Preparation of Weather-Resistant Nano-Coating Materials and Its Application in the Protection of Ancient Building Paintings
Abstract
1. Introduction
2. Test Section
2.1. Test Materials
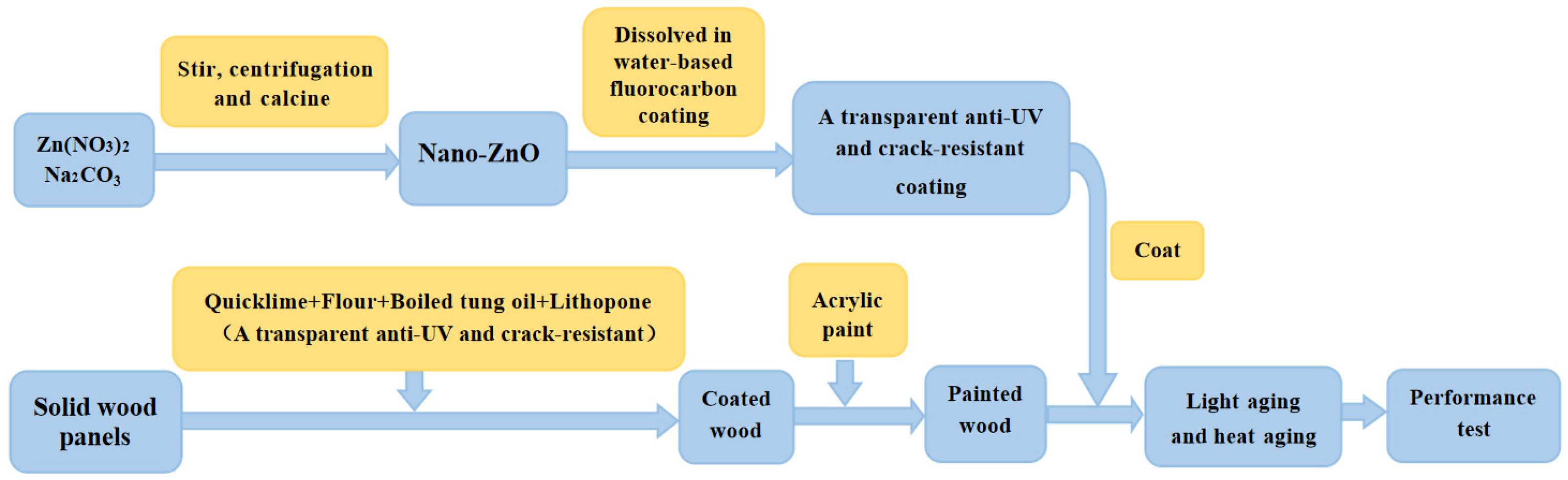
2.2. Preparation of Coating Materials
2.2.1. Preparation of Nano-Zinc Oxide
2.2.2. Preparation of Base Layer Material
2.2.3. Preparation of Transparent UV Resistant Crack-Resistant Coating Material
2.2.4. Plate Coating
- (1)
- Coat the glass slide
- (2)
- Coat the painted wooden board
2.3. Testing
3. Results and Discussion
3.1. Characterization of Homemade Nano-Zinc Oxide
3.1.1. Transmission Electron Microscopy Analysis
3.1.2. Ultraviolet-Visible Spectrophotometric Analysis
3.1.3. Anti-Settling Test
3.2. Performance Test of Transparent Uv Resistant Crack-Resistant Coating
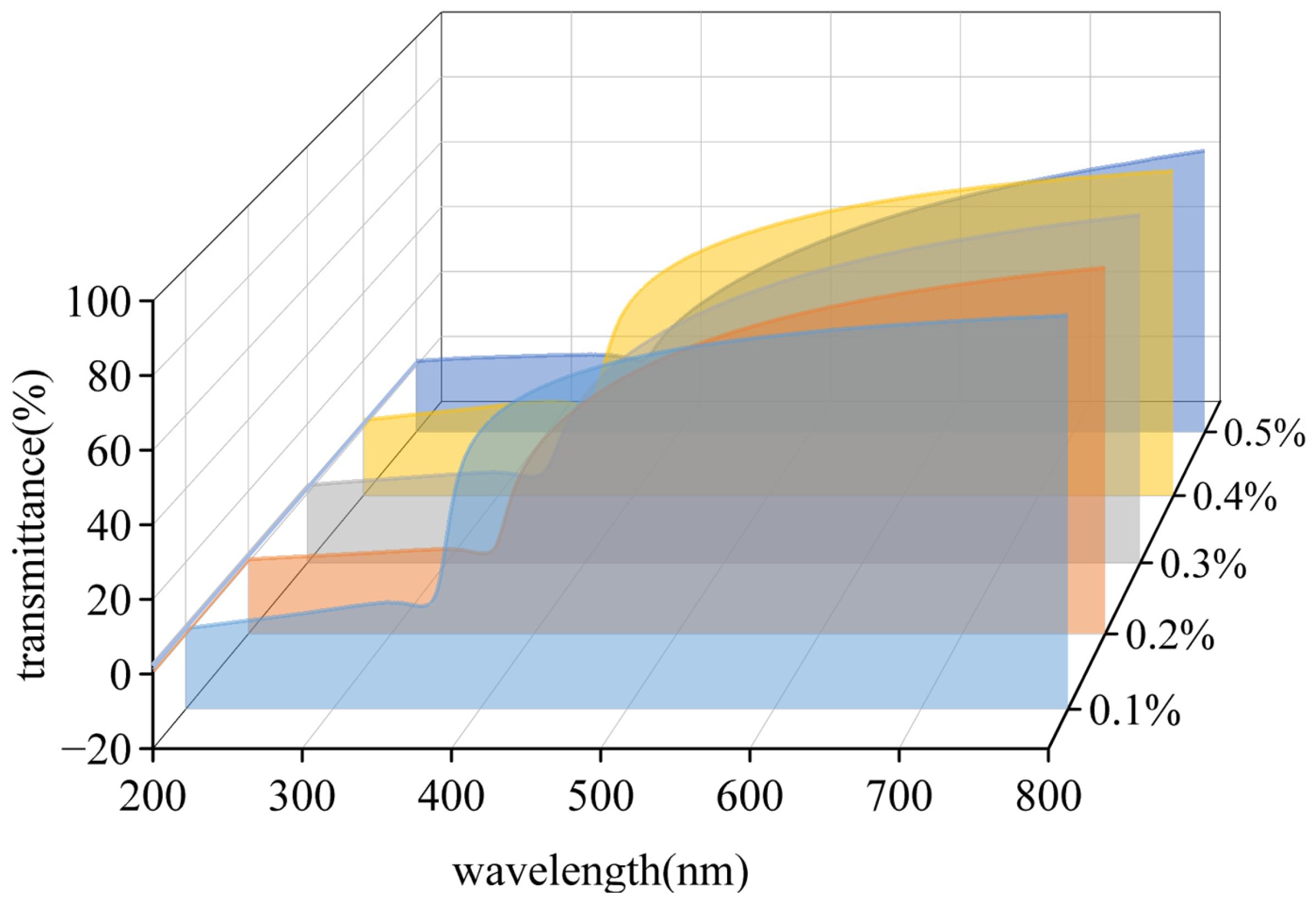
3.2.1. Transparency and Ultraviolet Absorption Capacity
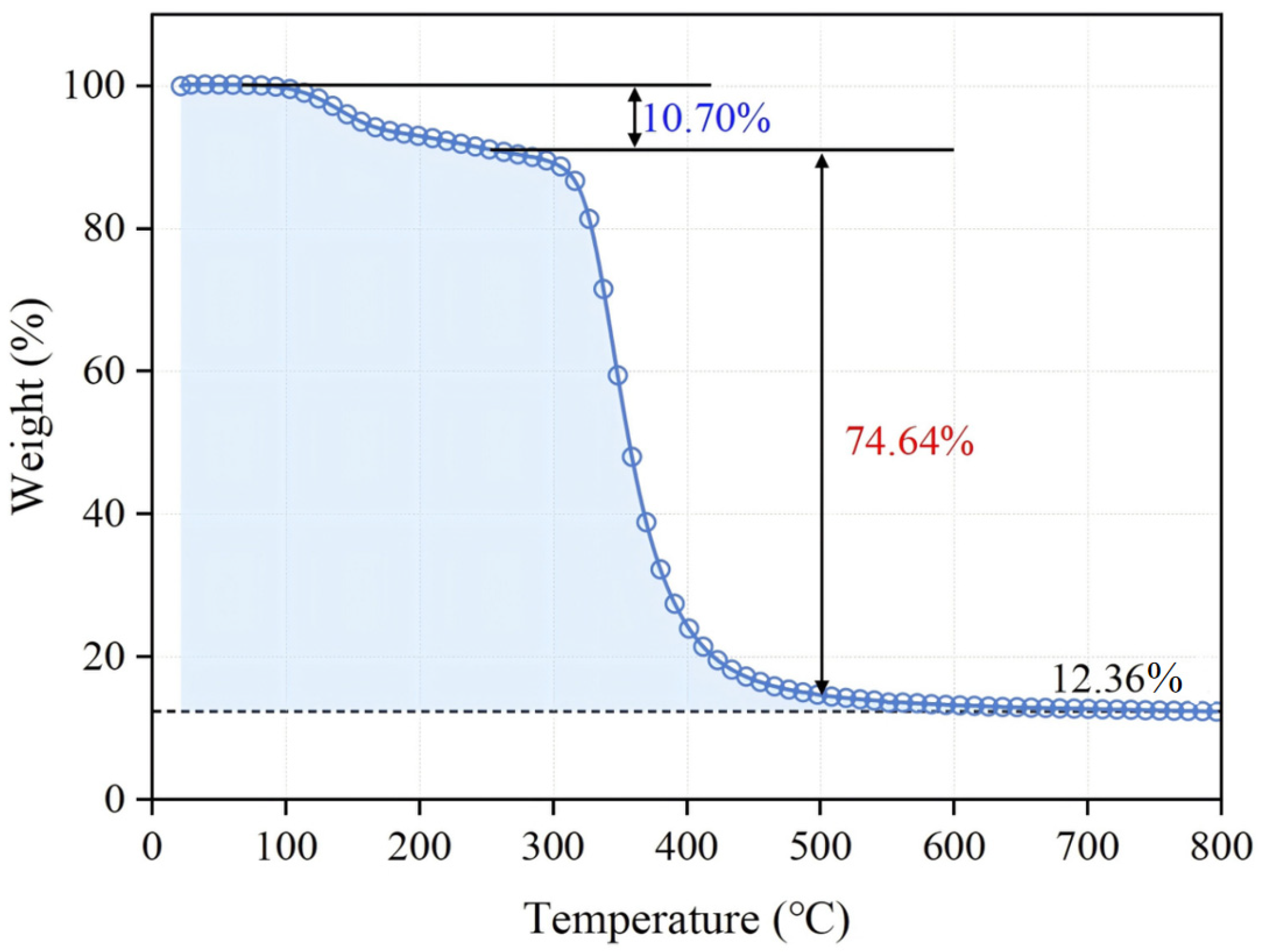
3.2.2. Thermal Performance Analysis of Coating Materials
- (1)
- Thermogravimetric (TG) analysis
- (2)
- Differential scanning calorimetry (DSC) analysis
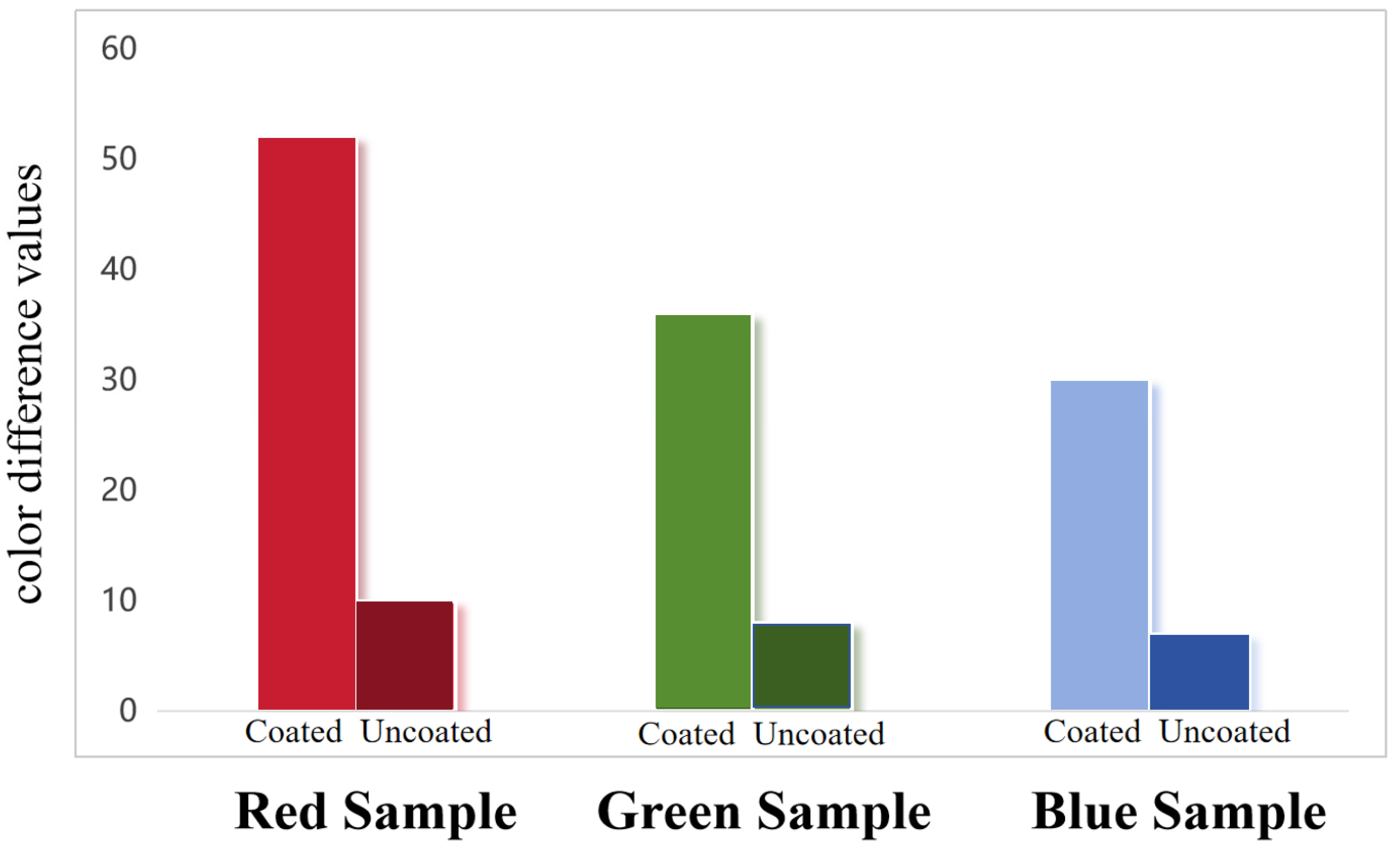
3.2.3. Ultraviolet Accelerated Aging Test of Painted Protective Coating
3.3. Conservation Experiment of Paintings in Changwu Zhaoren Temple and Xianyang Confucian Temple
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.; Cao, K.; Tang, Y.; Zhang, J.; Meng, X.; Ao, T.; Zhang, H. Study on Weathering Corrosion Characteristics of Red Sandstone of Ancient Buildings Under the Perspective of Non-Destructive Testing. J. Build. Eng. 2024, 85, 108520. [Google Scholar] [CrossRef]
- Yang, X.; Li, C.; Wang, L.; Yang, C.; Zhang, S.; Gao, J.; Qiu, J. The Characteristics of Ancient Residence Wood from the Qing Dynasty in Yunnan Province. Coatings 2024, 14, 200. [Google Scholar] [CrossRef]
- Qian, C.; Li, M.; Liao, H.; Zhang, C.; Li, H. Research on the Vibration Fatigue Characteristics of Ancient Building Wood Materials. Buildings 2024, 14, 2840. [Google Scholar] [CrossRef]
- Hang, T.T.X.; Dung, N.T.; Truc, T.A.; Duong, N.T.; Van Truoc, B.; Vu, P.G.; Hoang, T.; Thanh, D.T.M.; Olivier, M.-G. Effect of silane modified nano ZnO on UV degradation of polyurethane coatings. Prog. Org. Coat. 2015, 79, 68–74. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Tran, H.N.; Plamondon, C.O.; Tuduri, L.; Vo, D.-V.N.; Nanda, S.; Mishra, A.; Chao, H.-P.; Bajpai, A. Recent progress in the preparation, properties and applications of superhydrophobic nano-based coatings and surfaces: A review. Prog. Org. Coat. 2019, 132, 235–256. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation. Sci. Rep. 2023, 13, 9793. [Google Scholar] [CrossRef]
- Yang, H.; Han, K.; Fu, P.; Zhao, P.; Li, Y. Evaluation of the Reinforcement Performance of Protective Materials for Commonly Used Pigment Layers in Ancient Architectural Color Paintings. Sci. Conserv. Archaeol. 2024, 36, 94–106. [Google Scholar]
- Knežević, N.Ž.; Ilić, N.; Djokic, V.; Petrović, R.; Janaćković, D. Mesoporous Silica and Organosilica Nanomaterials as UV-Blocking Agents. ACS Appl. Mater. Interfaces 2018, 10, 20231–20236. [Google Scholar] [CrossRef]
- A Carboxylated Modified Nano-Titanium Dioxide UV Shielding Agent and Its Preparation Method. X Technology. 2021. Available online: https://www.xjishu.com/zhuanli/25/202110797499.html (accessed on 23 September 2025).
- Zhou, J.; Tan, Z.; Liu, Z.; Jing, M.; Liu, W.; Fu, W. Preparation of transparent fluorocarbon/TiO2-SiO2 composite coating with improved self-cleaning performance and anti-aging property. Appl. Surf. Sci. 2017, 396, 161–168. [Google Scholar] [CrossRef]
- Materials Characterization. Available online: https://www.sciencedirect.com/journal/materials-characterization (accessed on 23 September 2025).
- Liu, H. Research on the Functionalization of Nano-Zinc Oxide and the Preparation and Properties of Its UV—Shielding Composites; South China University of Technology: Guangzhou, China, 2022. [Google Scholar]
- Li, D.; Yang, C.; Li, P.; Yu, L.; Zhao, S.; Li, L.; Kang, H.; Yang, F.; Fang, Q. Synthesis and Properties of the Novel High—Performance Hydroxyl—Terminated Liquid Fluoroelastomer. Polymers 2023, 15, 2574. [Google Scholar] [CrossRef]
- Fan, T.; Feng, X.; Yang, J. Protection Practice and Reflection of Ancient Architectural Color Paintings in Jiangsu. Sci. Res. Chin. Cult. Relics 2022, 4, 29–40. [Google Scholar]
- Wadhwa, P.; Sharma, S.; Sahu, S.; Sharma, A.; Kumar, D. A review of nanoparticles characterization techniques. Curr. Nano-mater. 2022, 5, 7. [Google Scholar] [CrossRef]
- Doni, M.; Fierascu, I.; Fierascu, R.C. Recent Developments in Materials Science for the Conservation and Restoration of Historic Artifacts. Appl. Sci. 2024, 14, 11363. [Google Scholar] [CrossRef]
- Elkady, F.M.; Badr, B.M.; Saied, E.; Hashem, A.H.; Abdulrahman, M.S.; Alkherkhisy, M.M.; Selim, T.A.; Alshabrmi, F.M.; Alatawi, E.A.; Alkhayl, F.F.A.; et al. Mycosynthesis of zinc oxide nanoparticles using Mucor racemosus with their antimicrobial, antibiofilm, anticancer and antioxidant activities. Sci. Rep. 2025, 15, 1–22. [Google Scholar] [CrossRef]
- Guo, X.; Guo, R.; Fang, M.; Wang, N.; Liu, W.; Pei, H.; Liu, N.; Mo, Z. A novel composite protective coating with UV and corrosion resistance: Load floating and self-cleaning performance. Ceram. Int. 2022, 48, 17308–17318. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Docio, C.M.Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelanyet, S.Z. Chapter 15—Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; ScienceDirect. Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar]
- Fu, P.; Teri, G.-L.; Chao, X.-L.; Li, J.; Li, Y.-H.; Yang, H. Modified Graphene-FEVE Composite Coatings: Application in the Repair of Ancient Architectural Color Paintings. Coatings 2020, 10, 1162. [Google Scholar] [CrossRef]
- Al-Fadhily, Z.M.; Abdul-Hadi, M. A Novel Coating of Orthodontic Archwires with Chlorhexidine Hexametaphosphate Nanoparticles. Int. J. Biomater. 2023, 2023, 9981603. [Google Scholar] [CrossRef]
- Fistos, T.; Fierascu, I.; Doni, M.; Chican, I.E.; Fierascu, R.C. A Short Overview of Recent Developments in the Application of Polymeric Materials for the Conservation of Stone Cultural Heritage Elements. Materials 2022, 15, 6294. [Google Scholar] [CrossRef]
- Han, K.; Liu, J.; Hao, F.; Wang, J.; Yuan, J.; Pan, Z.; Pan, M. An adaptive waterborne fluorocarbon coatings with Anti-Flashing Rust, Antibiofouling, and Self-Repairing properties. Chem. Eng. J. 2024, 495, 153644. [Google Scholar] [CrossRef]
- Pino, F.; Fermo, P.; La Russa, M.; Ruffolo, S.; Comite, V.; Baghdachi, J.; Pecchioni, E.; Fratini, F.; Cappelletti, G. Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Sci. Pollut. Res. 2017, 24, 12608–12617. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.V.; Bodi, G.; Cernescu, A.; Spiridon, I.; Nicolescu, A.; Drobota, M.; Cotofana, C.; Simionescu, B.C.; Olaru, M. Protective coatings for ceramic artefacts exposed to UV ageing. NPJ Mater. Degrad. 2023, 7, 1–13. [Google Scholar] [CrossRef]
- Cinteză, L.O.; Tănase, M.A. Multifunctional ZnO Nanoparticle: Based Coatings for Cultural Heritage Preventive Conservation. In Thin Films; IntechOpen eBooks: London, UK, 2021. [Google Scholar]

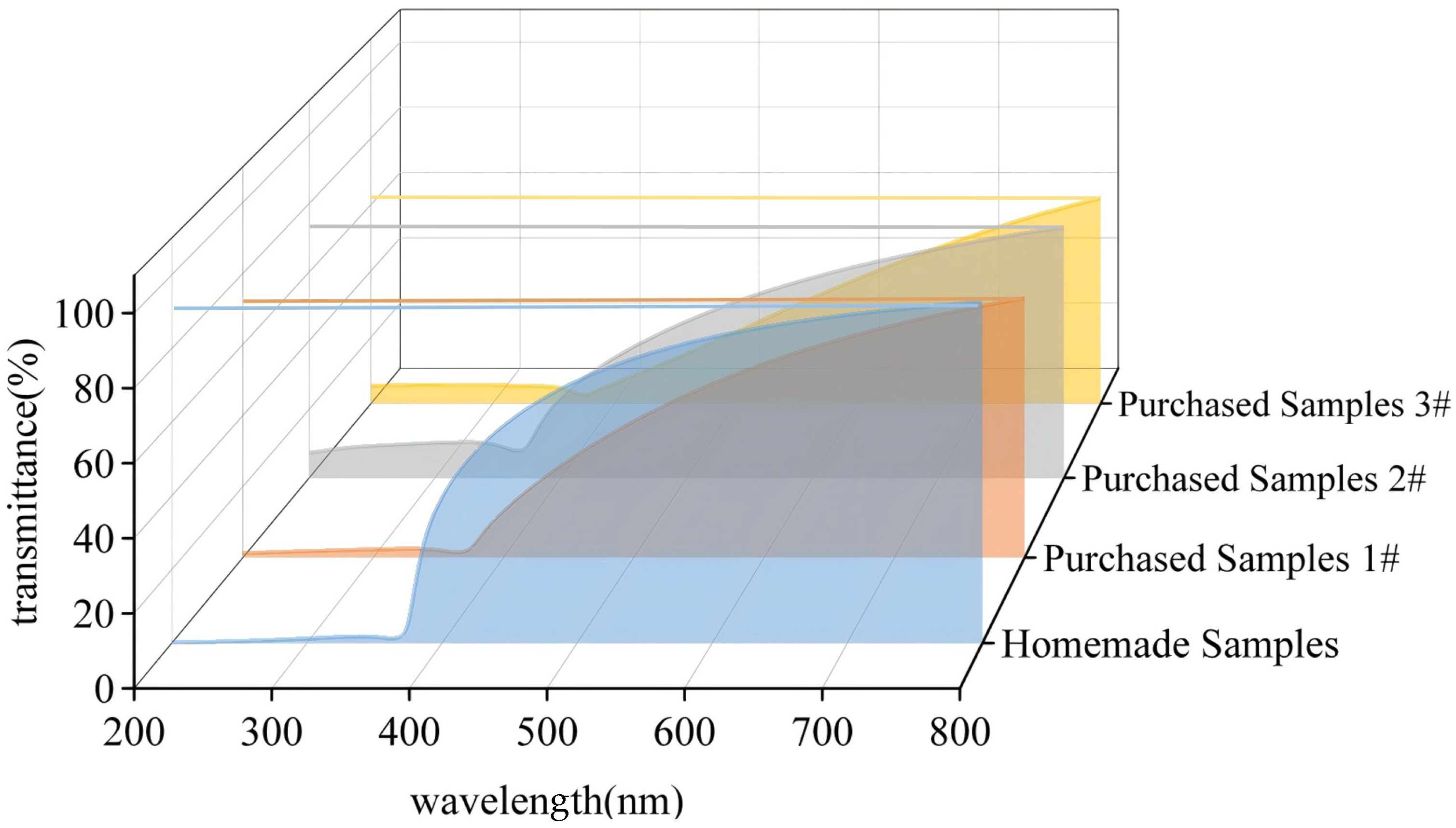



| Name of Sample | Grain Size Z-Mean | PDI | Settling Time/ Complete Settling Time (h) |
|---|---|---|---|
| Homemade Samples | 150 ± 20 | 0.18 | 120/576 |
| Purchased Samples 1# | 300 ± 50 | 0.28 | 3/24 |
| Purchased Samples 2# | 400 ± 50 | 0.36 | 1/11 |
| Purchased Samples 3# | 500 ± 20 | 0.45 | 0.5/6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zeng, J.; Zhang, W.; Huang, S.; Yang, H.; Wang, T.; Yu, X.; Cao, J. Preparation of Weather-Resistant Nano-Coating Materials and Its Application in the Protection of Ancient Building Paintings. Coatings 2025, 15, 1161. https://doi.org/10.3390/coatings15101161
Yang J, Zeng J, Zhang W, Huang S, Yang H, Wang T, Yu X, Cao J. Preparation of Weather-Resistant Nano-Coating Materials and Its Application in the Protection of Ancient Building Paintings. Coatings. 2025; 15(10):1161. https://doi.org/10.3390/coatings15101161
Chicago/Turabian StyleYang, Jinglong, Jiahe Zeng, Weihong Zhang, Siping Huang, Haoming Yang, Tao Wang, Xinjie Yu, and Jing Cao. 2025. "Preparation of Weather-Resistant Nano-Coating Materials and Its Application in the Protection of Ancient Building Paintings" Coatings 15, no. 10: 1161. https://doi.org/10.3390/coatings15101161
APA StyleYang, J., Zeng, J., Zhang, W., Huang, S., Yang, H., Wang, T., Yu, X., & Cao, J. (2025). Preparation of Weather-Resistant Nano-Coating Materials and Its Application in the Protection of Ancient Building Paintings. Coatings, 15(10), 1161. https://doi.org/10.3390/coatings15101161







