First-Principles Study on Desolvation and Capacitive Performance of Bispyrrolidinium Cations in Pristine/Oxygen-Functionalized Bilayer Graphene Flat Pores
Abstract
1. Introduction
2. Calculation Method
- The total energy error of the system was controlled to be less than 0.05 kcal/mol;
- The total stress tensor was reduced to 0.1 GPa;
- The maximum ionic displacement was limited within 0.01 Å;
- The convergence accuracy of the forces acting on atoms was set to 0.5 kcal/mol/Å.
3. Results and Discussion
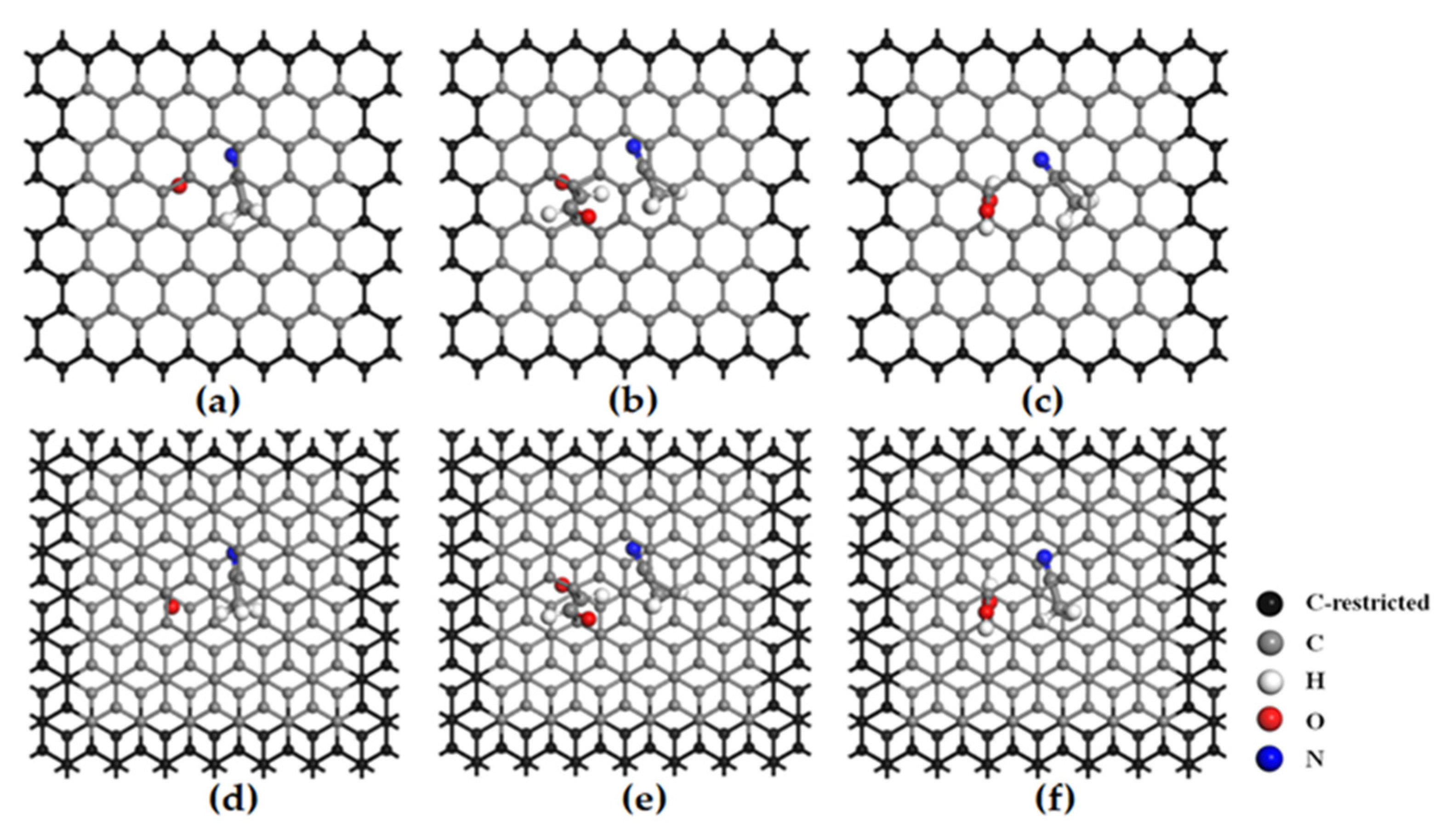
3.1. Reaction Principle
- A denotes SBP+;
- A(AN) represents bispyrrolidine cation complexes;
- FP refers to flat pores of pristine bilayer graphene;
- OFP denotes oxygen-containing functionalized flat pores of bilayer graphene, including hydroxyl-functionalized flat pores (HFP), carbonyl-functionalized flat pores (CFP), and aldehyde-functionalized flat pores (AFP);
- A(FP/OFP) stands for the intercalation compound of SBP+ in FP or OFP;
- AN(FP/OFP) represents the intercalation compound of AN molecules in FP or OFP;
- {A(AN)FP/OFP} denotes the intercalation compound of SBP+ complexes in FP or OFP.
- EA(AN) represents the energy of bispyrrolidine cation complexes;
- EOFP denotes the energy of oxygen-containing functionalized flat pores of bilayer graphene;
- EFP refers to the energy of flat pores of pristine bilayer graphene;
- EA(OFP) stands for the energy of the intercalation compound of bispyrrolidine cations in OFP;
- EA(FP) represents the energy of the intercalation compound of bispyrrolidine cations in FP;
- EAN denotes the energy of AN molecules;
- EAN(OFP) refers to the energy of the intercalation compound of AN molecules in OFP;
- EA(AN)FP stands for the energy of the intercalation compound of bispyrrolidine cation complexes in FP;
- EA represents the energy of bispyrrolidine cation;
- EA(AN)OFP denotes the energy of the intercalation compound of bispyrrolidine cation complexes in OFP.
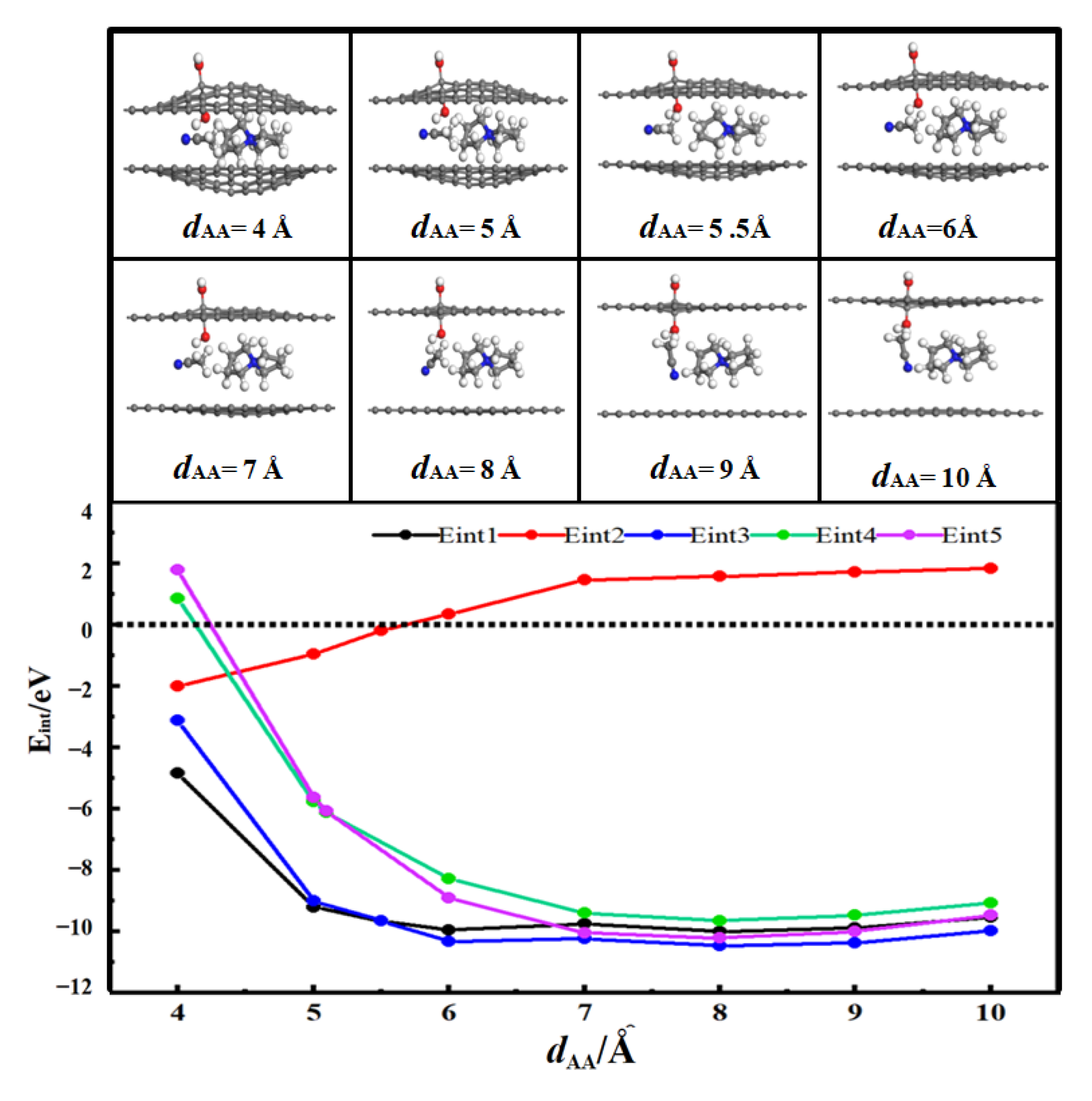
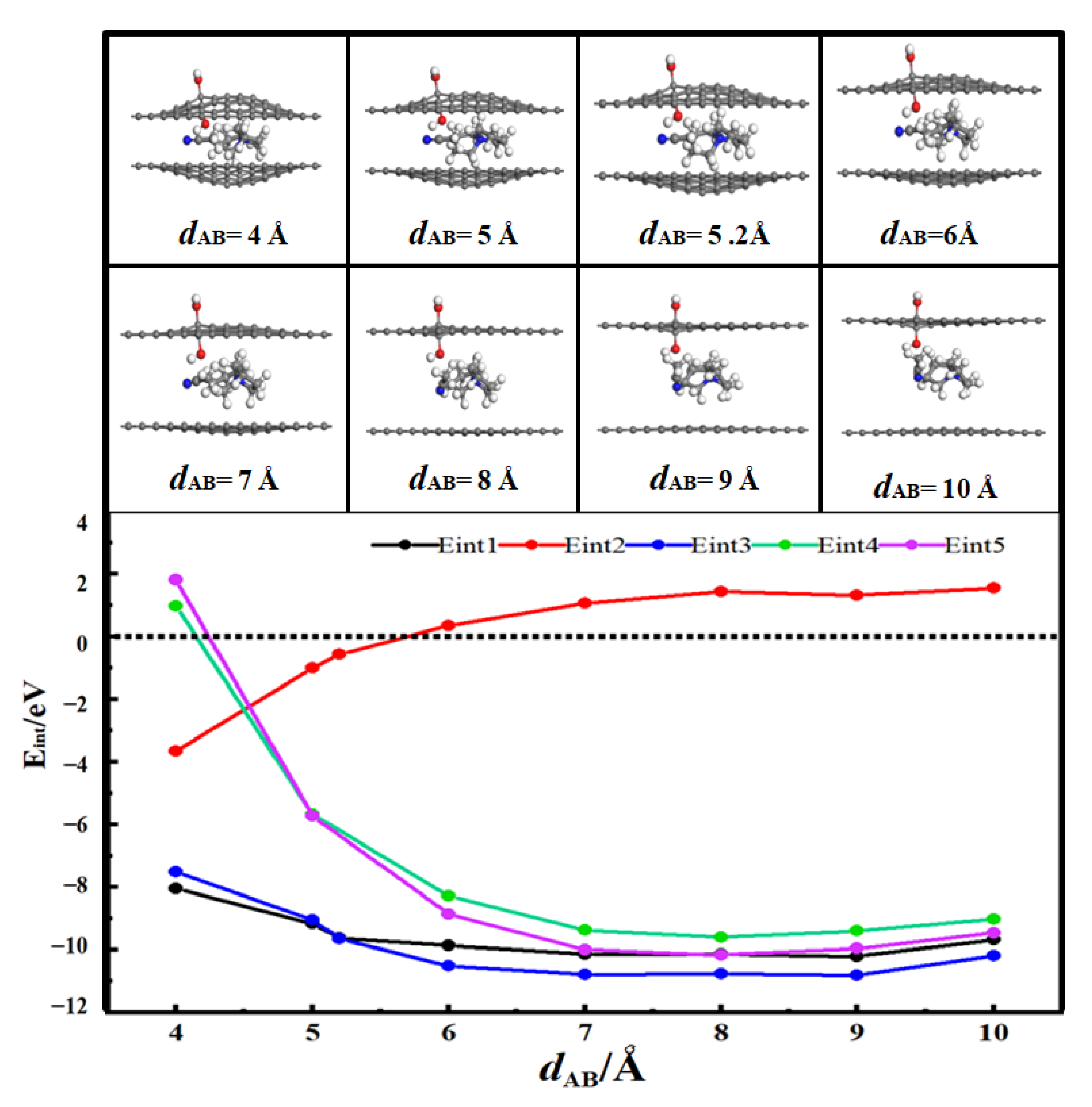
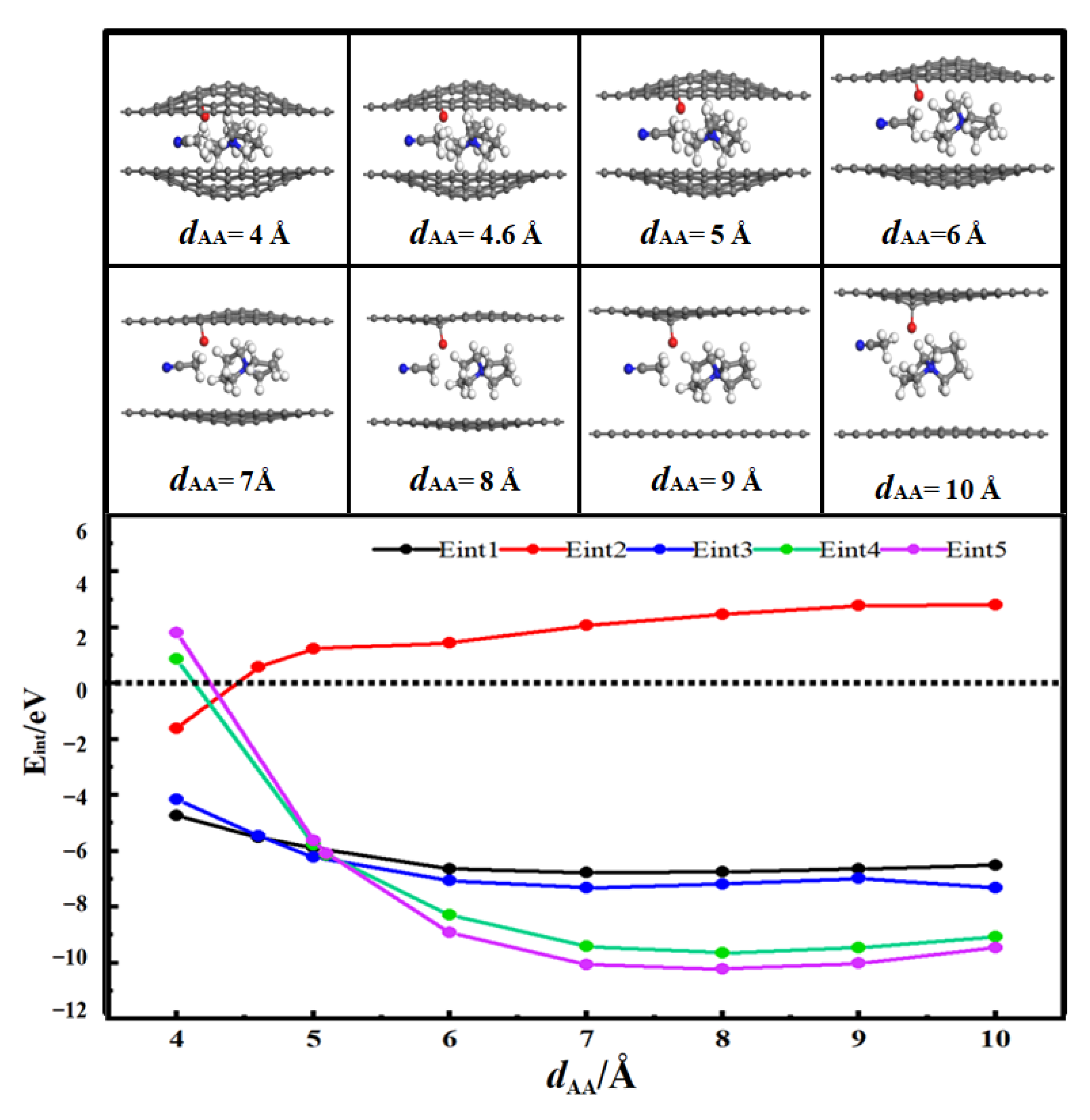
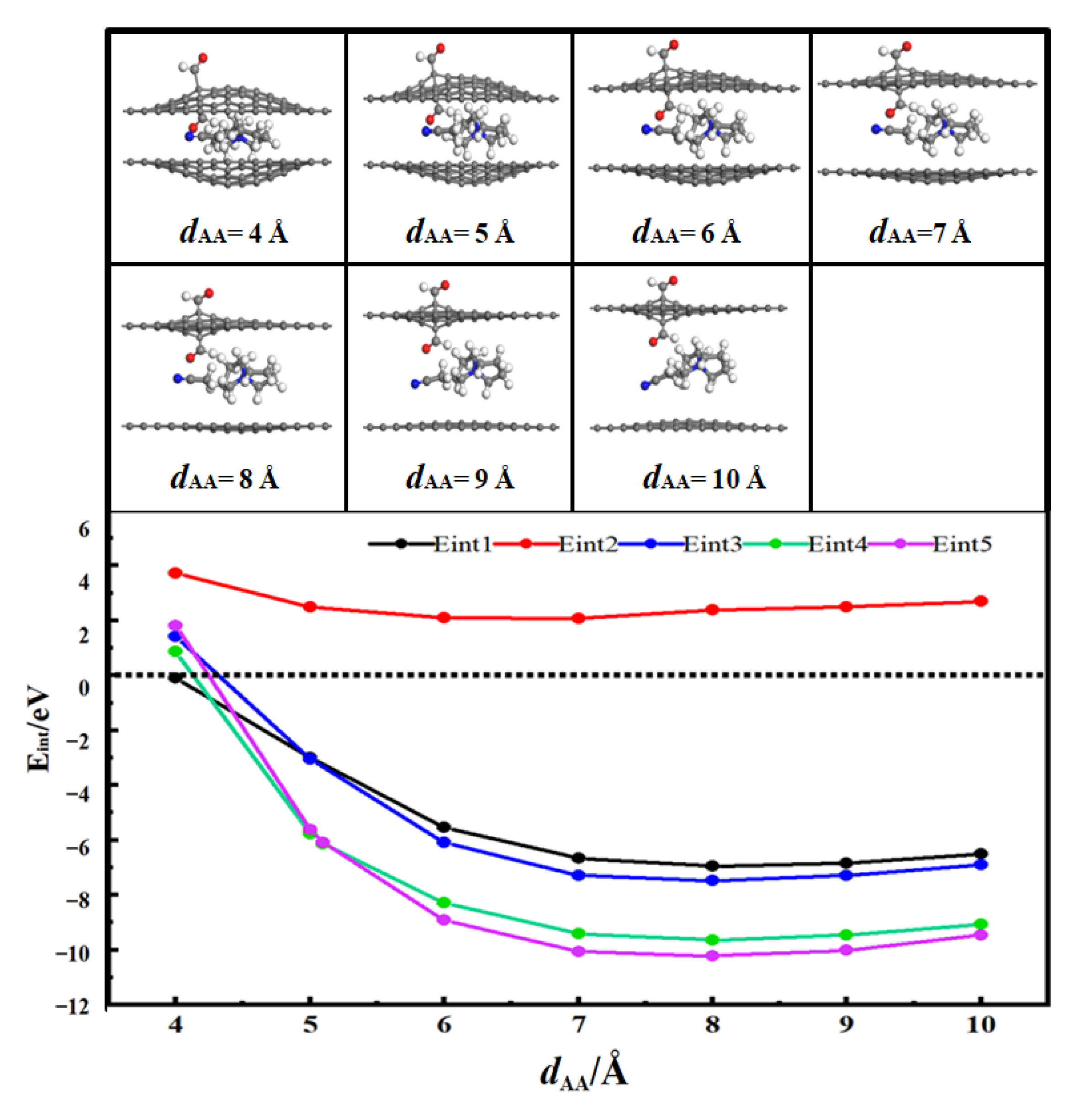
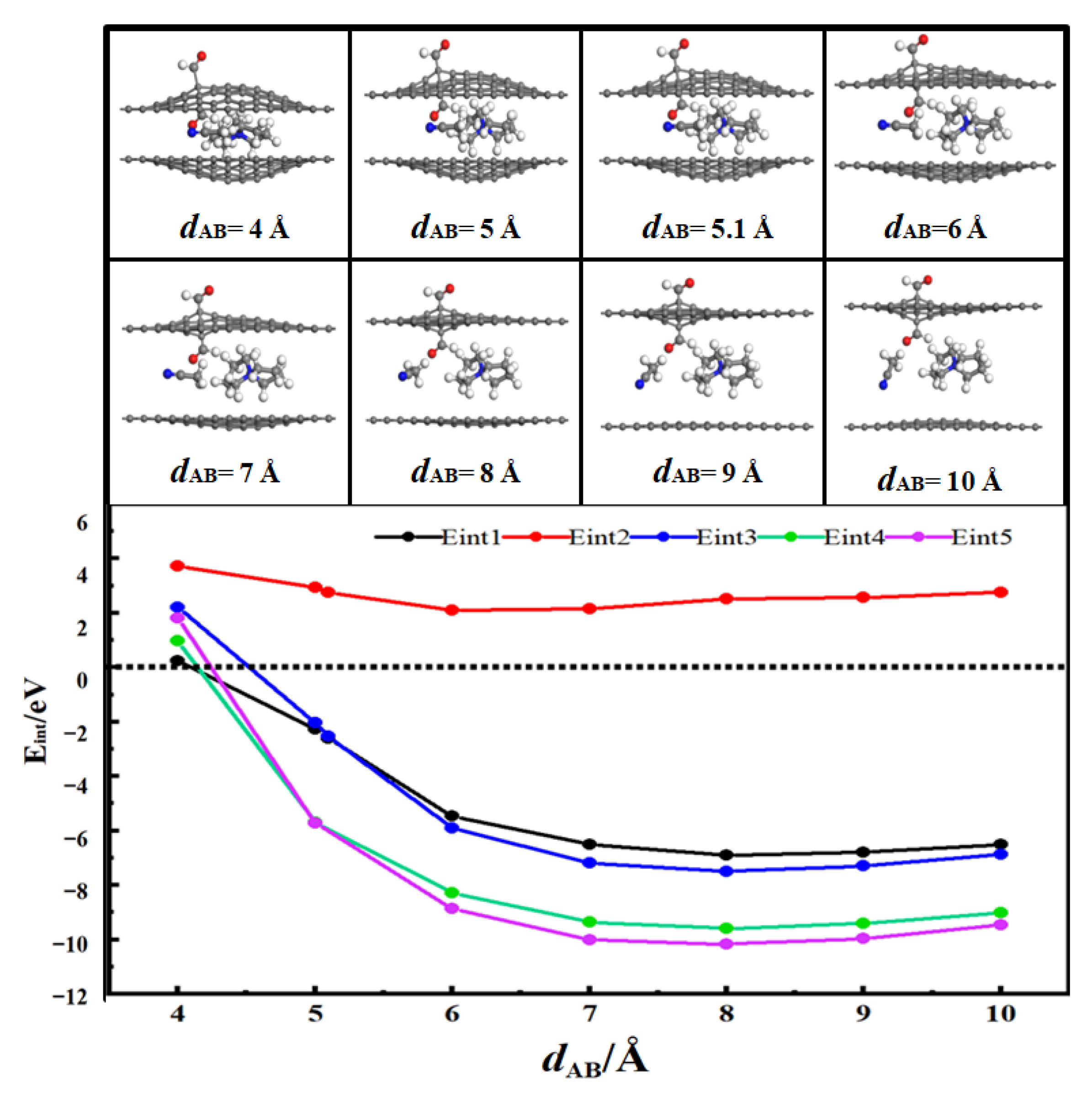
3.2. Desolvation of SBP+ Complexes
3.2.1. Desolvation of SBP+ Complexes by Hydroxylated-Flat Pores
3.2.2. Desolvation of SBP+ Complexes by Carbonylated-Flat Pores
3.2.3. Desolvation of SBP+ Complexes by Aldehydized-Flat Pores
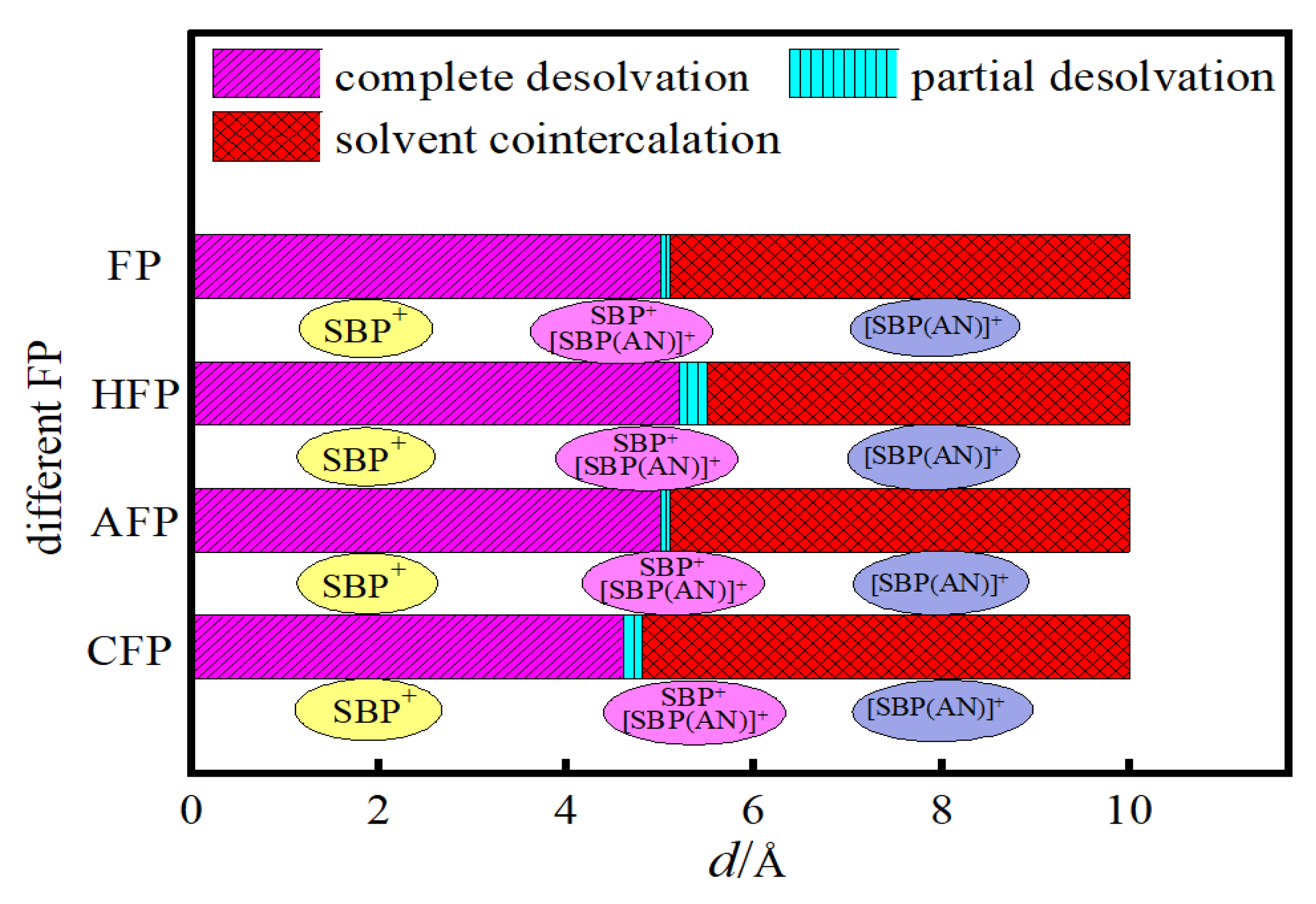
3.2.4. Analysis of the Influence of Differently Functionalized Bilayer Graphene on the Desolvation Size of SBP+
- For flat pores (FP), the complete desolvation size of the SBP+ complex is 5.0 Å, and the partial desolvation size ranges from 5.0 to 5.1 Å;
- For hydroxyl-functionalized flat pores (HFP), the complete desolvation size is 5.2 Å, and the partial desolvation size ranges from 5.2 to 5.5 Å;
- For aldehyde-functionalized flat pores (AFP), the complete desolvation size is 5.0 Å, and the partial desolvation size ranges from 5.0 to 5.1 Å;
- For carbonyl-functionalized flat pores (CFP), the complete desolvation size is 4.6 Å, and the partial desolvation size ranges from 4.6 to 4.8 Å.
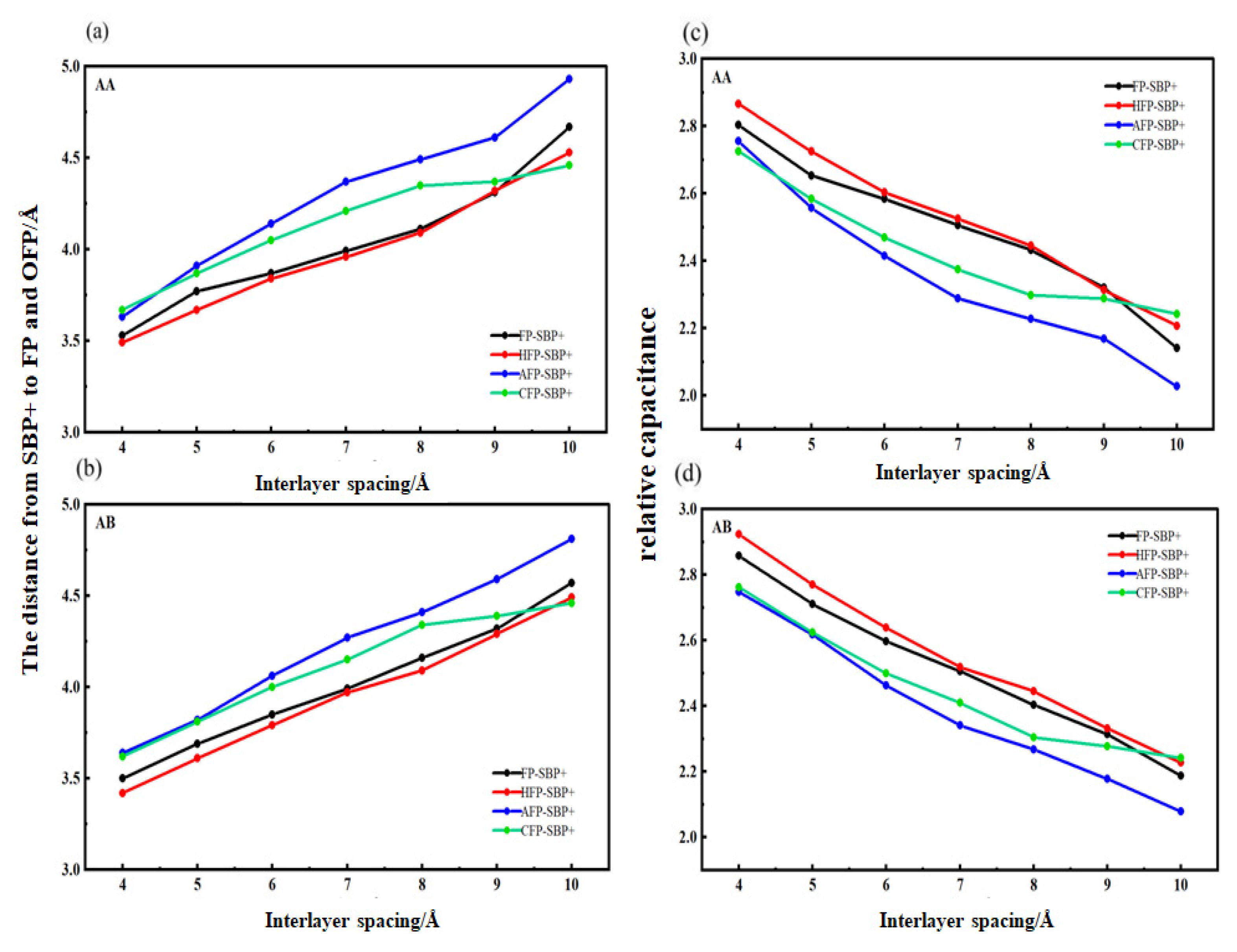
3.3. Analysis of the Relative Capacitance of SBP+ Embedded in Bilayer Graphene Flat Pores with Different Functional Groups
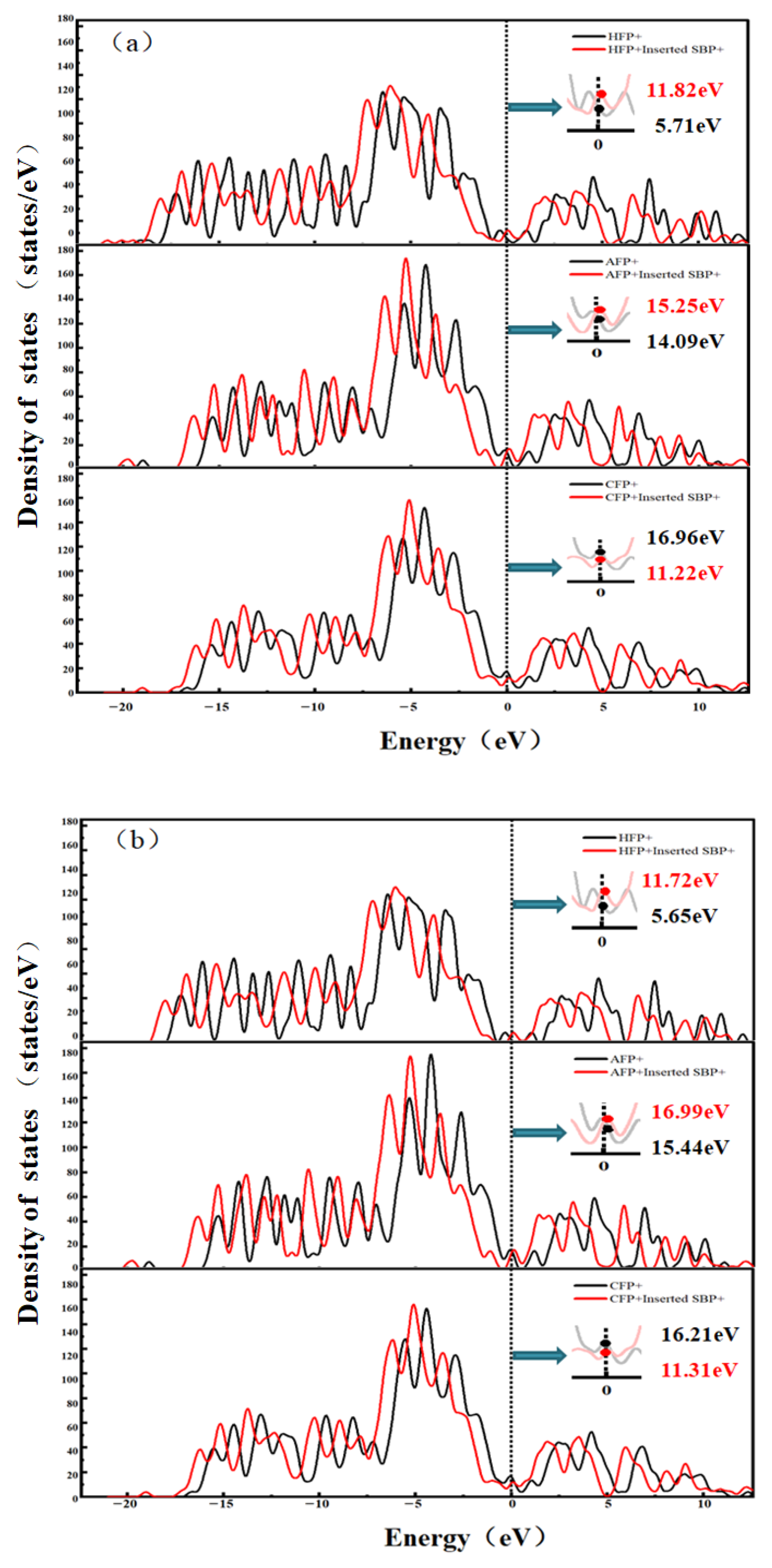
3.4. Density of States Analysis of SBP+ After Desolvation in Functionalized Flat Pores
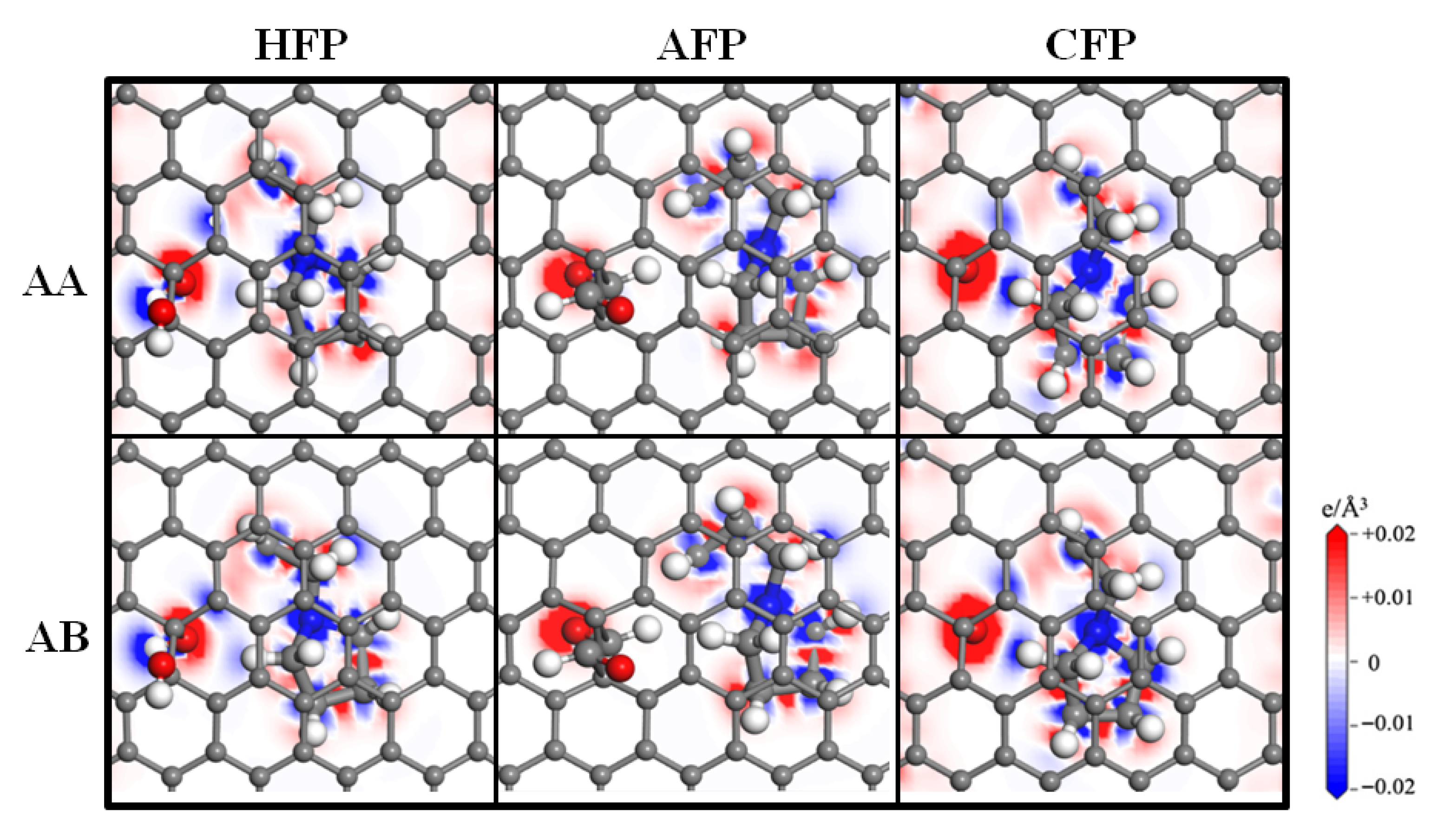
3.5. Charge Density Difference Analysis of SBP+ After Desolvation
4. Conclusions
- Critical desolvation diameters and capacitance effects: The minimum pore sizes enabling complete [SBP(AN)]+ desolvation (averaged across AA/AB stackings) are 5.0 Å (FP), 5.2 Å (HFP), 5.0 Å (AFP), and 4.6 Å (CFP), with partial desolvation ranges of 5.0~5.1 Å, 5.2~5.5 Å, 5.0~5.1 Å, and 4.6~4.8 Å, respectively. Hydroxyl functionalization expands the critical desolvation diameter by 0.2 Å and increases relative capacitance by 2%~3% (max 1.03× vs. FP) by enhancing SBP+ storage capacity. Carbonyl groups reduce the critical diameter by 0.4 Å and hinder SBP+ intercalation, lowering capacitance, while aldehyde groups show no significant impact on desolvation size or capacitance.
- Conductivity regulation via functional groups: DOS analysis reveals that embedding desolvated SBP+ enhances the conductivity of HFP and AFP (Fermi level peak increases by 2.0~10.1 states/eV) but reduces that of CFP (Fermi level peak decreases by 4.9~5.7 states/eV). AA- and AB-stacking configurations do not alter the trend of conductivity changes but tune ion transport properties—AA-stacked HFP favors diffusion, while AB-stacked HFP strengthens adsorption.
- Charge transfer mechanism: Charge density difference and Bader charge analysis confirm that SBP+ acts as an electron donor, transferring 0.67~0.76 e to OFP. Electron transfer primarily occurs between SBP+ and oxygen atoms in functional groups (oxygen gains 0.37~0.63 e), with negligible interaction between SBP+ and the carbon basal plane.
- This work provides quantitative guidance for electrode optimization: hydroxyl-functionalized bilayer graphene with 5.2 Å pores is preferred for high-performance supercapacitors. Future studies should extend to mixed electrolytes and experimental validation to further improve practical applicability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Advanced Materials for Electrochemical Capacitors: From Fundamentals to Applications. Nat. Mater. 2022, 21, 799–814. [Google Scholar]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X. Carbon-Based Nanomaterials for Supercapacitor Electrodes: Design Strategies and Performance Optimization. Chem. Soc. Rev. 2022, 51, 3872–3915. [Google Scholar]
- Burke, A. Ultracapacitor Technology: Recent Advances, Challenges, and Future Perspectives. J. Power Sources 2023, 568, 233245. [Google Scholar]
- Singh, P.; Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. Electrode Materials for Electrochemical Supercapacitors: Recent Developments and Future Trends. Chem. Soc. Rev. 2021, 50, 6888–6926. [Google Scholar]
- Beguin, F.; Presser, V.; Balduccl, A. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Stoller, M.D.; Zhu, Y.; Ruoff, R.S. Graphene-Based Ultracapacitors: Recent Progress in Electrode Design and Performance. Nano Lett. 2021, 21, 9234–9245. [Google Scholar]
- Rajagopal, A.; Kuriqi, A.; Gogotsi, Y. Functionalized Carbon Materials for High-Energy Supercapacitors: Synthesis and Mechanisms. Chem. Mater. 2022, 34, 8976–9002. [Google Scholar]
- Khan, M.K.; Kim, H.Y. Functionalized Graphene-Based Nanocomposites for Supercapacitor Application. Int. J. Electrochem. Sci. 2011, 6, 5840–5853. [Google Scholar] [CrossRef]
- Wang, H.; Yoshio, M. Effect of cation on the performance of AC/graphite capacitor. Electrochem. Commun. 2008, 10, 382–386. [Google Scholar] [CrossRef]
- Yang, S.B.; Liu, X.L.; Zhang, X.; Tang, S.W. Insights into the effect of hydroxyl-, epoxy-, and carboxyl-pores on the desolvation of K+ with water as a solvent: A first-principles study. J. Phys. Condens. Matter 2021, 33, 445201. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Liu, Z.; Lu, X.; Zhang, S.; Yan, Y.; Chi, C.; Huangfu, C.; Wang, G.; Gao, P.; Chi, W.; et al. Dual Molecules Cooperatively Confined In-Between Edge-oxygen-rich Graphene Sheets as Ultrahigh Rate and Stable Electrodes for Supercapacitors. J. Mater. Chem. A 2024, 12, 28122–28130. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.E.; Schultz, P.A.; Desjarlais, M.P.; Mattsson, T.R.; Leung, K. Designing meaningful density functional theory calculations in materials science-a primer. Model. Simul. Mater. Sci. Eng. 2004, 13, R1. [Google Scholar] [CrossRef]
- Armand, M.; MacFarlane, D.R.; Forsyth, M. Ionic Liquids for Electrochemical Energy Storage: Recent Breakthroughs and Future Challenges. Nat. Mater. 2023, 22, 545–556. [Google Scholar]
- Prehal, C.; Koczwara, C.; Jäckel, N.; Schreiber, A.; Burian, M.; Nitsch, H.A.; Hartmann, M.A.; Presser, V.; Paris, O. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat. Energy 2017, 2, 16215. [Google Scholar] [CrossRef]
- Sahu, S.; Zwolak, M.P. Ionic selectivity and filtration from fragmented dehydration in multilayer graphene nanopores. Nanoscale 2017, 9, 18532–18539. [Google Scholar] [CrossRef]
- Niketa, A.K.; Kumar, S. Electrostatic Modulation for Enhanced Ion Selectivity in Gate-All-Around Multilayer Stacked Graphene Nanopore. ACS Nano 2024, 9, 54919–54926. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J.; Perdew, J.P. Density Functional Theory: From Fundamentals to Materials Applications. Phys. Rev. Lett. 2021, 127, 156401. [Google Scholar]
- Frauenheim, T.; Köhler, T.; Aradi, B. Density-Functional Tight-Binding: Recent Developments for Materials Simulation. J. Comput. Chem. 2023, 44, 1890–1908. [Google Scholar]
- Dubey, P. Electrolytic Study of Pineapple Peel Derived Porous Carbon for All-Solid-State Supercapacitors. ChemistrySelect 2021, 6, 11736–11746. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.27. [Google Scholar] [CrossRef]
- Lee, G.S.; Lee, B.K.; Kim, J.Y.; Cho, K.J. Ozone Adsorption on Graphene: Ab Initio Study and Experimental Validation. J. Phys. Chem. C 2009, 113, 14225–14229.28. [Google Scholar] [CrossRef]
- Liu, F.; Yang, S.; Zhang, X.; Tang, S.; Xia, Y. Insight into the Desolvation of Quaternary Ammonium Cation with Acetonitrile as a Solvent in Hydroxyl-Flat Pores: A First-Principles Calculation. Materials 2023, 16, 3858. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zhou, M.; Zhang, C. Metal-Embedded Graphene: A Possible Catalyst with High Activity. J. Phys. Chem. C 2009, 113, 20156–20160. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, F.M.; Peeters, F.M. Adsorption of molecules on graphene: The role of the substrate. Phys. Rev. B 2008, 77, 125418. [Google Scholar]
- Yang, S.B.; Shan, X.Y.; Li, S.N. Insight into the Adsorption and Diffusion Behaviors of Na on XC3 (X=B, N and P) Doping Graphene Surfaces: A First Principle Study. Mater. Rep. B Res. Pap. 2019, 33, 1640–1645. [Google Scholar]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, T.; Jónsson, H. A fast and robust algorithm for Bader charge allocation. J. Chem. Phys. 2006, 124, 064108. [Google Scholar]
- Dobrota, M.; Kokalj, A. Insights into the Interaction of Graphene Oxide with Organic Cations. J. Phys. Chem. C 2017, 121, 26698–26708. [Google Scholar]

| Structure | FP | HFP | AFP | CFP | ||||
|---|---|---|---|---|---|---|---|---|
| AA | AB | AA | AB | AA | AB | AA | AB | |
| FP-4@ SBP+ | 2.83 | 2.86 | 2.87 | 2.92 | 2.75 | 2.75 | 2.72 | 2.76 |
| FP-5@ SBP+ | 2.65 | 2.71 | 2.72 | 2.77 | 2.56 | 2.62 | 2.58 | 2.62 |
| FP-6@ SBP+ | 2.58 | 2.59 | 2.60 | 2.64 | 2.42 | 2.46 | 2.47 | 2.50 |
| FP-7@ SBP+ | 2.51 | 2.51 | 2.53 | 2.52 | 2.29 | 2.34 | 2.38 | 2.41 |
| FP-8@ SBP+ | 2.43 | 2.40 | 2.44 | 2.44 | 2.23 | 2.27 | 2.29 | 2.30 |
| FP-9@ SBP+ | 2.32 | 2.31 | 2.31 | 2.33 | 2.17 | 2.18 | 2.29 | 2.28 |
| FP-10@ SBP+ | 2.14 | 2.19 | 2.21 | 2.23 | 2.03 | 2.08 | 2.24 | 2.24 |
| Charge/e | HFP | AFP | CFP |
|---|---|---|---|
| AA | +0.757 | +0.760 | +0.677 |
| AB | +0.732 | +0.755 | +0.668 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Cao, Y.; Li, S.; Qi, X.; Liu, B. First-Principles Study on Desolvation and Capacitive Performance of Bispyrrolidinium Cations in Pristine/Oxygen-Functionalized Bilayer Graphene Flat Pores. Coatings 2025, 15, 1299. https://doi.org/10.3390/coatings15111299
Liu F, Cao Y, Li S, Qi X, Liu B. First-Principles Study on Desolvation and Capacitive Performance of Bispyrrolidinium Cations in Pristine/Oxygen-Functionalized Bilayer Graphene Flat Pores. Coatings. 2025; 15(11):1299. https://doi.org/10.3390/coatings15111299
Chicago/Turabian StyleLiu, Fudong, Yi Cao, Sinan Li, Xin Qi, and Bing Liu. 2025. "First-Principles Study on Desolvation and Capacitive Performance of Bispyrrolidinium Cations in Pristine/Oxygen-Functionalized Bilayer Graphene Flat Pores" Coatings 15, no. 11: 1299. https://doi.org/10.3390/coatings15111299
APA StyleLiu, F., Cao, Y., Li, S., Qi, X., & Liu, B. (2025). First-Principles Study on Desolvation and Capacitive Performance of Bispyrrolidinium Cations in Pristine/Oxygen-Functionalized Bilayer Graphene Flat Pores. Coatings, 15(11), 1299. https://doi.org/10.3390/coatings15111299






