Active Surfaces in Sensor Technologies Utilizing Ceramic Nanotube-Conducting Polymer Composites Containing Embedded Gold Nanoparticles
Abstract
1. Introduction
2. Materials, Technology, and Characterization Methods
2.1. Sample Preparation
2.2. Characterization Equipment
3. Results and Discussion
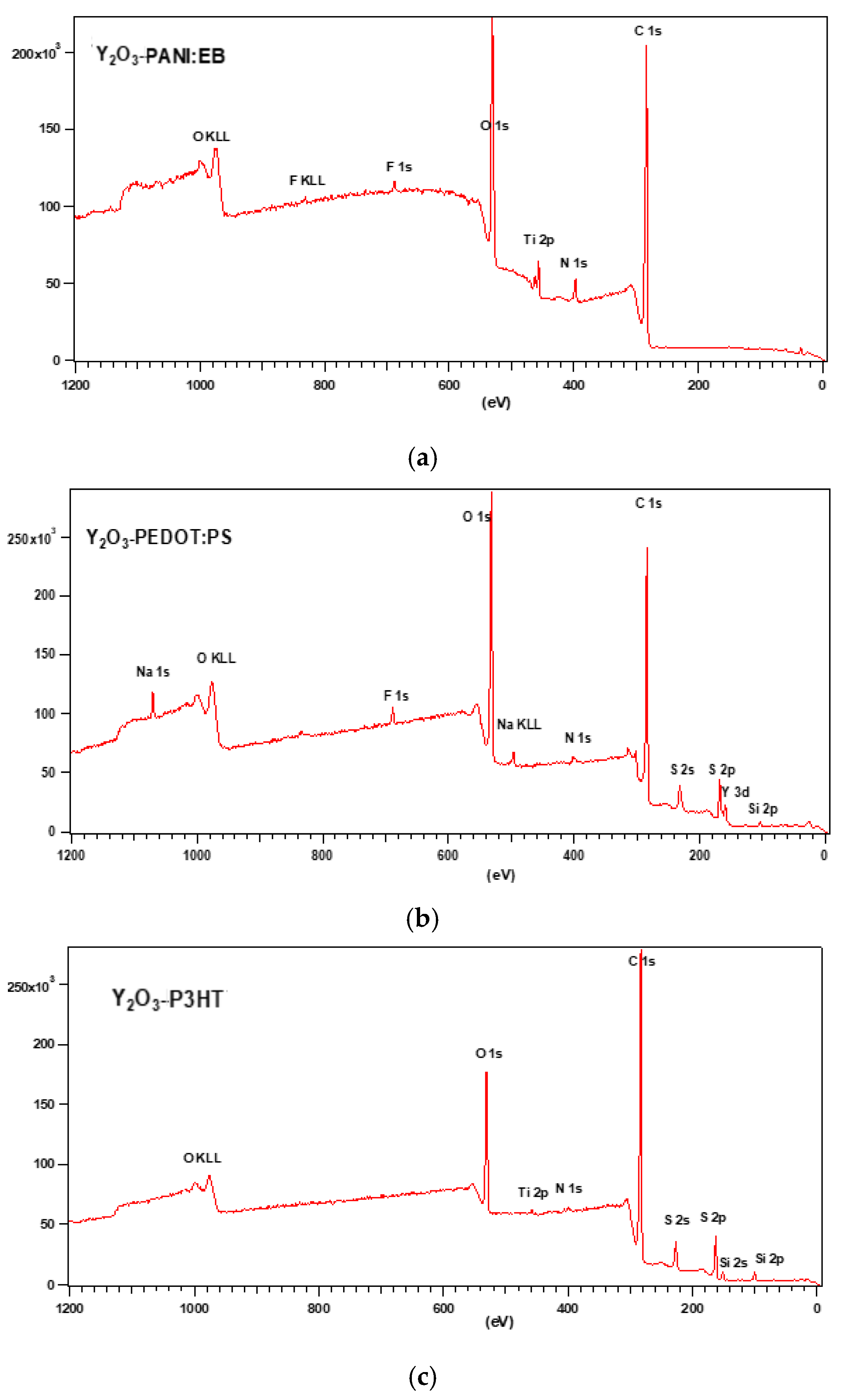
3.1. XPS and EDS Analysis
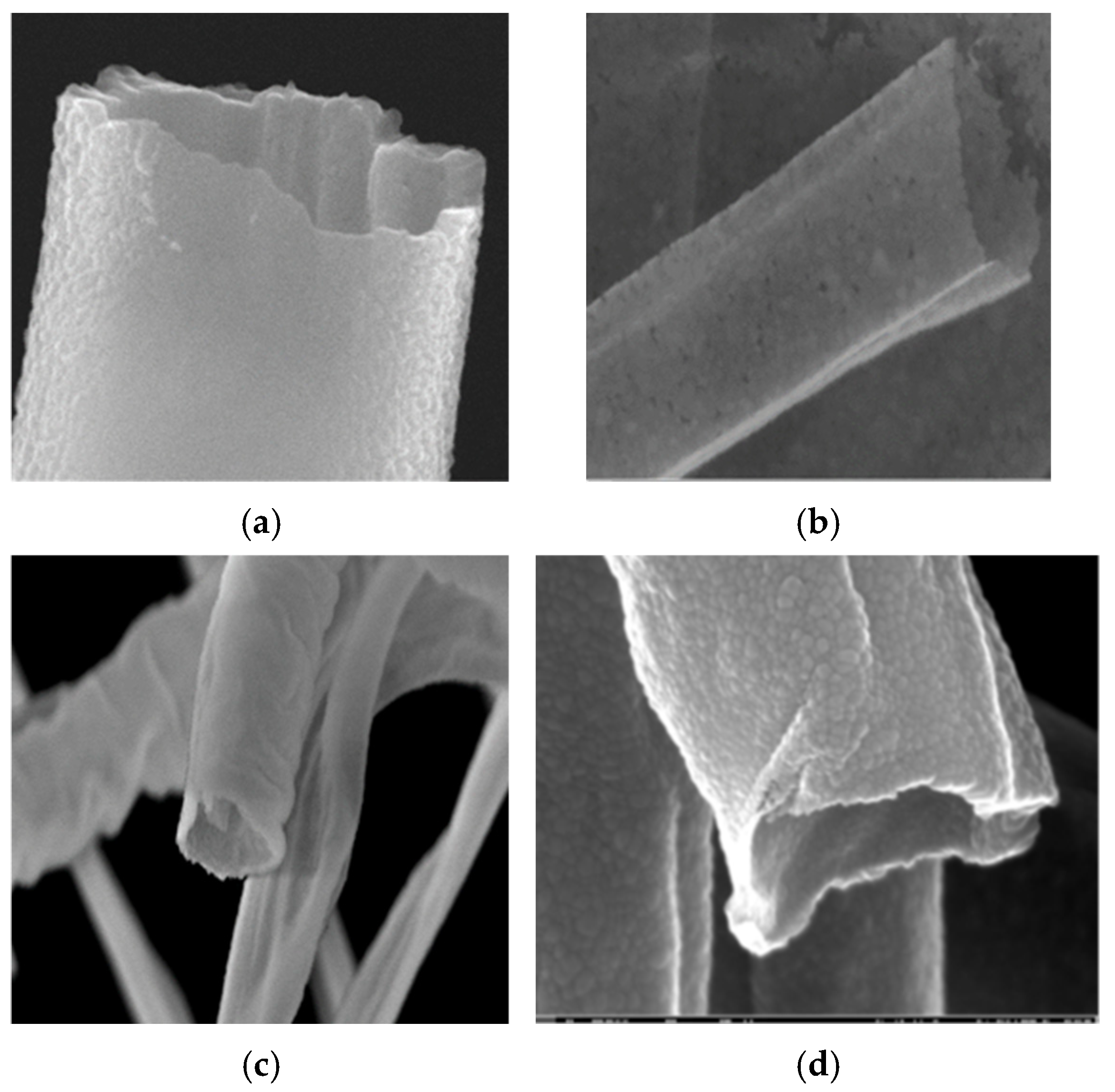
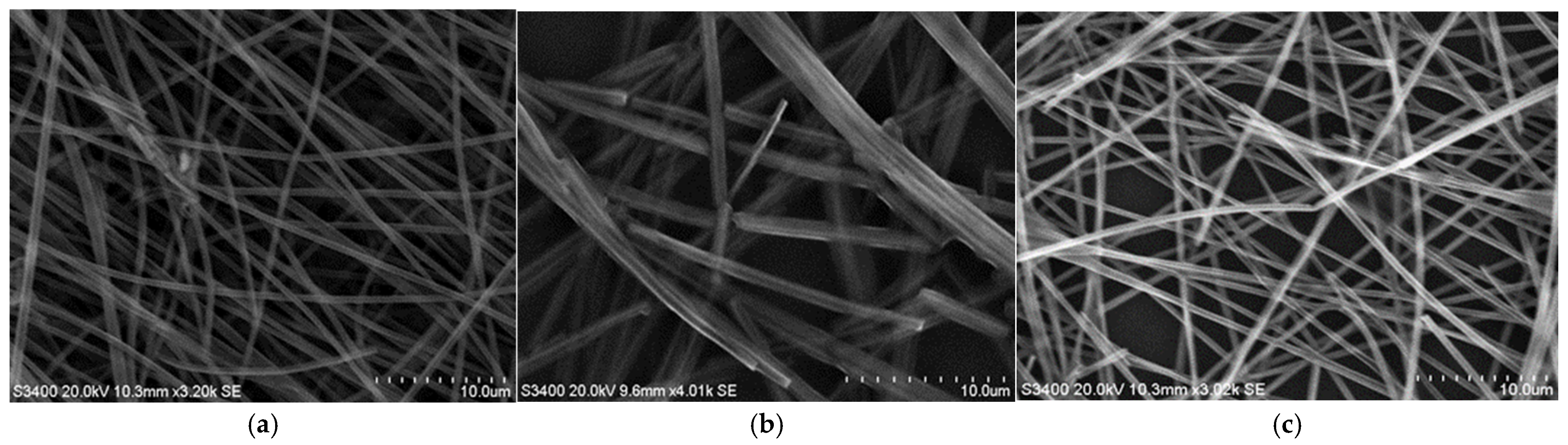
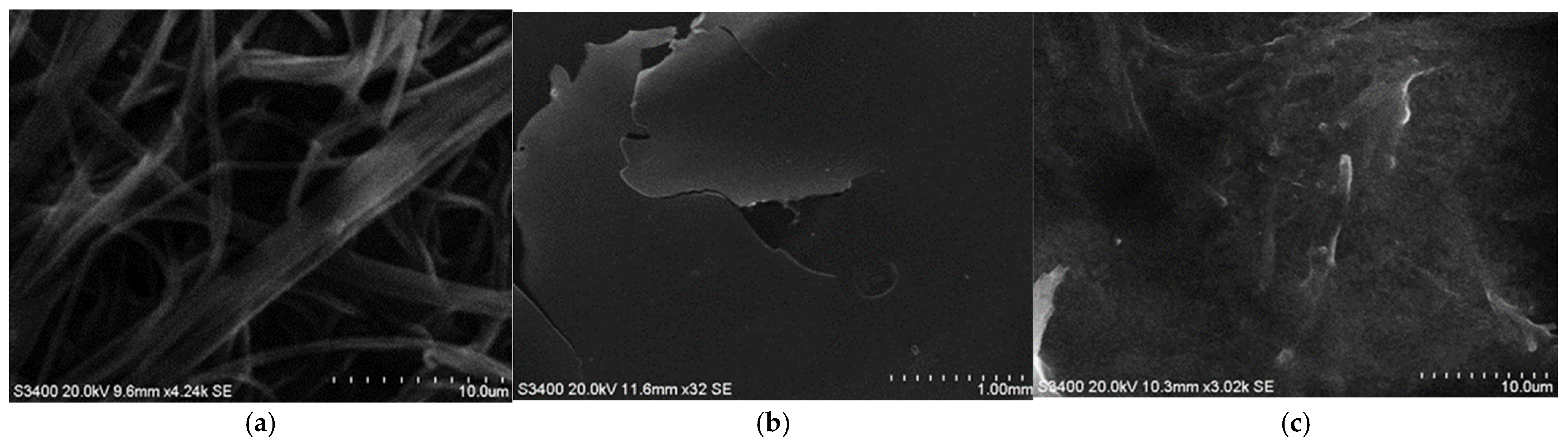
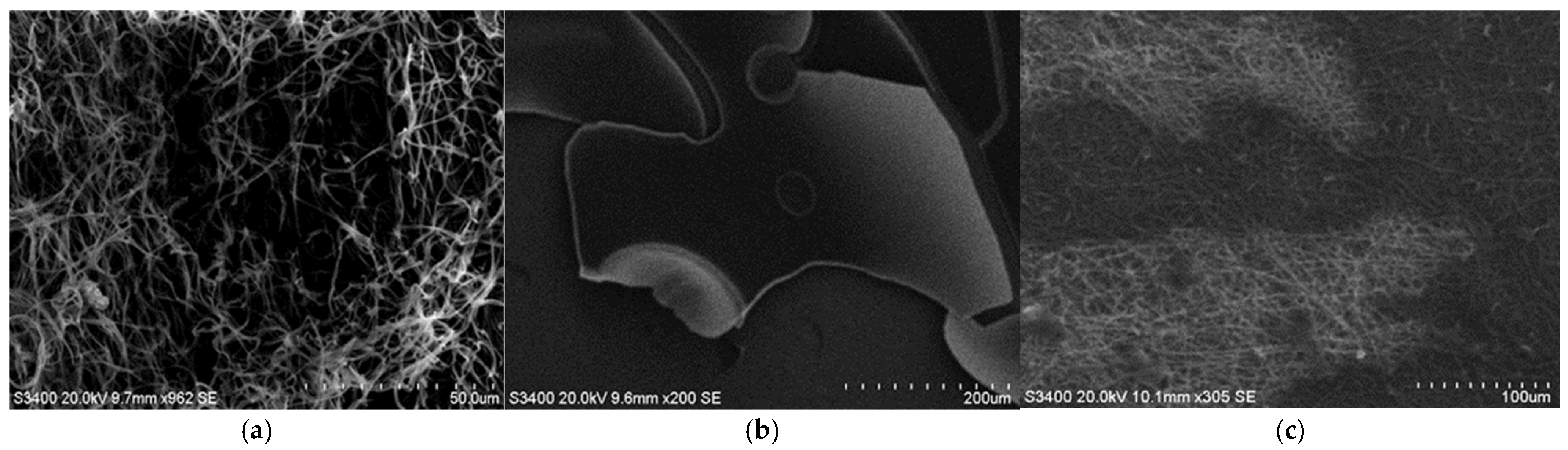
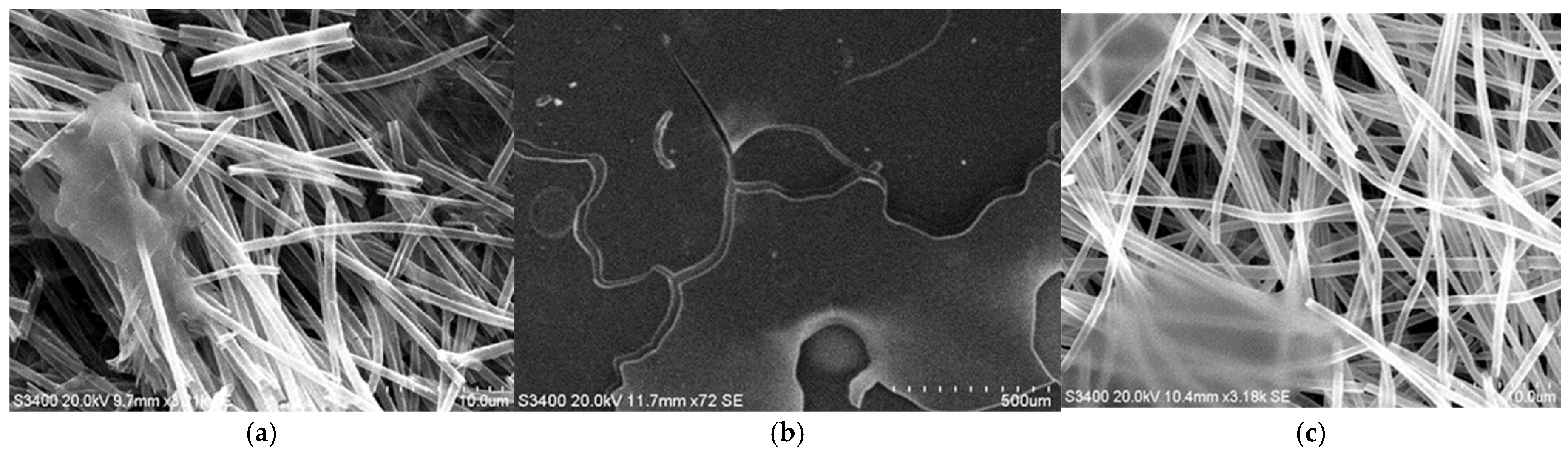
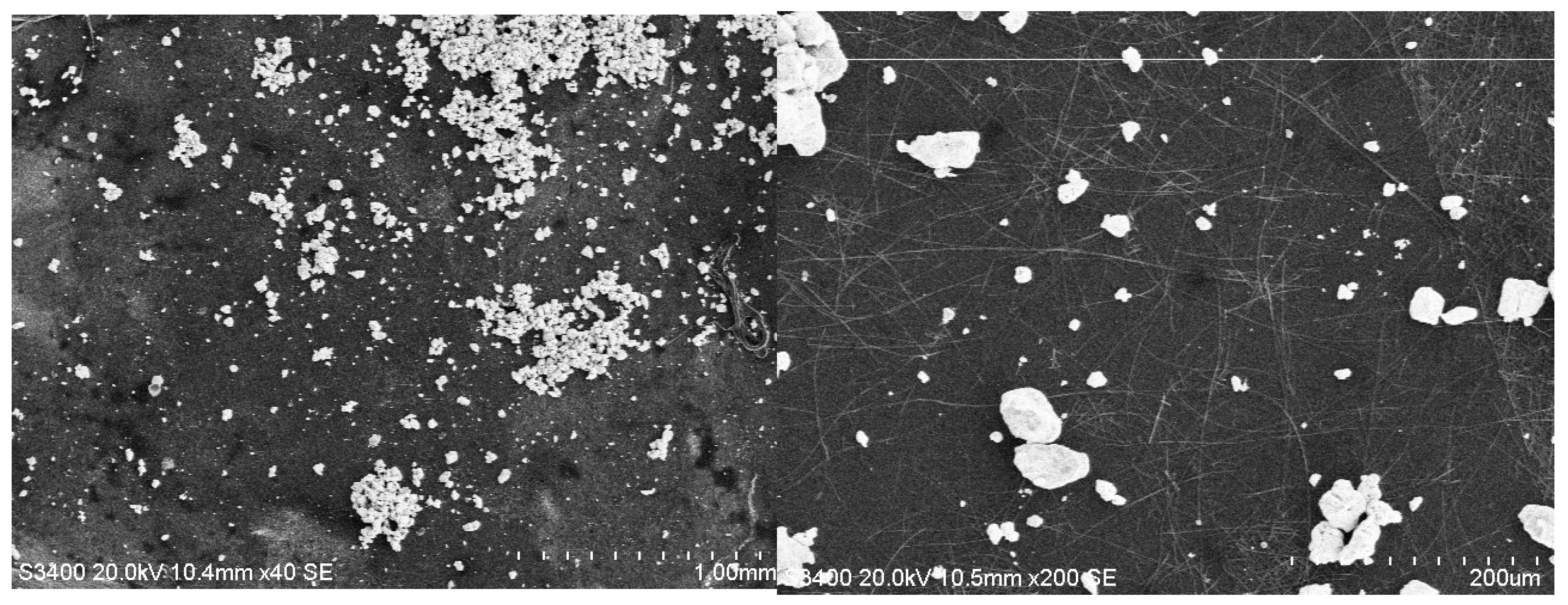
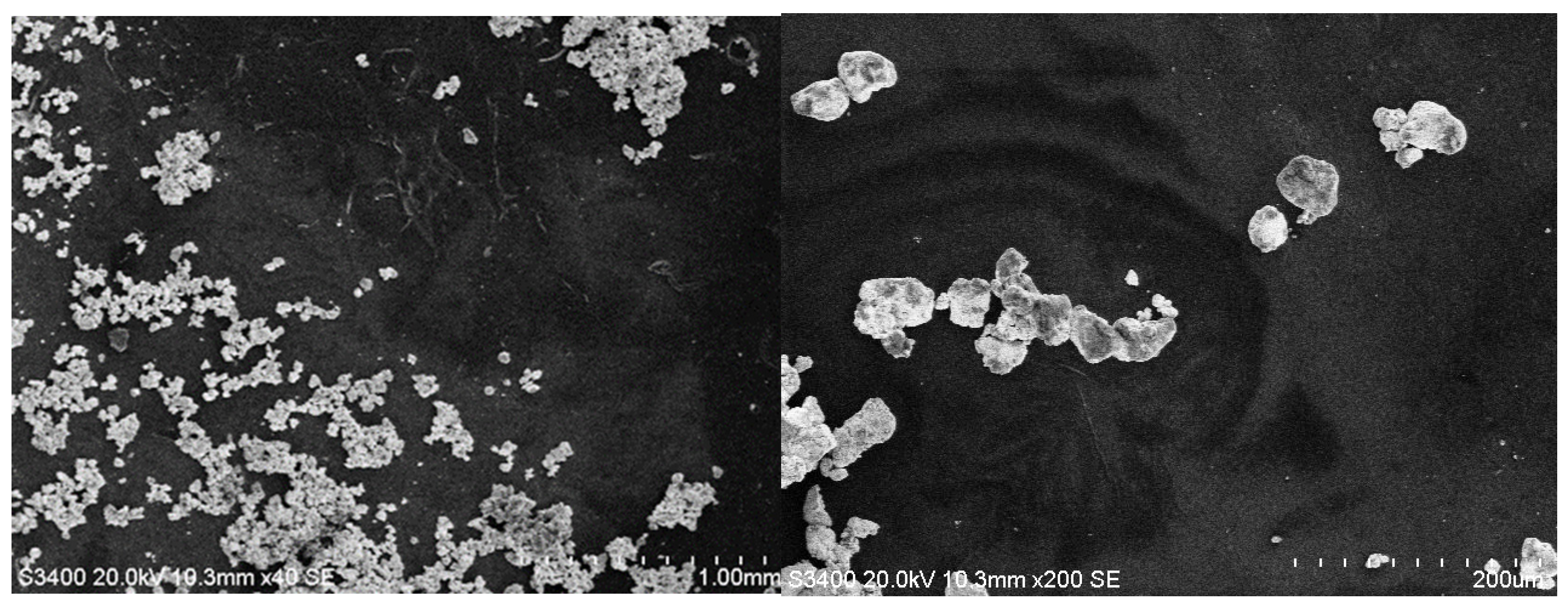
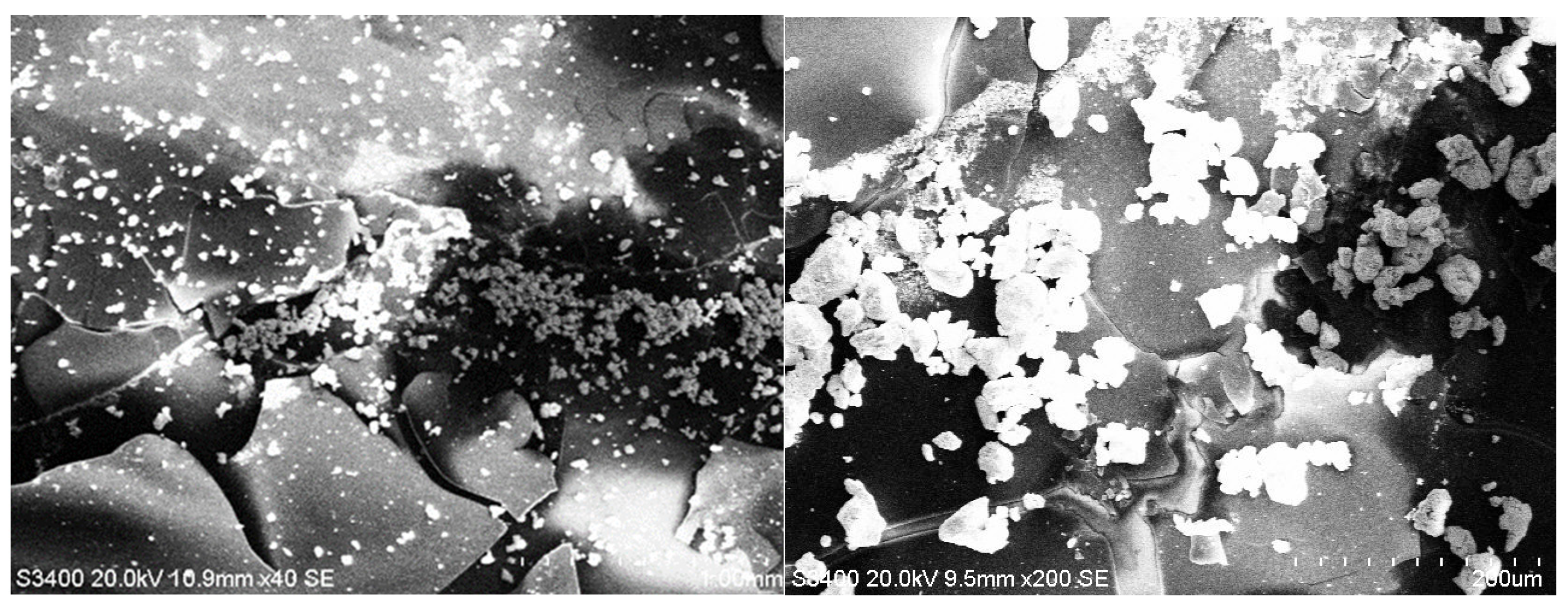
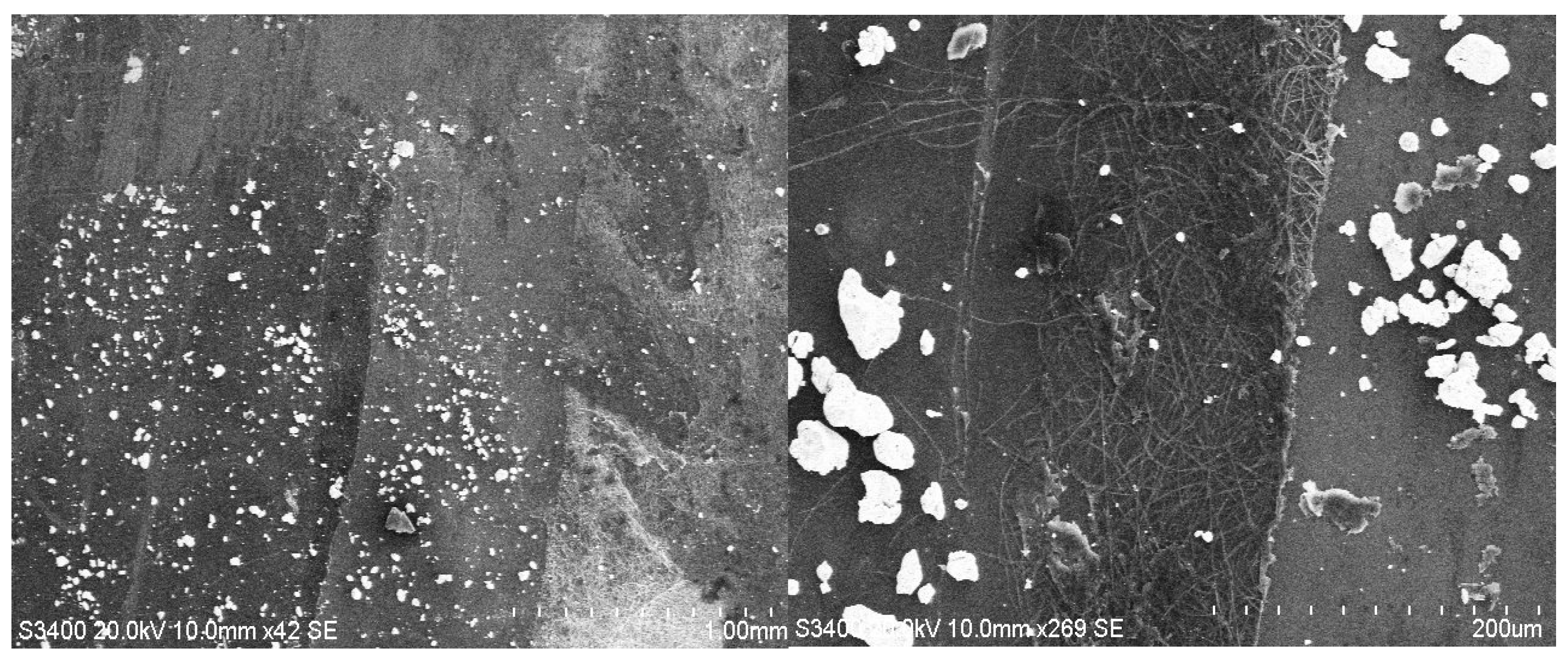
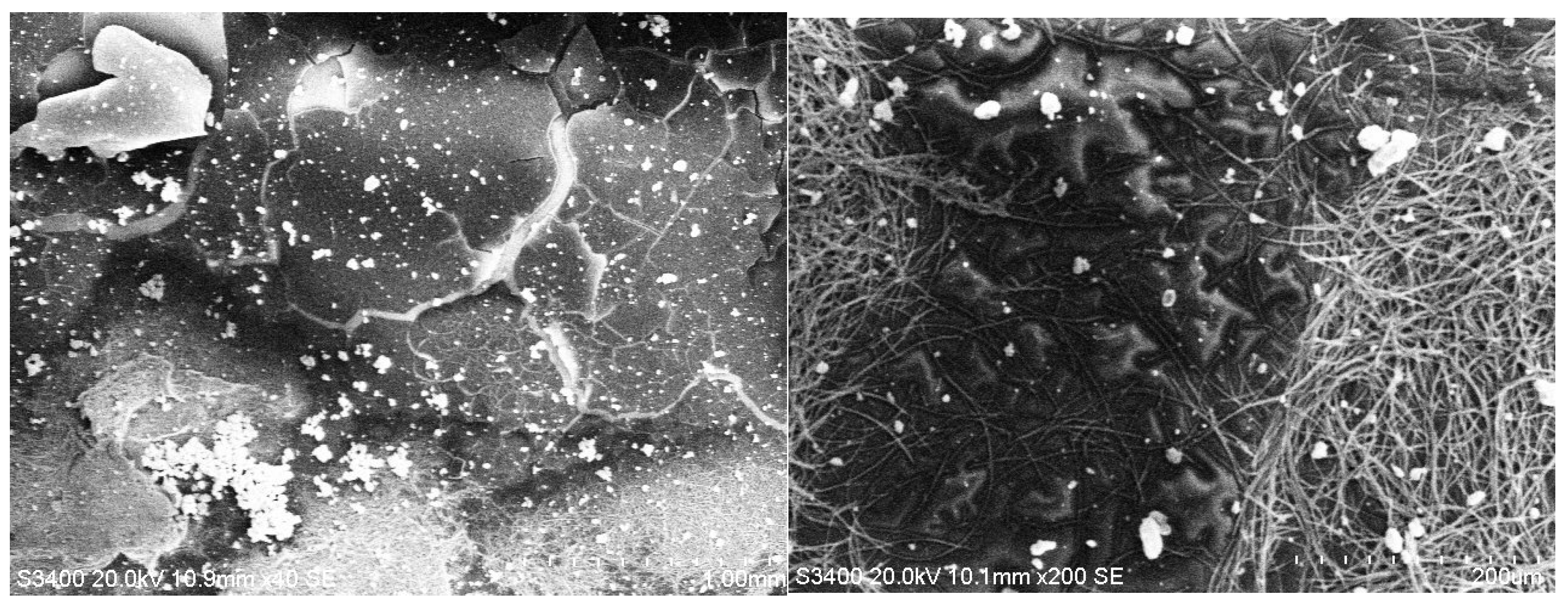
3.2. SEM Analysis
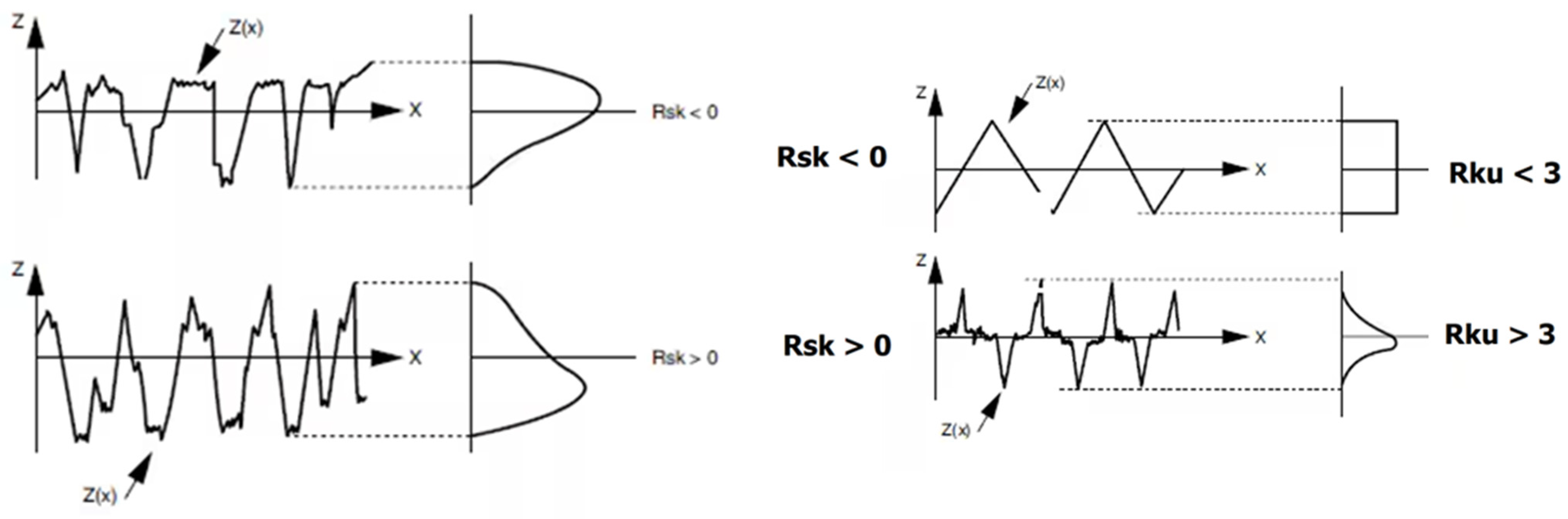
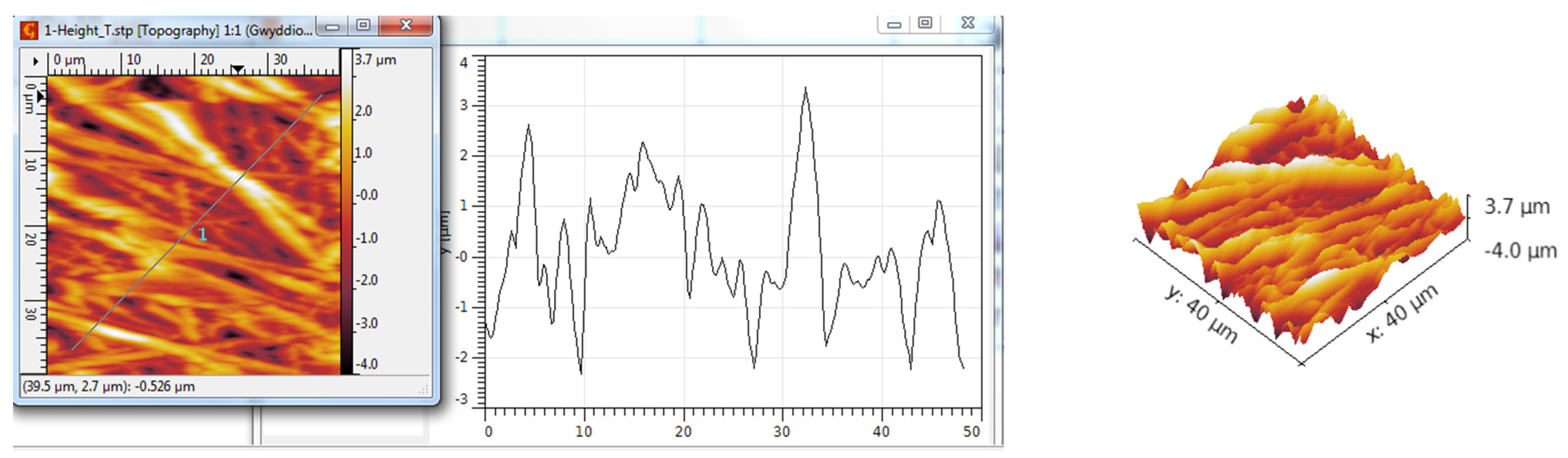
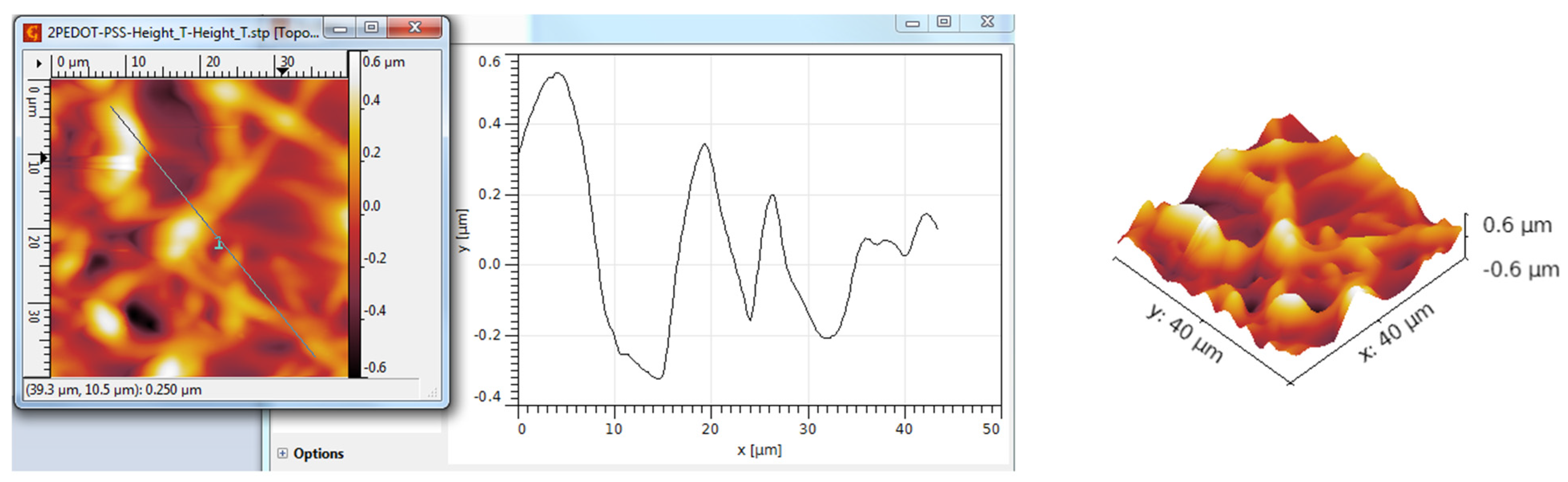
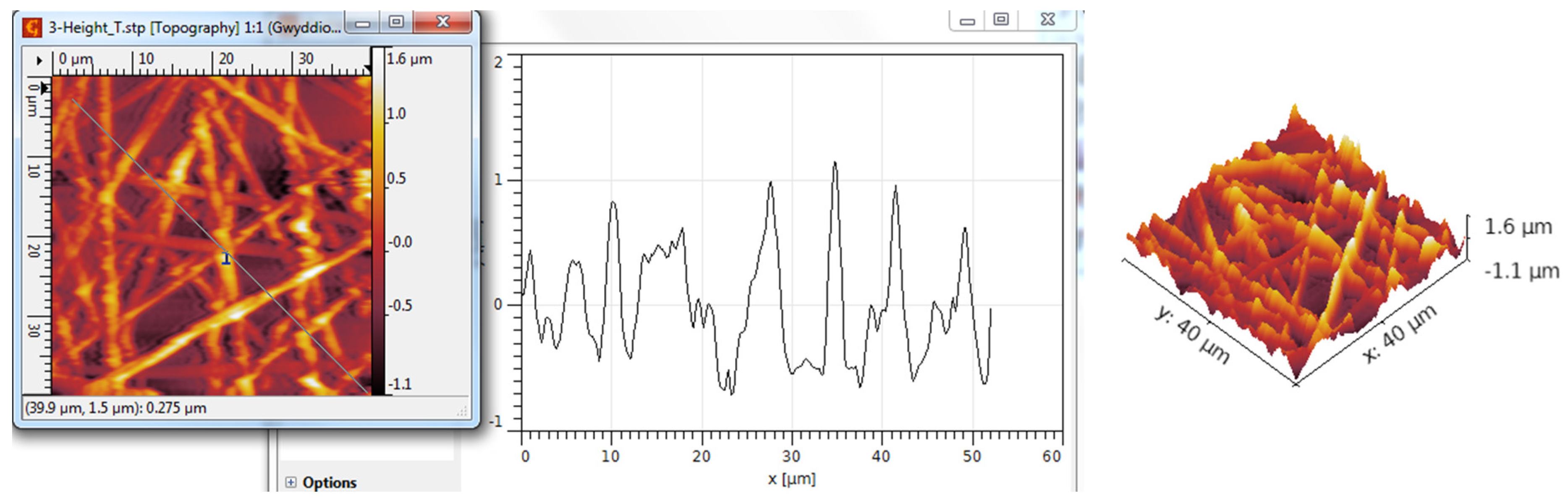
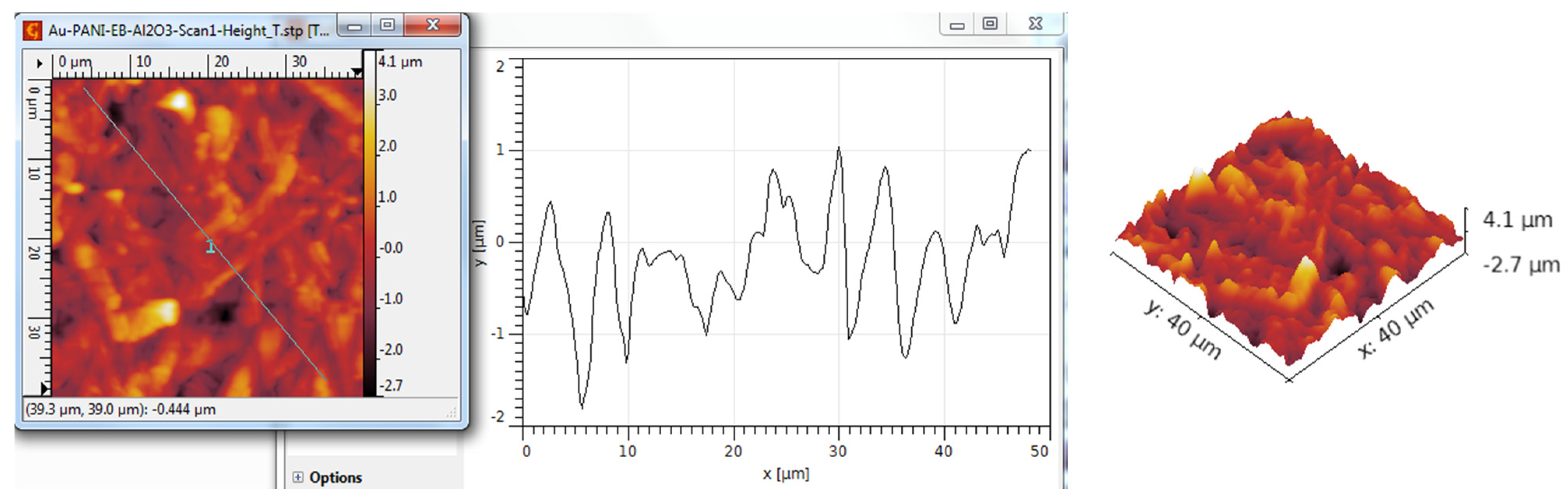
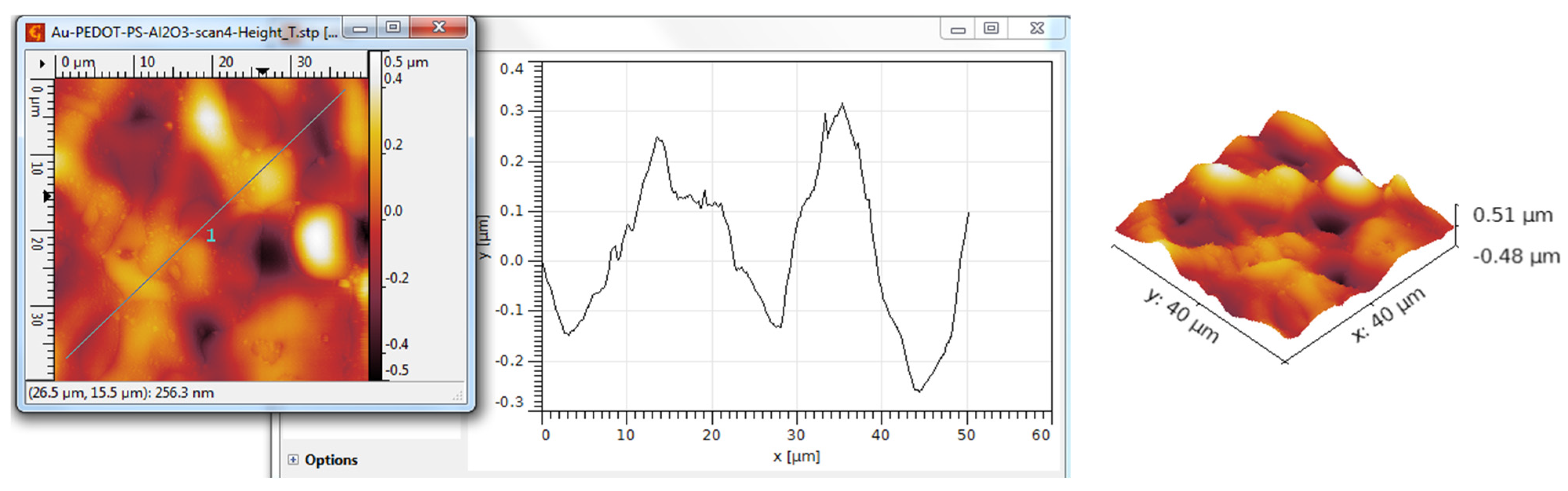
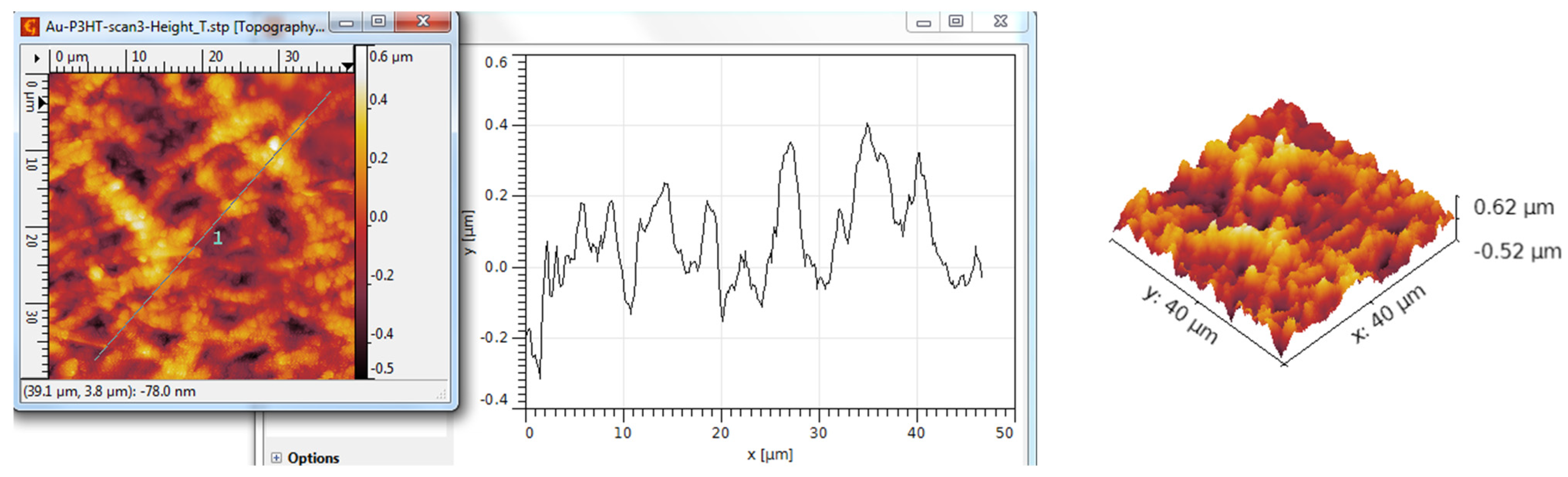
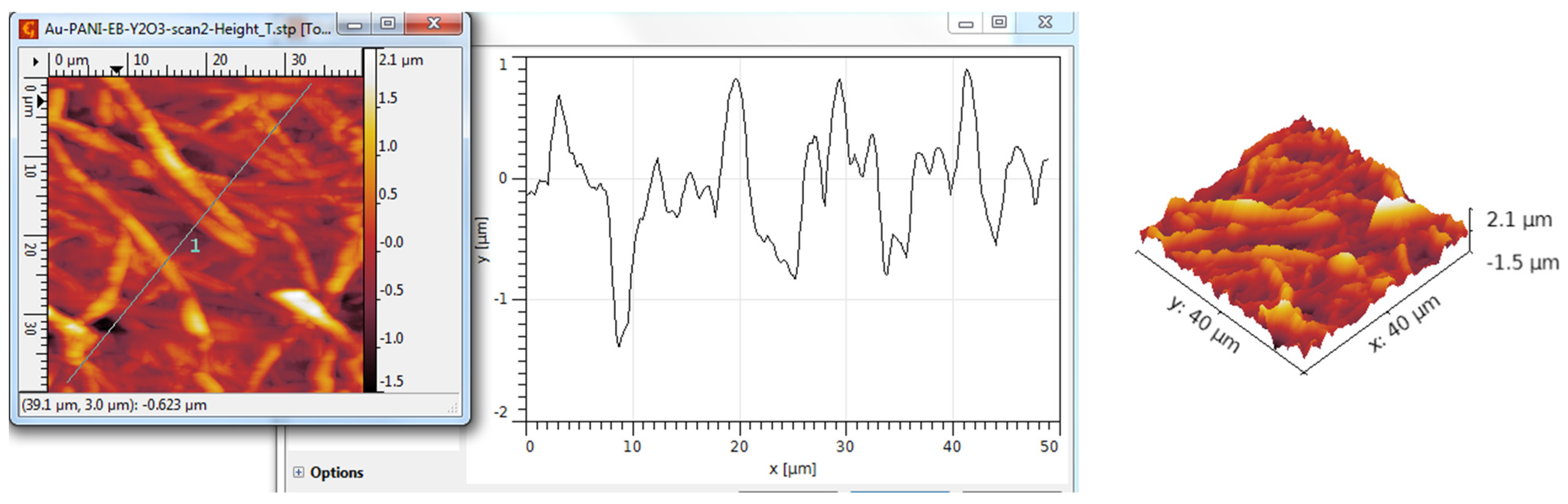
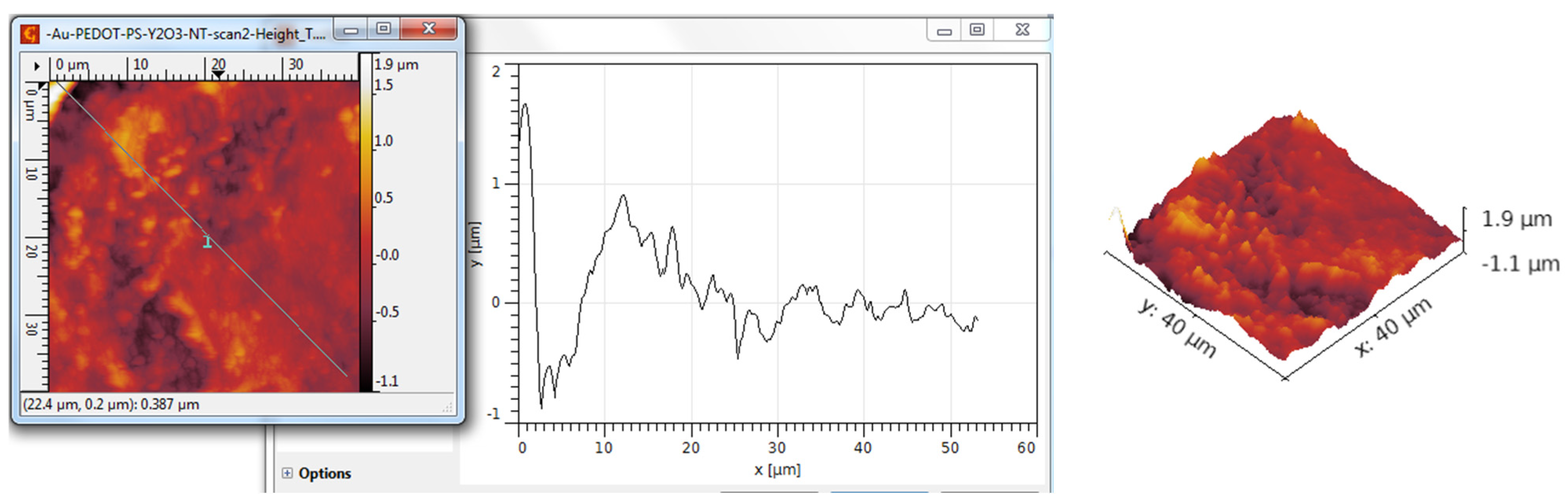
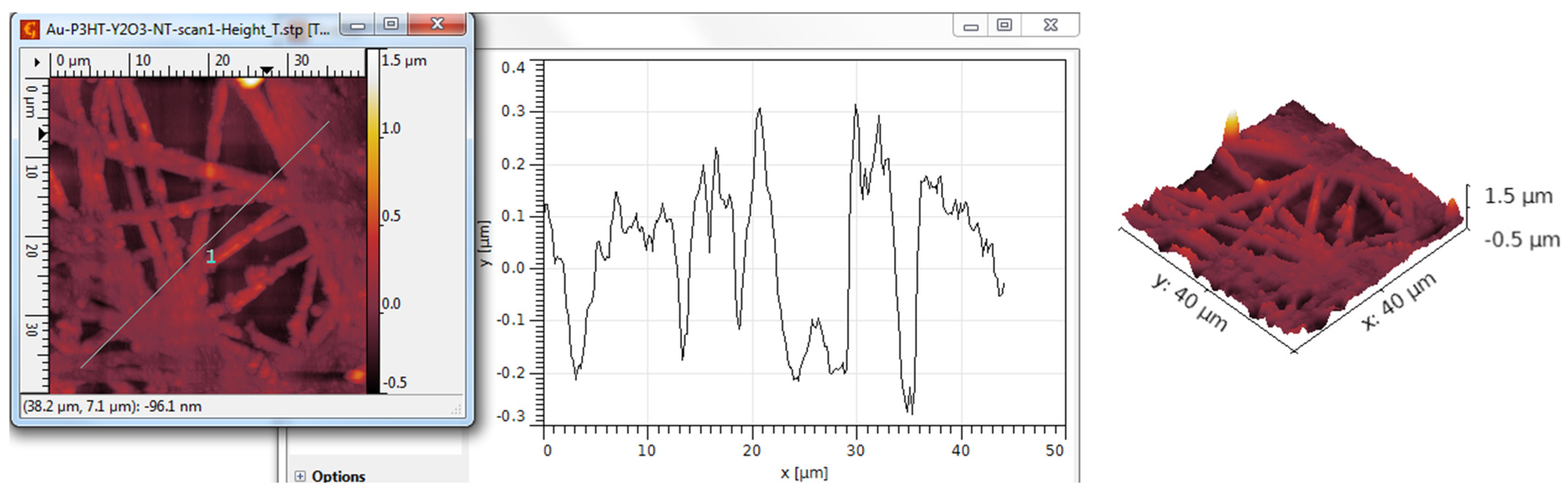
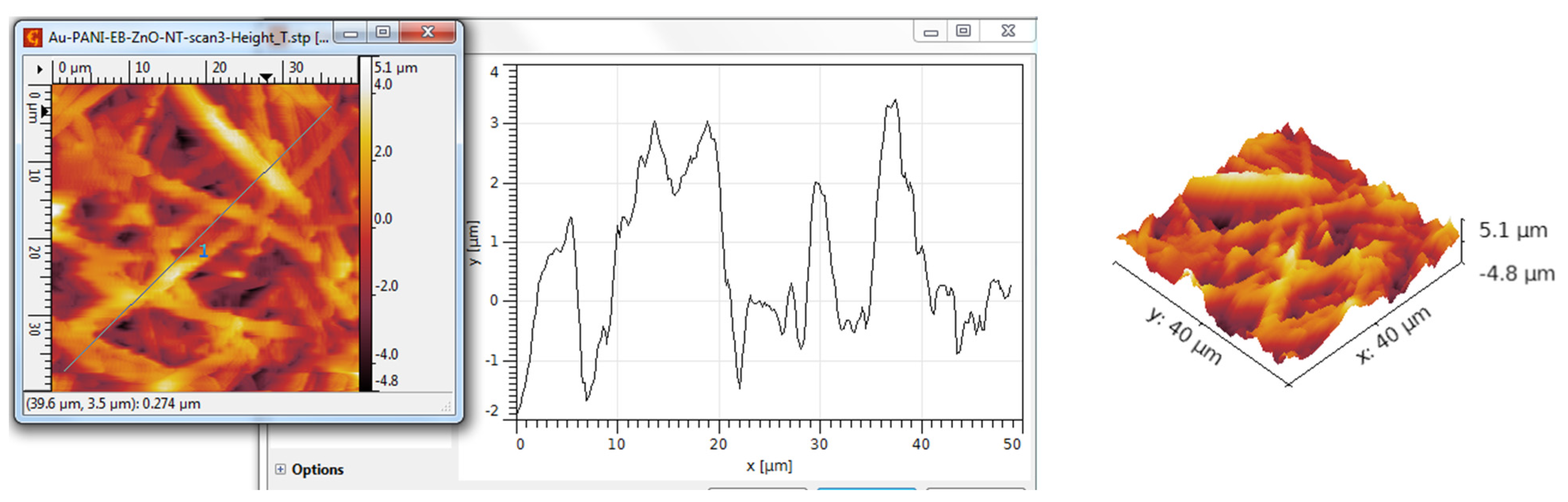
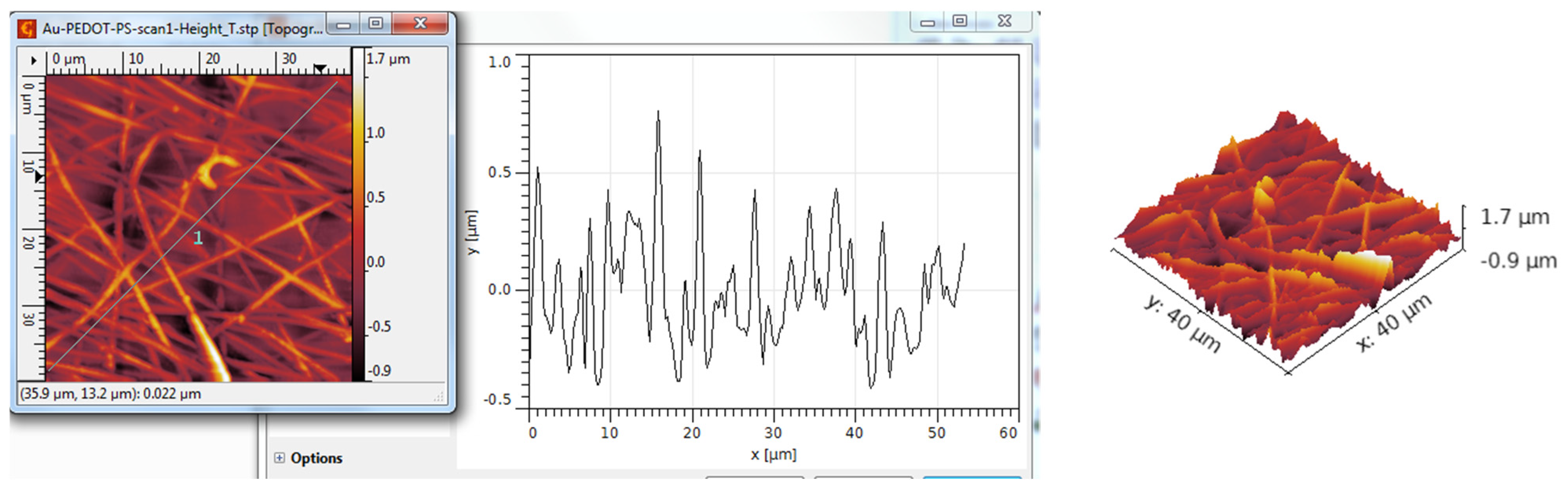
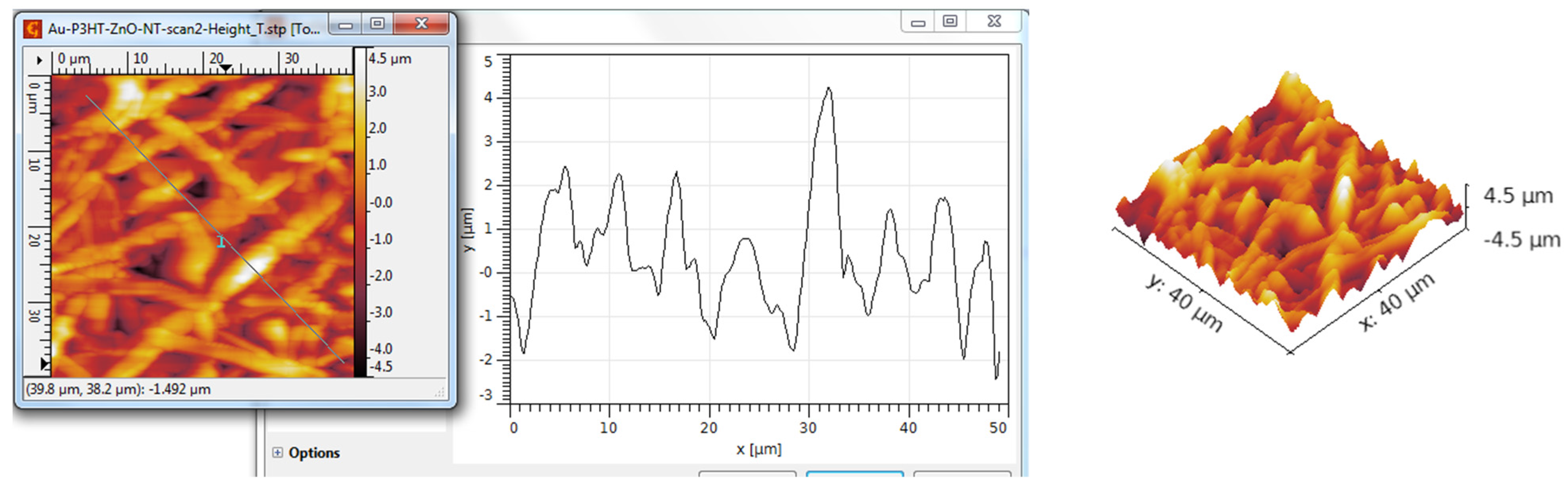
3.3. AFM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Iacob Dinu, I.; Constantin, A.; Apetrei, C. Development of Polypyrrole Modified Screen-Printed Carbon Electrode Based Sensors for Determination of L-Tyrosine in Pharmaceutical Products. J. Mol. Sci. 2021, 22, 7528. [Google Scholar] [CrossRef]
- Zhiyang, L.Z.; Leung, C.; Gao, F.; Gu, Z. Effects of Nanowire Length and Surface Roughness on the Electrochemical Sensor Properties of Nafion-Free, Vertically Aligned Pt Nanowire Array Electrodes. Sensors 2015, 15, 22473–22489. [Google Scholar] [CrossRef]
- Miller, C.; Keattch, O.; Shergill, R.; Patel, B. Evaluating diverse electrode surface patterns of 3D printed carbon thermoplastic electrochemical sensors. Analyst 2024, 149, 1502–1508. [Google Scholar] [CrossRef]
- Kılıç, Y.; Manickham, P.; Bhansali, S. Brief Fine Polishing of Thin-film Gold Electrode Sensors Leads to Better Reproducibility than Electrochemical Pretreatment. Int. J. Electrochem. Sci. 2020, 15, 5067–5075. [Google Scholar] [CrossRef]
- Sarmphim, P.; Teeparuksapun, K.; Wunsri, S.; Sorngodchagorn, S.; Duangsiri, W.; Tapparak, P.; Sirisathitkul, C. Facile Fabrication of Screen-Printed Carbon Electrodes for Electrochemical Sensors. Int. J. Nanoelectron. Mater. 2021, 14, 1–10. Available online: https://ijneam.unimap.edu.my/index.php/vol-14-no-1-january-2021 (accessed on 17 July 2025).
- González-Martínez, E.; Saem, S.; Beganovic, N.; Moran-Mirabal, J. Electrochemical Nano-Roughening of Gold Microstructured Electrodes for Enhanced Sensing in Biofluids. Angew. Chem. 2023, 62, e202218080. [Google Scholar] [CrossRef]
- Ostertag, B.; Porshinsky, E.; Nawarathne, C.; Ross, A. Surface-Roughened Graphene Oxide Microfibers Enhance Electrochemical Reversibility. Langmuir 2024, 40, 12124–12136. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoon, S.; Kim, D. Effect of surface roughness on the extinction-based localized surface plasmon resonance biosensors. Appl. Opt. 2008, 47, 5886–5892. [Google Scholar] [CrossRef] [PubMed]
- AL-Mosht, S.; Al-Fandi, M.; Al-Ebbini, L. Nanoarchitectonics of composite biosensor for early detection of hepatocellular carcinoma. Appl. Phys. A 2022, 128, 536. [Google Scholar] [CrossRef]
- El-Said, W.; Saleh, T.; Al-Bogami, A.; Wani, M.; Choi, J. Development of Novel Surface-Enhanced Raman Spectroscopy-Based Biosensors by Controlling the Roughness of Gold/Alumina Platforms for Highly Sensitive Detection of Pyocyanin Secreted from Pseudomonas aeruginosa. Biosensors 2024, 14, 399. [Google Scholar] [CrossRef]
- Shergill, R.S.; Miller, C.L.; Patel, B.A. Influence of instrument parameters on the electrochemical activity of 3D printed carbon thermoplastic electrodes. Sci. Rep. 2023, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Ganbavle, V.V.; Rajpure, K.Y.; Deshmukh, H.P.; Mujawar, S.H. Fast response and highly selective nitrogen dioxide gas sensor based on Zinc Stannate thin films. Mater. Sci. Energy Technol. 2020, 3, 36–42. [Google Scholar] [CrossRef]
- Ghaderi, A.; Sabbaghzadeh, J.; Dejam, L.; Pour, G.; Moghimi, E.; Matos, R.; da Fonseca Filho, H.; Țălu, S.; Salehishayegan, A.; Aval, L.; et al. Nanoscale morphology, optical dynamics and gas sensor of porous silicon. Sci. Rep. 2024, 14, 3677. [Google Scholar] [CrossRef]
- Abdelkarem, K.; Saad, R.; El Sayed, A.M.; Fathy, M.I.; Shaban, M.; Hamdy, H. Design of high-sensitivity La-doped ZnO sensors for CO2 gas detection at room temperature. Sci. Rep. 2023, 13, 18398. [Google Scholar] [CrossRef] [PubMed]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review. Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. A portable wireless intelligent electrochemical sensor based on layer-by-layer sandwiched nanohybrid for terbutaline in meat products. Food Chem. 2022, 371, 131140. [Google Scholar] [CrossRef]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. Detection and electrocatalytic mechanism of zearalenone using nanohybrid sensor based on copper-based metal-organic framework/magnetic Fe3O4-graphene oxide modified electrode. Food Chem. 2022, 370, 131024. [Google Scholar] [CrossRef]
- Kumar, N.; Navani, N.K.; Manhas, S.K. Effect of Metal Oxide Nanoparticles on Carbon Nanotube Device Characteristics. J. Electron. Mater. 2021, 50, 528–536. [Google Scholar] [CrossRef]
- Hovancová, J.; Šišoláková, I.; Oriňaková, R.; Oriňak, A. Nanomaterial-based electrochemical sensors for detection of glucose and insulin. J. Solid. State Electrochem. 2017, 21, 2147–2166. [Google Scholar] [CrossRef]
- Tee, S.Y.; Teng, C.P.; Ye, E. Metal nanostructures for non-enzymatic glucose sensing. Mater. Sci. Eng. C 2017, 70, 1018–1030. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, M.; Qu, F.; Shen, G.; Yu, R. Enzyme-functionalized gold nanowires for the fabrication of biosensors. Bioelectrochemistry 2007, 71, 211–216. [Google Scholar] [CrossRef]
- Hira, S.A.; Yusuf, M.; Annas, D.; Saravanan Nagappan, S.; Song, R.; Park, S.; Park, K. Recent Advances on Conducting Polymer-Supported Nanocomposites for Nonenzymatic Electrochemical Sensing. Ind. Eng. Chem. Res. 2021, 60, 13425–13437. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ataei, A. Decoration of Metal Nanoparticles and Metal Oxide Nanoparticles on Carbon Nanotubes. Adv. J. Chem. 2024, 7, 122–145. [Google Scholar]
- Wang, H.; Li, P.; Sun, G.; Jiang, Z. Influence of substrate surface roughness on the properties of a planar-type CO2 sensor using evaporated Li3PO4 film. In Proceedings of the 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems 2013, Suzhou, China, 7–10 April 2013. [Google Scholar] [CrossRef]
- MenshykauIan, D.; Compton, R. Influence of Electrode Roughness on Cyclic Voltammetry. J. Phys. Chem. C 2008, 112, 14428–14438. [Google Scholar] [CrossRef]
- Treebupachatsakul, T.; Shinnakerdchoke, S.; Pechprasarn, S. Analysis of Effects of Surface Roughness on Sensing Performance of Surface Plasmon Resonance Detection for Refractive Index Sensing Application. Sensors 2021, 21, 6164. [Google Scholar] [CrossRef] [PubMed]
- Possan, A.; Menti, C.; Beltrami, M.; Santos, A.; Roesch-Ely, M.; Missell, F. Effect of surface roughness on performance of magnetoelastic biosensors for the detection of Escherichia coli. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 541–547. [Google Scholar] [CrossRef]
- Alvez, H.; de Barros, T.; Nascimento, D.; Silva, D.; Peixoto e Silva, M.; do Nascimento, J.; Fontana, E.; Martins-Filho, J. Influence of surface roughness on the sensitivity of a D-shaped optical fiber-based refractive index sensor. Sens. Actuators A Phys. 2022, 344, 113702. [Google Scholar] [CrossRef]
- Saitta, L.; Celano, G.; Tosto, C.; Arcadio, F.; Zeni, L.; Sergi, C.; Cennamo, N.; Cicala, G. The effect of surface roughness on the performance of 3D printed surface plasmon resonance sensors for refractive index measurements. Int. J. Adv. Manuf. Technol. 2024, 132, 5503–5519. [Google Scholar] [CrossRef]
- Hashim, J.; Salim, M.; Qazi, H.; Noor, M.; Azmi, A.; Ibrahim, M.; Manap, H. Effect of Surface Roughness on Sensitivity of Unclad Fiber-Optic Sensors. J. Phys. Conf. Ser. 2023, 2550, 12018. [Google Scholar] [CrossRef]
- Zhang, J.; Kuang, Z.; Li, H.; Li, S.; Xia, F. Electrode surface roughness greatly enhances the sensitivity of electrochemical non-enzymatic glucose sensors. J. Electroanal. Chem. 2022, 919, 116541. [Google Scholar] [CrossRef]
- Dutta, G.; Fernandes, F.; Estrela, P.; Moschou, D.; Bueno, P. Impact of surface roughness on the self-assembling of molecular films onto gold electrodes for label-free biosensing applications. Electrochim. Acta 2021, 378, 138137. [Google Scholar] [CrossRef]
- Golba, S.; Kubisztal, J. The Influence of Roughness on the Properties of Electroactive Polypyrrole. Molecules 2024, 29, 5436. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.; Gateman, S.; Sifakis, J.; Pollegioni, L.; Mauzeroll, J. Enhancement of the Enzymatic Biosensor Response through Targeted Electrode Surface Roughness. J. Electrochem. Soc. 2018, 165, G3074. [Google Scholar] [CrossRef]
- Surdo, S.; Barillaro, G. Impact of Fabrication and Bioassay Surface Roughness on the Performance of Label-Free Resonant Biosensors Based On One-Dimensional Photonic Crystal Microcavities. ACS Sens. 2020, 5, 2894–2902. [Google Scholar] [CrossRef]
- Dobri, G.; Banu, A.; Donath, C.; Neacsu, E.; Anastasescu, M.; Maxim, M.; Vasilescu, C.; Preda, L.; Marcu, M. Effect of Surface Roughness on the Electrochemical Behavior and Corrosion Resistance of TiTaNbZrAg Alloy with Different Amounts of Tantalum in Bulk Composition. Materials 2024, 17, 5217. [Google Scholar] [CrossRef] [PubMed]
- Enculescu, M.; Costas, A.; Evanghelidis, A.; Enculescu, I. Fabrication of ZnO and TiO2 Nanotubes via Flexible Electrospun Nanofibers for Photocatalytic Applications. Nanomaterials 2021, 11, 1305. [Google Scholar] [CrossRef]
- Ramasamy, P.; Lim, D.H.; Kim, J.; Kim, J. A general approach for synthesis of functional metal oxide nanotubes and their application in dye-sensitized solar cells. RSC Adv. 2014, 4, 2858–2864. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.Y.; Feng, Y.; Yuan, Z.Y.; Su, B.L. One-Dimensional Metal Oxide Nanotubes, Nanowires, Nanoribbons, and Nanorods: Synthesis, Characterizations, Properties and Applications. Crit. Rev. Solid. State Mater. Sci. 2012, 37, 1–74. [Google Scholar] [CrossRef]
- Muench, F.; Sun, L.; Kottakkat, T.; Antoni, M.; Schaefer, S.; Kunz, U.; Molina-Luna, K.; Duerrschnabel, M.; Kleebe, H.; Ayata, S.; et al. Free-Standing Networks of Core-Shell Metal and Metal Oxide Nanotubes for Glucose Sensing. ACS Appl. Mater. Interfaces 2017, 9, 771–781. [Google Scholar] [CrossRef]
- Trandabat, F.; Ciobanu, R.; Schreiner, O.; Aradoaei, M.; Aradoaei, S. Manufacturing of TiO2, Al2O3 and Y2O3 Ceramic Nanotubes for Application as Electrodes for Printable Electrochemical Sensors. Crystals 2024, 14, 454. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. Pure Appl. Chem. 1991, 63, 711–734. [Google Scholar] [CrossRef]
- Coelho, D.; Luiz, G.; Machado, S. Estimating the Electrochemically Active Area: Revisiting a Basic Concept in Electrochemistry. J. Braz. Chem. Soc. 2021, 32, 1912–1917. [Google Scholar] [CrossRef]
- Surface Roughness Parameters. Available online: https://www.keyence.eu/ss/products/microscope/roughness/line/tab03_b.jsp (accessed on 17 July 2025).
- Schreiner, O.; Trandabat, A.F.; Ciobanu, R.; Schreiner, T. TiO2 Ceramic Nanotubes—Conducting Polymer Assemblies with Embedded Gold Particles for Potential Use as Chemosensors in the Detection of Oral Diseases. Chemosensors 2025, 13, 117. [Google Scholar] [CrossRef]
- Trandabat, A.F.; Ciobanu, R.; Schreiner, O.; Schreiner, T.; Aradoaei, D. Ceramic Nanotubes—Conducting Polymer Assemblies with Potential Application as Chemosensors for Breath Hydrogen sulfide Detection in Chronic Kidney Disease. Chemosensors 2024, 12, 198. [Google Scholar] [CrossRef]
- Umeda, H.; Mezaki, Y.; Oshio, A.; Kaneko, Y.; Okamoto, R.; Kusumoto, S.; Kunimura, S. Gold Nanoparticles Produced by Low-temperature Heating of the Dry Residue of a Droplet of an HCl Acidic Solution of HAuCl4·4H2O in a Low Vacuum. Anal. Sci. 2021, 37, 1427–1432. [Google Scholar] [CrossRef]
- Ii, K.; Kurita, Y.; Kida, N.; Kunimura, S. Preparation of gold nanoparticles using low-temperature heating of the dry residue of a droplet of an HAuCl4 solution in air. Anal. Sci. 2024, 40, 213–217. [Google Scholar] [CrossRef]
- ISO 21920-2:2021 (Previously ISO 4287:1997). Available online: https://www.iso.org/standard/72226.html (accessed on 17 July 2025).
- Zhang, R.; Sun, D.; Zhang, R.; Lin, W.; Macias-Montero, M.; Patel, J.; Askari, S.; McDonald, C.; Mariotti, D.; Maguire, P. Gold nanoparticle-polymer nanocomposites synthesized by room temperature atmospheric pressure plasma and their potential for fuel cell electrocatalytic application. Sci. Rep. 2017, 7, 46682. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Hoffmann, M.; Seçkin, S.; König, T.; Hermes, I.; Rossner, C.; Fery, A. Toward coupling across inorganic/organic hybrid interfaces: Polyaniline-coated gold nanoparticles with 4-aminothiophenol as gold-anchoring moieties. Colloid. Polym. Sci. 2024, 303, 1743–1751. [Google Scholar] [CrossRef]
- Lai, L.; Irene, E. Area evaluation of microscopically rough surfaces. J. Vac. Sci. Technol. B 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Sekretareva, A.; Vagin, M.; Volkov, A.; Zozoulenko, I.; Eriksson, M. Evaluation of the Electrochemically Active Surface Area of Microelectrodes by Capacitive and Faradaic Currents. ChemElectroChem 2019, 6, 4411–4417. [Google Scholar] [CrossRef]
- Connor, P.; Schuch, J.; Kaiser, B.; Jaegermann, W. The determination of electrochemical active surface area and specific capacity revisited for the system MnOx as an oxygen evolution catalyst. Z. Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.; Zhang, Z.; Baranova, E. Comparison of electrochemical active surface area methods for various nickel nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Serapinienė, B.; Gudavičiūtė, L.; Tutlienė, S.; Grigucevičienė, A.; Selskis, A.; Juodkazytė, J.; Ramanauskas, R. On the Electrochemically Active Surface Area Determination of Electrodeposited Porous Cu 3D Nanostructures. Coatings 2023, 13, 1335. [Google Scholar] [CrossRef]
- Queiroz, M.A.R.; Vasconcellos, S.; Ribeiro, M.A.; Luz, P.; de Moura Souza, F.; dos Santos, M.; Guimarães, M.; Salgado, J.; Pedicini, R.; Ribeiro, J. Determination of the electrochemically active surface area by CO and hydrogen of PtSnRuTa/C-based electrocatalysts and their relationship with catalytic activity against alcohol oxidation. Chem. Pap. 2022, 76, 4597–4613. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Wegner, J.; Anwar, M.; Raza-Khan, A.; Franzka, S.; Kleszczynski, S.; Čolić, V. The determination of the electrochemically active surface area and its effects on the electrocatalytic properties of structured nickel electrodes produced by additive manufacturing. Electrochim. Acta 2024, 476, 143663. [Google Scholar] [CrossRef]
- Watzele, S.; Hauenstein, P.; Liang, Y.; Xue, S.; Fichtner, J.; Garlyyev, B.; Scieszka, D.; Claudel, F.; Maillard, F.; Bandarenka, A. Determination of electroactive surface area of Ni-, Co-, Fe-, and Ir-based oxide electrocatalysts. ACS Catal. 2019, 9, 9222–9230. [Google Scholar] [CrossRef]
- Lai, T.; Shu, H.; Yao, B.; Lai, S.; Chen, T.; Xiao, X.; Wang, Y. A Highly Selective Electrochemical Sensor Based on Molecularly Imprinted Copolymer Functionalized with Arginine for the Detection of Chloramphenicol in Honey. Biosensors 2023, 13, 505. [Google Scholar] [CrossRef]
- Tan, C.; Yin, H.; Brooks, V.; Arumugam, P.U.; Siddiqui, S. A Study of the Effect of Electrochemical Roughening of Platinum on the Sensitivity and Selectivity of Glutamate Biosensors. J. Electrochem. Soc. 2022, 169, 037510. [Google Scholar] [CrossRef]
- Darvishi, S.; Ensafi, A.A.; Mousaabadi, K.Z. Design and fabrication of electrochemical sensor based on NiO/Ni@C-Fe3O4/CeO2 for the determination of niclosamide. Sci. Rep. 2024, 14, 7576. [Google Scholar] [CrossRef]
- Huang, J.; Liu, P.; Wang, Y.; Dai, K.; Dou, Q.; Yin, Y.; Wang, X.; You, Z. Double-kill contribution of high-roughness high-density porous carbon electrodes to mechanically self-sensing supercapacitors. Nano Res. 2024, 17, 6157–6167. [Google Scholar] [CrossRef]
- Liu, J.; Shen, J.; Ji, S.; Zhanga, Q.; Zhao, W. Research progress of electrode materials for non-enzymatic glucose electrochemical sensors. Sens. Diagn. 2023, 2, 36–45. [Google Scholar] [CrossRef]
- Zhong, N.; Zhu, X.; Liao, Q.; Wang, Y.; Chen, R.; Sun, Y. Effects of surface roughness on optical properties and sensitivity of fiber-optic evanescent wave sensors. Appl. Opt. 2013, 52, 3937–3945. [Google Scholar] [CrossRef] [PubMed]
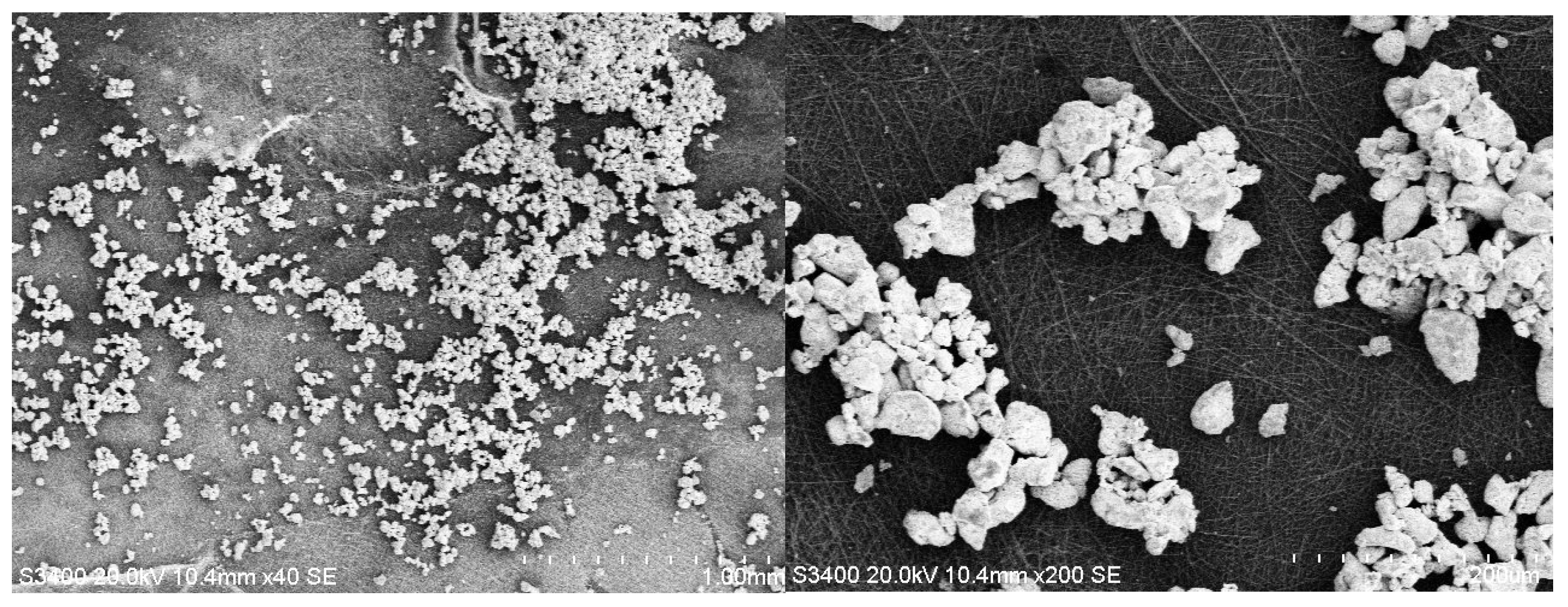
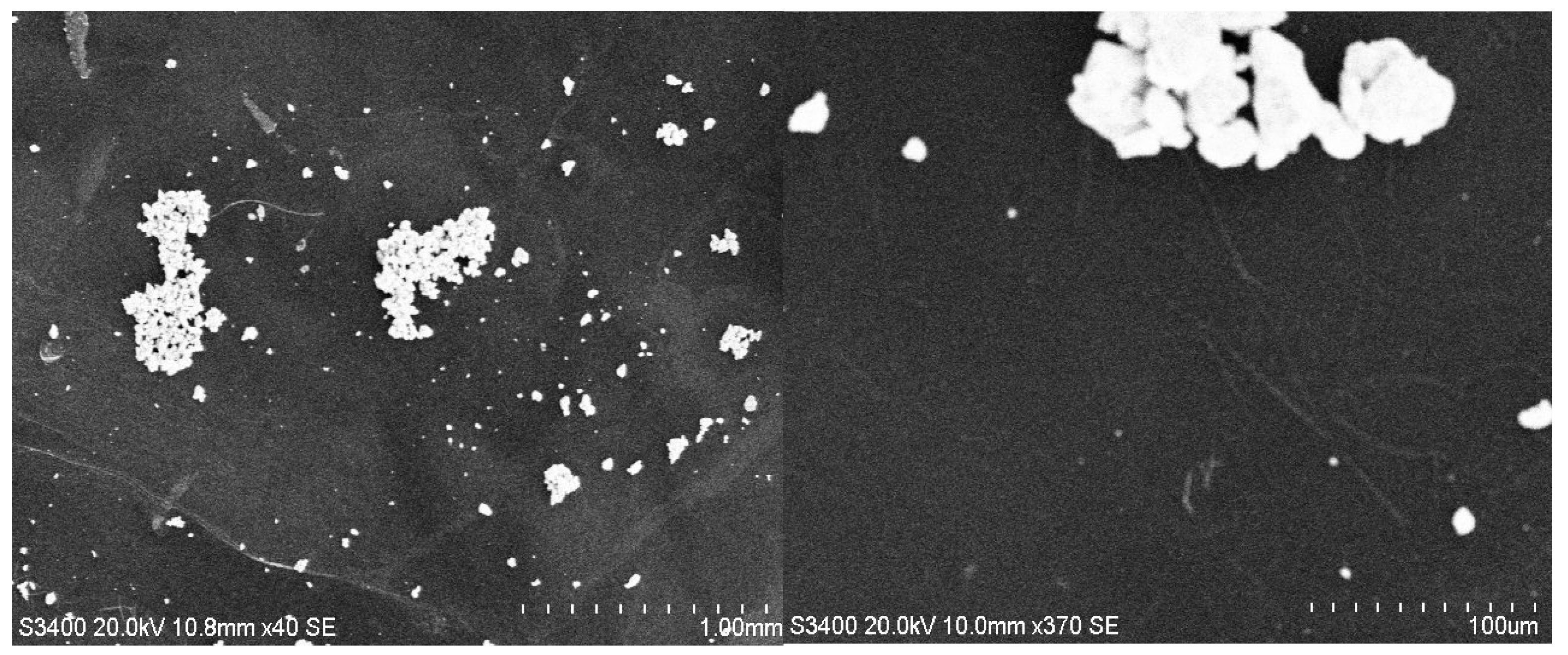





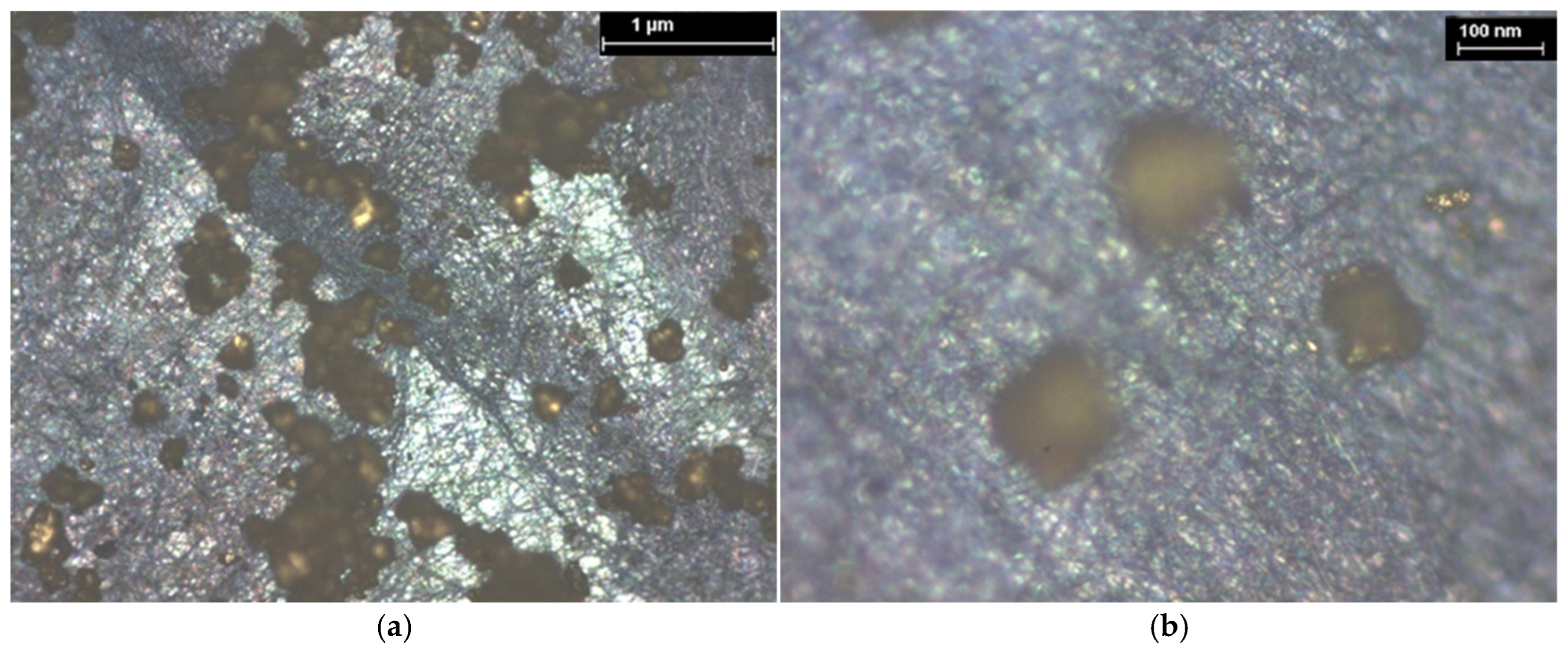
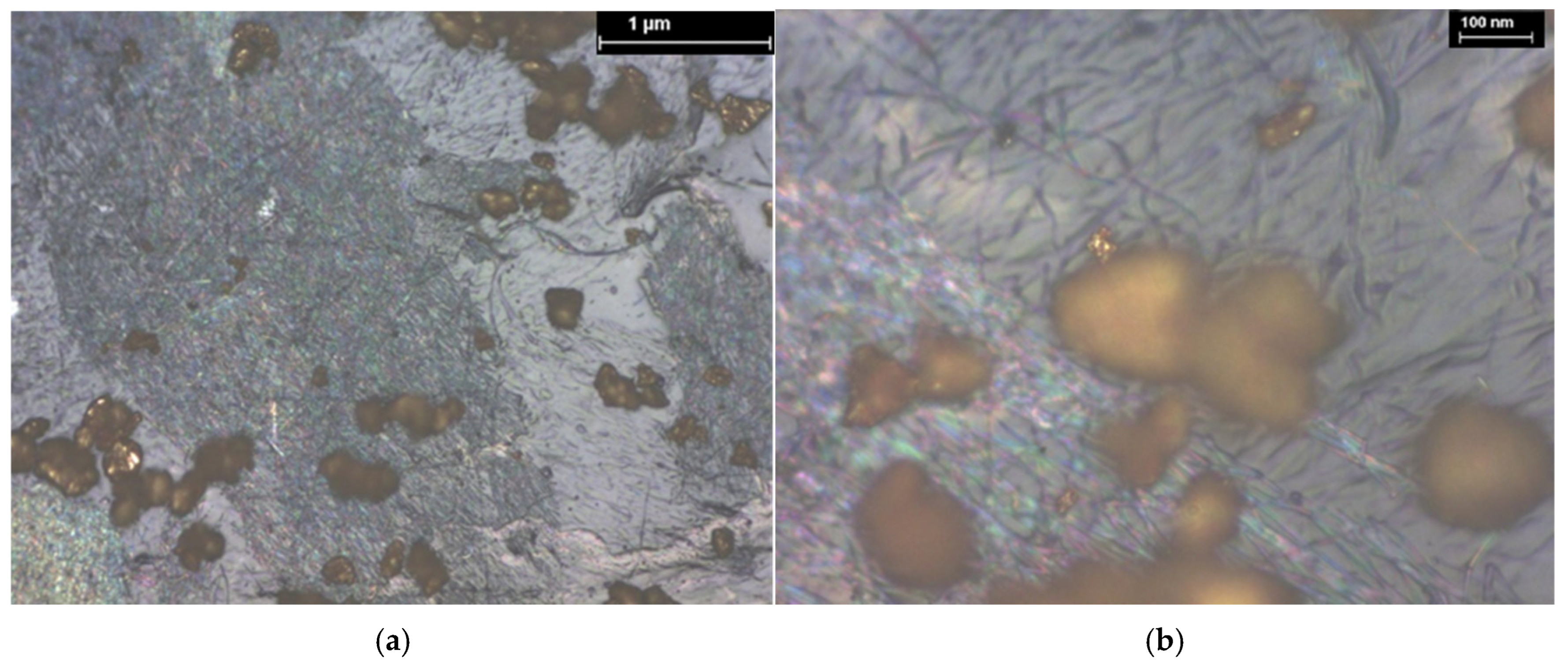
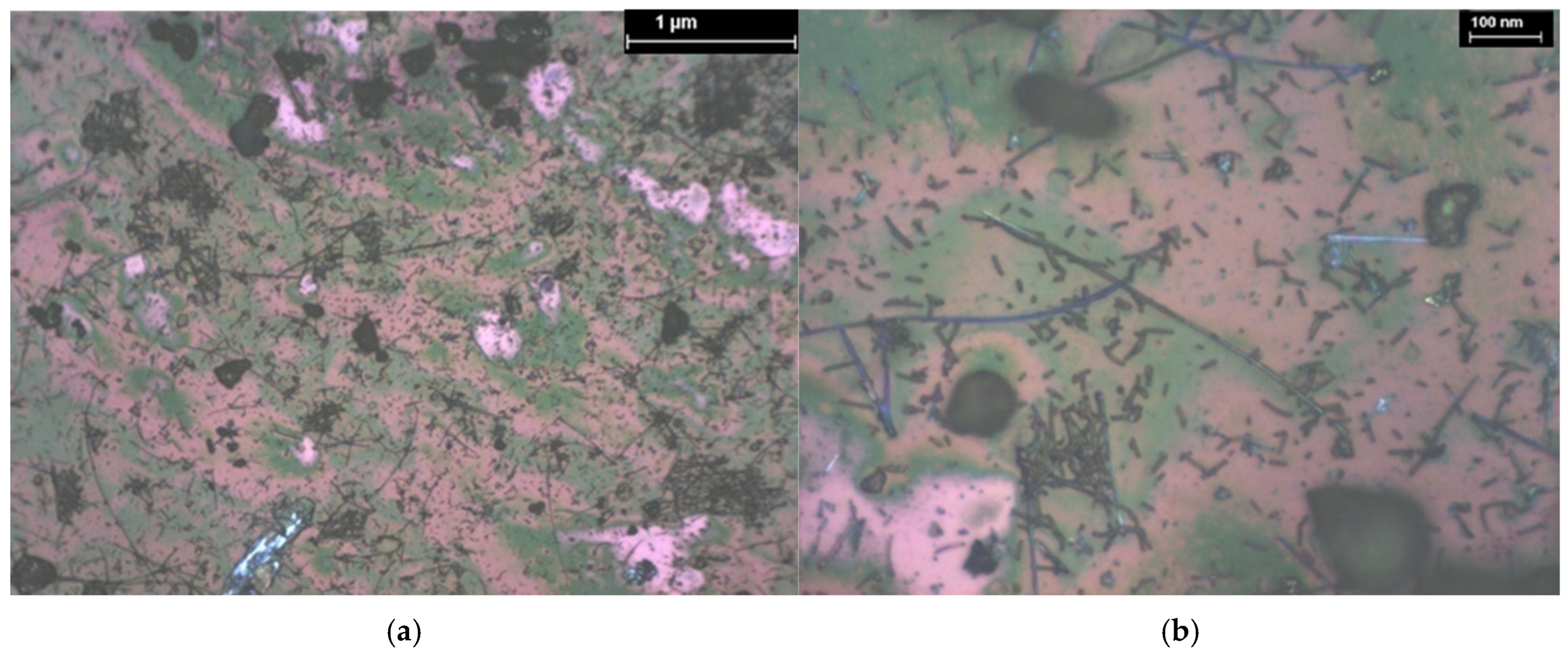
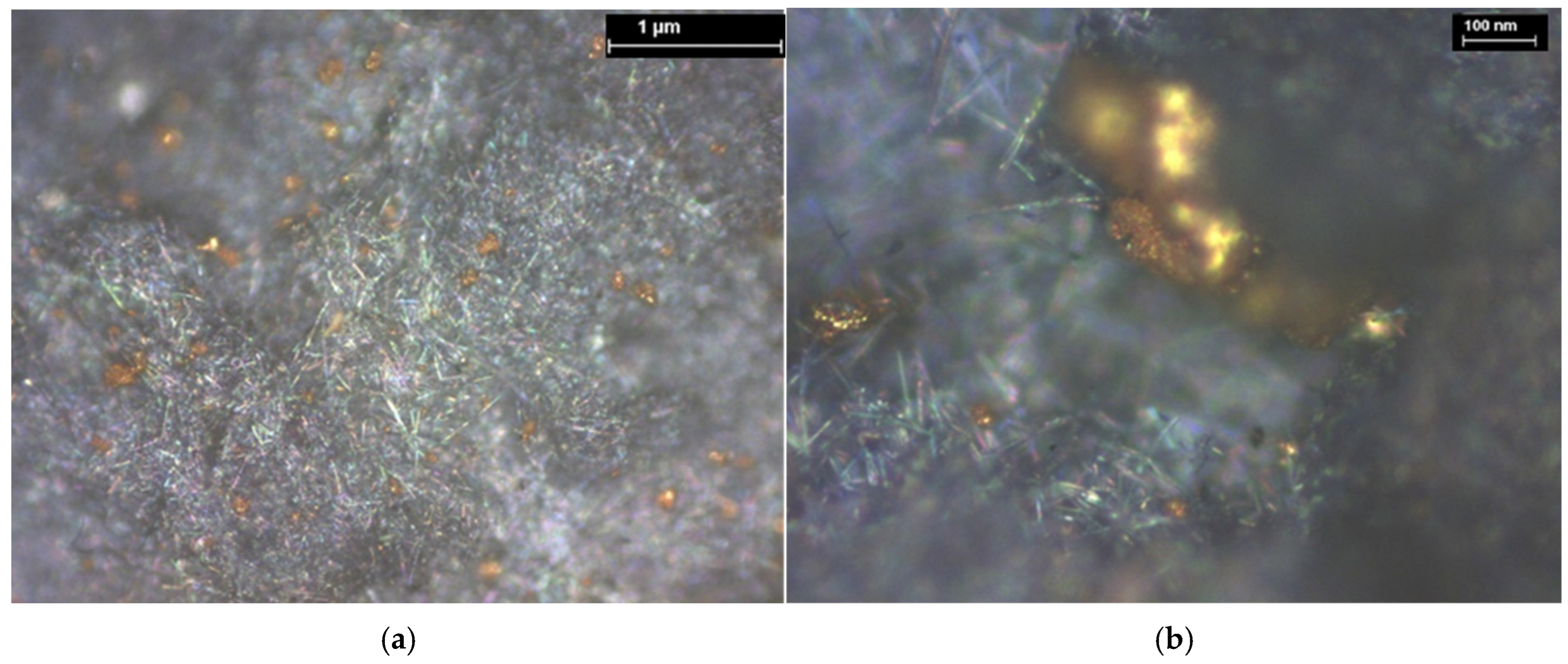
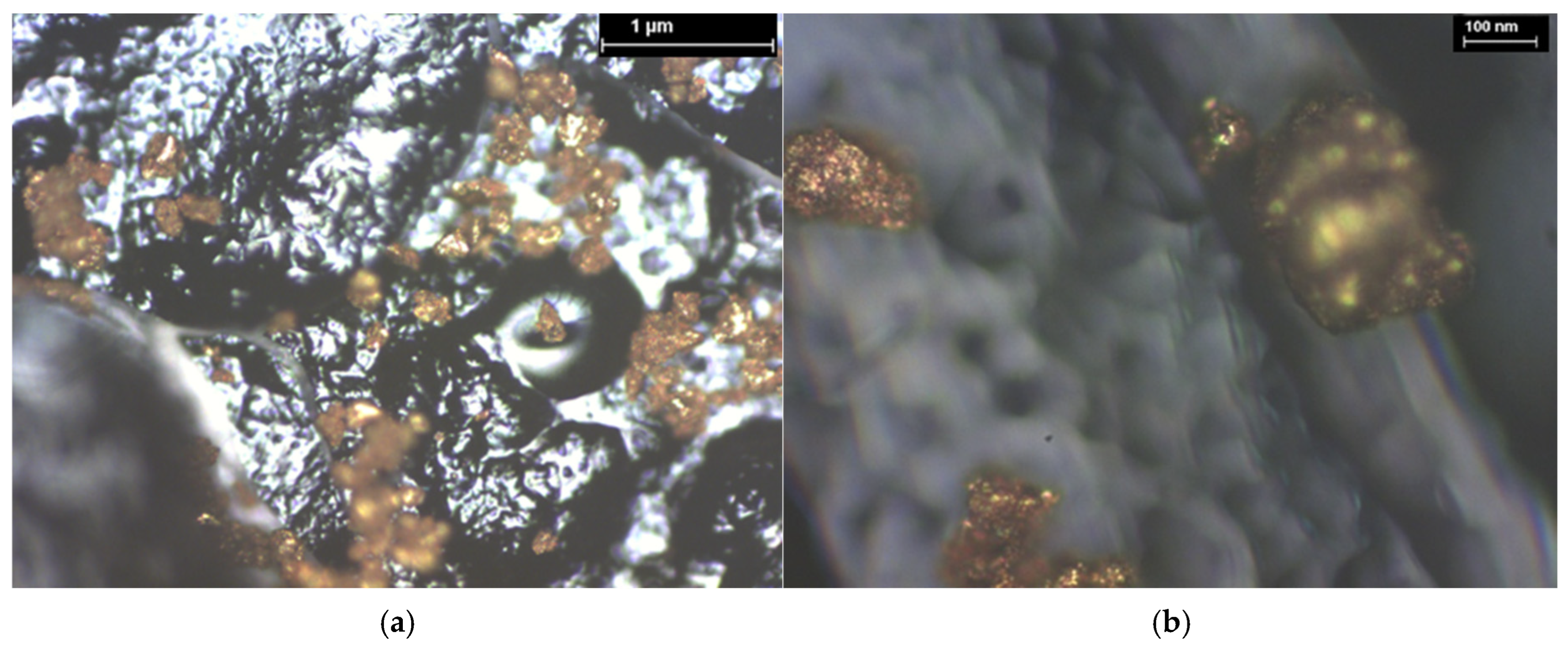
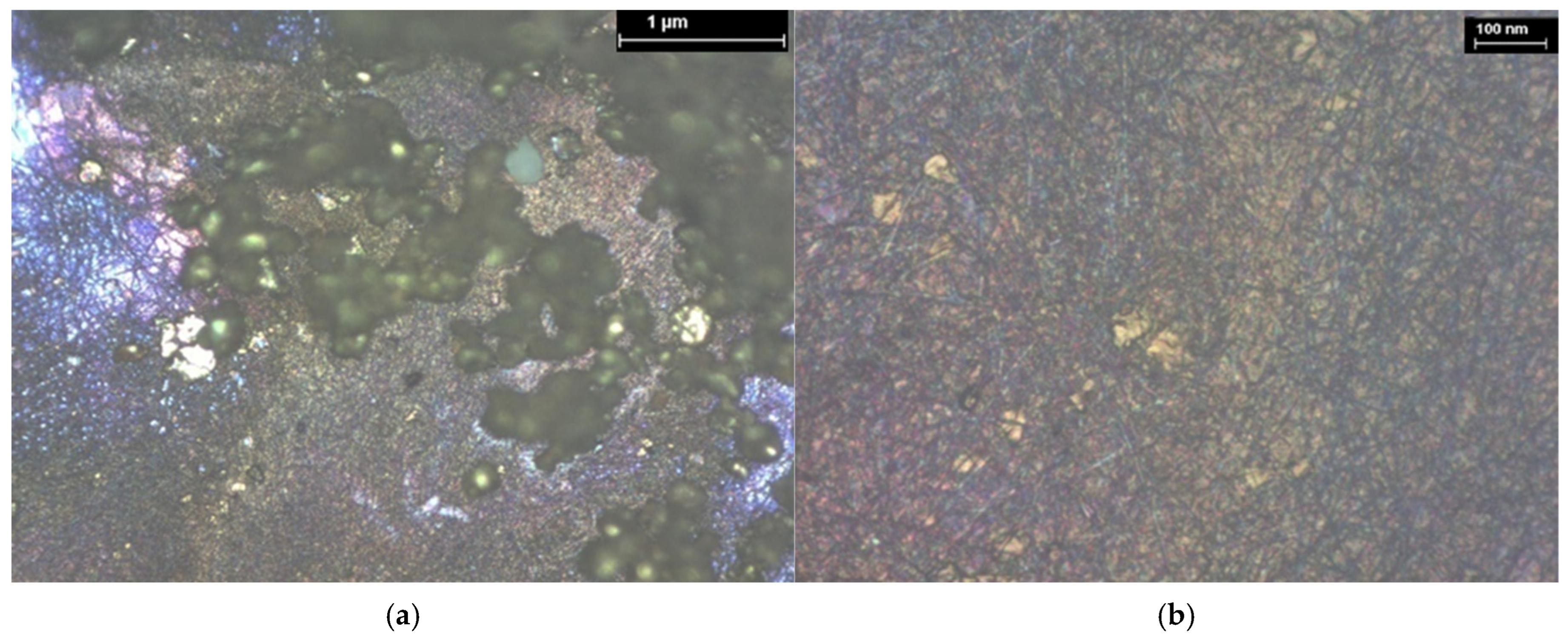
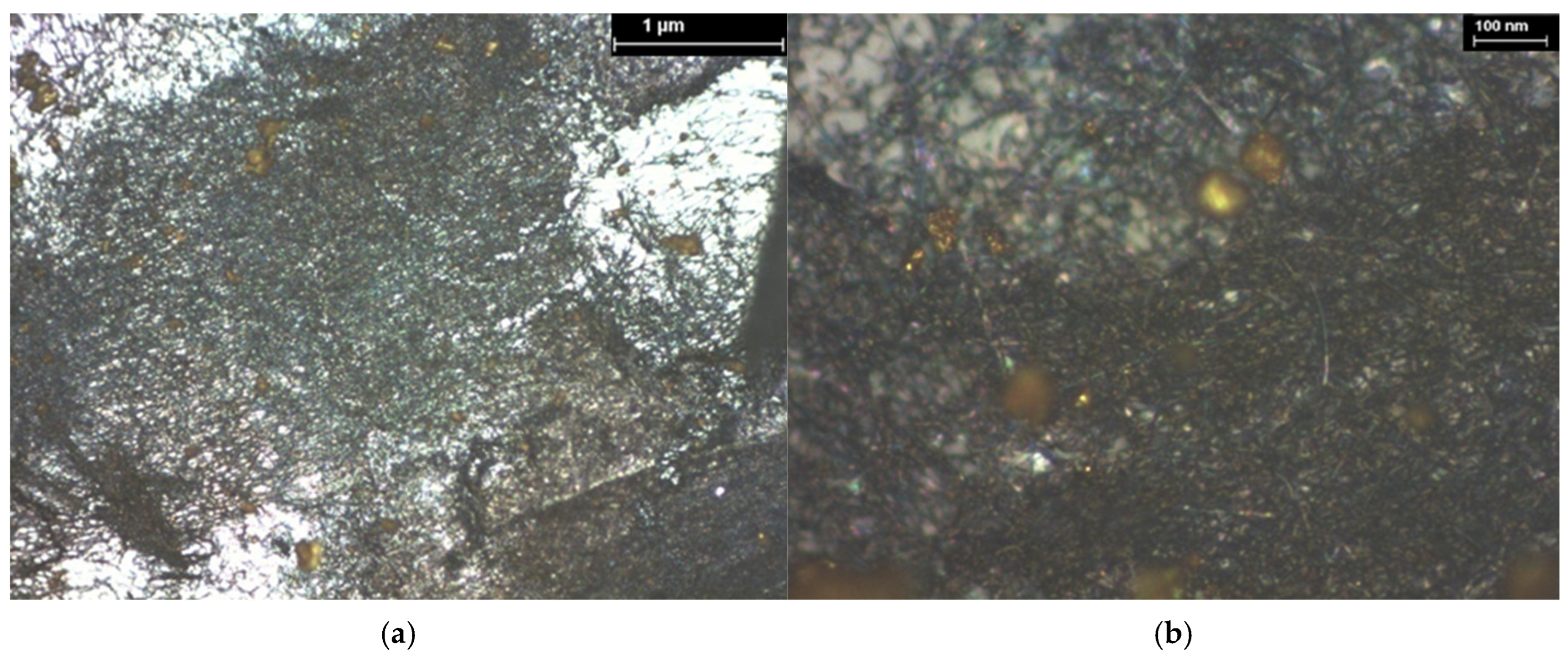
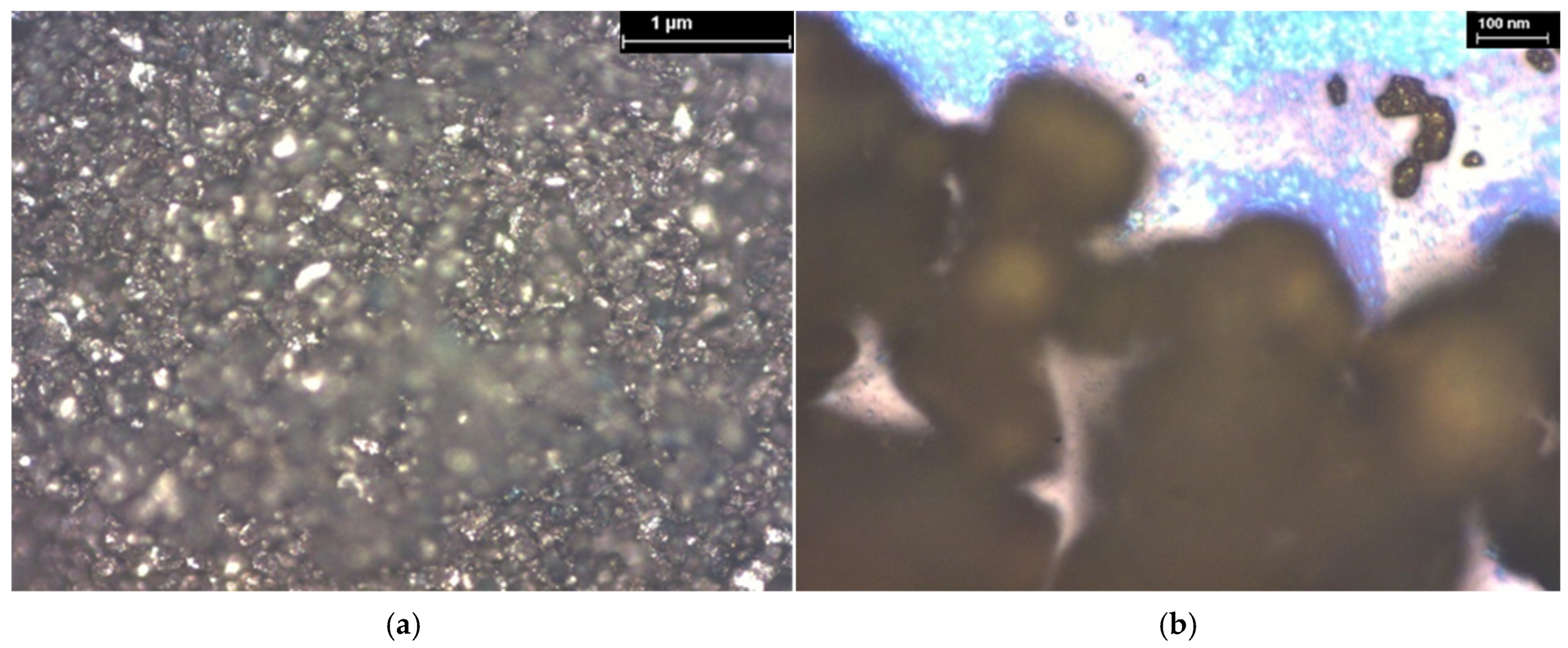
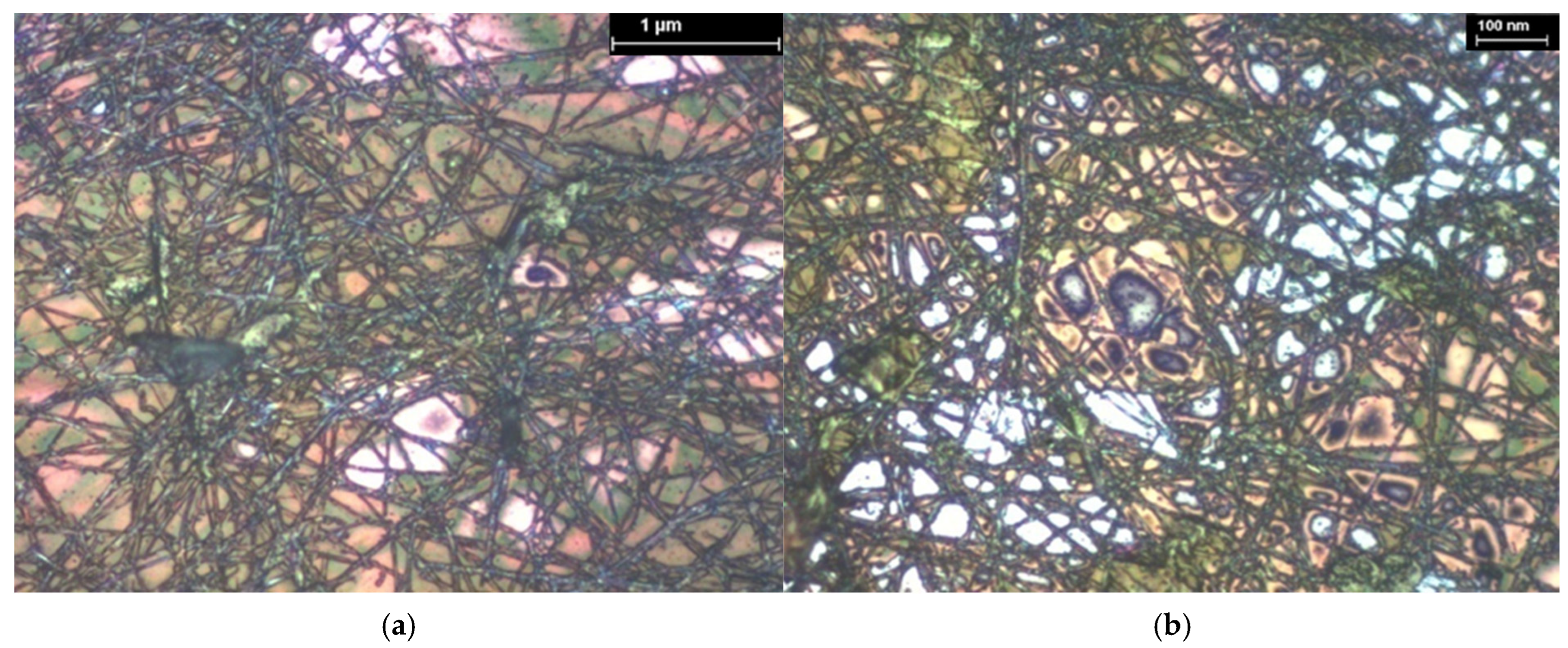
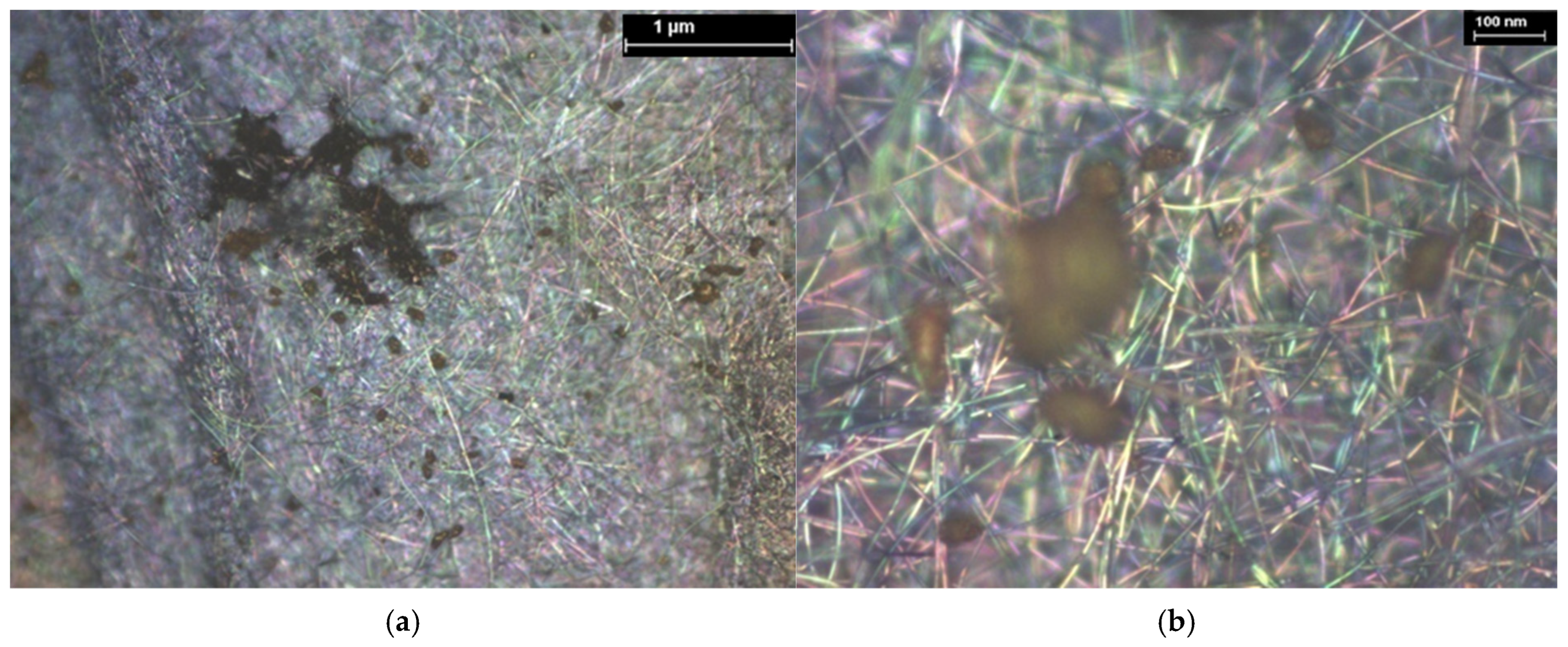
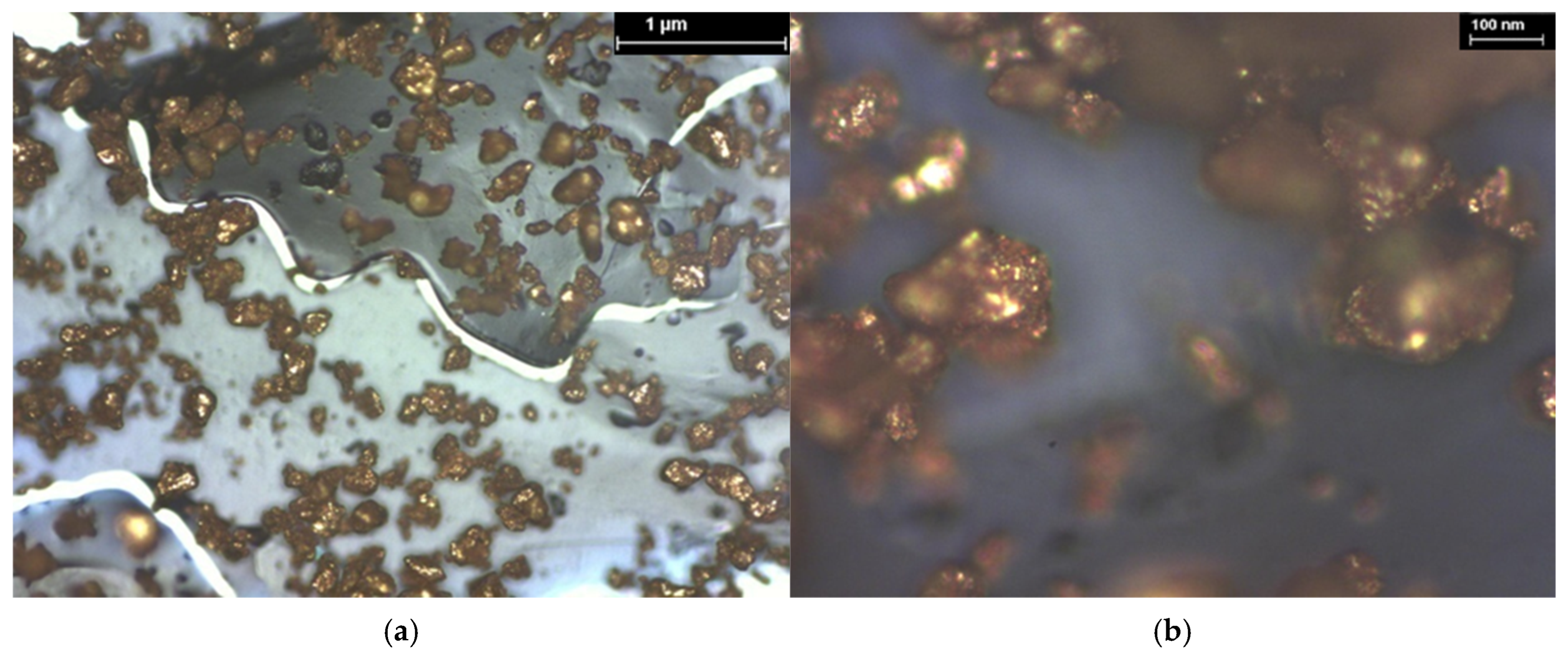

| Hybrid Structure | Weight Percentage (%) |
|---|---|
| TiO2/PANI:EB+Au | 1.66 ± 0.14 |
| TiO2/PEDOT:PSS+Au | 0.64 ± 0.11 |
| TiO2/P3HT+Au | 0.92 ± 0.08 |
| Al2O3/PANI:EB+Au | 0.22 ± 0.04 |
| Al2O3/PEDOT:PSS+Au | 1.78 ± 0.16 |
| Al2O3/P3HT+Au | 0.89 ± 0.12 |
| Y2O3/PANI:EB+Au | 0.37 ± 0.07 |
| Y2O3/PEDOT:PSS+Au | 0.54 ± 0.08 |
| Y2O3/P3HT+Au | 0.26 ± 0.03 |
| ZnO/PANI:EB+Au | 0.46 ± 0.09 |
| ZnO/PEDOT:PSS+Au | 1.85 ± 0.18 |
| ZnO/P3HT+Au | 1.66 ± 0.15 |
| Hybrid Structure | Type of Distribution/Area Covered |
|---|---|
| TiO2/PANI:EB+Au | uniform/large |
| TiO2/PEDOT:PSS+Au | uniform/very reduced |
| TiO2/P3HT+Au | uniform/reduced |
| Al2O3/PANI:EB+Au | uneven/very reduced |
| Al2O3/PEDOT:PSS+Au | uniform/very large |
| Al2O3/P3HT+Au | uniform/large |
| Y2O3/PANI:EB+Au | uniform/quite large |
| Y2O3/PEDOT:PSS+Au | uniform/large |
| Y2O3/P3HT+Au | uniform/very reduced |
| ZnO/PANI:EB+Au | uniform/reduced |
| ZnO/PEDOT:PSS+Au | uniform/very large |
| ZnO/P3HT+Au | relatively uniform/very large |
| Scanned Material | Ra (nm) | RMS (nm) | RSk | RKu |
|---|---|---|---|---|
| TiO2 nanotubes—PANI:EB | 715 | 947 | −0.18 | 4.04 |
| TiO2 nanotubes—PANI:EB/Au | 948 | 1197 | 0.28 | 2.97 |
| TiO2 nanotubes—PEDOT:PSS | 105 | 131 | −0.15 | 3.04 |
| TiO2 nanotubes—PEDOT:PSS/Au | 79 | 107 | 0.16 | 4.42 |
| TiO2 nanotubes—P3HT | 298 | 362 | 0.17 | 2.53 |
| TiO2 nanotubes—P3HT/Au | 341 | 420 | 0.42 | 2.89 |
| Al2O3 nanotubes—PANI:EB— | 702 | 881 | 0.08 | 3.44 |
| Al2O3 nanotubes—PANI:EB/Au | 1053 | 1321 | 0.03 | 3.12 |
| Al2O3 nanotubes—PEDOT:PSS | 194 | 273 | 0.16 | 3.97 |
| Al2O3 nanotubes—PEDOT:PSS/Au | 115 | 146 | 0.22 | 3.35 |
| Al2O3 nanotubes—P3HT | 123 | 198 | 0.62 | 5.86 |
| Al2O3 nanotubes—P3HT/Au | 134 | 166 | 0.02 | 2.69 |
| Y2O3 nanotubes—PANI:EB | 362 | 464 | 0.31 | 3.16 |
| Y2O3 nanotubes—PANI:EB/Au | 368 | 465 | 0.50 | 3.84 |
| Y2O3 nanotubes—PEDOT:PSS | 218 | 289 | 0.34 | 5.44 |
| Y2O3 nanotubes—PEDOT:PSS/Au | 269 | 342 | 0.74 | 4.21 |
| Y2O3 nanotubes—P3HT | 132 | 166 | 0.46 | 5.86 |
| Y2O3 nanotubes—P3HT/Au | 172 | 257 | 0.52 | 7.11 |
| ZnO nanotubes—PANI:EB | 1086 | 1413 | −0.21 | 2.89 |
| ZnO nanotubes—PANI:EB/Au | 1134 | 1418 | −0.11 | 2.87 |
| ZnO nanotubes—PEDOT:PSS | 196 | 265 | −0.13 | 4.23 |
| ZnO nanotubes—PEDOT:PSS/Au | 332 | 466 | 0.46 | 5.86 |
| ZnO nanotubes—P3HT | 956 | 1191 | −0.15 | 2.88 |
| ZnO nanotubes—P3HT/Au | 1042 | 1327 | −0.03 | 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandabat, A.F.; Ciobanu, R.C.; Schreiner, O.D. Active Surfaces in Sensor Technologies Utilizing Ceramic Nanotube-Conducting Polymer Composites Containing Embedded Gold Nanoparticles. Coatings 2025, 15, 1211. https://doi.org/10.3390/coatings15101211
Trandabat AF, Ciobanu RC, Schreiner OD. Active Surfaces in Sensor Technologies Utilizing Ceramic Nanotube-Conducting Polymer Composites Containing Embedded Gold Nanoparticles. Coatings. 2025; 15(10):1211. https://doi.org/10.3390/coatings15101211
Chicago/Turabian StyleTrandabat, Alexandru Florentin, Romeo Cristian Ciobanu, and Oliver Daniel Schreiner. 2025. "Active Surfaces in Sensor Technologies Utilizing Ceramic Nanotube-Conducting Polymer Composites Containing Embedded Gold Nanoparticles" Coatings 15, no. 10: 1211. https://doi.org/10.3390/coatings15101211
APA StyleTrandabat, A. F., Ciobanu, R. C., & Schreiner, O. D. (2025). Active Surfaces in Sensor Technologies Utilizing Ceramic Nanotube-Conducting Polymer Composites Containing Embedded Gold Nanoparticles. Coatings, 15(10), 1211. https://doi.org/10.3390/coatings15101211






