Abstract
Due to their low elasticity modulus, significant fatigue strength, and good formability, titanium and titanium alloys have shown a continuous growth trend in various fields of application. However, the passivation film on the surface of titanium and titanium alloys may dissolve, leading to corrosion under certain environmental conditions. Surface modification of these materials has become an indispensable and critical step in meeting the requirements of various operating conditions of material performance. Compared to other surface treatment techniques, plasma surface treatment has advantages such as high efficiency, wide applicability, environmental friendliness, flexibility and controllability, and low-temperature treatment. This article focuses on the topic of plasma surface modification technology for titanium and titanium alloys and highlights the key limitations of Plasma chemical heat treatment, Physical Vapor Deposition (PVD), plasma-enhanced chemical vapor deposition (PECVD), Plasma immersion ion implantation (PIII), and plasma spraying (PS). The current research status of surface modification methods in improving the surface properties of titanium and titanium alloys and the prospects of surface modification technology for titanium alloys are also discussed.
1. Introduction
1.1. Corrosion Behavior of Titanium and Titanium Alloys
Titanium and titanium alloys are characterized by their lightweight, high strength, and excellent corrosion resistance, particularly in saline or seawater environments and marine atmospheric conditions [1,2,3,4,5]. Consequently, titanium is considered a leading lightweight structural material and is often referred to as the “marine metal,” establishing its importance as a strategic resource [6,7,8,9,10,11,12]. Moreover, titanium alloys’ remarkable biocompatibility makes them invaluable in fields such as biomedical sciences and medicine [13,14,15,16,17,18]. Titanium is a transition metal found in the fourth period and Group IVB of the periodic table. It is widely used as a structural metal in engineering due to its abundance of approximately 0.42% in the Earth’s crust, highlighting its plentiful availability as a resource [19,20].
During the use of titanium and titanium alloy products, they are often affected by corrosion factors in the environment. In the marine environment, the accumulation of chloride ions may lead to local acidification, which in turn can cause crevice corrosion [21,22,23]. At the same time, titanium and titanium alloys in the ocean are often affected by deep-sea high pressure or hydrostatic pressure, which can change the electrode potential of the material, increase the rate of hydrogen evolution reaction, accelerate hydrogen-induced cracking stress corrosion, and greatly reduce the stability of the passivation film on the surface of titanium alloys. In the human environment, due to the synergistic effect of body fluid corrosion and micro abrasive wear, the passivation film on the surface of titanium alloys is more easily damaged than expected, resulting in the much lower corrosion resistance of titanium alloy implants than expected [24,25]. Therefore, solving the problem of the insufficient corrosion resistance of titanium alloy products in marine and human environments is of great significance for extending the service life of titanium alloy products and reducing product maintenance and renewal costs [26,27]. Various surface technologies for titanium and titanium alloys have been widely used to improve corrosion resistance [28,29].
Figure 1 illustrates several titanium alloy workpieces and implants used in the marine industry. Titanium and titanium alloys are particularly susceptible to issues such as pitting corrosion, stress corrosion, and galvanic corrosion during operation [30,31,32,33,34,35]. For example, as a submersible descends to greater ocean depths, it is exposed to extreme hydrostatic pressure, which can compromise its corrosion resistance by promoting pitting and damaging the protective oxide layer on the surface. Additionally, factors such as seawater salinity, oxygen content, pH, and temperature vary with depth, resulting in different effects on the corrosion behavior of titanium [36,37,38,39,40].
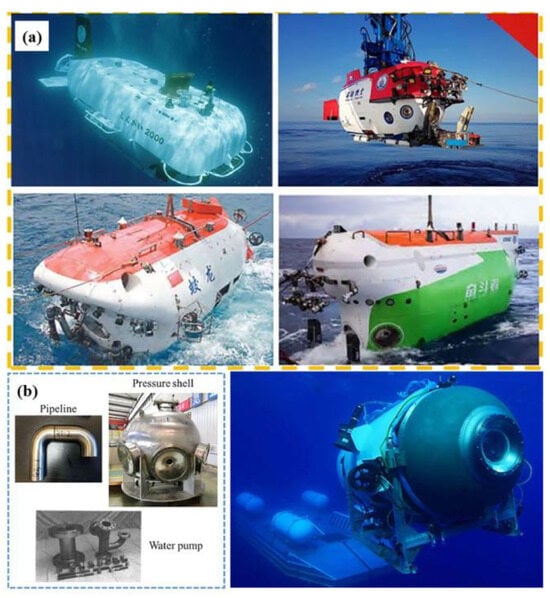
Figure 1.
(a) Pictures of a manned submersible fabricated using Ti alloys; (b) pictures of a Ti alloy-based pressure shell, pipeline, and water pump for marine equipment (adapted from [41], with permission).
Titanium and its alloys are widely utilized in marine engineering due to their low density, excellent corrosion resistance, good heat resistance, ease of welding, and high impact strength, earning them the designation “marine metals” [42,43,44,45,46]. They are extensively applied in components such as ship hull valves, engine pumps, submersible pressure hulls, seawater desalination equipment, propellers, and marine pipelines [47,48,49]. The surface seawater contains nearly saturated gases such as O2 and CO2, along with various inorganic salts, primarily NaCl, and has a pH of approximately 8.2, making it a complex corrosive electrolyte. When titanium and its alloys are exposed to seawater, their surfaces are prone to the accumulation of inorganic salt deposits like NaCl, which can lead to electrochemical corrosion [50,51,52,53]. As a submersible descends to greater ocean depths, the material is subjected to immense hydrostatic pressure, which compromises its corrosion resistance by promoting pitting and damaging the passive film on the surface [54,55,56]. Figure 2 illustrates the corrosion mechanism of a dual-phase titanium alloy in seawater, primarily focusing on the formation and dissolution process of the passive film on the alloy surface [57]. The titanium alloy matrix undergoes hydration reaction in seawater, and TiO2 is continuously generated on the alloy surface:
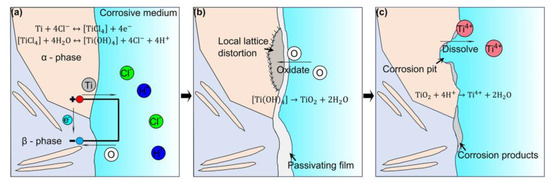
Figure 2.
Growth and dissolution process of passivated film on the surface of biphasic titanium alloy in sea water (adapted from [57], with permission).
When the externally applied anode potential exceeds a certain value, the passive film on the surface will be damaged, and a dissolution reaction will occur:
Titanium alloys are known for their high strength, toughness, excellent corrosion resistance, and good biocompatibility and mechanical compatibility, which make them widely used in biomedical applications [58,59,60,61,62,63,64]. They have become the primary material for medical surgical implants, with well-established applications, particularly in the fields of dentistry and orthopedics [65,66,67,68,69]. Figure 3a,b presents schematic diagrams of a titanium alloy dental implant. Generally, the corrosion resistance and chemical inertness of titanium are sufficient to meet the requirements for implants in the human body [70]. However, due to the complexity of the human body environment, implants are subject to higher stability requirements [71,72,73,74,75,76]. In particular, the oral environment is more demanding, as the pH in the mouth fluctuates with behaviors such as eating, food residues, dental plaque, and other factors, which impose stricter corrosion resistance requirements on the implants. As a result, the actual lifespan of the implant is often shorter than its predicted lifespan [77,78,79]. Once titanium alloy undergoes corrosion in the human body’s physiological environment, aluminum (Al) and vanadium (V) ions diffuse into the surrounding tissues, which may lead to toxic side effects and impair normal bodily functions [80]. Therefore, creating a dense passivation layer on the surface of a titanium alloy is an effective approach to minimize its toxicity.
Titanium alloys exhibit more significant corrosion in fluoride-containing and acidic fluoride saliva solutions compared to environments with only fluoride ions. Notable localized corrosion occurred, with fluoride ions potentially causing the rupture of the protective passive film on the titanium’s surface, ultimately leading to pitting corrosion [81,82]. Ferreira et al. [83] proposed a synergistic effect between corrosion and mechanical wear of titanium alloy implants in Ringer’s solution, with experimental validation conducted using the ASTM-F67 Gr1 titanium alloy. The results indicate that higher-friction loads increase the corrosion degradation rate of titanium alloys. Therefore, the combined effects of corrosion and wear on implant lifespan should also be considered when applying titanium alloy implants. Corne et al. [84] conducted a 16 h fretting wear test on Ti6Al4V dental implants in a human saliva environment. The results indicate that the implants were severely corroded due to fretting, and that titanium–niobium alloys and β-titanium alloys exhibited better corrosion resistance compared to Ti6Al4V alloys. Figure 3 shows a schematic diagram of a dental implant and a bone implant being corroded in the human environment. Therefore, strengthening the traditional Ti6Al4V titanium alloy is necessary to meet the demands of the human bodily fluid environment [85].
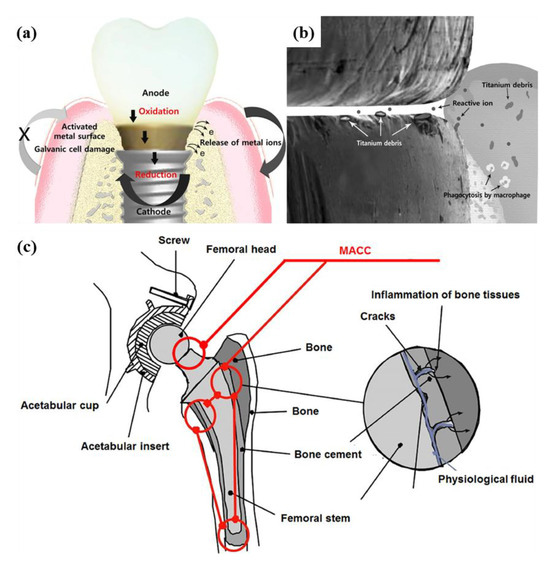
Figure 3.
(a,b) Schematic representation of Ti debris and reactive ions related to galvanic corrosion in the implant-attached gingival connection; (c) mechanical-assisted crevice corrosion (MACC) of total hip replacement implants (adapted [86] from and [2], with permission).
The study of early corrosion principles of metals, especially titanium and titanium alloy workpieces, at the nanoscale using theoretical models is of great significance [87]. Wang et al. [88] explored the principle of stress corrosion cracking induced by hydrides in titanium alloys using first principles and explained the formation of titanium hydride at the atomic scale, making important contributions to the theoretical research of stress corrosion cracking in titanium alloys. Xu et al. [89] conducted molecular dynamics simulations on titanium. The simulation results indicate that screw dislocations may contribute to the formation of microstructures containing high-density twin interfaces and be beneficial for mechanical properties. The interaction between dislocations may form various substructures, which may further accumulate as defects and ultimately lead to the nucleation and growth of cracks during deformation. This provides a theoretical basis for understanding the atomic mechanisms of deformation and phase transformation in titanium and titanium alloys.
Surface modification of titanium alloys has become an indispensable and critical step in meeting the requirements of various operating conditions on material performance. Traditional methods, such as polymer coating, can also enhance the corrosion resistance of titanium alloys [90,91,92,93]. Hussain et al. [94] prepared epoxy anti-corrosive coating containing nano-silica by the sol–gel method to improve the mechanical and microstructure of the coating. The experimental results indicate that nano-silica improves the mechanical and microstructural properties of the coating, thereby enhancing its corrosion resistance. The low-frequency impedance modulus of the epoxy-based anti-corrosion coating containing 2 wt% nano-silica is increased by up to six orders of magnitude and has the widest capacitance loop in the Nyquist plot. This reflects the potential of polymer coatings for protecting metal substrates. Meng et al. [95] prepared waterborne acrylic anti-corrosive coatings containing different proportions of yttrium oxide/titanium dioxide by the sol–gel method to further improve the corrosion resistance of the coating. The research results indicate that the addition of oxide particles reduces the volatility of the resin-curing reaction. Y2O3 and TiO2 particles reduce the diffusion rate of electrolyte into the coating through pore filling, thereby reducing the possibility of resin degradation in the corrosion coating. The 1:4 weight ratio of the yttrium oxide/titanium dioxide coating exhibits better electrochemical performance and better corrosion resistance.
Compared with other surface treatment techniques, plasma surface treatment has advantages such as high efficiency, wide applicability, environmental friendliness, flexibility and controllability, and low-temperature treatment. This article focuses on the topic of plasma surface modification technology for titanium and titanium alloys and provides an overview of Plasma chemical heat treatment, Physical Vapor Deposition (PVD), plasma-enhanced chemical vapor deposition (PECVD), plasma ion implantation (PIII) and plasma spraying (PS).
1.2. Plasma Generation and Its Influence on Surface Treatment
Plasma is the fourth state of matter (excluding solid, liquid, and gas), composed of ionized gases containing free electrons, ions, and neutral particles. It ionizes gases through high temperatures or strong electric fields, accounting for over 99% of visible matter in the universe. Plasma is an ionized gaseous substance composed of positively and negatively charged ions, generated by the ionization of atoms and atomic clusters through partial electron removal. It is a macroscopically electrically neutral ionized gas with a scale larger than the Debye length. Plasma, considered the fourth state of matter (alongside solid, liquid, and gas), is widely present in nature [96,97,98]. Plasma, as the fourth state of matter, has significant differences in energy state compared to ordinary gases, mainly reflected in high ionization degree, collective behavior, and complex energy distribution. As an excellent conductor, plasma can be influenced by magnetic fields, which can be used to capture, move, or accelerate it. Therefore, the development of plasma physics has introduced new ideas and methods for advancing material surface-strengthening technologies [99]. Plasma is produced by a variety of methods, the most common of which, as well as in laboratory and industrial applications, are gas discharge, laser, and microwave for plasma generation [100,101].
When two electrodes are placed in a gas containing free-charged particles and a voltage is applied, a current will flow through the gas, resulting in the generation of plasma. This phenomenon is known as gas discharge [102]. Gas discharge is primarily classified into dark discharge, glow discharge, arc discharge, corona discharge, spark discharge, and high-frequency discharge, depending on factors such as applied voltage, power frequency, current magnitude, and gas pressure. Among these, glow discharge and arc discharge have been particularly applied and developed for material surface strengthening due to their advantages of low gas pressure, low energy consumption, and ease of control [103,104]. When a high DC voltage is applied between two electrodes in a thin gas, electrons are accelerated in the electric field, gaining high kinetic energy. These high-energy electrons collide inelastically with neutral gas molecules, exciting them. The resulting electrons are further accelerated, initiating an avalanche ionization process that leads to glow discharge. This phenomenon allows for ion diffusion and deposition onto the substrate surface, serving as the basis for plasma chemical heat treatment and magnetron sputtering. If the gas is ionized by high voltage or the electrodes short-circuit, the cathode emits intense hot electrons at high temperatures. A large number of electrons are then emitted and bombard the anode, sustaining stable discharge with low voltage and high current. During this discharge process, significant heat is generated, fully ionizing the gas and forming a thermally balanced plasma, which is characteristic of arc discharge. The high plasma density of this discharge phenomenon enables rapid coating deposition or spraying, which is the primary reason for the high coating efficiency of cathodic arc ion plating and plasma spraying [105].
When a short-pulse laser with extremely high power density is focused on a small area, the target surface absorbs a large amount of energy in a very short time, causing a rapid temperature increase. This leads to melting, vaporization, and eventually the formation of a highly ionized plasma plume consisting of electrons, ions, and unionized particles that expand outward, creating laser plasma [90]. This type of plasma is highly controllable, allowing for precise control over its generation position and characteristics by adjusting the laser’s energy, pulse width, and wavelength. For example, laser shock plasma nitridation and laser pulse deposition are achieved through laser shock, enabling the preparation of high-precision and high-quality surface strengthening layers [106].
When a microwave of a specific frequency is transmitted into a resonance cavity filled with low-pressure gas, the alternating electromagnetic field of the microwave oscillates a small number of free electrons in the gas, causing them to gain energy. These energized electrons then ionize the gas by colliding with neutral particles. Once the ionization process reaches equilibrium, a microwave plasma is formed [107]. This method avoids electrode corrosion, melting, and contamination of the electrode material by the plasma, allowing for the production of very pure plasma. It is commonly used in plasma-enhanced chemical vapor deposition (PECVD) technology and plays a key role in the preparation of diamond films and carbon nanotubes [108].
2. Plasma Surface Strengthening Method
To further enhance the corrosion resistance of titanium alloys in complex and harsh service environments, surface strengthening is an effective approach [51,60,109]. Over time, various surface-strengthening methods have been developed to improve the corrosion resistance of titanium alloys. Initially, due to technological and theoretical limitations, traditional techniques such as electroplating, gas carburizing, and gas nitriding were the primary methods for surface strengthening of titanium alloys [110,111,112]. With the gradual maturation of plasma theory and technology; however, techniques like plasma chemical heat treatment, plasma Physical Vapor Deposition, plasma-enhanced chemical vapor deposition, ion implantation, and plasma spraying have been widely studied and applied [113]. These advanced techniques help prevent the titanium alloy substrate from exposure to corrosive environments, significantly improving the service life of titanium alloy components [114,115,116].
2.1. Plasma Chemical Heat Treatment
Chemical heat treatment is a metal heat treatment process that uses chemical reactions, sometimes combined with physical methods, to alter the chemical composition and microstructure of the surface layer of metal workpieces [117]. This process offers better technical and economic benefits compared to homogeneous materials. Typically, the workpiece is placed in an environment containing active media and is heated to a specific temperature, allowing for the elements in the medium to diffuse into the surface of the workpiece [118,119]. After chemical heat treatment, the workpiece can be considered a special composite material, with the core retaining its original composition while the surface layer becomes enriched with alloying elements. The interface between the core and surface layer forms a tightly bonded crystalline structure [120,121].
The most common method of chemical heat treatment is nitriding. Plasma nitriding (PN) is a chemical heat-treatment process performed in a low-vacuum nitrogen-containing atmosphere, where the furnace body acts as the anode and the workpiece to be treated serves as the cathode [122,123,124,125,126]. A DC voltage of several hundred volts is applied between the anode and cathode, generating a glow discharge to carry out the nitriding process [127,128,129]. Depending on the process, a diffusion layer ranging from several micrometers to hundreds of micrometers in thickness may form on the surface of titanium alloy workpieces, along with a ceramic-phase compound layer several micrometers to tens of micrometers thick. With the continuous development of plasma nitriding technology, many novel plasma nitriding methods have emerged that can further enhance the uniformity of the microstructure and properties of the plasma nitrided layer, improving the industrial application value of plasma nitriding for surface strengthening titanium alloys [130,131,132]. Compared to conventional chemical heat treatment using gases or liquids, using plasma for chemical heat treatment can significantly improve the efficiency of diffusion of alloying elements into the substrate, reduce the treatment temperature, increase the diffusion rate, and save energy [120,133]. Daniel Toboła et al. [134] used gas nitriding to strengthen Ti6Al4V, and, after treatment at 540 °C for 24 h, only a 50 nm thick compound layer was obtained. Li et al. [135] used plasma nitriding to treat Ti6Al4V at 510 °C for only 1 h and obtained a compound layer with a thickness of 2.5 μm. The use of plasma method significantly improves the diffusion rate of nitrogen in titanium alloys, saving time and economic costs.
Active Screen Plasma Nitriding (ASPN) can transfer the high potential from the material to the active screen, thereby reducing damage caused by arc striking on the workpiece surface [136]. Hollow Cathode Plasma Nitriding (HCPN) allows for active ions to move freely on the surface of the hollow cathode without sputtering on the sample’s surface. A large number of electrons oscillate between two cathode plates under the influence of electric potential, with low electron loss, resulting in the formation of a high-quality nitrided layer [137,138]. Laser Shock Plasma Nitriding (LSPN) is a nitriding method that utilizes shockwaves induced by a high-intensity laser to generate plastic deformation on the metal surface, increasing dislocation density and producing residual compressive stress, which enhances nitrogen adsorption and diffusion rates. Laser shock peening can significantly improve surface hardness and slow the decline in hardness across the cross-section [139]. Table 1 presents some relevant studies on plasma nitriding and its polarization behavior in common corrosive environments, such as natural seawater, simulated body fluids (SBF), phosphate-buffered saline (PBS), Hank’s solution, Ringer’s solution, etc. Corrosion resistance is indicated by corrosion current density (Icorr) and corrosion potential (Ecorr).

Table 1.
Improvement in corrosion resistance of titanium and titanium alloy by plasma nitriding.
According to the research results, a dense diffusion layer of a certain thickness can be formed on the surface of titanium alloys after plasma nitriding treatment. If the nitriding temperature is elevated or a hollow cathode method is used, a ceramic-like compound layer can further form on the surface. These dense passive layers can effectively block the corrosion of ions in the corrosive medium, significantly reducing the corrosion current density, as observed in the dynamic potential polarization results. The corrosion current density decreases by about two orders of magnitude in a seawater environment and by at least one order of magnitude in various biological environments. Zhang et al. [135] formed a TiN compound layer on the surface of the titanium alloy at a lower diffusion temperature through a unique double-barrel HCPN, while the conventional plasma nitriding process often takes nearly 900 °C to form [141]. This demonstrates the positive influence of hollow cathode effect on assisted diffusion.
Zhang et al. [150] proposed that, during the plasma nitriding process of TA17 titanium alloy, the surface Al element will diffuse inward. Prior to diffusion, micro-deformation of the sample surface can reduce the nucleation barrier of TiN, which is beneficial for the formation of nanocrystalline structures. M. Tarnowski et al. [144] believe that the surface of Ti6Al7Nb titanium alloy active screen after plasma nitriding is composed of a nanocrystalline TiN layer, Ti2N layer, and α-Ti (N) layer. Sun et al. [151] believe that plasma nitriding has an insignificant effect on the surface roughness of TC6 titanium alloy. The treated surface can form a TiNxOy passivation film, significantly reducing the corrosion rate and polarization reaction.
Figure 4 shows the corrosion behavior of the nitrided Layer of Ti6Al4V Alloy by Hollow Cathodic Plasma Source Nitriding. It shows that a dense and hard passivation layer is formed on the surface after plasma nitriding with a hollow cathode. The Ti-N nitrided layer comprises a mixture of TiN, Ti2N, and α-Ti (N) phases. The compound layer consists of a top layer of nanocrystalline TiN surface, a sublayer of crystalline TiN, an intermediate layer of Ti2N, and a bottom layer of interstitial solid solution α-Ti (N). This passivation layer has high resistance to pitting corrosion and has high-charge transfer resistance and low capacitance, which can hinder the penetration and migration of reactive ions, thereby significantly improving corrosion resistance.
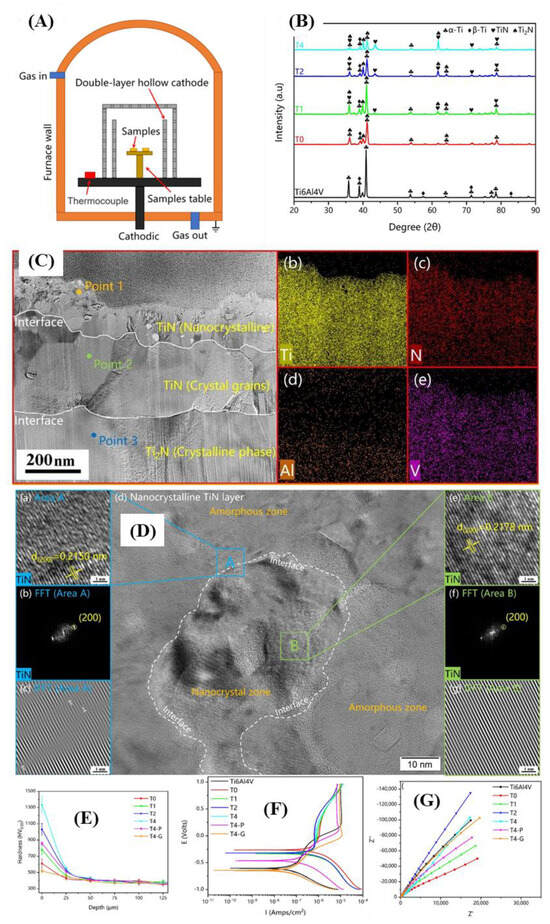
Figure 4.
Corrosion behavior of nitrided layer of Ti6Al4V titanium alloy by Hollow Cathodic Plasma Source Nitriding (HCPSN) (adapted from [135], with permission): (A) schematic diagrams of the HCPSN device; (B) the XRD patterns of the untreated and nitride Ti6Al4V samples; (C) the TEM image; (D) the HRTEM image; (E) the cross-sectional hardness profile of different samples; (F) the polarization curves in Hank’s solution; (G) Nyquist plots.
Plasma nitriding is widely used due to its high diffusion efficiency, energy-saving and environmental protection abilities, and wide material adaptability [152]. However, it also faces some challenges in practical applications [153]. Traditional thermocouple contact temperature measurement is difficult to accurately reflect the true temperature of the workpiece in vacuum and glow environments. The edge effect leads to inconsistency in hardness between the corners of the workpiece and other parts. It is difficult to control and measure the temperature when mixing workpieces with different structures. Arc discharge on the surface of the parts can cause plasma instability or damage to the surface of high-purity workpieces. Solving these problems is the development trend for improving the accuracy and application scope of plasma nitriding.
2.2. Physical Vapor Deposition
Physical Vapor Deposition (PVD) refers to a process in which a material is vaporized into dynamic molecules or partially ionized into plasma under vacuum conditions, subsequently deposited as a thin film with specific functions on the substrate surface through a low-pressure gas or plasma process [154,155,156,157,158]. PVD encompasses techniques such as vacuum evaporation, magnetron sputtering, and cathodic arc ion plating. Magnetron sputtering and cathodic arc ion plating are techniques for surface deposition using plasma [159,160]. The use of plasma glow discharge can significantly improve the uniformity and mechanical properties of coatings. H. GARBACZ et al. [161] prepared Al coatings on Ti6Al4V using vacuum evaporation and magnetron sputtering. The results show that the Al coating prepared by magnetron sputtering had a uniform microstructure and exhibited higher microhardness. This indicates that Al coatings deposited by magnetron sputtering are more suitable for the fabrication of Ti-Al intermetallic compound coatings.
By selecting different target materials, gas environments, and adjusting process parameters, a wide range of thin films can be deposited, including metal films, alloy films, compounds, ceramics, semiconductors, and polymer films [162,163,164,165]. The deposition of dense protective layers containing elements such as Ti, Al, and N on the surface of titanium alloys effectively prevents surface corrosion [166]. Additionally, their oxidation products, such as dense Al2O3 and TiO2, significantly enhance the corrosion resistance of titanium alloy surfaces [167,168]. Table 2 shows some relevant studies on PVD and data on the dynamic potential polarization tests in the studies.

Table 2.
Improvement of corrosion resistance of titanium alloy by Physical Vapor Deposition.
Li et al. [173] demonstrated that the TiN/TaN coating prepared by magnetron sputtering (MS) effectively reduces vacancy defects and prevents the penetration of corrosive ions into Ti6Al4V substrates. The protective efficiency of the coating can reach up to 97.8%. Tao et al. [180] showed that the use of metal-assisted ion plating (MAIP) technology at a bias of −100 V facilitates the preparation of TiN coatings with the lowest roughness and porosity on titanium plates, thereby improving their corrosion resistance. The thickness of these coatings ranges from a few tenths of microns to several microns. It results show that the corrosion current density can be reduced by up to two orders of magnitude, while the corrosion potential shifts positively, significantly improving the corrosion resistance of titanium alloy components in marine or body fluid environments and extending their service life.
2.2.1. Magnetron Sputtering
The principle of Magnetron Sputtering (MS) involves electrons, influenced by an electric field, colliding with Ar atoms as they travel toward the substrate [182,183,184,185]. This collision ionizes the Ar atoms, generating Ar positive ions and additional electrons. The newly generated electrons move toward the substrate, while the Ar ions, accelerated by the electric field, are directed toward the cathode target [182]. Upon striking the target surface with high energy, these ions cause the target material to undergo sputtering [186,187]. Plasma-Enhanced Magnetron Sputtering (PEMS) improves the standard magnetron sputtering process by utilizing the plasma-enhanced effect. In PEMS, plasma is generated within the sputtering chamber, introducing high-energy ions that increase both ion density and energy, thus enhancing the quality and growth rate of the thin film [188,189].
The coating containing the Ti-N component prepared by MS has a high density and low defect rate, which can effectively block the penetration of corrosive media. Cui et al. [172] employed MS to deposit nanocrystalline TiZrN gradient coatings on Ti6Al4V and conducted electrochemical corrosion tests in Hank’s solution, both with and without calf serum, as shown in Figure 5. The results demonstrate that, compared to TiN coatings, the nanocrystalline TiZrN coatings exhibited superior hardness and enhanced corrosion resistance in physiological environments. The strengthening effect of this coating is due to the solid solution-strengthening effect of Zr in TiN and the rapid formation of a dense nitrogen oxide layer containing Zr and Ti on the surface-hardening layer during the corrosion process.
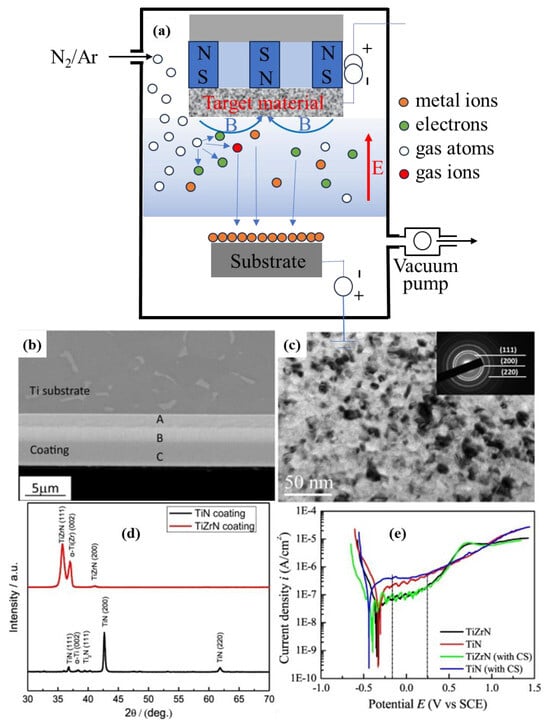
Figure 5.
(a) Schematic diagram of MS equipment; cross-sectional image (b) and nanocrystalline structure (c) of TiZrN coating; (d) XRD patterns of the nanocrystalline TiN- and TiZrN-graded coating on the Ti alloy; (e) potentiodynamic polarization curves of the TiN- and TiZrN-coated specimens in Hank’s solution with and without calf serum at 37 °C (adapted from [172], with permission).
Adjusting different process parameters can enable magnetron sputtering technology to prepare coatings with different properties. Niu et al. [190] employed magnetron sputtering technology to deposit TiAlN coatings on Ti6Al4V at various nitrogen gas flow rates. The experimental results demonstrate that the nitrogen flow rate had a significant impact on the microstructure of the films. Coatings deposited at flow rates between 12 and 16 sccm exhibited high density and uniformity. However, higher nitrogen flow rates led to target poisoning, causing defects such as pores and cracks. Moreover, both the deposition rate and the corresponding film thickness decreased as the nitrogen flow rate increased. Lü et al. [191] employed magnetron sputtering to deposit TiAlN coatings on Ti6Al4V at various nitrogen flow rates. The results show that the nitrogen flow rate significantly influenced the microstructure of the films. Coatings deposited at flow rates between 12 and 16 sccm exhibited high density and uniformity. In contrast, higher nitrogen flow rates caused target poisoning, leading to defects such as pores and cracks. Additionally, both the deposition rate and film thickness decreased with increasing nitrogen flow rate. This study provides valuable insights into optimizing the gas environment for producing high-quality ceramic phase coatings via magnetron sputtering. Lü et al. [192] used pulsed electric field (PEF) to suppress the columnar grain growth of TiAlN coatings and replaced the traditional heating method in the vacuum chamber with PEF, thereby enhancing the coating preparation speed and effectiveness, as shown in Figure 6. The results show that the discontinuities of PEF block the growth process of TiN columnar crystals and form a typical nanocrystalline TiN structure. Compared with the columnar crystal structure, the nanocrystalline structure is more beneficial to release the internal stress, block the crack propagation, improve the bonding force between the coating and the matrix, and improve the mechanical properties of the coating. This provides a feasible solution for improving the bonding force and other properties of the magnetron sputtering coating.
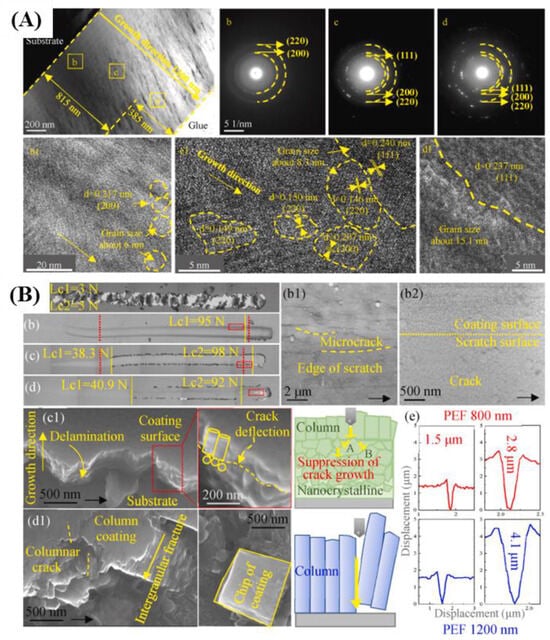
Figure 6.
(A) Cross-sectional TEM at different locations of the PEF coating with a thickness of 1200 nm; (B) scratch morphologies of the RT coating and the PEF coatings (adapted from [192], with permission).
The coatings prepared by magnetron sputtering have the advantages of strong adhesion, good density, and good coating uniformity, and are often used in practical production to prepare high-quality coatings. However, in practical applications, magnetron sputtering technology still has some shortcomings [193], such as the concentration of magnetic field distribution in specific circular areas on the surface of the target material, resulting in low utilization rate of the target material. Excessive reaction gas can cover the surface of the target material and form compounds, leading to ‘target poisoning’. These issues constrain the production efficiency of magnetron sputtering technology, making it difficult to achieve rapid large-scale production in industrial production.
2.2.2. Cathodic Arc Ion Plating
Cathodic arc ion plating (AIP) is a technique that utilizes arc discharge to directly evaporate metal from a solid cathode target [194,195,196,197]. The evaporated material consists of ions of cathode substances emitted from the cathodic luminous spot, which form a plasma in space and later deposit as a thin film on the substrate surface [197,198,199,200,201]. Multi-arc ion plating (MAIP) integrates the characteristics of evaporation, sputtering, glow discharge, and vacuum evaporation [198,202]. MAIP offers advantages such as a high electron ionization rate, high ion bombardment energy, and ease of operation. Films produced using the MAIP technique exhibit superior density and adhesion strength [203,204].
The coating containing TiN prepared using AIP has good adhesion, extremely high hardness, and excellent high-temperature oxidation resistance. Zhang et al. [167] prepared a 4 μm thick TiAlN coating on a Ti6Al4V substrate using AIP to mitigate hot salt corrosion on the titanium alloy surface. The results indicate that the 12 μm thick TiAlN coating was able to form protective Al2O3 and TiO2 layers on the surface, preventing internal oxidation and accelerating the corrosion as well as the cyclic wear of the coating. Consequently, the coating demonstrated excellent resistance to hot salt corrosion.
How to optimize the parameters of cathodic arc ion plating process to obtain coatings with the best corrosion resistance and mechanical properties has been a research topic for researchers. Zhang et al. [205] fabricated TiAlN monolayer coatings and Ti/TiAlN composite multilayer coatings with varying Ti layer thicknesses on Ti6Al4V using arc ion plating and investigated their high-temperature corrosion behavior in NaCl, O2, and H2O environments. The study suggested that the coatings enhanced the corrosion resistance of titanium alloys by inhibiting the active corrosion of the alloy through gaseous metal chlorides, hydrochloric acid, and chlorine during the corrosion process. Furthermore, defects in the coatings had a significant impact on their corrosion resistance. Li et al. [180] systematically investigated the effect of bias voltage on the surface microstructure of multiarc ion-plated TiN films and characterized the corrosion resistance of the coatings. The results are shown in Figure 7. The findings indicate that the surface microstructure of the TiN films strongly depends on the bias voltage. At a bias voltage of −100 V, the TiN film exhibited the best surface microstructure. The optimized TiN film demonstrated excellent corrosion resistance in a 0.5 M H2SO4 + 2 ppm HF solution at 80 °C and in air at 80 °C, with a corrosion current density of 0.87 μA/cm2. These results show that the TiN coating prepared by the cathode arc ion plating is a feasible method for improving the corrosion resistance of Ti bipolar plates.
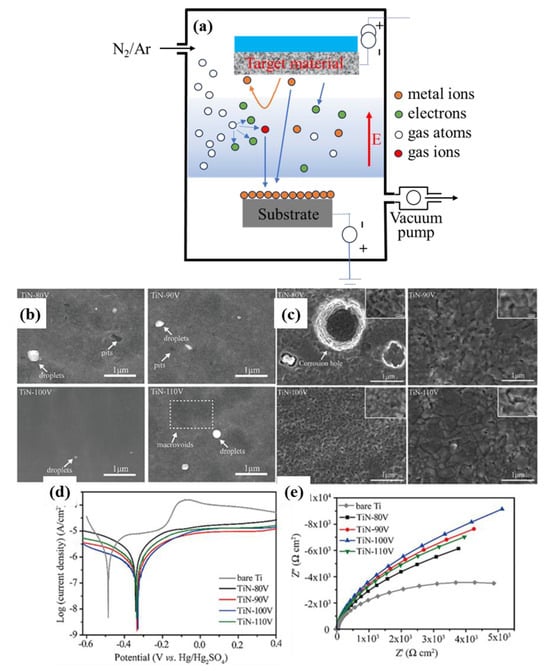
Figure 7.
(a) Schematic diagram of AIP equipment; (b) SEM images of TiN films prepared at different bias voltages; (c) SEM images of the TiN films prepared at different bias voltage after potentiodynamic polarization test at 70 °C; (d) potentiodynamic polarization curves; (e) Nyquist plots (adapted from [180], with permission).
Cathodic arc ion plating technology has significant advantages in large-scale industrial production due to its high ionization rate and deposition rate, high utilization rate of target materials, environmentally friendly process, and wide material adaptability [206]. However, compared to magnetron sputtering, the coating quality has slightly decreased. During arc discharge, the local instantaneous high temperature on the surface of the target material will produce tiny metal droplets which spray and deposit onto the substrate, resulting in micro-scale particle defects in the coating, affecting the density and corrosion resistance of the coating. Higher ion bombardment energy, while forming dense coatings, may also lead to significant residual stresses inside the coating, affecting its adhesion. Therefore, balancing the production efficiency and coating quality of cathodic arc ion plating is the future development direction of this technology.
2.2.3. Pulsed Laser Deposition
Pulsed laser deposition (PLD) is a surface strengthening technology that focuses a high-energy laser on a small area of the target material, causing part of the target material to evaporate or ionize. This material then moves away from the target and deposits onto the substrate, forming a thin film [207,208,209,210]. When the high-energy pulsed laser is focused on the surface of the target, the instantaneous power density in the spot region can reach 109–1011 W/cm2. The target absorbs the photon energy through reverse bremsstrahlung-induced radiation, and the electron lattice system of the surface completes heat conduction on a picosecond scale, raising the local temperature above the evaporation threshold [211,212]. The target material undergoes a phase explosion, generating a metal vapor cloud composed of atoms, ions, free electrons, and micron-sized droplets. This vapor cloud is further ionized under the continuous action of the laser, forming a high-temperature plasma fireball with a dense core [106]. Plasma-assisted pulsed laser deposition (PAPLD) is a technique that generates additional plasma by creating a discharge in the background gas between the PLD and substrate. The addition of the plasma background mitigates the deposition of large particles and eliminates the need for annealing treatments, thereby improving coating quality [213,214,215].
Slepička et al. [216] deposited diamond-like carbon (DLC) coatings on pure titanium and Ti90Al6V4 using vacuum evaporation and PLD, respectively. The results show that the DLC coating prepared by PLD exhibited better hydrophilicity and wrinkling morphology than that prepared by vacuum evaporation, which was more conducive to cell adhesion. Additionally, the antibacterial activity of the DLC coating prepared by vacuum evaporation was significantly higher, highlighting the potential of PLD technology for applications in titanium alloys for the biomedical field. Gnanavel et al. [217] also demonstrated that the corrosion resistance and biological activity of titanium alloy matrices can be enhanced by depositing hydroxyapatite (HA) coatings in the form of calcium phosphate on Ti-13Nb-13Zr β-titanium alloy via PLD.
Floroian et al. [218] deposited TiN and ZrN protective layers on the surface of Ti using nitrogen plasma-assisted pulsed laser deposition and studied their electrochemical characteristics in SBF. The results show that, after 32 days of electrochemical corrosion, the corrosion current density of ZrN coating only increased by about 10%, while the corrosion current density of Ti matrix increased by about 120%. ZrN coating has good corrosion resistance and can reduce the release of metal ions in solution, indicating the potential of PAPLD in the formation of ceramic phase implant coatings.
2.3. Plasma-Enhanced Chemical Vapor Deposition
Plasma-enhanced chemical vapor deposition (PECVD) is a technique that uses plasma discharge to activate reactive gases, enabling low-temperature chemical vapor deposition [219,220,221]. The significant advantage of PECVD lies in its ability to produce high-performance films without causing substantial environmental pollution or harmful effects on operators [203,222,223]. Therefore, compared to traditional coating preparation technologies, PECVD is widely used in the preparation of nanofilms, amorphous films, and pinhole-free films due to its green, reliable, and low-cost nature. These high-performance films offer several advantages over conventional film materials, such as a lower apparent Young’s modulus, greater deformability, and improved elasticity, toughness, and biocompatibility [224,225]. Bedarev et al. [226] compared carbon containing zirconia coatings prepared by traditional CVD and PECVD. The results indicate that the coatings prepared by PECVD have smaller grain sizes, thus exhibiting better thermal insulation properties. Joo et al. [227] compared the anti-hydration coatings prepared by traditional CVD and PECVD on MgO. The results indicate that PECVD is more effective in applying anti hydration coatings due to the plasma activation of MgO surface and reactants, thereby more effectively maintaining physical and chemical properties under conditions of 85 °C and 85% relative humidity. In addition, when used as a filler for thermal interface composite materials, MgO modified with PECVD exhibits approximately 1.5 times higher adhesion strength and thermal conductivity than bare MgO due to its increased affinity for hydrophobic adhesives. The author also believes that PECVD has more advantages in preparing diamond-like carbon (DLC) or nitride coatings compared to traditional CVD. As a result, PECVD shows significant potential for applications in the surface strengthening of titanium alloys [228].
The deposition of DLC coatings by PECVD has long been a prominent research focus for surface protection and has numerous applications in surface strengthening of titanium alloys. L. Mohan et al. [229] prepared DLC coatings with high hardness, excellent wear resistance, and good biocompatibility on Ti–15Mo–3Nb–3Al–0.2Si titanium alloy using PECVD. The results demonstrate that the coating formed a strong bond with the Ti matrix, showing significant potential for biomedical applications. Swiatek et al. [230] also prepared Si/Ag-doped DLC coatings on Ti6Al7Nb titanium alloy via PECVD and investigated the effects of different Ag concentrations on the mechanical and biological properties of the coatings. The findings indicate that silver was incorporated into the coating as Si-O-Ag groups. The inclusion of Ag enhanced the bactericidal activity, but reduced both hardness and biocompatibility. For medical antimicrobial coatings, DLC coatings containing 2.94 at.% Si and 1.36 at.% Ag exhibited the best performance.
PECVD can also be used to deposit intermetallic compound and metal oxide coatings with excellent performance on the surface of titanium alloys. R. Sitek et al. [231] prepared a Ti-Al intermetallic coating on Ti6Al2Mo2Cr titanium alloy using PECVD technology and deposited an alumina layer on the same alloy by applying Al(CH3)3 vapor at a temperature of 90 °C in a hydrogen atmosphere at 400 °C, thereby creating an intermetallic oxide composite coating. The relevant experimental results are shown in Figure 8. The findings demonstrate that the coating exhibited an amorphous nanocrystalline structure, which enhanced the corrosion resistance, formed a hydrogen barrier, and reduced hydrogen permeability.
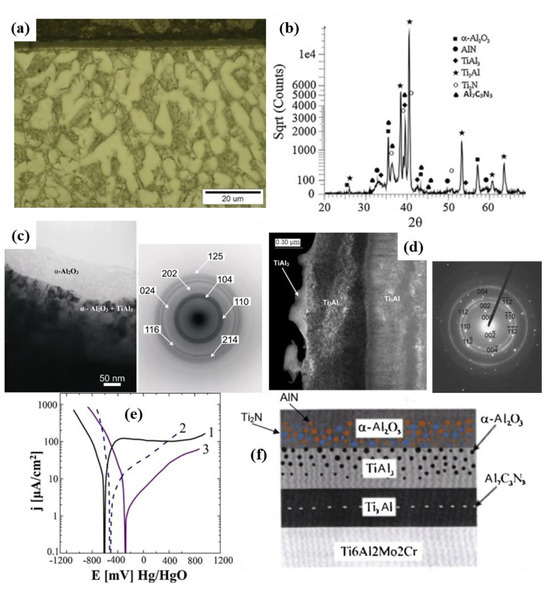
Figure 8.
(a) Microstructures of cross-section; (b) X-ray diffractograms; (c) TEM of outer layer of α-Al2O3 with TiAl3 phase; (d) microstructure of TiAl3 + Ti3Al phase; (e) polarization curve obtained in 0.1 M NaOH: (1) Ti6Al2Mo2Cr, (2) Ti6Al2Mo2Cr protected by the ceramic layer, (3) Ti6Al2Mo2Cr alloy covered with the ceramic layer, after the removal of part (about 0.5 μm) of the surface sub-layer; (f) simplified schematic representation (adapted from [231], with permission).
Compared with PVD, the gas-phase reactants of PECVD can form coatings on surfaces of complex shapes, deep holes, and grooves, making it widely used on workpieces with complex shapes. However, PECVD still has certain limitations in practical use. For example, gas-phase reactions may generate by-products and particle pollution. The equipment structure is complex and the maintenance cost is high. The process is complex and requires significant experience accumulation. Therefore, PECVD often requires a long period of research and exploration before it achieves practical application in industrial production.
2.4. Plasma Immersion Ion Implantation
Plasma Immersion Ion Implantation (PIII) is a vacuum injection technology that accelerates ions generated by an ion source using high voltage ranging from tens of thousands to hundreds of thousands of volts and then injects them at high speed into the surface of the target material [232,233]. The processing temperature of PIII can be reduced to below 200 °C, falling within the category of low-temperature modification. Additionally, it does not alter the macroscopic dimensions of the workpiece or affect the intrinsic properties of the base material. Therefore, compared to high-temperature surface treatment techniques such as chemical surface heat treatment, PIII has significant advantages and is an effective method for surface strengthening of precision components [203,233,234].
Coatings prepared by PIII often exhibit excellent biocompatibility, making them widely used in the biological and medical fields. Zhao et al. [235] performed N-PIII on a Ti6Al4V substrate to prepare TiN gradient coatings. These coatings do not significantly alter the surface hydrophilicity, but do increase surface roughness and corrosion resistance. Experimental results indicate that this structure can stimulate the response of pre-osteoblasts and fibroblasts and induce early bone formation. The coatings exhibit good corrosion resistance and biocompatibility, playing an important role in the process of new bone formation in vivo.
Figure 9 shows the effect of nitrogen ion implantation on the corrosion resistance of Ti-35Nb-7Zr-5Ta titanium alloy [236]. Corrosion tests in physiological saline solution demonstrated that the implanted surfaces exhibited high corrosion resistance. The corrosion resistance increased with the implantation dose until the saturated dose was reached. Flow rates higher than the saturation dose led to a decrease in corrosion resistance to levels similar to those of lower doses. The resistance of all nitrogen-implanted surfaces was higher than that of non-implanted surfaces.
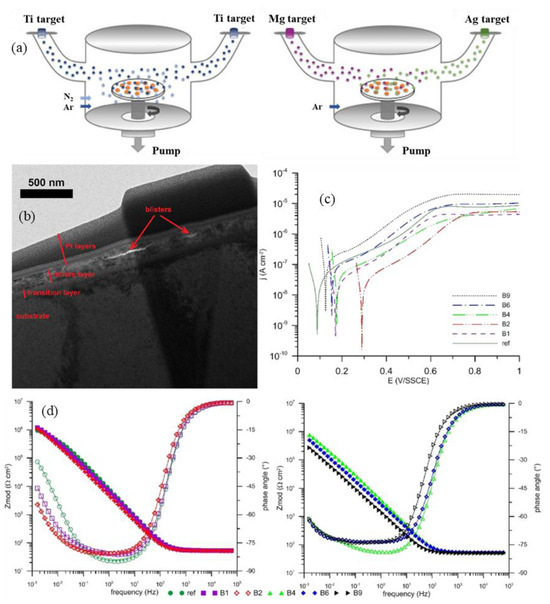
Figure 9.
(a) PIII equipment schematic diagram; (b) SEM top view micrographs of the PIII samples; (c) TEM cross-section image of the implanted zone; (d) potentiodynamic curves of the implanted Ti-35Nb-7Zr-5Ta alloy in a physiological solution (adapted from [236], with permission).
PIII can achieve all-round injection and uniform ion implantation on complex shaped workpieces. And the equipment is relatively simple, the process cost is low, and it can achieve mass production, which has many practical applications in industrial production. However, this process still has problems such as thin injection layer, uneven injection dose, and uneven plasma energy, so it is often used in conjunction with other surface-strengthening techniques.
2.5. Plasma Spraying
Plasma spraying (PS) technology is a technique that uses a plasma torch to generate a DC arc, which heats and melts materials before high-speed spraying them onto a metal surface to form a coating [160,237,238,239]. The plasma spraying process is stable, easy to operate, has a large equipment capacity and output power, fast powder flight speed, and high deposition efficiency, enabling large-scale continuous production. Nelson et al. [240] used traditional flame spraying to mix Ti6Al4V powder with bioactive glass to prepare a titanium alloy bioactive glass composite coating, significantly improving the bioactivity of the titanium alloy. Smith et al. [241] compared the coatings prepared by plasma spraying and traditional thermal spraying. The coating prepared by PS has higher density and uniformity, stable composition, and layered or columnar microstructure, making it advantageous in the preparation of ceramic, metal, and composite coatings. This technology can produce relatively thick coatings with considerable control over coating thickness [242,243]. The center temperature of the plasma flame is very high, capable of melting almost all ceramic and metallic powders; therefore, the selection range of materials for plasma spraying is very broad. Plasma-sprayed coatings typically have micro/nanometer-scale surface roughness, and such structures have been shown to promote the integration of implants with host bone. Consequently, PS-enhanced titanium alloys exhibit excellent biological performance [244].
PS has broad research and application prospects, especially in the field of human implants. Anand et al. [245] prepared TiB-TiN composite coating on a Ti6Al4V substrate using plasma spraying, which has good friction and corrosion properties and has promising prospects in the application of wear-resistant and load-bearing implants. Alontseva et al. [246] investigated the corrosion resistance and biocompatibility of micro-plasma-sprayed titanium and tantalum coatings on a Ti6Al4V titanium alloy. Compared to the untreated Ti6Al4V alloy, both titanium and tantalum-coated samples exhibited enhanced corrosion resistance. The tantalum coating demonstrated superior cell proliferation and osteogenic differentiation abilities, along with the highest corrosion resistance. The titanium coating followed closely, showing good osteogenic properties and enhanced roughness, which is crucial for cell behavior and attachment. These coatings also exhibited excellent tensile adhesion strength, indicating strong bonding with the substrate. The osteoinductive properties of the tantalum coating, combined with its ability to enhance cell proliferation and its lack of cytotoxicity, make the tantalum porous coating containing MPS a promising candidate for implant development, particularly for bone grafts requiring high biocompatibility and corrosion resistance.
Hydroxyapatite coatings prepared using plasma spraying (PS) have been widely used in load-bearing titanium alloy implants due to their high biological activity, mass production advantages, and extensive research and development in recent years. Cao et al. [247] synthesized Mg and Sr co-substituted hydroxyapatite ((Mg, Sr)-HA) nano-sized powder on Ti6Al4V to further enhance its biological function. The results indicate that the coating formed a thin melt layer with nanoparticles during spraying, demonstrating high bonding strength and improved biological function, with promising applications in dental and orthopedic fields. Singh et al. [248] investigated the impact of varying plasma power on the corrosion resistance of the coating, with relevant findings shown in Figure 10. It shows that increasing plasma power significantly reduced microcracks, pores, and other defects, decreased coating porosity, and provided a smaller path for simulated body fluid electrolyte to permeate through the coating, thereby enhancing its corrosion resistance.
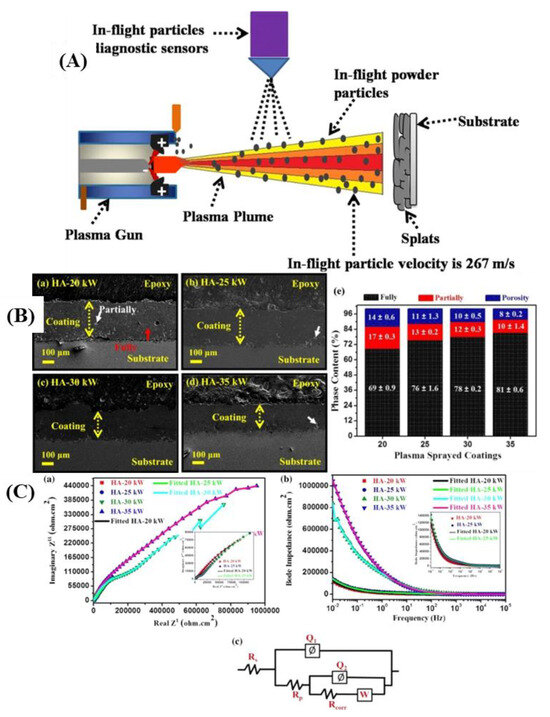
Figure 10.
(A) Setup of plasma-sprayed coating along with Accura spray in-flight diagnostic sensor; (B) FE-SEM images of the cross-sectional surface of plasma sprayed HA coatings and the porosity, partially melted and fully melted regions in plasma sprayed HA coatings; (C) EIS results for coatings prepared at different power (adapted from [248], with permission).
PS can spray almost all materials with melting points using extremely high flame flow temperatures while depositing coatings with diverse functions at lower temperatures through cooling systems. This technology has been widely studied in the field of functional coatings [249]. However, due to the limitations of relatively low adhesion, the presence of pores and oxide inclusions in the coating, and issues such as noise and radiation during the spraying process, it is often necessary to select suitable material systems and optimize process parameters for specific working conditions.
Compared to traditional surface strengthening techniques, plasma surface strengthening technology has many advantages, but a single plasma modification method still has many shortcomings [250]. The hardened layer generated by plasma chemical heat treatment may have uneven thickness, and its mechanical properties are often not as good as those of the coating hardened layer [251,252,253]. The problem of insufficient adhesion between coatings prepared by PVD, PECVD, and PS and the substrate has also restricted the development of coating technology [254,255,256,257,258,259]. PIII has a slow processing speed and high cost, making it unsuitable for large-scale production [257]. Industrial application scenarios, advantages, and disadvantages of various plasma technologies are shown in Table 3.

Table 3.
Industrial application scenarios, advantages, and disadvantages of various plasma technologies.
2.6. Duplex Treatment
Currently, the enhancement of titanium alloy corrosion resistance using the single-strengthening technique is no longer sufficient. Attention has shifted to the application of various surface technology composite treatments, aiming to achieve multi-layered and gradient structures of titanium alloy surface modification layers, as well as the incorporation of multiple alloying elements [260,261,262]. This approach is designed to compensate for the shortcomings of a single technology or meet the requirements of titanium alloys in demanding conditions such as heavy loads, high speeds, high temperatures, and other harsh service environments [263,264,265,266]. Table 4 below illustrates the impact of composite surface treatments on the corrosion resistance of titanium alloys. This includes techniques not described in the text, such as plasma electrolytic oxidation (PEO), atomic layer deposition (ALD), triode plasma oxynitriding (TPON).

Table 4.
Some duplex treatment methods to improve the corrosion resistance of titanium alloys.
The effect of plasma nitriding can be improved through certain pretreatment, such as specific heat treatment or surface micro-deformation. Zhang et al. [279] investigated the synergistic effect of shot peening (SP) on PN titanium alloy, with the related characterization shown in Figure 11. The results indicate that SP refined the surface grains, promoted PN, and resulted in the formation of a thicker nitride layer on the titanium alloy surface. Compared to the as-received titanium alloy, the surface microhardness increased by 51.4%, and the wear rate decreased by two orders of magnitude. SP-PN treatment enhanced the electrochemical corrosion resistance of the titanium alloy, highlighting its effectiveness in improving the wear and corrosion properties of medical titanium alloys. This method has emerged as an innovative approach for preparing wear-resistant and corrosion-resistant titanium alloys for medical applications.
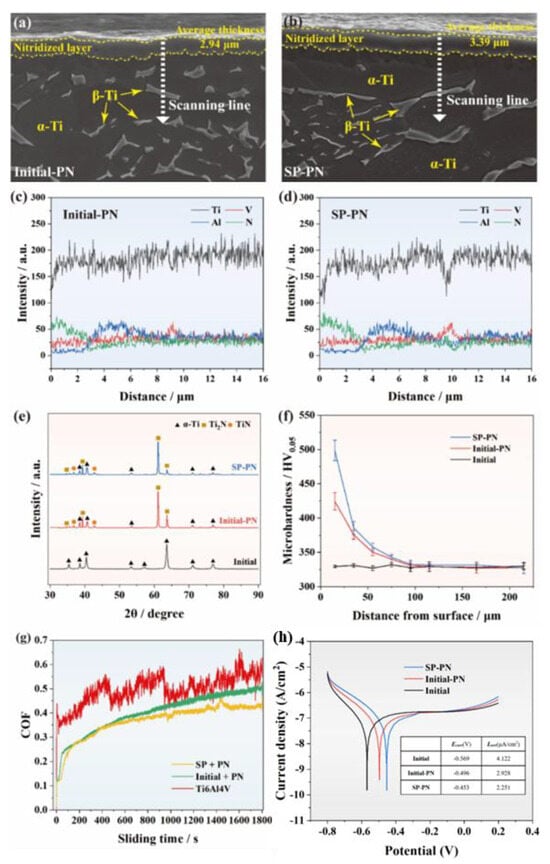
Figure 11.
(a,b) SEM morphology; (c,d) EDS scanning; (e) X-ray diffractograms; (f) cross-sectional microhardness; (g) friction coefficients; (h) polarization curves of the Initial, Initial-PN, and SP-PN samples (adapted from [279], with permission).
Pretreatment using other techniques or preparing composite coatings using multiple techniques before coating preparation can significantly improve the drawbacks of single-technique coating preparation, thereby enabling titanium alloy products to have a wider range of application scenarios. The combination of duplex surface treatment technologies breaks through the bottleneck of single-plasma-treatment technology in improving corrosion resistance and improves biocompatibility or bactericidal performance. Dong [272] performed nitriding pretreatment before depositing TiSiCN coating, which improved the load-bearing capacity and stability of the coating, thereby enhancing the adhesion between the coating and the substrate. Zhao et al. [273] used a combination of PVD and plasma electrolytic oxidation to prepare a composite reinforcement layer that improved the corrosion resistance of Ti6Al4V while also exhibiting effective antibacterial properties against Escherichia coli. Esfahani et al. [269] addressed the degradation and adhesion issues of TiN ceramic phase coatings by employing a dual-phase triode TPON + TiN treatment process to fabricate TiN coatings on ALM-Ti6Al4V. This process involves ion oxygen and nitrogen diffusion followed by TiN deposition. The results indicate that, compared to the Ti alloy substrate, the anodic current density of the coated samples decreased to 0.75 V (Ag/AgCl), demonstrating enhanced corrosion resistance of the dual-phase TiN coating. Both the substrate and the dual-phase TiN coating deposited on it exhibited excellent localized corrosion resistance in a 25% FBS + PBS solution. Sliding corrosion experiments revealed that, after sliding, a protective layer gradually formed within the wear track of the dual-phase TiN coating, enabling it to withstand higher contact pressures in biomedical applications. This composite treatment process further improves the corrosion resistance of titanium alloy surface and expands the application scenarios of titanium alloy. Liu et al. [270] prepared Cu-TiN nanocomposite coatings on titanium alloys using magnetron sputtering. Based on this coating, PIII was employed to implant Mg or Ca ions with the same dose into the Cu-TiN coatings to further enhance their biological performance. The corrosion resistance, protein adhesion rate, in vitro cell proliferation, and antibacterial activity of Mg+-implanted Cu-TiN and Ca+-implanted Cu-TiN were compared, with the related characterization results shown in Figure 12. The results indicate that the Mg+-implanted Cu-TiN exhibited superior corrosion behavior, higher protein adsorption capacity, ideal cell proliferation and adhesion, and improved antibacterial properties. It demonstrated an improved combination of osteogenic and antibacterial activities, providing a promising option for surface modification of biomedical materials.
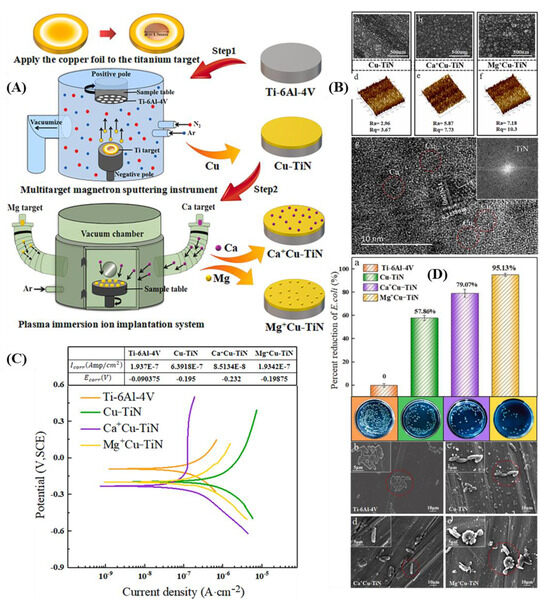
Figure 12.
(A) The flow chart of samples making; (B) SEM images, AFM surface topography, and HRTEM image; (C) potentiodynamic polarization curves of samples; (D) antibacterial rates and re-cultured E. coli colonies on agar plates of four samples, SEM images (adapted from [270], with permission).
Plasma surface modification technology can also be used to further strengthen non-plasma technologies in order to achieve the complementarity of various technologies. Joo et al. [278] further strengthened the wollastonite coating prepared by the sol–gel method through PEO pretreatment of Ti6Al4V. This technology enhances the biological activity and improves the stability of dental implants by forming a duplex treatment strengthening layer with a thickened wollastonite coating, providing valuable insights into the effects of PEO treatment and wollastonite coating on the surface of dental implants. Ren et al. [280] successfully prepared a self-lubricating plasma electrolytic oxidation polytetrafluoroethylene (PEO-PTFE) composite coating on Ti surface using PEO and impregnation/sintering duplex treatment. This composite coating is more durable than a single PTFE coating, improving the service life of the workpiece. Xu et al. [277] successfully developed a biomimetic HPO coating based on MOF (UiO-66 (Ce)) on TA1 using PEO and chemical etching combined with the solvothermal method. This coating can significantly improve the antibacterial efficiency and corrosion resistance of titanium alloy surfaces, and can replace traditional toxic anti-fouling agents containing copper and silver, achieving the inhibition of marine biofilms without producing ecological toxicity.
3. Conclusions and Prospects
Titanium and titanium alloys have been widely used in marine engineering and medical implants due to their high strength, corrosion resistance, and biocompatibility, and have shown greater potential in fields such as new energy and deep-sea engineering. But the increasingly complex working conditions require higher requirements for its surface comprehensive performance, especially corrosion resistance, which requires better surface technology. The main conclusions and prospects are as follows:
(1) Plasma surface engineering technology has many advantages that cannot be compared to other technologies, such as low processing temperature, environmental friendliness, and the ability to obtain excellent surface properties with fewer materials. It has obvious energy-saving, material-saving, and pollution-reducing effects. In the modern era of insufficient resources, the development of plasma surface engineering technology will gain new opportunities and vitality by continuously improving and perfecting plasma surface engineering technology, solving the problems of uniformity and internal surface treatment in large-scale processing and mass production in industry, effectively improving the corrosion resistance of titanium alloys, and promoting the application of titanium alloy components under extreme working conditions.
(2) Different plasma surface modification technologies have made some progress in improving the corrosion resistance of titanium alloys, but there are still some difficulties regarding these surface modifications, such as insufficient thickness, various difficult-to-meet biological performance requirements, and an inability to cope with extreme working conditions such as high load or high temperature. Therefore, the composite treatment of multiple strengthening technologies and the research and preparation of multi-component gradient composite coatings will become the main development direction of surface modification.
(3) In the future, research on surface the corrosion resistance enhancement of titanium and titanium alloys will continue to deepen by exploring their corrosion mechanisms in different environments, in order to cope with more severe service environments. Therefore, theoretical models, such as first principles and molecular dynamics, are used to explore the early corrosion mechanism of titanium and titanium alloy workpieces and to predict the performance of new plasma surface technologies, which can provide a theoretical basis for enhancing the corrosion resistance of titanium and titanium alloy surfaces.
Author Contributions
Writing—review and editing, Y.L.; Writing—original draft, M.J.; Supervision and Writing—review and editing, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Provincial Natural Science Foundation (No. ZR2024ME101) and the National Natural Science Foundation of China (No. 52175192).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to give thanks for the technical assistance from the state key laboratory of tribology in advanced equipment. We would like to express our heartfelt gratitude to Lu Jinpeng, Yan Jiwen, Dou Haichun, and Wang Zhengwei for their invaluable support in data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PN | Plasma nitriding |
| PVD | Physical Vapor Deposition |
| MS | Magnetron Sputtering |
| AIP | Cathodic arc ion plating |
| PLD | Pulsed Laser Deposition |
| PAPLD | Plasma Assisted Pulsed Laser Deposition |
| CVD | Chemical Vapor Deposition |
| PECVD | Plasma-enhanced Chemical Vapor Deposition |
| PIII | plasma immersion ion implantation |
| PS | Plasma spraying |
| PEO | Plasma electrolytic oxidation |
References
- Muñoz, J.M.J.; Tiburcio, C.G.; Ramirez, C.T.M.; Ramirez, M.G.C.; Zamora, M.A.B.; Hurtado, G.S.; Banda, M.L.; Lopez, F.E.; Mendoza, D.N.; Calderon, F.A. Corrosion of Anodized Titanium Alloys. Coatings 2024, 14, 809. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; He, Y. Tribocorrosion and Surface Protection Technology of Titanium Alloys: A Review. Materials 2023, 17, 65. [Google Scholar] [CrossRef]
- Pang, J.; Blackwood, D.J. Corrosion of Titanium Alloys in High Temperature near Anaerobic Seawater. Corros. Sci. 2016, 105, 17–24. [Google Scholar] [CrossRef]
- Yang, X.; Lin, B.; Zhang, H.; Tang, J.; Zhou, T.; Wang, Y.; Zheng, H.; Kuang, Y. Influence of Stress on the Corrosion Behavior of Ti Alloys: A Review. J. Alloys Compd. 2024, 985, 173346. [Google Scholar] [CrossRef]
- Lei, Z.; Minghao, S.; Zhengwei, W.; Zhehao, Z.; Yongyong, H.; Jiwen, Y.; Jinpeng, L.; Jianxun, Q.; Yang, L. Comparison of Tribological Properties of Nitrided Ti-N Modified Layer and Deposited TiN Coatings on TA2 Pure Titanium. Tribol. Int. 2022, 174, 107712. [Google Scholar]
- Ciszak, C.; Popa, I.; Brossard, J.-M.; Monceau, D.; Chevalier, S. NaCl-Induced High-Temperature Corrosion of β21S Ti Alloy. Oxid. Met. 2017, 87, 729–740. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Jia, C.; Chang, H.; Zhang, W.; Bai, Y.; Li, S.; Zhang, L.C.; Yuan, W. Corrosion Behavior of Laser Powder Bed Fusion Additive Manufacturing Produced TiNi Alloy by Micro-Arc Oxidation. npj Mater. Degrad. 2024, 8, 13. [Google Scholar] [CrossRef]
- Shuo, W.; Yujie, N.; Hui, X.; Huayang, T.; Jiangming, L.; Bin, L. Study on the Galvanic Corrosion Behavior of Copper-Nickel/Titanium Alloys under Simulated Seawater Environment. J. Solid State Electrochem. 2023, 28, 2315–2329. [Google Scholar]
- Zhang, C.; Lu, Z.; Li, F.; Jia, L.; Yang, Z.; Chen, G.; Yu, Q.; Dong, R.; Cai, M. Corrosion and Lubrication Properties of a Halogen-Free Gemini Room-Temperature Ionic Liquid for Titanium Alloys. Tribol. Int. 2021, 156, 106850. [Google Scholar] [CrossRef]
- Liu, H.; Shi, L.; Liu, H.; Ren, L.; Yang, K. Corrosion behavior of laser powder bed fusion prepared antibacterial Cu-bearing titanium alloy. Mater. Lett. 2023, 331, 133496. [Google Scholar] [CrossRef]
- Fuyue, W.; Xiangjie, W.; Qiang, Y.; Jianzhong, C. Corrosion Behavior of TC4 Titanium Alloys in Al–Li Alloy Melt. Metals 2021, 11, 794. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Yang, J.; Liu, X.; Zhang, J.; Ye, R. Corrosion Behavior of TC18 Titanium Alloy with Different Initial Microstructures in Salt Spray. J. Mater. Res. Technol. 2025, 35, 1619–1628. [Google Scholar] [CrossRef]
- Ji, H.; Xie, X.; Jiang, Z.; Wu, X. Wear and Corrosion of Titanium Alloy Spinal Implants In Vivo. Sci. Rep. 2024, 14, 16847. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Pequegnat, A.; Wang, J.; Zhou, Y.N.; Khan, M.I. Corrosion Performance of Medical Grade NiTi after Laser Processing. Surf. Coat. Technol. 2017, 324, 478–485. [Google Scholar] [CrossRef][Green Version]
- Rundora, N.R.; Merwe, J.W.V.d.; Klenam, D.E.P.; Bodunrin, M.O. Enhanced Corrosion Performance of Low-Cost Titanium Alloys in a Simulated Diabetic Environment. Mater. Corros. 2023, 74, 1486–1498. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; Merwe, J.W.v.d.; Alaneme, K.K.; Oganbule, C.; Klenam, D.E.P.; Mphasha, N.P. Corrosion Behavior of Titanium Alloys in Acidic and Saline Media. Corros. Rev. 2020, 38, 25–47. [Google Scholar] [CrossRef]
- Yu, W.X.; Dong, Z.S.; Qiang, L.; Qing, F.A.; Ling, L.J. Corrosion Behavior on Titanium Alloys as OCTG in Oil Fields. Mater. Sci. Forum 2021, 1032, 195–200. [Google Scholar] [CrossRef]
- Hua, Y.; Bai, Y.; Ye, Y.; Xue, Q.; Liu, H.; Chen, R.; Chen, K. Hot Corrosion Behavior of TC11 Titanium Alloy Treated by Laser Shock Processing. Appl. Surf. Sci. 2013, 283, 775–780. [Google Scholar] [CrossRef]
- Manikandan, C.B.; Selvakumar, N. Analyzing the Surface Roughness and Corrosion Resistance of YSZ-Coated Grade V Titanium Alloy by RF Sputtering. J. Mater. Eng. Perform. 2024, 30, 2380–2389. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Chen, C.; Zhong, W. Effect of Graphene Oxide on Corrosion Resistance and Biological Activity of Micro Arc Oxidation Ceramic Layer on Titanium Alloy. Mater. Lett. 2022, 327, 133056. [Google Scholar] [CrossRef]
- Fuyang, G.; Zhijie, S.; Shengli, Y.; Peng, J.; Zhiqian, L. Stress Corrosion Characteristics of Electron Beam Welded Titanium Alloys Joints in NaCl Solution. Mater. Charact. 2022, 192, 112126. [Google Scholar] [CrossRef]
- Ye, Q.W.; Li, Y.; Zhang, M.Y.; Zhang, S.Z.; Bi, Y.J.; Gao, X.P.; He, Y.Y. Electrochemical Behavior of (Cr, W, Al, Ti, Si)N Multilayer Coating on Nitrided AISI 316L Steel in Natural Seawater. Ceram. Int. 2020, 46, 22404–22418. [Google Scholar] [CrossRef]
- Naghashzadeh, A.R.; Shafyei, A.; Sourani, F. Nanoindentation and Tribological Behavior of TiN-TiCN-TiAlN Multilayer Coatings on AISI D3 Tool Steel. J. Mater. Eng. Perform. 2022, 31, 4335–4342. [Google Scholar] [CrossRef]
- Shi, Y.; Joseph, S.; Saunders, E.A.; Sandala, R.S.; Walker, A.; Lindley, T.C.; Dye, D. AgCl-Induced Hot Salt Stress Corrosion Cracking in a Titanium Alloy. Corros. Sci. 2021, 187, 109497. [Google Scholar] [CrossRef]
- Li, Y.; Ye, Q.; Zhu, Y.; Zhang, L.; He, Y.; Zhang, S.; Xiu, J. Microstructure, Adhesion and Tribological Properties of CrN/CrTiAlSiN/WCrTiAlN Multilayer Coatings Deposited on Nitrocarburized AISI 4140 Steel. Surf. Coat. Technol. 2019, 362, 27–34. [Google Scholar] [CrossRef]
- Dong, F.; Li, X.; Zhang, L.; Ma, L.; Li, R. Cavitation Erosion Mechanism of Titanium Alloy Radiation Rods in Aluminum Melt. Ultrason. Sonochem. 2016, 31, 150–156. [Google Scholar] [CrossRef]
- Li, Y.; Bi, Y.; Zhang, M.; Zhang, S.; Gao, X.; Zhang, Z.; He, Y. Hollow Cathodic Plasma Source Nitriding of AISI 4140 Steel. Surf. Eng. 2020, 37, 351–359. [Google Scholar] [CrossRef]
- Małkiewicz, K.; Sztogryn, M.; Mikulewicz, M.; Wielgus, A.; Kamiński, J.; Wierzchoń, T. Comparative Assessment of the Corrosion Process of Orthodontic Archwires Made of Stainless Steel, Titanium–Molybdenum and Nickel–Titanium Alloys. Arch. Civ. Mech. Eng. 2018, 18, 941–947. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Y.; Zhang, Z.H.; Zhang, S.Z.; Ren, P.; Qiu, J.X.; Wang, W.W.; Bi, Y.J.; He, Y.Y. Friction and Wear Behavior of Duplex-Treated AISI 316L Steels by Rapid Plasma Nitriding and (CrWAlTiSi)N Ceramic Coating. Results Phys. 2021, 24, 104132. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, L.; Xin, C.; Li, N.; Zhao, B.; Li, L. Effect of Molybdenum Content on Corrosion Resistance and Corrosion Behavior of Ti-Mo Titanium Alloy in Hydrochloric Acid. Mater. Today Commun. 2023, 34, 105032. [Google Scholar] [CrossRef]
- Faraji, M.; Pezzato, L.; Yazdanpanah, A.; Nardi, G.; Esmailzadeh, M.; Calliari, I. Effect of Natural Inhibitors on the Corrosion Properties of Grade 2 Titanium Alloy. Materials 2024, 17, 5202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Song, Y.; Dong, K.; Shan, D.; Han, E.H. Effect of Passive Film on the Galvanic Corrosion of Titanium Alloy Ti60 Coupled to Copper Alloy H62. Mater. Corros. 2019, 70, 1745–1754. [Google Scholar] [CrossRef]
- Benea, L.; Răvoiu, A.; Celis, J.-P. Anticorrosion Performance of the Electrochemically Grown Mixed Porous Oxide Films on Titanium Alloy in Biological Solution. ACS Biomater. Sci. Eng. 2019, 5, 5925–5934. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.-N.; Ali, M.M.; Kh, M.M. Biocompatibility, Corrosion, and Wear Resistance of β Titanium Alloys for Biomedical Applications. Appl. Phys. A 2020, 126, 942. [Google Scholar] [CrossRef]
- Nichul, U.; Hiwarkar, V. Carbon Dot Complimentary Green Corrosion Inhibitor for Crystallographically Textured Beta C Titanium Alloy for Marine Application: A State of Art. J. Alloys Compd. 2023, 962, 171116. [Google Scholar] [CrossRef]
- Hailan, S.; Daoxin, L.; Xiaohua, Z.; Weidong, Z.; Zhen, L.; Mengyao, L.; Yuting, H. Effect of Plasma Electrolytic Oxidation on the Hot Salt Corrosion Fatigue Behavior of the TC17 Titanium Alloy. Mater. Corros. 2021, 73, 558–572. [Google Scholar] [CrossRef]
- Ruixia, Z.; Steven, M.; Nicholas, W.; Hongyu, G.; Hao, Z.; Xiaoning, H.; Haifeng, Q.; Zhencheng, R.; Xianfeng, Z.; Doll, G.L.; et al. Effects of Laser Shock Peening on the Corrosion Behavior and Biocompatibility of a Nickel-Titanium Alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1854–1863. [Google Scholar]
- Çomaklı, O.; Yazıcı, M.; Atmaca, A.; Yetim, T. The Electrochemical and Tribocorrosion Behavior of Hybrid Ceramic Film-Coated Ti Alloys. Ceram. Int. 2024, 50, 7988–7997. [Google Scholar] [CrossRef]
- Cao, H.; Dong, F.; Zhang, Q.; Wu, H. Chemical Corrosion Resistance Mechanism of Titanium Alloy Radiation Rods with Self-Protected Structure. Ultrason. Sonochem. 2025, 113, 107224. [Google Scholar] [CrossRef]
- Ionescu, F.; Reclaru, L.; Ardelean, L.C.; Blatter, A. Comparative Analysis of the Corrosion Resistance of Titanium Alloys Intended to Come into Direct or Prolonged Contact with Live Tissues. Materials 2019, 12, 2841. [Google Scholar] [CrossRef]
- Li, Z.; Fan, L.; Ma, L.; Duan, T.; Zhang, H.; Hou, J.; Sun, M. Perspective Review on Factors That Influence the Stress Corrosion of Ti Alloys for Deep-Sea Applications. J. Mater. Sci. Technol. 2025, 222, 228–249. [Google Scholar] [CrossRef]
- Yang, X.; Du, C.; Wan, H.; Liu, Z.; Li, X. Influence of Sulfides on the Passivation Behavior of Titanium Alloy TA2 in Simulated Seawater Environments. Appl. Surf. Sci. 2018, 458, 198–209. [Google Scholar] [CrossRef]
- Feifei, H.; Yuxiang, Z.; Meng, Y.; Lei, W.; Ying, J. Synergistic Effects of Hydrostatic Pressure and Dissolved Oxygen on the SCC Behavior of Hydrogenated Ti6Al4V Alloy in Deep-Sea Environment. Metals 2023, 13, 449. [Google Scholar]
- Cheng, K.-Y.; Nargaraj, R.; Bijukumar, D.; Mathew, M.T.; McNallan, M. Improvement of Tribocorrosion Behavior on Titanium Alloy by Carbide-Derived Carbon (CDC). Surf. Coat. Technol. 2020, 392, 125692. [Google Scholar] [CrossRef]
- Li, X.-X.; Chen, L.-Y.; Hu, W.-B.; Wan, S.; Song, L.-F.; Wang, Y.-P.; Liao, B.-K.; Guo, X.-P. Corrosion and Passive Behavior of SLM and Wrought TA15 Titanium Alloys in Hydrochloric Acid Solutions. J. Iron Steel Res. Int. 2024, 14, 1356–1370. [Google Scholar] [CrossRef]
- Ma, L.; Li, W.; Yang, Y.; Ma, Y.; Luo, K.; Jia, B.; Xu, Z.; Yu, Z. Corrosion Behavior of NiTi Alloys Fabricate by Selective Laser Melting Subjected to Femtosecond Laser Shock Peening. Coatings 2021, 11, 1078. [Google Scholar] [CrossRef]
- Ran, L.; Yingshuang, L.; Dalei, Z. Multiscale Characterization of Erosion of TA2 Titanium Alloy Welded Joints. Front. Mater. 2022, 9, 910319. [Google Scholar] [CrossRef]
- López, M.F.; Gutiérrez, A.; Jiménez, J.A. In Vitro Corrosion Behaviour of Titanium Alloys Without Vanadium. Electrochim. Acta 2002, 47, 1359–1364. [Google Scholar] [CrossRef]
- Królikowska, M.M. Influence of Silicon and Chromium on the Na2SO4-Induced Hot Corrosion Behavior of Titanium Alloys. Crystals 2023, 13, 948. [Google Scholar] [CrossRef]
- Li, A.; Wang, Q.; Chen, R.; Ding, X.; Su, Y.; Fu, H. Application of Alloying for Enhancing the Corrosion Resistance of Titanium Alloys: A Review. Mater. Today Commun. 2025, 42, 111111. [Google Scholar] [CrossRef]
- Huang, J.; Shen, L.; Yu, S.; Yu, X.; Liu, G.; Fan, D. Corrosion Characterization of in Situ Nitrogen Reinforced Titanium Alloy Arc Cladding Layer. J. Mater. Eng. Perform. 2023, 33, 10546–10559. [Google Scholar] [CrossRef]
- Hailan, S.; Daoxin, L.; Xiaohua, Z.; Tianyi, J.; Weidong, Z. Effect of Pre-Hot Salt Corrosion on Hot Salt Corrosion Fatigue Behavior of the TC11 titanium alloy at 500 °C. Int. J. Fatigue 2022, 163, 107055. [Google Scholar] [CrossRef]
- Li, H.; Ma, G.; Wang, Z. Corrosion Behavior of ZrO2-TiO2 Composite Coatings Produced on Titanium Alloy Via Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2023, 469, 129814. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, S.; Samuel Lim, C.V.; Zhou, X.; Chen, X.; Hinton, B.R.W.; Boyer, R.R.; Williams, J.C.; Wu, X. The Mechanism of Aqueous Stress-Corrosion Cracking of α + β Titanium Alloys. Corros. Sci. 2017, 125, 29–39. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, J.; Guo, Y.; Wen, L. The Influence of Surface Nanocrystallization of TA2 Titanium Alloy on Its Corrosion Resistance. Coatings 2024, 14, 1114. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Lu, X.; Zhang, S.; Sun, Z.; Gao, F.; Li, J.; Lai, M. Influences of Microstructures and Macrozones on the Stress Corrosion Cracking Sensitivity of a Near Alpha Titanium Alloy. Corros. Sci. 2024, 232, 112015. [Google Scholar] [CrossRef]
- Lu, W.; Liu, Y.; Wu, X.; Liu, X.; Wang, J. Corrosion Behavior and Microstructural Effects on Passivation Film Mechanisms in Forged Ti-5Al-5Mo-5V-1Cr-1Fe Titanium Alloy Under Laser Surface Remelting. Corros. Sci. 2024, 241, 112542. [Google Scholar] [CrossRef]
- Costa, T.N.Q.; Dotta, T.C.; Galo, R.; Soares, M.E.d.C.; Pedrazzi, V. Effect of Tribocorrosion on Surface-Treated Titanium Alloy Implants: A Systematic Review with Meta-Analysis. J. Mech. Behav. Biomed. Mater. 2023, 145, 106008. [Google Scholar] [CrossRef]
- Escobar Claros, C.A.; Contri Campanelli, L.; Moreira Jorge, A.; Leprêtre, J.-C.; Bolfarini, C.; Roche, V. Corrosion Behaviour of Biomedical β-Titanium Alloys with the Surface-Modified by Chemical Etching and Electrochemical Methods. Corros. Sci. 2021, 188, 109544. [Google Scholar] [CrossRef]
- Mahadule, D.; Khatirkar, R.K.; Gupta, S.K.; Gupta, A.; Dandekar, T.R. Microstructure Evolution and Corrosion Behaviour of a High Mo Containing α + β Titanium Alloy for Biomedical Applications. J. Alloys Compd. 2022, 912, 165240. [Google Scholar] [CrossRef]
- Yang, X.; Hutchinson, C.R. Corrosion-Wear of β-Ti alloy TMZF (Ti-12Mo-6Zr-2Fe) in Simulated Body Fluid. Acta Biomater. 2016, 42, 429–439. [Google Scholar] [CrossRef]
- Angela, X.; Mostafa, A.; Remya, A.R.; Apurwa, S.; Barão, V.A.; Sukotjo, C.; Mathew, M.T. Peri-Implantitis in Relation to Titanium Corrosion: Current Status and Future Perspectives. J. Bio-Tribo-Corros. 2022, 8, 46. [Google Scholar]
- Burkov, A.A.; Chigrin, P.G.; Dvornik, M.I. Electrospark CuTi Coatings on Titanium Alloy Ti6Al4V: Corrosion and Wear Properties. Surf. Coat. Technol. 2023, 469, 129796. [Google Scholar] [CrossRef]
- Diomidis, N.; Mischler, S.; More, N.S.; Roy, M.; Paul, S.N. Fretting-Corrosion Behavior of β Titanium Alloys in Simulated Synovial Fluid. Wear 2011, 271, 1093–1102. [Google Scholar] [CrossRef]
- Noel, S.; Long, V.; Otgonsuren, L.; Sara, K. Computational Design of Corrosion-Resistant and Wear-Resistant Titanium Alloys for Orthopedic Implants. Mater. Today Commun. 2022, 33, 104465. [Google Scholar]
- Xiaofeng, Z.; Hui, L.; Susu, L.; Abdulrahman, A.-A.; Huan, L.; Yang, H.; Ling, R.; Hui, Y.; Ke, Y. Corrosion Behavior of Laser Powder Bed Fusion Manufactured Cu-Bearing Titanium Alloy in Artificial Saliva. Mater. Lett. 2023, 349, 134817. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Su, B.; Luo, L.; Wang, L.; Su, Y.; Xu, Y.; Huang, H.; Guo, J.; Fu, H. Investigation on Corrosion Behavior of Zr-Bearing TA10-Based Titanium Alloys. Corros. Sci. 2023, 221, 111354. [Google Scholar] [CrossRef]
- He, S.; Zhu, J.; Jing, Y.; Long, S.; Tang, L.; Cheng, L.; Shi, Z. Effect of 3D-Printed Porous Titanium Alloy Pore Structure on Bone Regeneration: A Review. Coatings 2024, 14, 253. [Google Scholar] [CrossRef]
- Sari, M.; Kristianto, N.A.; Chotimah; Ana, I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion Behavior of β Titanium Alloys for Biomedical Applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, Y.; Wang, Q.; Du, H.; Qiao, L. Effect of Povidone–Iodine Deposition on Tribocorrosion and Antibacterial Properties of Titanium Alloy. Appl. Surf. Sci. 2016, 363, 432–438. [Google Scholar] [CrossRef]
- Jingtao, Z.; Liping, N.; Jingwen, Z.; Yinglong, L. Investigation on Ultrasonic Cavitation Erosion of Aluminum–Titanium Alloys in Sodium Chloride Solution. Crystals 2021, 11, 1299. [Google Scholar] [CrossRef]
- Qiao, Q.; Cristino, V.A.M.; Tam, L.M.; Kwok, C.T. Laser Surface Alloying of Titanium Alloy with Silver: Microstructure, Hardness and Corrosion Property. Surf. Coat. Technol. 2023, 458, 129357. [Google Scholar] [CrossRef]
- Hrir, H.; Layachi, O.A.; Boudouma, A.; Bouari, A.E.; Sidimou, A.A.; Marrakchi, M.E.; Khoumri, E. Electrochemical Corrosion Behavior of α-Titanium Alloys in Simulated Biological Environments (Comparative Study). RSC Adv. 2024, 14, 38110–38119. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Olguín-Coca, J.; López-Léon, L.D.; Flores-De los Rios, J.P.; Almeraya-Calderón, F. Electrochemical Noise Analysis of the Corrosion of Titanium Alloys in NaCl and H2SO4 Solutions. Metals 2021, 11, 105. [Google Scholar]
- Koike, M.; Mitchell, R.J.; Horie, T.; Hummel, S.K.; Okabe, T. Biofilm Accumulation on Additive Manufactured Ti-6Al-4V Alloy Surfaces. J. Oral Sci. 2022, 64, 139–144. [Google Scholar] [CrossRef]
- Ritika, M.; Yingmei, H.; Nijhuis, C.A.; Nikolaos, S.; Castro Neto, A.H.; Vinicius, R. Graphene Nanocoating Provides Superb Long-Lasting Corrosion Protection to Titanium Alloy. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2021, 37, 1553–1560. [Google Scholar]
- Il, S.D.; Bong, L.J. Localized Corrosion and Repassivation Behaviors of Additively Manufactured Titanium Alloys in Simulated Biomedical Solutions. npj Mater. Degrad. 2023, 7, 44. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, H.; Sun, D.; Zhou, S.; Gao, Y.; Gao, K.; Fan, L. Markedly Improved Tensile Property and Corrosion Resistance of ZTC4 Titanium Alloy by Hot Pressing. Mater. Lett. 2024, 357, 135814. [Google Scholar] [CrossRef]
- Zhou, Y.; Jing, Z.; Jun, H. Study on Surface Properties of Nanosecond Laser Textured Plasma Nitrided Titanium Alloy. Mater. Today Commun. 2022, 31, 103746. [Google Scholar] [CrossRef]
- Mabilleau, G.; Bourdon, S.; Joly-Guillou, M.L.; Filmon, R.; Baslé, M.F.; Chappard, D. Influence of Fluoride, Hydrogen Peroxide and Lactic Acid on the Corrosion Resistance of Commercially Pure Titanium. Acta Biomater. 2006, 2, 121–129. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; He, X.; Li, M.; Huang, X.; Hang, R.; Tang, B. Wear and Corrosion Resistance of Anti-Bacterial Ti–Cu–N Coatings on Titanium Implants. Appl. Surf. Sci. 2014, 317, 614–621. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Almeida, S.M.A.; Soares, R.B.; Juliani, L.; Bracarense, A.Q.; Lins, V.d.F.C.; Junqueira, R.M.R. Synergism Between Mechanical Wear and Corrosion on Tribocorrosion of a Titanium Alloy in a Ringer Solution. J. Mater. Res. Technol. 2019, 8, 1593–1600. [Google Scholar] [CrossRef]
- Corne, P.; March, P.D.; Cleymand, F.; Geringer, J. Fretting-Corrosion Behavior on Dental Implant Connection in Human Saliva. J. Mech. Behav. Biomed. Mater. 2019, 94, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pound Bruce, G. Galvanic Corrosion of Nitinol Under Deaerated and Aerated Conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1322. [Google Scholar]
- Min, K.S. Oral Galvanism Related to Dental Implants. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 36. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Losada-Pérez, P.; Tiringer, U.; Taheri, P.; Desta, D.; Xie, C.; Crespo, D.; Mol, A.; Milošev, I.; Kokalj, A.; et al. Study of Mercaptobenzimidazoles as Inhibitors for Copper Corrosion: Down to the Molecular Scale. J. Electrochem. Soc. 2021, 168, 1504. [Google Scholar] [CrossRef]
- Wang, C.-M.; Cao, S.; Yang, F.-X.; Ma, Y.-J.; Su, H.; Fu, A.-Q.; Zhang, S.-Z.; Yang, R.; Hu, Q.-M. Insights into the Formation of Titanium Hydrides from First Principles Calculations. Acta Mater. 2024, 275, 119921. [Google Scholar] [CrossRef]
- Xu, D.; Meng, Z.; Guo, H.; Bao, Q.; Wang, M.; Wang, H.; Li, X.; Zhang, J.; Zhao, Z.; Wang, Q.; et al. Molecular Dynamics Simulation of the Atomic Mechanisms of Deformation and Phase Transformation in Titanium Alloys. Metall. Mater. Trans. A 2025, 18, 1971–1982. [Google Scholar] [CrossRef]
- Kranthi Kiran, A.S.; Kizhakeyil, A.; Ramalingam, R.; Verma, N.K.; Lakshminarayanan, R.; Kumar, T.S.S.; Doble, M.; Ramakrishna, S. Drug Loaded Electrospun Polymer/Ceramic Composite Nanofibrous Coatings on Titanium for Implant Related Infections. Ceram. Int. 2019, 45, 18710–18720. [Google Scholar] [CrossRef]
- Rikhari, B.; Saranya, K.; Kalaiyarasan, M.; Rahaman, M.; Periyasami, G.; Pandiaraj, S.; Thiruvengadam, M.; Pugalmani, S.; Rajakumar, G. Bioactive Conductive Polymer-Coated Titanium to Support Osseointegration. Biomass Convers. Biorefinery 2024, 14, 10699–10712. [Google Scholar] [CrossRef]
- Gevrek, T.N.; Yu, K.; Kizhakkedathu, J.N.; Sanyal, A. Thiol-Reactive Polymers for Titanium Interfaces: Fabrication of Antimicrobial Coatings. ACS Appl. Polym. Mater. 2019, 1, 1308–1316. [Google Scholar] [CrossRef]
- Wang, B.; Wei, S.; Huang, W.; Wang, Y.; Liang, Y.; Li, J.; Shi, R.; Guo, L.; Xue, J. Preparation, Anticorrosion Mechanism, and Application of Nano-Ti Polymer-Containing Coating. Eur. Polym. J. 2023, 195, 112200. [Google Scholar] [CrossRef]
- Hussain, Z.; Lin, Z.; Pan, H.; Huang, Y.; Tang, F.; Jiang, L. Synergizing Empirical and AI Methods to Examine Nano-Silica’s Microscale Contribution to Epoxy Coating Corrosion Resistance. Ceram. Int. 2024, 50, 47172–47191. [Google Scholar] [CrossRef]
- Meng, X.; Hou, L.; Wang, Z.; Liu, W.; Hao, H.; Lv, X.; An, J. Waterborne Acrylic Anti-Corrosion Coatings with Different Proportions of Yttrium Oxide/Titanium Dioxide Fillers. Prog. Org. Coat. 2025, 208, 109423. [Google Scholar] [CrossRef]
- Kamat, A.M.; Copley, S.M.; Segall, A.E.; Todd, J.A. Laser-Sustained Plasma (LSP) Nitriding of Titanium: A Review. Coatings 2019, 9, 283. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Zhang, Y.; Wang, X. Electrochemical Characteristics of Iridium Coating by Double Glow Plasma Discharge Process on Titanium Alloy Substrates. Surf. Eng. 2019, 35, 954–961. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Luo, J.; Zhang, S.; Qiu, J.; He, Y. Microstructure, Mechanical and Adhesive Properties of CrN/CrTiAlSiN/WCrTiAlN Multilayer Coatings Deposited on Nitrided AISI 4140 steel. Mater. Charact. 2018, 147, 353–364. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; He, Y. Solid Lubrication System and Its Plasma Surface Engineering: A Review. Lubricants 2023, 11, 473. [Google Scholar] [CrossRef]
- Dufour, T. From Basics to Frontiers: A Comprehensive Review of Plasma-Modified and Plasma-Synthesized Polymer Films. Polymers 2023, 15, 3607. [Google Scholar] [CrossRef]
- Anders, A. Plasma and Ion Sources in Large Area Coating: A Review. Surf. Coat. Technol. 2005, 200, 1893–1906. [Google Scholar] [CrossRef]
- Lu, S.; Sun, X.; Zhang, B.; Wu, J. Review of Cathode Plasma Electrolysis Treatment: Progress, Applications, and Advancements in Metal Coating Preparation. Materials 2024, 17, 3929. [Google Scholar] [CrossRef]
- Grigoriev, S.; Sotova, C.; Vereschaka, A.; Uglov, V.; Cherenda, N. Modifying Coatings for Medical Implants Made of Titanium Alloys. Metals 2023, 13, 718. [Google Scholar] [CrossRef]
- Loskutova, T.; Scheffler, M.; Pavlenko, I.; Zidek, K.; Pohrebova, I.; Kharchenko, N.; Smokovych, I.; Dudka, O.; Palyukh, V.; Ivanov, V.; et al. Corrosion Resistance of Coatings Based on Chromium and Aluminum of Titanium Alloy Ti-6Al-4V. Materials 2024, 17, 3880. [Google Scholar] [CrossRef] [PubMed]
- Bobzin, K.; Öte, M.; Schein, J.; Zimmermann, S.; Möhwald, K.; Lummer, C. Modelling the Plasma Jet in Multi-Arc Plasma Spraying. J. Therm. Spray Technol. 2016, 25, 1111–1126. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Tehrani, Z.P.; Ohannessian, N.; Schneider, C.W.; Pergolesi, D.; Lippert, T. A Practical Guide to Pulsed Laser Deposition. Chem. Soc. Rev. 2023, 52, 2294. [Google Scholar] [CrossRef]
- Kilicaslan, A.; Zabeida, O.; Bousser, E.; Schmitt, T.; Klemberg-Sapieha, J.E.; Martinu, L. Hard Titanium Nitride Coating Deposition Inside Narrow Tubes Using Pulsed DC PECVD Processes. Surf. Coat. Technol. 2019, 377, 124894. [Google Scholar] [CrossRef]
- Pillaca, E.J.D.M.; Ramírez, M.A.; Gutierrez Bernal, J.M.; Lugo, D.C.; Trava-Airoldi, V.J. DLC Deposition Inside of a Long Tube by Using the Pulsed-DC PECVD Process. Surf. Coat. Technol. 2019, 359, 55–61. [Google Scholar] [CrossRef]
- Afzali, P.; Ghomashchi, R.; Oskouei, R.H. On the Corrosion Behaviour of Low Modulus Titanium Alloys for Medical Implant Applications: A Review. Metals 2019, 9, 878. [Google Scholar] [CrossRef]
- Gurappa, I. Protection of titanium alloy components against high temperature corrosion. Mater. Sci. Eng. A 2003, 356, 372–380. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Zou, X.; Zhang, Z.; Liu, G.; Wang, Z.; Zhou, E.; Fan, Y.; Xu, D.; Wang, F. Riboflavin Facilitates Microbial Corrosion of NiTi Alloy by Human Intestinal Microbiota. Corros. Sci. 2024, 236, 112234. [Google Scholar] [CrossRef]
- Yilong, Y.; Ya, Z.; Xuhe, L.; Haoming, Z. Study on Chemical Corrosion Properties of Titanium Alloy in 2A14 Aluminum Melt. Mater. Res. Express 2023, 10, 106512. [Google Scholar] [CrossRef]
- Lian, Z.W.; Xin, S.W.; Guo, P.; Wang, H.; Qiang, F.; Tu, X.Y.; Fang, H.L. Synergistic Effect of Cr and Fe Elements on Stress Corrosion Fracture Toughness of Titanium Alloy. Met. Mater. Int. 2024, 10, 1087–1095. [Google Scholar] [CrossRef]
- Dong, K.; Song, Y.; Bian, G.; Cai, Y.; Han, E.-H. Tribocorrosion Behavior of TC18 Titanium Alloy: A Discussion About the Interaction Between Galvanic Corrosion and Wear. Tribol. Int. 2024, 192, 109292. [Google Scholar] [CrossRef]
- Fekry Amany, M.; Filippova Inna, V.; Medany Shymaa, S.; Abdel Gawad Soha, A.; Filippov Lev, O. Use of a Natural Rock Material as a Precursor to Inhibit Corrosion of Ti Alloy in an Aggressive Phosphoric Acid Medium. Sci. Rep. 2024, 14, 9807. [Google Scholar] [CrossRef]
- Grabarczyk, J.; Batory, D.; Kaczorowski, W.; Pązik, B.; Januszewicz, B.; Burnat, B.; Czerniak-Reczulska, M.; Makówka, M.; Niedzielski, P. Comparison of Different Thermo-Chemical Treatments Methods of Ti-6Al-4V Alloy in Terms of Tribological and Corrosion Properties. Materials 2020, 13, 5192. [Google Scholar] [CrossRef] [PubMed]
- Aires, M.M.; Naeem, M.; Melo-Silveira, R.F.; Macedo, A.J.; de Souza, I.V.; Rocha, H.A.O.; de Sousa, R.R.M.; Júnior, C.A. A Comparative Investigation of Plasma Nitriding and Oxy-Nitriding Treatment on Cells and Bacterial Interaction on Titanium Surfaces. Mater. Chem. Phys. 2025, 339, 130716. [Google Scholar] [CrossRef]
- Kovács, D.; Quintana, I.; Dobránszky, J. Effects of Different Variants of Plasma Nitriding on the Properties of the Nitrided Layer. J. Mater. Eng. Perform. 2019, 28, 5485–5493. [Google Scholar] [CrossRef]
- Weicheng, K.; Zhou, Y.; Jun, H. Characterization and Tribological Performance of Titanium Nitrides in Situ Grown on Ti6Al4V Alloy by Glow Discharge Plasma Nitriding. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2022, 37, 76–84. [Google Scholar]
- Liu, G.; Wang, E.; Gao, W.; Huang, Z.; Wei, B.; Yang, Y.; Yan, M.; Fu, Y.-D. Modification Mechanism of Ti-6Al-4V Alloy with Pre-Coated Ti-Cu-Al Multilayer Film Treated by Ion Nitriding: Experiments and First-Principles Calculations. Surf. Interfaces 2023, 40, 103004. [Google Scholar] [CrossRef]
- Ohtsu, N.; Takeda, S.; Endo, R.; Miura, K.; Kiba, T. Comparison of Open-Atmosphere Nitriding on Various Metal Surfaces Triggered by a Focused Pulsed Laser Irradiation. Surf. Coat. Technol. 2023, 454, 129190. [Google Scholar] [CrossRef]
- Fernandes, F.D.; de Oliveira Velloso, V.M.; Ferreira, B.; Landers, R.; Martins, G.V.; Barboza, M.J.R. Enhanced Tribological Behavior of Titanium Grade 2 Through Gas Nitriding: Process, Characterization and Performance Evaluation. J. Mater. Eng. Perform. 2024, 14, 17073–17092. [Google Scholar] [CrossRef]
- Sowińska, A.; Czarnowska, E.; Tarnowski, M.; Witkowska, J.; Wierzchoń, T. Structure and Hemocompatibility of Nanocrystalline Titanium Nitride Produced Under Glow-Discharge Conditions. Appl. Surf. Sci. 2018, 436, 382–390. [Google Scholar] [CrossRef]
- Coelho, L.; Batista, A.C.; Nobre, J.P.; Marques, M.J. Rolling and Rolling-Sliding Contact Fatigue Failure Mechanisms in 32 CrMoV 13 Nitrided Steel—An Experimental Study. Appl. Sci. 2021, 11, 10499. [Google Scholar] [CrossRef]
- Adachi, S.; Yamaguchi, T.; Tanaka, K.; Nishimura, T.; Ueda, N. Effects of Solid-Solution Carbon and Eutectic Carbides in AISI 316L Steel-Based Tungsten Carbide Composites on Plasma Carburizing and Nitriding. Metals 2023, 13, 1350. [Google Scholar] [CrossRef]
- Heo, H.-S.; Kim, S.-J. Electrochemical Properties and Interfacial Contact Resistance of Plasma Ion Nitrided Titanium as PEMFC Bipolar Plate. Jpn. J. Appl. Phys. 2023, 62, 1018. [Google Scholar] [CrossRef]
- Rossi, S.; Fedrizzi, L.; Bacci, T.; Pradelli, G. Corrosion Behaviour of Glow Discharge Nitrided Titanium Alloys. Corros. Sci. 2003, 45, 511–529. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Wang, L. Surface Properties of Nitrided Layer on AISI 316L Austenitic Stainless Steel Produced by High Temperature Plasma Nitriding in Short Time. Appl. Surf. Sci. 2014, 298, 243–250. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Xiu, J.; Wang, W.; Zhu, Y.; Hu, B. Wear and Corrosion Properties of AISI 420 Martensitic Stainless Steel Treated by Active Screen Plasma Nitriding. Surf. Coat. Technol. 2017, 329, 184–192. [Google Scholar] [CrossRef]
- Sun, F.; Cheng, W.; Zhao, B.; Lin, Z. Fatigue Properties of Plasma Nitriding for Dental Implant Application. J. Prosthet. Dent. 2024, 131, e321–e329. [Google Scholar] [CrossRef]
- Sun, J.; Yao, Q.T.; Zhang, Y.H.; Du, X.D.; Wu, Y.C.; Tong, W.P. Simultaneously Improving Surface Mechanical Properties and in Vitro Biocompatibility of Pure Titanium Via Surface Mechanical Attrition Treatment Combined with Low-Temperature Plasma Nitriding. Surf. Coat. Technol. 2017, 309, 382–389. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Ye, Z.; Jin, W.; Chen, Z.; Hu, Y.; Wu, J.; Chen, D.; Bai, B.; Wang, X.; et al. Improved Dry Sliding Wear Behavior of TA1 Titanium by Low-Temperature Plasma Nitriding by CCPN Method. Vacuum 2024, 221, 112945. [Google Scholar] [CrossRef]
- Miao, B.; Chai, Y.; Wei, K.; Hu, J. A Novel Duplex Plasma Treatment Combining Plasma Nitrocarburizing and Plasma Nitriding. Vacuum 2016, 133, 54–57. [Google Scholar] [CrossRef]
- Toboła, D.; Morgiel, J.; Maj, Ł. TEM Analysis of Surface Layer of Ti–6Al–4V ELI Alloy After Slide Burnishing and Low-Temperature Gas Nitriding. Appl. Surf. Sci. 2020, 515, 145942. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, M.; Zhang, Z.; Yi, X.; Yan, J.; Zhou, Z.; Fang, D.; He, Y.; Li, Y. Corrosion Behavior of Nitrided Layer of Ti6Al4V Titanium Alloy by Hollow Cathodic Plasma Source Nitriding. Materials 2023, 16, 2961. [Google Scholar] [CrossRef]
- Borowski, T. Enhancing the Corrosion Resistance of Austenitic Steel Using Active Screen Plasma Nitriding and Nitrocarburising. Materials 2021, 14, 3320. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhehao, Z.; Yongjie, B.; Minyi, Z.; Yang, L.; Fushuai, Z.; Shangzhou, Z.; Yongyong, H. Properties of Stainless-Steel Surface After Hollow Cathode Assisted Plasma Nitriding. Mater. Res. Express 2020, 7, 116524. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Shao, M.; He, Y.; Li, Y.; Li, Y.; Chen, G.; Luo, J. Nitrogen–Carbon-Induced Spinodal Structure in Plasma Nitrocarburized 38CrMoAl with Hollow Cathode Discharging. J. Mater. Sci. 2024, 59, 13305–13322. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, Z.; Meng, D.; Li, Y.; Jiang, X.; Li, J.; Wang, J.; Jiao, L.; Zhao, B.; Wang, L.; et al. Microstructure Evolution and Vacuum Tribological Properties of Surface Nitriding Layer on a TiZrAlV Alloy Fabricated by Laser Nitriding. J. Mater. Res. Technol. 2023, 24, 928–939. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Formation, Tribological and Corrosion Properties of Thicker Ti-N Layer Produced by Plasma Nitriding of Titanium in a N2-NH3 Mixture Gas. Surf. Coat. Technol. 2020, 393, 125846. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Yi, X.; Yan, J.; Xiu, J.; Fang, D.; Shao, M.; Ren, P.; He, Y.; Qiu, J. Improved Seawater Corrosion Resistance of Electron Beam Melting Ti6Al4V Titanium Alloy by Plasma Nitriding. Vacuum 2023, 216, 112463. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C. Effect of Gas Composition on Corrosion Behavior and Growth of Apatite on Plasma Nitrided Titanium Alloy Beta-21S. Appl. Surf. Sci. 2013, 268, 288–296. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Tamborim, S.M.; dos Santos, C.B.; Müller, I.L. Tribological, Electrochemical and Tribo-Electrochemical Characterization of Bare and Nitrided Ti6Al4V in Simulated Body Fluid Solution. Corros. Sci. 2011, 53, 1786–1793. [Google Scholar] [CrossRef]
- Tarnowski, M.; Borowski, T.; Skrzypek, S.; Kulikowski, K.; Wierzchoń, T. Shaping the Structure and Properties of Titanium and Ti6Al7Nb Titanium Alloy in low-Temperature Plasma Nitriding Processes. J. Alloys Compd. 2021, 864, 158896. [Google Scholar] [CrossRef]
- Kamiński, J.; Sitek, R.; Adamczyk-Cieślak, B.; Kulikowski, K. Impact of Glow-Discharge Nitriding Technology on the Properties of 3D-Printed Grade 2 Titanium Alloy. Materials 2024, 17, 4592. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Characterization and Electrochemical Performance of Plasma-Nitrided Titanium Alloy for Bipolar Plates. Eng. Res. Express 2024, 6, 5533. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L. Corrosion Resistance and Electrical Conductivity of Plasma Nitrided Titanium. Int. J. Hydrogen Energy 2021, 46, 11084–11091. [Google Scholar] [CrossRef]
- Hussein, M.A.; Yilbas, B.; Kumar, A.M.; Drew, R.; Al-Aqeeli, N. Influence of Laser Nitriding on the Surface and Corrosion Properties of Ti-20Nb-13Zr Alloy in Artificial Saliva for Dental Applications. J. Mater. Eng. Perform. 2018, 27, 4655–4664. [Google Scholar] [CrossRef]
- El-Hossary, F.M.; Negm, N.Z.; Abd El-Rahman, A.M.; Raaif, M.; Seleem, A.A.; Abd El-Moula, A.A. Tribo-Mechanical and Electrochemical Properties of Plasma Nitriding Titanium. Surf. Coat. Technol. 2015, 276, 658–667. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, K.; Gao, Y. Columnar and Nanocrystalline Combined Microstructure of the Nitrided Layer by Active Screen Plasma Nitriding on Surface-Nanocrystalline Titanium Alloy. Appl. Surf. Sci. 2023, 617, 156614. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, Z.; Wang, J.; Si, C. The Effects of Plasma Nitriding on the Microstructure, Friction Wear, and Electrochemical Properties of TC6 Titanium Alloy. J. Mater. Sci. 2025, 60, 3974–3987. [Google Scholar] [CrossRef]
- Yang, L.; Zhengwei, W.; Minghao, S.; Zhehao, Z.; Chengxu, W.; Jiwen, Y.; Jinpeng, L.; Lei, Z.; Bing, X.; Yongyong, H.; et al. Characterization and Electrochemical Behavior of a Multilayer-Structured Ti–N Layer Produced by Plasma Nitriding of Electron Beam Melting TC4 Alloy in Hank’s Solution. Vacuum 2023, 208, 111737. [Google Scholar]
- Xing, Y.-Z.; Wang, G.; Zhang, Y.; Chen, Y.-N.; Dargusch, M. Development in Plasma Surface Diffusion Techniques of Ti-6Al-4V Alloy: A Review. Int. J. Adv. Manuf. Technol. 2017, 92, 1901–1912. [Google Scholar] [CrossRef]
- Stuart, B.W.; Stan, G.E. Physical Vapour Deposited Biomedical Coatings. Coatings 2021, 11, 619. [Google Scholar] [CrossRef]
- Rajput, S.S.; Gangopadhyay, S.; Yaqub, T.B.; Cavaleiro, A.; Fernandes, F. Room and High Temperature Tribological Performance of CrAlN(Ag) Coatings: The Influence of Ag Additions. Surf. Coat. Technol. 2022, 450, 129011. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.X.; Qiu, J.; Chen, M.; Wang, J.; Hu, J. Antibacterial Property and Bioadaptability of Ti6Al4V Alloy with a Silvered Gradient Nanostructured Surface Layer. Rare Met. 2021, 41, 621–629. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, H.; Zhao, R.; Yu, G.; Chen, J.; Yin, F. Microstructure, Mechanical and Corrosion Performance of Magnetron Sputtered (Al0.5CoCrFeNi)Nx High-Entropy Alloy Nitride Films. J. Alloys Compd. 2023, 968, 172158. [Google Scholar] [CrossRef]
- Shakib, S.E.; Babakhani, A.; Kashefi Torbati, M. Nanomechanical Assessment of Tribological Behavior of TiN/TiCN Multi-Layer Hard Coatings Deposited by Physical Vapor Deposition. J. Mater. Res. Technol. 2023, 25, 1344–1354. [Google Scholar] [CrossRef]
- Vengesa, Y.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O.; Keshavarz, M.K. Investigation of Corrosion and Tribological Characteristics of Annealed CrN/CrAlN Coatings Deposited by CAE-PVD. Ceram. Int. 2023, 49, 3016–3029. [Google Scholar] [CrossRef]
- Major, L.; Krawiec, H.; Lackner, J.M.; Dyner, M.; Grysakowski, B.; Major, B. Nanoscale Characterization of Corrosion Mechanisms in Advanced Zr/ZrxN and Zr/ZrxN+a-C:H Nano-Multilayer Coatings for Medical Tools. Mater. Charact. 2020, 168, 110565. [Google Scholar] [CrossRef]
- Garbacz, H.; WieciŃSki, P.; Adamczyk-CieŚLak, B.; Mizera, J.; KurzydŁOwski, K.J. Studies of Aluminium Coatings Deposited by Vacuum Evaporation and Magnetron Sputtering. J. Microsc. 2010, 237, 475–480. [Google Scholar] [CrossRef]
- Wang, B.; Fu, X.; Song, S.; Chu, H.O.; Gibson, D.; Li, C.; Shi, Y.; Wu, Z. Simulation and Optimization of Film Thickness Uniformity in Physical Vapor Deposition. Coatings 2018, 8, 325. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Dai, S.; Zhu, L. Research Progress in Electrospark Deposition Coatings on Titanium Alloy Surfaces: A Short Review. Coatings 2023, 13, 1473. [Google Scholar] [CrossRef]
- Dongguang, L.; Ruan, C.F.; Peng, Z.; Haoran, M.; Yan, L.; Jiangping, T. Structural, Interface Texture and Toughness of TiAlN/CNx Multilayer Films. Mater. Charact. 2021, 178, 111301. [Google Scholar]
- Wang, Y.; Zhang, J.; Wang, Y.; Wang, C.; Guo, W.; Lu, X.; Sui, Y.; Lan, J. Inhibiting Tribocorrosion Damage of Cr/CrxN Coatings by Multi-Layer Design. Ceram. Int. 2021, 47, 842–850. [Google Scholar] [CrossRef]
- Bondarev, A.V.; Kiryukhantsev-Korneev, P.V.; Levashov, E.A.; Shtansky, D.V. Tribological Behavior and Self-Healing Functionality of TiNbCN-Ag Coatings in Wide Temperature Range. Appl. Surf. Sci. 2017, 396, 110–120. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, Y.; Wang, Y.; Niu, Y.; Xin, L.; Li, Y.; Su, J.; Zhu, S.; Wang, F. Corrosion Behaviors of Nitride Coatings on Titanium Alloy in NaCl-Induced Hot Corrosion. Acta Metall. Sin. 2021, 34, 1434–1446. [Google Scholar] [CrossRef]
- Andrei, S.; Mart, V.; Maksim, A.; Jakob, K.; Kristjan, J.; Priit, K.; Heinar, V.; Vytenis, J.; Rimtautas, L.; Regita, B.; et al. Effect of Laser Heat Treatment on AlxTi1−xN-Based PVD Coatings, Deposited on Carbon and Tool Steel Substrates. Surf. Coat. Technol. 2022, 446, 128771. [Google Scholar]
- Ananthakumar, R.; Subramanian, B.; Kobayashi, A.; Jayachandran, M. Electrochemical Corrosion and Materials Properties of Reactively Sputtered TiN/TiAlN Multilayer Coatings. Ceram. Int. 2011, 38, 477–485. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, W.; Cui, W.; Qin, G. Corrosion Resistance and Antibacterial Activity of Ti-N-O Coatings Deposited on Dental Titanium Alloy. Surf. Coat. Technol. 2021, 419, 127296. [Google Scholar] [CrossRef]
- Cui, W.-F.; Niu, F.-J.; Tan, Y.-L.; Qin, G.-W. Microstructure and Tribocorrosion Performance of Nanocrystalline TiN Graded Coating on Biomedical Titanium Alloy. Trans. Nonferrous Met. Soc. China 2019, 29, 1026–1035. [Google Scholar] [CrossRef]
- Cui, W.; Cheng, J.; Liu, Z. Bio-Tribocorrosion Behavior of a Nanocrystalline TiZrN Coating on Biomedical Titanium Alloy. Surf. Coat. Technol. 2019, 369, 79–86. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Li, X.; Zhou, X.; Liu, L.; Li, X.; Jiang, X.; Xiong, C.; Chen, Y.; You, F. Effect of N2 Ratio on the Conductivity and Corrosion Resistance of TiN/TaN Coating on TC4 Bipolar Plates for PEMFC. Mater. Today Commun. 2025, 42, 111415. [Google Scholar] [CrossRef]
- Anusha Thampi, A.T.; Bendavid, A.; Martin, P.J.; Vaithilingam, V.; Bean, P.A.; Evans, M.D.M.; Subramanian, B. Biomineralisation with Saos-2 Bone Cells on TiSiN Sputtered Ti Alloys. Colloids Surf. B Biointerfaces 2017, 155, 1–10. [Google Scholar]
- Zhang, M.; Gao, A.; Ma, S.; Xu, K.; Chu, P.K. Corrosion Resistance of Ti-Si-N Coatings in Blood and Cytocompatibility with Vascular Endothelial Cells. Vacuum 2016, 128, 45–55. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Huang, J. Multilayered TiAlN Films on Ti6Al4V Alloy for Biomedical Applications by Closed Field Unbalanced Magnetron Sputter Ion Plating Process. Mater. Sci. Eng. C 2016, 59, 669–676. [Google Scholar] [CrossRef]
- Hussein, M.A.; Kumar, A.M.; Ankah, N.; Abdul Azeem, M. Thermal Treatment Effect on the Surface and in Vitro Corrosion Characteristics of Arc Deposited TiN Coating on Ti Alloy for Orthopedic Applications. Ceram. Int. 2021, 47, 23203–23213. [Google Scholar] [CrossRef]
- Liang, C.-L.; Cheng, G.-A.; Zheng, R.-T.; Liu, H.-P. Fabrication and Performance of TiN/TiAlN Nanometer Modulated Coatings. Thin Solid Film. 2011, 520, 813–817. [Google Scholar] [CrossRef]
- Ke, L.; Fuliang, M.; Ming, L.; Minpeng, D.; Yebiao, Z.; Yongxin, W.; Xuedong, W.; Xiang, L.; Jinlong, L. Structure and Tribocorrosion Behavior of TiAlCN Coatings with Different Al Contents in Artificial Seawater by Multi-Arc Ion Plating. Surf. Topogr.-Metrol. Prop. 2021, 9, 5004. [Google Scholar]
- Li, T.; Yan, Z.; Liu, Z.; Yan, Y.; Chen, Y. Surface Microstructure and Performance of TiN Monolayer Film on Titanium Bipolar Plate for PEMFC. Int. J. Hydrogen Energy 2021, 46, 31382–31390. [Google Scholar] [CrossRef]
- SUN, Z.; HE, G.; MENG, Q.; LI, Y.; TIAN, X. Corrosion Mechanism Investigation of TiN/Ti Coating and TC4 Alloy for Aircraft Compressor Application. Chin. J. Aeronaut. 2020, 33, 1824–1835. [Google Scholar] [CrossRef]
- Vargas, S.; Galeano-Osorio, D.S.; Castano, C.E. Controlling Preferential Growth of Chromium—Nitrogen R-HiPIMS and R-DCMS Films by Substrate Magnetic Biasing. Appl. Surf. Sci. 2021, 569, 151113. [Google Scholar] [CrossRef]
- Mejía V., H.D.; Perea, D.; Bejarano, G. Development and Characterization of TiAlN (Ag, Cu) Nanocomposite Coatings Deposited by DC Magnetron Sputtering for Tribological Applications. Surf. Coat. Technol. 2020, 381, 125095. [Google Scholar]
- Kiryukhantsev-Korneev, P.V.; Kuptsov, K.A.; Tabachkova, N.Y.; Andreev, N.V.; Sagalova, T.B.; Golizadeh, M.; Bondarev, A.V. Studying the Diffusion-Barrier Properties, Thermal Stability and Oxidation Resistance of TiAlSiCN, TiAlSiCN/AlOx, and TiAlSiCN/SiBCN Coatings. Prot. Met. Phys. Chem. Surf. 2021, 57, 1008–1024. [Google Scholar] [CrossRef]
- Yaqub, T.B.; Yaqoob, K.; Mukhtar, A.; Fernandes, F.; Bondarev, A.; Ferreira, F.; Al-Rjoub, A.; Cavaleiro, A. Vacuum Tribological Properties of W-S-N Coatings Synthesized by Direct Current Magnetron Sputtering. Coatings 2022, 12, 1646. [Google Scholar] [CrossRef]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Pana, I.; Braic, V.; Vladescu, A.; Ion, R.; Parau, A.C.; Zoita, N.C.; Dinu, M.; Kiss, A.E.; Cimpean, A.; Braic, M. SiC- and Ag-SiC-Doped Hydroxyapatite Coatings Grown Using Magnetron Sputtering on Ti Alloy for Biomedical Application. Coatings 2022, 12, 195. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Zhang, Y.; Yu, X.; Zhou, Y.; Gao, P. Aluminum Coating of Twinning-Induced Plasticity Plates Via Plasma-Enhanced Magnetron Sputtering as a Potential Industrial Technique. Surf. Coat. Technol. 2021, 411, 126937. [Google Scholar] [CrossRef]
- Babur, M.Z.; Iqbal, Z.; Shafiq, M.; Naz, M.Y.; Makhlouf, M.M. Comparative Study of PVD Titanium Nitride Coating with Cathodic Cage Plasma Nitriding of Austenitic 201 Stainless Steel for Enhanced Tribological Properties. Appl. Phys. A 2021, 26, 954. [Google Scholar] [CrossRef]
- Niu, X.; Dong, G.; Wei, S.; Wang, Y.; Wang, B.; Tian, H. Effects of Nitrogen Flow Rate on the Microstructure and Mechanical and Tribological Properties of TiAlN Films Prepared Via Reactive Magnetron Sputtering. Ceram. Int. 2023, 49, 19885–19894. [Google Scholar] [CrossRef]
- Lü, W.; Li, G.; Zhou, Y.; Liu, S.; Wang, K.; Wang, Q. Effect of High Hardness and Adhesion of Gradient TiAlSiN Coating on Cutting Performance of Titanium Alloy. J. Alloys Compd. 2020, 820, 153137. [Google Scholar] [CrossRef]
- Lü, W.; Li, G.; Li, X.; Liu, S.; Deng, J.; Wang, Q. Effect of Pulsed Electric Field on the Nanocrystalline Growth of Magnetron-Sputtered TiAlN Hard Coatings. Ceram. Int. 2024, 50, 920–926. [Google Scholar] [CrossRef]
- Lofaj, F.; Kabátová, M.; Kvetková, L.; Dobrovodský, J.; Girman, V. Hybrid PVD-PECVD W-C:H Coatings Prepared by Different Sputtering Techniques: The Comparison of Deposition Processes, Composition and Properties. Surf. Coat. Technol. 2019, 375, 839–853. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.W.; Zhang, Z.H.; Shao, M.H.; Lu, J.P.; Yan, J.W.; Zhang, L.; He, Y.Y. Microstructure and Tribological Properties of Multilayered ZrCrW(C)N Coatings Fabricated by Cathodic Vacuum-Arc Deposition. Ceram. Int. 2022, 48, 36655–36669. [Google Scholar] [CrossRef]
- Wongpanya, P.; Silawong, P.; Photongkam, P. Adhesion and Corrosion of Al–N Doped Diamond-Like Carbon Films Synthesized by Filtered Cathodic Vacuum Arc Deposition. Ceram. Int. 2022, 48, 20743–20759. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.-B.; Gong, C.-Z.; Liu, X.-L.; Li, J. Effect of Plasma Nitriding Substrate Current Density on the Adhesion Strength of in Situ PVD TiN Coatings. J. Vac. Sci. Technol. A 2023, 41, 033405. [Google Scholar] [CrossRef]
- Wongpanya, P.; Silawong, P.; Photongkam, P. Nanomechanical Properties and Thermal Stability of Al–N-Co-Doped DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition. Surf. Coat. Technol. 2021, 424, 127655. [Google Scholar] [CrossRef]
- Tian, C.; Xiang, Y.; Zou, C.; Yu, Y.; Abudouwufu, T.; Yang, B.; Fu, D. Mechanical and Tribological Properties of CrWN/MoN Nano-Multilayer Coatings Deposited by Cathodic Arc Ion Plating. Coatings 2024, 14, 367. [Google Scholar] [CrossRef]
- Ding, F.; Wei, X.; Cao, J.; Ma, Y.; Su, H.; Zhao, T.; You, J.; Lv, Y. Thermal Corrosion Properties of Composite Ceramic Coating Prepared by Multi-Arc Ion Plating. Coatings 2024, 14, 1150. [Google Scholar] [CrossRef]
- Vengesa, Y.; Fattah-alhosseini, A.; Elmkhah, H.; Imantalab, O. Influence of Post-Deposition Annealing Temperature on Morphological, Mechanical and Electrochemical Properties of CrN/CrAlN Multilayer Coating Deposited by Cathodic Arc Evaporation- Physical Vapor Deposition Process. Surf. Coat. Technol. 2022, 432, 128090. [Google Scholar] [CrossRef]
- Yang, J.; Cao, H.; Li, Y.; Liu, F.; Tang, Y.; Zhao, N.; Qi, F.; Ouyang, X. Microstructure, Mechanical Properties, and Corrosion Resistance of TiSiN Coating Prepared by FCVA Technique with Different N2 Flow Rates. Vacuum 2023, 209, 111811. [Google Scholar] [CrossRef]
- Pooneh, P.; Hassan, E.; Yousef, M. Correlation Between Nanoindentation Response and Wear Characteristics of CrN-Based Coatings Deposited by an Arc-PVD Method. Int. J. Appl. Ceram. Technol. 2022, 19, 2598–2612. [Google Scholar]
- Chaochao, J.; Qiaoqin, G.; Jianping, L.; Yongchun, G.; Zhong, Y.; Wei, Y.; Dapeng, X.; Bo, Y. Microstructure and Properties of CrN Coating Via Multi-Arc Ion Plating on the Valve Seat Material Surface. J. Alloys Compd. 2022, 891, 160859. [Google Scholar]
- Wang, Y.; Gao, Y.; Fan, Y. Interfacial Modification of Ti3AlC2/Cu Composites by Multi-Arc Ion Plating Titanium. Coatings 2022, 12, 1754. [Google Scholar] [CrossRef]
- Zhang, M.; Xin, L.; Ding, X.; Zhu, S.; Wang, F. Effects Ti/TiAlN Composite Multilayer Coatings on Corrosion Resistance of Titanium Alloy in Solid NaCl-H2O-O2 at 600 °C. J. Alloys Compd. 2018, 734, 307–317. [Google Scholar] [CrossRef]
- Nolan, D.; Huang, S.W.; Leskovsek, V.; Braun, S. Sliding Wear of Titanium Nitride Thin Films Deposited on Ti–6Al–4V Alloy by PVD and Plasma Nitriding Processes. Surf. Coat. Technol. 2006, 200, 5698–5705. [Google Scholar] [CrossRef]
- Mohammed, F.K.; Ramizy, A.; Ahmed, N.M.; Yam, F.K.; Hassan, Z.; Beh, K.P. Characteristics of Aluminium Nitride Thin Film Prepared by Pulse Laser Deposition with Varying Laser Pulses. Opt. Mater. 2024, 153, 115622. [Google Scholar] [CrossRef]
- Gatabi, J.R.; Rahman, S.; Amaro, A.; Nash, T.; Rojas-Ramirez, J.; Pandey, R.K.; Droopad, R. Tuning Electrical Properties of PZT Film Deposited by Pulsed Laser Deposition. Ceram. Int. 2017, 43, 6008–6012. [Google Scholar] [CrossRef]
- Dellasega, D.; Russo, V.; Pezzoli, A.; Conti, C.; Lecis, N.; Besozzi, E.; Beghi, M.; Bottani, C.E.; Passoni, M. Boron Films Produced by High Energy Pulsed Laser Deposition. Mater. Des. 2017, 134, 35–43. [Google Scholar] [CrossRef]
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef]
- Dumitrescu, L.N.; Ionita, E.-R.; Birjega, R.; Lazea-Stoyanova, A.; Ionita, M.-D.; Epurescu, G.; Banici, A.-M.; Brajnicov, S.; Andrei, F.; Matei, A. Kaolinite Thin Films Grown by Pulsed Laser Deposition and Matrix Assisted Pulsed Laser Evaporation. Nanomaterials 2022, 12, 546. [Google Scholar] [CrossRef]
- Antonova, K.; Duta, L.; Szekeres, A.; Stan, G.E.; Mihailescu, I.N.; Anastasescu, M.; Stroescu, H.; Gartner, M. Influence of Laser Pulse Frequency on the Microstructure of Aluminum Nitride Thin Films Synthesized by Pulsed Laser Deposition. Appl. Surf. Sci. 2017, 394, 197–204. [Google Scholar] [CrossRef]
- Bhuyan, H.; Escalona, M.; Villegas, R.; Mal, E.; Cisternas, M.; Saikia, P.; Bora, B.; Kausik, S.S.; Wyndham, E.; Favre, M. Effect of RF Acetylene Plasma on the Composition and Dynamics of a Titanium Plasma Plume in a Plasma Enhanced Pulsed Laser Deposition System. Opt. Laser Technol. 2025, 181, 111803. [Google Scholar] [CrossRef]
- Liu, X.; Xu, W.; Xu, M.; Hao, X.; Feng, X. Epitaxial CuO Thin Films Prepared on MgAl2O4 (110) by RF-Plasma Assisted Pulsed Laser Deposition. Vacuum 2019, 169, 108932. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, D.; Wu, L.; Xu, N.; Sun, J.; Wu, J. Structure and Optical Properties of ZrxHf1−xO2 Films Deposited by Pulsed Laser Co-Ablation of Zr and Hf Targets with the Assistance of Oxygen Plasma. Ceram. Int. 2022, 48, 587–596. [Google Scholar] [CrossRef]
- Slepička, P.; Hurtuková, K.; Rimpelová, S.; Trhoňová, Š.; Martan, J.; Procházka, M.; Švorčík, V.; Slepičková Kasálková, N. Ti and TiAlV Foils Enhanced with PLD and Flash-Deposited Carbon: On Cytocompatibility and Antibacterial Activity. J. Adv. Join. Process. 2025, 11, 100274. [Google Scholar] [CrossRef]
- Gnanavel, S.; Ponnusamy, S.; Sivakumar, K.; Priyadarshini, D. Electrochemical and Biological Behaviour of Near β Titanium Alloy for Biomedical Implant Applications. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 236, 233–241. [Google Scholar] [CrossRef]
- Floroian, L.; Craciun, D.; Socol, G.; Dorcioman, G.; Socol, M.; Badea, M.; Craciun, V. Titanium Implants’ Surface Functionalization by Pulsed Laser Deposition of TiN, ZrC and ZrN Hard Films. Appl. Surf. Sci. 2017, 417, 175–182. [Google Scholar] [CrossRef]
- Gürsoy, M. Fabrication of pH-Responsive PDPAEMA Thin Film Using a One-Step Environmentally Friendly Plasma Enhanced Chemical Vapor Deposition. Coatings 2024, 14, 347. [Google Scholar] [CrossRef]
- Parastoo, M.; Rasoul, S.-M.; Hossein, A. Preparation of Titanium Nitride/Oxynitride Nanotube Array Via Ammonia-Free PECVD Method for Enhancing Supercapacitor Performance. J. Alloys Compd. 2022, 904, 163895. [Google Scholar]
- Cichomski, M.; Burnat, B.; Prowizor, M.; Jedrzejczak, A.; Batory, D.; Piwoński, I.; Kozłowski, W.; Szymanski, W.; Dudek, M. Tribological and Corrosive Investigations of Perfluoro and Alkylphosphonic Self-Assembled Monolayers on Ti Incorporated Carbon Coatings. Tribol. Int. 2019, 130, 359–365. [Google Scholar] [CrossRef]
- Zilong, Z.; Qiang, M.; Wenping, L.; Jinjiao, X.; Hao, L.; Yan, Q.; Shiwei, Z. Effect of CrAl Interlayer on Adhesion Strength of CrAlN Coating. Surf. Eng. 2020, 36, 438–446. [Google Scholar]
- Braceras, I.; Ibáñez, I.; Dominguez-Meister, S.; Velasco, X.; Brizuela, M.; Garmendia, I. Electro-Tribological Properties of Diamond Like Carbon Coatings. Friction 2020, 8, 451–461. [Google Scholar] [CrossRef]
- Tahir, N.A.M.; Abdollah, M.F.B.; Tamaldin, N.; Zin, M.R.B.M.; Amiruddin, H. Optimisation of Graphene Grown from Solid Waste Using CVD Method. Int. J. Adv. Manuf. Technol. 2020, 106, 211–218. [Google Scholar] [CrossRef]
- Çelik, A.; Demirdelen, M.; Tüzemen, Ş.M.; Kovacı, H. Improving the Corrosion Resistance of Ti6Al4V Alloy Through TiN/Polyaniline Hybrid Films Produced by Plasma Nitriding and PECVD. Appl. Surf. Sci. 2025, 692, 162697. [Google Scholar] [CrossRef]
- Bedarev, V.; Maaß, P.A.; Thewes, A.; Böke, M.; von Keudell, A. Plasma Deposited Carbon Containing Zirconia Films as Thermal Barriers. J. Vac. Sci. Technol. A 2023, 41, 3001. [Google Scholar] [CrossRef]
- Joo, S.H.; Kim, Y.K.; Lee, S.-J.; Kim, J.-P. Dry Media Reaction-Based Anti-Hydration Coating on Magnesium Oxide Microspheres for Thermal Interfacial Applications. Appl. Surf. Sci. 2024, 672, 160855. [Google Scholar] [CrossRef]
- Nass, K.C.F.; Radi, P.A.; Leite, D.M.G.; Massi, M.; da Silva Sobrinho, A.S.; Dutra, R.C.L.; Vieira, L.; Reis, D.A.P. Tribomechanical and Structural Properties of a-SiC:H Films Deposited Using Liquid Precursors on Titanium Alloy. Surf. Coat. Technol. 2015, 284, 240–246. [Google Scholar] [CrossRef]
- Mohan, L.; Anandan, C.; Grips, V.K.W. Corrosion Behavior of Titanium Alloy Beta-21S Coated with Diamond Like Carbon in Hank’s Solution. Appl. Surf. Sci. 2012, 258, 6331–6340. [Google Scholar] [CrossRef]
- Swiatek, L.; Olejnik, A.; Grabarczyk, J.; Jedrzejczak, A.; Sobczyk-Guzenda, A.; Kaminska, M.; Jakubowski, W.; Szymanski, W.; Bociaga, D. Multi-Doped Diamond Like-Carbon Coatings (DLC-Si/Ag) for Biomedical Applications Fabricated Using the Modified Chemical Vapour Deposition Method. Diam. Relat. Mater. 2016, 67, 54–62. [Google Scholar] [CrossRef]
- Sitek, R.; Bolek, T.; Mizera, J. Microstructure and Properties of Ti-Al Intermetallic/Al2O3 Layers Produced on Ti6Al2Mo2Cr Titanium Alloy by PACVD Method. Appl. Surf. Sci. 2018, 437, 19–27. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Yi, J.; Dong, F.; Chen, P. Overview of Surface Modification Techniques for Titanium Alloys in Modern Material Science: A Comprehensive Analysis. Coatings 2024, 14, 148. [Google Scholar] [CrossRef]
- Song, X.; Zhao, M.; Li, D. Controllable Ag/Ta Ratios of Co-Implanted TiN Films on Titanium Alloys for Osteogenic Enhancement and Antibacterial Responses. Surf. Coat. Technol. 2022, 436, 128294. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Z.; Yu, B.; Ruan, Q.; Chu, P.K. Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy. Coatings 2020, 10, 313. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, S.M.; Wong, H.M.; Wu, S.; Hu, T.; Yeung, K.W.; Chu, P.K. Effects of Carbon and Nitrogen Plasma Immersion Ion Implantation on in Vitro and in Vivo Biocompatibility of Titanium Alloy. ACS Appl. Mater. Interfaces 2013, 5, 1510. [Google Scholar] [CrossRef]
- Vlcak, P.; Fojt, J.; Weiss, Z.; Kopeček, J.; Perina, V. The Effect of Nitrogen Saturation on the Corrosion Behaviour of Ti-35Nb-7Zr-5Ta Beta Titanium Alloy Nitrided by Ion Implantation. Surf. Coat. Technol. 2019, 358, 144–152. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, B.; Huang, J.; Zhou, M.; Wang, L.; Xi, Y.; Jia, H.; Xu, S.; Liu, H.; et al. A Review of Research on Improving Wear Resistance of Titanium Alloys. Coatings 2024, 14, 786. [Google Scholar] [CrossRef]
- Ghorbel, H.F.; Guidara, A.; Danlos, Y.; Bouaziz, J.; Coddet, C. Alumina-Fluorapatite Composite Coating Deposited by Atmospheric Plasma Spraying: An Agent of Cohesion Between Bone and Prostheses. Mater. Sci. Eng. C 2017, 71, 1090–1098. [Google Scholar] [CrossRef]
- Adachi, S.; Ueda, N. Formation of S-Phase Layer on Plasma Sprayed AISI 316L Stainless Steel Coating by Plasma Nitriding at Low Temperature. Thin Solid Film. 2012, 523, 11–14. [Google Scholar] [CrossRef]
- Nelson, G.M.; Nychka, J.A.; McDonald, A.G. Flame Spray Deposition of Titanium Alloy-Bioactive Glass Composite Coatings. J. Therm. Spray Technol. 2011, 20, 1339–1351. [Google Scholar] [CrossRef]
- Smith, M.F.; Hall, A.C.; Fleetwood, J.D.; Meyer, P. Very Low Pressure Plasma Spray—A Review of an Emerging Technology in the Thermal Spray Community. Coatings 2011, 1, 117–132. [Google Scholar] [CrossRef]
- Heimann, R.B. The Nature of Plasma Spraying. Coatings 2023, 13, 622. [Google Scholar] [CrossRef]
- Wang, G.; Liu, J.; Yang, J.; Liu, S.; Bu, L.; Chen, J. Study of the Performance of Laser Melting Wear-Resistant Coatings on TC4 Titanium Alloy Surfaces. Coatings 2024, 14, 730. [Google Scholar] [CrossRef]
- Bobzin, K.; Öte, M.; Schein, J.; Zimmermann, S. Numerical Study on Plasma Jet and Particle Behavior in Multi-arc Plasma Spraying. J. Therm. Spray Technol. 2017, 26, 811–830. [Google Scholar] [CrossRef]
- Anand, A.; Das, M.; Kundu, B.; Balla, V.K.; Bodhak, S.; Gangadharan, S. Plasma-Sprayed Ti6Al4V Alloy Composite Coatings Reinforced with In Situ Formed TiB-TiN. J. Therm. Spray Technol. 2017, 26, 2013–2019. [Google Scholar] [CrossRef]
- Alontseva, D.; Safarova, Y.; Voinarovych, S.; Obrosov, A.; Yamanoglu, R.; Khoshnaw, F.; Yavuz, H.I.; Nessipbekova, A.; Syzdykova, A.; Azamatov, B.; et al. Biocompatibility and Corrosion of Microplasma-Sprayed Titanium and Tantalum Coatings versus Titanium Alloy. Coatings 2024, 14, 206. [Google Scholar] [CrossRef]
- Cao, L.; Ullah, I.; Li, N.; Niu, S.; Sun, R.; Xia, D.; Yang, R.; Zhang, X. Plasma Spray of Biofunctional (Mg, Sr)-Substituted Hydroxyapatite Coatings for Titanium Alloy Implants. J. Mater. Sci. Technol. 2019, 35, 719–726. [Google Scholar] [CrossRef]
- Singh, S.; Pandey, K.K.; Keshri, A.K. Effect of Plasma Power on Corrosion Behaviour of Plasma Sprayed Hydroxyapatite Coatings. Met. Mater. Int. 2021, 27, 4455–4462. [Google Scholar] [CrossRef]
- Qadir, D.; Sharif, R.; Nasir, R.; Awad, A.; Mannan, H.A. A Review on Coatings Through Thermal Spraying. Chem. Pap. 2024, 78, 71–91. [Google Scholar] [CrossRef]
- Zhao, W.; Kong, D. Microstructure, Bonding Strength, and Friction–Wear Performance of AlCrN/Nitrided Layer Composite Coating on H13 Hot Work Mould Steel. Int. J. Appl. Ceram. Technol. 2019, 16, 951–965. [Google Scholar] [CrossRef]
- Broch, M.; Fontoura, C.P.; Lima, A.O.; Ordoñez, M.F.; Machado, I.F.; Aguzzoli, C.; Farias, M.C. Scratch Response of Hollow Cathode Radiofrequency Plasma-Nitrided and Sintered 316L Austenitic Stainless Steel. Coatings 2024, 14, 334. [Google Scholar] [CrossRef]
- Abakay, E.; Armağan, M.; Avcu, Y.Y.; Guney, M.; Yousif, B.F.; Avcu, E. Advances in Improving Tribological Performance of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Critical Review. Front. Mater. 2024, 11, 1452288. [Google Scholar] [CrossRef]
- Dalibon, E.L.; Moreira, R.D.; Guitar, M.A.; Trava-Airoldi, V.J.; Brühl, S.P. Influence of the Substrate Pre-Treatment on the Mechanical and Corrosion Response of Multilayer DLC Coatings. Diam. Relat. Mater. 2021, 118, 108507. [Google Scholar] [CrossRef]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M. Review of Growth Defects in Thin Films Prepared by PVD Techniques. Coatings 2020, 10, 447. [Google Scholar] [CrossRef]
- Yaqoob, S.; Ghani, J.A.; Jouini, N.; Juri, A.Z. Performance Evaluation of PVD and CVD Multilayer-Coated Tools in Machining High-Strength Steel. Coatings 2024, 14, 865. [Google Scholar] [CrossRef]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Pavol, Š.; Miroslav, B.; Ernest, G.; František, T.; Roman, P. Wear of AlCrN and CrAlSiN Coatings Applied to Nonstandard Involute Gears. Lubricants 2021, 9, 54. [Google Scholar]
- Filho, E.A.M.; Naeem, M.; Díaz-Guillén, J.C.; Sousa, E.M.; Costa, T.H.C.; Iqbal, J.; Sousa, R.R.M. Microstructure, Mechanical, and Tribological Properties of Transition Metal (Nb, V, W) Nitride Coating on AISI-1045 Steel by Cathodic Cage Plasma Deposition. J. Vac. Sci. Technol. A 2024, 42, 053105. [Google Scholar] [CrossRef]
- Rahimi, E.; Nijdam, T.; Jahagirdar, A.; Broitman, E.; Mol, A. A Combined Microstructural, Electrochemical and Nanomechanical Study of the Corrosion and Passivation Properties of a Cr/CrN Multilayer Coating. Corros. Sci. 2025, 252, 112943. [Google Scholar] [CrossRef]
- Qi, J.L.; Wang, L.P.; Hao, J.; Zhang, Y.; He, X.J.; Pang, H.P.; Zhang, K.; Wen, M. Self-Adaptive Root-Like Capillary Diffusion Channels Enabled by TaN/Zr3N4 Nanomultilayered Architecture: Achieving Superior Corrosion Resistance. Mater. Des. 2023, 225, 111555. [Google Scholar] [CrossRef]
- Naeem, M.; Awan, S.; Shafiq, M.; Raza, H.A.; Iqbal, J.; Díaz-Guillén, J.C.; Sousa, R.R.M.; Jelani, M.; Abrar, M. Wear and Corrosion Studies of Duplex Surface-Treated AISI-304 Steel by a Combination of Cathodic Cage Plasma Nitriding and PVD-TiN Coating. Ceram. Int. 2022, 48, 21473–21482. [Google Scholar] [CrossRef]
- Tjandra, J.; Alabort, E.; Barba, D.; Pedrazzini, S. Corrosion, Fatigue and Wear of Additively Manufactured Ti Alloys for Orthopaedic Implants. Mater. Sci. Technol. 2023, 39, 2951–2965. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Barboza, A.P.M.; Batista, R.J.C.; Neves, B.R.A.; Müller, I.L. Corrosion, Wear and Wear–Corrosion Behavior of Graphite-Like a-C:H Films Deposited on Bare and Nitrided Titanium Alloy. Diam. Relat. Mater. 2013, 31, 58–64. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, P.; Wu, L.; Zhang, X.; Wei, P.; Zhao, J. Corrosion-Induced Deceleration-to-Acceleration of Fatigue Crack Growth for Deep-Sea Ti6Al4V ELI Titanium Alloy. Eng. Fract. Mech. 2023, 281, 109160. [Google Scholar] [CrossRef]
- Tang, J.; Luo, H.; Qi, Y.; Xu, P.; Ma, S.; Zhang, Z.; Ma, Y. The Effect of Cryogenic Burnishing on the Formation Mechanism of Corrosion Product Film of Ti-6Al-4V Titanium Alloy in 0.9% NaCl Solution. Surf. Coat. Technol. 2018, 345, 123–131. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, K.; Gao, Y. Low-Temperature Plasma Nitriding at 500 °C on Surface-Nanocrystalline Ti–4Al–2V Alloy. Mater. Chem. Phys. 2023, 306, 128080. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Hu, J. Performances Investigation of Ti6Al4V Alloy Modified by Plasma Nitriding + Plasma Enhanced Chemical Vapor Deposition and Laser Remelting Process in Simulated Body Fluid. Met. Mater. Int. 2020, 27, 4757–4767. [Google Scholar] [CrossRef]
- Esfahani, E.A.; Bukuaghangin, O.; Banfield, S.; Vangölü, Y.; Yang, L.; Neville, A.; Hall, R.; Bryant, M. Surface Engineering of Wrought and Additive Layer Manufactured Ti-6Al-4V Alloy for Enhanced Load Bearing and Bio-Tribocorrosion Applications. Surf. Coat. Technol. 2022, 442, 128139. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, M.; Song, X.; Li, D. The Comparative Biological Properties of Mg+ or Ca+ Implanted Cu–TiN Nanocomposite Coatings on Titanium Alloys. Vacuum 2021, 194, 110618. [Google Scholar] [CrossRef]
- Kuang, X.; Li, L.; Wang, L.; Li, G.; Huang, K.; Xu, Y. The Effect of N+ Ion-Implantation on the Corrosion Resistance of HiPIMS-TiN Coatings Sealed by ALD-Layers. Surf. Coat. Technol. 2019, 374, 72–82. [Google Scholar] [CrossRef]
- Dong, M.; Zhu, Y.; Wang, C.; Shan, L.; Li, J. Structure and Tribocorrosion Properties of Duplex Treatment Coatings of TiSiCN/Nitride on Ti6Al4V Alloy. Ceram. Int. 2019, 45, 12461. [Google Scholar] [CrossRef]
- Zhao, C.; Gao, W.; Wang, J.; Ju, J.; Li, J. A Novel Biomedical TiN-Embedded TiO2 Nanotubes Composite Coating with Remarkable Mechanical Properties, Corrosion, Tribocorrosion Resistance, and Antibacterial Activity. Ceram. Int. 2023, 49, 15629–15640. [Google Scholar] [CrossRef]
- Cheraghali, B.; Ghasemi, H.M.; Abedini, M.; Yazdi, R. A Functionalized Duplex Coating on CP-Titanium for Biomedical Applications. Surf. Coat. Technol. 2020, 399, 126117. [Google Scholar] [CrossRef]
- Sypniewska, J.; Szkodo, M.; Majkowska-Marzec, B.; Mielewczyk-Gryń, A.D. Exploring the Composition and Corrosion Resistance in Hybrid-Modified Ti13Nb13Zr Alloy. MRS Commun. 2024, 14, 1439–1445. [Google Scholar] [CrossRef]
- Çaha, I.; Alves, A.C.; Chirico, C.; Pinto, A.M.; Tsipas, S.; Gordo, E.; Toptan, F. Improved Tribocorrosion Behavior on bio-Functionalized β-Type Titanium Alloy by the Pillar Effect Given by TiN Reinforcements. Surf. Coat. Technol. 2021, 415, 127122. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, Z.; He, X.; Wang, J.; Zheng, M.; Wang, R.; Wu, X. In-Situ Growth of PEO/UiO-66(Ce) Nanozyme Coatings on Titanium Alloy for Antibacterial Applications. Surf. Coat. Technol. 2025, 515, 132627. [Google Scholar] [CrossRef]
- Joo, S.Y.; Nisar, S.S.; Lee, J.K.; Choe, H.-C. Calcium Silicate Ceramic Coatings on the Plasma Electrolytic Oxidized Ti-6Al-4V Alloy Using Spin Coating Method. Surf. Coat. Technol. 2024, 479, 130575. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Sun, W.; Si, C.; Wang, J. Shot Peening Pre-Treatment for Nano-Nitriding Layer Formation to Enhance Wear and Corrosion Resistance in Medical Titanium Alloys. Mater. Lett. 2024, 368, 136669. [Google Scholar] [CrossRef]
- Ren, L.; Wang, T.; Chen, Z.; Li, Y.; Qian, L. Self-Lubricating PEO–PTFE Composite Coating on Titanium. Metals 2019, 9, 170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).