Investigation on the Interfacial Behavior of Thiols on Silver Surface by DFT Study and MD Simulation
Abstract
1. Introduction
2. Details of the Theoretical Calculation
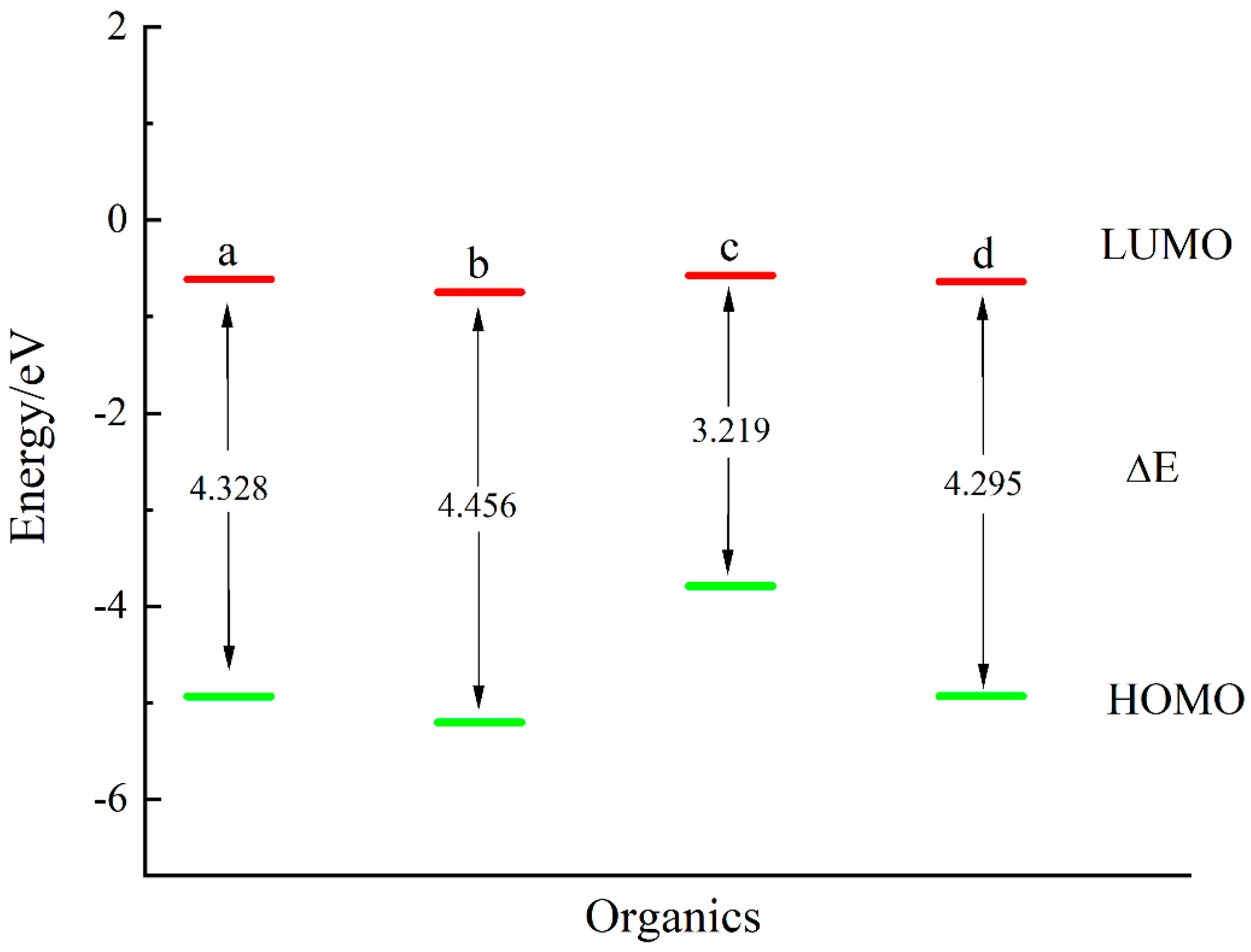
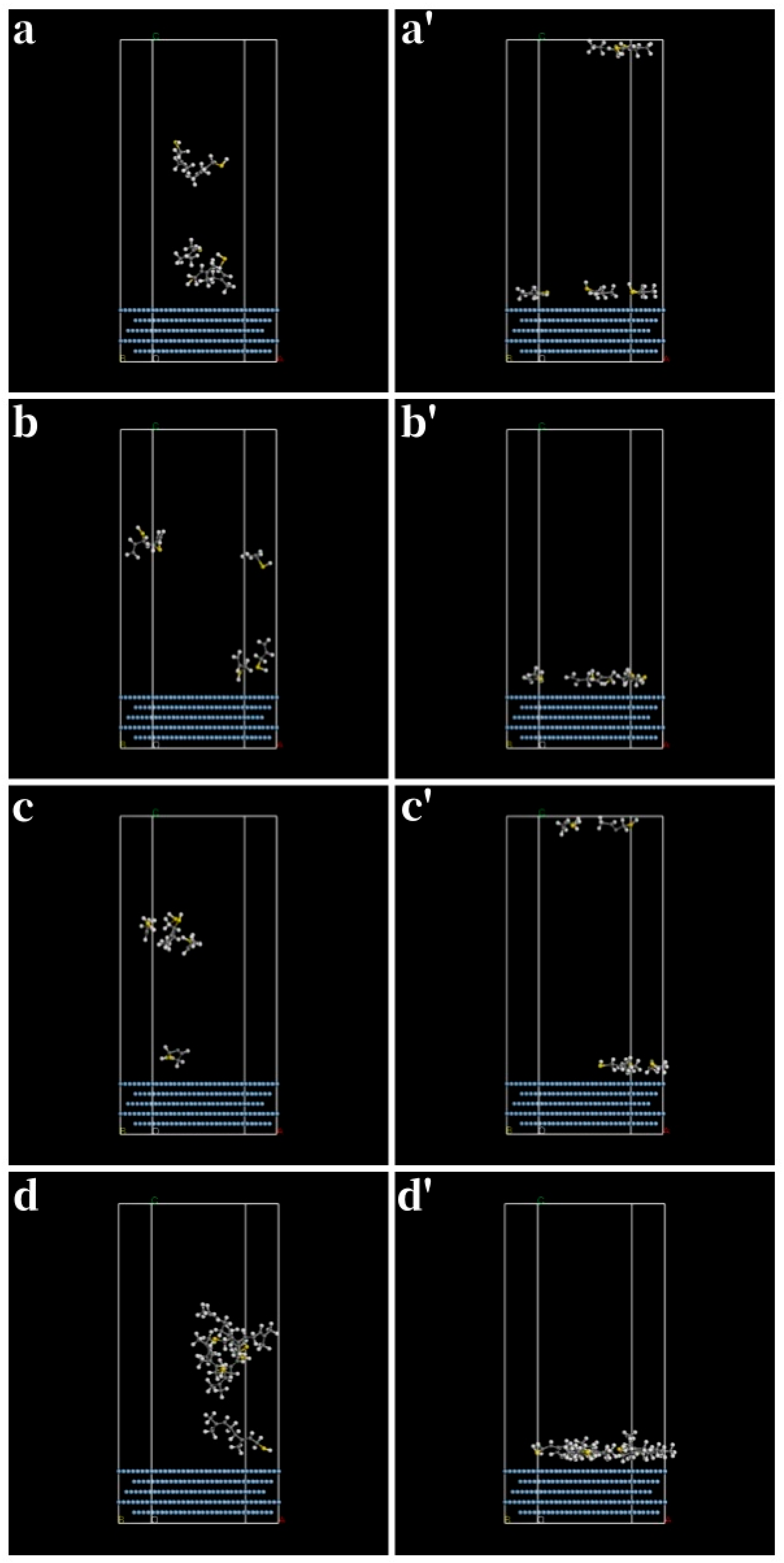
3. Results and Discussion
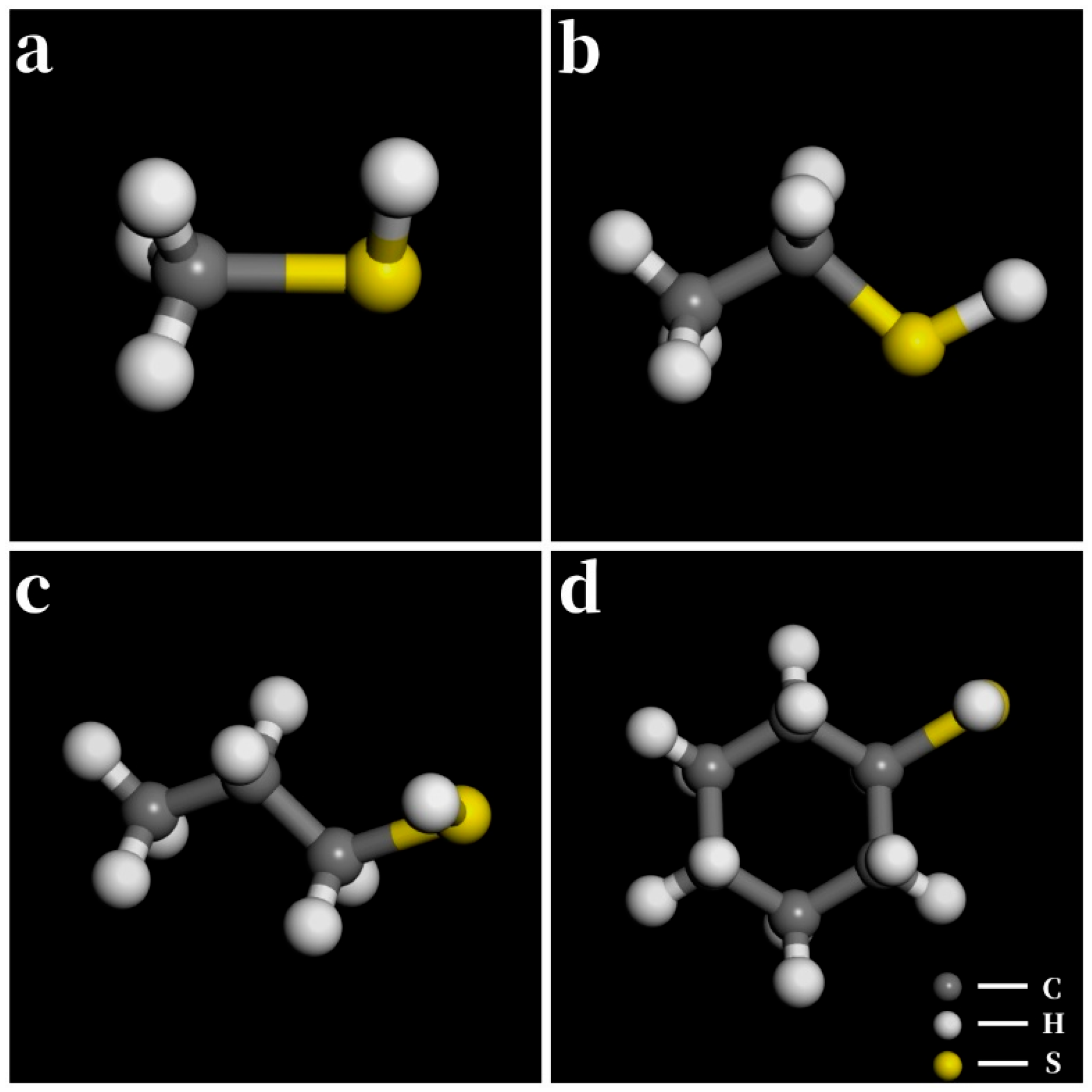
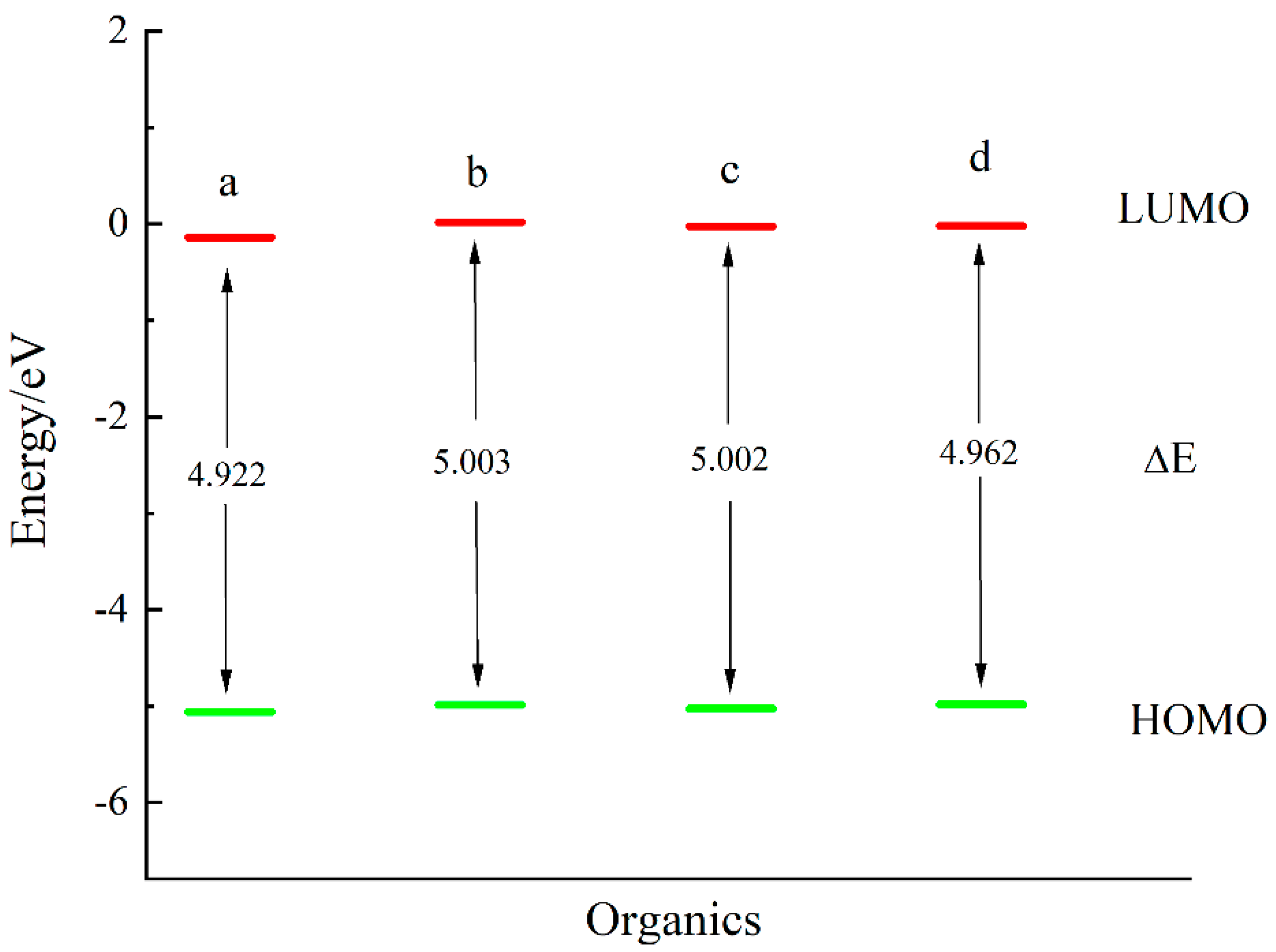
3.1. Short-Chain Saturated Aliphatic Thiols
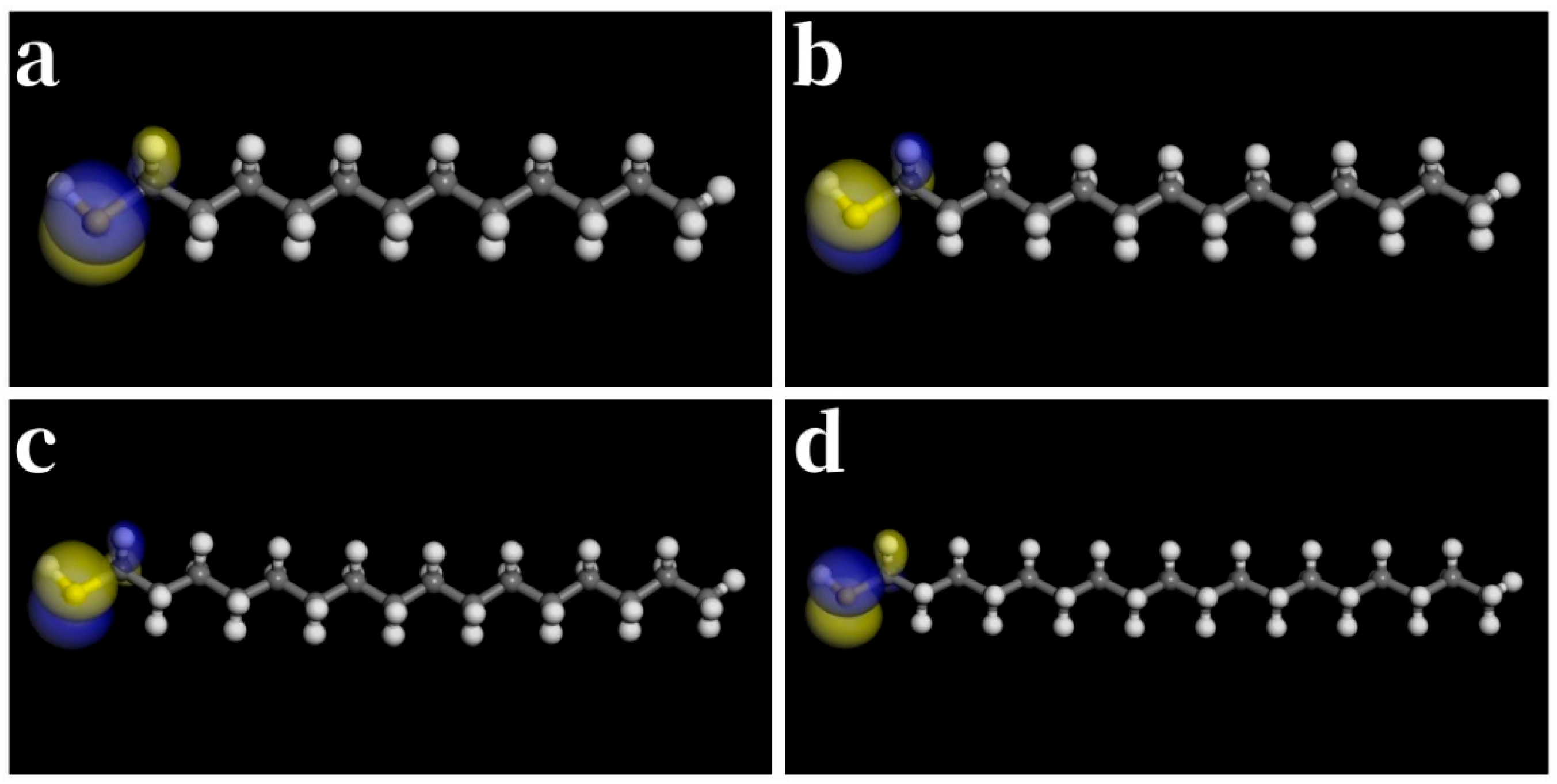
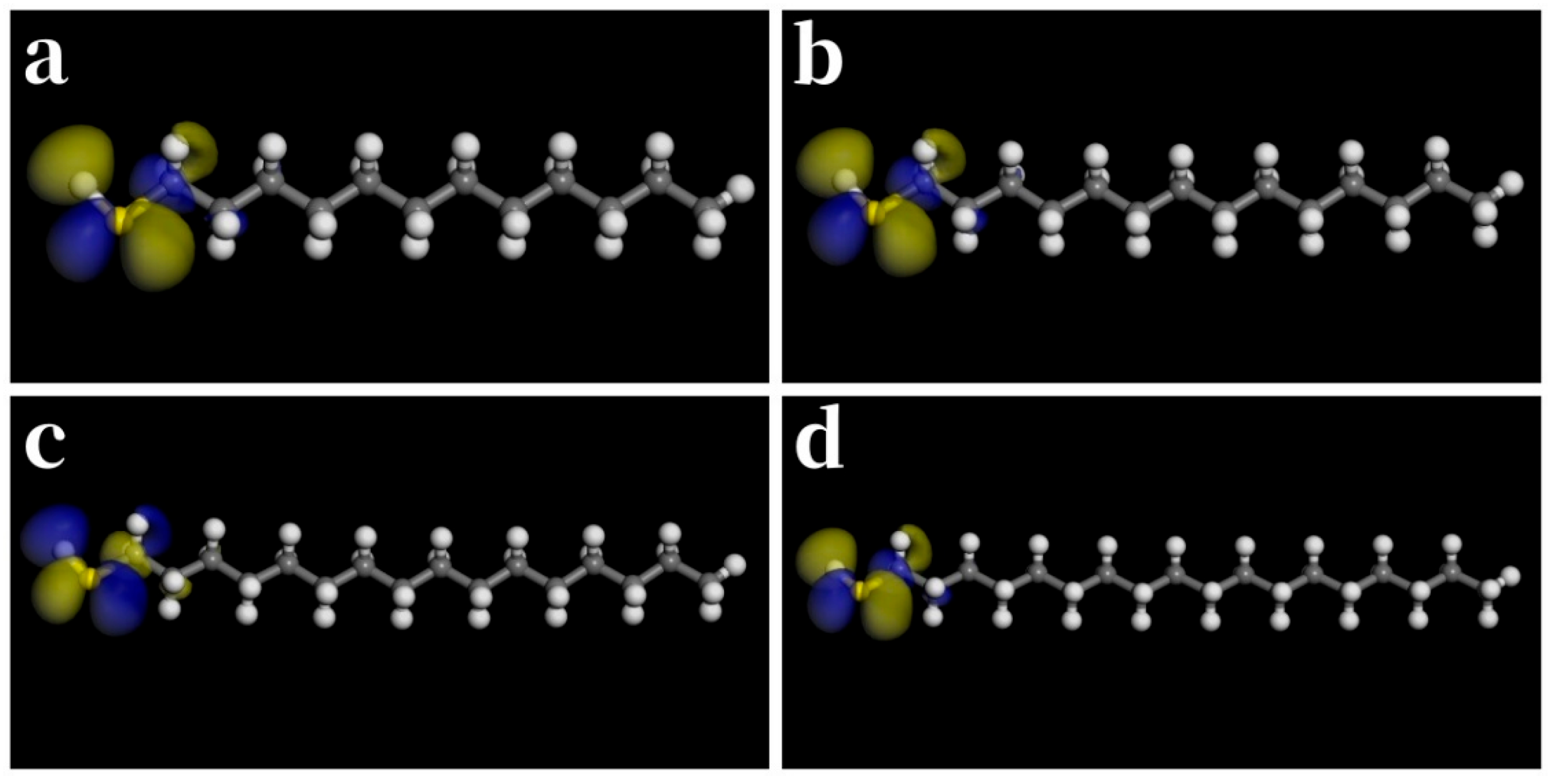
3.2. Long-Chain Saturated Aliphatic Thiols
3.3. Aromatic Thiols
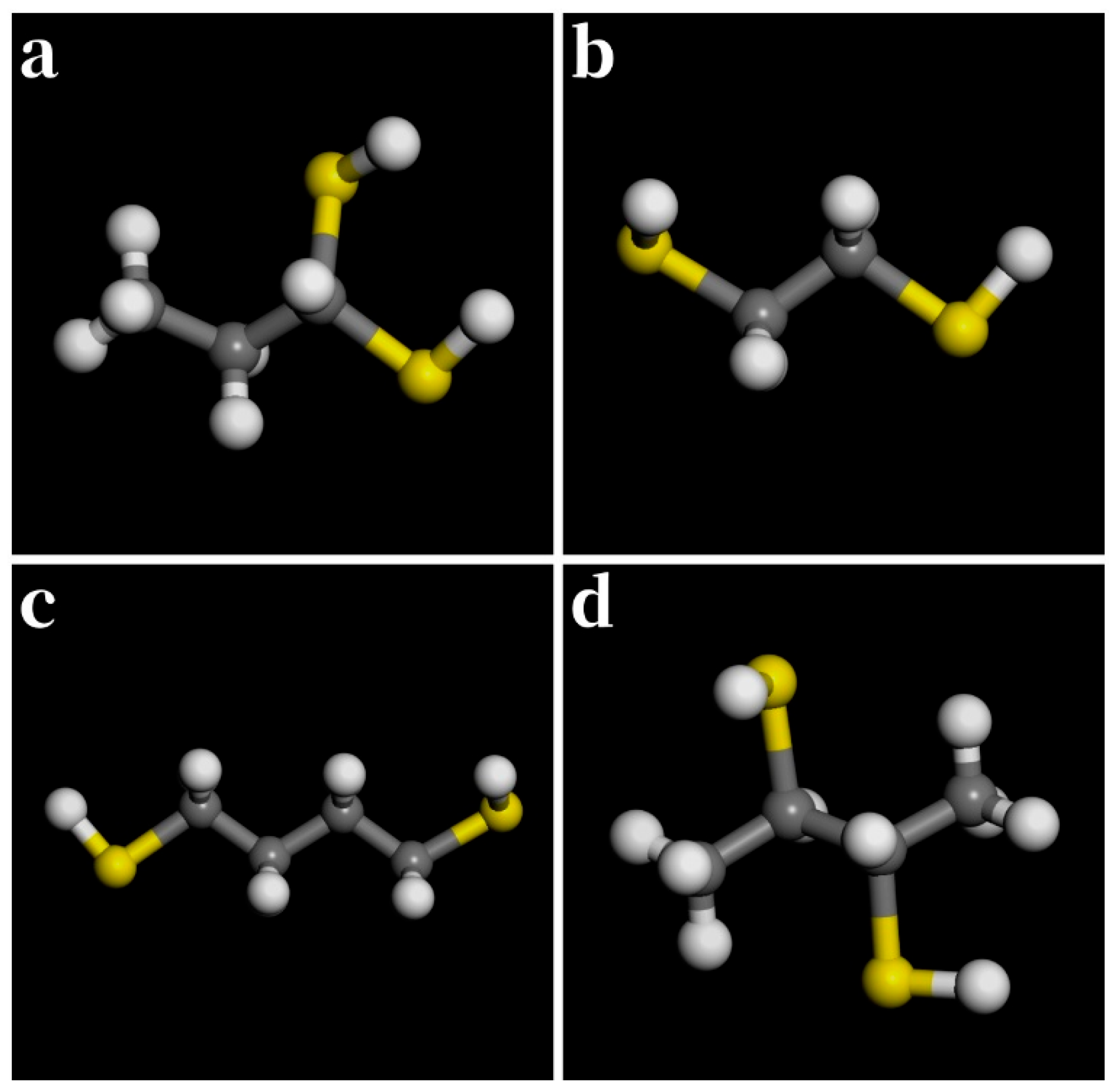
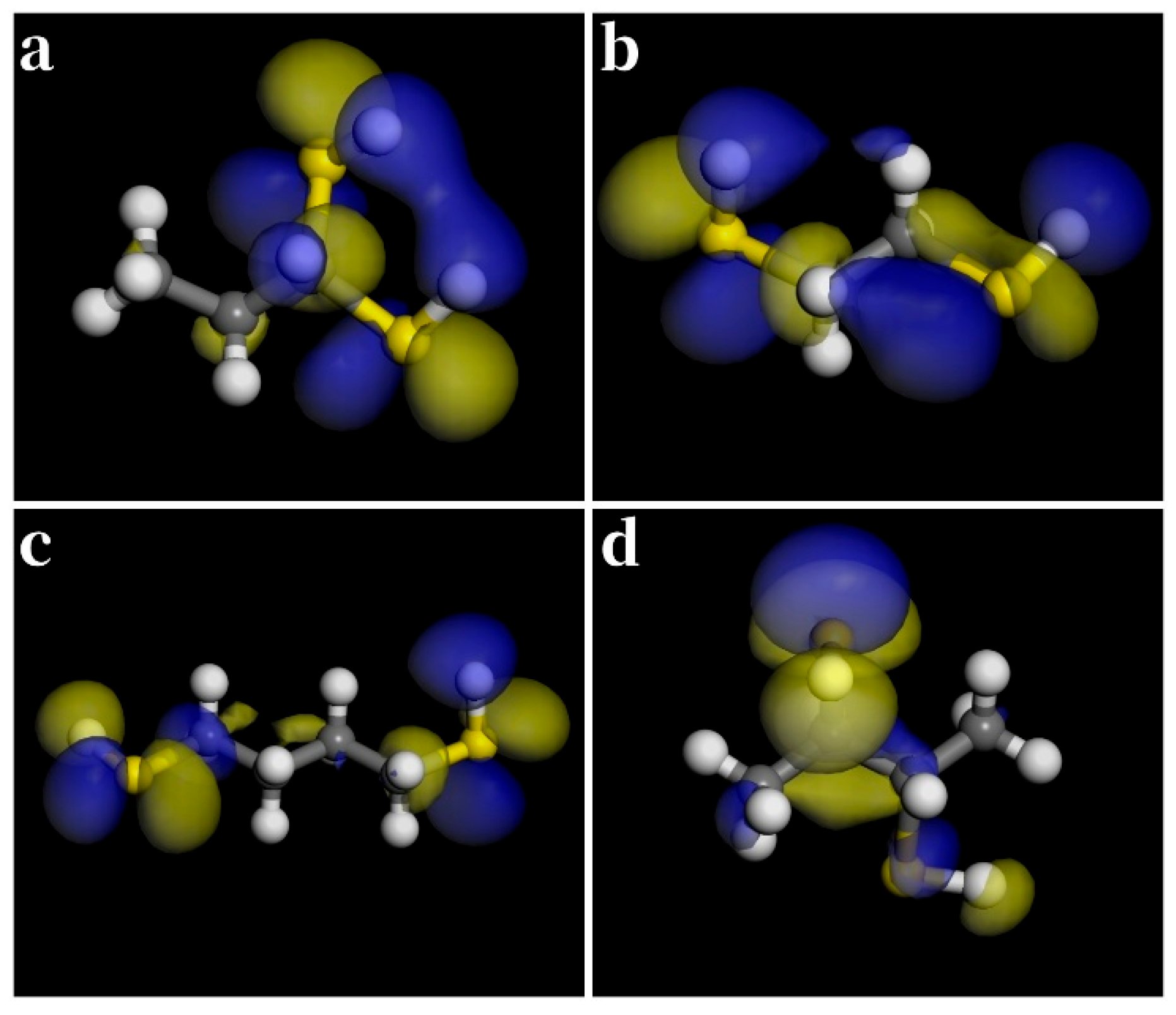
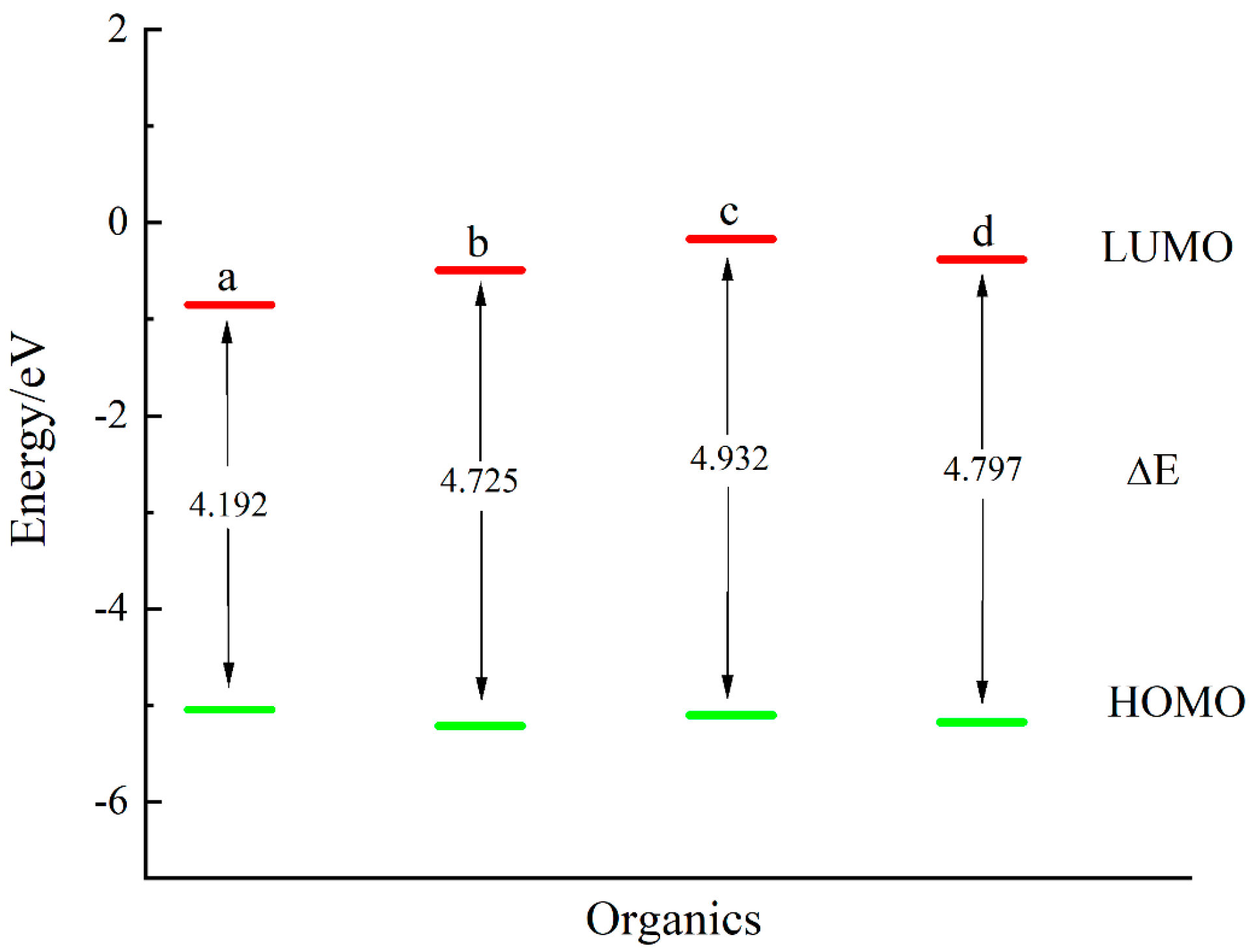
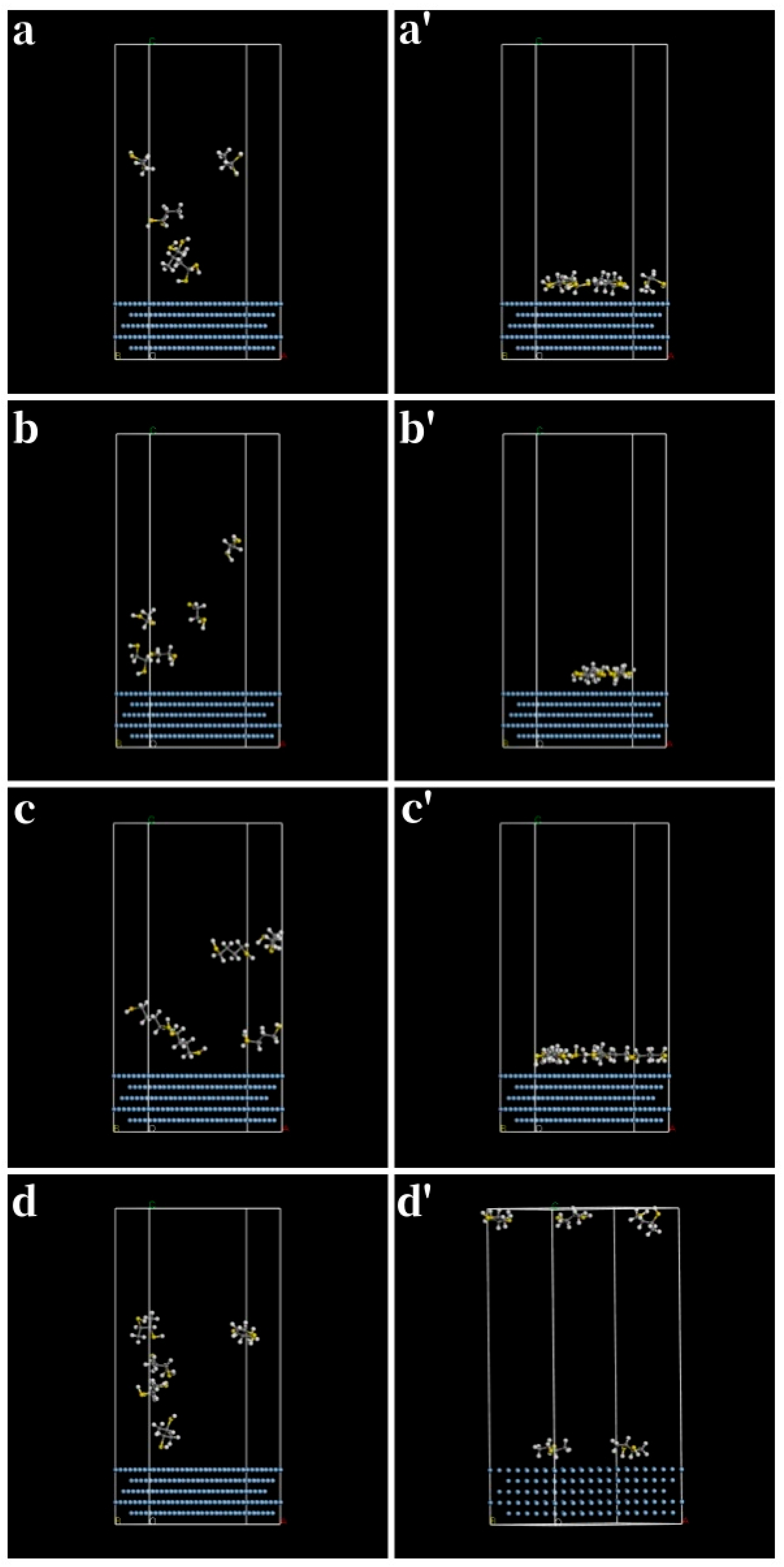
3.4. Binary Thiols
3.5. Unsaturated Aliphatic Thiols
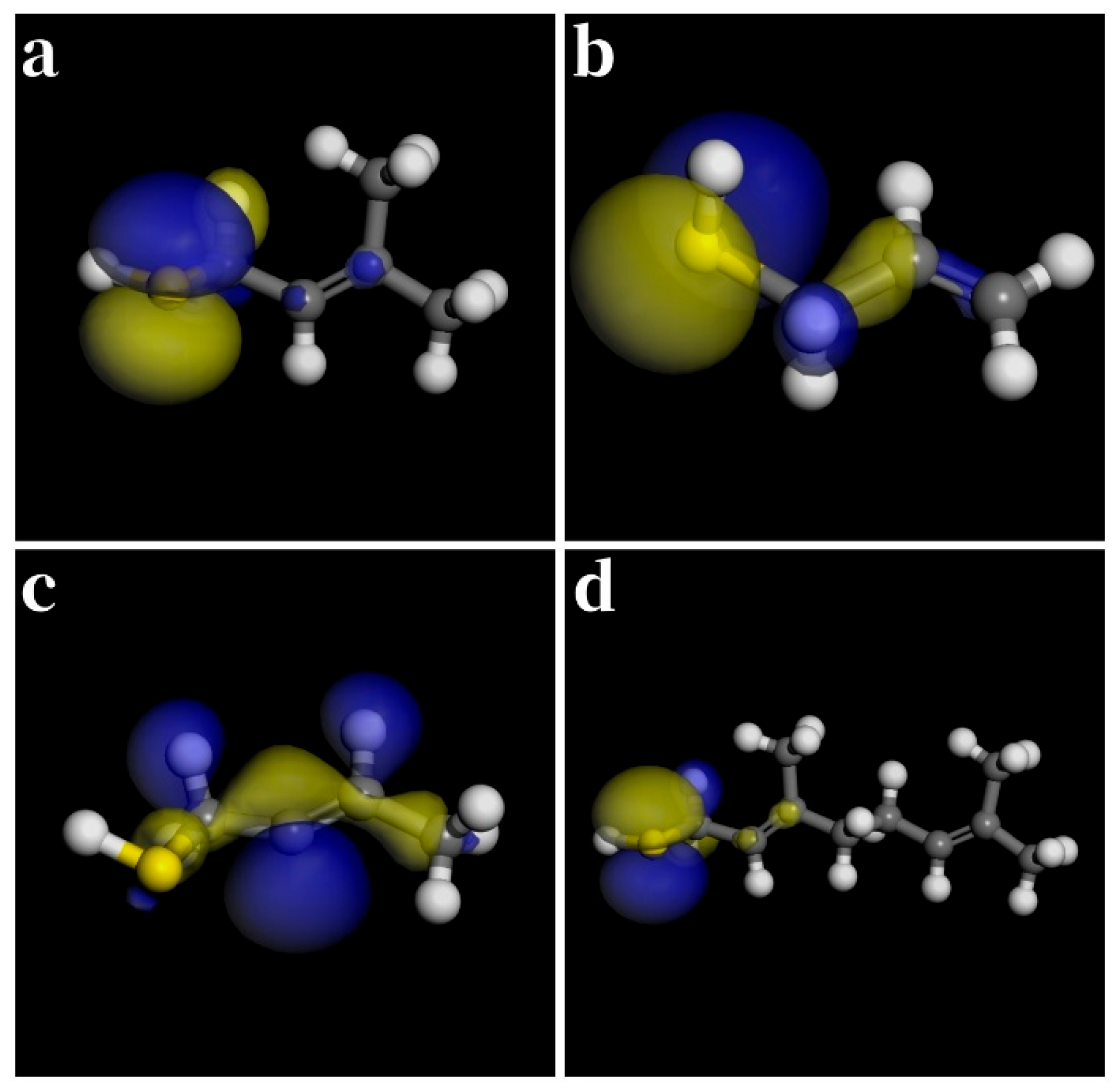
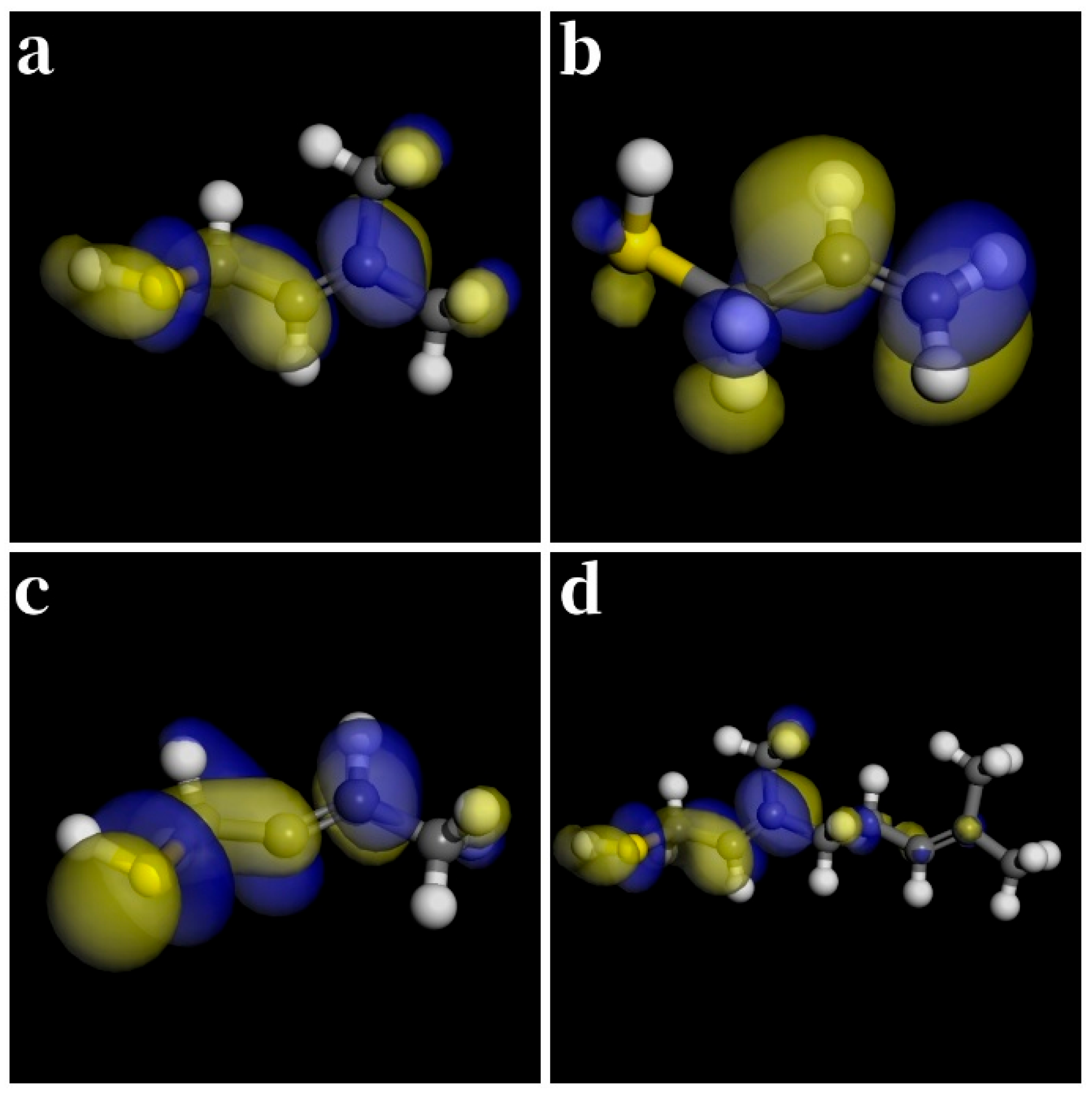
3.6. Charge Density Difference
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, N.V.; Tung, D.H.; Thao, T.T.; Hoa, V.X.; Thoan, N.H.; Tan, P.T.; Minh, P.N.; Fal, J.; Żyła, G.; Van Trinh, P.; et al. High thermal conductivity of green nanofluid containing Ag nanoparticles prepared by using solution plasma process with Paramignya trimera extract. J. Therm. Anal. Calorim. 2023, 148, 7579–7590. [Google Scholar] [CrossRef]
- Long, X.; Yu, D.; Han, J.; Huang, Z.; Xiao, J.; Feng, G.; Zhu, J.; Yang, K. High-performance Ag-TiO2 nanoparticle composite catalyst synthesized by pulsed laser ablation in liquid: Properties, mechanism and preparation studies. Opt. Express 2024, 32, 21304. [Google Scholar] [CrossRef]
- Wang, K.; Li, X.; Fan, H.; Xiong, S.; Liu, B.; Shuai, C. Preparation of Conductive Silver Paste with Mixed Nanostructured Silver Powders. J. Mater. Eng. Perform. 2025, 34, 9645–9653. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, M.; Lu, B.; Zhang, D.; He, H. Synthesis of Silver Nanoparticle/Multi-Walled Carbon Nanotube Composites and Their Application in Electronic Pastes. Nanomaterials 2025, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, M.E.; Kalita, G.; Sharma, S.; Tanemura, M. Chemical vapor deposition of graphene on silver foil as a tarnish-resistant coating. Phys. Status Solidi (RRL) Rapid Res. Lett. 2013, 7, 1076–1079. [Google Scholar] [CrossRef]
- Elechiguerra, J.L.; Larios-Lopez, L.; Liu, C.; Garcia-Gutierrez, D.; Camacho-Bragado, A.; Yacaman, M.J. Corrosion at the Nanoscale: The Case of Silver Nanowires and Nanoparticles. Chem. Mater. 2005, 17, 6042–6052. [Google Scholar] [CrossRef]
- Reed, J.C.; Zhu, H.; Zhu, A.Y.; Li, C.; Cubukcu, E. Graphene-Enabled Silver Nanoantenna Sensors. Nano Lett. 2012, 12, 4090–4094. [Google Scholar] [CrossRef]
- Bahmani, E.; Zakeri, A.; Sabour Rouh Aghdam, A. Protection of silver electrodeposit surfaces against accelerated and natural atmospheric corrosion by Ge incorporation in Ag structure; a microstructural investigation. Surf. Interfaces 2021, 26, 101288. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Tarnishing (Ag2S) Layer on Silver-Plated Electrical Contacts: Its Influence on Electrical Contact Resistance. IEEE Trans. Compon. Packag. Manuf. Technol. 2023, 13, 45–58. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, X. The research progress on corrosion and protection of silver layer. SN Appl. Sci. 2019, 1, 464. [Google Scholar] [CrossRef]
- Wu, M.; Yu, X.; Huang, J. Nano-TiO2 film enables silver artefacts to regenerate. Bull. Mater. Sci. 2022, 45, 115. [Google Scholar] [CrossRef]
- Fratoddi, I.; Marghella, G.; Venditti, I.; Ferro, D.; Russo, M.V. Organometallic Pt(II) containing polymer as silver protection against sulfide tarnishing. J. Appl. Polym. Sci. 2013, 128, 304–309. [Google Scholar] [CrossRef]
- Paussa, L.; Guzman, L.; Marin, E.; Isomaki, N.; Fedrizzi, L. Protection of silver surfaces against tarnishing by means of alumina/titania-nanolayers. Surf. Coat. Technol. 2011, 206, 976–980. [Google Scholar] [CrossRef]
- Tyurina, S.A.; Chavushyan, S.L.; Makarova, A.V.; Khvostov, R.E.; Yudin, G.A. Research and Analysis of Methods for Preventing Silver Alloys from Tarnishing. Inorg. Mater. Appl. Res. 2021, 12, 1615–1622. [Google Scholar] [CrossRef]
- Sasaki, T.; Kawamura, M.; Abe, Y.; Kim, K.H. Suppression of property changes in Ag thin films by introducing organic monolayers. Vacuum 2015, 121, 317–319. [Google Scholar] [CrossRef]
- Wei, H.; Huang, Z.; Qin, Z. Anti-corrosion effect of thiol self-assembled monolayers on Ag plate. In Proceedings of the 2013 IEEE 15th Electronics Packaging Technology Conference (EPTC 2013), Singapore, 11–13 December 2013; pp. 555–559. [Google Scholar] [CrossRef]
- Burleigh, T.D.; Shi, C.; Kilic, S.; Kovacik, S.; Thompson, T.; Enick, R.M. Self-Assembled Monolayers of Perfluoroalkyl Amideethanethiols, Fluoroalkylthiols, and Alkylthiols for the Prevention of Silver Tarnish. Corrosion 2002, 58, 49–56. [Google Scholar] [CrossRef]
- Liang, C.; Yang, C.; Huang, N. Tarnish protection of silver by octadecanethiol self-assembled monolayers prepared in aqueous micellar solution. Surf. Coat. Technol. 2009, 203, 1034–1044. [Google Scholar] [CrossRef]
- Evesque, M.; Keddam, M.; Takenouti, H. The formation of self-assembling membrane of hexadecane-thiol on silver to prevent the tarnishing. Electrochim. Acta 2004, 49, 2937–2943. [Google Scholar] [CrossRef]
- Wang, N.-N.; Li, Q.-Z.; Yu, Z.-W. Hydrogen Bonding Interactions in Three 2-Mercaptoethanol Systems: An Excess Infrared Spectroscopic Study. Appl. Spectrosc. 2009, 63, 1356–1362. [Google Scholar] [CrossRef]
- Shwetha, K.M.; Praveen, B.M.; Devendra, B.K.; Saleh, D.I.; Rathod, M.R. Investigations of a new derivative as an effective mild steel corrosion inhibitor in acidic medium using experimental, electrochemical and DFT simulation methods. Inorg. Chem. Commun. 2025, 177, 114289. [Google Scholar] [CrossRef]
- Fadhil, A.A.; Khadom, A.A.; Liu, H.; Fu, C.; Wang, J.; Fadhil, N.A.; Mahood, H.B. (S)-6-Phenyl-2,3,5,6-tetrahydroimidazo[2,1-b] thiazole hydrochloride as corrosion inhibitor of steel in acidic solution: Gravimetrical, electrochemical, surface morphology and theoretical simulation. J. Mol. Liq. 2019, 276, 503–518. [Google Scholar] [CrossRef]
- Li, X.; Wu, D.; Luo, Q.; Yin, R.; An, J.; Liu, S.; Wang, D. Fabrication of CPAN/Ag/AgCl composites and their efficient visible-light photocatalytic activity. J. Alloys Compd. 2017, 702, 585–593. [Google Scholar] [CrossRef]
- Liu, R.; Li, S.; Yu, X.; Zhang, G.; Ma, Y.; Yao, J. Facile synthesis of a Ag nanoparticle/polyoxometalate/carbon nanotube tri-component hybrid and its activity in the electrocatalysis of oxygen reduction. J. Mater. Chem. 2011, 21, 14917–14924. [Google Scholar] [CrossRef]
- Choudhury, A. Polyaniline/silver nanocomposites: Dielectric properties and ethanol vapour sensitivity. Sens. Actuators B Chem. 2009, 138, 318–325. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Chen, L.; Huang, X. Ag-enhanced SEI formation on Si particles for lithium batteries. Electrochem. Commun. 2003, 5, 935–939. [Google Scholar] [CrossRef]
- Zhou, N.; Yang, T.; Jiao, K.; Song, C.-X. Electrochemical Deoxyribonucleic Acid Biosensor Based on Multiwalled Carbon Nanotubes/Ag-TiO2 Composite Film for Label-Free Phosphinothricin Acetyltransferase Gene Detection by Electrochemical Impedance Spectroscopy. Chin. J. Anal. Chem. 2010, 38, 301–306. [Google Scholar] [CrossRef]
- Chung, D.; Khosla, A.; Gray, B.L.; Parameswaran, A.M.; Ramaseshan, R.; Kohli, K. Investigations of Flexible Ag/AgCl Nanocomposite Polymer Electrodes for Suitability in Tissue Electrical Impedance Scanning (EIS). J. Electrochem. Soc. 2014, 161, B3071–B3076. [Google Scholar] [CrossRef]
- Yang, H.; Liu, H.R.; Guo, Z.C.; Chen, B.M.; Zhang, Y.C.; Xu, R.D. Electrochemical behaviors of Pb-0.3%Ag-0.06%Ca rolled alloy anode during and after zinc electrowinning—Tafel investigations. In Frontiers of Manufacturing Science and Measuring Technology Iii, Pts 1–3; Sung, W.P., Kao, J.C.M., Chen, R., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2013; Volume 401, p. 779. [Google Scholar]
- Kaya, Ş.; Çağlar, A.; Akdemir, M.; Kivrak, H.D.; Horoz, S.; Kaya, M. Exploring the Triple Applications of Ag/PMAc-g-CNT Nanocomposites in Enhancing HER, OER and Supercapacitor Performance. Waste Biomass Valorization 2024, 15, 2781–2792. [Google Scholar] [CrossRef]
- Eberhardt, D.; Santos, E.; Schmickler, W. Hydrogen evolution on silver single crystal electrodes—First results. J. Electroanal. Chem. 1999, 461, 76–79. [Google Scholar] [CrossRef]
- Neumaier, M.; Weigend, F.; Hampe, O.; Kappes, M.M. Binding energy and preferred adsorption sites of CO on gold and silver–gold cluster cations: Adsorption kinetics and quantum chemical calculations. Faraday Discuss. 2008, 138, 393–406. [Google Scholar] [CrossRef]
- Al-Saadi, A.A. Computational study of SERS effects in some aliphatic and cyclic carboxylic acids with silver nanomaterials. J. Phys. Conf. Ser. 2020, 1564, 012008. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Sun, D.; Ye, X.; Yi, X.; Fang, W.; Luo, Z. Effect of pulse electroplating parameters on the morphology and corrosion resistance of Ag plating coating. Surf. Topogr. Metrol. Prop. 2024, 12, 025030. [Google Scholar] [CrossRef]
- Morovati, H.; Noorbala, M.R.; Namazian, M.; Zare, H.R.; Dehghani-Firouzabadi, A.A. Experimental and theoretical investigation of two thioether-based Schiff bases as anti-corrosion agents for carbon steel in HCl electrolyte. Anti-Corros. Methods Mater. 2024, 71, 81–91. [Google Scholar] [CrossRef]
- Ajagekar, A.; You, F. New frontiers of quantum computing in chemical engineering. Korean J. Chem. Eng. 2022, 39, 811–820. [Google Scholar] [CrossRef]
- Bernal, D.E.; Ajagekar, A.; Harwood, S.M.; Stober, S.T.; Trenev, D.; You, F. Perspectives of quantum computing for chemical engineering. AIChE J. 2022, 68, e17651. [Google Scholar] [CrossRef]
- Korniy, S.A. Quantum-Chemical Calculations of the Stability of Zeolite–Phosphate Complexes as Pigments of Paint Coatings. Mater. Sci. 2022, 58, 12–19. [Google Scholar] [CrossRef]
- Garrido, C.; Diaz-Fleming, G.; Campos-Vallette, M.M. SERS spectrum of gallic acid obtained from a modified silver colloid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 163, 68–72. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Chaudhuri, R.K.; Chattopadhyay, S. Theoretical investigations of electronic spectra of silver atom using all-electron scalar relativistic basis. AIP Adv. 2022, 12, 125019. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Sundaram, M. A study on the gas film formation in electrochemical discharging processes by molecular dynamics simulation. Manuf. Lett. 2024, 41, 351–356. [Google Scholar] [CrossRef]
- Taghavi Kalajahi, S.; Mofradnia, S.R.; Yazdian, F.; Rasekh, B.; Neshati, J.; Taghavi, L. Computational evaluation of multifunctional nanostructured inhibitors to control microbiologically influenced corrosion: DFT calculations and MD simulations. J. Appl. Electrochem. 2023, 53, 2359–2373. [Google Scholar] [CrossRef]
- Karaseov, P.A.; Karabeshkin, K.; Mongo, E.; Titov, A.; Ullah, M.; Kuronen, A.; Djurabekova, F.; Nordlund, K. Experimental study and MD simulation of damage formation in GaN under atomic and molecular ion irradiation. Vacuum 2016, 129, 166–169. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Zou, Q.; Li, Y.; Lei, M.; Wang, W.; Li, Y. Experimental study and molecular dynamics simulation of Ag/Ti diffusion bonding. Surf. Interfaces 2024, 55, 105296. [Google Scholar] [CrossRef]
- Zhao, Y.; Shibahara, M.; Fan, X.; Liu, C.; Li, J. Molecular dynamics study of high temperature Ag-Cu-Sn liquid metal infiltration between Ag-Cu alloys: Influences of adsorption and dissolution. Mater. Today Commun. 2024, 40, 110167. [Google Scholar] [CrossRef]
- Anjum, Q.; Nasir, N.; Cheema, S.A.; Imran, M.; Rahman, A.R.; Tanveer, Z.; Amin, N.; Anjam, Y.N. Multiscale modeling investigation into the thermal conductivity dynamics of graphene-silver nano-composites: A molecular dynamic study. Dig. J. Nanomater. Biostruct. 2022, 17, 557–568. [Google Scholar] [CrossRef]
- Shi, D.W.; He, L.M.; Kong, L.G.; Lin, H.; Hong, L. Superheating of Ag nanowires studied by molecular dynamics simulations. Model. Simul. Mater. Sci. Eng. 2008, 16, 025009. [Google Scholar] [CrossRef]
- Nourmohamadi, H.; Esrafili, M.D.; Aghazadeh, V.; Rezai, B. The influence of Ag+ cation on elemental sulfur passive layer and adsorption behavior of chalcopyrite toward Fe3+ and Fe2+ ions: Insights from DFT calculations and molecular dynamics simulations. Phys. B Condens. Matter 2022, 627, 413611. [Google Scholar] [CrossRef]
- Huang, H.; Bu, F. Correlations between the inhibition performances and the inhibitor structures of some azoles on the galvanic corrosion of copper coupled with silver in artificial seawater. Corros. Sci. 2020, 165, 108413. [Google Scholar] [CrossRef]
- Pergolese, B.; Muniz-Miranda, M.; Bigotto, A. Surface enhanced Raman spectroscopic studies on 1H-1,2,4-triazole adsorbed on silver colloidal nanoparticles. Vib. Spectrosc. 2008, 48, 202–205. [Google Scholar] [CrossRef]
- Liu, A.; Ren, X.; Yang, Q.; Sokolowski, J.; Guo, J.; Li, Y.; Gao, L.; An, M.; Wu, G. Theoretical and Experimental Studies of the Prevention Mechanism of Organic Inhibitors on Silver Anti-Tarnish. J. Electrochem. Soc. 2018, 165, H725. [Google Scholar] [CrossRef]
- Aeberhard, P.C.; Arey, J.S.; Lin, I.-C.; Rothlisberger, U. Accurate DFT Descriptions for Weak Interactions of Molecules Containing Sulfur. J. Chem. Theory Comput. 2009, 5, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Li, X.; Blowers, P. Adsorption Energies of Mercury-Containing Species on CaO and Temperature Effects on Equilibrium Constants Predicted by Density Functional Theory Calculations. Langmuir 2009, 25, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Dong, H.; Li, Y.; Si, Z.; Yan, F. Highly Stable N3-Substituted Imidazolium-Based Alkaline Anion Exchange Membranes: Experimental Studies and Theoretical Calculations. Macromolecules 2014, 47, 208–216. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Král, P. Metallopyrrole-Capped Carbon Nanocones. J. Phys. Chem. B 2006, 110, 21480–21486. [Google Scholar] [CrossRef]
- Mahammedi, N.A.; Ferhat, M.; Belkada, R. Prediction of indirect to direct band gap transition under tensile biaxial strain in type-I guest-free silicon clathrate Si46: A first-principles approach. Superlattices Microstruct. 2016, 100, 296–305. [Google Scholar] [CrossRef]
- Jabłońska, A.; Ponikiewski, Ł.; Ejsmont, K.; Herman, A.; Dołęga, A. Syntheses, spectroscopic and structural properties of phenoxysilyl compounds: X-ray structures, FT-IR and DFT calculations. J. Mol. Struct. 2013, 1054–1055, 359–366. [Google Scholar] [CrossRef]
- Pan, J.; Sun, Z.; Zhu, H.; Cao, H.; Wang, B.; Zhao, J.; Yan, F. Synthesis and characterization of main-chain type polyimidazolium-based alkaline anion exchange membranes. J. Membr. Sci. 2020, 610, 118283. [Google Scholar]
- Zhang, Z.; Deng, L.; Zhou, X. Adsorption behavior of fluorides on Ag(111) and its doped surfaces: A first-principles calculation. Chem. Eng. Sci. 2023, 280, 119071. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L. Implications for electronic structures of S-doped graphene nanoribbons from a DFTB algorithm at atomic scale. Mater. Today Commun. 2024, 41, 110382. [Google Scholar] [CrossRef]




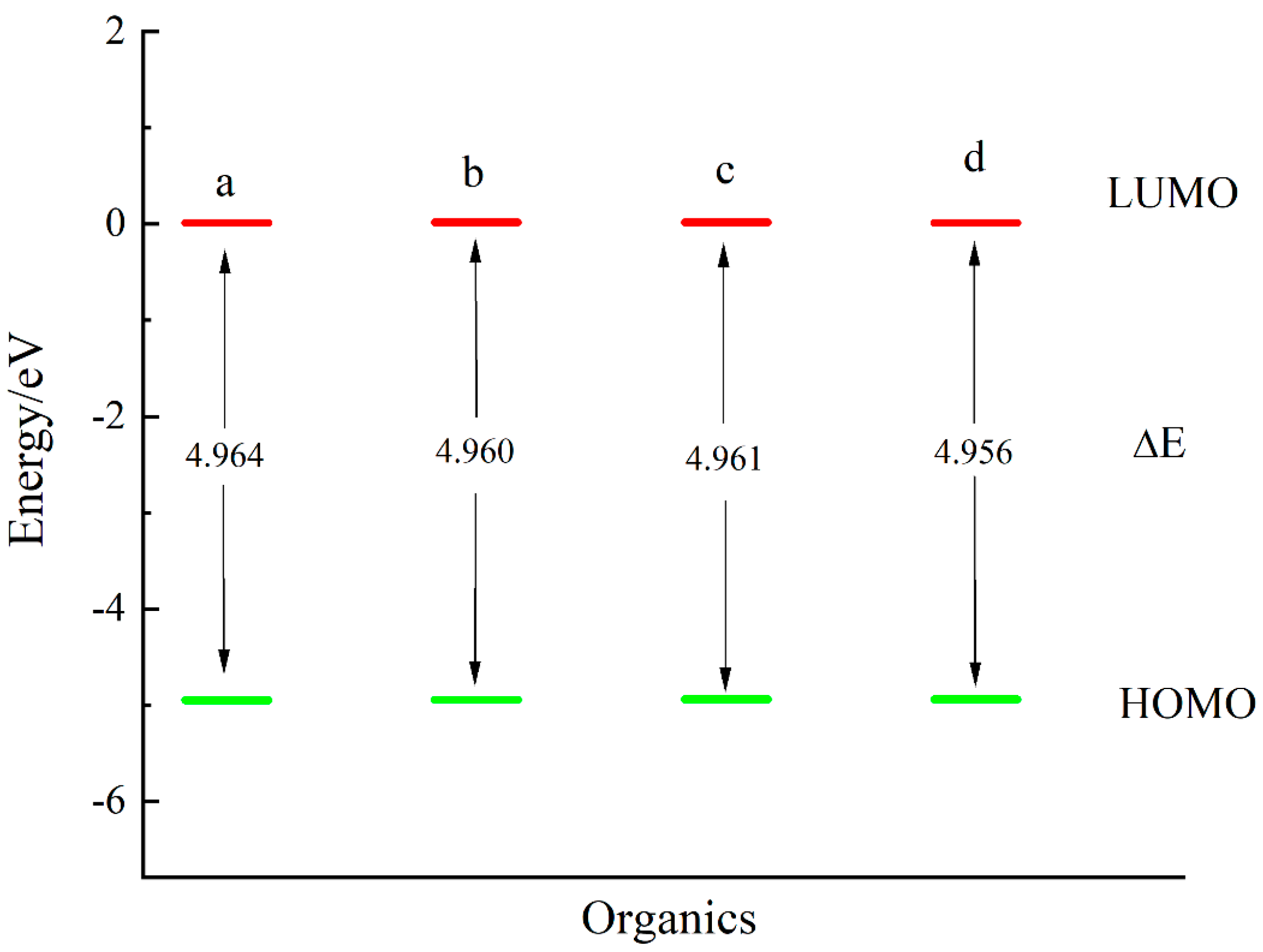
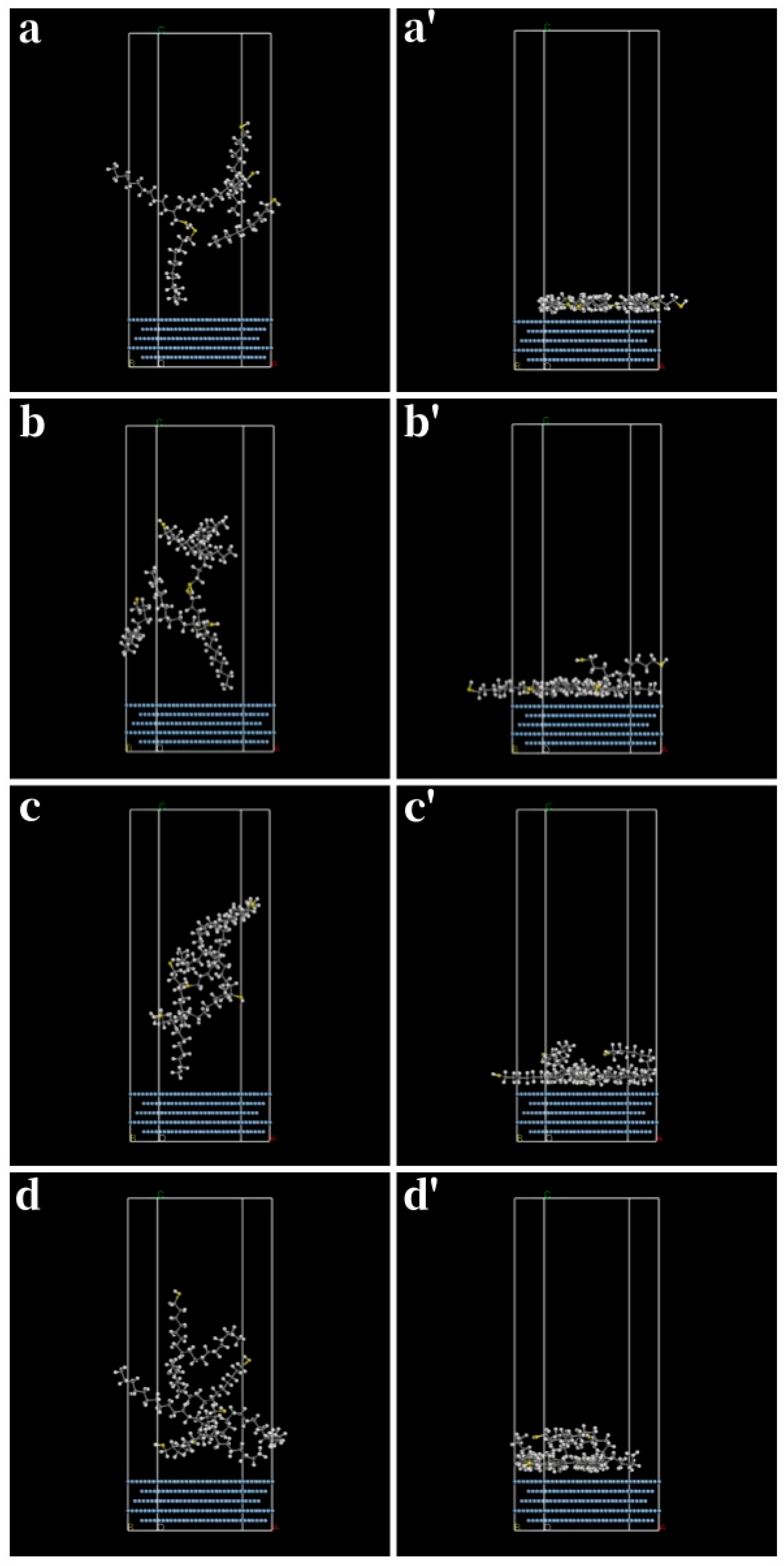
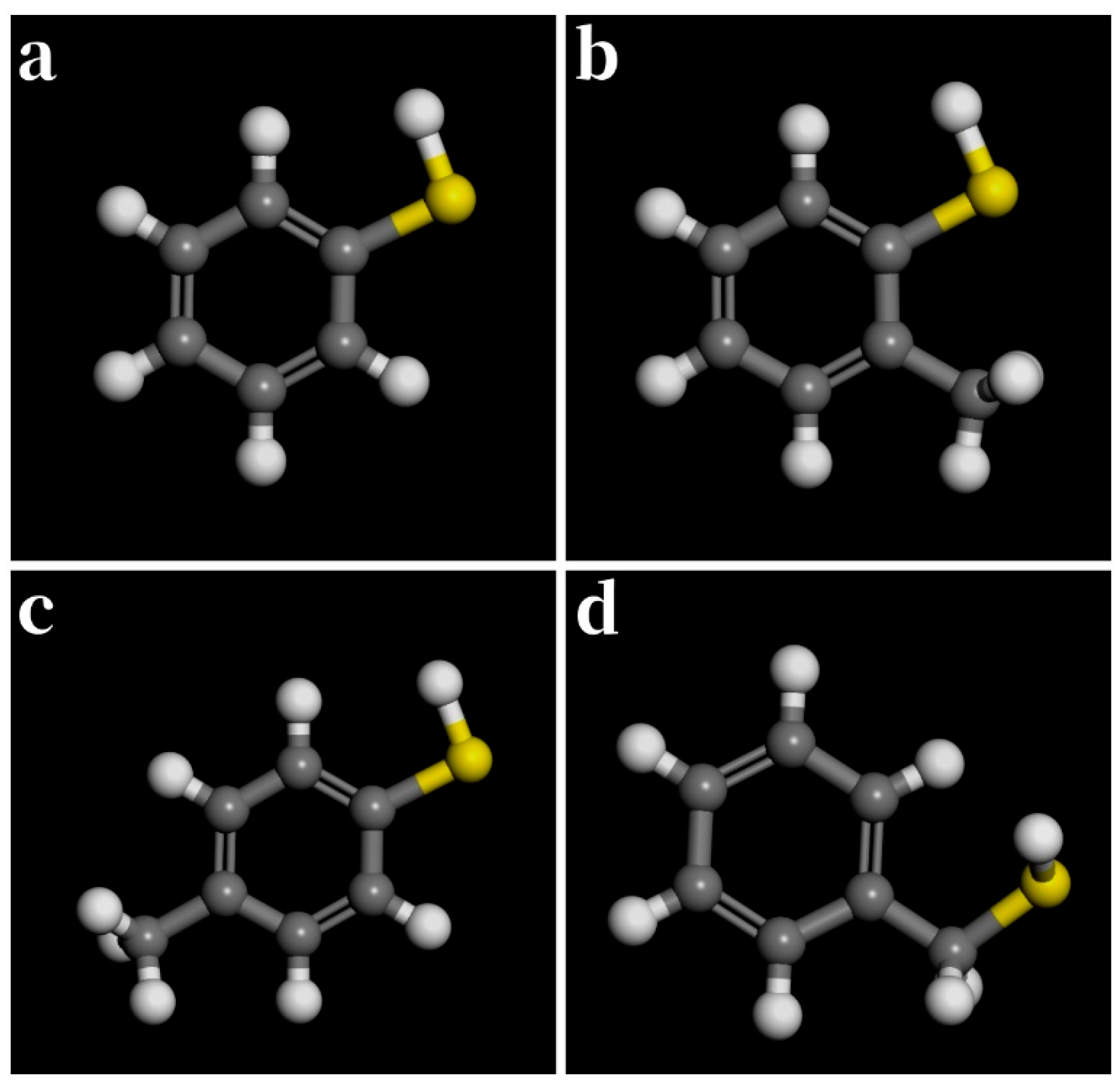
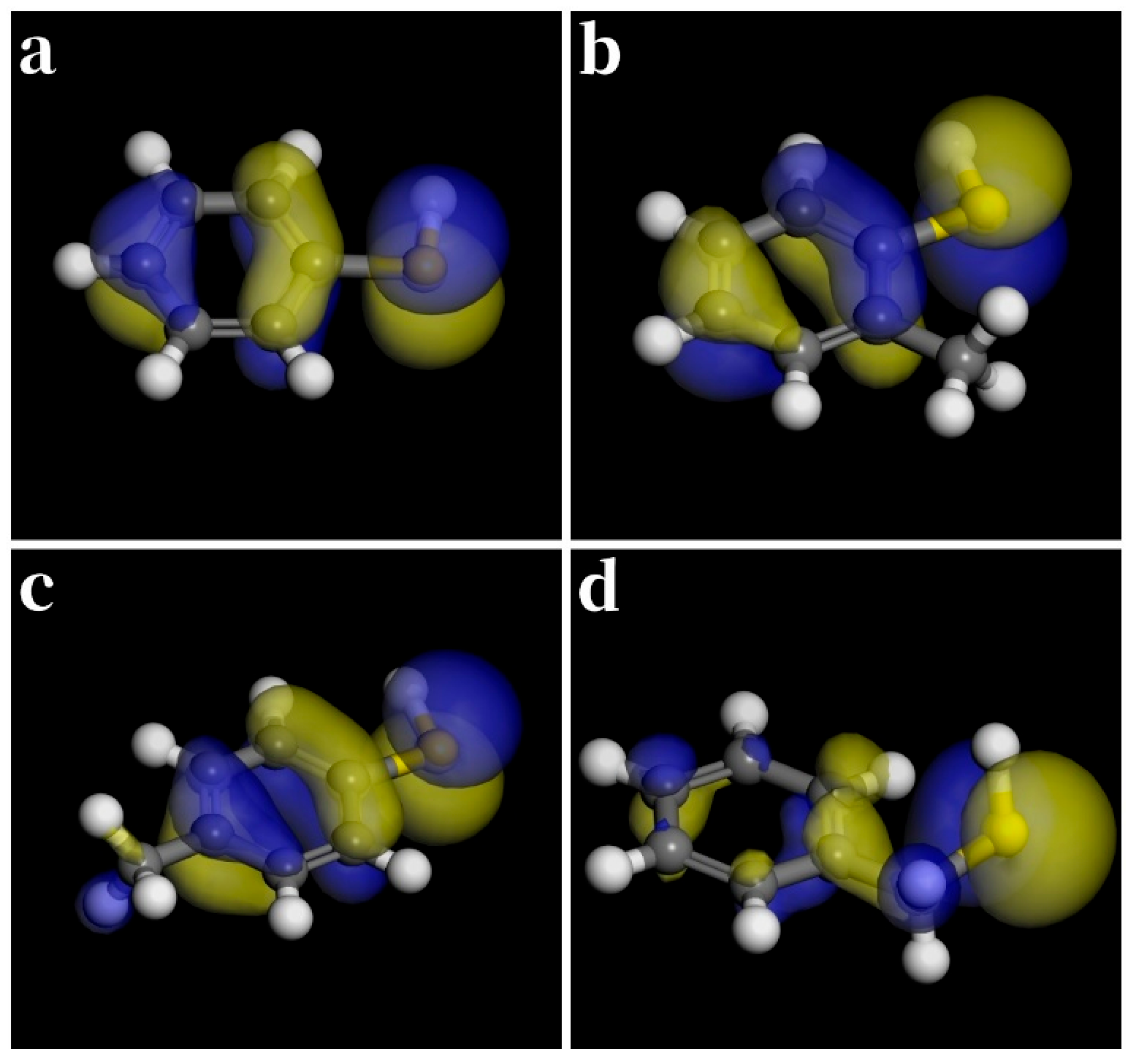






| Thiol | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| Methanethiol | 1092.059 | 1202.212 | −3.916 | −106.236 |
| Ethanethiol | 2064.127 | 2164.656 | 4.608 | −105.138 |
| Propanethiol | 1082.434 | 1196.981 | 20.959 | −135.506 |
| Cyclohexanethiol | 976.303 | 1199.927 | −43.810 | −179.813 |
| Thiol | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| 1-dodecanethiol | 1525.875 | 1865.724 | 2.601 | −342.450 |
| 1-tetradecanethiol | 3275.309 | 3628.980 | −17.562 | −336.109 |
| 1-hexadecanethiol | 823.026 | 1206.617 | −46.832 | −336.759 |
| 1-octadecanethiol | 824.203 | 1205.771 | −44.534 | −337.035 |
| Thiol | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| Benzenethiol | 1581.795 | 1712.713 | 52.569 | −183.488 |
| 2-methylthiophenol | 2025.625 | 2158.365 | 77.864 | −210.603 |
| 4-methylthiophenol | 1968.059 | 2160.374 | 19.712 | −212.027 |
| Phenylmethanethiol | 2057.943 | 2158.380 | 102.544 | −202.982 |
| Thiol | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| 1,1-propanedithiol | 1294.410 | 1482.010 | −21.534 | −166.065 |
| Ethanedithiol | 2207.431 | 2318.614 | 32.025 | −143.208 |
| 1,4-butanedithiol | 1733.393 | 1943.528 | −10.408 | −199.727 |
| 2,3-butanedithiol | 1777.018 | 1947.296 | 13.088 | −183.366 |
| Thiol | (kJ/mol) | (kJ/mol) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|
| 3-methyl-2-buten-1-thiol | 1818.892 | 1997.182 | 2.392 | −180.682 |
| 2-propene-1-thiol | 2407.443 | 2484.687 | 42.869 | −120.113 |
| 2-butene-1-thiol | 2789.694 | 2870.316 | 67.257 | −147.880 |
| Thiogeraniol | 2285.626 | 2552.269 | 14.764 | −281.407 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; An, Y.; Zhai, H.; Gao, B.; Liu, A. Investigation on the Interfacial Behavior of Thiols on Silver Surface by DFT Study and MD Simulation. Coatings 2025, 15, 1134. https://doi.org/10.3390/coatings15101134
Gao W, An Y, Zhai H, Gao B, Liu A. Investigation on the Interfacial Behavior of Thiols on Silver Surface by DFT Study and MD Simulation. Coatings. 2025; 15(10):1134. https://doi.org/10.3390/coatings15101134
Chicago/Turabian StyleGao, Wenjing, Yukun An, Hongjia Zhai, Boyu Gao, and Anmin Liu. 2025. "Investigation on the Interfacial Behavior of Thiols on Silver Surface by DFT Study and MD Simulation" Coatings 15, no. 10: 1134. https://doi.org/10.3390/coatings15101134
APA StyleGao, W., An, Y., Zhai, H., Gao, B., & Liu, A. (2025). Investigation on the Interfacial Behavior of Thiols on Silver Surface by DFT Study and MD Simulation. Coatings, 15(10), 1134. https://doi.org/10.3390/coatings15101134







