Abstract
This study aims to enhance the corrosion resistance and bioactivity of zinc surfaces through the development of chitosan–pistachio shell (CPM) coatings reinforced with Nb2CTx MXene. The approach introduces a sustainable pathway by incorporating waste pistachio shells as a natural, eco-friendly additive within a biopolymer matrix. Comprehensive structural and surface characterizations confirmed the homogeneous dispersion of Nb2CTx and the successful fabrication of the hybrid coating. Electrochemical analyses in simulated body fluid demonstrated that the CPM coatings markedly improved the corrosion protection of zinc by shifting the corrosion potential to more noble values, reducing current density and increasing polarization resistance. Impedance results further indicated enhanced charge transfer resistance and stable diffusion-controlled behavior. The coatings also exhibited stronger adhesion, higher hydrophilicity, and improved surface compatibility. After immersion in simulated body fluid, the formation of a dense apatite layer on the CPM surface confirmed the coating’s excellent bioactivity. These findings demonstrate that Nb2CTx-reinforced CPM coatings significantly enhance the functional performance of zinc, combining corrosion resistance, biocompatibility, and mechanical stability. Moreover, the use of pistachio shell waste underscores the potential of sustainable biomaterials in developing environmentally friendly coatings for biomedical applications.
1. Introduction
Traditional stainless steel, titanium, and cobalt–chromium alloys, which are used as implant materials in the biomedical field, are also preferred in specific areas such as orthopedic implants and stent applications. Due to the low degradation tendencies of these commonly preferred materials, a second surgical operation may be required in the long term, leading to clinical problems [1]. For this reason, in research on metallic implants, scientists have focused on biodegradable metals. Among these, metals such as iron and zinc (Zn), especially magnesium, are also attracting attention. Although Mg has high biocompatibility, it can cause adverse effects during use due to its uncontrollable degradation rate and H2 gas formation (which can lead to local infection) without surface treatment [2]. On the other hand, Zn and its alloys stand out due to their controlled biodegradation rates, biocompatibility, and being an essential element in the body. Although Zn has a longer biodegradation characteristic, it has limited biological interaction when in contact with surrounding tissues and does not show the desired bonding at the implant-tissue interface [3]. Therefore, surface modification of Zn-based implants, especially coating strategies that improve interaction with surrounding tissues, is of great importance [4,5]. In these surface modification processes, chitosan, a natural polysaccharide exhibiting behaviors such as biocompatibility, biodegradability, and antimicrobial effect, serves as an ideal matrix in coating strategies for biomedical implants, particularly due to its properties [6]. However, the biodegradation times of pure chitosan coatings are quite rapid, allowing them to detach from the surface before fulfilling their intended function, leading to problems with adhesion to the implant surface, and limiting long-term protection and bioactivity [7]. Therefore, improving the coating properties by adding functional additives to the pure chitosan matrix is among the researchers’ goals. In a study conducted by Szőke et al., to reduce the corrosion sensitivity of Zn, ammonium tungstate (PTA) and chitosan coatings were investigated [8]. With the contribution of PTA, the swelling rate of chitosan was significantly reduced, and it was also observed that the coatings exposed to a corrosive environment for 3 days still adhered to the surface. This was found to be due to chitosan, which forms strong ionic crosslinks with PTA, increasing corrosion resistance from 29% to 98%.
Following research in the field of materials science, a breakthrough is happening in the field of biomaterials with the discovery of new types of 2D materials. One of these, MXenes, holds promise in the biomedical field due to their unique and distinct properties [9]. This new type of 2D material, discovered in a 2011 study by Naguib et al., was essentially synthesized for the first time by etching the Ti3AlC2 MAX-phase with hydrofluoric acid (i.e., chemically selectively etching the Al layer), resulting in layered, conductive 2D structures terminated with functional groups like -OH and -F on the Ti3C2 surface [10]. This discovery laid the foundation for MXene (referred to as Ti3C2Tx MXene) research. Although there are numerous types of MXenes in the literature, niobium-based Nb2CTx MXene stands out as an additive material that can enhance biological functions within coatings due to its hydrophilic surface, functional groups, and biocompatibility potential [11]. The dispersion of 2D Nb2CTx MXene nanosheets in a chitosan matrix can increase the density of the coating, thereby contributing to the alloy surface’s resistance to corrosion [12]. Simultaneously, recent studies demonstrating that MXenes promote cell proliferation and osteogenic differentiation suggest that such hybrid films could be effective not only in corrosion control but also in enhancing biological and antibacterial activity [13,14]. A Ti3C2Tx/polyvinyl alcohol composite coating was prepared by Wang et al. to improve the corrosion sensitivity of AZ31B Mg alloy [15]. Corrosion tests conducted in a 3.5% NaCl electrolyte showed that the corrosion rate of the composite coating was approximately five orders of magnitude lower than that of the Mg alloy substrate. It has been stated that this could have occurred both because the layered structure of Ti3C2Tx reduces the diffusion path of corrosive environments and because PVA entering between the lamellae of Ti3C2Tx reduces the natural defects of Ti3C2Tx. Tang et al., investigated the biological effects and corrosion resistance of Nb2CTx MXene coatings on Zn alloy [11]. Along with the coating, the release of metallic ions from the Zn substrate was regulated, leading to a positive response on HGF-1 cells. Furthermore, it has been stated that coatings exhibiting over 99% antibacterial efficacy against both E. coli and S. aureus could enable the control of Zn degradation, increase antibacterial activity, and promote bone regeneration as a promising strategy. The synthesis of Zn on its own (without any binder like chitosan, etc.) and the absence of pistachio shell in the coating composition also highlight another unique aspect of our study.
On the other hand, due to the increased use of materials, researchers working on implants are turning toward the use of sustainable and environmentally friendly additives [16]. The potential for agricultural waste to be transformed into high-value biomaterials is quite intriguing in this regard, creating advantages both economically and environmentally [17,18]. Balasundar et al., investigated the potential use of pistachio shell reinforcements as a biocomposite. The study concluded that pistachio shell waste could exhibit the necessary quality to serve as a bio-filler in a polymer matrix, with a thermal degradation temperature of 274.2 °C and small particle sizes (3.626 nm) [19]. At this point, nut shells (walnut shell, pistachio shell, nut shell, etc.) are promising due to their lignocellulosic structure and diverse mineral content, and they are attracting attention for their potential to be converted into biomaterials [20,21,22]. Therefore, the use of agro-wastes such as pistachio shells to increase biological interaction, and their use in the coating matrix, which can trigger bioactivity on the coating surface and increase osteogenic interaction, emerges as one of the hypothesis questions of this research. Thus, it may be possible to develop environmentally friendly and functional coatings with contributions that can promote sustainably sourced biological interactions.
With this study, the application of Nb2CTx MXene and pistachio shell-added composite coatings to the surface of Zn, which is being considered for use as a biomaterial, aims to achieve three goals: (i) to keep the biodegradation rate at a clinically appropriate level by providing controlled corrosion on the Zn surface; (ii) to obtain bioactive surfaces that can provide optimal interaction with surrounding tissues; (iii) to offer a sustainable and economical coating composition solution by using agro-wastes like pistachio shells as an additive. In this study, pistachio shell and Nb2CTx MXene coatings in a chitosan matrix were successfully applied to Zn surfaces using the dip coating method, and the surface and structural characterizations of the coatings, as well as the adhesion interaction at the coating–metal interface, were investigated. The in vitro electrochemical corrosion sensitivities and in vitro bioactivity behaviors of the coatings were revealed and discussed in detail.
2. Materials and Methods
2.1. Coating Synthesis
Nb2CTx MXene was produced using commercial Nb2AlC MAX (325 mesh with purity: 99+%, Nanografi, Ankara, Turkey) phase powder. MXene synthesis was carried out according to previous studies [23]. Briefly, 1 g of Nb2AlC powder was etched in 20 mL of 49% HF solution for 24 h at 60 °C using a magnetic stirrer (YELLOW LINE–MSH B). The resulting solution was then centrifuged 3 times with deionized water to neutralize it to a pH of ~6–7, yielding Nb2CTx MXene powder. Pistachio shells were thoroughly washed, dried at 60 °C for 24 h, and ground using an agate mortar to obtain a fine powder with an average particle size of 50–60 µm. The powder was then sterilized at 121 °C for 20 min, and subjected to alkaline treatment (1 wt.% NaOH, 2 h) to improve surface wettability and dispersion within the chitosan matrix. For the preparation of the chitosan-based coating, 1% (w/v) low molecular weight chitosan (CAS No: 9012-76-4, Sigma, St. Louis, MO, USA) was dissolved in 1% (v/v) acetic acid solution, and pistachio shell powder was added at a concentration of 5% (w/v) of the total solution. The chitosan–pistachio shell–Nb2CTx MXene (CPM) solution was homogenized in a magnetic stirrer at 500 rpm for 2 h. The coatings were applied to Zn substrates with dimensions of 10 × 10 mm2 using the dip coating method (MTI, PTL-MM01, Richmond, CA, USA); the samples were immersed in the solution for 5 min and withdrawn at a speed of 2 mm·s−1. All samples were stabilized at 37 °C for 12 h for analysis/tests. The following abbreviations were used for the samples to make them more understandable: uncoated, C, CP, and CPM for the uncoated Zn, chitosan, chitosan–pistachio-shell, and chitosan–pistachio shell–Nb2CTx MXene-coated samples, respectively.
2.2. Characterization
The surface morphology and chemical composition of the coatings were examined using various characterization techniques. Zn surfaces and coatings were observed using high-resolution scanning electron microscopy (SEM, Zeiss, Sigma-300, Jena, Germany) to evaluate surface topography and coating homogeneity; elemental distribution and chemical composition were determined by energy-dispersive X-Ray spectroscopy (EDS, Ametek, EDAX, Pleasanton, CA, USA). The crystal structure and phase analysis of the coatings were scanned using X-ray diffraction (XRD, PANalytical, Empyrean, Malvern, UK, Cu Kα, λ = 1.5406 Å) over a 2θ range of 10–60°, and the crystallinity/amorphous nature of the coatings and the presence of MXene additive were examined. Possible chemical interactions between the Zn surface and functional groups and coatings were analyzed using Fourier transform infrared spectroscopy (FTIR, Thermo Scientific, Nicolet 6700, Waltham, MA, USA) in the range of 4000–400 cm−1. High-resolution transmission electron microscopy (HRTEM, JEOL 2100, Peabody, MA, USA, 200 kV) was used to examine the nano structural properties of both Nb2CTx MXene and the coating layers. Selected area electron diffraction (SAED) patterns, high-resolution lattice images, and layer thicknesses were examined in HR-TEM measurements. X-ray photoelectron spectroscopy (XPS, Thermo Scientific, K-Alpha, Waltham, MA, USA) was used to determine the chemical bonding states on the surfaces of the coatings. The surface wetness and hydrophilic/hydrophobic properties of the coatings were evaluated by measuring the contact angle (CA, Attension Theta Flex, Västra Frölunda, Sweden in SBF medium) using the sessile drop method. Contact angle measurements were performed using SBF instead of distilled water to better simulate physiological conditions relevant to biomedical applications. Coating adhesion was evaluated by a tape test according to ASTM D3359-09 standard [24]; cross-scratches were made on the surface of each sample, 3 M adhesive tape was applied, and after removal, the integrity of the coating with the substrate was examined using an optical microscope at 200× magnifications (OM, Model: XJL17, AOB, Istanbul, Turkey). Surface roughness measurements of the sample groups before coating were taken using contact profilometer (Time, TR-200, Beijing, China), and were compared with the adhesion strengths after coating.
2.3. Electrochemical In Vitro Corrosion Tests
The electrochemical in vitro corrosion sensitivities of the coatings were investigated using a three-electrode system in SBF (Simulated Body Fluid) medium. In the study, uncoated Zn and samples with different coatings were used as the working electrode, platinum wire as the counter electrode, and Ag/AgCl as the reference electrode. First, open-circuit potential (OCP) measurements were performed for 3600 s until the samples reached a stable potential. After ensuring potential stability, potentiodynamic polarization measurements (PDP) were performed using a potentiostat (Emstat 4R, Palmsens, Houten, The Netherlands) over a range of −250 mV to +250 mV at a scan rate of 1 mV·s−1. Electrochemical impedance spectroscopy (EIS) measurements were performed over a frequency range of 100 kHz–10 mHz with an AC signal amplitude of 10 mV, and the resulting Nyquist and Bode diagrams were used to evaluate corrosion resistance and coating barrier properties. Measurements were performed at body temperature (37 ± 0.5 °C) and in SBF electrolyte with a pH of 7.4. The obtained EIS data were fitted using EC-Lab Demo v10.40 software, appropriate equivalent circuit diagrams were derived, and the corrosion behavior was quantitatively analyzed by iterating the chi-square (χ2) value to <10−3.
2.4. In Vitro Bioactivity Tests
The apatite-like tissue formation abilities on the surfaces of the samples were evaluated according to Kokubo and Takadama’s SBF (Simulated Body Fluid) protocol [25]. Each coated and uncoated Zn sample was placed vertically in sterile tubes and contacted with 20 mL of SBF solution. The samples were incubated at 37 ± 0.5 °C under static conditions for 7 days. The SBF solution was replaced with fresh solution every 24 h to maintain ionic balance. At the end of the test, the samples were washed with distilled water, dried in a desiccator without heat, and the surface morphology was examined using SEM. The bioactivity of the samples was analyzed comparatively based on their Ca/P ratios and apatite-like layer density.
3. Results and Discussion
Figure 1 shows the SEM surface morphologies of the samples and the EDS analysis results of the marked areas. When the SEM and EDS analyses presented in the figure are evaluated together, it is seen that the uncoated surface (Figure 1a) exhibits a relatively flat topography but contains surface defects and microscopic irregularities in certain regions. This situation suggests that the uncoated structure may be more vulnerable to corrosion and the effects of the biological environment. As seen in Figure 1b, the surface of the C coating appears more compact, homogeneous, and contains clusters. It is anticipated that the coating will successfully cover the Zn surface, providing both a protective barrier effect and a positive contribution to biocompatibility [26]. In the CP coating (Figure 1c), it is noteworthy that the surface morphology gains more distinct topographic features, which could provide an advantage for cell interactions in a biological environment. The most striking structure is observed in the CPM coating (Figure 1d), where the surface gains a more integrated, dense, and layered form, and the MXene particles, which are visible in the SEM image, are embedded in the surface and are approximately 15–20 μm in size. The presence of Nb2CTx MXene is expected to strengthen the surface interactions of the coating [15]. When examining EDS analyzes, intense Zn and O peaks were observed on the uncoated surface (Figure 1(#1)), while the C signal was strengthened in the C and CP coatings due to the organic structure (Figure 1(#2,#3)). In the CPM coating, in addition to Zn, O, and C, distinct Nb peaks appear in the range of 0.67–2.0 keV (Figure 1(#4)). The Nb reflections in these energy regions confirm that the Nb2CTx MXene particles are successfully integrated into the coating. On the other hand, the presence of micropores on the surface of the CPM sample is thought to play a significant role in increasing the interaction between the coating and the surrounding tissues [27]. Therefore, it is predicted that the CPM coating, exhibiting functional surface properties with both morphological integrity and a clear Nb signal in EDS, will lead to morphological success in biomedical applications.
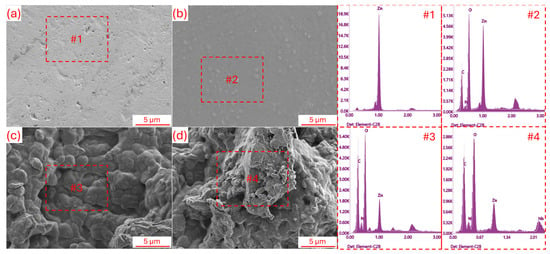
Figure 1.
SEM surface morphology and EDS elemental analysis results for; (a) Uncoated, (b) C, (c) CP, and (d) CPM samples.
Figure 2 presents the XRD and FTIR analysis results for the samples. The XRD patterns (Figure 2a), sharp diffraction peaks characteristic of Zn is observed in the uncoated sample: these peaks, appearing around ~2θ = 31.8° (100), 34.5° (002), 36.3° (101), 47.5° (102), and 56.6° (102), correspond to the hexagonal Zn structure [28]. In coating C, the amorphous character is more prominent than the crystalline structure; a distinct broad peak is observed around ~2θ = 20°, which reflects the typical semi-crystalline polysaccharide backbone structure of chitosan [29]. In the CP coating, weak peaks around ~15–16° represent the (110) plane of the cellulose-I structure, a broad peak in the range of ~20–22° represents the combined semi-crystalline structure of hemicellulose and chitosan, and a weak signal around ~26° represents lignin and aromatic additives [30,31]. In the case of CPM, a new peak observed in the XRD pattern, particularly at low angles (~2θ = 9° (002) plane), confirms the presence of MXene layers; these peaks indicate the lamellar structure of Nb2CTx MXene [32]. In addition, the sharp peaks around 31.8°, 34.5°, 36.3°, 47.5°, and 56.6° for Zn showed a loss of intensity, indicating that the coating successfully covered the Zn surface and gave the structure an amorphous character. After XRD analysis, it is predicted that uncoated Zn will have high crystallinity, C and CP coatings will tend toward a more amorphous structure, and the addition of MXene (CPM) can contribute to both the emergence of new phases and making the coating more homogeneous and functional.
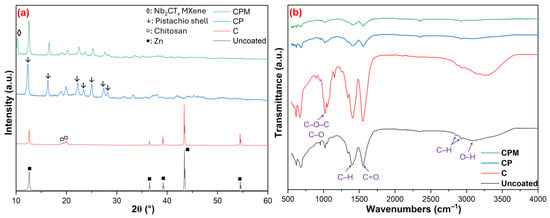
Figure 2.
Structural ((a): XRD) and functional ((b): FTIR) analysis results for samples.
FTIR results presented in Figure 2b, the following are observed in the Uncoated sample: O–H stretching at ~3300–3400 cm−1, aliphatic C–H stretching at ~2920–2850 cm−1, C=O or H–O–H bending of water at ~1630–1650 cm−1, C–H bending at ~1400–1450 cm−1, and C–O stretching at ~1000–1100 cm−1. In coating C, O–H and N–H stretching vibrations were observed at ~3200–3500 cm−1, aliphatic C–H stretching vibrations at ~2920–2870 cm−1, Amide I (C=O) at ~1650 cm−1, Amide II (N–H bending) at ~1550 cm−1, CH2 bending vibrations at ~1420–1380 cm−1, and C–O–C vibrations belonging to the polysaccharide backbone at ~1150–1070 cm−1. In the CP coating, hydroxyl and amine stretches were detected at ~3400 cm−1, aliphatic C–H at ~2920–2850 cm−1, carbonyl vibrations at ~1650 cm−1, aromatic ring vibrations at ~1510–1550 cm−1 and Amide II, CH2 bends at ~1420 cm−1, lignin-specific C–O–C aromatic stretches at ~1240–1270 cm−1, and polysaccharide C–O–C bands at ~1020–1100 cm−1 [30]. In the CP coating, the intensity of these characteristic bands changed and peak shifts were observed, indicating the formation of hydrogen bonds between Chitosan and the lignocellulosic components of pistachio shells (especially between O–H and phenolic or carbonyl groups). In the CPM spectrum, weakened O–H/N–H at ~3400 cm−1, C–H at ~2920–2850 cm−1, weakened Amide I at ~1650 cm−1, Amide II at ~1550 cm−1, CH2 bends at ~1420 cm−1, polysaccharide bands at ~1000–1100 cm−1, and signals indicating Nb–C vibrations at <700 cm−1 are observed [33]. In the CPM spectrum, smoother and suppressed bands are noticeable; this indicates that the surface is covered with Nb2CTx MXene and the organic groups are partially shielded. Specifically, the peaks in the 1000–1200 cm−1 region are associated with C–O–C stretching vibrations, and these signals are weakened by the binding of MXene to the surface. These results indicate that both physical and chemical interactions occur during the coating process, and a more homogeneous and functional surface is obtained with the addition of MXene.
Figure 3 shows the SEM-EDS results for Nb2CTx MXene and the HR-TEM results for the CPM coating film, respectively. The characterization results presented in the figure strongly support the potential of the CPM coating in the field of biomaterials. Firstly, the SEM-EDS analysis of Nb2CTx powder (Figure 3a) clearly reveals the dominant presence of the Nb element, confirming the successful synthesis of Nb2CTx MXene. This structure extends the known biocompatibility advantage of Nb in the biomedical field. The HR-TEM image (Figure 3b) shows that the chitosan, pistachio shell, and Nb2CTx components are integrated together, indicating a homogeneous distribution of the organic and inorganic phases. These types of hybrid structures play a critical role both in terms of mechanical integrity and functional surface interactions within the biological environment [34]. The atomic resolution image obtained by HR-TEM (Figure 3c) shows the (330) planes with a d-spacing of 0.839 nm, indicating that the crystalline structure of Nb2CTx is preserved; this enhances the electrochemical stability of the coating and its ability to interact with biological fluids. HAADF-STEM and EDS elemental mapping results (Figure 3d) clearly show that the elements Nb, C, and O are homogeneously distributed across the surface [35]. This uniform distribution is crucial for controlled ion release in biological environments and balanced bio interactions with the surface. These results indicate that the CPM coating can exhibit superior properties in terms of both corrosion resistance and biocompatibility in biomaterial applications, and that Nb2CTx has the potential to support biomedical processes such as osteointegration and cell adhesion due to its functional contributions to the coating structure.
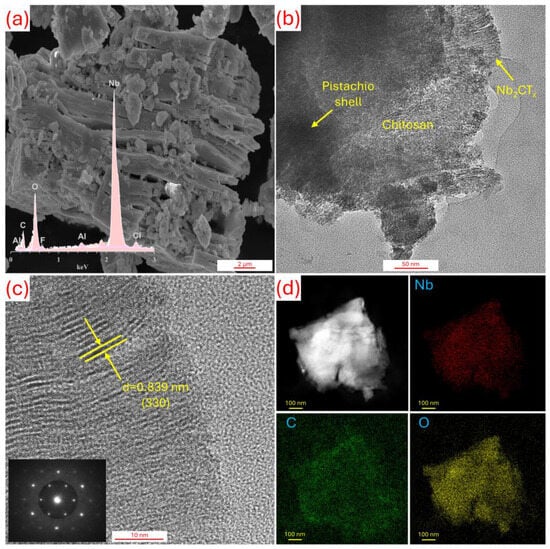
Figure 3.
(a) SEM-EDS results for Nb2CTx MXene with (b–d) HR-TEM analysis for CPM sample.
The XPS analysis results obtained from the CPM film are shared in Figure 4. The presented XPS spectra (Figure 4a–d) clearly reveal the surface chemistry of the composite coating and its significance for biomaterial applications: the Nb3d region shows three components—a high-binding energy peak (~210 eV, 3d3/2) and a slightly lower one (~206 eV, 3d5/2) corresponding to Nb2O5, along with a lower-binding energy signal (~203–204 eV) from Nb–C/Nb2C—indicating that the MXene is protected but partial oxidation (Nb→NbOx) and the presence of oxygenated terminations are present on the surface (Figure 4a) [36]. The C1s spectrum presented in Figure 4b distinguishes typical organic components; the main intense peak at ~284.5–285 eV represents the C–C/C–H carbon skeleton, the component with intermediate binding energy (~285.5–286.5 eV) represents oxygen/nitrogen functionalities bound in C–O/C–N types, and the higher energy (~around 288 eV) indicates carbonyl/carboxylate (O–C=O) contributions; this confirms that the polysaccharide/amine groups of chitosan and the lignocellulosic carbonyl/ester groups of pistachio shells are protected on the surface [37]. The O1s spectrum (ranging from ~530–533 eV) distinguish signals from both metal oxide/Nb–O (lower BE), C–O/OH (intermediate Be), and adsorbed water/organic O (higher eV), confirming both the MXene terminations (–O, –OH) and the organic matrix oxygen content; they can also indicate residual Al oxide/bonds from the MAX phase if weak Al–O components are present (Figure 4c) [38]. The signal observed in F1s (around ~684–686 eV) indicates etching/fluoridation residues or F-terminated Nb–F species, which are typical byproducts of the MXene preparation process (Figure 4d) [37]. This surface composition offers both positive and stimulating aspects: preserved amine and hydroxyl/C–O groups provide suitable chemical sites for cell adhesion, protein adsorption, and functionalization; surface oxides (NbOx) and MXene cores can contribute mechanical stability, conductivity, and potential antimicrobial effects [39]. On the other hand, F residues and the level of surface oxidation should be minimized (through washing, thermal/chemical passivation, or reduction steps) as they can have negative impacts on biocompatibility or long-term stability if left uncontrolled. Furthermore, the surface distributions of the particles embedded within chitosan, which are difficult to measure from SEM micrographs, were compositionally revealed by XPS results. XPS results shows there is electron distribution and strong surface interactions (hydrogen bonds, partial charge transfer) between MXene and the chitosan–pistachio shell matrix, which results in a multi-functional interface suitable for cell adhesion, surface functionalization, and biomedical applications.
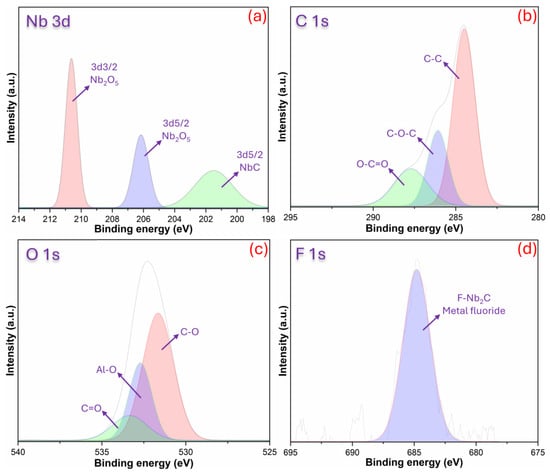
Figure 4.
XPS results for CPM film with (a) Nb3d, (b) C1s, (c) O1s, and (d) F1s.
Figure 5 evaluates the adhesion strength of uncoated Zn, C, CP, and CPM coatings, considering the mechanical and chemical interactions of the interfacial bonds, and revealing the deformation, scratch marks, and separation modes on the surfaces. In Figure 5a, a scratch test was not applied to the uncoated Zn, and it only represents the natural morphology of the uncoated surface. This indicates that the surface has a rather rough morphology. Figure 5b, coating C shows the scratch mark with the labels “scratch track” and “coating”; it is observed that the chitosan forms partial chemical bonds with Zn through hydrogen bonds and amino groups, but offers moderate adhesion strength (level 2B) with slight deformation during scratching, indicating that the organic matrix limits mechanical interlocking [40]. The CP coating in Figure 5c exhibits partial detachment, while it is thought that the cellulosic fibers of the pistachio shell increase mechanical anchoring in the hybrid structure, the local delamination results in lower adhesion behavior (level 1B) compared to coating C due to stress concentration [41]. Figure 5d, CPM provides a homogeneous appearance with minimal separation; the covalent/ionic bonds and high surface area of Nb2CTx MXene maximize interfacial energy, resulting in the highest adhesion strength (level 4B), and the coating’s integrity is maintained during scratch testing, confirming superior mechanical stability. The adhesion measurements, along with the surface roughness results obtained before the coating processes, showed average surface roughness (Ra) values of 2.15 ± 0.15 μm, 2.33 ± 0.37 μm, and 2.24 ± 0.77 μm for the C, CP, and CPM coatings, respectively. The surface roughness being quite close to each other in terms of Ra values, so different adhesion results can be obtained between the coating composition and the substrate due to chemical bonds instead of surface roughness effect [42]. Furthermore, it is thought that the integration of chitosan in a viscous state into the lamellar arrangement of MXene structures may be the reason for this [43]. The adhesion strength order is CP < C < CPM, and it is believed that strengthening the MXene interface bonds (chemical affinity and mechanical interlocking) could benefit long-term reliability in biomedical implants.
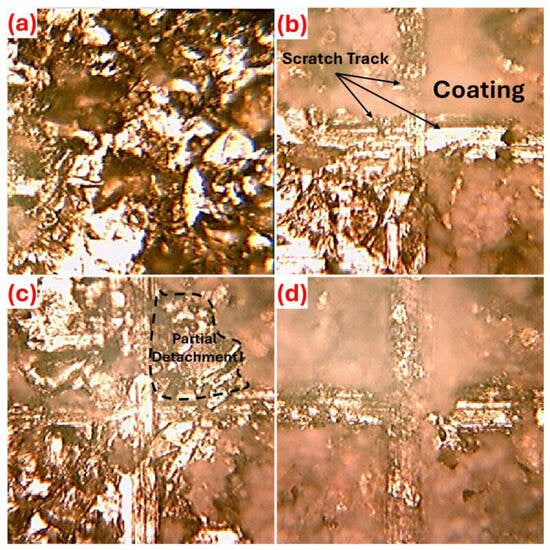
Figure 5.
Adhesion strength between coating—substrate in terms of ASTM D3359-09 standard [24], (a) uncoated, (b) C, (c) CP, and (d) CPM samples.
Figure 6 shows the electrochemical in vitro corrosion results (OCP and PDP) obtained for the samples. The OCP data in Figure 6a reveals the thermodynamic tendency and short-term stability of uncoated and coated surfaces. Here, a more positive (nobler) potential indicates better protective behavior. As seen in Table 1 and Figure 6a, the uncoated Zn initially had a potential close to the most negative value, around EOCP = −0.769 V (Ag/AgCl), and although it shifted slightly in the positive direction over time, it still remained the most active. The C coating shifted the EOCP to ≈ −0.699 V, making the surface more noble; the CP became even more positive, reaching EOCP to = ≈ −0.559 V, providing an additional barrier/chemical effect; and the most positive change was observed in the CPM containing Nb2CTx, with the EOCP increasing to ≈ −0.458 V. OCP curves remain relatively stable over time across all coatings; particularly, the more positive and stable OCP exhibited by CPM indicates the formation of a long-term protective film on the surface that is non-continuous or impermeable [44]. The OCP results clearly support the noble behavior of the Zn surface by the coatings and that CPM provides the most effective protective effect. When considering this situation in terms of biomedical implant coatings, it offers the potential for a more positive and stable potential, which could slow down uncontrolled ion release on the implant surface, leading to a safer and more biocompatible interaction with the tissue. On the other hand, as can be seen in Figure 6b, the PDP data quantifies kinetic information and the actual corrosion rate. According to the Tafel results in Table 1, the uncoated surface has the highest corrosion current density with Icorr = 5.66 × 10−2 A·cm−2, while the current density decreases in the order of C → 4.15 ×10−2 A·cm−2, CP → 3.34 × 10−2 A·cm−2, and CPM → 1.48 × 10−2 A·cm−2. This decrease is consistent with a positive shift in Ecorr values: Ecorr increased from −0.769 V for the uncoated sample to −0.458 V for the CPM. Polarization resistance (Rp) follows the same trend: uncoated = 626.0 Ω, C = 721.3 Ω, CP = 846.0 Ω, and especially CPM = 2518 Ω. The dramatic increase in Rp in CPM indicates that the coating formed a strong barrier against ion transfer [45]. Corrosion rates (mm·year−1) also decrease in parallel: Uncoated 0.848 mm·year−1 → C 0.613 mm·year−1 → CP 0.501 mm·year−1 → CPM 0.221 mm·year−1; in other words, the corrosion rate with CPM is reduced by approximately four times. βa/βc values obtained from Tafel curves, there is a significant increase in CPM, especially on the cathodic slope (for the βc calculation, the E windows CPM was selected between −1.69 V and −1.36 V on the cathodic branch, βc = 0.535 V·decade−1); this suggests that the coating slows down the cathodic process (e.g., O2 reduction or proton reactions), thus facilitating protection of surfaces [46]. In other words, inhibition of cathodic O2 reduction leads to decreases the overall cathodic current density, thereby reducing the driving force for anodic dissolution. This slower cathodic kinetics promotes the stabilization of surface films, as the lower reduction rate delays aggressive Cl− ions. The denser microstructure and MXene barrier effect limit ionic transport, which further enhances CPM film stability. Thus, the inhibition of cathodic O2 reduction indirectly facilitates by creating favorable environment for protective films. In conclusion, the PDP/Tafel data quantitatively confirms that the coatings significantly reduce the corrosion kinetics and that the performance ranking is uncoated < C < CP < CPM; mechanistically, the chitosan barrier effect, the contribution of the pistachio shell’s organic matrix, and the increase in coating density/film integrity by Nb2CTx MXene can be interpreted as the main reasons for this improvement, which restricts ion diffusion. This improvement contributes to both extending implant life and preventing toxic effects in surrounding tissues by providing long-term corrosion resistance and controlled safe biodegradation in biomedical implants.
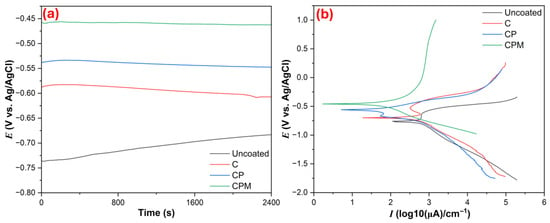
Figure 6.
(a) OCP immersion and (b) PDP curves of samples in SBF electrolyte.

Table 1.
Tafel values obtained from PDP curves.
Figure 7 presents the EIS data of the samples in SBF, proposes equivalent circuit diagrams for each sample group, and calculated the data. The equivalent circuit diagrams were defined Rs+QZn/RZn, Rs+Qc/(Rc+QZn/RZn), Rs+QCP,CPM/(RCP,CPM)+QZn/RZn, and Rs+QCP,CPM/(RCP,CPM)+QZn/RZn for uncoated, C, CP, and CPM samples, respectively. When we analyze the EIS data, the values obtained from Table 2 and the Nyquist plots (Figure 7a) reflect the corrosion behavior of the coatings. The low charge transfer resistance and relatively low low-frequency impedance observed in uncoated Zn indicate an active surface behavior that provides an easy electrical path for metal dissolution (Zn → Zn2+). But these terms (charge transfer resistance and low-frequency impedance) were increased with C coating, forming a barrier film that limits electron flow and anodic/cathodic reactions, but coatings may have still been porous [47]. CP composites reached a very high, dramatically hindering both charge transfer and ion diffusion. The large semicircle in the Nyquist plot and the high low-frequency impedance in the Bode plots (Figure 7c,d) indicate that CP significantly reduced Zn dissolution through both its compact barrier effect and pore filling/conductive pathway blocking mechanisms (the cross-linking role of lignocellulosic components in pistachio shells). But the Bode phase angle and the peak in low-frequency impedance indicate that the addition of Nb2CTx MXene creates an extra tortuosity/extended diffusion path on the surface for CPM coating. Furthermore, the Warburg (W) element observed in the equivalent circuit (Figure 7b) is more pronounced for CPM, highlighting the importance of diffusion-controlled behavior [48]. The C coating can be estimated to have reduced the corrosion current, CPM, and CP also reduced much higher, meaning CP is the most effective physical barrier. However, the high low-frequency impedance and phase angle of CPM suggest that it offers an interface more resistant to long-term ion diffusion, and that MXene gains additional protective functions through surface functionalization [49]. From a mechanism perspective, the rate-limiting step in uncoated Zn is direct charge transfer and oxidation, while in the C coating, ion movement and water/Cl− penetration are slowed down due to the barrier film. In the CP coating, the lignocellulosic filler and crosslinks of the chitosan matrix block the pores, suppressing Zn dissolution both physically and chemically; and in the CPM, the multilayered, conductive but physically obstructive structure formed by the MXene layers further extends the diffusion path and reduces the access of pitting-initiating Cl−. From a biomaterial coating perspective, the numerical EIS data indicates that chitosan–pistachio shell-based coatings (especially CP and CPM) significantly protect as physical barrier zinc surface against corrosion, and the combination of potential antimicrobial benefits with the extra diffusion barrier provided by MXene is promising for biomedical surface applications. However, further in vitro and in vivo tests are needed regarding long-term stability, surface oxidation, potential -F residues, and biocompatibility.
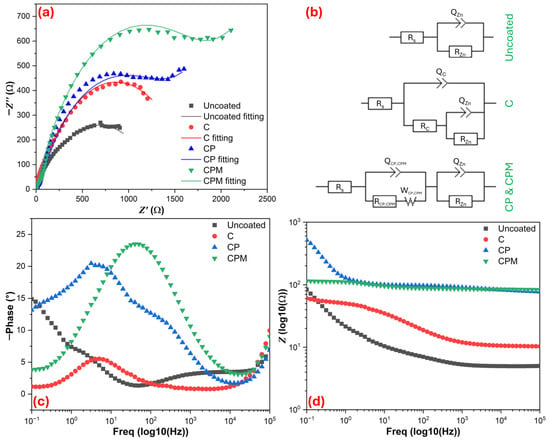
Figure 7.
Electrochemical impedance spectroscopy results for samples; (a) Nyquist curves, (b) proposed equivalent circuit diagrams for Nyquist curves, (c,d) Bode plots.

Table 2.
Parameters for equivalent circuit elements from EIS Nyquist fittings.
The CA test results for the samples are presented visually in Figure 8, and these results are of significant importance during potential implant-tissue interactions [50]. When the contact angle images provided in the figure are examined, the surface wettability properties of the coatings and, consequently, their hydrophilic-hydrophobic character are clearly revealed. The contact angle measured on the uncoated Zn surface (Figure 8a) was 77.51°, indicating that the surface exhibits a moderately hydrophobic character. In coating C (Figure 8b), the contact angle decreased to 45.91°, indicating that the surface became more hydrophilic; this suggests increased interaction with the SBF solution due to the hydrophilic functional groups of chitosan [51]. The decrease in the contact angle to 39.82° in the CP coating (Figure 8c) indicates that the surface wettability is further decreased and the potential for interaction with biological fluids is still limited. The lowest contact angle was measured on the CPM coating (Figure 8d) at 19.26°. This value indicates that the surface has become extremely hydrophilic and exhibits strong wettability with SBF fluid. This significant increase in surface hydrophilicity is extremely important, especially in terms of protein adsorption, cell adhesion, and biocompatibility [52]. The addition of Nb2CTx MXene, surface function feature, resulting in a surface property that is highly advantageous for osteointegration and tissue compatibility in biomedical applications [53,54]. Overall, the contact angle results clearly show a systematic increase in surface hydrophilicity depending on the coating type, with the CPM coating having the highest potential for interaction with biological fluids.
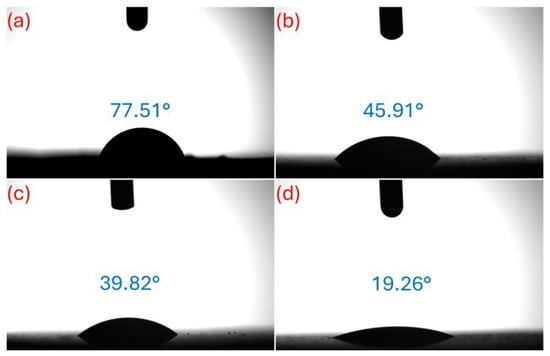
Figure 8.
CA test results of samples; (a) uncoated, (b) C, (c) CP, and (d) CPM.
Figure 9 shows the SEM surface morphologies of the samples after 7 days of immersion in SBF at 37 °C. Surface morphology studies reveal the apatite formation potential of uncoated Zn, C, CP, and CPM coatings after the SBF (Simulated Body Fluid) immersion bioactivity test. The uncoated Zn surface shown in Figure 9a exhibits a relatively irregular and rough structure after testing, with a limited number of small crystal-like deposits observed; this indicates the low biocompatibility of the pure metallic substrate and insufficient nucleation sites for apatite, as the natural oxide layer of Zn restricts bioactivity. In contrast, in Figure 9b, the C coating exhibits a more homogeneous surface morphology and shows the formation of scattered, spherical apatite layers on the surface; the amino and hydroxyl groups of Chitosan appear to have increased bioactivity by promoting the adsorption of calcium and phosphate ions, but the deposits are relatively sparse and small-scale (evident at the 5 μm bar). In Figure 9c, the CP (Chitosan–Pistachio shell) coating exhibits denser and clustered apatite crystals, with the natural porous structure of the pistachio shell contributing to this. Flower-like morphologies and increased coating thickness are observed on the surface, confirming that the organic-inorganic hybrid structure accelerates apatite mineralization by enhancing the ion exchange capacity [22]. The CPM coating in Figure 9d exhibits the most pronounced bioactivity due to the integration of the two-dimensional layered structure of Nb2CTx MXene; dense, hierarchical nanoparticle deposits and extensive apatite coating (spheres and fibrillar structures covering almost the entire surface) are observed on the surface, and the high surface area and conductivity of Nb2CTx have maximized bioactivity by catalyzing calcium phosphate nucleation. These types of morphologies can exhibit superior performance in terms of osseointegration by accelerating biological processes such as ion exchange and protein adsorption [25]. Generally, the more complex and particle-rich the surface morphology, the faster and more intense the nucleation and growth of apatite in the SBF environment. The bioactivity order of the coatings progresses as Zn < C < CP < CPM, and the addition of MXene dramatically increases surface reactivity, making it promising for orthopedic implant applications; this morphological evolution enhances the potential for in vivo osseointegration and highlights the clinical superiority of composite materials.
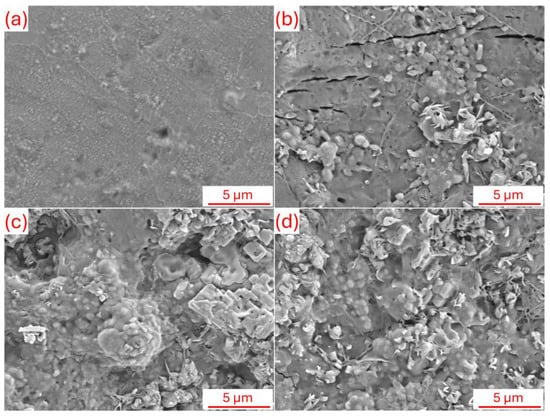
Figure 9.
SEM surface morphology after bioactivity (SBF immersion) tests; (a) uncoated, (b) C, (c) CP, and (d) CPM.
4. Conclusions
Chitosan–pistachio shell hybrid coatings reinforced with Nb2CTx MXene were applied to Zn surfaces to investigate their potential for use as biomaterial coatings, focusing on improving corrosion resistance and surface bioactivity. The findings demonstrate that the coatings provided significant improvements in both structural integrity and biological performance can be summarized as follows: (i) SEM-EDS, XRD, FTIR, HRTEM, and XPS analyzes showed that Nb2CTx MXene was homogeneously distributed within the chitosan–pistachio shell matrix and formed integrated hybrid coatings on the Zn surface. Adhesion tests (ASTM D3359: 4B) revealed that the coatings adhered to the Zn surface with high stability. (ii) CPM coating significantly reduced the degradation rate by exhibiting a corrosion rate approximately 4 times lower (0.221 mm·year−1) compared to uncoated Zn. CPM exhibited a more noble open-circuit potential (−0.4585 V), lower corrosion current density (1.48 × 10−2 A·cm−2), and higher polarization resistance (2518 Ω) compared to uncoated Zn. The hybrid coating has developed a diffusion-controlled protection mechanism by providing high load transfer resistance (≈1.25 × 103 Ω). (iii) Contact angle measurements decreased from 77.5° for uncoated Zn to 19.3° for CPM, indicating an increase in hydrophilicity. After 7 days of SBF incubation, a dense layer of apatite formed on the CPM surface and the coating’s biological activity was proven. The novelty of this work lies in the use of pistachio shell waste as a functional, eco-friendly additive in MXene-reinforced hybrid coatings, offering a sustainable approach for biomedical surface modification. This strategy not only valorizes an agricultural by-product but also expands the design space for polymer–MXene composites in biomedical applications. Future research should focus on stabilizing MXene within the polymeric matrix, optimizing coating composition and processing methods, and conducting comprehensive in vitro and in vivo studies. Overall, this work provides a foundation for environmentally friendly, high-performance coatings for biomedical metals, combining corrosion protection, bioactivity, and sustainability.
Author Contributions
Conceptualization, M.T. and F.C.T.; methodology, M.T. and F.C.T.; formal analysis, M.T. and F.C.T.; investigation, M.T. and F.C.T.; resources, M.T.; data curation, M.T. and F.C.T.; writing—original draft preparation, M.T. and F.C.T.; writing—review and editing, M.T.; visualization, M.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, Q.; Thouas, G.A. Metallic Implant Biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Topuz, M.; Akinay, Y.; Karatas, E.; Cetin, T. Ti3C2Tx MXene-Functionalized Hydroxyapatite/Halloysite Nanotube Filled Poly– (Lactic Acid) Coatings on Magnesium: In Vitro and Antibacterial Applications. J. Magnes. Alloys 2024, 12, 3758–3771. [Google Scholar] [CrossRef]
- Liu, Y.; Du, T.; Qiao, A.; Mu, Y.; Yang, H. Zinc-Based Biodegradable Materials for Orthopaedic Internal Fixation. J. Funct. Biomater. 2022, 13, 164. [Google Scholar] [CrossRef] [PubMed]
- Pesode, P.; Barve, S. Surface Modification of Biodegradable Zinc Alloy for Biomedical Applications. Bionanoscience 2023, 13, 1381–1398. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. In-vitro corrosion and bioactivity behavior of tailored calcium phosphate- containing zinc oxide coating prepared by plasma electrolytic oxidation. Corros. Sci. 2020, 173, 108781. [Google Scholar] [CrossRef]
- Szőke, Á.F.; Szabó, G.; Simó, Z.; Hórvölgyi, Z.; Albert, E.; Végh, A.G.; Zimányi, L.; Muresan, L.M. Chitosan Coatings Ionically Cross-Linked with Ammonium Paratungstate as Anticorrosive Coatings for Zinc. Eur. Polym. J. 2019, 118, 205–212. [Google Scholar] [CrossRef]
- Pozzo, L.d.Y.; da Conceição, T.F.; Spinelli, A.; Scharnagl, N.; Pires, A.T.N. Chitosan Coatings Crosslinked with Genipin for Corrosion Protection of AZ31 Magnesium Alloy Sheets. Carbohydr. Polym. 2018, 181, 71–77. [Google Scholar] [CrossRef]
- Szőke, Á.F.; Szabó, G.S.; Hórvölgyi, Z.; Albert, E.; Gaina, L.; Muresan, L.M. Eco-Friendly Indigo Carmine-Loaded Chitosan Coatings for Improved Anti-Corrosion Protection of Zinc Substrates. Carbohydr. Polym. 2019, 215, 63–72. [Google Scholar] [CrossRef]
- Akinay, Y.; Topuz, M.; Gunes, U.; Gokdemir, M.E.; Cetin, T. Review: Recent Developments in 2D MXene-Filled Bioinspired Composites for Biomedical Applications. J. Mater. Sci. 2025, 60, 10670–10699. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, Y.; Chen, Y.; Wang, J.; Huang, C.; Yang, H.; Wang, Q.; Bai, Y.; Li, P.; Gu, X.; et al. Fabrication of Nb2C MXene Coated Zinc for Improved Degradation, Antibacterial Activity, and Osteogenesis for Guided Bone Regeneration Applications. J. Mater. Sci. Technol. 2025, 237, 201–218. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Hart, P.; Scarpa, F.; Thakur, V.K. Sustainable MXene-Chitosan/Chitin Composites for Interdisciplinary Applications in Water Purification, Bio-Medical, Bio-Sensing and Electronic Fields. Mater. Today Sustain. 2024, 25, 100671. [Google Scholar] [CrossRef]
- Akinay, Y.; Karatas, E.; Ruzgar, D.; Akbari, A.; Baskin, D.; Cetin, T.; Kazici, H.C.; Topuz, M. Cytotoxicity and Antibacterial Activity of Polyhedral Oligomeric Silsesquioxane Modified Ti3C2Tx MXene Films. Sci. Rep. 2025, 15, 8463. [Google Scholar] [CrossRef] [PubMed]
- Topuz, M.; Karatas, E.; Ruzgar, D.; Akinay, Y.; Cetin, T. Ti3C2Tx MXene/Halloysite Nanotube Functionalized Films for Antibacterial Applications. J. Biomater. Sci. Polym. Ed. 2025, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, H.; Tang, A.; Lu, X.; Yuan, N.; Ding, J. Ti3C2Tx-Based Composite Coating on AZ31B Mg Alloy Surface for Improved Anti-Corrosion/Wear-Reducing Properties. Mater. Today Commun. 2023, 35, 105664. [Google Scholar] [CrossRef]
- Darmenbayeva, A.; Zhussipnazarova, G.; Rajasekharan, R.; Massalimova, B.; Zharlykapova, R.; Nurlybayeva, A.; Mukazhanova, Z.; Aubakirova, G.; Begenova, B.; Manapova, S.; et al. Applications and Advantages of Cellulose–Chitosan Biocomposites: Sustainable Alternatives for Reducing Plastic Dependency. Polymers 2024, 17, 23. [Google Scholar] [CrossRef]
- Barczewski, M.; Matykiewicz, D.; Krygier, A.; Andrzejewski, J.; Skórczewska, K. Characterization of Poly(Lactic Acid) Biocomposites Filled with Chestnut Shell Waste. J. Mater. Cycles Waste Manag. 2018, 20, 914–924. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Balasundar, P.; Narayanasamy, P.; Senthil, S.; Abdullah Al-Dhabi, N.; Prithivirajan, R.; Shyam Kumar, R.; Ramkumar, T.; Subrahmanya Bhat, K. Physico-Chemical Study of Pistachio (Pistacia Vera) Nutshell Particles as a Bio-Filler for Eco-Friendly Composites. Mater. Res. Express 2019, 6, 105339. [Google Scholar] [CrossRef]
- Altun, M.; Celebi, M.; Ovali, S. Preparation of the Pistachio Shell Reinforced PLA Biocomposites: Effect of Filler Treatment and PLA Maleation. J. Thermoplast. Compos. Mater. 2022, 35, 1342–1357. [Google Scholar] [CrossRef]
- Balart, J.F.; García-Sanoguera, D.; Balart, R.; Boronat, T.; Sánchez-Nacher, L. Manufacturing and Properties of Biobased Thermoplastic Composites from Poly(Lactid Acid) and Hazelnut Shell Wastes. Polym. Compos. 2018, 39, 848–857. [Google Scholar] [CrossRef]
- Topuz, M.; Topuz, F.C.; Dikici, B.; Seifzadeh, D. Sustainable Walnut Shell-Filled Polylactic Acid–Hydroxyapatite Hybrid Coatings for Enhanced Corrosion Resistance and Bioactivity of Magnesium Biomaterials. J. Appl. Polym. Sci. 2025, 142, e57321. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Banat, F.; Hasan, S.W. Recent Advances in Niobium MXenes: Synthesis, Properties, and Emerging Applications. Matter 2022, 5, 546–572. [Google Scholar] [CrossRef]
- ASTM D3359-09; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2012.
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Yang, Y.; Boccaccini, A.R. A New Strategy for Developing Chitosan Conversion Coating on Magnesium Substrates for Orthopedic Implants. Appl. Surf. Sci. 2019, 466, 854–862. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Killion, J.A.; Lyons, J.G.; Higginbotham, C.L.; Devine, D.M. Halloysite Nanotube Reinforced Polylactic Acid Composite. Polym. Compos. 2017, 38, 2166–2173. [Google Scholar] [CrossRef]
- Vourlias, G. Application of X-Rays Diffraction for Identifying Thin Oxide Surface Layers on Zinc Coatings. Coatings 2020, 10, 1005. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of Chitosan Characteristics on Polymer Properties. I: Crystallographic Properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Flores-Hernandez, C.G.; Del Real, A.; Cornejo-Villegas, M.d.L.Á.; Millán-Malo, B.; Fonseca-Hernández, G.A.; Rivera-Armenta, J.L. Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite. Polymers 2025, 17, 1907. [Google Scholar] [CrossRef]
- Ahmed, J.; Farouk, M.; El-Aassar, M.R.; Omran, K.A.; Mohamed, F.M. Fabricating a Pistachio Nut Shells Activated Carbon + CNTs @ Chitosan (PAC-CNTs @Cs) Composite for Pesticides Attenuation from Agricultural Wastewater. Desalination Water Treat. 2024, 320, 100899. [Google Scholar] [CrossRef]
- Gul, S.; Serna, M.I.; Zahra, S.A.; Arif, N.; Iqbal, M.; Akinwande, D.; Rizwan, S. Un-Doped and Er-Adsorbed Layered Nb2C MXene for Efficient Hydrazine Sensing Application. Surf. Interfaces 2021, 24, 101074. [Google Scholar] [CrossRef]
- Din Babar, Z.U.; Fatheema, J.; Arif, N.; Anwar, M.S.; Gul, S.; Iqbal, M.; Rizwan, S. Magnetic Phase Transition from Paramagnetic in Nb2AlC-MAX to Superconductivity-like Diamagnetic in Nb2C-MXene: An Experimental and Computational Analysis. RSC Adv. 2020, 10, 25669–25678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Naleway, S.E.; Wang, B. Biological and Bioinspired Materials: Structure Leading to Functional and Mechanical Performance. Bioact. Mater. 2020, 5, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Ryu, J.W.; Jeon, H.J.; Mishra, R.K.; Choi, Y.; Gwag, J.S. Emerging Role of Nb2CTx MXene in Sensors: The Roadmap from Synthesis to Health and Environmental Monitoring. Sensors 2025, 25, 3691. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wen, J.; Zhao, J.; Ma, X.; Gao, H.; Zhang, X. A Safe Etching Route to Synthesize Highly Crystalline Nb2CTx MXene for High Performance Asymmetric Supercapacitor Applications. Electrochim. Acta 2020, 337, 135803. [Google Scholar] [CrossRef]
- Wu, L.; Fan, T.; Wei, S.; Xu, Y.; Zhang, Y.; Ma, D.; Shu, Y.; Xiang, Y.; Liu, J.; Li, J.; et al. All-Optical Logic Devices Based on Black Arsenic–Phosphorus with Strong Nonlinear Optical Response and High Stability. Opto-Electron. Adv. 2022, 5, 200046. [Google Scholar] [CrossRef]
- Dong, H.; Xiao, P.; Jin, N.; Wang, B.; Liu, Y.; Lin, Z. Molten Salt Derived Nb2CTx MXene Anode for Li-ion Batteries. ChemElectroChem 2021, 8, 957–962. [Google Scholar] [CrossRef]
- Mozafari, M.; Soroush, M. Surface Functionalization of MXenes. Mater. Adv. 2021, 2, 7277–7307. [Google Scholar] [CrossRef]
- Baskar, S.; Subbiah, G.; Guntaj, J.; Kumar, M.; Kaliappan, N. Development and Characterization of an Epoxy Matrix Composite Reinforced with Al2O3 Embedded Banyan Fibers for Secondary Structural Applications. Results Eng. 2025, 26, 105587. [Google Scholar] [CrossRef]
- Suriani, M.J.; Rapi, H.Z.; Ilyas, R.A.; Petrů, M.; Sapuan, S.M. Delamination and Manufacturing Defects in Natural Fiber-Reinforced Hybrid Composite: A Review. Polymers 2021, 13, 1323. [Google Scholar] [CrossRef]
- Li, P.; Qian, J.; Zhang, W.; Schille, C.; Schweizer, E.; Heiss, A.; Klotz, U.E.; Scheideler, L.; Wan, G.; Geis-Gerstorfer, J. Improved biodegradability of zinc and its alloys by sandblasting treatment. Surf. Coat. Technol. 2021, 405, 126678. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, A.A. MXene-Embedded Electrospun Polymeric Nanofibers for Biomedical Applications: Recent Advances. Micromachines 2023, 14, 1477. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Xiao, T.; Cong, L.; Ling, Y.; Lu, Z.; Fukushima, C.; Tsuchitori, I.; Bazzaoui, M. Using Atomic Force Microscopy to Measure Thickness of Passive Film on Stainless Steel Immersed in Aqueous Solution. Sci. Rep. 2019, 9, 13094. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, A.A.; Sánchez-Duarte, R.G.; Orozco-Carmona, V.M.; Devora-Isiordia, G.E.; Villegas-Peralta, Y.; Álvarez-Sánchez, J. Chitosan and Its Derivatives as a Barrier Anti-Corrosive Coating of 304 Stainless Steel against Corrosion in 3.5% Sodium Chloride Solution. Coatings 2024, 14, 1244. [Google Scholar] [CrossRef]
- Khalaj, G.; Pouraliakbar, H.; Arab, N.; Nazerfakhari, M. Correlation of Passivation Current Density and Potential by Using Chemical Composition and Corrosion Cell Characteristics in HSLA Steels. Measurement 2015, 75, 5–11. [Google Scholar] [CrossRef]
- Venkatraman, M.S.; Cole, I.S.; Emmanuel, B. Corrosion under a Porous Layer: A Porous Electrode Model and Its Implications for Self-Repair. Electrochim. Acta 2011, 56, 8192–8203. [Google Scholar] [CrossRef]
- Singh, M.B.; Gabriel, B.I.; Venkatraman, M.S.; Cole, I.S.; Moorthy, C.G.; Emmanuel, B. Theory of Impedance for Initial Corrosion of Metals under a Thin Electrolyte Layer: A Coupled Charge Transfer-Diffusion Model. J. Chem. Sci. 2022, 134, 32. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Wang, S.; Liu, C.; Shi, H.; Liang, L.; Pi, K. Surface Functionalization of Ti3C2Tx and Its Application in Aqueous Polymer Nanocomposites for Reinforcing Corrosion Protection. Compos. Part B Eng. 2021, 217, 108900. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The Impact of Contact Angle on the Biocompatibility of Biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Ying, W.S.; Razzaghi, M.; Sharif, S.; Ismail, A.F.; Berto, F. Improved Bacteriostatic and Anticorrosion Effects of Polycaprolactone/Chitosan Coated Magnesium via Incorporation of Zinc Oxide. Materials 2021, 14, 1930. [Google Scholar] [CrossRef]
- Sutthiwanjampa, C.; Hong, S.; Kim, W.J.; Kang, S.H.; Park, H. Hydrophilic Modification Strategies to Enhance the Surface Biocompatibility of Poly(Dimethylsiloxane)-Based Biomaterials for Medical Applications. Adv. Mater. Interfaces 2023, 10, 2202333. [Google Scholar] [CrossRef]
- Gayathri, V.G.; Richard, B.; Chacko, J.T.; Bayry, J.; Abdul Rasheed, P. Non-Ti MXenes: New Biocompatible and Biodegradable Candidates for Biomedical Applications. J. Mater. Chem. B 2024, 13, 1212–1228. [Google Scholar] [CrossRef]
- Saxena, A.; Tyagi, A.; Vats, S.; Gupta, I.; Gupta, A.; Kaur, R.; Tiwary, S.K.; Elzatahry, A.A.; Singh, M.; Karim, A. MXene-Integrated Composites for Biomedical Applications: Synthesis, Cancer Diagnosis, and Emerging Frontiers. Small Sci. 2025, 5, 2400492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).