1. Introduction
Magnesium alloys are increasingly considered promising biodegradable implants materials, owing to their appropriate mechanical properties, natural biocompatibility, and gradual degradability within physiological environments [
1,
2,
3]. However, it is difficult to control their degradation behavior. Excessive corrosion in bodily fluids often leads to premature degradation of mechanical properties and uncontrolled hydrogen release, hindering its clinical application. To address this challenge, various surface modification techniques have been developed, among which micro-arc oxidation (MAO) has gained particular attention [
4,
5,
6,
7]. Compared to bare alloys, the MAO coating forms an in situ ceramic oxide layer on the magnesium substrate, significantly reducing the corrosion rate. Yet, the intrinsic formation of micro-pores and cracks during MAO processing creates channels for corrosive, limiting long-term protection [
8,
9,
10,
11,
12].
Researchers have focused on implementing pore-sealing treatments on MAO coating surfaces to augment corrosion resistance, such as multi-stage immersion sealing, polymer infiltration, poly(L-lactic acid) (PLLA), Layer-by-layer (LBL) self-assembly, sol–gel, and electrophoretic deposition (EPD) [
13,
14,
15,
16,
17,
18]. The corrosion resistance enhancement efficacy of these sealing methods critically depends on the thickness, integrity, and interfacial quality of the sealing layer. Insufficient thickness fails to establish continuous barrier protection, while excessive thickness induces stress-driven delamination or cytotoxicity risks [
9,
10,
11].
In recent years, high-intensity pulsed ion beam (HIPIB) has been introduced as a novel modification strategy for MAO coatings. The ultrafast deposition of energy produces transient surface melting and resolidification, sealing microstructural defects and forming a denser surface layer. A growing body of studies has shown that HIPIB can markedly enhance the corrosion resistance of MAO coatings. Han et al. [
19] employed HIPIB with intensities ranging from 100 to 350 A/cm
2 to improve the corrosion resistance of the MAO coating. The results showed that the surface of the MAO coatings melts and forms a remelted layer by HIPIB, and localized ablation occurs at higher irradiation intensities, which reduces the overall thickness of the MAO coatings once the ablation threshold is surpassed. Shao et al. [
20] developed the temperature and stress fields for MAO coatings by HIPIB to investigate the relationship between energy density, remelting, and ablation. The findings revealed that the surface temperature of the MAO coating reached its melting point at 4 J/cm
2, causing it to melt. The polarization curve results showed HIPIB can enhance the corrosion resistance, and the best corrosion resistance was observed at an energy density of 4 J/cm
2. Our previous studies [
21] have reported that HIPIB also improves biological performance, including higher cell viability, enhanced osteogenic activity, and even antibacterial effects. Its findings suggest that HIPIB not only extends the service life of magnesium implants but also promotes favorable biological responses at the tissue-implant interface.
Despite these promising outcomes, the mechanism underlying the biological properties of HIPIB-modified MAO coatings remains unclear. Therefore, this study aims to systematically investigate the effect of HIPIB irradiation on MAO coatings with particular emphasis on the mechanism of biological performance improvement and reveal the links between irradiation-induced surface modifications and cellular responses. The findings are anticipated to shed light on the underlying mechanisms, facilitating the advancement of magnesium alloys as dependable biodegradable implants.
2. Materials and Methods
2.1. Production of MAO Coatings
The MAO coatings were fabricated on AZ31 magnesium alloy substrates (25 mm × 25 mm × 5 mm) using a 20 kW MAO power supply, with the AZ31 alloy serving as the anode and stainless steel as the cathode. The MAO process was conducted under the following parameters: positive voltage of 360 V, negative voltage of 60 V, duty cycle of 30%, and frequency of 800 Hz. The electrolyte consisted of sodium phosphate (Na
3PO
4, 10 g/L), potassium hydroxide (KOH, 2 g/L), potassium fluoride (KF, 4 g/L), and HA (Ca
5(PO
4)
3(OH), 4 g/L). After oxidation, the specimens were thoroughly rinsed with deionized water, dried, and stored for subsequent use [
20].
2.2. HIPIB Irradiation Process
The MAO coatings on magnesium alloy were irradiated under an energy density of 4 J/cm2 with 2 shots by a TEMP-6 HIPIB system. The ion beam, composed primarily of carbon ions, was accelerated at voltages ranging from 200 to 250 kV with a pulse width of 75 ns. The irradiation was carried out over an area of 1 cm2 in a vacuum chamber at a pressure below 10−2 Pa.
2.3. Surface Characterization and Hydrogen Evolution Test
The surface and cross-sectional morphologies of the modified coatings were characterized by a SUPRA55 SAPPHIRE scanning electron microscope (SEM, ZEISS, Oberkochen, Germany). The phase structure was analyzed by grazing-incidence XRD (Cu Kα, 2°). The hydrogen gas release (n = 3) during the degradation of MAO coatings in simulated body fluid (SBF) was measured by using a GC7900 type gas chromatography. Sampling was performed every 24 h, with a total of 12 measurements conducted over the experimental period for each sample. The wettability of the coatings was assessed using a water contact angle meter (LSA MOB-M). A 5 μL droplet of deionized water was placed at 3 randomly selected positions on each surface, and 3 measurements were recorded at each position. The mean contact angle value was calculated to represent the wettability of the sample. Potentiodynamic polarization curves were recorded using an electrochemical workstation (CHI660E, Chenhua, Shanghai, China) to evaluate the corrosion resistance. A conventional three-electrode system was employed, with the sample (exposed area: 1.00 cm2) as the working electrode, a platinum sheet (area: 1.00 cm2) as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The scan rate for the polarization curves was 5 mV/s. The electrochemical measurements and hydrogen evolution experiments were conducted in SBF, with both the solution maintained at 37 °C to simulate physiological conditions.
2.4. Cell Culture and Observation by SEM
MC3T3-E1 pre-osteoblastic cells were maintained in α-MEM supplemented with 10% fetal bovine serum (FBS, Gibco, Billings, MT, USA) and 1% penicillin-streptomycin (Beyotime) at 37 °C under 5% CO2. Cells were passaged at 80-90% confluence and seeded into 6-well plates at a density of 1 × 105 cells/well 24 h prior to treatment. Experimental groups included a blank control (untreated cells), a pristine sample group, and an irradiated sample group. Stock solutions of test materials were diluted in complete medium to working concentrations and applied to the cells. Samples were harvested at 1, 2, and 3 days post-treatment for subsequent analyses. The cells were cultured on the material for 1 day, and then the samples were processed as follows: First, the cells were washed three times with phosphate-buffered saline (PBS) and fixed with 3% glutaraldehyde for 3 h. Subsequently, fixed samples were rinsed once with distilled water to remove residual fixative. And then a graded ethanol series (50%, 70%, 90%, and 100% ethanol) was used for dehydration, with each concentration applied for 15 min. After dehydration, a small amount of hexamethyldisilazane (HMDS) was added to the samples, which were then left undisturbed in a fume hood overnight to air-dry. The dried samples were sputter-coated with a thin layer of gold to enhance conductivity for SEM observation.
2.5. Cell Viability Assay (CCK-8)
Cell viability was evaluated using the CCK-8 kit (Beyotime). Following treatment, cells were washed twice with PBS, and a mixture of fresh medium and CCK-8 reagent (10:1 v/v) was added to each well (100 μL/well). After 2 h of incubation at 37 °C, absorbance was measured at 450 nm using a microplate reader (BioTek Synergy H1). Each experiment was performed in triplicate.
Cell viability (%) was calculated as shown in Formula (1):
2.6. Cell Cycle Analysis by Flow Cytometry
For cell cycle profiling, cells were trypsinized, fixed in 70% ethanol at 4 °C for ≥2 h, and stained with propidium iodide (PI)/RNase A solution (BD Biosciences; 10 μg/mL PI, 100 μg/mL RNase A) for 30 min at 37 °C. Filtered cell suspensions were analyzed using a flow cytometer (BD FACSCanto II, Franklin Lakes, NJ, USA), and phase distributions (G0/G1, S, G2/M) were quantified with FlowJo software (v10.8.1). All experiments were performed in triplicate, and data are expressed as mean ± SD. Statistical significance was determined by one-way ANOVA with Tukey’s post hoc test (GraphPad Prism 9.0; p < 0.05).
2.7. Alkaline Phosphatase (ALP) Activity Assay
An alkaline phosphatase activity assay (Beyotime, Shanghai, China) was employed to determine ALP levels in both pristine and irradiated MAO coatings. On days 7 and 14 of culture, the cell samples were rinsed twice with PBS and stored at −80 °C overnight. They were subsequently lysed with 0.2% Triton X-100, followed by incubation at 4 °C for 40 min. The lysates were centrifuged at 2500× g for 5 min at 4 °C, and 50 μL of p-nitrophenyl phosphate disodium (p-Npp) was added to an equal volume of the collected supernatant for a 30 min reaction. The reaction was terminated with 100 μL of 2 N NaOH. Afterwards, 100 μL aliquots of the final mixture were transferred into 96-well plates, and absorbance was recorded at 405 nm using a microplate reader (Elx800, BioTek, Winooski, VT, USA). Protein concentration, determined with a BCA protein assay kit (Pierce, Thermo Scientific, Waltham, MA, USA), was used to normalize ALP activity. Each experiment was repeated 3 times.
3. Results
3.1. Microstructural Characterization
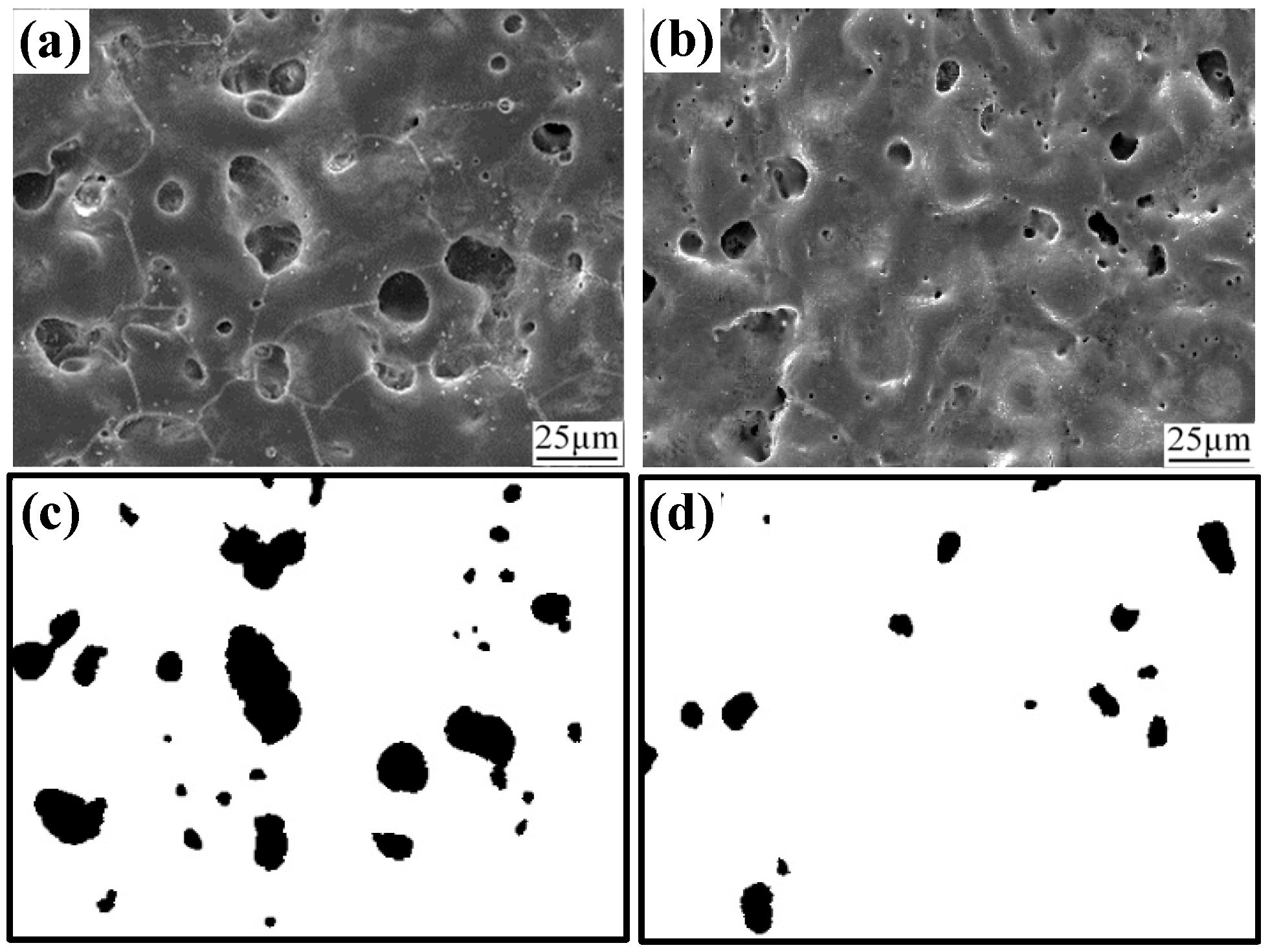
Figure 1 presents the surface characteristics of pristine and irradiated MAO coatings. The pristine MAO coating exhibits numerous pores and micro-cracks of varying size, with a porosity of 10.21%. After HIPIB irradiation, both the number and size of surface pores were markedly reduced, resulting in a decreased porosity of 2.77%. These structural and compositional changes originate from the unique physical effects associated with HIPIB. The ultra-high energy deposition within nanoseconds produces localized surface melting of the MAO layer. The ion-beam impact further generates dynamic perturbations within the molten pool, which promote the lateral flow and diffusion of molten oxides into adjacent pores and micro-cracks. This rapid melt–flow–solidification cycle effectively seals the structural defects and yields a denser remelting layer.
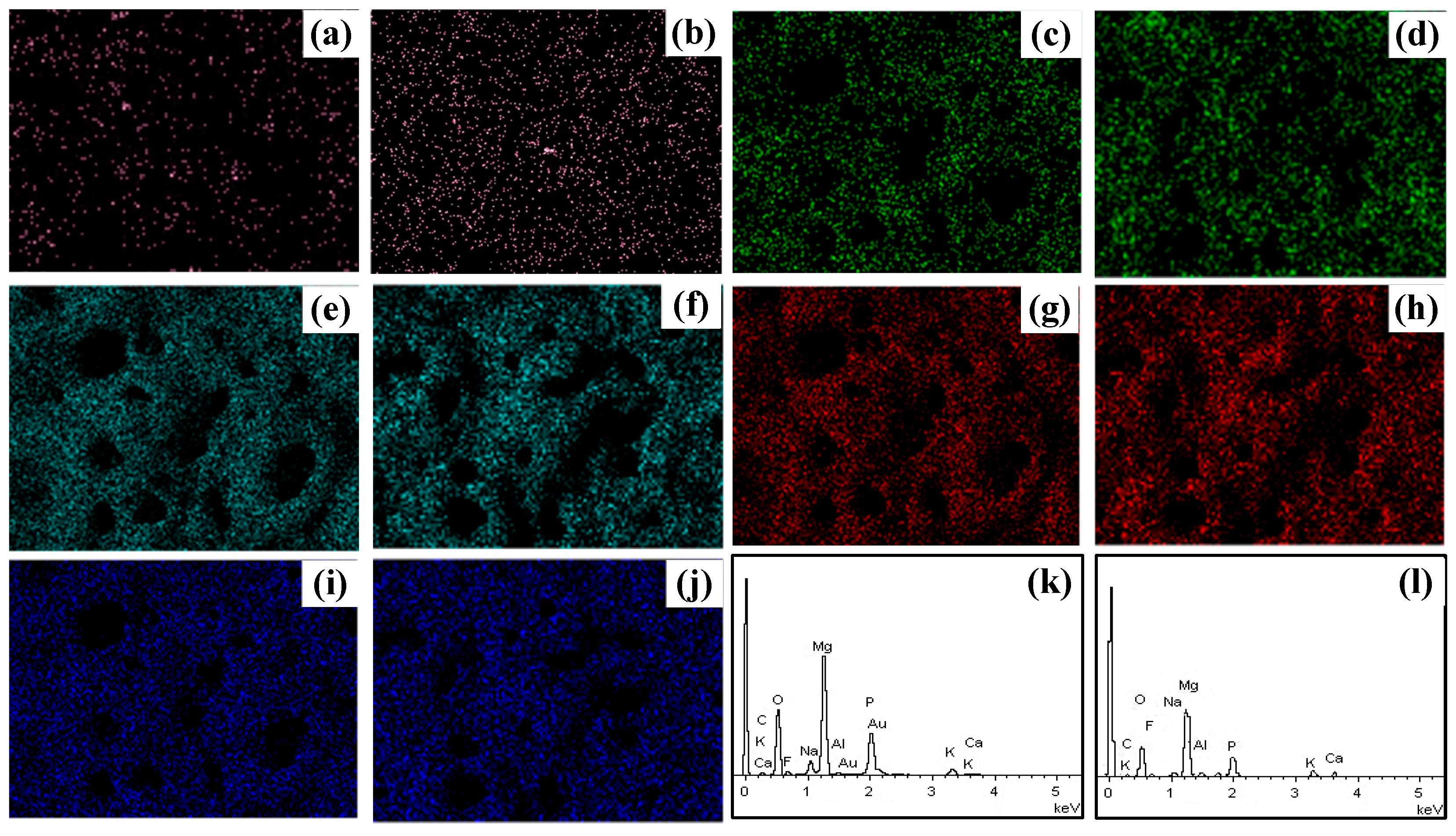
Figure 2 shows the EDS of the pristine and irradiated coating surfaces. The EDS results of the pristine and irradiated MAO coatings show that the contents of Mg, O, Na, and P remained relatively unchanged before and after irradiation, whereas the Ca content increased significantly. The original MAO film exhibited a relatively low Ca content of only 0.08 at.%, while the Ca content increased to 0.31 at.% by HIPIB irradiation. This phenomenon can be attributed to the selective ablation effect induced by HIPIB and promotes the migration of Ca towards the outermost layer of the film, resulting in a higher concentration of Ca at the surface.
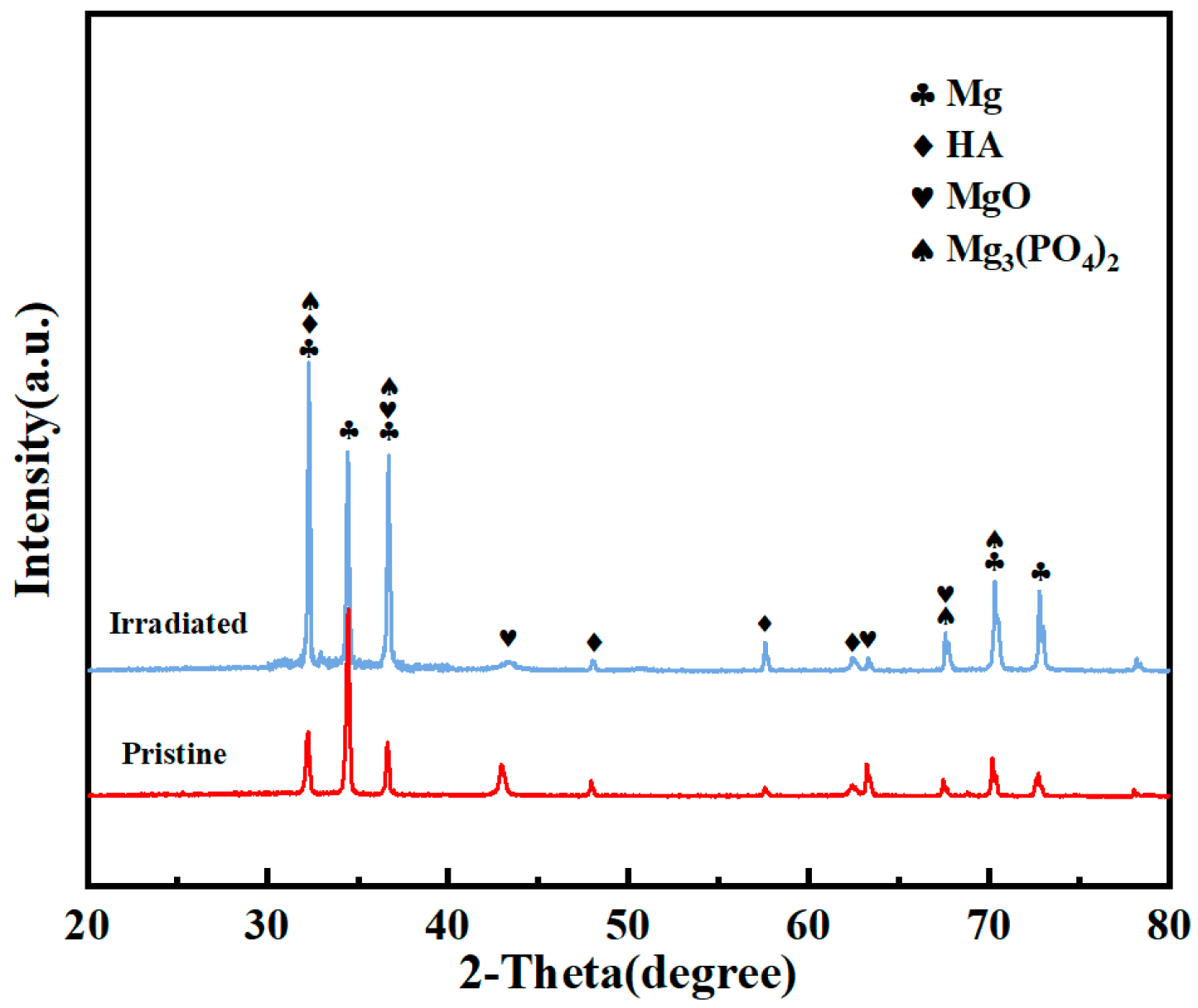
Figure 3 presents the X-ray diffraction pattern of the pristine and irradiated MAO coatings. The phase composition of the irradiated coating remains largely unchanged compared to the pristine MAO coating. The phase structure of the irradiated MAO coating primarily consists of MgO, Mg
3(PO
4)
2, and hydroxyapatite. The presence of these phases suggests that the irradiation process does not significantly alter the fundamental crystalline structure of the MAO coating. MgO, as a stable oxide phase, serves as a protective layer, while Mg
3(PO
4)
2 and HA contribute to the bioactive properties of the coating.
3.2. Polarization Curve
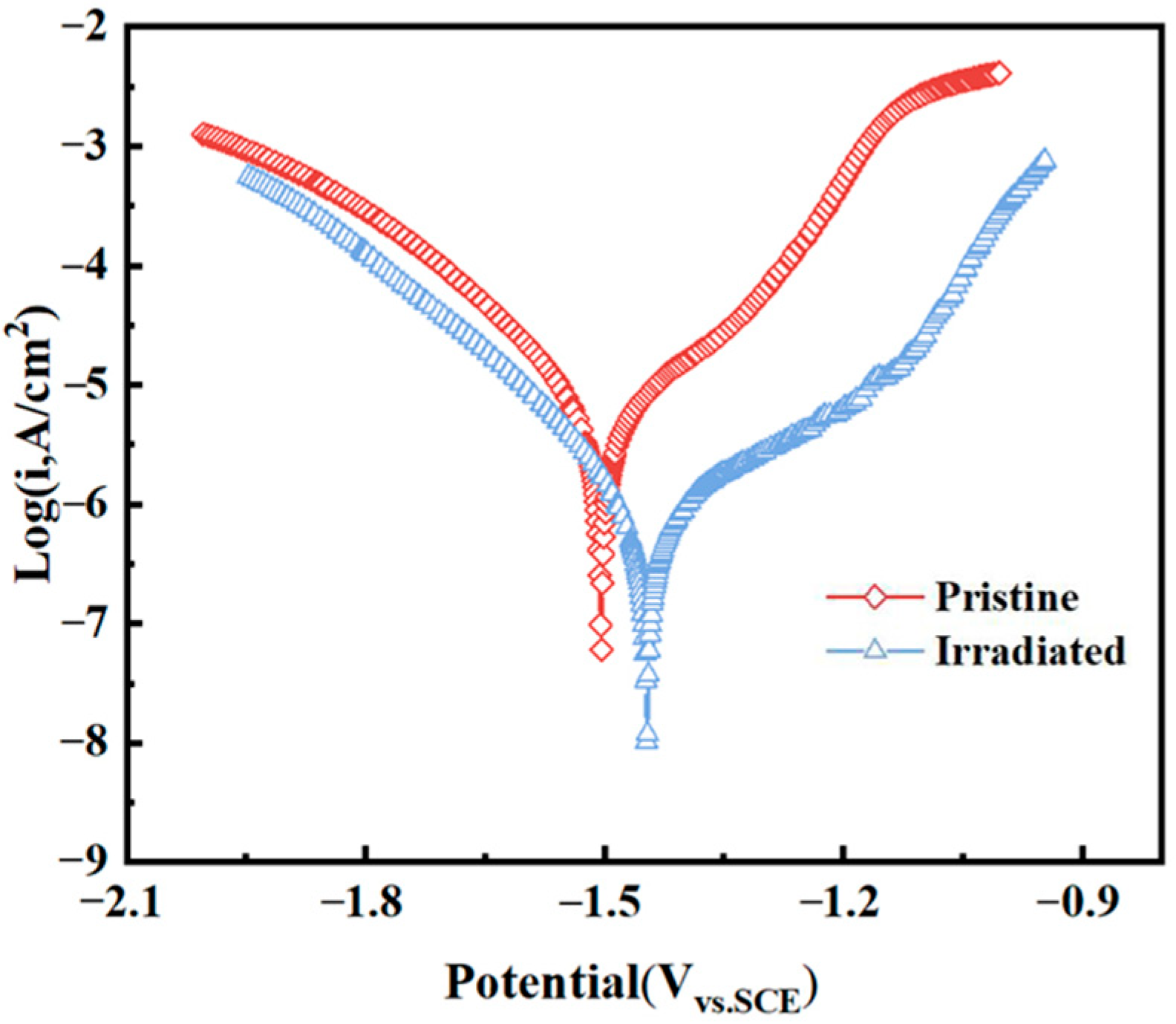
Figure 4 shows the potentiodynamic polarization curve for pristine and irradiated MAO coatings at 4 J/cm
2 with 2 shots in SBF solution. It can be observed that the pristine MAO coating undergoes a typical active-dissolution process. After HIPIB irradiation, the corrosion mode shifts to pitting with a distinct passivation stage. The corrosion potential increases from −1.504 V to −1.447 V, the corrosion current density decreases to 7.89 × 10
−7 A·cm
−2, and a clear passive region emerges. Passivation breakdown occurs at −1.132 V with a breakdown current density of 1.68 × 10
−6 A·cm
−2. HIPIB irradiation partially remelts and rapidly resolidifies the outer MAO layer, healing microcracks and pores and producing a denser, more uniform remelting layer. These structural refinements promote the formation and stability of a protective passive layer, which accounts for the lower corrosion current and the emergence of a passivation plateau.
3.3. Roughness and Contact Angle
Figure 5 presents the roughness and contact angle of pristine and irradiated MAO coatings. After irradiation, the surface roughness increased significantly, from an average value of 2.29 Ra for the pristine MAO coating to 7.57 Ra. Concurrently, the contact angle rose from 76.06° to 102.11°, indicating a transition of the coating surface from hydrophilic to hydrophobic. The pronounced increase in roughness is attributed to localized melting and rapid solidification by HIPIB irradiation, which produces surface undulations, contributes to the enhanced water contact angle. The densified and roughened remelted layer serves as a more effective barrier against ionic penetration, while the hydrophobic surface reduces electrolyte wetting and diminishes the effective contact area between the corrosive medium and the substrate, resulting in enhanced corrosion resistance. In addition to enhancing corrosion resistance, the synergistic modification of surface roughness and wettability is expected to improve the biological response of the MAO coating. The increased roughness provides favorable anchoring sites for cell adhesion, while moderate hydrophobicity can promote protein adsorption and regulate cell behavior.
3.4. Hydrogen Evolution Test
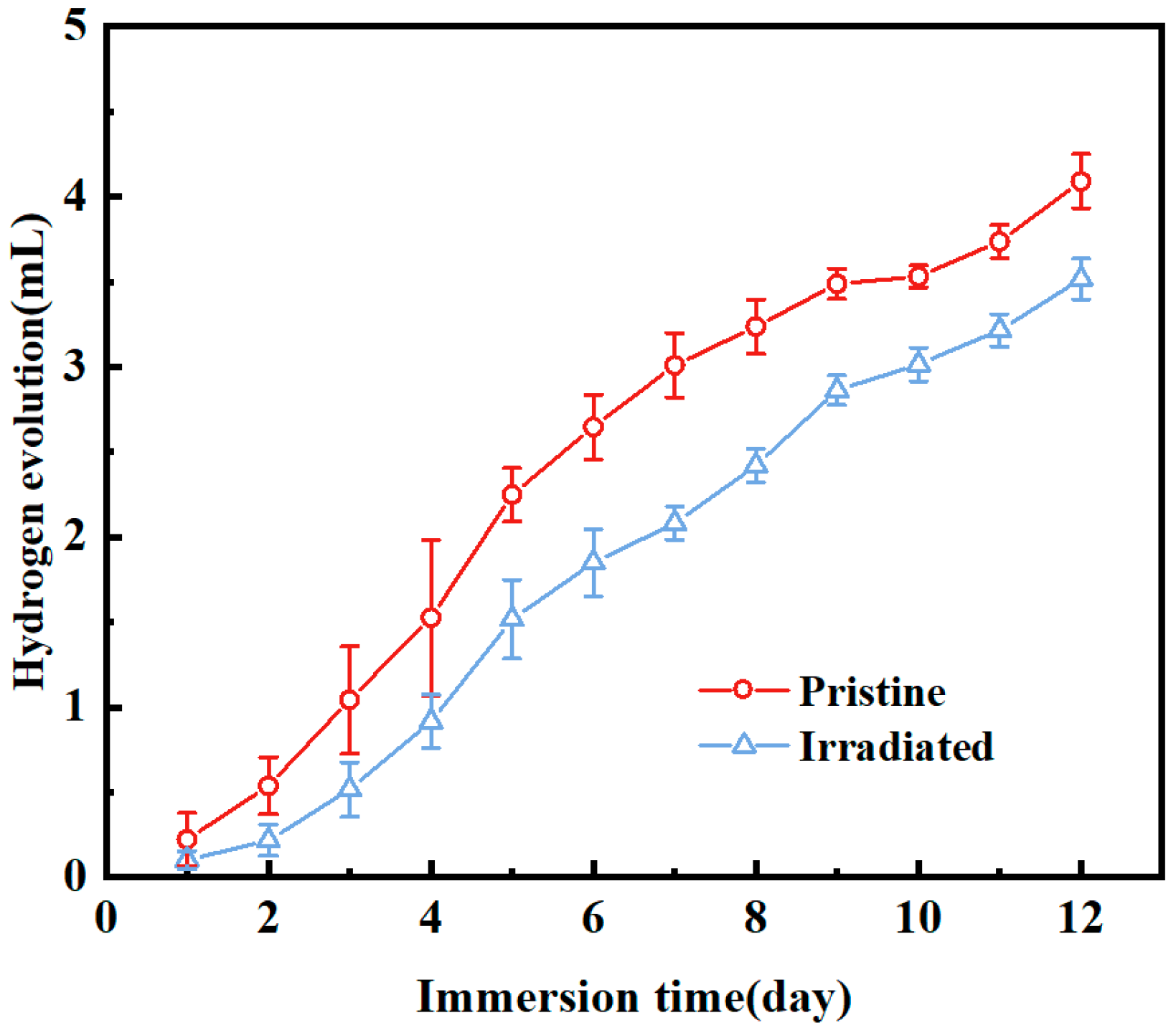
Figure 6 presents hydrogen release volume of pristine and irradiated MAO coatings immersed in SBF. Both coatings exhibited cumulative hydrogen release over time. After 24 h of immersion, the pristine MAO coating generated 0.22262 mL of hydrogen, while the irradiated sample produced only 0.10156 mL, representing a reduction of 54.3%. By 288 h, total hydrogen release amounted to 4.09074 mL for the pristine surface compared with 3.51569 mL for the irradiated one, indicating a 14% decrease. This suppression of hydrogen evolution can be attributed to the structural changes induced by irradiation, which likely improve the stability and protective capacity of the coating, thereby mitigating the potential risks of local alkalization from hydrogen accumulation under physiological conditions.
3.5. Cell Growth Morphology and Activity Growth Rate
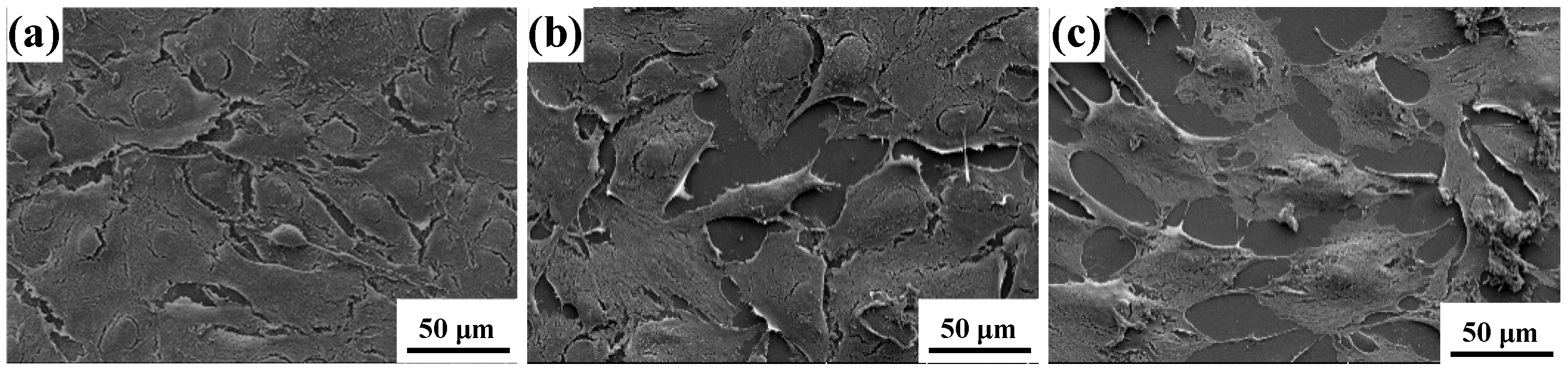
Figure 7 displays the Cell growth morphology on blank, pristine and irradiated MAO coatings. In the blank group, cells appear relatively evenly distributed; however, most of them do not establish close contact with the substrate, leaving visible gaps at the interface. This suggests that the cells remain in the early adhesion stage, with limited cytoskeletal extension. On the pristine MAO coating surface, a portion of the cells display partial spreading compared with the blank group, and cell–substrate attachment is somewhat tighter. Nonetheless, the majority of cells still retain circular outlines with noticeable gaps, indicating that the restricted surface roughness or limited chemical activity of the coating may hinder optimal cell growth and morphological development. In contrast, cells on the HIPIB-modified surface exhibit flattened, polygonal morphologies, with elongated pseudopodia and the formation of intercellular networks. The cell edges closely adhere to the substrate, showing firm anchorage. These features indicate that HIPIB irradiation effectively enhances the surface properties of the MAO coating, thereby facilitating cell spreading, strengthening adhesion, and improving overall cellular compatibility.
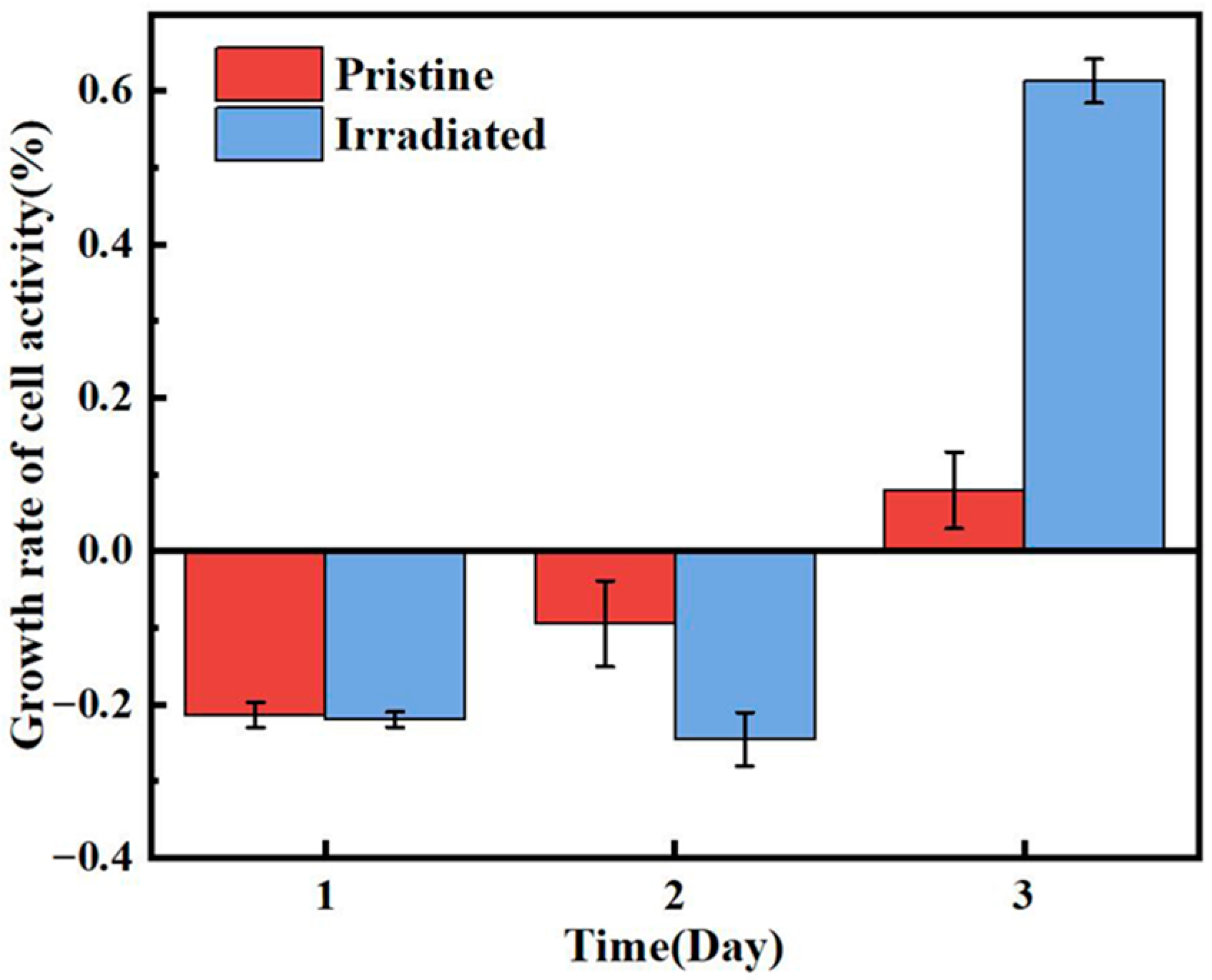
Figure 8 presents the growth rate of cell activity for pristine and irradiated MAO coatings. In the initial phase, both the pristine MAO coating and the irradiated coating exhibited negative cell growth rates, with the modified coating showing a more significant inhibitory effect on cell activity during the early period, which were −9.5% and −24.58%, respectively, on the second day. However, the HIPIB-modified coating demonstrated a significant promotion of cell proliferation on day 3, and the cell growth rate increased significantly to 61.29%, with a marked increase in cell growth rate. In contrast, the pristine MAO coating still showed limited growth, with a growth rate of only 7.9%. The initial negative growth rates can be attributed to the time required for cells to adapt to the modified surface characteristics. However, as cells adapted to the modified surface, the enhanced surface features began to facilitate better cell attachment, spreading, and proliferation, leading to the significant improvement in cell growth rate observed on Day 3 for the HIPIB-modified coating.
3.6. ALP Activity
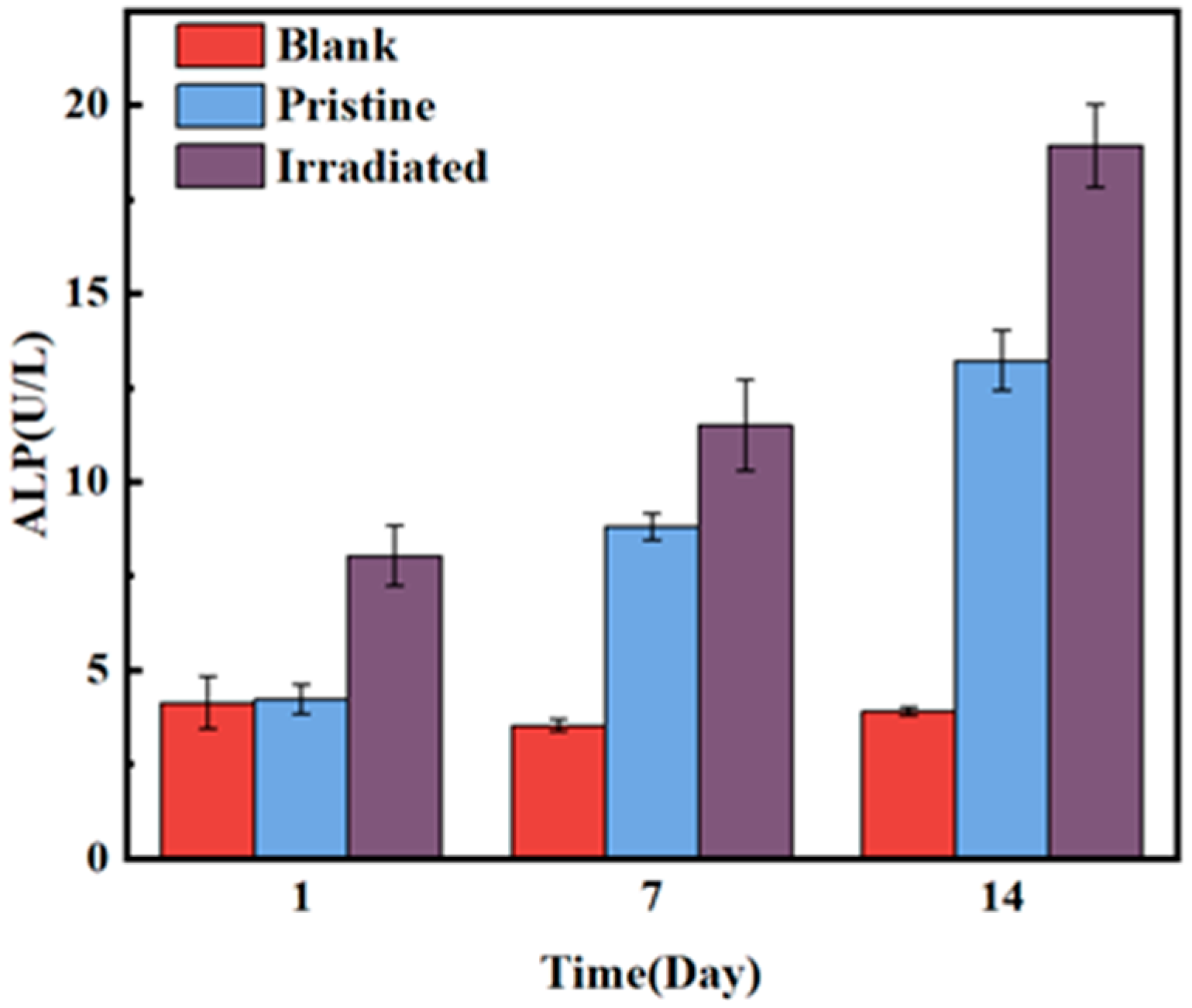
Figure 9 displays the time-dependent ALP activity for blank, pristine and irradiated MAO coatings. The ALP activity of the blank group showed minimal change over time, only 3.9 on the 14th day. In contrast, the ALP activity of both the pristine MAO coating and the HIPIB-irradiated MAO coating gradually increased over the course of the experiment. The ALP activity of the pristine MAO coating began to rise on Day 1 and reached 13.22 on Day 14, which was significantly higher than the blank group, but still lower than the irradiated group (18.91). This indicates that the irradiated MAO coating effectively promotes osteogenic differentiation. This enhancement is likely due to the increased surface roughness and hydrophobicity, which facilitated cell attachment and proliferation. Furthermore, after HIPIB irradiation, calcium accumulation occurred on the surface of the MAO coating, further enhancing osteogenic differentiation and leading to the significant increase in ALP activity.
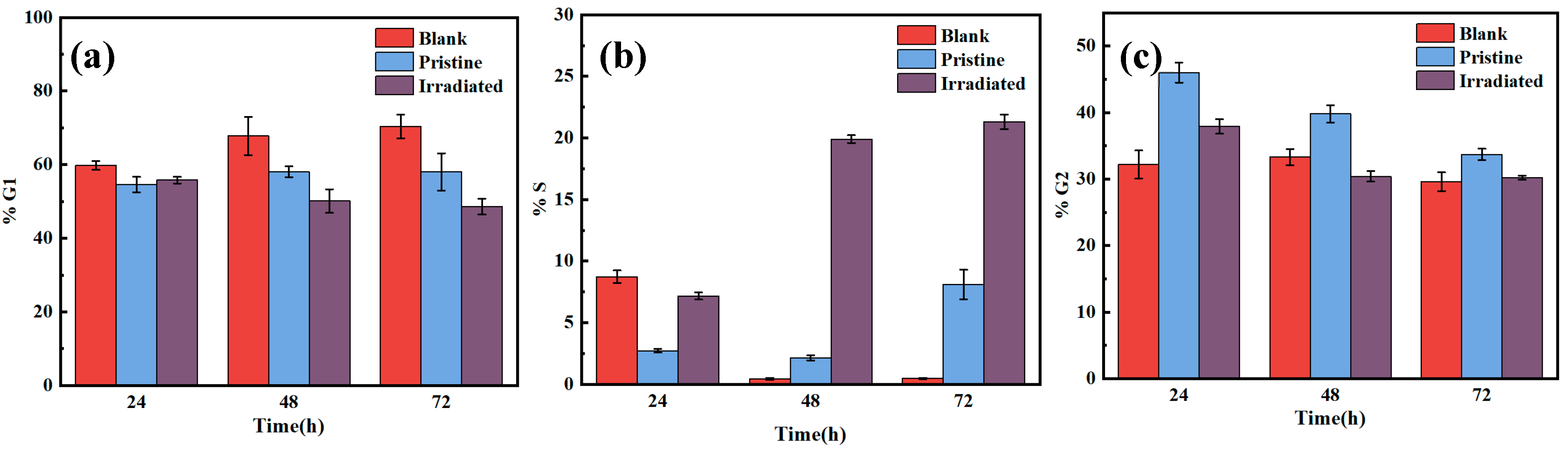
3.7. Cell Cycle Process
Figure 10 shows the cell cycle distribution for blank, pristine and irradiated MAO coatings at different time points (24, 48, 72 h). The data were categorized into G1, S, and G2 phases to assess the effects of different coatings on cell cycle progression. At 24 h, the blank control group exhibited the highest percentage of cells in the G1 phase (59.8%), indicating that the majority of cells were in the early stages of the cell cycle, with 54.6% and 55.8%, respectively. Both the pristine and irradiated MAO coating groups showed lower G1 cell percentages, suggesting that the coatings facilitated cell progression to subsequent phases. By 48 h, the G1 phase percentage decreased in both the pristine and irradiated groups, with the irradiated group showing a more pronounced reduction. This trend continued at 72 h, where the irradiated group exhibited the lowest G1 phase percentage, reducing to 48.6%, indicating accelerated cell cycle progression compared to the blank and pristine groups. The percentage of cells in the S phase was minimal at 24 h for all groups. At 48 h, the irradiated group showed an increase in S-phase cells to 19.9%, which was much higher than the 8.09% of the original MAO film, indicating that the irradiated coating promoted DNA synthesis and cell proliferation. This trend was sustained at 72 h, where the irradiated group had a higher percentage of cells in the S phase compared to both the blank and pristine groups, further supporting the role of the irradiated coating in enhancing cell proliferation. For G2 phase, the percentage of cells in the G2 phase was the lowest in the blank group at 24 h. The pristine and irradiated groups showed a slight increase in G2-phase cells, reaching 39.8% and 37.9%, respectively, indicating that the modified coating facilitated the transition from S phase to G2, preparing cells for mitosis. At 72 h, the irradiation group showed the lowest proportion of G2-phase cells, which may be due to the accelerated cell cycle progression with which some cells passed through the G2 phase. The data from
Figure 8 demonstrate that the HIPIB-modified MAO coating significantly accelerates cell cycle progression compared to both the blank control and pristine MAO coatings. The irradiated coating promoted a faster transition from the G1 phase to the S and G2 phases, leading to enhanced cell proliferation. The increase in S-phase and G2-phase cells in the irradiated group suggests that HIPIB modification improves the cell growth environment, facilitating DNA synthesis and cell division.
4. Discussion
HIPIB irradiation dramatically reduces the number and size of surface pores, leading to a decrease in porosity from 10.21% in the pristine MAO coating to 2.77% in the irradiated coating. The Ca content increases from 0.08 at.% to 0.31 at.%, indicating a redistribution of elements during irradiation. The selective ablation effect induced by the ion bombardment further drives Ca migration to the outermost layer, contributing to the increased Ca content at the surface. These surface modifications are crucial for improving corrosion resistance and providing an optimal environment for cellular interactions. As shown by the potentiodynamic polarization curves, the irradiated MAO coating demonstrates enhanced corrosion resistance, transitioning from an active-dissolution process in the pristine MAO coating to pitting corrosion with a clear passivation stage by irradiation. The corrosion potential shifts positively, and the corrosion current density decreases significantly, confirming that HIPIB irradiation produces a more stable passive layer. These structures also contribute to a reduced hydrogen release rate, further enhancing the stability of the coating in physiological environment, thereby mitigating the potential risks of local alkalization from hydrogen accumulation under physiological conditions. The increased surface roughness (from 2.29 Ra to 7.57 Ra) and the transition from hydrophilic to hydrophobic characteristics in the irradiated coating (contact angle increase from 76.06° to 102.11°) play a key role in enhancing cell adhesion. The rougher and hydrophobic surface provides favorable anchoring sites for cells, while the reduced electrolyte wetting minimizes ionic penetration, thus improving corrosion resistance. Moreover, this synergistic modification of surface properties enhances protein adsorption, regulates cell behavior, and facilitates cell spreading and proliferation. Cell growth and morphology data further support the positive impact of HIPIB. Although both the pristine and irradiated coatings initially showed negative cell growth rates, the irradiated coating promoted significant cell proliferation by Day 3, achieving a 61.29% growth rate. ALP activity in the irradiated coating group significantly increased over time, reaching 18.91 on Day 14, compared to 13.22 in the pristine MAO group and 3.9 in the blank control. This enhancement is likely due to both the increased surface roughness and the higher Ca content, which supports the formation of a more favorable microenvironment for osteogenic differentiation. Flow cytometry analysis of the cell cycle revealed that HIPIB significantly accelerated cell cycle progression. The irradiated group showed a faster transition from the G1 phase to the S and G2 phases, with a corresponding increase in S-phase cells, indicating enhanced DNA synthesis and cell division. The observed decrease in G1-phase cells, particularly at 72 h, further supports the conclusion that the irradiated coating facilitates cell cycle progression and promotes cellular proliferation.
5. Conclusions
The enhanced biological properties of irradiated MAO coatings are driven by a combination of structural, compositional, and surface energy modifications. These changes not only improve the corrosion resistance of the coating but also significantly enhance cell adhesion, proliferation, and osteogenic differentiation. The increase in surface roughness, hydrophobicity, and calcium content, coupled with the healing of surface defects and the formation of a denser layer, creates an ideal microenvironment for cell growth and bone formation. HIPIB thus offers a promising approach to enhance the biological performance of MAO coatings, making them more suitable for applications in biomedical implants.
Author Contributions
Conceptualization, Y.S. and X.H.; methodology, X.H.; software, Y.S. and Y.J.; validation, X.H., Y.S. and Y.J.; formal analysis, Y.S.; investigation, X.H.; resources, Y.W.; data curation, X.H.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S. and X.H.; visualization, Y.S. and X.H.; supervision, Y.W. and X.H.; project administration, Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (NSFC) under Grant no. 52175378.
Data Availability Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amukarimi, S.; Zadshakoyan, M.; Mobasherpour, I. Fabrication and characterization of strontium-and copper-doped bioactive glasses modified biodegradable MgO-PCL coated magnesium biomaterials. Appl. Phys. A-Mater. 2024, 130, 698. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.; Hamzah, E.; Ebrahimi-Kahrizsangi, R.; Daroonparvar, M.; Medraj, M. Fabrication and characterization of hydrophobic microarc oxidation/poly-lactic acid duplex coating on biodegradable Mg–Ca alloy for corrosion protection. Vacuum 2016, 125, 185–188. [Google Scholar] [CrossRef]
- Chen, J.; Liang, S.; Fu, D.; Fan, W.; Lin, W.; Ren, W.; Zou, L.; Cui, X. Design and in situ prepare a novel composite coating on Mg alloy for active anti-corrosion protection. J. Alloys Compd. 2020, 831, 154580. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, R.; Qi, H.; Chen, D.; Zhou, S.; Wang, X.; Li, W. Influence of voltage modes on microstructure and corrosion resistance of micro-arc oxidation coating on magnesium alloy. J. Adhes. Sci. Technol. 2023, 37, 2232–2246. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Wu, M.; Yao, W.; Wu, J.; Zhou, Y.; Yuan, Y.; Xie, Z.; Pan, F. Preparation and corrosion behavior of MAO based MOFs coatings with excellent film-forming and adhesion properties. Surf. Coat. Technol. 2024, 493, 131246. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Xie, Z.; Yuan, Y.; Jiang, B.; Pan, F. In situ growth of Mg-Zn-Al LDHs by ZIF-8 carrying Zn source and micro-arc oxidation integrated coating for corrosion and protection of magnesium alloys. Surf. Coat. Technol. 2022, 451, 129032. [Google Scholar] [CrossRef]
- Yan, H.; Qi, Y.; Cui, X.; Li, C. Micro-arc oxidation phenomenon for producing coatings of AZ31B mg alloy using Na3PO4 with varying KOH concentrations and voltages: A study. Coatings 2023, 13, 1370. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, X.; Yuan, Y.; Jian, F.; Tang, H. Preparation of superhydrophobic hydroxyapatite coating on AZ31 mg alloy by combining micro-arc oxidation and liquid-phase deposition. Coatings 2025, 15, 675. [Google Scholar] [CrossRef]
- Ryu, H.; Hong, S. Corrosion resistance and antibacterial properties of Ag-containing MAO coatings on AZ31 magnesium alloy formed by microarc oxidation. J. Electrochem. Soc. 2010, 157, C131–C136. [Google Scholar] [CrossRef]
- Liu, R.; Xu, D.; Liu, Y.; Wu, L.; Yong, Q.; Xie, Z. Enhanced corrosion protection for MAO coating on magnesium alloy by the synergism of LDH doping with deposition of 8HQ inhibitor film. Ceram. Int. 2023, 49, 30039–30048. [Google Scholar] [CrossRef]
- Liu, J.; Yin, H.; Xu, Z.; Shao, Y.; Wang, Y. Improving the corrosion resistance of micro-arc oxidization film on AZ91D mg alloy through silanization. Metals 2024, 14, 569. [Google Scholar] [CrossRef]
- Li, C.; Gao, L.; Fan, X.; Zeng, R.; Chen, D.; Zhi, K. In vitro degradation and cytocompatibility of a low temperature in-situ grown self-healing Mg-Al LDH coating on MAO-coated magnesium alloy AZ31. Bioact. Mater. 2020, 5, 364–376. [Google Scholar] [CrossRef]
- Cui, L.; Zeng, R.; Guan, S.; Qi, W.; Zhang, F.; Li, S.; Han, E. Degradation mechanism of micro-arc oxidation coatings on biodegradable Mg-Ca alloys: The influence of porosity. J. Alloys Compd. 2017, 695, 2464–2476. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Dou, B.; Zeng, X.; Lin, X. Deposition time effects on structure and corrosion resistance of duplex Mao/Al coatings on AZ31B mg alloy. Anti-Corros. Methods Mater. 2017, 64, 357–364. [Google Scholar]
- Wu, M.; Li, B. Microstructure, Mao performance, interfacial characteristics and corrosion behavior of FSW joint of Al–Mg-Sc alloy. Mater. Chem. Phys. 2025, 329, 130046. [Google Scholar] [CrossRef]
- Wei, P.; Huang, J.; Chen, L.; Hu, C.; He, W.; Yuan, Z.; Chen, D. Effect of P/W/Ce-based inhibitor on the corrosion resistance and self-healing behavior of micro-arc oxidation on magnesium alloy. Surf. Interfaces 2025, 72, 107315. [Google Scholar] [CrossRef]
- Ungan, G.; Cakir, A. Investigation of MgO effect on bioactivity of coatings produced by MAO. Surf. Coat. Technol. 2015, 282, 52–60. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, R.; Yu, F.; Zhou, Y.; Yao, W.; Yuan, Y.; Xie, Z.; Ma, Y.; Andrej, A.; Wu, L. Preparation and corrosion resistance mechanism of magnesium–lithium alloy micro-arc oxidation/quaternary ldhs@ GO self-healing composite film. Acta Met. Sin-Engl. 2025, 38, 1545–1558. [Google Scholar] [CrossRef]
- Han, X.; Zhu, F.; Zhu, X.; Lei, M.; Xu, J. Electrochemical properties of microarc oxidation films on a magnesium alloy modified by high-intensity pulsed ion beam. Surf. Coat. Technol. 2011, 206, 874–878. [Google Scholar] [CrossRef]
- Shao, Y.; Han, X.; Ma, C.; Wei, Y.; Zhu, X.; Xu, J. The thermal dynamic simulation and microstructure characterization of micro-arc oxidation (MAO) films on magnesium AZ31 irradiated by high-intensity pulsed ion beam. Appl. Surf. Sci. 2025, 682, 161705. [Google Scholar] [CrossRef]
- Han, X.; Shao, Y.; Shan, Y.; Xu, J.; Zhu, X.; Wei, Y. The bioactivities of irradiated MAO coatings on magnesium alloy by high-intensity pulsed ion beam. Mater. Lett. 2025, 402, 139381. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).