Silicon Oxycarbide Coatings Produced by Remote Hydrogen Plasma CVD Process from Cyclic Tetramethylcyclotetrasiloxane
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
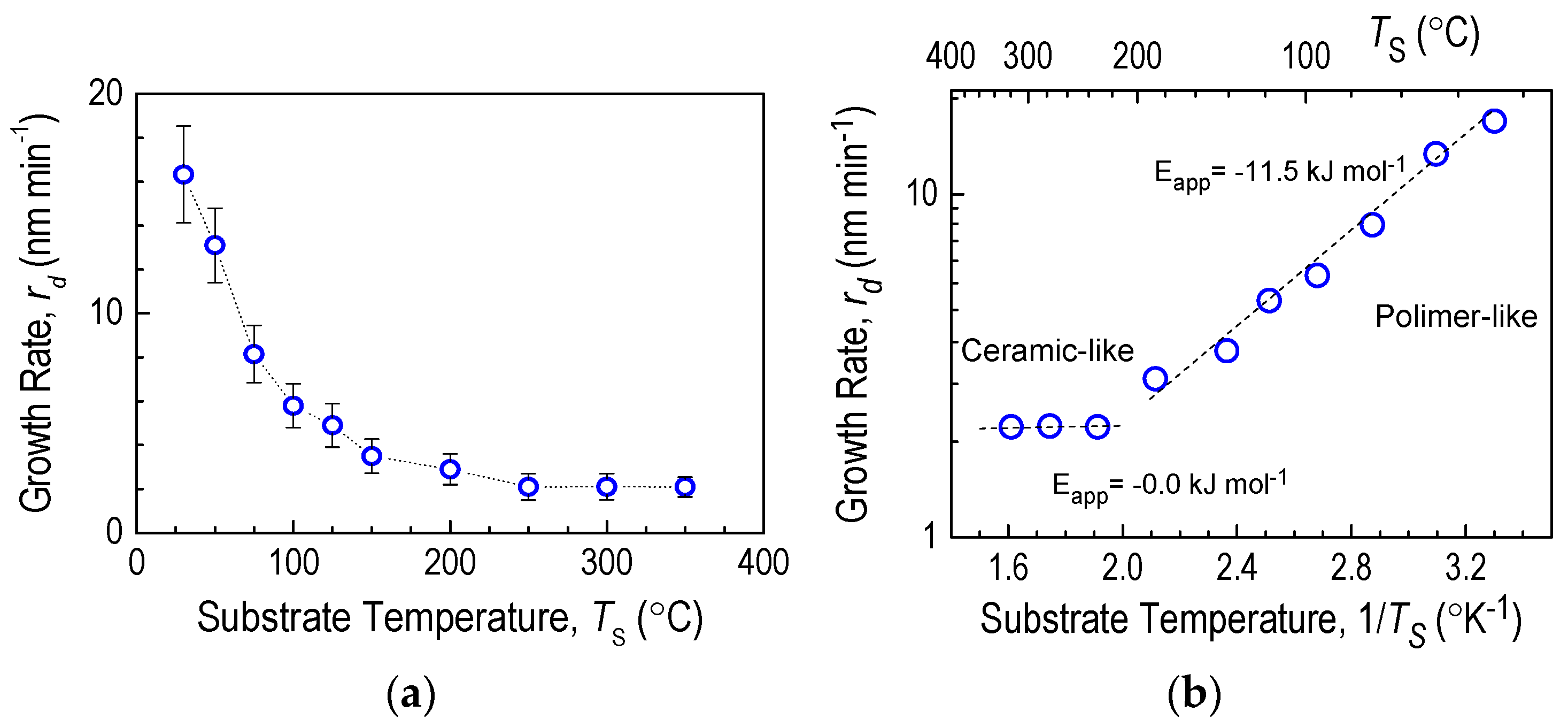
3.1. Influence of Substrate Temperature on Deposition Kinetics
3.2. Chemical Structure of CVD Layers
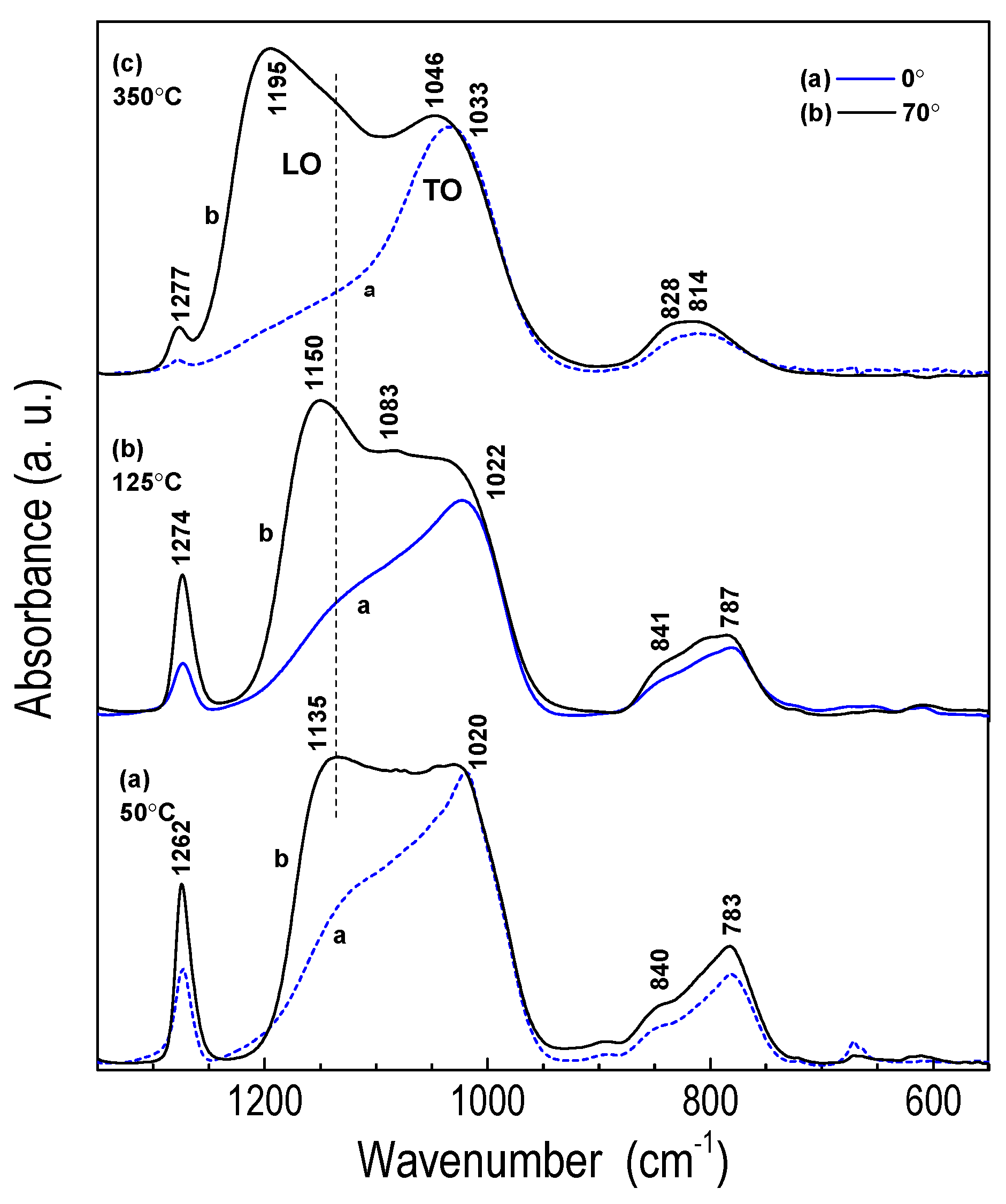
3.2.1. FTIR Analysis of Layer Structure
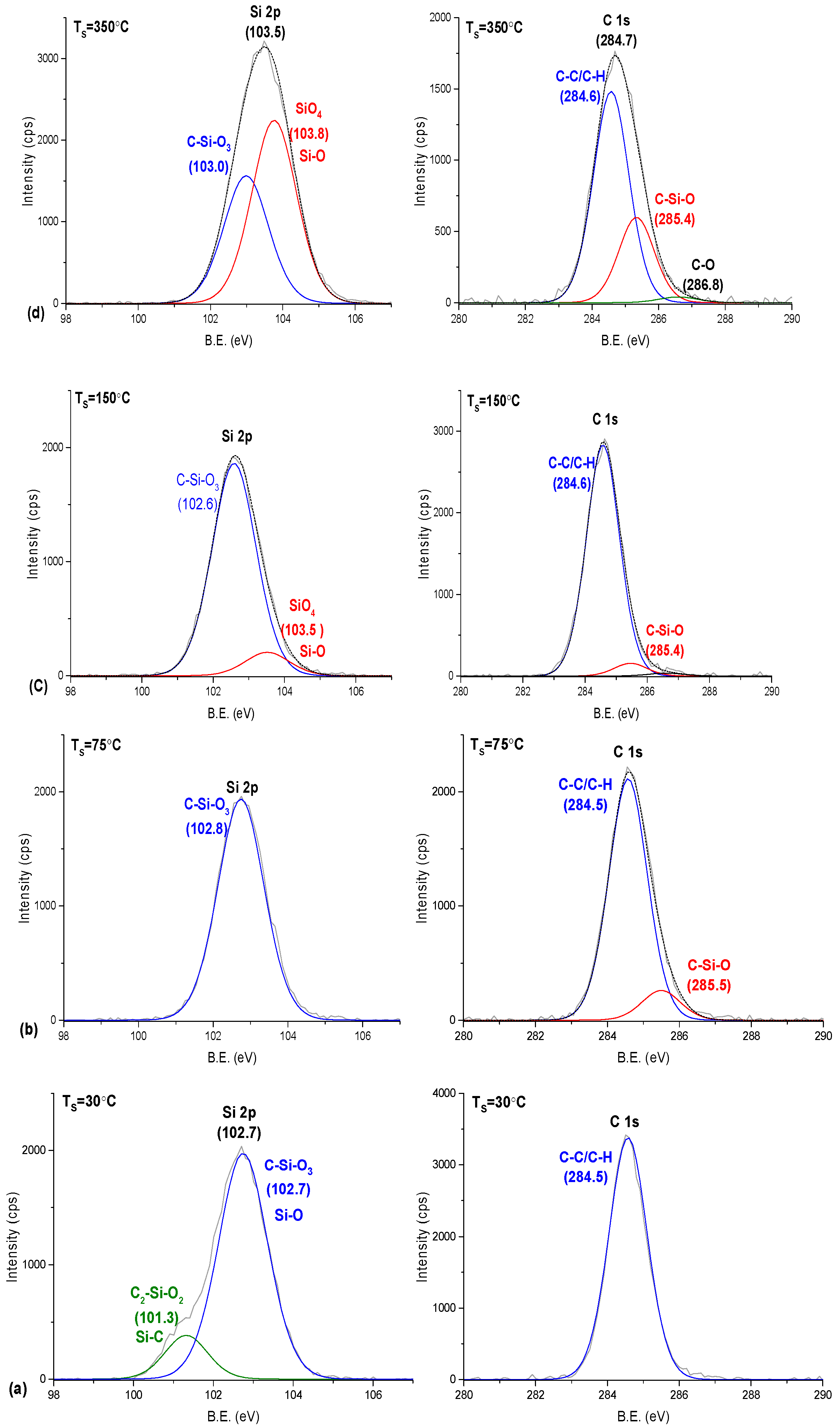
3.2.2. Chemical Composition Analysis by XPS
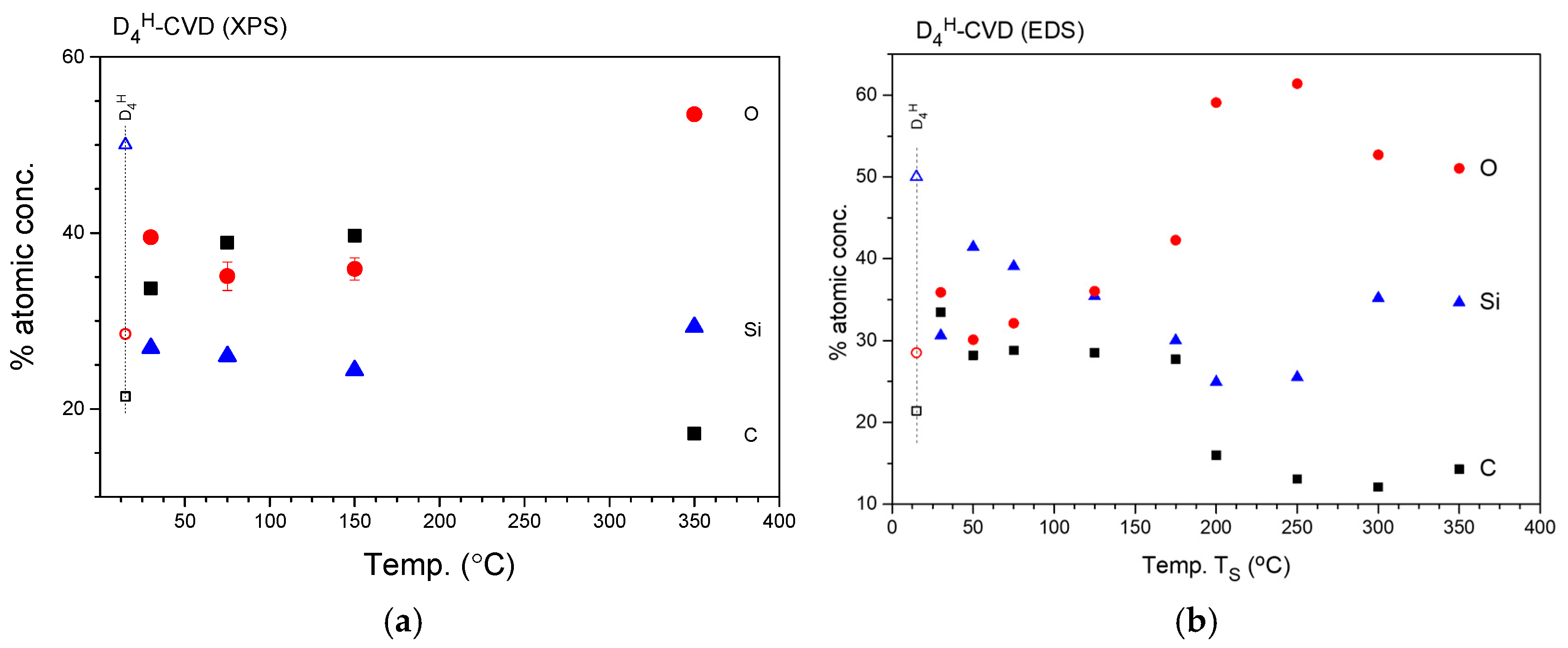
3.2.3. Elemental Composition of the Layers
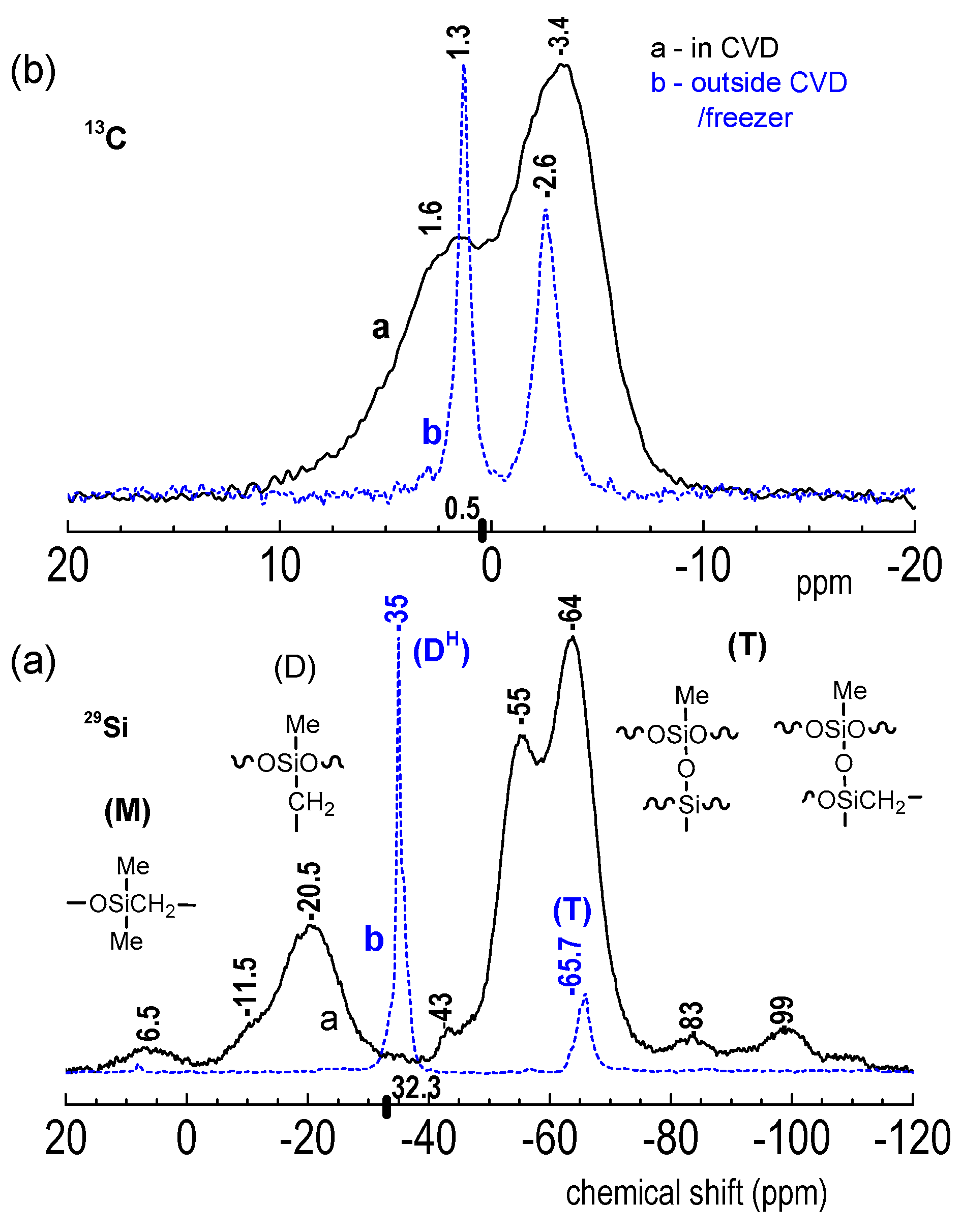
3.2.4. 29Si and 13C NMR Spectroscopy of D4H-CVD and D4H-Freezer Films
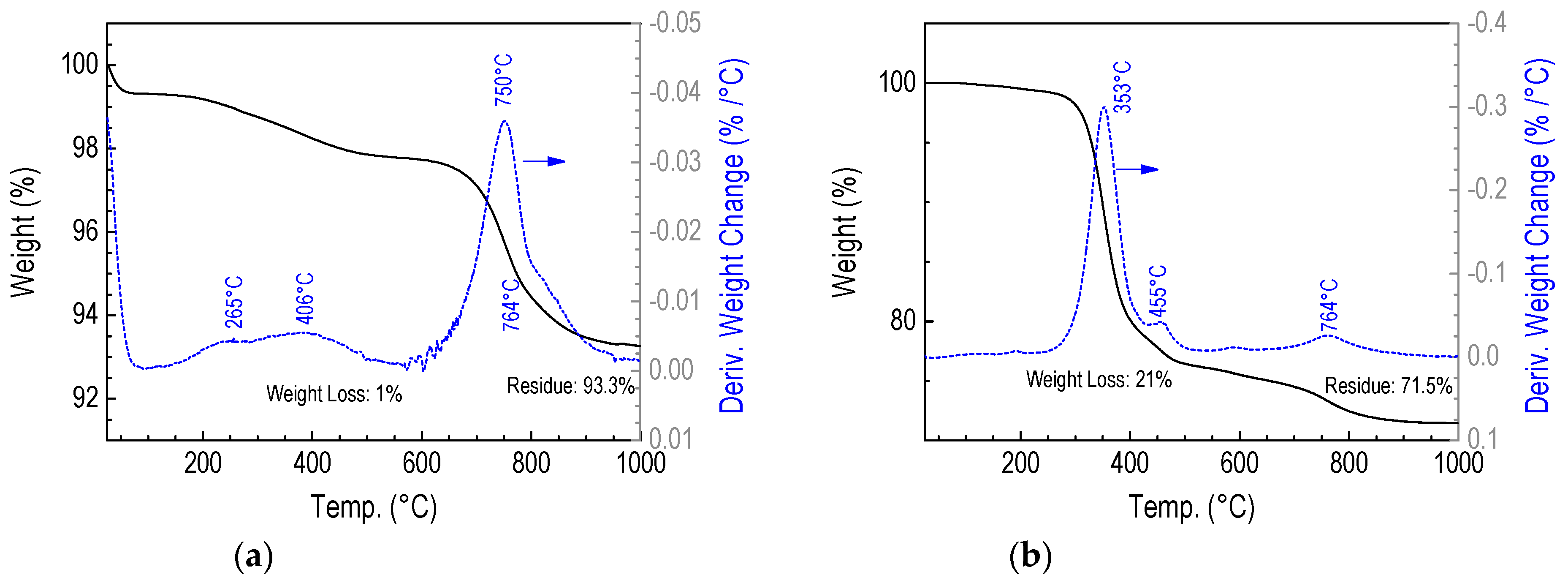
3.2.5. Thermogravimetric Studies of D4H-CVD and D4H-Freezer Films
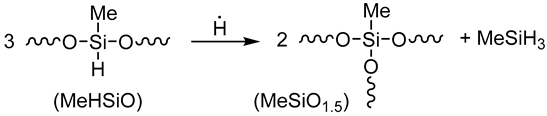
3.2.6. Elementary Chemical Reactions Occurring in the RHP-CVD Process with the Participation of the D4H Precursor
- Activation step
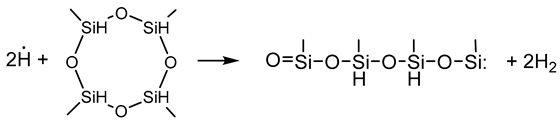

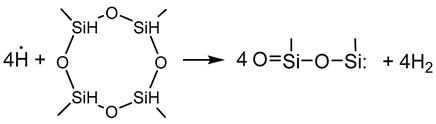
- Growth and networking stage
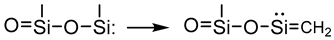
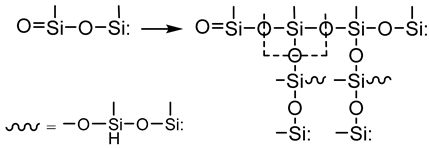

3.2.7. Analysis of Surface Morphology
- Scanning electron microscopy (SEM)
- Atomic force microscopy (AFM)
- Conformal step coverage
3.3. Physical and Physicochemical Properties of D4H-CVD Layers
- Density of D4H-CVD
- Refractive index of CVD layers
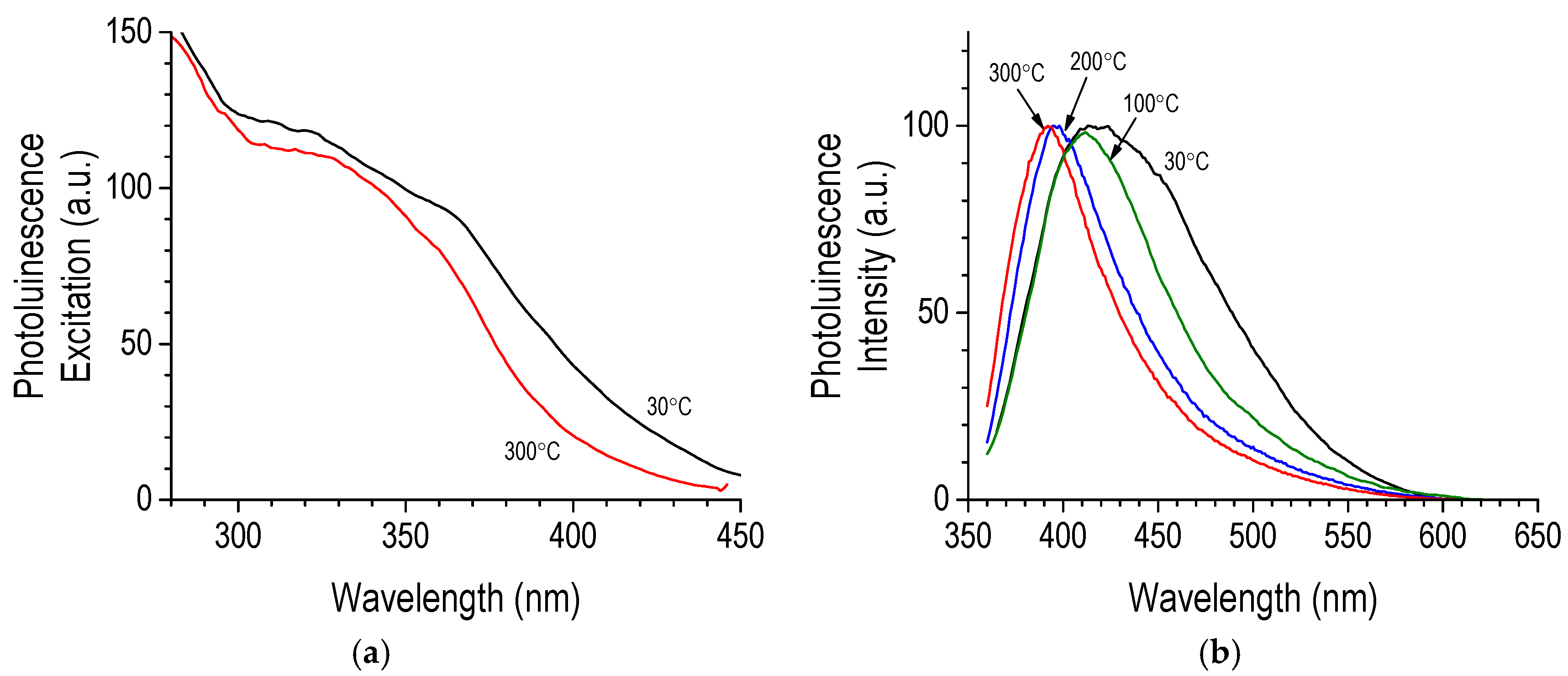
- Photoluminescence of CVD layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CVD | Chemical Vapor Deposition |
| RHP | Remote Hydrogen Plasma |
| AFM | Atomic Force Microscopy |
| HTP | Hydride Transfer Process |
| PL | Photoluminescence |
| XPS | X-ray Photoelectron Spectroscopy |
| D4H | 1,3,5,7-tetracyclotetrasiloxane |
| TMDSO | 1,1,3,3-tetramethyldisiloxane |
| TGA | Thermogravimetric analysis |
| EDS | Energy Dispersive X-ray Spectroscopy |
| SEM | Scanning Electron Microscopy |
| M, D, T, Q | Si structures with a specific number of adjacent oxygen atoms, one, two, three and four, respectively |
References
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based polymer-derived ceramics: Synthesis properties and applications-a review dedicated to Prof. Dr. Fritz Aldinger on the occasion of his 65th birthday. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef]
- Godavarthi, S.; Wang, C.; Verdonck, P.; Matsumoto, Y.; Koudriavtsev, I.; Dutt, A.; Tielens, H.; Baklanov, M. Study of porogen removal by atomic hydrogen generated by hot wire chemical vapor deposition for the fabrication of advanced low-k thin films. Thin Solid Film. 2015, 575, 103–106. [Google Scholar] [CrossRef]
- Islam, M.Z.; Fu, Y.; Deb, H.; Hasan, M.K.; Dong, Y.; Shi, S. Polymer-based low dielectric constant and loss materials for high-speed communication network: Dielectric constants and challenges. Eur. Polym. J. 2023, 200, 112543. [Google Scholar] [CrossRef]
- Salehi, M.M.; Mohammadi, M.; Akbarzadeh, A.R.; Babamoradi, M.; Maleki, A.; Zare, E.N. Advancements in preceramic inorganic polymers for environmental applications: Properties, synthesis, and potential uses. RSC Adv. 2025, 15, 25514–25541. [Google Scholar] [CrossRef]
- Mazo, M.A.; Padilla, I.; López-Delgado, A.; Tamayo, A.; Rubio, J. Silicon Oxycarbide and Silicon Oxycarbonitride Materials under Concentrated Solar Radiation. Materials 2021, 14, 1013. [Google Scholar] [CrossRef]
- Chen, J.; King, S.W.; Muthuswamy, E.; Koryttseva, A.; Wu, D.; Navrotsky, A. Thermodynamic Stability of Low-k Amorphous SiOCH Dielectric Films. J. Am. Ceram. Soc. 2016, 99, 2752–2759. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Wah Wong, M. Generating amorphous SiOC:H Structure: A novel Unit-Cell algorithm and its application in low-k porous material simulations. Comput. Mater. Sci. 2024, 242, 113093. [Google Scholar] [CrossRef]
- Yuan, C.; van der Sluis, O.; Zhang, G.Q.; Ernst, L.J.; Driel, W.; van silfhout, R.; Thijsse, B. Chemical–mechanical relationship of amorphous/porous low-dielectric film materials. Comput. Mater. Sci. 2007, 42, 606–613. [Google Scholar] [CrossRef]
- Ramos-Serrano, J.R.; Matsumoto, Y.; Ávila, A.; Romero, G.; Meneses, M.; Morales, A.; Luna, J.A.; Flores, J.; Minquiz, G.M.; Moreno-Moreno, M. Luminescence Study of Hydrogenated Silicon Oxycarbide (SiOxCy:H) Thin Films Deposited by Hot Wire Chemical Vapor Deposition as Active Layers in Light Emitting Devices. Inorganics 2024, 12, 298. [Google Scholar] [CrossRef]
- Grill, A. From tribological coatings to low-k dielectrics for ULSI interconnects. Thin Solid Film. 2001, 398–399, 527–532. [Google Scholar] [CrossRef]
- Du, P.; Wang, X.; Lin, I.K.; Zhang, X. Effects of composition and thermal annealing on the mechanical properties of silicon oxycarbide films. Sens. Actuators A Phys. 2012, 176, 90–98. [Google Scholar] [CrossRef]
- Plummer, J.D. Silicon VLSI Technology: Fundamentals, Practice, and Modeling; Pearson Education India: Delhi, India, 2009. [Google Scholar]
- Arango Ospina, M.; Xie, F.; Gonzalo de Juan, I.; Riedel, R.; Ionescu, E.; Boccaccini, A. Review: Silicon oxycarbide based materials for biomedical applications. Appl. Mater. Today 2019, 18, 100482. [Google Scholar] [CrossRef]
- Zhuo, R.; Colombo, P.; Pantano, C.; Vogler, E.A. Silicon oxycarbide glasses for blood-contact applications. Acta Biomater. 2005, 1, 583–589. [Google Scholar] [CrossRef]
- Böke, F.; Giner, I.; Keller, A.; Grundmeier, G.; Fischer, H. Plasma-Enhanced Chemical Vapor Deposition (PE-CVD) yields better Hydrolytical Stability of Biocompatible SiOx Thin Films on Implant Alumina Ceramics compared to Rapid Thermal Evaporation Physical Vapor Deposition (PVD). ACS Appl. Mater. Interfaces 2016, 8, 17805–17816. [Google Scholar] [CrossRef]
- Mizerska, U.; Rubinsztajn, S.; Chojnowski, J.; Cypryk, M.; Uznanski, P.; Walkiewicz-Pietrzykowska, A.; Fortuniak, W. Self-Restructuring of Polyhydromethylsiloxanes by the Hydride Transfer Process: A New Approach to the Cross-Linking of Polysiloxanes and to the Fabrication of Thin Polysiloxane Coatings. Materials 2022, 15, 6981. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Uznanski, P.; Walkiewicz-Pietrzykowska, A. Silicon Oxycarbide Films Produced by Remote Microwave Hydrogen Plasma CVD using a Tetramethyldisiloxane Precursor: Growth Kinetics, Structure, Surface Morphology, and Properties. Chem. Vap. Depos. 2015, 21, 307–318. [Google Scholar] [CrossRef]
- Uznanski, P.; Walkiewicz-Pietrzykowska, A.; Jankowski, K.; Zakrzewska, J.; Wrobel, A.M.; Balcerzak, J.; Tyczkowski, J. Atomic Hydrogen Induced Chemical Vapor Deposition of Silicon Oxycarbide Thin Films Derived from Diethoxymethylsilane Precursor. Appl. Organomet. Chem. 2020, 34, e5674. [Google Scholar] [CrossRef]
- Agnieszka, W.-P.; Pawel, U.; Aleksander, M.W. Silicon Carbide, Silicon Carbonitride, and Silicon Oxycarbide Thin Films Formed by Remote Hydrogen Microwave Plasma CVD. Curr. Org. Chem. 2017, 21, 2229–2239. [Google Scholar] [CrossRef]
- Uznanski, P.; Glebocki, B.; Walkiewicz-Pietrzykowska, A.; Zakrzewska, J.; Wrobel, A.M.; Balcerzak, J.; Tyczkowski, J. Surface modification of silicon oxycarbide films produced by remote hydrogen microwave plasma chemical vapour deposition from tetramethyldisiloxane precursor. Surf. Coat. Technol. 2018, 350, 686–698. [Google Scholar] [CrossRef]
- Walkiewicz-Pietrzykowska, A.; Jankowski, K.; Kurjata, J.; Dolot, R.; Brzozowski, R.; Zakrzewska, J.; Uznanski, P. Silicon Oxycarbide Thin Films Produced by Hydrogen-Induced CVD Process from Cyclic Dioxa-Tetrasilacyclohexane. Materials 2025, 18, 2911. [Google Scholar] [CrossRef]
- King, S.W.; French, M.; Bielefeld, J.; Lanford, W.A. Fourier transform infrared spectroscopy investigation of chemical bonding in low-k a-SiC:H thin films. J. Non-Cryst. Solids 2011, 357, 2970–2983. [Google Scholar] [CrossRef]
- Raynaud, P.; Despax, B.; Segui, Y.; Caquineau, H. FTIR Plasma Phase Analysis of Hexamethyldisiloxane Discharge in Microwave Multipolar Plasma at Different Electrical Powers. Plasma Process. Polym. 2005, 2, 45–52. [Google Scholar] [CrossRef]
- Vautrin-Ul, C.; Roux, F.; Boisse-Laporte, C.; Pastol, J.L.; Chausse, A. Hexamethyldisiloxane (HMDSO)-plasma-polymerised coatings as primer for iron corrosion protection: Influence of RF bias. J. Mater. Chem. 2002, 12, 2318–2324. [Google Scholar] [CrossRef]
- Wu, Q.; Gleason, K.K. Plasma-Enhanced CVD of Organosilicate Glass (OSG) Films Deposited from Octamethyltrisiloxane, Bis(trimethylsiloxy)methylsilane, and 1,1,3,3-Tetramethyldisiloxane. Plasmas Polym. 2003, 8, 31–41. [Google Scholar] [CrossRef]
- Launer, P.; Arkles, B. Infrared Analysis of Organosilicon Compounds. In Silicon Compounds: Silanes & Silicones; Gelest Inc.: Glen Rock, PA, USA, 2013; pp. 175–178. [Google Scholar]
- Smith, A.L. Infrared spectra-structure correlations for organosilicon compounds. Spectrochim. Acta 1960, 16, 87–105. [Google Scholar] [CrossRef]
- Yu, S.; Tu, R.; Goto, T. Preparation of SiOC nanocomposite films by laser chemical vapor deposition. J. Eur. Ceram. Soc. 2015, 36, 403–409. [Google Scholar] [CrossRef]
- Ma, J.; Shi, L.; Shi, Y.; Luo, S.; Xu, J. Pyrolysis of polymethylsilsesquioxane. J. Appl. Polym. Sci. 2002, 85, 1077–1086. [Google Scholar] [CrossRef]
- Ellul, R.; Potzinger, P.; Reimann, B. Reaction of hydrogen atoms with hexamethyldisilane. J. Phys. Chem. 1984, 88, 2793–2796. [Google Scholar] [CrossRef]
- Fabry, L.; Potzinger, P.; Reimann, B.; Ritter, A.; Steenbergen, H.P. Gas-phase homolytic substitution reactions of hydrogen atoms at silicon centers. Organometallics 1986, 5, 1231–1235. [Google Scholar] [CrossRef]
- Yajima, S.; Hasegawa, Y.; Hayashi, J.; Iimura, M. Synthesis of continuous silicon carbide fibre with high tensile strength and high Young’s modulus. J. Mater. Sci. 1978, 13, 2569–2576. [Google Scholar]
- Birot, M.; Pillot, J.-P.; Dunogues, J. Comprehensive Chemistry of Polycarbosilanes, Polysilazanes, and Polycarbosilazanes as Precursors of Ceramics. Chem. Rev. 1995, 95, 1443–1477. [Google Scholar] [CrossRef]
- Wróbel, A.M.; Walkiewicz-Pietrzykowska, A.; Klemberg-Sapieha, J.E.; Nakanishi, Y.; Aoki, T.; Hatanaka, Y. Remote Hydrogen Plasma Chemical Vapor Deposition from (Dimethylsilyl)(trimethylsilyl)methane. 1. Kinetics of the Process; Chemical and Morphological Structure of Deposited Silicon−Carbon Films. Chem. Mater. 2003, 15, 1749–1756. [Google Scholar] [CrossRef]
- Baxamusa, S.H.; Gleason, K.K. Thin Polymer Films with High Step Coverage in Microtrenches by Initiated CVD. Chem. Vap. Depos. 2008, 14, 313–318. [Google Scholar] [CrossRef]
- Wrobel, A.M.; Walkiewicz-Pietrzykowska, A.; Uznanski, P. Remote hydrogen microwave plasma chemical vapor deposition from methylsilane precursors. 2. Surface morphology and properties of deposited a-SiC:H films. Thin Solid Film. 2014, 564, 232–240. [Google Scholar] [CrossRef]
- Wróbel, A.M.; Walkiewicz-Pietrzykowska, A.; Wickramanayaka, S.; Hatanaka, Y. Mechanism of Amorphous Silica Film Formation from Tetraethoxysilane in Atomic Oxygen-Induced Chemical Vapor Deposition. J. Electrochem. Soc. 1998, 145, 2866–2876. [Google Scholar] [CrossRef]
- Coyopol, A.; Garcia-Salgado, G.; Díaz-Becerril, T.; Vásquez-Agustín, M.A.; Romano-Trujillo, R.; López, R.; Rosendo, E.; Nieto-Caballero, F.G.; Morales-Ruiz, C.; Morales-Sanchez, A. Strong white light emission from SiCxOy films grown by HFCVD technique. Opt. Mater. 2020, 99, 109551. [Google Scholar] [CrossRef]
- Oh, T. Photoluminescence characteristics of SiOC thin films deposited by using inductively coupled plasma chemical vapor deposition with annealing. Inf. Int. Interdiscip. J. 2013, 16, 1391–1397. (In Japanese) [Google Scholar]
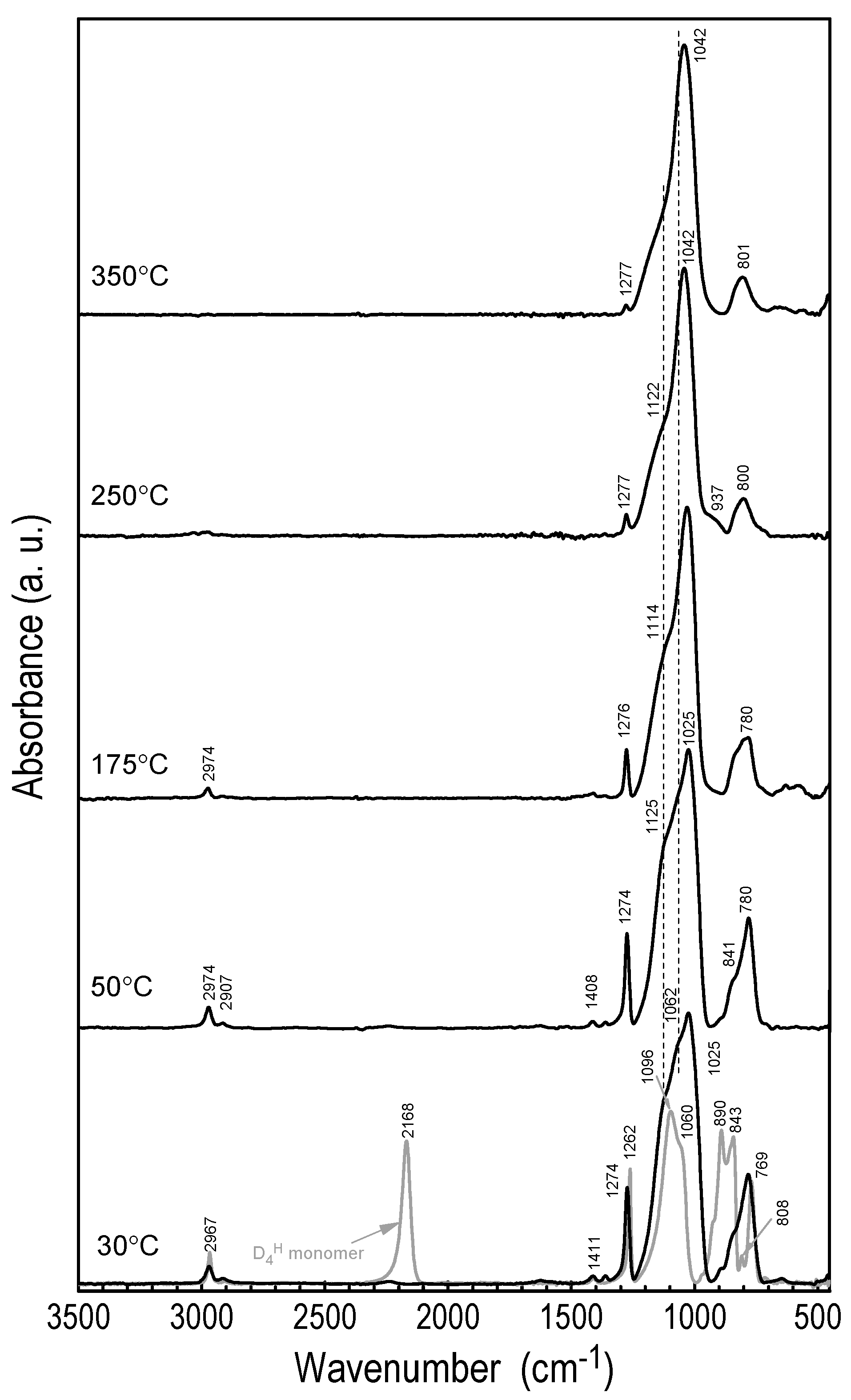
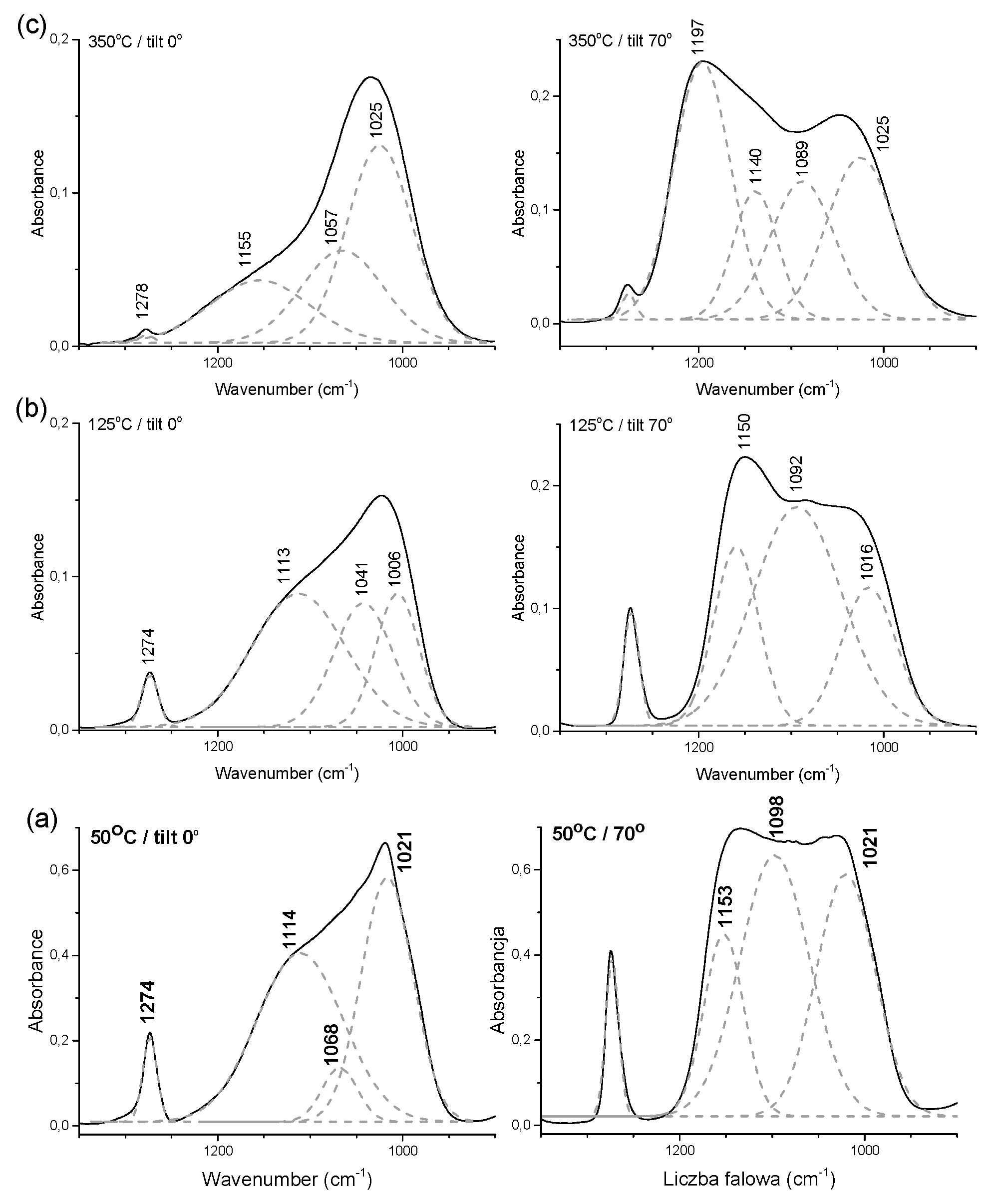
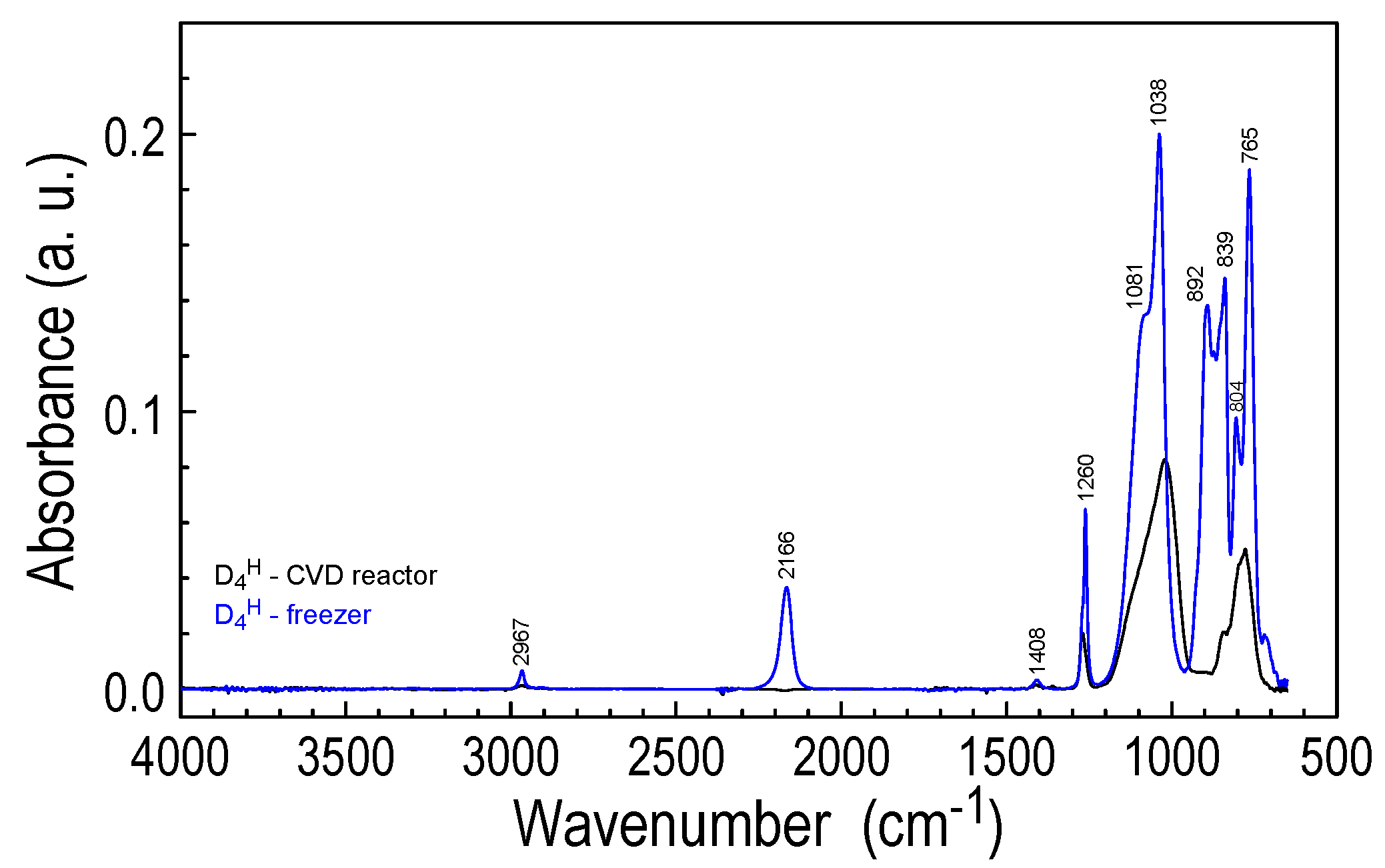





| Vibration Assignment * | Band Position (cm−1) | ||||
|---|---|---|---|---|---|
| D4H (Precursor) | CVD-30 °C TS = 30 °C | CVD-175 °C TS = 175 °C | CVD-250 °C TS = 250 °C | CVD-350 °C TS = 350 °C | |
| νas(CH3) | 2967 | 2967 | 2974 | - | - |
| νs(CH3) | 2907 | 2907 | 2916 | - | - |
| νas(Si–H) | 2168 | 2240 | - | - | - |
| δas(CH3) in Si-(CH3) | 1411 | 1411 | 1413 | - | - |
| δ(Si–CH2–) | - | 1361 | 1364 | - | |
| δs(CH3) in Si-(CH3) | 1262 | 1274 | 1276 | 1277 | 1277 |
| νas(Si–O–Si) | 1096 | 1125 sh | 1114 sh | 1122 sh | 1155 sh |
| νas(Si–O–Si) | 1060 sh | 1062 sh | - | - | - |
| νs(Si–O–Si) | - | 1025 | 1030 | 1042 | 1042 |
| δ(Si–H) | 918 sh | - | - | - | - |
| –O–SiHCH3 | 890 | 890 | - | - | - |
| ρ(CH3) CHw Si–(CH3) | 843 | 840 sh | 832 sh | 831 sh | - |
| Si–C (carbide) | 800 | 801 | |||
| 808 | |||||
| νs(Si–C) in Si–(CH3) | 769 | 780 | 780 | - | - |
| Electron Level | Number of Component Bands | Binding Energy (eV) | Half-Width (eV) | Assigned Structure |
|---|---|---|---|---|
| Si(2p) | 4 | 101.3 102.7 103.5 103.8 | 1.1 1.1 1.2 | Si–C Si–O2– Si–O3– Si–O4– |
| C(1s) | 2 | 284.5 285.4 | 1.2 1.2 | CHx, C–C C–O–Si |
| O(1s) | 1 | 532.4 | 1.2–1.4 | O–Si |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkiewicz-Pietrzykowska, A.; Jankowski, K.; Brzozowski, R.; Zakrzewska, J.; Uznański, P. Silicon Oxycarbide Coatings Produced by Remote Hydrogen Plasma CVD Process from Cyclic Tetramethylcyclotetrasiloxane. Coatings 2025, 15, 1179. https://doi.org/10.3390/coatings15101179
Walkiewicz-Pietrzykowska A, Jankowski K, Brzozowski R, Zakrzewska J, Uznański P. Silicon Oxycarbide Coatings Produced by Remote Hydrogen Plasma CVD Process from Cyclic Tetramethylcyclotetrasiloxane. Coatings. 2025; 15(10):1179. https://doi.org/10.3390/coatings15101179
Chicago/Turabian StyleWalkiewicz-Pietrzykowska, Agnieszka, Krzysztof Jankowski, Romuald Brzozowski, Joanna Zakrzewska, and Paweł Uznański. 2025. "Silicon Oxycarbide Coatings Produced by Remote Hydrogen Plasma CVD Process from Cyclic Tetramethylcyclotetrasiloxane" Coatings 15, no. 10: 1179. https://doi.org/10.3390/coatings15101179
APA StyleWalkiewicz-Pietrzykowska, A., Jankowski, K., Brzozowski, R., Zakrzewska, J., & Uznański, P. (2025). Silicon Oxycarbide Coatings Produced by Remote Hydrogen Plasma CVD Process from Cyclic Tetramethylcyclotetrasiloxane. Coatings, 15(10), 1179. https://doi.org/10.3390/coatings15101179






