Enhancing Energy Harvesting in Plant Microbial Fuel Cells with SnS-Coated 304 Stainless Steel Electrodes
Abstract
1. Introduction
2. Experimental Details
2.1. SnS Film Obtention
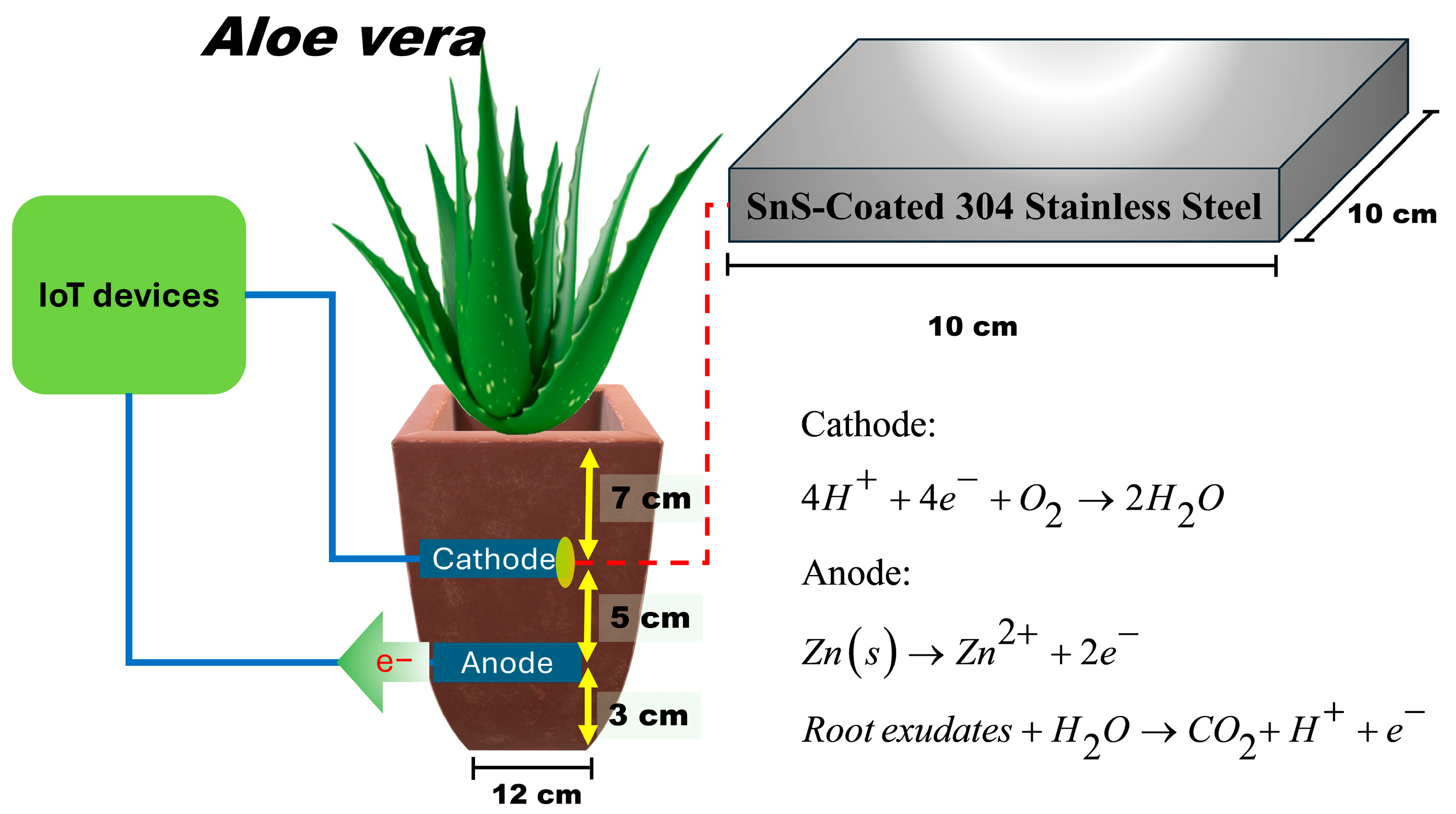
2.2. PMFC Construction
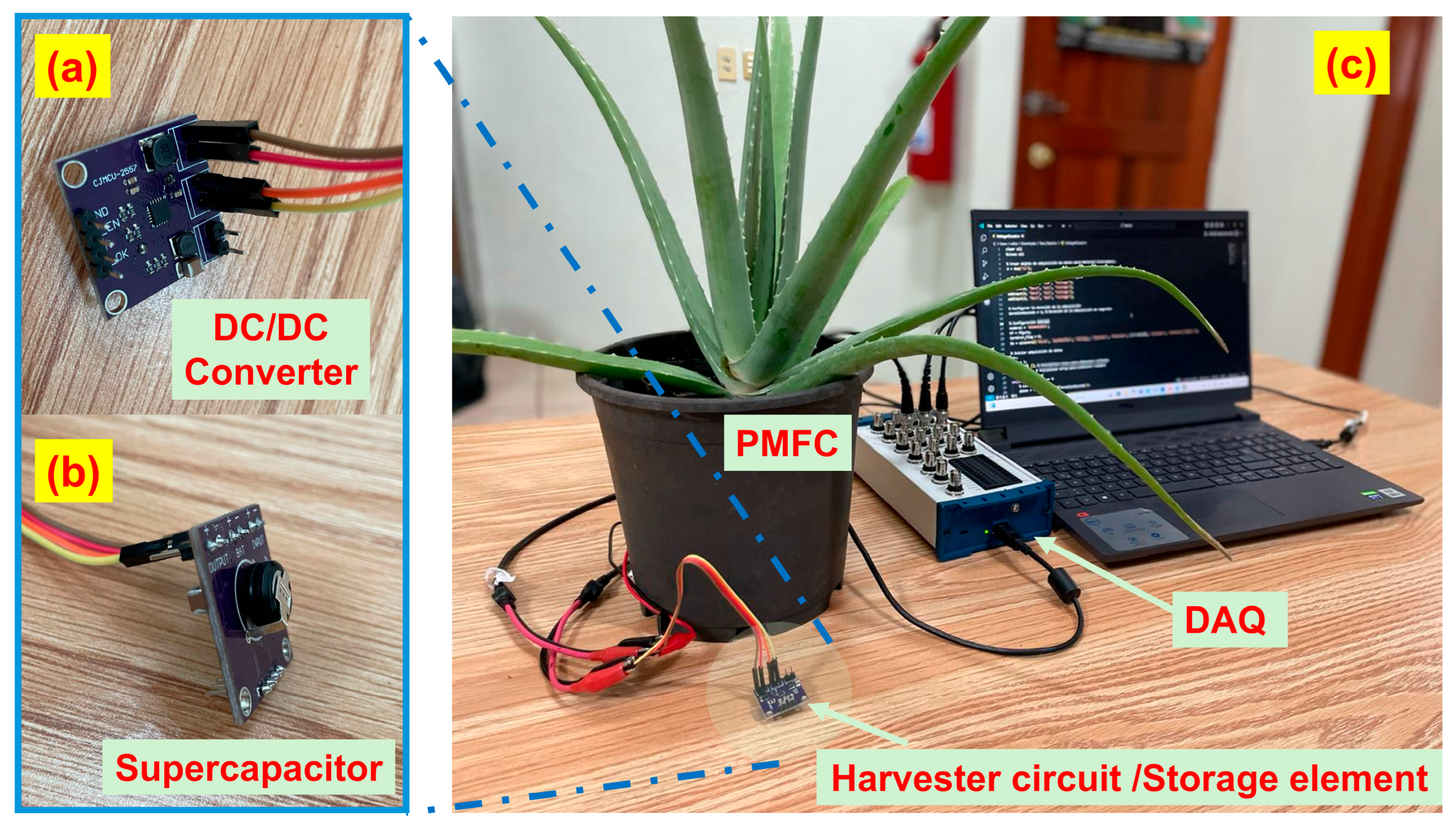
2.3. Evaluation of PMFC Energy Storage Using SnS Thin Film-Coated Electrodes
2.4. Characterization Techniques
2.5. Data Treatment
3. Results and Discussions
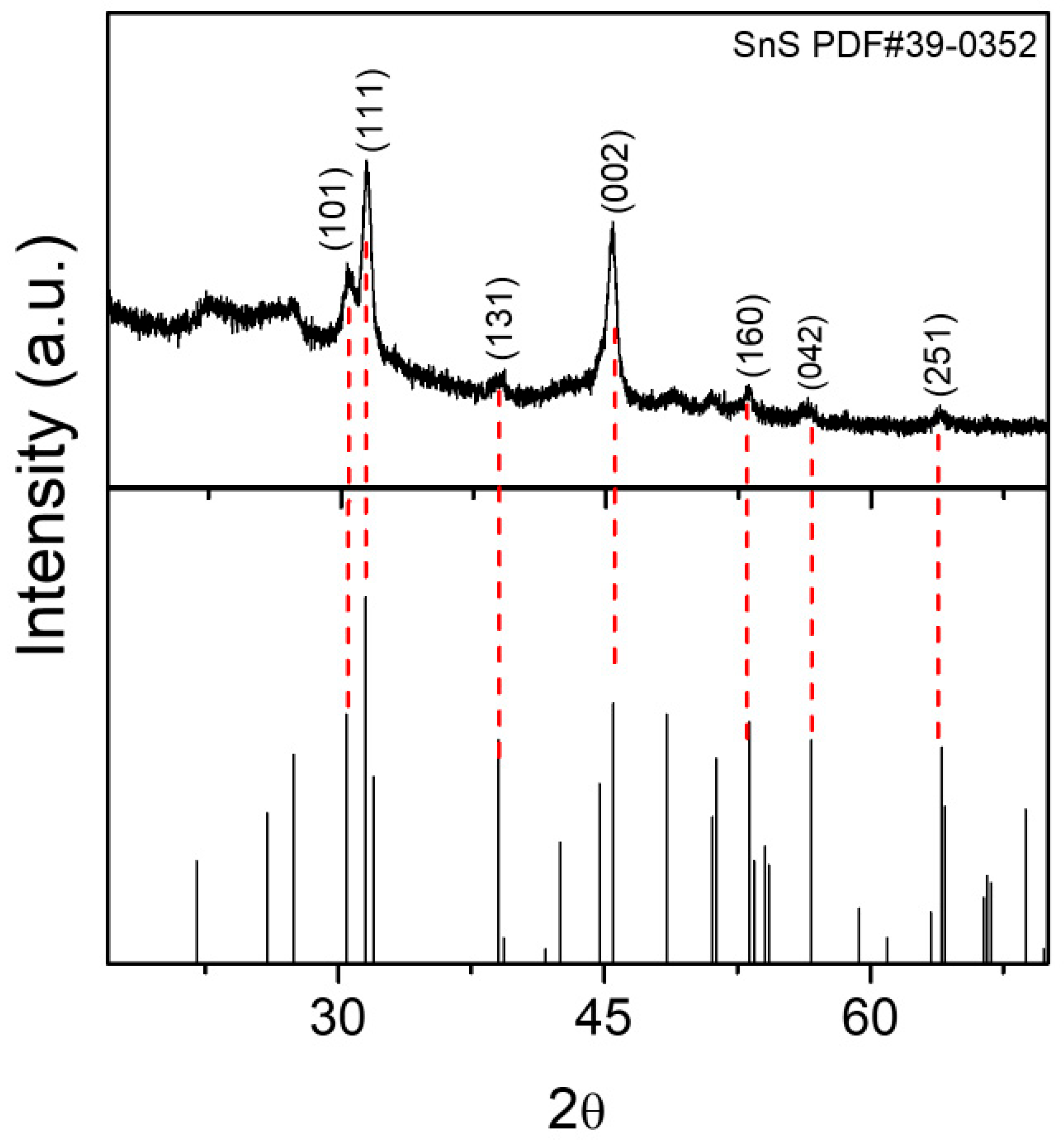
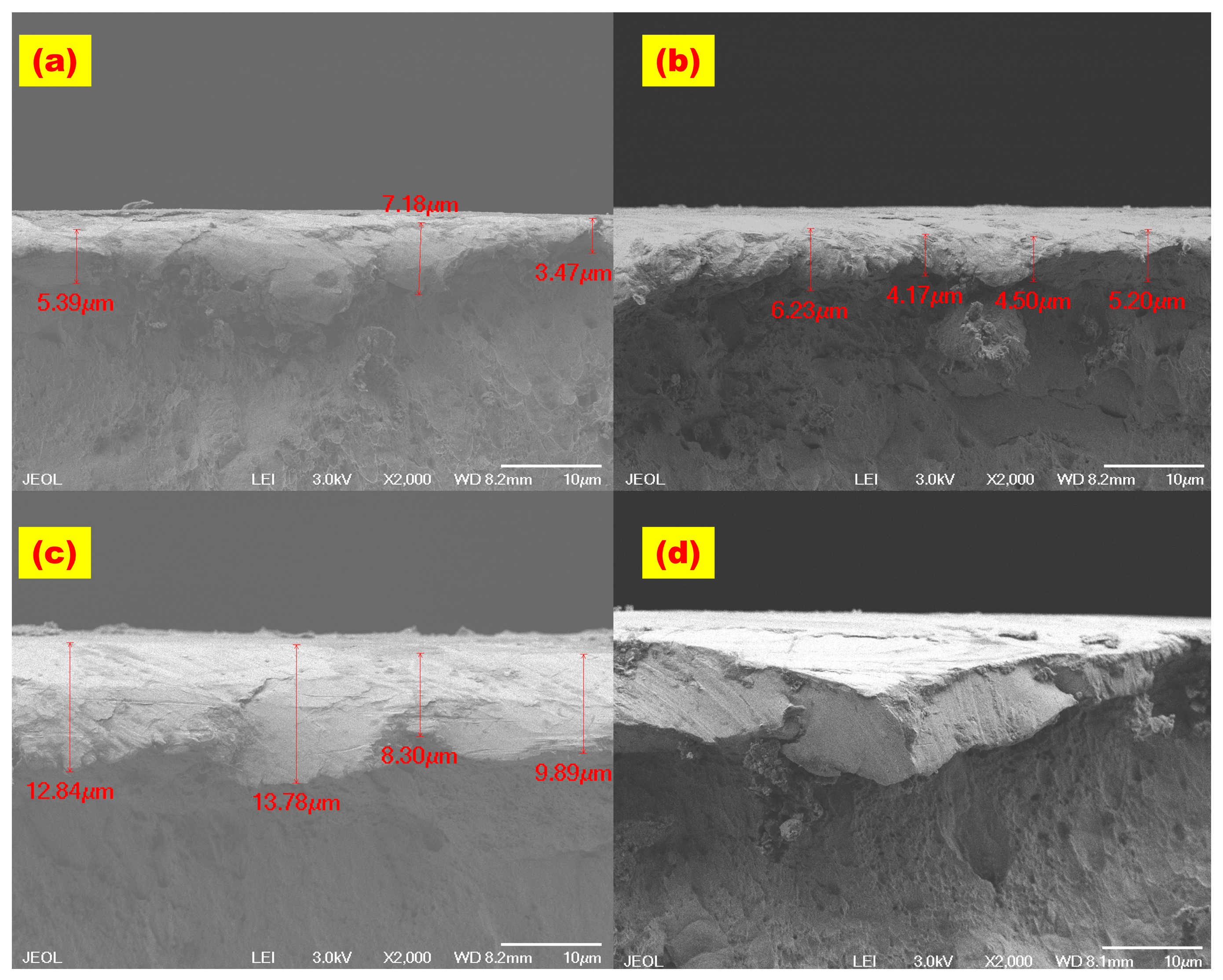
3.1. Structural and Morphology Analysis
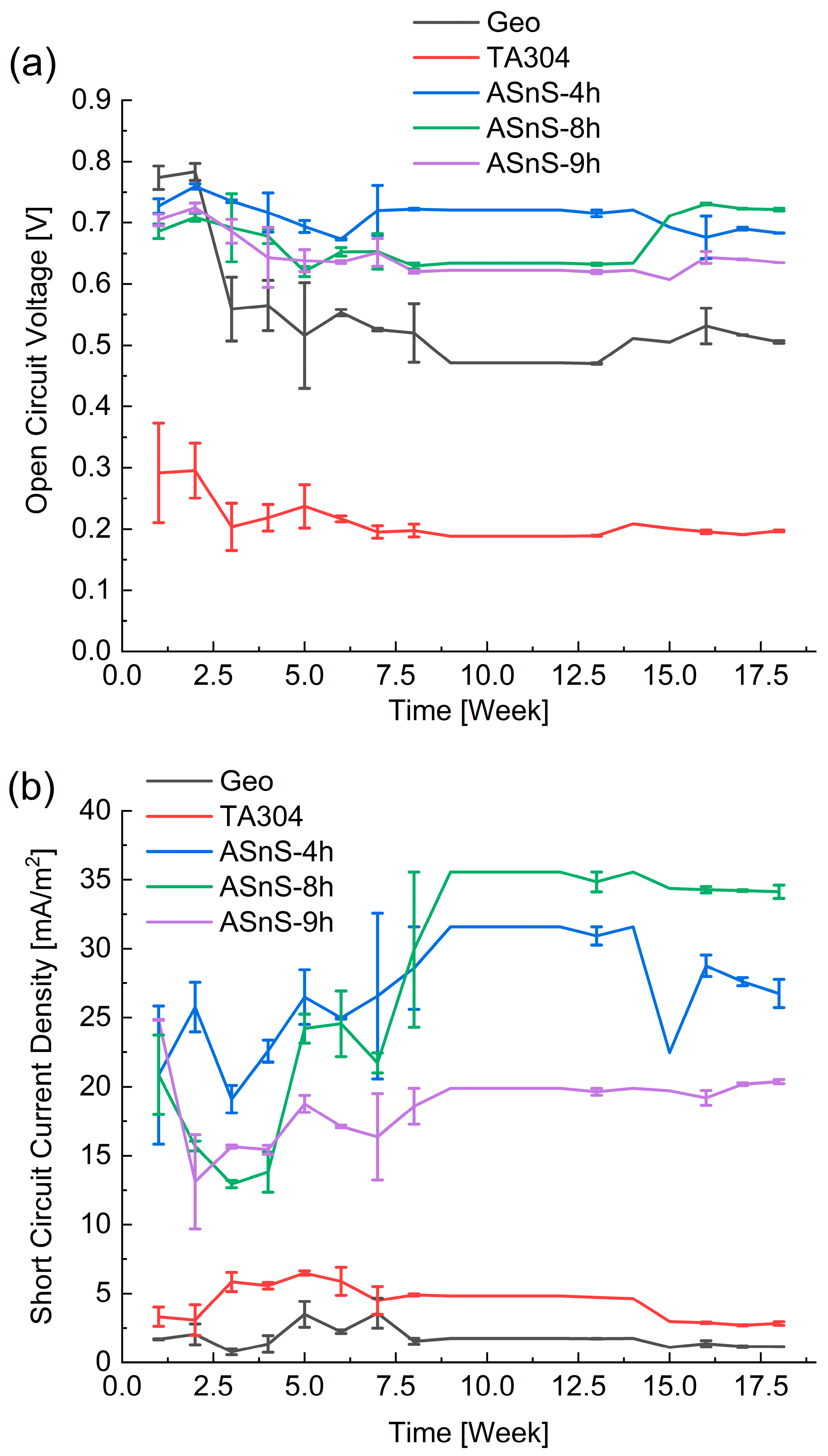
3.2. Electrical Measurements of SnS Thin Films in PMFC
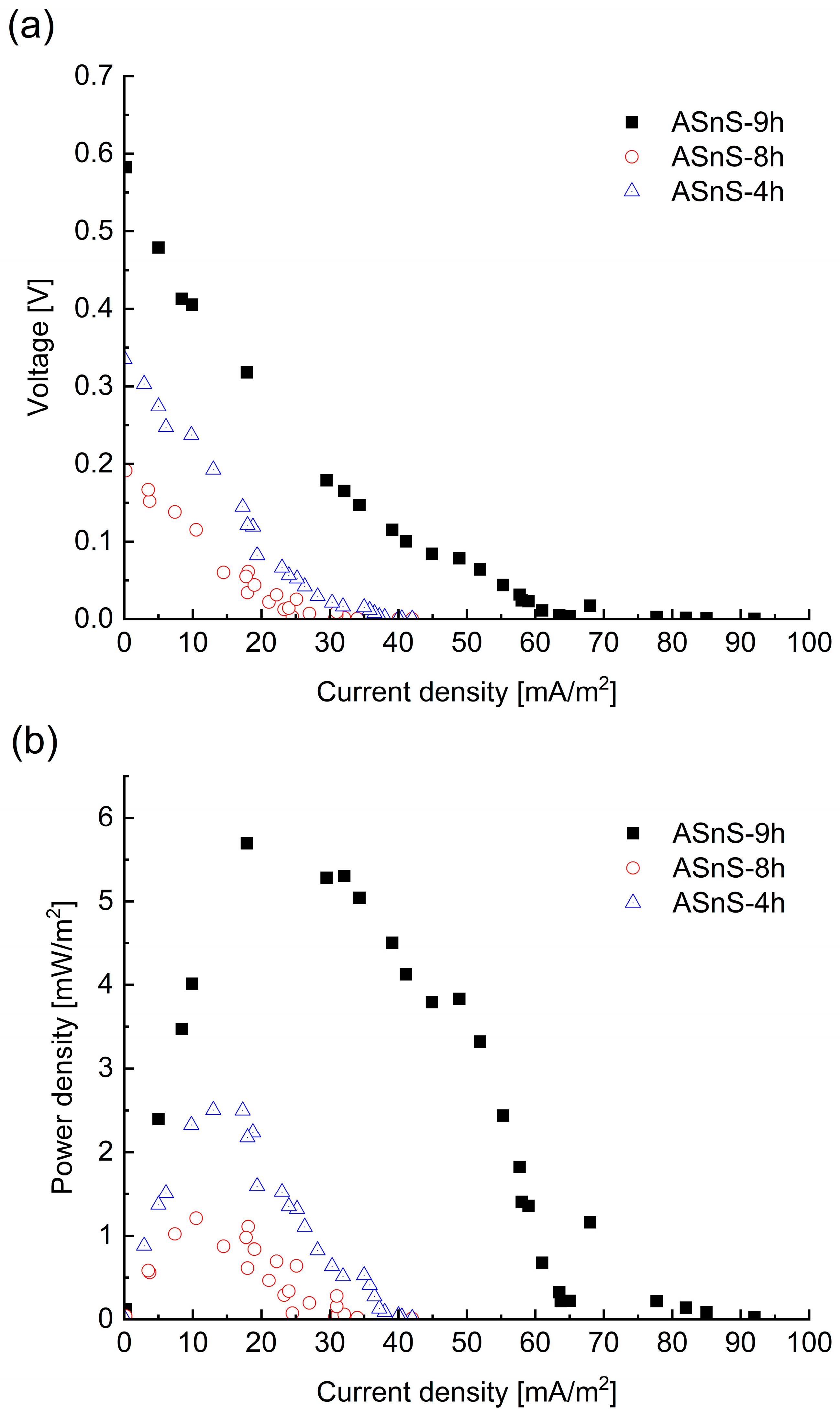
3.3. Polarization Curves of the PMFC
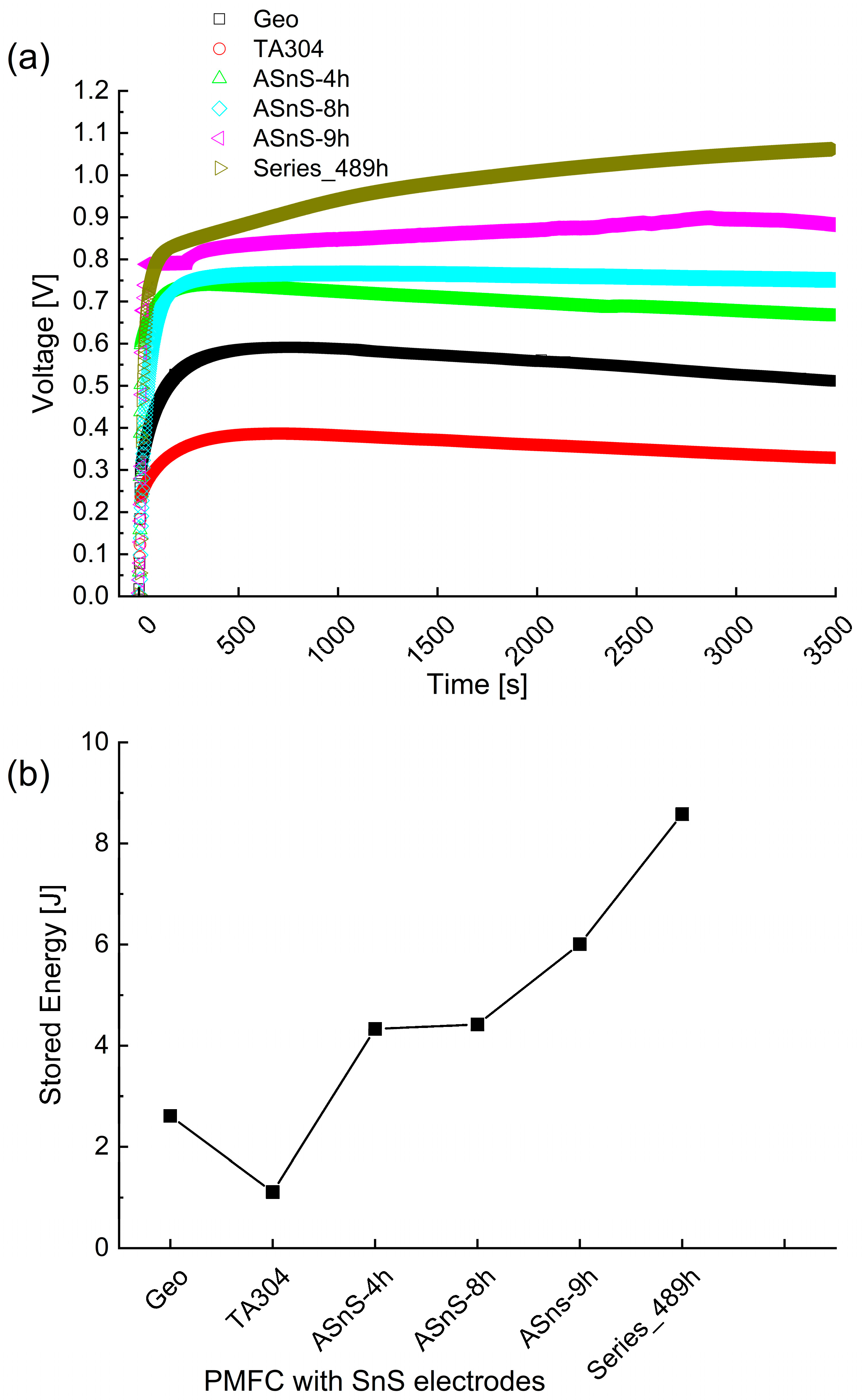
3.4. Applied Energy of the PMFC Configurations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmers, R.A.; Rothballer, M.; Strik, D.P.B.T.B.; Engel, M.; Schulz, S.; Schloter, M.; Hartmann, A.; Hamelers, B.; Buismanet, C. Microbial community structure elucidates performance of Glyceria maxima plant microbial fuel cell. Appl. Microbiol. Biotechnol. 2012, 94, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Helder, M.; Chen, W.-S.; van der Harst, E.J.M.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Buisman, C.J.N.; Potting, J. Electricity production with living plants on a green roof: Environmental performance of the plant-microbial fuel cell. Biofuels Bioprod. Biorefining 2013, 7, 52–64. [Google Scholar] [CrossRef]
- Chung-Yu Guan Chang-Ping, Y. Evaluation of plant microbial fuel cells for urban green roofs in a subtropical metropolis. Sci. Total Environ. 2021, 765, 142786. [Google Scholar] [CrossRef]
- Ayala-Ruiz, D.; Castillo-Atoche, A.; Ruiz-Ibarra, E.; Osorio de la Rosa, E.; Vázquez-Castillo, J. A Self-Powered PMFC-Based Wireless Sensor Node for Smart City Applications. Wirel. Commun. Mob. Comput. 2019, 2019, 8986302. [Google Scholar] [CrossRef]
- Castillo-Atoche, A.; Vázquez-Castillo, J.; Osorio-de-la-Rosa, E.; Heredia-Lozano, J.C.; Viñas-Avilés, J.; Quijano-Cetina, R.; Estrada-López, J.J. An Energy-Saving Data Statistics-Driven Management Technique for Bio-Powered Indoor Wireless Sensor Nodes. IEEE Trans. Instrum. Meas. 2021, 70, 9507010. [Google Scholar] [CrossRef]
- Osorio-de-la-Rosa, E.; Valdez-Hernández, M.; Vázquez-Castillo, J.; Franco-de-la-Cruz, A.; Woo-García, R.M.; Castillo-Atoche, A.; La Rosa, R. Plant microbial fuel cells as a bioenergy source used in precision beekeeping. Sustain. Energy Technol. Assess. 2023, 60, 103499. [Google Scholar] [CrossRef]
- Maddalwar, S.; Nayak, K.K.; Kumar, M.; Singh, L. Plant microbial fuel cell: Opportunities, challenges, and prospects. Bioresour. Technol. 2021, 341, 125772. [Google Scholar] [CrossRef]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energy 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Rusyn, I.; Medvediev, O.; Valko, B. Enhancement of bioelectric parameters of multi-electrode plant–microbial fuel cells by combining of serial and parallel connection. Int. J. Environ. Sci. Technol. 2021, 18, 1323–1334. [Google Scholar] [CrossRef]
- Gilani, S.R.; Yaseen, A.; Rza, S.A.; Zahra, M.; Mahmood, Z.J. Photocurrent Generation through Plant Microbial Fuel Cell by Varying Electrode Materials. J. Chem. Soc. Pak. 2016, 38, 17. [Google Scholar]
- Cheng, C.; Hu, Y.; Shao, S.; Yu, J.; Zhou, W.; Zhang, L. Simultaneous Cr(VI) reduction and electricity generation in Plant-Sediment Microbial Fuel Cells (P-SMFCs): Synthesis of non-bonding Co3O4 nanowires onto cathodes. Environ. Pollut. 2019, 247, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Apollon, W.; Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Kamaraj, S.-K.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A. Performance of electrical energy monitoring data acquisition system for plant-based microbial fuel cell. J. Exp. Biol. Agric. Sci. 2022, 10, 387–395. [Google Scholar] [CrossRef]
- Azri, Y.M.; Tou, I.; Sadi, M. Electrodes materials evaluation in plant microbial fuel cells: A comparison of graphite and stainless steels. Biofuels 2023, 14, 1077–1086. [Google Scholar] [CrossRef]
- Ancona, V.; Cavone, C.; Grenni, P.; Gagliardi, G.; Cosentini, C.; Borello, D.; Barra Caracciolo, A. Plant microbial fuel cells for recovering contaminated environments. Int. J. Hydrogen Energy 2024, 72, 1116–1126. [Google Scholar] [CrossRef]
- Bhowmick, G.D.; Das, S.; Ghangrekar, M.M.; Mitra, A.; Banerjee, R. Improved Wastewater Treatment by Combined System of Microbial Fuel Cell with Activated Carbon/TiO2 Cathode Catalyst and Membrane Bioreactor. J. Inst. Eng. India Ser. A 2019, 100, 675–682. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Park, M.; Choi, J.; Kim, D.-W. Ultra-Small SnS Quantum Dots Anchored onto Nitrogen-Enriched Carbon Nanospheres as an Advanced Anode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 7114–7124. [Google Scholar] [CrossRef]
- Tripathi, A.M.; Mitra, S. Tin sulfide (SnS) nanorods: Structural, optical and lithium storage property study. RSC Adv. 2014, 4, 10358–10366. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; Wang, G.; Lin, Y.; Ou, X.; Wang, J.; Zhao, B.; Liu, M.; Lin, Z.; Huang, K. SnS nanoparticles electrostatically anchored on three-dimensional N-doped graphene as an active and durable anode for sodium-ion batteries. Energy Environ. Sci. 2017, 10, 1757–1763. [Google Scholar] [CrossRef]
- Kul, M. Electrodeposited SnS film for photovoltaic applications. Vacuum 2014, 107, 213–218. [Google Scholar] [CrossRef]
- Specialty Steel Industry of North America. Designer Handbook. In The Care and Cleaning of Stainless Steel; Specialty Steel Industry of North America: Washington, DC, USA, 2001. [Google Scholar]
- Peña-Méndez, Y.; Gamboa, S.A.; López-Martínez, S.D.; Kharissov-Ildusovich, B.; Gómez-Vidales, V. Photoelectrocatalytic hydrogen production on SnS films prepared by chemical bath. Int. J. Hydrogen Energy 2024, 70, 606–613. [Google Scholar] [CrossRef]
- Patishtán Pérez, J.; Rodríguez García, R.; Zavala García, F.; Jasso Cantú, D. Conductancia estomática y asimilación neta de CO2 en sábila (Aloe vera Tourn) bajo sequía. Rev. Fitotec. Mex. 2010, 33, 305–314. [Google Scholar] [CrossRef]
- Rodriguez Martinez, R.D.; Alvarez Bermudez, M.E. Production of electrical energy from living plants in microbial fuel cells. Clean Energy 2023, 7, 408–416. [Google Scholar] [CrossRef]
- Habeeb, F.; Shakir, E.; Bradbury, F.; Cameron, P.; Taravati, M.R.; Drummond, A.J.; Gray, A.I.; Ferro, V. Screening methods used to determine the anti-microbial properties of Aloe vera inner gel. Methods 2007, 42, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Chen, Z.; Zhao, F. Energy from plants and microorganisms: Progress in plant-microbial fuel cells. ChemSusChem 2012, 5, 1006–1011. [Google Scholar] [CrossRef]
- BQ25570. Available online: https://www.ti.com/document-viewer/bq25570/datasheet (accessed on 15 July 2024).
- Data Acquisition. Available online: https://www.ni.com/en/support/documentation/cable-accessory-guide/daq-multifunction-i-o-cable-accessory-compatibility/main-page---daq-multifunction-i-o-cable-and-accessory-compatibil/62xx-models.html#group2 (accessed on 15 July 2024).
- Md Khudzari, J.; Kurian, J.; Gariépy, Y.; Tartakovsky, B.; Raghavan, G.S.V. Effects of salinity, growing media, and photoperiod on bioelectricity production in plant microbial fuel cells with weeping alkaligrass. Biomass Bioenergy 2018, 109, 1–9. [Google Scholar] [CrossRef]
- Adesiji, N.E.; Adeoye, M.; Omojokun, A.O.; Fatile, J.A. The effect of electrodes on the voltage generation of microbial fuel cell. Niger. J. Pure Appl. Phys. 2021, 10, 8–11. [Google Scholar] [CrossRef]
- Sophia Carmalin, A.; Sreeja, S. Green energy generation from plant microbial fuel cells (PMFC) using compost and a novel clay separator. Sustain. Energy Technol. Assess. 2017, 21, 59–66. [Google Scholar] [CrossRef]
- Doglioni, M.; Nardello, M.; Brunelli, D. Plant Microbial Fuel Cells: Energy Sources and Biosensors for battery-Free Smart Agriculture. IEEE Trans. AgriFood Electron. 2024, 2, 460–470. [Google Scholar] [CrossRef]
- Osorio, E.; Vázquez-Castillo, J.; Castillo-Atoche, A.; Heredia-Lozano, J.; Becerra-Nuñez, G.; Barbosa, R. Arrays of Plant Microbial Fuel Cells for Implementing Self-Sustainable Wireless Sensor Networks. IEEE Sens. J. 2021, 21, 1965–1975. [Google Scholar] [CrossRef]
- Jafary, T.; Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Hghparast, F.; Wan Daud, W.R. Assessment of bioelectricity production in microbial fuel cells through series and parallel connections. Energy Convers. Manag. 2013, 75, 256–262. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Clomera, J.A.A.; Garcia, K.V.; Ravara, G.R.; Salamat, E.J.G. Stacking of aquatic plant-microbial fuel cells growing water spinach and water lettuce. OP Conf. Ser. Earth Environ. Sci. 2018, 191, 012054. [Google Scholar] [CrossRef]

| Electrode Material Cathode/Anode | Open-Circuit Voltage (V) | Current Density (mA/m2) | Power Density (mW/m2) | Evaluation Time (Days) | Reference |
|---|---|---|---|---|---|
| Carbon/Carbon | 0.06 to 0.72 | Not specified | Not specified | 14 | [29] |
| Carbon/Copper | 0.02 to 0.67 | Not specified | Not specified | 14 | [29] |
| Carbon/Zinc | 0.11 to 0.78 | Not specified | Not specified | 14 | [29] |
| Carbon felts | 0.220 | Not specified | Not specified | Varies | [14] |
| Carbon cloth | 0.114 ± 5.89 | Not specified | Not specified | Varies | [14] |
| Graphite felt | 0.0614 | Not specified | 121.7 | Varies | [14] |
| Carbon plate | 0.543 | Not specified | Not specified | 40 | [14] |
| Activated Carbon/TiO2 composite | 0.536 ± 25 | Not specified | 1.02 W/m3 | 200 | [15] |
| Hydrated carbon cloth/carbon brush | Not specified | Not specified | 69.32 to 222.54 | Not specified | [30] |
| Manganese-based catalyzed carbon/carbon felt | 0.600 | 90 | 1.5 | 114 | [28] |
| Graphite felts | 0.050 to 0.391 | Not specified | Not specified | 32 | [23] |
| Graphite granules | Not specified | Not specified | 15.84 | 367 | [14] |
| Graphite | 0.400 | 20.4 | 37 | 20 | [13] |
| Co3O4 nanowires | Not specified | Not specified | 75.12 ± 2.9 | Not specified | [11] |
| Stainless Steel 316 | 0.150 | 5.33 | 23.0 | 25 | [13] |
| Stainless Steel 436 | 0.106 | 2.2 | 10.5 | 25 | [13] |
| Stainless Steel 304 | 0.600 | 92.8 | 5.8 | 120 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Regalado, N.; Peña-Méndez, Y.; Osorio-de-la-Rosa, E.; Gómez-de-la-Fuente, I.; Valdez-Hernández, M.; López-Huerta, F. Enhancing Energy Harvesting in Plant Microbial Fuel Cells with SnS-Coated 304 Stainless Steel Electrodes. Coatings 2025, 15, 1130. https://doi.org/10.3390/coatings15101130
Rodríguez-Regalado N, Peña-Méndez Y, Osorio-de-la-Rosa E, Gómez-de-la-Fuente I, Valdez-Hernández M, López-Huerta F. Enhancing Energy Harvesting in Plant Microbial Fuel Cells with SnS-Coated 304 Stainless Steel Electrodes. Coatings. 2025; 15(10):1130. https://doi.org/10.3390/coatings15101130
Chicago/Turabian StyleRodríguez-Regalado, Nestor, Yolanda Peña-Méndez, Edith Osorio-de-la-Rosa, Idalia Gómez-de-la-Fuente, Mirna Valdez-Hernández, and Francisco López-Huerta. 2025. "Enhancing Energy Harvesting in Plant Microbial Fuel Cells with SnS-Coated 304 Stainless Steel Electrodes" Coatings 15, no. 10: 1130. https://doi.org/10.3390/coatings15101130
APA StyleRodríguez-Regalado, N., Peña-Méndez, Y., Osorio-de-la-Rosa, E., Gómez-de-la-Fuente, I., Valdez-Hernández, M., & López-Huerta, F. (2025). Enhancing Energy Harvesting in Plant Microbial Fuel Cells with SnS-Coated 304 Stainless Steel Electrodes. Coatings, 15(10), 1130. https://doi.org/10.3390/coatings15101130








