The Application of Carbon-Based Materials in Cathodes for High-Performance K-Se Batteries: A Review
Abstract
1. Introduction
2. Mechanism, Challenges, and Optimization Strategies of Potassium–Selenium Batteries
2.1. The Mechanism and Challenges of Potassium–Selenium Batteries
2.2. The Optimization Strategies of Potassium–Selenium Batteries
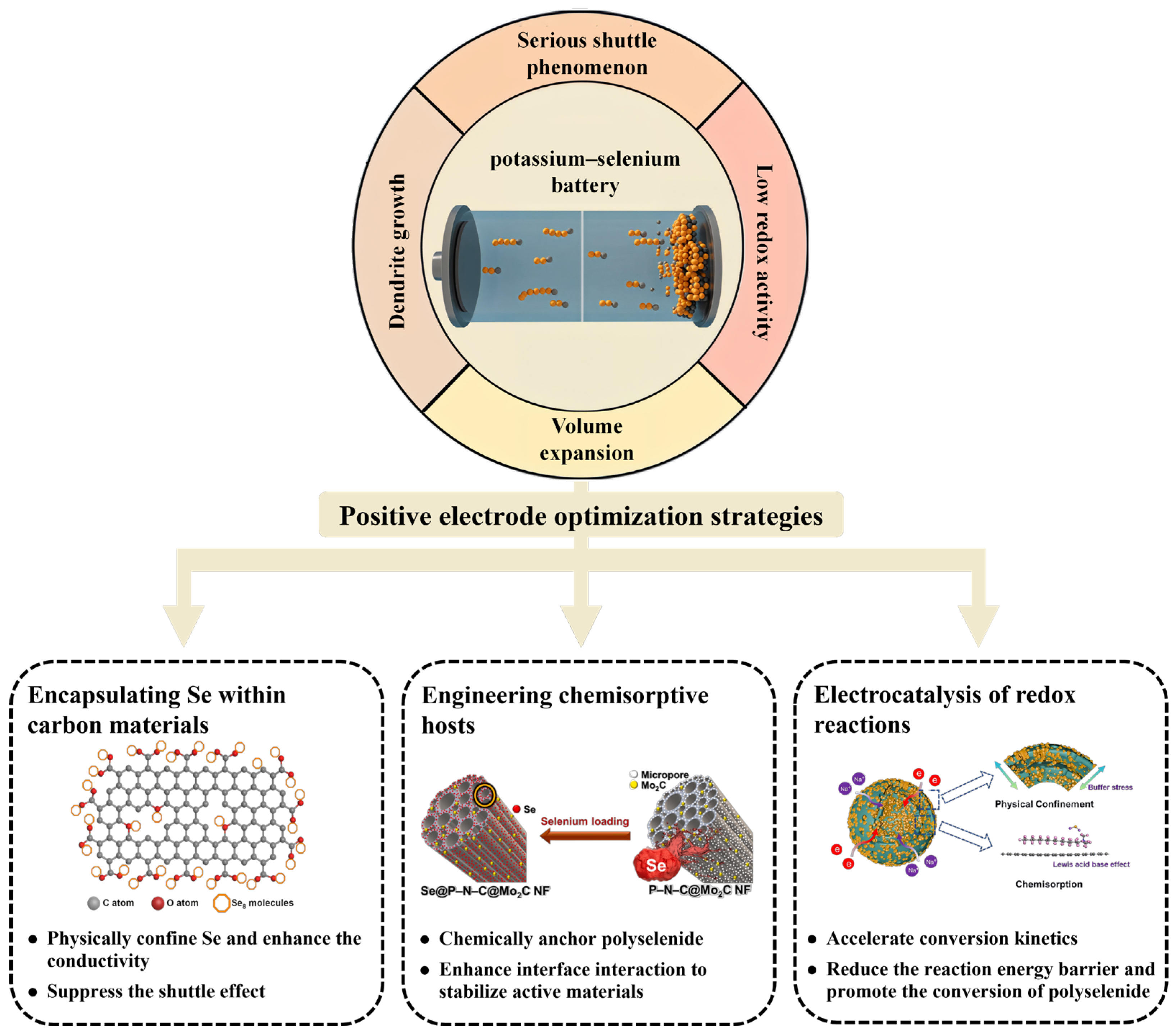
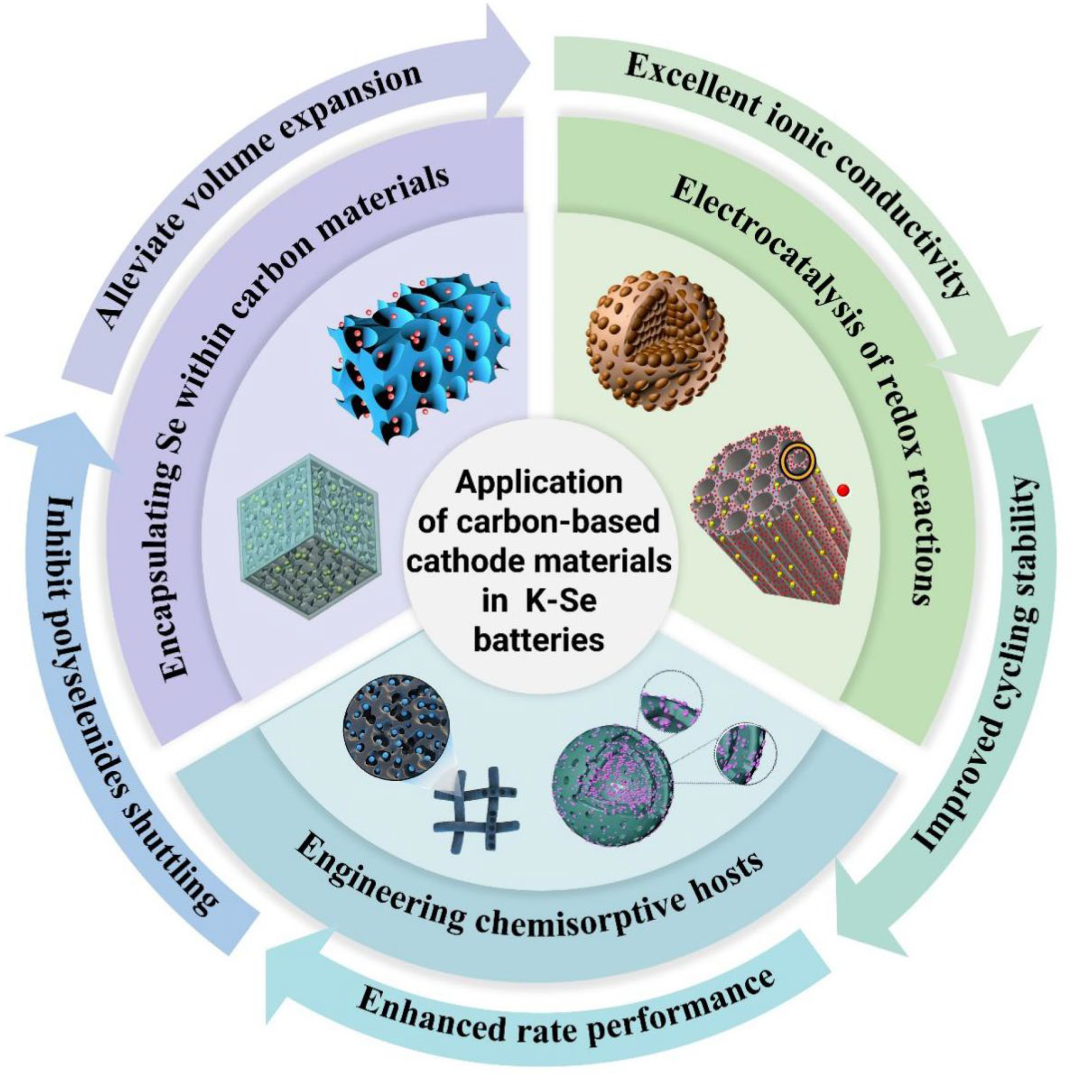
3. Application of Carbon-Based Materials in Cathodes for Potassium–Selenium Batteries
3.1. Encapsulating Se Within Carbon Materials
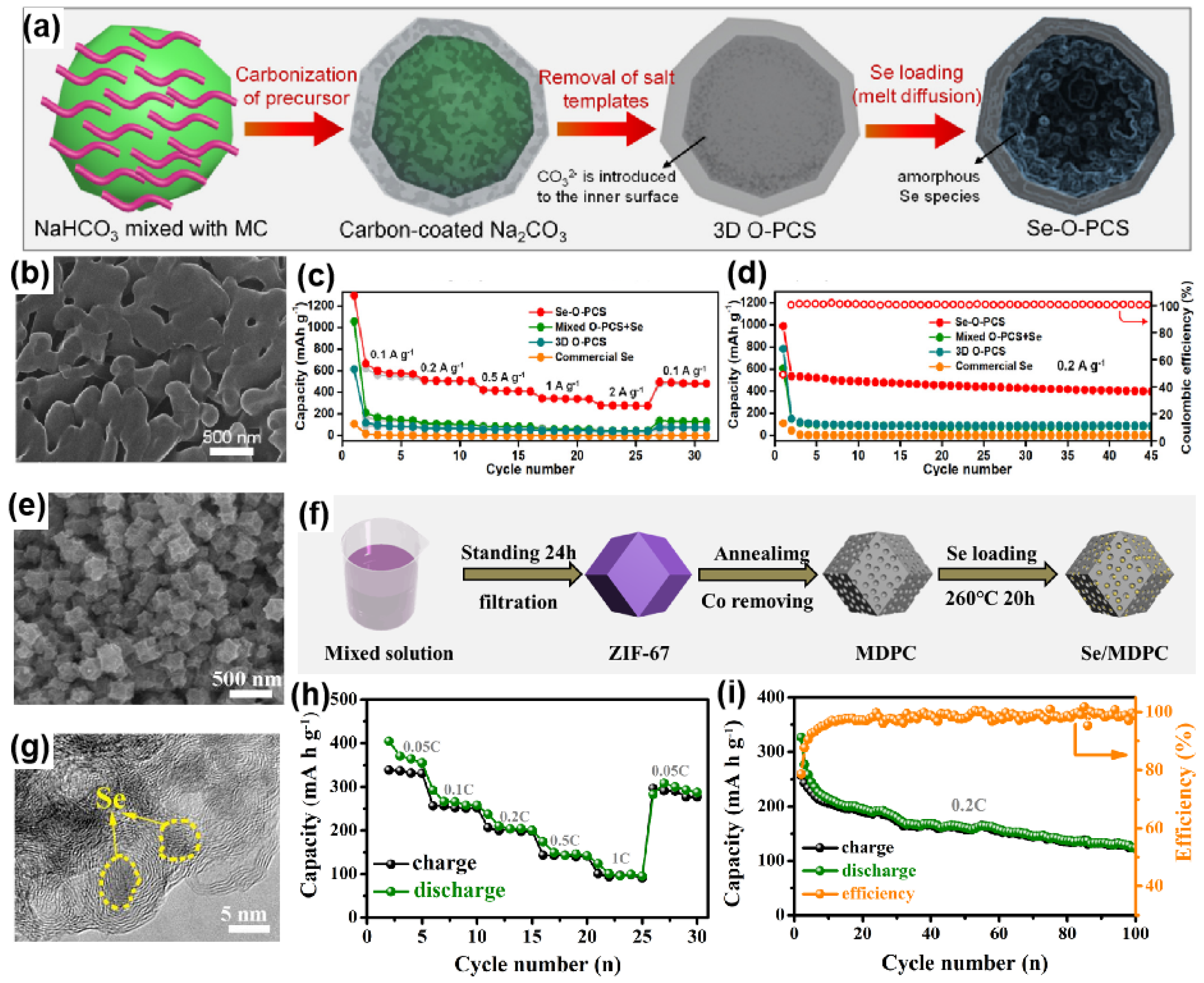
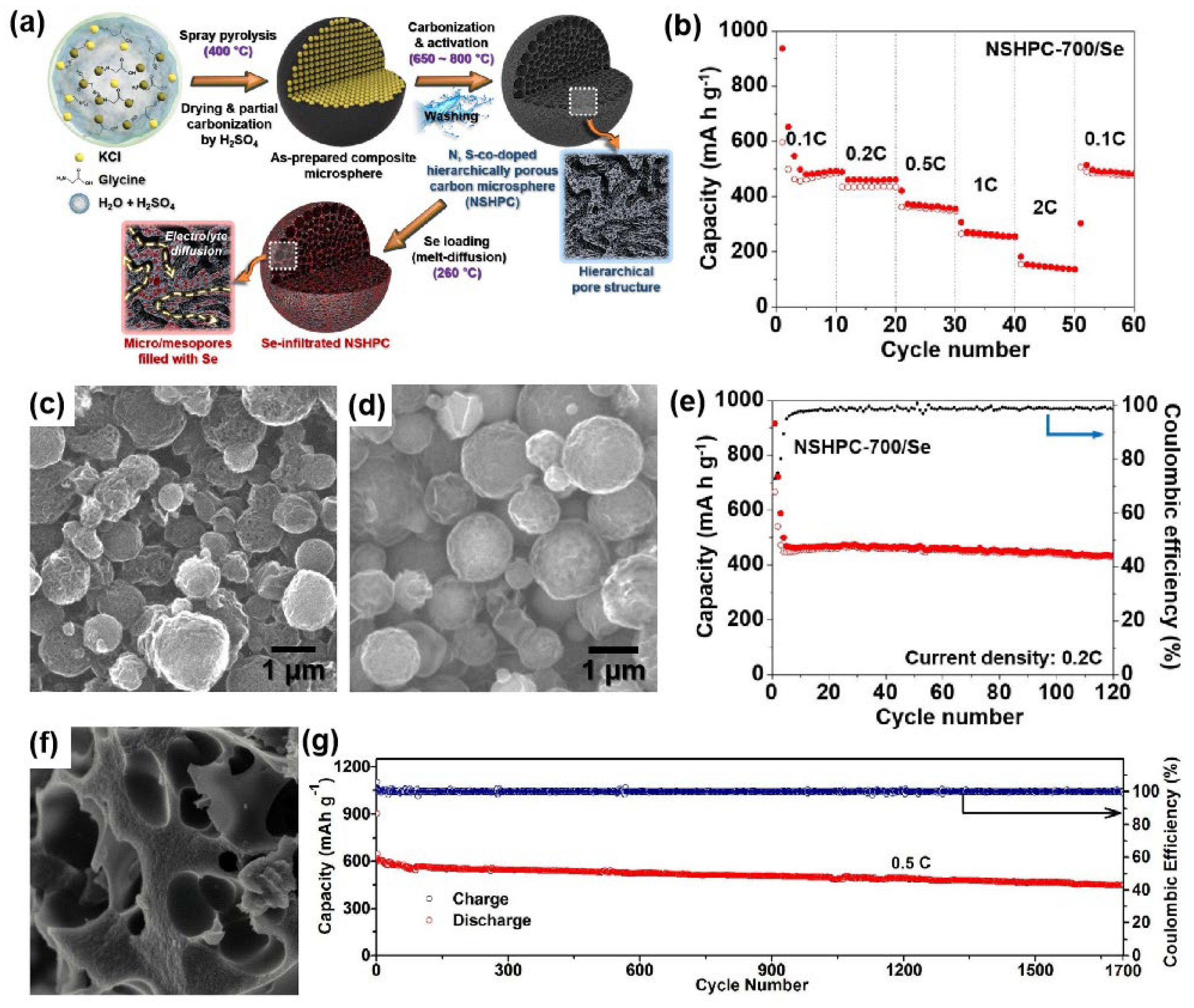
3.1.1. Se/Porous Carbon Composites
3.1.2. Se/Hollow Carbon Composites
3.1.3. Se/Heteroatom-Doped Carbon Composites
3.1.4. Se/Biomass-Derived Carbon Composites
3.1.5. Small-Molecular Se/Carbon Composites
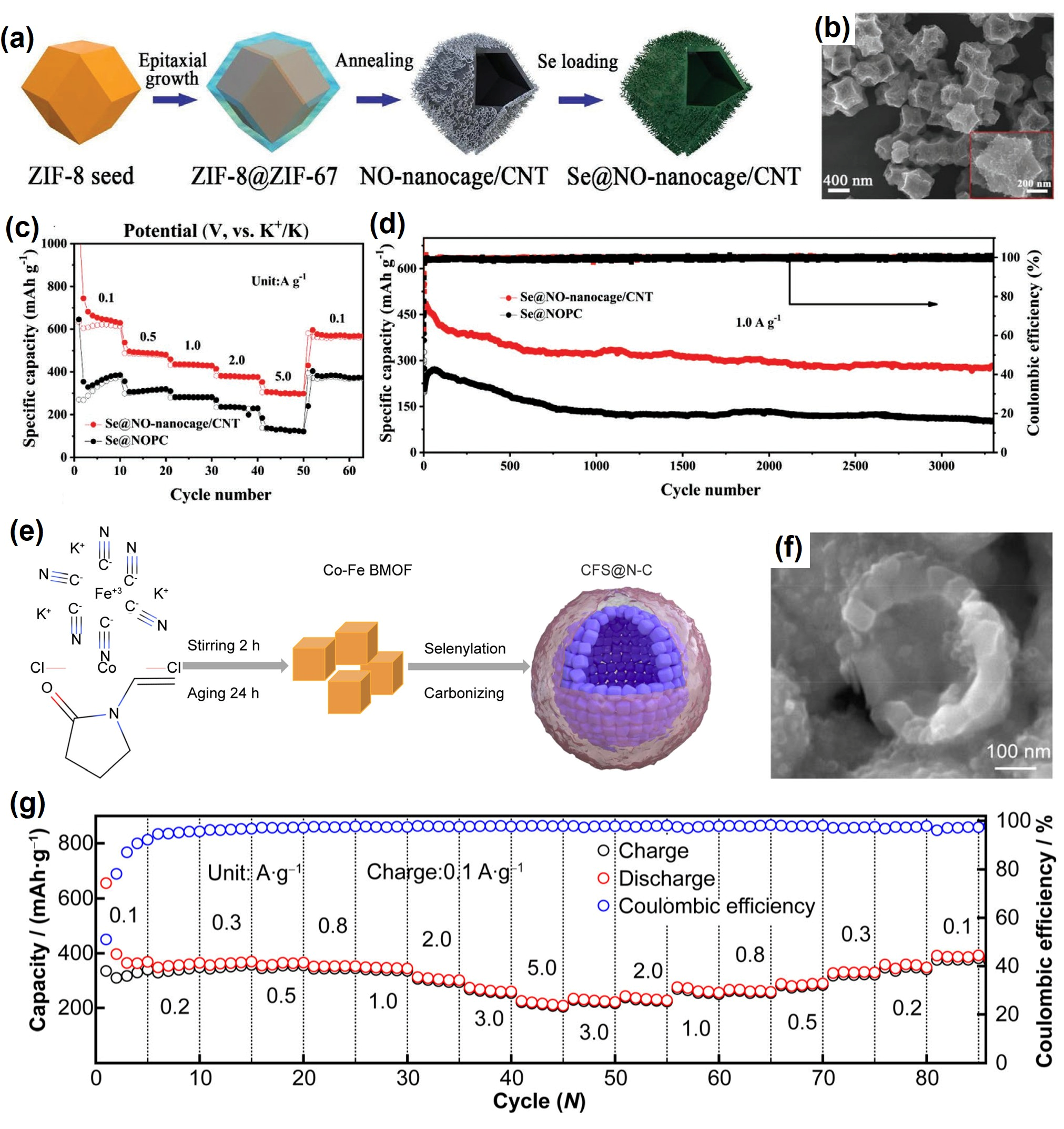
3.2. Engineering Chemisorptive Hosts
3.3. Electrocatalysis of Redox Reactions
3.4. Summary
4. Conclusions and Outlook
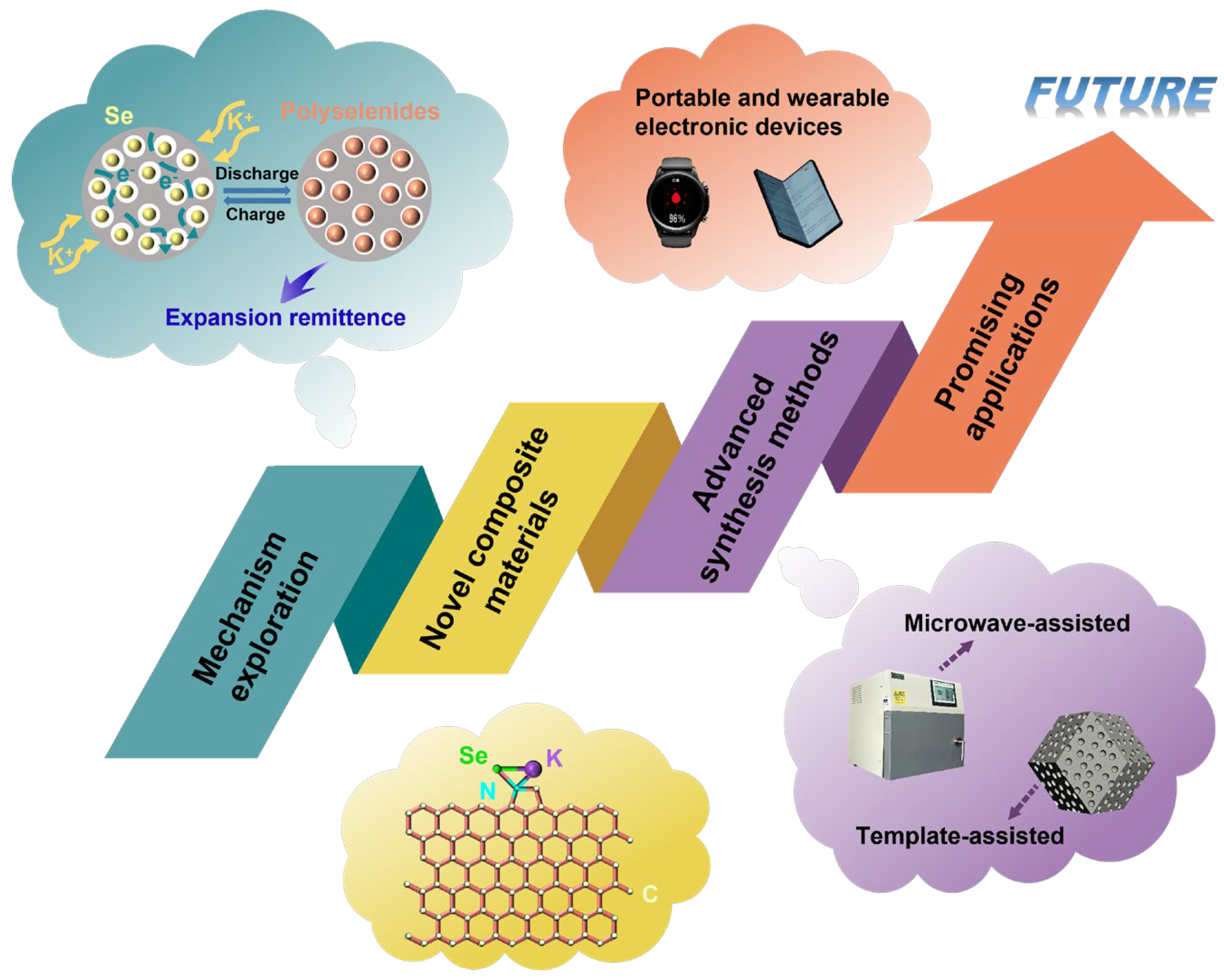
- Mechanism exploration. In K-Se battery cathodes, carbon-based materials are primarily used as Se host matrices, conductive frameworks, and polyselenide adsorption media, effectively enhancing battery performance through synergistic physical confinement, chemical anchoring, and conductivity enhancement mechanisms. Nevertheless, current studies do not clearly establish the quantitative relationship between carbon pore structures and selenium loading capacity, the differential adsorption energies of polyselenide at heteroatom doping sites, and the mechanistic links between Se phase transition pathways (particularly intermediate states during Se8 to K2Se conversion) and the structural stability of carbon-based materials. Therefore, it is imperative to employ in situ characterization techniques (e.g., in situ XRD and Raman spectra) to track the evolution of Se species and polyselenide, combined with theoretical simulations (such as density functional theory calculations of adsorption energies) [65,66], to illustrate the dynamic interactions between carbon-based materials and Se, thereby providing a foundation for designing high-performance materials.
- Novel composite materials. Currently, the applications of carbon-based materials in cathodes for potassium-selenium batteries primarily include Se/porous carbon composites, Se/heteroatom-doped carbon composites, Se/hollow carbon composites, and Se/biomass-derived carbon composites. Although current carbon-based materials exhibit substantial diversity and have successfully elevated battery performance, the development of novel carbon composite materials is still essential for achieving superior electrochemical properties. For instance, the hybridization of carbon materials with graphene, metal-based materials (e.g., MOFs and MXenes), and carbides or nitrides enables the fabrication of carbon materials with enhanced electrochemical activity. Moreover, using artificial intelligence (AI) to accelerate the selection of high-performance materials has emerged as a leading frontier in the field [66]. These novel composite materials can simultaneously provide efficient ion/electron transport pathways and effectively inhibit polyselenide shuttling via chemical anchoring, thereby significantly enhancing overall stability. Furthermore, through precise morphological control, further performance optimization can be achieved, endowing these materials with significant potential for future applications.
- Advanced synthesis methods. The melt-diffusion method has been widely adopted for preparing Se/carbon composites due to its operational simplicity, cost-effectiveness, and scalability for large-scale production. The uniformity and loading capacity of Se can be significantly enhanced through precise regulation of the porous structure of the carbon host and optimization of the melt-diffusion temperature. Recently, researchers have proposed some effective ways to optimize the melt-diffusion method. For example, microwave-assisted melting significantly reduces both the reaction duration and Se volatilization. Another approach is template-guided melting, which demonstrates considerable potential by enhancing confinement effects through predesigned pore architectures. Furthermore, the development of novel carbon-based composites will significantly advance their synthetic applications and performance optimization. Beyond the melt-diffusion method, alternative synthetic approaches including electrospinning have been systematically investigated. Comprehensively evaluating the advantages and limitations of different methods and selecting the optimal strategy are expected to drive significant progress in the application of carbon-based materials for K-Se batteries.
- Promising applications. With the advancement of flexible energy storage technologies, potassium–selenium batteries are expected to become a new focus in portable and wearable electronic devices due to their excellent energy density and low-cost K resource advantages. By constructing a self-supporting flexible cathode in the carbon framework, the excellent flexibility and conductivity of carbon materials can be efficiently used to provide high endurance power for thin wearable and foldable-screen devices. Notably, in view of the fact that the ionic conductivity of the K-Se battery is less affected by low temperature, the system can be applied to wearable monitoring equipment in extreme low-temperature conditions. Therefore, with the continuous progress in the preparation technologies of carbon-based cathode materials, flexible potassium–selenium batteries are expected to reach large-scale production and further accelerate the commercial advancement of wearable electronics. Moreover, K-Se batteries could be coupled with energy harvesting technologies (such as capturing biomechanical and environmental energy), and K-Se batteries can greatly extend the runtime of wearable biometric sensors and other sustainable devices. This integration could enhance functionality and reliability while providing sustainability and energy efficiency. As carbon-based cathode materials advance, scalable production of flexible K-Se batteries is expected, accelerating the commercialization of next-generation wearable technology and promoting a more sustainable future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Rangarajan, S.S.; Sunddararaj, S.P.; Sudhakar, A.; Shiva, C.K.; Subramaniam, U.; Collins, E.R.; Senjyu, T. Lithium-Ion Batteries—The Crux of Electric Vehicles with Opportunities and Challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Yao, Q.; Zhu, C. Advanced Post-Potassium-Ion Batteries as Emerging Potassium-Based Alternatives for Energy Storage. Adv. Funct. Mater. 2020, 30, 2005209. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Wu, N.; Shen, J.; Li, Q.; Li, S.; Guo, D.; Li, J.; Liu, G.; Zhao, J.; Cao, A.; Mi, H.; et al. Synergistic Bimetallic Interaction and Regulated Void Size in Isocubanite CuFe2S3 Enables UltraFast and Durable Sodium Storage. ACS Sustain. Chem. Eng. 2025, 13, 5546–5556. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Wang, F.; Wang, H.-B.; Kong, C.-Y.; Wang, G.-X.; Liu, X.-M.; Liu, Y. Shining light on fillers uniform dispersion of PVDF/garnet composite electrolytes for high-performance solid-state Li batteries: Fundamentals, progress and perspectives. Rare Met. 2025, 44, 5957–5979. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Wang, L.; Tong, X.; Dou, S.X.; Wang, Z.M. Advanced High-Performance Potassium-Chalcogen (S, Se, Te) Batteries. Small 2021, 17, 2004369. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, Y.; Sun, Y.; Rong, J.; Li, H.; Niu, L. Advanced Anode Materials of Potassium Ion Batteries: From Zero Dimension to Three Dimensions. Nano-Micro Lett. 2020, 13, 12. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jian, Z.; Ji, X. Potassium Secondary Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4404–4419. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, H.; Moon, S.O.; Jo, C.; Park, H.S. Carbon-based materials for potassium-ion battery anodes: Storage mechanisms and engineering strategies. J. Energy Chem. 2025, 105, 764–796. [Google Scholar] [CrossRef]
- Deng, Q.; Pei, J.; Fan, C.; Ma, J.; Cao, B.; Li, C.; Jin, Y.; Wang, L.; Li, J. Potassium salts of para-aromatic dicarboxylates as the highly efficient organic anodes for low-cost K-ion batteries. Nano Energy 2017, 33, 350–355. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci. Adv. 2019, 5, eaav7412. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhang, Q.; Wang, H.; Pang, W.K.; Liu, H.K.; Konstantinov, K.; Guo, Z. A new energy storage system: Rechargeable potassium-selenium battery. Nano Energy 2017, 35, 36–43. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, W.; Pan, Y.; Sun, C.-F. Approaching the voltage and energy density limits of potassium–selenium battery chemistry in a concentrated ether-based electrolyte. Chem. Sci. 2020, 11, 6045–6052. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, H.; Wang, G.; Wang, C.; Ni, Y.; Nan, C.-W.; Fan, L.-Z. Challenges, interface engineering, and processing strategies toward practical sulfide-based all-solid-state lithium batteries. InfoMat 2022, 4, e12292. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Kim, J.; Agostini, M.; Xiong, S.; Matic, A.; Hwang, J.-Y. Recent Developments and Future Challenges in Designing Rechargeable Potassium-Sulfur and Potassium-Selenium Batteries. Energies 2020, 13, 2791. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Celeste, A.; Park, H.; Kansara, S.; Zumpano, R.; Piacentini, V.; Brutti, S.; Matic, A.; Agostini, M.; et al. Prospects of Alkali Metal–Se Batteries and Beyond: From Redox Mechanisms to Electrode Design. ACS Energy Lett. 2025, 10, 2512–2531. [Google Scholar] [CrossRef]
- Zhou, X.; Rui, X.; Yu, Y. High Energy Density Na (K)-Se Batteries: Some Challenges and Potential Solutions. Acc. Mater. Res. 2023, 4, 467–471. [Google Scholar] [CrossRef]
- Huang, X.L.; Guo, Z.; Dou, S.X.; Wang, Z.M. Rechargeable Potassium-Selenium Batteries. Adv. Funct. Mater. 2021, 31, 2102326. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.; Li, C.; Barakat, T.; Sun, M.; Wang, Y.; Wu, L.; Wang, L.; Xia, L.; Hu, Z.-Y.; et al. Three-dimensional ordered hierarchically porous carbon materials for high performance Li-Se battery. J. Energy Chem. 2022, 68, 624–636. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Sun, X.; Gou, H.; Zhang, C.; Wang, G. Carbon host pore structure regulation boosting fast and stable Se-K electrochemistry towards Se-K ion capacitors. Carbon 2023, 203, 141–151. [Google Scholar] [CrossRef]
- Gao, Y.-M.; Liu, Y.; Feng, K.-J.; Ma, J.-Q.; Miao, Y.-J.; Xu, B.-R.; Pan, K.-M.; Akiyoshi, O.; Wang, G.-X.; Zhang, K.-K.; et al. Emerging WS2/WSe2@graphene nanocomposites: Synthesis and electrochemical energy storage applications. Rare Met. 2024, 43, 1–19. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, M.; Xu, R.; Zeng, S.; Yang, H.; Ye, S.; Liu, F.; Wu, X.; Yu, Y. CNT Interwoven Nitrogen and Oxygen Dual-Doped Porous Carbon Nanosheets as Free-Standing Electrodes for High-Performance Na-Se and K-Se Flexible Batteries. Adv. Mater. 2018, 30, 1805234. [Google Scholar] [CrossRef]
- Lim, J.B.; Kim, H.J.; Na, J.H.; Kim, J.K.; Jeong, S.-Y.; Park, S.-K. Hierarchical nitrogen-doped multichannel carbon nanofibers for efficient potassium-selenium batteries. Rare Met. 2025, 44, 3839–3851. [Google Scholar] [CrossRef]
- Du, Y.; Ma, S.; Dai, J.; Lin, J.; Zhou, X.; Chen, T.; Gu, X. Biomass Carbon Materials Contribute Better Alkali-Metal-Selenium Batteries: A Mini-Review. Batteries 2022, 8, 123. [Google Scholar] [CrossRef]
- Zhou, L.; Cui, Y.; Kong, D.; Feng, W.; Gao, X.; Yan, Y.; Ren, H.; Hu, H.; Xue, Q.; Yan, Z.; et al. Amorphous Se species anchored into enclosed carbon skeleton bridged by chemical bonding toward advanced K-Se batteries. J. Energy Chem. 2021, 61, 319–326. [Google Scholar] [CrossRef]
- Khan, M.; Ding, X.; Zhao, H.; Wang, Y.; Zhang, N.; Chen, X.; Xu, J. Recent Advancements in Selenium-Based Cathode Materials for Lithium Batteries: A Mini-Review. Electrochem 2022, 3, 285–308. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Yao, Y.; Jiang, Y.; Xu, R.; Wang, H.; Wu, X.; Yu, Y. Integrating Conductivity, Captivity, and Immobility Ability into N/O Dual-Doped Porous Carbon Nanocage Anchored with CNT as an Effective Se Host for Advanced K-Se Battery. Adv. Funct. Mater. 2020, 30, 2003871. [Google Scholar] [CrossRef]
- Qiu, R.; Fei, R.; Zhang, T.; Liu, X.; Jin, J.; Fan, H.; Wang, R.; He, B.; Gong, Y.; Wang, H. Biomass-derived, 3D interconnected N-doped carbon foam as a host matrix for Li/Na/K-selenium batteries. Electrochim. Acta 2020, 356, 136832. [Google Scholar] [CrossRef]
- Xu, R.; Yao, Y.; Wang, H.; Yuan, Y.; Wang, J.; Yang, H.; Jiang, Y.; Shi, P.; Wu, X.; Peng, Z.; et al. Unraveling the Nature of Excellent Potassium Storage in Small-Molecule Se@Peapod-Like N-Doped Carbon Nanofibers. Adv. Mater. 2020, 32, 2003879. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Wu, H.; Xie, B.; Wang, D.; Wang, R.; Zhang, X.; Piao, Y.; Diao, G.; Chen, M. Encapsulation of Se in dual-wall hollow carbon spheres: Physical confinement and chemisorption for superior Na-Se and K-Se batteries. Carbon 2022, 187, 354–364. [Google Scholar] [CrossRef]
- Cho, S.W.; Choi, H.H.; Senthamaraikannan, T.G.; Lim, D.-H.; Park, G.D.; Cho, C.; Jeong, S.M.; Saroha, R.; Cho, J.S. Hierarchical porous one-dimensional N-doped C framework comprising ultrafine Mo2C catalysts for stable Na/K-Se batteries: Experimental and theoretical investigations. Chem. Eng. J. 2025, 512, 162456. [Google Scholar] [CrossRef]
- Zeng, L.; Zeng, W.; Jiang, Y.; Wei, X.; Li, W.; Yang, C.; Zhu, Y.; Yu, Y. A Flexible Porous Carbon Nanofibers-Selenium Cathode with Superior Electrochemical Performance for Both Li-Se and Na-Se Batteries. Adv. Energy Mater. 2015, 5, 1401377. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, C.; Lu, W.; Wang, X.; Yue, H.; Zhang, D.; Xing, Z. A graphitized hierarchical porous carbon as an advanced cathode host for alkali metal-selenium batteries. Chem. Eng. J. 2022, 433, 133527. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Q.; Gao, W.; Yang, T.; Zhan, R.; Deng, J.; Guo, B.; Tao, M.; Liu, H.; Xu, M. Rechargeable K-Se batteries based on metal-organic-frameworks-derived porous carbon matrix confined selenium as cathode materials. J. Colloid Interface Sci. 2019, 539, 326–331. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, J.; Sun, J.; Duan, L.; Du, Y.; Bao, J.; Zhou, X. Zn-MOF-74-Derived Carbon Nanorods as an Efficient Se Host for K-Se Batteries. ACS Appl. Energy Mater. 2022, 5, 13023–13030. [Google Scholar] [CrossRef]
- Huang, H.; Luo, X.; Yao, Y.; Zhou, X.; Jiang, Y.; Guo, C.; Liu, J.; Wu, X.; Yu, Y. Binding Se into nitrogen-doped porous carbon nanosheets for high-performance potassium storage. InfoMat 2021, 3, 421–431. [Google Scholar] [CrossRef]
- Kim, J.K.; Kang, Y.C. Encapsulation of Se into Hierarchically Porous Carbon Microspheres with Optimized Pore Structure for Advanced Na-Se and K-Se Batteries. ACS Nano 2020, 14, 13203–13216. [Google Scholar] [CrossRef]
- Huang, X.; Deng, J.; Qi, Y.; Liu, D.; Wu, Y.; Gao, W.; Zhong, W.; Zhang, F.; Bao, S.; Xu, M. A highly-effective nitrogen-doped porous carbon sponge electrode for advanced K-Se batteries. Inorg. Chem. Front. 2020, 7, 1182–1189. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, L.; Yang, Z.; Chen, G.; Yue, H.; Zhang, D.; Sun, Z.; Li, F. An alkali metal-selenium battery with a wide temperature range and low self-discharge. J. Mater. Chem. A 2019, 7, 21774–21782. [Google Scholar] [CrossRef]
- Jia, X.-X.; Yu, X.-Z.; Lu, B.-A. Fe0.8CoSe2 nanosphere coated by N-doped carbon for ultra-high rate potassium selenium battery. Rare Met. 2021, 40, 2455–2463. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Deng, J.; Gao, W.; Liu, D.; Ma, Q.; Xu, M. A Se-hollow porous carbon composite for high-performance rechargeable K-Se batteries. Inorg. Chem. Front. 2019, 6, 2118–2125. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Y.; Huang, Z.; Song, W.; Zhong, C.; Ding, J.; Hu, W. Toward Theoretical Capacity and Superhigh Power Density for Potassium-Selenium Batteries via Facilitating Reversible Potassiation Kinetics. ACS Appl. Mater. Interfaces 2022, 14, 6828–6840. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Na, J.-H.; Park, S.-K. Monodisperse Hierarchical N-Doped Carbon Microspheres with Uniform Pores as a Cathode Host for Advanced K-Se Batteries. Batteries 2025, 11, 101. [Google Scholar] [CrossRef]
- Du, Y.; Fan, H.; Zhu, Y.; Zhang, X.; Wei, D.; Jin, C.; Cui, Y.; Lv, M. Anchoring active sulfur/selenium into enhanced carbon hosts with multiple chemical affinities for efficient K-S/Se batteries. Green Chem. 2025, 27, 2309–2318. [Google Scholar] [CrossRef]
- Li, D.; Wang, L.; Cheng, X.; Yao, Y.; Jiang, Y.; Shi, P.; Wu, Y.; Wu, X.; Ma, C.; Yu, Y. Manipulating selenium molecular configuration in N/O dual-doped porous carbon for high performance potassium-ion storage. J. Energy Chem. 2021, 62, 581–589. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Qi, H.; Li, Z.; Yue, H. N/O-doped porous carbon matrix for improved potassium-selenium battery cathode in different electrolyte systems. J. Alloys Compd. 2023, 930, 167395. [Google Scholar] [CrossRef]
- Ma, Q.; Chen, S.; Li, Y.; Yan, Z.; Liu, Y.; Fang, Z. Encapsulating Selenium into Biomass-Derived Nitrogen-Doped Porous Carbon As the Cathode for Sodium-Selenium and Potassium-Selenium Batteries. ACS Appl. Nano Mater. 2024, 7, 16599–16608. [Google Scholar] [CrossRef]
- Cai, R.; Chen, X.; Liu, P.; Chen, T.; Liu, W.; Fan, X.; Ouyang, B.; Liu, K. A Novel Cathode Based on Selenium Confined in Biomass Carbon and Graphene Oxide for Potassium-Selenium Battery. ChemElectroChem 2020, 7, 4477–4483. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Cao, J.; Liang, C.; Yu, K. N/S co-doped biomass-based porous carbon surface-embedded small-molecule selenium as cathode circumflex accent A for high-performance K-Se batteries. Electrochim. Acta 2022, 432, 141158. [Google Scholar] [CrossRef]
- Yang, W.; Jamil, S.; Xu, M. Hierarchical Porous Carbon Embedded MoSe2 Effectively Improve Cycle Stability in K-Se Battery. ChemistrySelect 2023, 8, e202300470. [Google Scholar] [CrossRef]
- Ding, Y.; Cai, J.; Sun, Y.; Shi, Z.; Yi, Y.; Liu, B.; Sun, J. Bimetallic Selenide Decorated Nanoreactor Synergizing Confinement and Electrocatalysis of Se Species for 3D-Printed High-Loading K-Se Batteries. ACS Nano 2022, 16, 3373–3382. [Google Scholar] [CrossRef]
- Zhou, L.; Li, B.; Hu, H.; Liu, H.; Nan, J.; Wu, W.; Xu, H.; Cai, T.; Liu, P.; Li, X.; et al. Regulating Redox Kinetics by a W2N Electrocatalyst toward High-Performance K-Se Batteries. Energy Fuels 2023, 37, 18103–18110. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Xiong, J.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Three-dimensional hierarchical C-Co-N/Se derived from metal-organic framework as superior cathode for Li-Se batteries. J. Power Sources 2017, 363, 103–109. [Google Scholar] [CrossRef]
- Wu, T.; Ding, Z.; Jing, M.; Zou, G.; Hou, H.; Tian, Y.; Jiang, Y.; Hong, W.; Ji, X. Chem-Bonding and Phys-Trapping Se Electrode for Long-Life Rechargeable Batteries. Adv. Funct. Mater. 2019, 29, 1809014. [Google Scholar] [CrossRef]
- Li, F.; Zhou, Z. Micro/Nanostructured Materials for Sodium Ion Batteries and Capacitors. Small 2018, 14, 1702961. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, T.; Gao, W.; Zhan, R.; Zhang, Y.; Bao, S.; Li, X.; Chen, Y.; Xu, M. Jackfruit-like electrode design for advanced Na-Se batteries. J. Power Sources 2019, 443, 227245. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, T.; Li, Y.; Hu, L.; Dai, C.; Zhang, Y.; Li, Y.; Liu, D.; Xu, M. Selenium Encapsulated into Metal–Organic Frameworks Derived N-Doped Porous Carbon Polyhedrons as Cathode for Na–Se Batteries. ACS Appl. Mater. Interfaces 2017, 9, 41339–41346. [Google Scholar] [CrossRef]
- Dong, W.; Chen, H.; Xia, F.; Yu, W.; Song, J.; Wu, S.; Deng, Z.; Hu, Z.-Y.; Hasan, T.; Li, Y.; et al. Selenium clusters in Zn-glutamate MOF derived nitrogen-doped hierarchically radial-structured microporous carbon for advanced rechargeable Na–Se batteries. J. Mater. Chem. A 2018, 6, 22790–22797. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, D.; Tan, H.; Feng, Y.; Rui, X.; Yu, Y. Advances in K-Q (Q = S, Se and SexSy) batteries. Mater. Today 2020, 39, 9–22. [Google Scholar] [CrossRef]
- Luo, C.; Xu, Y.; Zhu, Y.; Liu, Y.; Zheng, S.; Liu, Y.; Langrock, A.; Wang, C. Selenium@Mesoporous Carbon Composite with Superior Lithium and Sodium Storage Capacity. ACS Nano 2013, 7, 8003–8010. [Google Scholar] [CrossRef]
- Huang, X.L.; Zhou, C.; He, W.; Sun, S.; Chueh, Y.-L.; Wang, Z.M.; Liu, H.K.; Dou, S.X. An Emerging Energy Storage System: Advanced Na-Se Batteries. ACS Nano 2021, 15, 5876–5903. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.-Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium–Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Yu, L.; You, Y.; Cong, H.-P.; Yin, Y.-X.; Du, X.-L.; Guo, Y.-G.; Yu, S.-H.; Cui, Y.; Goodenough, J.B. The Electrochemistry with Lithium versus Sodium of Selenium Confined to Slit Micropores in Carbon. Nano Lett. 2016, 16, 4560–4568. [Google Scholar] [CrossRef] [PubMed]
- Antil, B.; Olhan, S.; Vander Wal, R.L. Production of Graphitic Carbon from Renewable Lignocellulosic Biomass Source. Minerals 2025, 15, 262. [Google Scholar] [CrossRef]
- Feng, J.; Yu, S.; Shi, C.; Tang, X.; Zhao, X.; Chen, S.; Song, J. Advanced Cathode Designs for High-Energy Lithium/Sodium–Selenium Battery. Adv. Funct. Mater. 2025, 35, 2422013. [Google Scholar] [CrossRef]




| Materials | Se Content (wt%)/Loading (mg cm–2) | Synthesis Method | Electrolyte a | Voltage Window (V) | Electrochemical Performance (DC b, CD c, CRR d, CN e) | Ref. |
|---|---|---|---|---|---|---|
| Encapsulating Se within Carbon Materials | ||||||
| HPC/Se | -/1–1.2 | Melt-diffusion | 1 M KPF6 in EC/DEC | 0.5–3.0 | 527, 0.1 C, ~85%, 100 | [34] |
| Se-O-PCS | 51/~1.0 | Template and melt-diffusion | 1 M KFSI in EC/DEC | 0.5–3.0 | 514, 0.2 C, ~80%, 45 | [26] |
| Se/MDPC | 53/1.0–1.8 | Melt-diffusion | 1 M KPF6 in EC/PC | 0.7–2.3 | ~280, 0.2C, ~50%, 100 | [35] |
| Se@HCR | 55/~0.8 | Solvothermal and melt-diffusion | 0.8 M KPF6 in EC/DEC | 0.5–3.0 | 581.4, 0.1 C, 86%, 200 | [36] |
| Se@N-HCNS | 45.6/- | Melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | 610, 0.3 C, ~89%, 100 | [37] |
| NSHPC-700/Se | 57–59/1.8–2.1 | Spray pyrolysis and melt-diffusion | 1 M KFSI in EC/DEC | 0.5–3.0 | 461, 0.2 C, ~95%, 120 | [38] |
| Se@NPCS | 60/- | Melt-diffusion | 1 M KPF6 in EC/DEC | 0.5–2.5 | 604, 0.5 C, ~52%, 300 | [39] |
| Se@h-NMCNF | 60/~1.9 | Electrospinning and melt-diffusion | 3 M KFSI in EC/DEC | - | 384, 0.5 C, 54.9%, 1000 | [24] |
| Se50/SO-HPC3 | 50/~1.5 | Melt-diffusion | 1 M KPF6 in EC/DEC | 0.5–2.5 | ~620, 0.5 C, ~88%, 1700 | [40] |
| Se@NO-nanocage/CNT | 49/- | Solution method and melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | 470, 1.5 C, ~82%, 200 | [28] |
| CFS@N-C | 55/0.8–1.0 | Self-assembly method | 4 M KFSI in DME | - | 392, 0.15 C, ~97%, 500 | [41] |
| Se-HPC | 42/0.5–1.0 | Freeze-drying process and melt-diffusion | 1 M KPF6 in EC/PC | 0.5–2.5 | ~580, 0.2 C, ~80%, 100 | [42] |
| Se@AHCS | 51.7/~1.0 | Melt-diffusion | 0.8 M KPF6 in EC/DEC | 0.5–2.8 | 547.8, 0.3 C, 59%, 300 | [43] |
| Se@NCHS | 60/~1.2 | Melt-diffusion | 3 M KFSI in EC/DEC | 0.5–3.0 | ~320, 0.5 C, ~62%, 500 | [44] |
| Se@NOPC-CNT | 60/~1.5 | Melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | ~420, 1.2 C, ~80%, 700 | [23] |
| MSTC@Se | 60/~1.5 | Melt-diffusion | 1 M KFSI in EC/DEC | 0.5–3.0 | ~300, 3 C, ~70%, 2000 | [45] |
| Se2–3/Se4–7@MMCFs | 49.4/1.2–1.5 | Electrospinning and melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | 443, 1.5 C, 90%, 2000 | [46] |
| Se/HHPC | 47/~1.5 | Melt-diffusion | 0.1 M KTFSI in DOL/DME | 0.5–2.5 | 589, 0.2 C, 39%, 200 | [47] |
| 3D-N-CPC/Se | 54/1.0–1.5 | Freeze-drying and pyrolysis process | 0.85 M KPF6 in EC/DEC | 0.5–3.0 | ~590, 2 C, ~39%, 800 | [48] |
| PC/Se/GO | 40/- | Melt-diffusion | 0.8 M KPF6 in EC/DEC | 0.5–2.5 | 426.3, 0.5 C, 74%, 150 | [49] |
| FNDPC@Se | 40/- | Melt-diffusion | 0.8 M KPF6 in EC/DEC | 0.5–3.0 | ~150, 3 C, ~72%, 500 | [29] |
| Se@NPCFs | 62/~1.5 | Electrospinning and melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | ~580, 0.75C, ~63%, 1670 | [30] |
| Se@PWC-NS | 9.75/~3.4 | Two-step carbonization and melt-diffusion | 1 M KTFSI in EC/DEC | 0.001–3.0 | 642.7, 0.2 C, 90%, 200 | [50] |
| C-PAN-Se | 40/0.3–0.5 | Mixed sintering | 1 M KPF6 in EC/PC | 0.7–2.3 | 652, 0.2 C, ~61%, 100 | [13] |
| Engineering Chemisorptive Hosts | ||||||
| C-DWHCSs/Se | 62/1.6–2.0 | Template and melt-diffusion | 1 M KPF6 in EC/DEC | 0.5–3.0 | 612.5, 0.2 C, 91%, 100 | [31] |
| Se@MoSe2-HPC | 35/- | Freeze-dried carbonization and melt-diffusion | 1 M KPF6 in EC/PC | 0.5–3.0 | 572.4, 1 C, 56%, 500 | [51] |
| Electrocatalysis of Redox Reactions | ||||||
| Se@P-N-C@Mo2C | 58/~1.0 | Electrospinning and melt-diffusion | 0.7 M KPF6 in EC/DEC | 0.5–3.0 | ~300, 1 C, ~78%, 220 | [32] |
| Se/CoNiSe2-NR | 76.05/1.0–1.5 (typical); 1.7–3.8 (3D printing) | Melt-diffusion and 3D printing | 1 M KPF6 in EC/DEC | 0.5–3.0 | ~450, 0.1 C, ~87%, 150 | [52] |
| Se-W2N/C | 45/~1.0 | Melt-diffusion | 1 M KTFSI in EC/DEC | 0.5–3.0 | 540.7, 0.15 C, 74%, 100 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liang, Y.; Gu, D.; Li, C.; Sui, Z.; Tang, X.; Sun, X.; Liu, Y. The Application of Carbon-Based Materials in Cathodes for High-Performance K-Se Batteries: A Review. Coatings 2025, 15, 1183. https://doi.org/10.3390/coatings15101183
Wang J, Liang Y, Gu D, Li C, Sui Z, Tang X, Sun X, Liu Y. The Application of Carbon-Based Materials in Cathodes for High-Performance K-Se Batteries: A Review. Coatings. 2025; 15(10):1183. https://doi.org/10.3390/coatings15101183
Chicago/Turabian StyleWang, Jingyang, Yanfang Liang, Dongqi Gu, Can Li, Zening Sui, Xibo Tang, Xiaobin Sun, and Yong Liu. 2025. "The Application of Carbon-Based Materials in Cathodes for High-Performance K-Se Batteries: A Review" Coatings 15, no. 10: 1183. https://doi.org/10.3390/coatings15101183
APA StyleWang, J., Liang, Y., Gu, D., Li, C., Sui, Z., Tang, X., Sun, X., & Liu, Y. (2025). The Application of Carbon-Based Materials in Cathodes for High-Performance K-Se Batteries: A Review. Coatings, 15(10), 1183. https://doi.org/10.3390/coatings15101183






