashkov, P.; Bonithon, R.; Blunn, G. Evaluation of Corrosion Performance of AZ31 Mg Alloy in Physiological and Highly Corrosive Solutions. ACS Appl. Bio. Mater. 2024, 7, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The Effect of Hydrogen Gas Evolution of Magnesium Implant on the Postimplantation Mortality of Rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mousavian, S.M.H.; Bautin, V.A.; Tabaian, S.H. Advanced Strategies for Mg-Based Biodegradable Implants: Alloying, Heat Treatment, and Coatings. J. Alloys Compd. 2025, 1040, 183575. [Google Scholar] [CrossRef]
- Panahi, Z.; Tamjid, E.; Rezaei, M. Surface Modification of Biodegradable AZ91 Magnesium Alloy by Electrospun Polymer Nanocomposite: Evaluation of in Vitro Degradation and Cytocompatibility. Surf. Coat. Technol. 2020, 386, 125461. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Zou, D.; Zu, H.; Li, W.; Zheng, Y. An Overview of Magnesium-Based Implants in Orthopaedics and a Prospect of Its Application in Spine Fusion. Bioact. Mater. 2024, 39, 456–478. [Google Scholar] [CrossRef]
- Ji, R.; Mo, F.; Liang, L.; Tian, C.; Dong, S.; Deng, C.; Wu, H.; Zhou, L.; Zhao, X. Biodegradable Polylactic Acid (PLA) Copolymer Materials: Structural Design, Synthesis Strategy, the Relationship between Structure and Performance, Industrial Application. Chem. Eng. J. 2025, 524, 169602. [Google Scholar] [CrossRef]
- Maadani, A.M.; Davoodian, F.; Salahinejad, E. Effects of PLGA Coating on Biological and Mechanical Behaviors of Tissue Engineering Scaffolds. Prog. Org. Coat. 2023, 176, 107406. [Google Scholar] [CrossRef]
- Shi, L.; Chen, S.; Zheng, F.; Liu, M.; Yang, H.; Zhang, B. Corrosion Resistance Evaluation of Biodegradable Magnesium Alloy Vascular Stents Optimized by Mechanical Adapted Polymer Coating Strategy. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130664. [Google Scholar] [CrossRef]
- Montero, C.; Ramírez, C.G.; Muñoz, L.; Sancy, M.; Azócar, M.; Flores, M.; Artigas, A.; Zagal, J.H.; Zhou, X.; Monsalve, A.; et al. Effect of Plasma Argon Pretreatment on the Surface Properties of AZ31 Magnesium Alloy. Materials 2023, 16, 2327. [Google Scholar] [CrossRef]
- Tian, P.; Liu, X. Surface Modification of Biodegradable Magnesium and Its Alloys for Biomedical Applications. Regen. Biomater. 2015, 2, 135–151. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kyzioł, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and Characterization of Electrospun Alginate Nanofibers Loaded with Ciprofloxacin Hydrochloride. Eur. Polym. J. 2017, 96, 350–360. [Google Scholar] [CrossRef]
- Kyzioł, A.; Mazgała, A.; Michna, J.; Regiel-Futyra, A.; Sebastian, V. Preparation and Characterization of Alginate/Chitosan Formulations for Ciprofloxacin-Controlled Delivery. J. Biomater. Appl. 2017, 32, 162–174. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Tosovic, J. Spectroscopic Features of Caffeic Acid: Theoretical Study. Kragujev. J. Sci. 2017, 39, 99–108. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, L. Effects of X-Shaped Reduction-Sensitive Amphiphilic Block Copolymer on Drug Delivery. Int. J. Nanomed. 2015, 10, 5309–5325. [Google Scholar] [CrossRef]
- Singh, G.; Tanurajvir, K.; Ravinder, K.; Kaur, A. Recent Biomedical Applications and Patents on Biodegradable Polymer-PLGA. Int. J. Pharmacol. Pharm. Sci. 2014, 1, 30–42. [Google Scholar]
- Jiang, W.; Tian, Q.; Vuong, T.; Shashaty, M.; Gopez, C.; Sanders, T.; Liu, H. Comparison Study on Four Biodegradable Polymer Coatings for Controlling Magnesium Degradation and Human Endothelial Cell Adhesion and Spreading. ACS Biomater. Sci. Eng. 2017, 3, 936–950. [Google Scholar] [CrossRef]
- Wu, G.; Feng, K.; Shanaghi, A.; Zhao, Y.; Xu, R.; Yuan, G.; Chu, P.K. Effects of Surface Alloying on Electrochemical Corrosion Behavior of Oxygen-Plasma-Modified Biomedical Magnesium Alloy. Surf. Coat. Technol. 2012, 206, 3186–3195. [Google Scholar] [CrossRef]
- Tiyyagura, H.R.; Puliyalil, H.; Filipič, G.; Kumar, K.C.; Pottathara, Y.B.; Rudolf, R.; Fuchs-Godec, R.; Mohan, M.K.; Cvelbar, U. Corrosion Studies of Plasma Modified Magnesium Alloy in Simulated Body Fluid (SBF) Solutions. Surf. Coat. Technol. 2020, 385, 125434. [Google Scholar] [CrossRef]
- Alberti, C.J.; Saito, E.; De Freitas, F.E.; Reis, D.A.P.; MacHado, J.P.B.; Dos Reis, A.G. Effect of Etching Temperature on Surface Properties of Ti6Al4V Alloy for Use in Biomedical Applications. Mater. Res. 2019, 22, e20180782. [Google Scholar] [CrossRef]
- Abdelrahim, R.A.; Badr, N.A.; Baroudi, K. The Effect of Plasma Surface Treatment on the Bioactivity of Titanium Implant Materials (in Vitro). J. Int. Soc. Prev. Community Dent. 2016, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Ben-Nissan, B.; Wang, H.A.; Juritza, A.; Swain, M.V.; Müller, W.H.; Chou, J.; Stamboulis, A.; Macha, I.J.; Taraschi, V. Mechanical Testing of Antimicrobial Biocomposite Coating on Metallic Medical Implants as Drug Delivery System. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109757. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured Amorphous Magnesium Phosphate/Poly (Lactic Acid) Composite Coating for Enhanced Corrosion Resistance and Bioactivity of Biodegradable AZ31 Magnesium Alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Zheng, Y.F.; Zhang, X.; Xi, T.F.; Wei, S.C. Surface Characteristics and Corrosion Behaviour of WE43 Magnesium Alloy Coated by SiC Film. Appl. Surf. Sci. 2012, 258, 3074–3081. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review: The THERMEC’2009 Biodegradable Metals. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]


| Element | Al | Zn | Mn | Si | Cu | Ca | Fe | Ni | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Content [wt.%] | 2.5 ÷ 3.5 | 0.7 ÷ 1.3 | 0.2 | 0.1 | 0.05 | 0.04 | 0.005 | 0.005 | Balance |
| Sample No. | Type of Surface Treatment |
|---|---|
| W0 | unmodified AZ31 magnesium alloy |
| W1 | after chemical and plasmochemical surface activation (SA) |
| W2 | SA + PLA coating |
| W3 | SA + PLA/CA coating |
| W4 | SA + PLGA coating |
| W5 | SA + PLGA/CA coating |
| Ion | Concentration, mM |
|---|---|
| sodium ion | 142.7 |
| potassium ion | 5.0 |
| magnesium ion | 1.5 |
| calcium ion | 2.6 |
| chloride | 187.8 |
| hydrogen carbonate | 1262.3 |
| hydrogen phosphate | 1.0 |
| sulphate | 0.5 |
| Tris | 0.05 |
| Element | Concentration, wt.% | ||||
|---|---|---|---|---|---|
| W0 | W1 | W2 | W4 | W5 | |
| Mg | 95.6 ± 0.1 | 80.1 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | ------------ |
| Al | 2.1 ± 0.1 | 2.9 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.1 | ------------ |
| Zn | 0.2 ± 0.1 | ------------ | ------------ | ------------ | ------------ |
| O | 2.1 ± 0.1 | 17.0 ± 0.1 | 16.8 ± 0.1 | 10.7 ± 0.1 | 21.4 ± 0.1 |
| C | ------------ | ------------ | 79.7 ± 0.1 | 86.1 ± 0.1 | 73.6 ± 0.1 |
| Series Name | Coefficient of Friction μ [-] | Friction Force (Ff) [N] |
|---|---|---|
| W0 | 0.34 ± 0.26 | 1.73 ± 0.06 |
| W1 | 0.30 ± 0.05 | 1.71 ± 0.07 |
| W2 | 0.43 ± 0.04 | 1.88 ± 0.09 |
| W3 | 0.56 ± 0.11 | 1.97 ± 0.08 |
| W4 | 0.53 ± 0.09 | 1.94 ± 0.08 |
| W5 | 0.69 ± 0.04 | 2.08 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyzioł, K.; Prażuch, J.; Gołąbczak, M.; Kyzioł, A.; Hebda, M.; Kluska, S. Surface Modification of AZ31 Mg Alloy Based on PLA or PLGA with Caffeic Acid for Bioengineering Applications. Coatings 2025, 15, 1309. https://doi.org/10.3390/coatings15111309
Kyzioł K, Prażuch J, Gołąbczak M, Kyzioł A, Hebda M, Kluska S. Surface Modification of AZ31 Mg Alloy Based on PLA or PLGA with Caffeic Acid for Bioengineering Applications. Coatings. 2025; 15(11):1309. https://doi.org/10.3390/coatings15111309
Chicago/Turabian StyleKyzioł, Karol, Janusz Prażuch, Marcin Gołąbczak, Agnieszka Kyzioł, Marek Hebda, and Stanisława Kluska. 2025. "Surface Modification of AZ31 Mg Alloy Based on PLA or PLGA with Caffeic Acid for Bioengineering Applications" Coatings 15, no. 11: 1309. https://doi.org/10.3390/coatings15111309
APA StyleKyzioł, K., Prażuch, J., Gołąbczak, M., Kyzioł, A., Hebda, M., & Kluska, S. (2025). Surface Modification of AZ31 Mg Alloy Based on PLA or PLGA with Caffeic Acid for Bioengineering Applications. Coatings, 15(11), 1309. https://doi.org/10.3390/coatings15111309








