Abstract
Through employing WO3 as a precursor, we successfully deposited a complete and continuous WC coating onto the surface of diamond particles by means of the salt bath method. Initially, a tungsten (W) layer forms on the diamond surface, which gradually transitions to a tungsten carbide (WC) coating as either the temperature is elevated or the duration of the process is prolonged. A thorough thermodynamic analysis was conducted to investigate this phase transition mechanism. At a lower synthesis temperature of 1000 °C, significant differences were observed in both the thickness and phase composition of the coatings formed on the (100) and (111) crystal planes of diamond. Specifically, the coating on the (100) plane exhibited earlier growth compared to that on the (111) plane, with WC phases appearing sooner within the coating’s composition. However, as the synthesis temperature increases, these differences in both thickness and phase composition between coatings on different diamond crystal faces tend to diminish, leading towards convergence. Furthermore, a detailed kinetic analysis of the coating growth process was conducted. It was found that the reduction reaction of carbon on WO3 led to the formation of the W coating, and the diffusion of carbon in the W coating resulted in the formation of the WC coating. The diffusion of carbon in the coating ensured its continuous growth, providing deeper insights into the mechanisms governing the deposition and transformation processes.
1. Introduction
Diamond, renowned for its exceptional hardness among natural materials, exhibits remarkable potential for applications in diverse fields such as cutting, grinding, and electronic devices due to its unique physical and chemical properties [1]. To reduce costs while enhancing adaptability for practical applications, metal-based diamond composite materials have been developed. However, the chemical inertness and poor wettability of diamond with metals pose significant challenges in achieving dense and stable metal-diamond composites. To address these limitations, various surface modification techniques for diamond have been explored [2,3]. Among these approaches, tungsten carbide (WC) has emerged as a promising coating material due to its compatibility with diamond surfaces.
Several methods have been investigated for depositing WC coatings on diamond surfaces, including sol–gel processing [4,5], magnetron sputtering [6,7,8], chemical vapor deposition (CVD) [9], high-pressure high-temperature (HPHT) synthesis [10,11,12], conventional sintering [13], hydrothermal crystal growth [14,15], spark plasma sintering (SPS) [16], and salt bath processing [17,18,19]. Notably, the salt bath method offers a cost-effective, environmentally friendly approach suitable for large-scale production. For example, Chen et al. [20] employed the salt bath technique to deposit coatings containing W and WC phases on diamond surfaces under conditions of 1050 °C for 2 h, with tungsten (W) as the primary coating component. Similarly, Dong et al. [] utilized the same method to achieve coatings comprising W, W2C, and WC phases at 1150 °C for 2 h, where WC was the dominant phase.
Previous studies reveal that variations in experimental temperature significantly influence the phase composition of W and WC coatings on diamond surfaces. Furthermore, the literature indicates differences in the growth behavior of W and WC coatings on distinct crystallographic planes of diamond particles [20]. Building on these findings, the W and WC coatings in this study are generated by the reduction of WO3, a commonly used industrial material [21,22,23], and this study investigates critical factors such as temperature and processing time that govern the phase transformation of coating components. Additionally, it provides an in-depth analysis of the growth mechanisms of tungsten carbide coatings on different diamond crystallographic planes and elucidates the underlying reasons for the observed differences in growth patterns across various planes.
2. Materials and Methods
Diamond particles (procured from Henan Huanghe Whirlwind International Co., Ltd., Xuchang, China, with an average diameter of 230 μm) served as the carbon source in this study. These particles were reacted with tungsten trioxide (WO3) in a molten salt mixture comprising potassium chloride (KCl) and sodium chloride (NaCl) (obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, with a purity of >99.0%). The molar ratio of NaCl to KCl was maintained at 1:1. Prior to the reaction, the diamond particles were immersed in dilute sulfuric acid and subjected to ultrasonic treatment for 30 min to remove residual surface impurities. Following this, the particles were rinsed twice with distilled water and dried thoroughly.
The WO3 was mixed with the chloride salts and ground continuously for 30 min, with a mass ratio of WO3 to the chloride salts maintained at 1:0.90. To ensure the reaction proceeded to completion, an excess of tungsten trioxide was used. The cleaned diamond particles were then added to this mixture and stirred in a small amount of ethanol until uniform dispersion was achieved. The mass ratio of diamond particles to WO3 was set at 1:1.94. The reactants are placed in a tubular furnace. To avoid the influence of residual O2 during the experiment, a vacuum pump is used to evacuate the gas inside the alumina tube to 10−1 MPa before the experiment begins. During both the heating and cooling processes, a high flow rate of Ar gas is continuously introduced into the tube to maintain a slight positive pressure inside, with an Ar gas flow rate of 0.1 L/min. Therefore, we consider that the reaction and cooling process are carried out in an oxygen-free environment. Due to equipment limitations, different heating rates are used at different temperature ranges, as shown in Table 1. According to the experimental design, the samples are held at different temperatures for different durations, as shown in Table 2, to prepare coatings with different thicknesses and compositions. After the reaction ends, the specimens are allowed to cool to room temperature in an Ar atmosphere. For comparison, coatings were also deposited onto CVD diamond wafers (China Suzhou Magic Stone Co., Ltd., Suzhou, China) using the same method.

Table 1.
Temperature rise rate of experimental equipment.

Table 2.
Experimental temperature and holding time for each sample.
The phase composition of the diamond with coating was analyzed using X-ray diffraction (XRD, Haoyuan DH-2700BH, Dandong, Liaoning). An X-ray diffractometer with a copper target Kα ray (λ = 0.15418 nm) was used for testing under θ-2θ scanning mode. Then, using Jade 6.0 software, the XRD results of the samples were analyzed quantitatively by intensity ratio (RIR method), and the same sample was analyzed multiple times to obtain the average value and error of the coating composition content. The microstructure and thickness of the coatings were examined using a scanning electron microscope (SEM) equipped with energy-dispersive spectroscopy (EDS, TESCAN MIRA LMS, Brno, Czech Republic). Both XRD and SEM experiments were conducted at room temperature.
3. Results
As depicted in Figure 1, it was observed that with increasing the coating temperature and holding time, the color of coating on diamond surface transitioned from purple (b) to black (d–g) and finally to gray-white (h). The purple adhesive particles on diamond particles could be removed via ultrasonic cleaning, as shown in Figure 1c, which are identified as WO2 by XRD, aligning with findings reported in the literature [24]. And through XRD testing, the black coating is a W coating, and the grayish-white coating is a WC coating. The change in the material phases caused the color variation in the diamond coatings.
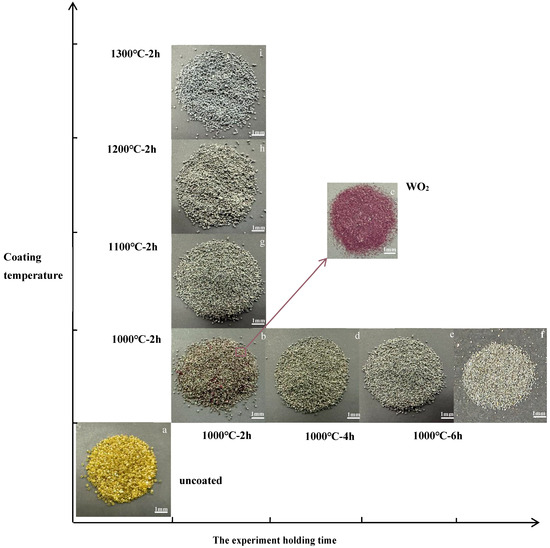
Figure 1.
Digital photos of the diamond: (a) uncoated diamonds, (b) diamonds coated at 1000 °C for 2 h, (c) adhesive particles separated from the surface of diamond particles in (b,d) diamonds coated at 1000 °C for 4 h, (e) diamonds coated at 1000 °C for 6 h, (f) diamonds coated at 1000 °C for 8 h, (g) diamonds coated at 1100 °C for 2 h, (h) diamonds coated at 1200 °C for 2 h (i) diamonds coated at 1300 °C for 2 h.
As shown in Figure 2a,b, when the coating temperature is 1000 °C and 1050 °C, the XRD spectrum exhibits peaks at 31.513°, 35.639°, 39.568°, 40.264°, 48.300°, 58.274°, 73.104°, 75.38°, and 87.021°. Among these, the peaks at 40.264°, 58.274°, and 87.021° correspond to W (PDF#04-0806), while the peaks at 31.513°, 35.639°, 48.300°, and 73.104° correspond to WC (PDF#72-0097). The peak at 39.568° corresponds to W2C (PDF#35-0776), and the peak at 75.38° corresponds to diamond (PDF#06-0675). When the experimental holding time is extended, after extending the time from 4 h to 5 h, the WC ratio also increased from 16.1% to 17.9%. When the holding time is further extended to 6 h, additional peaks appear at 64.016° and 84.069°, both corresponding to WC. At the same time, the WC content in the coating of the sample at 1000-6 h also increased to 49.9%. Therefore, under a coating temperature of 1000 °C and 1050 °C, the primary component of the coating is W, with small amounts of WC, W2C, and diamond present. Notably, W2C acts as an unstable intermediate phase and occupies a very small proportion in the coating.
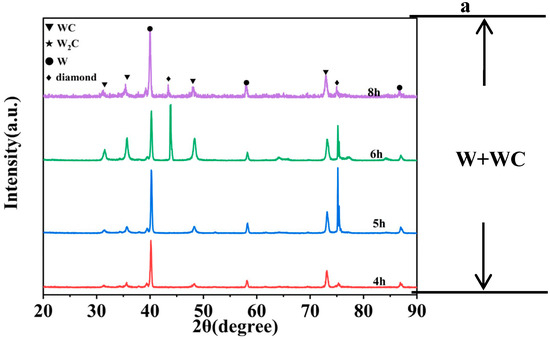
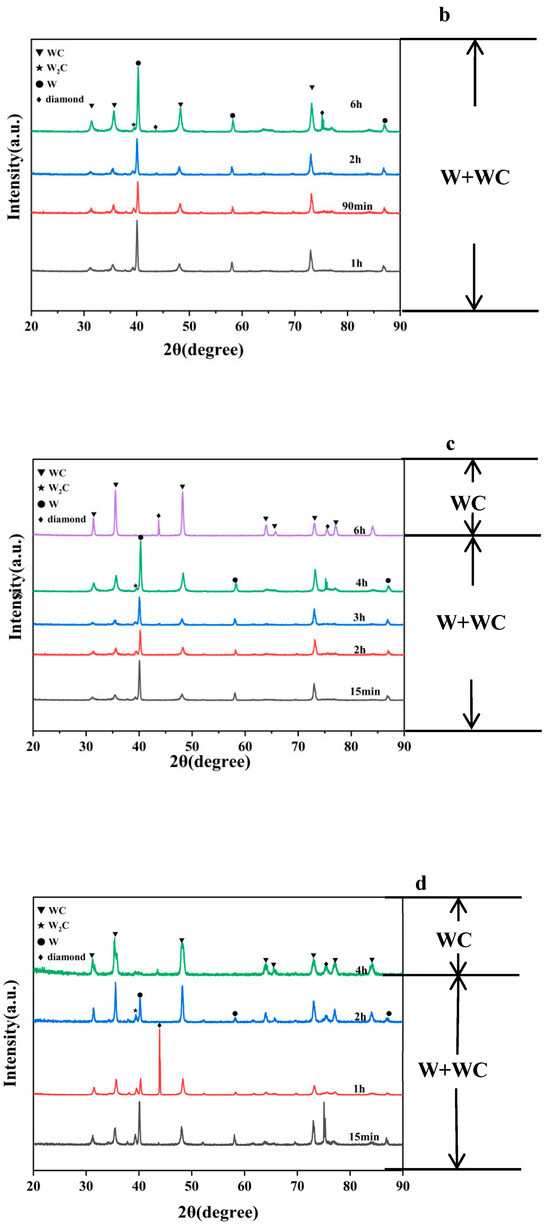
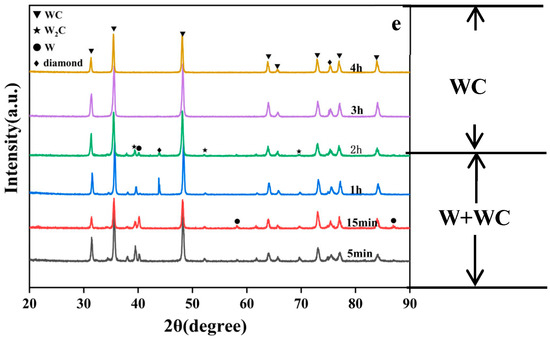
Figure 2.
XRD pattern of diamond particles with coatings produced at (a) 1000 °C, (b) 1050 °C, (c) 1100 °C, (d) 1200 °C, and (e) 1300 °C with varying holding times.
As shown in Figure 2c–e, in the following groups, the peak positions in the XRD spectra are consistent with those observed in Figure 2a: 1100-15 min, 1100-2 h, 1100-3 h, 1100-4 h, 1200-15 min, 1200-1 h, 1200-2 h, 1300-5 min, 1300-15 min, 1300-1 h, 1300-2 h. For this reason, when the temperature rises, coatings formed over shorter periods still contain W. Among these, when the temperature was 1100 °C and the time was extended from 15 min to 4 h, the WC ratio also increased from 25.2% to 45.7%. Similarly, when the temperature was 1200 °C and the time was extended from 15 min to 2 h, the WC ratio also increased from 37.9% to 71.6%. These samples all conform to the pattern where increasing the experimental time leads to an increase in the WC ratio within the coating. But when the temperature continues to rise, as in the following group:1100-6 h, 1200-4 h, 1300-3 h, 1300-4 h, the diffraction peaks corresponding to W and W2C disappear entirely, indicating that the coating has been fully converted into WC.
Therefore, across various experimental temperatures, the coatings underwent a transformation from W to WC, with rising temperatures significantly accelerating the rate of phase transformation.
Figure 3 illustrates the proportion of WC in the coatings synthesized under different experimental temperatures and holding times. The dashed line in the figure represents a WC content ratio of 50%. From the figure, it can be observed that under 1000 °C, the WC content in the coating is consistently below 50%, which means that at an experimental temperature of 1000 °C, the main component of the coating is tungsten (W). At experimental temperatures of 1100 °C and 1200 °C, the curves intersect with the 50% WC ratio line. This indicates that when the experimental time is shorter, the coating is dominated by W, while as the coating time increases, the coating composition becomes dominated by WC. Therefore, as the experimental time progresses, the coating composition transitions from the W phase to the WC phase. When the experimental temperature rises to 1300 °C, the WC content in the coating consistently exceeds 50%, meaning that under an experimental temperature of 1300 °C, the coating is predominantly composed of WC.
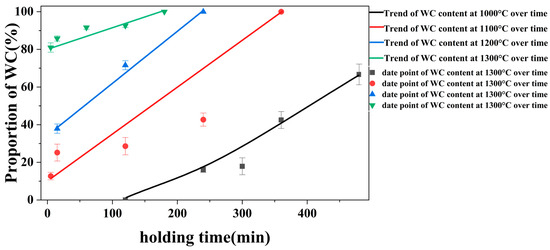
Figure 3.
Proportion of WC in the coatings synthesized under different experimental temperatures and durations.
Figure 4 presents the SEM images of coated diamond particles synthesized at 1050 °C for varying holding times: 30 (a), 60 (b), 90 (c), and 120 min (d). The SEM analysis reveals distinct morphological differences in coating coverage between the (100) and (111) crystal faces. At a holding time of 30 min (Figure 4a), the (100) crystal face exhibits complete coating coverage, while the (111) face shows negligible coating formation. As the holding time increases to 60 min (Figure 4b), initial coating nucleation is observed on the (111) face, though a continuous layer has not yet formed. When the holding time exceeds 90 min (Figure 4c,d), the diamond particles are fully encapsulated by the coating layer. Similar observations of crystallographically dependent coating behavior have been reported in previous studies on diamond surface coatings [25,26,27].
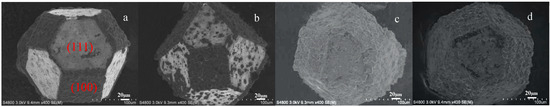
Figure 4.
Diamond with coating synthesized at 1050 °C with 30 (a), 60 (b), 90 (c), and 120 min (d).
Figure 5a shows the SEM image of a diamond particle coated at 1000 °C for 5 h. Cross-sectional views of the coatings on the (100) and (111) faces under identical conditions are presented in Figure 5b,c, respectively. The (100) face is entirely covered by a thick, uniform coating layer, whereas only a thin coating is observed on the (111) face. Quantitative analysis of the SEM images reveals a significant discrepancy in coating thickness: 5.05 ± 0.25 μm for the (100) face and 0.56 ± 0.16 μm for the (111) face. These results indicate that complete coverage of the diamond particle is achievable under these conditions, accompanied by substantial differences in coating thickness between the two crystallographic faces. To further investigate the phase composition of the coatings on different faces, XRD analysis was performed on coated CVD wafers, as shown in Figure 5d. The XRD results demonstrate distinct phase distributions: the (111) face coating consists exclusively of tungsten (W), while the (100) face coating contains a small fraction of WC phase. This finding confirms that the WC phase observed on the diamond particles in Figure 2a is exclusively associated with the (100) crystal face.
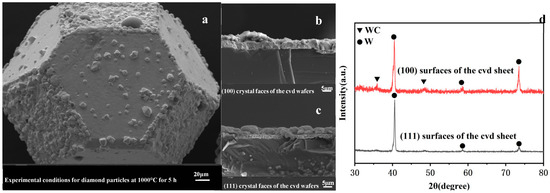
Figure 5.
Surface morphology and phase composition of diamond particle and CVD wafer coatings under experimental conditions of 1000 °C for 5 h: (a) SEM image of a diamond particle, (b,c) SEM images of the (100) and (111) crystal faces of the CVD wafer, (d) XRD spectrum of the CVD wafer.
Figure 6a presents the SEM image of a diamond particle coated at 1300 °C for 2 h. Cross-sectional images of the coatings on the (100) and (111) faces under identical conditions are displayed in Figure 6b and Figure 6c, respectively. Both crystallographic faces exhibit complete coverage by thick coating layers, with measured thicknesses of 5.56 ± 0.21 μm for the (100) face and 4.38 ± 0.19 μm for the (111) face. The coating thicknesses on both faces are relatively comparable under these synthesis conditions. Furthermore, XRD analysis shown in Figure 6d indicates that the phase compositions of the coatings on both faces are analogous, primarily consisting of WC (tungsten carbide) and W2C (tungsten sesquicarbide), with a minor presence of elemental tungsten (W). These results suggest that a uniform coating in terms of both composition and thickness can be achieved on diamond particle surfaces following 2 h of treatment at 1300 °C.
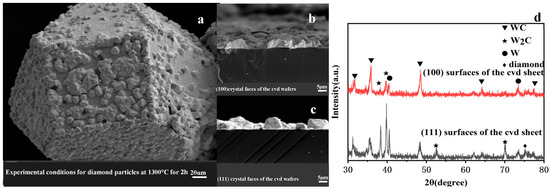
Figure 6.
Surface morphology and phase composition of diamond particle and CVD wafer coatings under experimental conditions of 1300 °C for 2 h: (a) SEM image of a diamond particle, (b,c) SEM images of the (100) and (111) crystal faces of the CVD wafer, (d) XRD spectrum of the CVD wafer.
4. Discussion
To further elucidate the coating growth mechanism, thermodynamic calculations were conducted to analyze possible reactions occurring during the coating process. Using thermodynamic modeling, the entire sequence of reactions involved in synthesizing WC coatings through the interaction of tungsten trioxide with diamond at 1000 °C and 1300 °C was systematically investigated:
WO3(s) + C (s) = WO2(s) + CO (g), ΔGT = 1000 °C = −15.588 kcal, ΔGT = 1300 °C = −26.686 kcal
4WO3(s) + 2C (s) = 4WO2(s) + 2CO2 (g), ΔGT = 1000 °C = −18.663 kcal, ΔGT = 1300 °C = −28.556 kcal
WO2(s) + 2C (s) = W (s) + 2CO (g), ΔGT = 1000 °C = −20.947 kcal, ΔGT = 1300 °C = −20.502 kcal
WO2(s) + C (s) = W (s) + CO2 (g), ΔGT = 1000 °C = −8.433 kcal, ΔGT = 1300 °C = −45.319 kcal
2W(s) + C(s) = W2C(s), ΔGT = 1000 °C = −4.599 kcal, ΔGT = 1300 °C = −4.251 kcal
W (s) + C (s) = WC (s), ΔG = T=1000 °C−8.488 kcal, ΔGT = 1300 °C = −8.219 kcal
W2C (s) + C (s) = 2WC (s), ΔGT = 1000 °C = −12.297 kcal, ΔGT = 1300 °C = −12.367 kcal
The experimental observations align with these thermodynamic predictions. Initially, the reduction of tungsten trioxide produces tungsten dioxide (WO2), which further reacts with carbon to form metallic tungsten (W). This intermediate tungsten phase is then converted into tungsten carbide (WC) through subsequent reactions.
The difference in surface energies between the (100) and (111) planes of diamond influences their reactivity during the reduction and carburization steps. At lower temperatures, the sequential nature of nucleation on these planes is evident, as shown in Figure 5. In contrast, at elevated temperatures, sufficient thermal energy is available to drive simultaneous reactions on both planes, leading to concurrent nucleation across different faces, as demonstrated in Figure 6.
Thermodynamic analysis reveals that coating growth requires direct contact between the oxide species and the carbon source. Once a continuous tungsten layer forms on the diamond surface, the oxide becomes isolated from the diamond, theoretically limiting further reaction and, consequently, the thickness of the coating. However, experimental results contradict this hypothesis. For instance, after reacting for four hours at 1100 °C, the (111) plane of diamond was fully covered by a 1.22 ± 0.10 μm thick tungsten coating (see Figure 2c). Extending the reaction time to six hours resulted in a coating thickness of 8.13 ± 0.30 μm, indicating continued growth even after the diamond surface was fully encapsulated. This suggests that alternative carbon sources, potentially from the diffusion of carbon through the tungsten layer, sustain the reaction and enable further coating growth.
Energy-dispersive spectroscopy (EDS) mapping of a representative tungsten coating on diamond, as shown in Figure 7, revealed significant carbon distribution within the tungsten layer. Furthermore, an EDS line scan was performed on the W-coated diamond, and the results showed that carbon diffused from the diamond to the coating surface. The presence of carbon could be observed in the surface region of the coating; thus, it can be inferred that the reduction reaction of WO3 can occur on the coating surface, leading to the thickening of the coating. This observation, consistent with findings reported elsewhere [28], implies that carbon can migrate from the diamond substrate into the coating, providing a sustained carbon source for continued growth. These results highlight the dynamic interplay between thermodynamic driving forces and kinetic factors in determining the ultimate thickness and composition of the coatings.
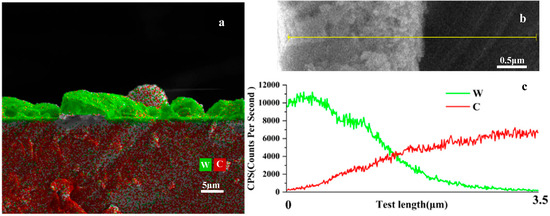
Figure 7.
(a) EDS image of the CVD chip coating reacted for 5 h at 1000 °C, (b): Enlarged view of the coating cross-section, (c): EDS line scan results corresponding to (b).
Similarly, upon the formation of a continuous WC shell, if carbon diffusion is impeded, the growth of the coating, as described in Equations (1) and (2), would terminate. The thickness of the WC coating on the (111) face of diamond synthesized at 1300 °C for one hour was measured to be 1.98 ± 0.10 μm; however, this thickness increased to 4.38 ± 0.19 μm when the reaction time was extended to two hours. Raman spectroscopy analysis of a representative WC coating on diamond is presented in Figure 8. The peaks observed at 1293.89 cm−1 and 1440.68 cm−1 correspond to the D and G bands of disordered carbon, respectively [29]. The presence of amorphous carbon on the surface of the coating suggests that WC does not act as a barrier to carbon diffusion at elevated temperatures, thereby enabling continuous thickening of the coating over time.
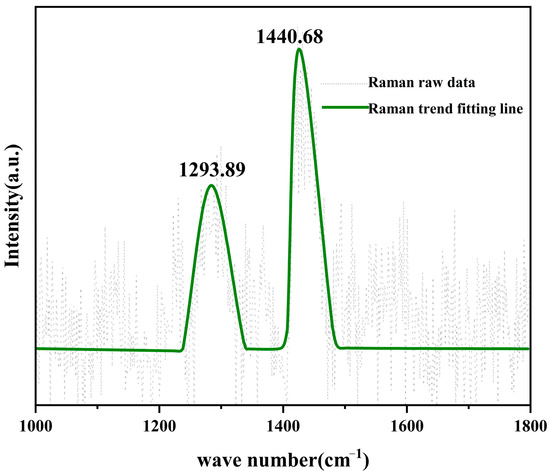
Figure 8.
Raman spectrum of the coating obtained after reacting for 2 h at 1300 °C.
This observation aligns with previous studies that have reported the phenomenon of amorphous carbon diffusing into WC at the interface between iron alloy and WC [30], which is consistent with the EDS results presented in this study.
5. Conclusions
In this study, WC coatings were successfully deposited onto diamond particles using WO3 as a precursor through the salt bath method under varying temperatures and coating durations. The deposition process initially resulted in the formation of a W coating on the diamond surface, which progressively transformed into a WC coating with increasing temperature or extended coating time.
The thickness and growth rate of the WC coatings on the (100) and (111) crystal planes were significantly influenced by both temperature and coating duration. Notably, at a lower synthesis temperature (1000 °C), distinct variations were observed in both the thickness and phase composition of the coatings on the (100) and (111) planes of diamond. However, as the synthesis temperature increased, the differences in both thickness and phase composition between the coatings on different crystal faces of diamond diminished, approaching convergence. This phenomenon can be attributed to differences in nucleation mechanisms: at lower temperatures, nucleation follows a sequential mechanism, whereas at elevated temperatures, sufficient activation energy enables concurrent initiation of reactions across different crystal faces.
Furthermore, an extension of the coating time permitted continuous thickening of the WC coatings. This behavior is explained by the fact that both W and WC do not act as diffusion barriers to carbon at high temperatures, allowing the reduction process of WO3 to proceed on the surface of the existing coatings over time, thereby facilitating progressive thickness enhancement.
Author Contributions
S.W.: Data curation, Formal analysis, Writing—original draft; Q.M.: Conceptualization, Writing—review and editing; X.M., M.Y., S.H. and Y.Q.: Supervision, Investigation, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
Deep Earth Probe and Mineral Resources Exploration-National Science and Technology Major Project-2024ZD1000908.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, Y.; Zang, J.; Su, S.; Zhang, C.; Zhao, L.; Yuan, Y.; Wang, Y.; Lu, J.; Xu, X.; Zhang, P. Mechanochemical grinding diamond film using titanium-coated diamond active abrasives prepared by vacuum micro-evaporation coating. Appl. Surf. Sci. 2023, 638, 158094. [Google Scholar] [CrossRef]
- Liu, J.; Liang, B.; Jiao, M. Low-Temperature Synthesis of TiC Coating on Diamond Surface by Thermal Explosion Reaction. J. Superhard Mater. 2024, 46, 106–111. [Google Scholar] [CrossRef]
- Banik, S.; Indhu, R.; Arunachalam, N.; Rao, M.R. Femtosecond laser-induced surface structuring for improved surface characteristics of single crystal diamond. Diam. Relat. Mater. 2025, 154, 112240. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Wang, D.; Wang, W.G.; Xiao, B.L.; Ma, Z.Y. Effect of Nanometer WC Coating on Thermal Conductivity of Diamond/6061 Composites. Acta Met. Sin. Engl. Lett. 2022, 36, 118–126. [Google Scholar] [CrossRef]
- Che, Z.; Li, J.; Wang, Q.; Wang, L.; Zhang, H.; Zhang, Y.; Wang, X.; Wang, J.; Kim, M.J. The formation of atomic-level interfacial layer and its effect on thermal conductivity of W-coated diamond particles reinforced Al matrix composites. Compos. Part A Appl. Sci. Manuf. 2018, 107, 164–170. [Google Scholar] [CrossRef]
- Chen, G.; Yang, W.; Xin, L.; Wang, P.; Liu, S.; Qiao, J.; Hu, F.; Zhang, Q.; Wu, G. Mechanical properties of Al matrix composite reinforced with diamond particles with W coatings prepared by the magnetron sputtering method. J. Alloys Compd. 2018, 735, 777–786. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Xin, L.; Tian, X.; Yang, W.; Chen, G.; Qiao, J.; Hu, F.; Zhang, Q.; Wu, G. Enhanced stability of the Diamond/Al composites by W coatings prepared by the magnetron sputtering method. J. Alloys Compd. 2018, 763, 305–313. [Google Scholar] [CrossRef]
- Ukhina, A.V.; Dudina, D.V.; Esikov, M.A.; Samoshkin, D.A.; Stankus, S.V.; Skovorodin, I.N.; Galashov, E.N.; Bokhonov, B.B. The influence of morphology and composition of metal–carbide coatings deposited on the diamond surface on the properties of copper–diamond composites. Surf. Coatings Technol. 2020, 401, 126272. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Cai, Z.; Peng, C.; Feng, Y.; Zhang, L. Effects of dual-layer coatings on microstructure and thermal conductivity of diamond/Cu composites prepared by vacuum hot pressing. Surf. Coat. Technol. 2015, 277, 299–307. [Google Scholar] [CrossRef]
- Liang, B.; Luo, Y.; Zhang, W.; Zhang, J.; Jiao, M. Sputtering Coating on the Surface of Diamond Particles Using High Temperature Generated by Thermal Explosion Reaction. J. Superhard Mater. 2024, 46, 197–203. [Google Scholar] [CrossRef]
- Ukhina, A.; Dudina, D.; Bokhonov, B.; Savintseva, D.; Samoshkin, D.; Stankus, S. Morphological features and phase composition of W-containing coatings formed on diamond via its interaction with WO3. Diam. Relat. Mater. 2022, 123, 108876. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; Xu, Z.; Qu, X. Preparation of W-Plated Diamond and Improvement of Thermal Conductivity of Diamond-WC-Cu Composite. Metals 2021, 11, 437. [Google Scholar] [CrossRef]
- Xiang, D.; Yin, L.W.; Liu, K.G.; Jia, L.Q. Study of W Metallic Coating Layer on Diamond Surface by Hydrothermal Method. Adv. Mater. Res. 2009, 79–82, 699–702. [Google Scholar] [CrossRef]
- Xiang, D.; Jia, L.Q.; Liu, K.G. Structure and Performance of Chemical Duplex Plating Ti Metal Layer on Diamond Surface by Hydrothermal Method. Adv. Mater. Res. 2014, 936, 1676–1680. [Google Scholar] [CrossRef]
- Liu, D.-G.; Zheng, L.; Zhang, L.; Tan, X.-Y.; Luo, L.-M.; Ma, H.-R.; Liu, J.-Q.; Wu, Y.-C. Effect of W-coated diamond on the microstructure and thermal conductivity of diamond/W matrix composites for plasma-facing materials (PFMs). Fusion Eng. Des. 2019, 144, 141–147. [Google Scholar] [CrossRef]
- Dong, Y.H.; Zhang, R.Q.; Zhou, L.; Chu, A.M.; Luo, T.G.; Chen, D.Z.; Chen, Q.J.; Ye, Z.G.; Zhang, B.L. Formation Mechanism and Properties of Thickness-Controllable Tungsten Coating on Diamond Surface by Salt Bath Plating. Mater. Sci. Forum 2018, 933, 264–273. [Google Scholar] [CrossRef]
- Wei, C.; Cheng, J.; Li, J.; Chen, W.; Chen, P.; Luo, L.; Liu, J. Tungsten-coated diamond powders prepared by microwave-heating salt-bath plating. Powder Technol. 2018, 338, 274–279. [Google Scholar] [CrossRef]
- Okada, T.; Fukuoka, K.; Arata, Y.; Yonezawa, S.; Kiyokawa, H.; Takashima, M. Tungsten carbide coating on diamond particles in molten mixture of Na2CO3 and NaCl. Diam. Relat. Mater. 2015, 52, 11–17. [Google Scholar] [CrossRef]
- Chen, W.; Qian, J.; Peng, S.; Fan, L.; Zheng, H.; Zhang, Z.; Zheng, P.; Zheng, L.; Zhang, Y. Thermal properties of tungsten/tungsten carbide-coated double-size diamond/copper composite. Diam. Relat. Mater. 2023, 135, 109818. [Google Scholar] [CrossRef]
- Kozlov, D.A.; Shcherbakov, A.B.; Kozlova, T.O.; Angelov, B.; Kopitsa, G.P.; Garshev, A.V.; Baranchikov, A.E.; Ivanova, O.S.; Ivanov, V.K. Photochromic and Photocatalytic Properties of Ultra-Small PVP-Stabilized WO3 Nanoparticles. Molecules 2019, 25, 154. [Google Scholar] [CrossRef] [PubMed]
- Lisitsyna, L.A.; Popov, A.I.; Karipbayev, Z.T.; Mussakhanov, D.A.; Feldbach, E. Luminescence of MgF2-WO3 ceramics synthesized in the flux of 1.5 MeV electron beam. Opt. Mater. 2022, 133, 112999. [Google Scholar] [CrossRef]
- Zubkins, M.; Vibornijs, V.; Strods, E.; Aulika, I.; Zajakina, A.; Sarakovskis, A.; Kundzins, K.; Korotkaja, K.; Rudevica, Z.; Letko, E.; et al. A stability study of transparent conducting WO3/Cu/WO3 coatings with antimicrobial properties. Surfaces Interfaces 2023, 41, 103259. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y. Study on diffusion plated tungsten on diamond surface. Jingangshi Yu Moliao Moju Gongcheng/Diam. Abras. Eng. 2015, 35, 17–20. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Wang, D.; Ni, D.; Chen, L.; Ma, Z. Effect of nanometer TiC coated diamond on the strength and thermal conductivity of diamond/Al composites. Mater. Chem. Phys. 2016, 182, 256–262. [Google Scholar] [CrossRef]
- Xu, X.; Wan, B.; Li, W.; Liu, F.; Zhai, T.; Zhang, L.; Tang, G. Reaction mechanisms for Ti coatings on diamond. Carbon 2024, 226, 119206. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Tian, W.; Hu, J.; Liao, W. Preparation and properties of tungsten mi-cro-deposited on diamond (100)–(111) facets/Cu composites. Fuhe Cailiao Xuebao/Acta Mater. Compos. Sini-Ca 2022, 39, 6004–6016. [Google Scholar] [CrossRef]
- Mohammadzadeh, H.; Barati, M. A comprehensive evaluation of non-isothermal simultaneous reduction and carburization kinetics of W-Ni oxide nano-composite powder. Mater. Chem. Phys. 2021, 272, 125027. [Google Scholar] [CrossRef]
- Polyzos, I.; Bianchi, M.; Rizzi, L.; Koukaras, E.N.; Parthenios, J.; Papagelis, K.; Sordan, R.; Galiotis, C. Suspended monolayer graphene under true uniaxial deformation. Nanoscale 2015, 7, 13033–13042. [Google Scholar] [CrossRef] [PubMed]
- Cai, X. Investigation of Formation Mechanism and Mechanical Properties of the Tungsten Carbide Cemented Layer Produced by a Diffusion-Controlled Reaction[D].null. 2019. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?filename=1019858027.nh&dbname=CDFDTEMP (accessed on 20 October 2025). (In Chinese).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).