Physical Vapor Deposited TiN and TiAlN on Biomedical β-Type Ti-29Nb-13Ta-4.6Zr: Microstructural Characteristics, Surface Hardness Enhancement, and Antibacterial Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Physical Vapor Deposition (PVD) on TNTZ Samples
2.3. Microstructural Characterization
2.4. Atomic Force Microscopy
2.5. Hardness Measurement
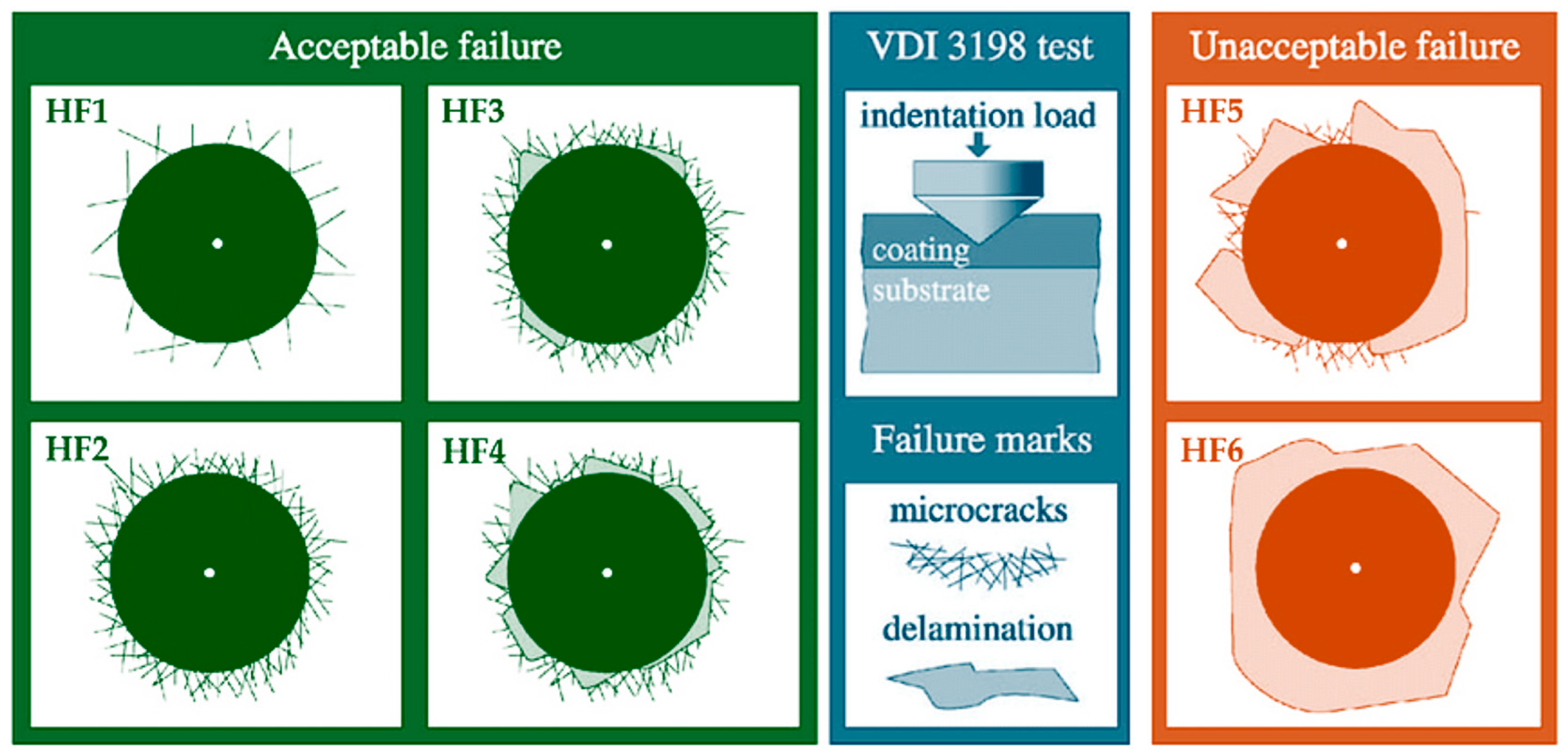
2.6. Adhesion Evaluation
2.7. Contact Angle Measurement
2.8. Bacterial Sensitivity Tests
3. Results and Discussion
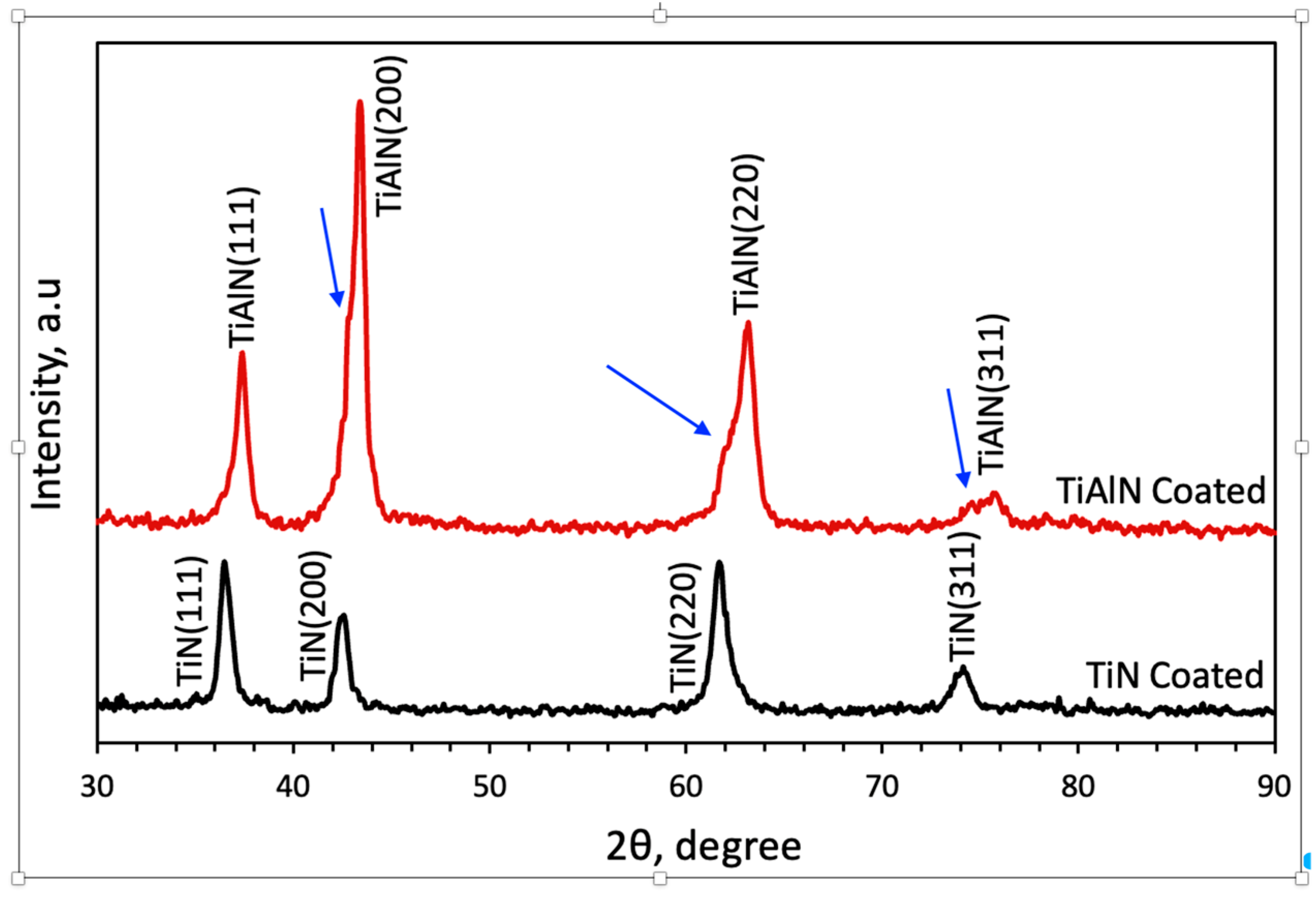
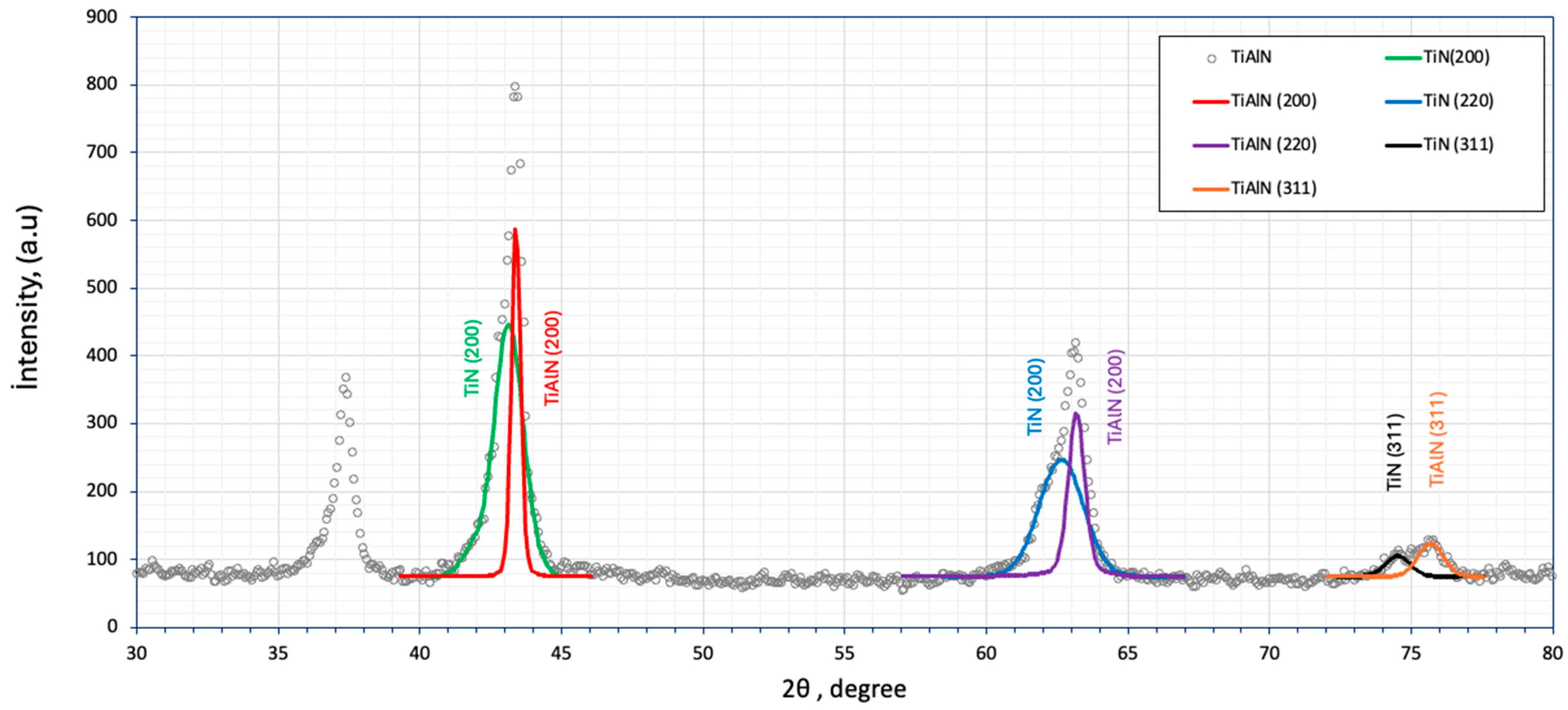
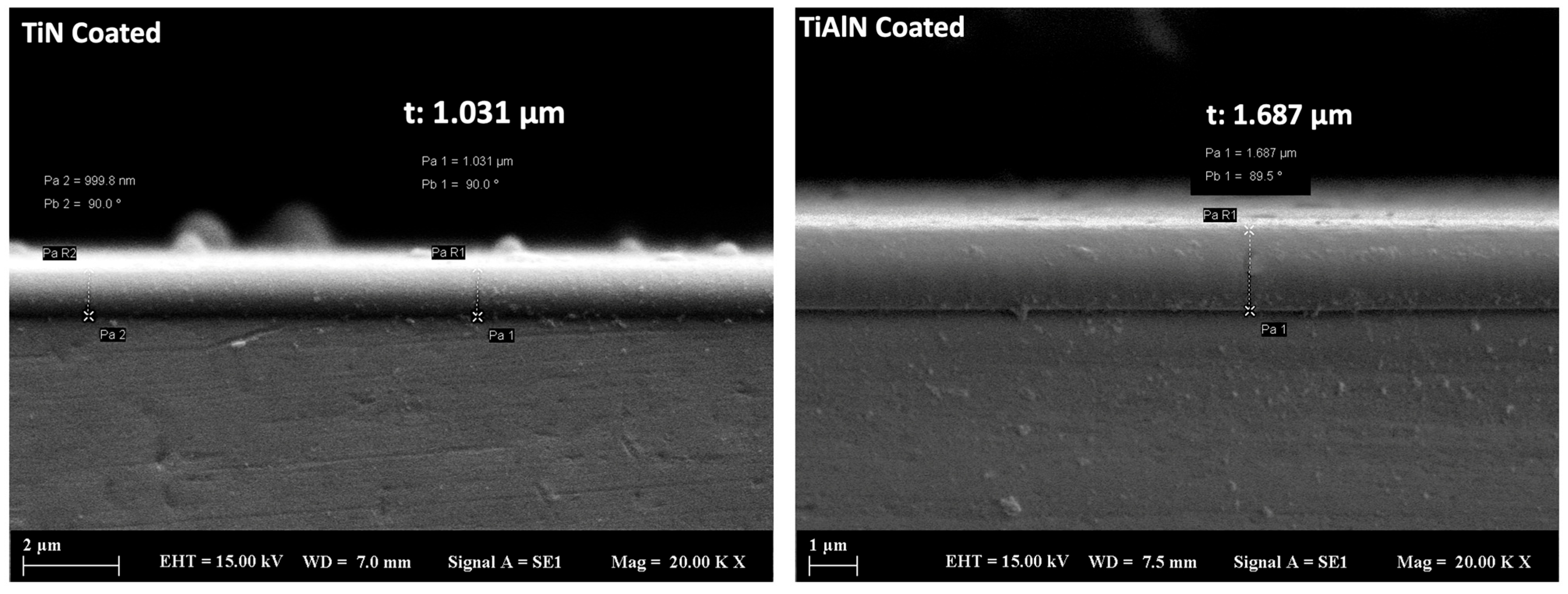
3.1. Microstructural Analysis
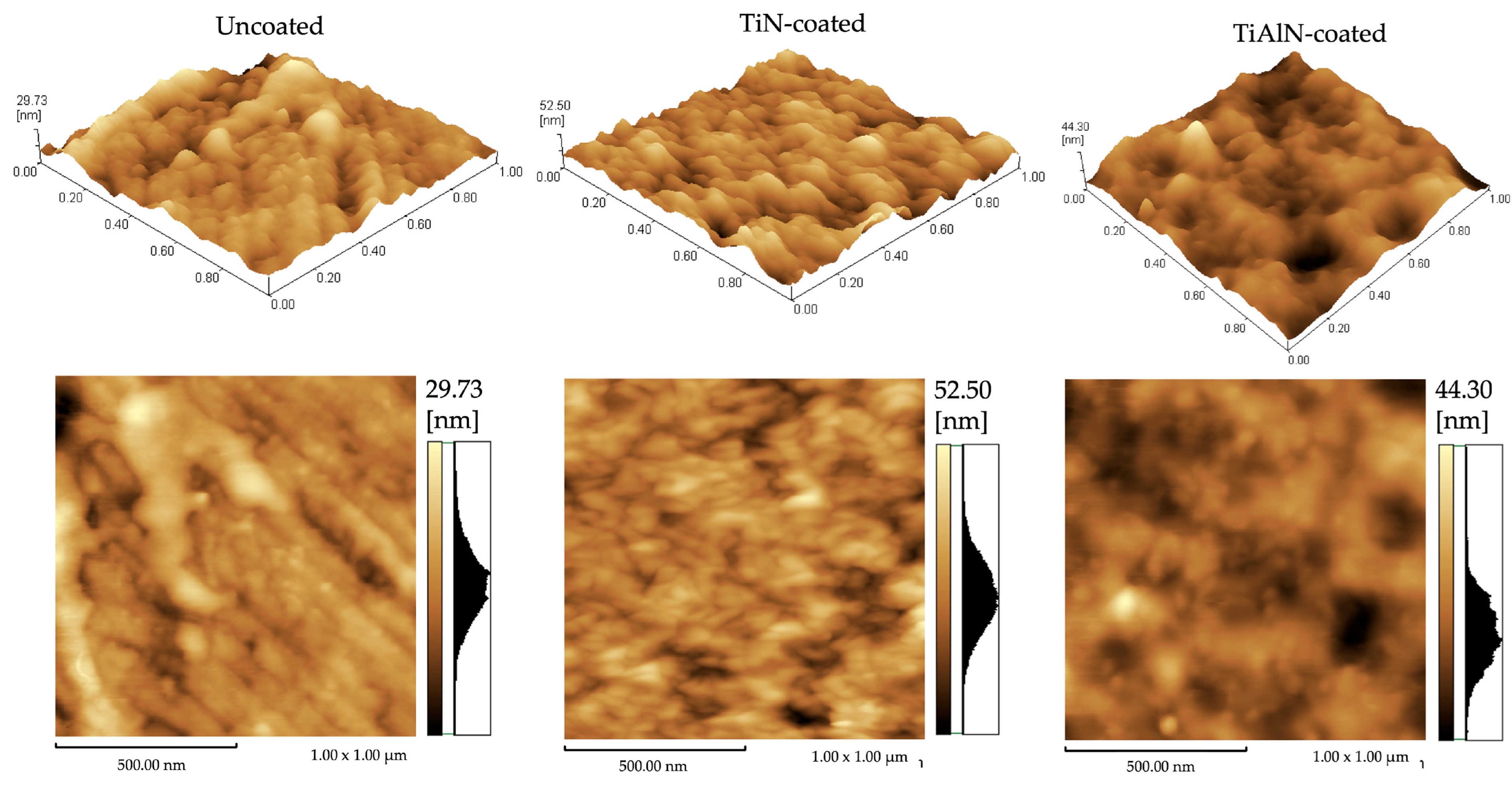
3.2. Coating Formation Mechanisms: Crystallite Size Estimation and AFM Topography
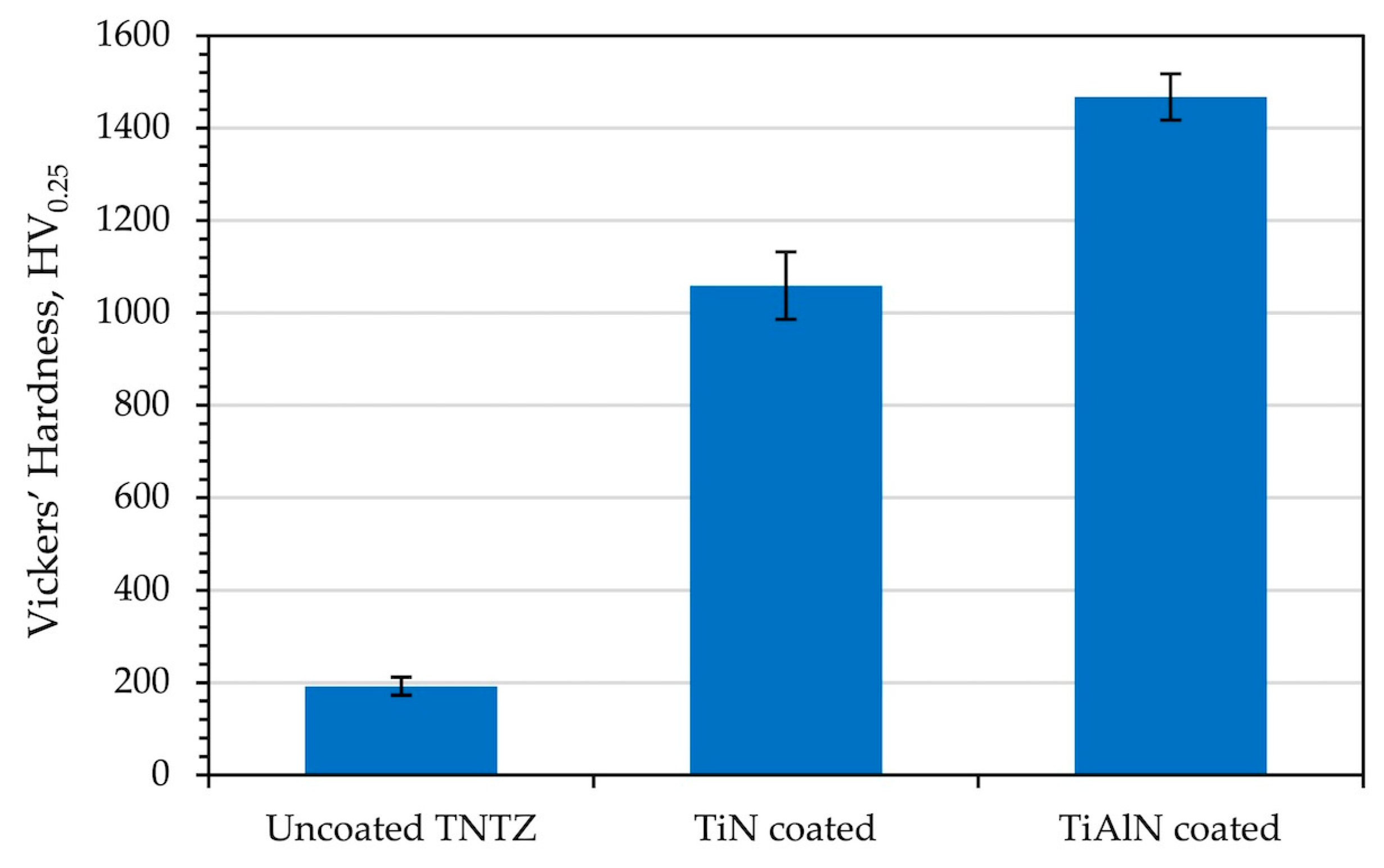
3.3. Surface Hardness
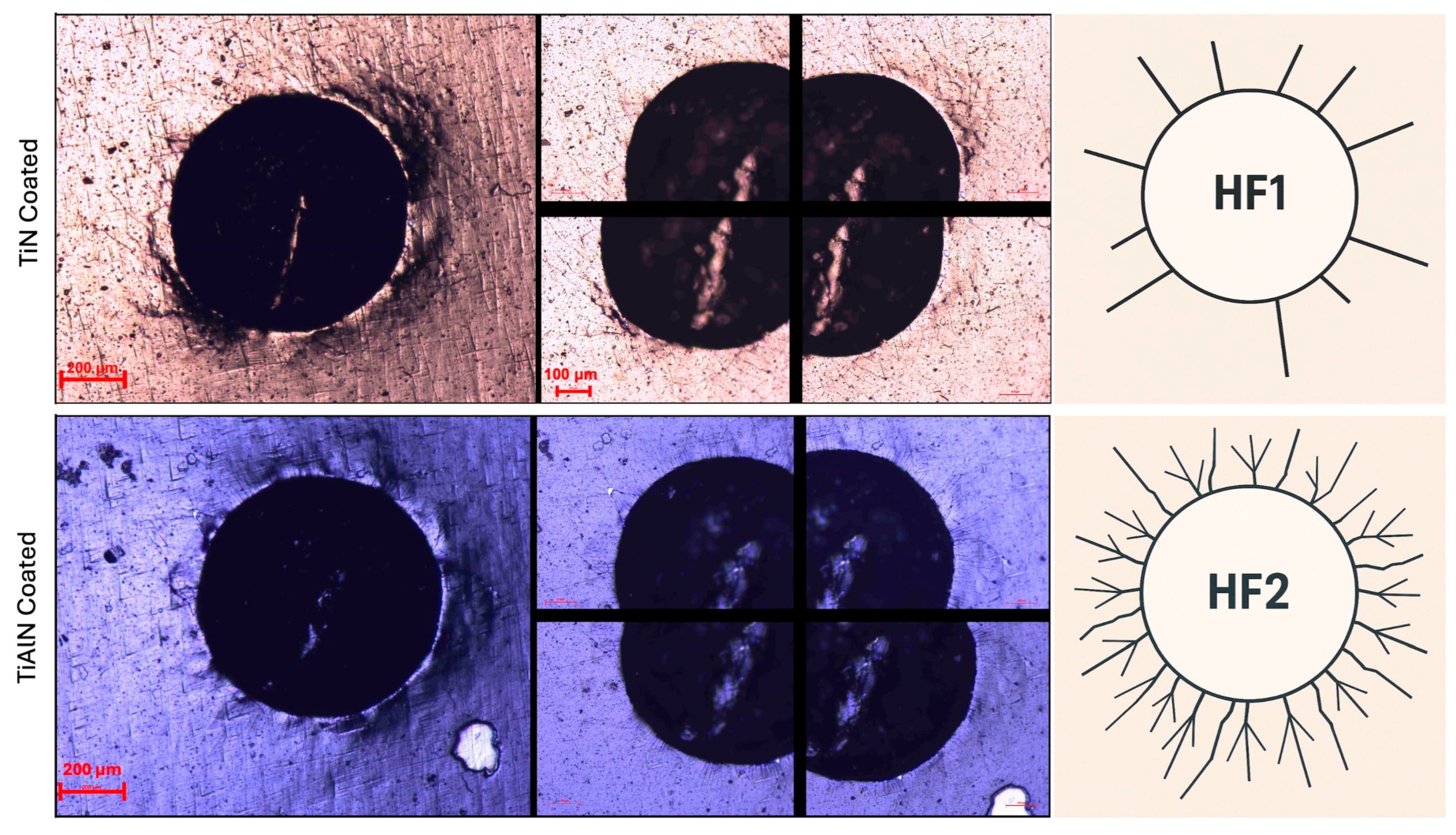
3.4. Coating Adhesion Performance
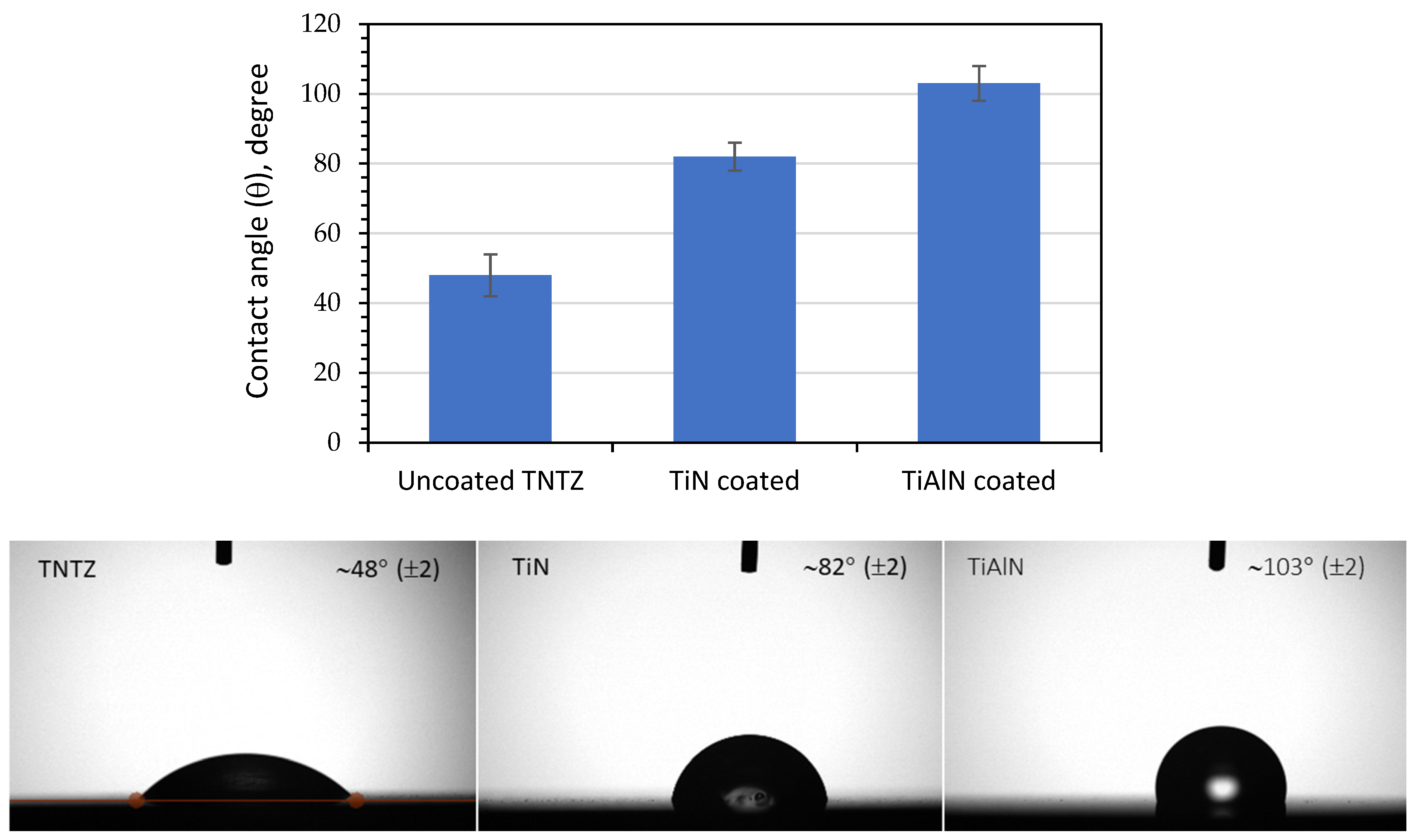
3.5. Surface Wettability
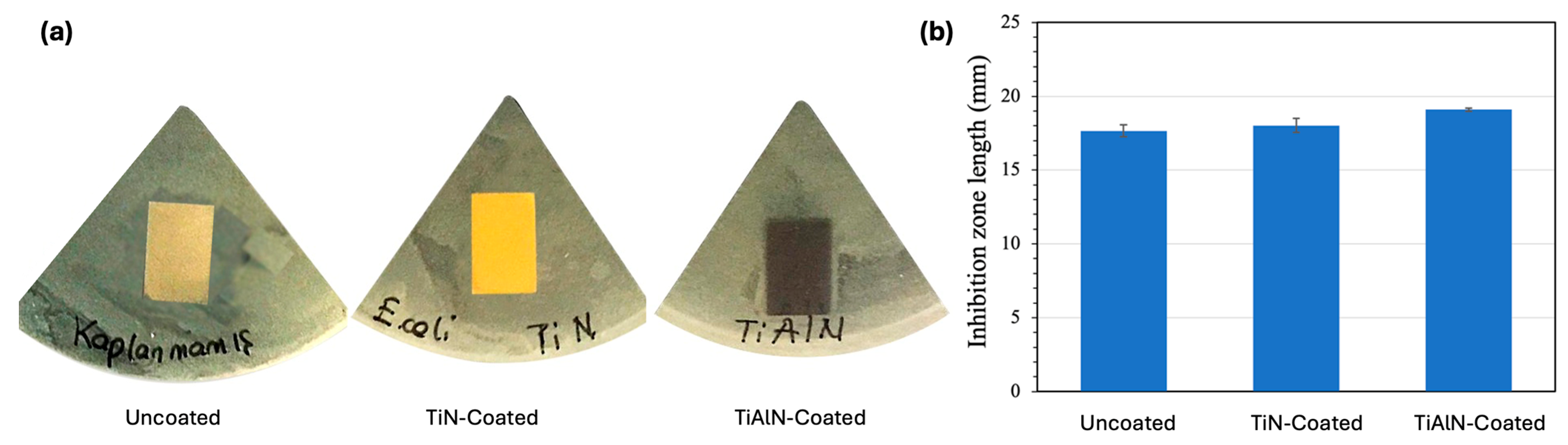
3.6. Bacterial Sensitivity
3.7. Comparative Analysis with Literature
4. Conclusions
5. Future Directions and Biomedical Validation
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, A.; Wang, J.; Abdel-Hady Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Banerjee, R.; Nag, S.; Fraser, H.L. A novel combinatorial approach to the development of beta titanium alloys for orthopedic implants. Mater. Sci. Eng. C 2005, 25, 282–289. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Niinomi, M. Development of new metallic alloys for biomedical applications. Acta Biomater. 2010, 6, 3888–3903. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Niinomi, M.; Akahori, T.; Takeda, J.; Toda, H. Relationships between tensile deformation behavior and microstructure in Ti–Nb–Ta–Zr system alloys. Mater. Sci. Eng. C 2005, 25, 363–369. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of Low Rigidity β-type Titanium Alloy for Biomedical Applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Li, J.; Hastings, G.W. Chapter 5 Oxide Bioceramics: Inert Ceramic Materials in Medicine and Dentistry. In Handbook of Biomaterial Properties; Murphy, W., Black, J., Hastings, G., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Colombo, D.A.; Mandri, A.D.; Echeverría, M.D.; Massone, J.M.; Dommarco, R.C. Mechanical and tribological behavior of Ti/TiN and TiAl/TiAlN coated austempered ductile iron. Thin Solid Film. 2018, 647, 19–25. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Nose, M.; Zhou, M.; Honbo, E.; Yokota, M.; Saji, S. Colorimetric properties of ZrN and TiN coatings prepared by DC reactive sputtering. Surf. Coat. Technol. 2001, 142–144, 211–217. [Google Scholar] [CrossRef]
- Shin, C.-S.; Gall, D.; Hellgren, N.; Patscheider, J.; Petrov, I.; Greene, J.E. Vacancy hardening in single-crystal TiNx(001) layers. J. Appl. Phys. 2003, 93, 6025–6028. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choe, H.C.; Brantley, W.A. Nanostructured thin film formation on femtosecond laser-textured Ti–35Nb–xZr alloy for biomedical applications. Thin Solid Film. 2011, 519, 4668–4675. [Google Scholar] [CrossRef]
- Safin Kaosar Saad, K.; Saba, T.; Bin Rashid, A. Application of PVD coatings in medical implantology for enhanced performance, biocompatibility, and quality of life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef]
- Amirtharaj Mosas, K.K.; Chandrasekar, A.R.; Dasan, A.; Pakseresht, A.; Galusek, D. Recent Advancements in Materials and Coatings for Biomedical Implants. Gels 2022, 8, 323. [Google Scholar] [CrossRef]
- Mani, S.P.; Kalaiarasan, M.; Ravichandran, K.; Rajendran, N. Corrosion resistant and conductive TiN/TiAlN multilayer coating on 316L SS: A promising metallic bipolar plate for proton exchange membrane fuel cell. J. Mater. Sci. 2021, 56, 10575–10596. [Google Scholar] [CrossRef]
- Kang, B.S.; Sul, Y.T.; Johansson, C.B.; Oh, S.J.; Lee, H.J.; Albrektsson, T. The effect of calcium ion concentration on the bone response to oxidized titanium implants. Clin. Oral Implant. Res. 2012, 23, 690–697. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Properties of single layer and gradient (Ti,Al)N coatings. Mater. Sci. Eng. A 2003, 361, 1–8. [Google Scholar] [CrossRef]
- Shugurov, A.R.; Kuzminov, E.D.; Garanin, Y.A. Structure and Mechanical Properties of Ti-Al-Ta-N Coatings Deposited by Direct Current and Middle-Frequency Magnetron Sputtering. Metals 2023, 13, 512. [Google Scholar] [CrossRef]
- Lisoń-Kubica, J.; Taratuta, A.; Wilk, K.; Kolasa, J.; Antonowicz, M.; Paszenda, Z.; Walke, W.; Gümüş, S.; Basiaga, M. The effect of TiN coating on the physicochemical properties of Ti-13Nb-13Zr alloy for biomedical applications. Langmuir 2025, 41, 13264–13271. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Grips, V.W.; Ezhil Selvi, V.; Barshilia, H.C.; Rajam, K.S. Electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive direct current magnetron sputtering. Thin Solid Film. 2006, 514, 204–211. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Oh, J.K.; Yegin, Y.; Yang, F.; Zhang, M.; Li, J.; Huang, S.; Verkhoturov, S.V.; Schweikert, E.A.; Perez-Lewis, K.; Scholar, E.A.; et al. The influence of surface chemistry on the kinetics and thermodynamics of bacterial adhesion. Sci. Rep. 2018, 8, 17247. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotech 2011, 6, 534. [Google Scholar] [CrossRef]
- Aissani, L.; Belgroune, A.; Saoudi, A.; Hmima, A.; Fellah, M.; Obrosov, A.; Alhussein, A. Tribo-mechanical performance and antibacterial activity in (Cu, Zr)-alloyed Ti(Al)N coatings synthesized by reactive magnetron sputtering. J. Mater. Sci. 2022, 57, 19612–19630. [Google Scholar] [CrossRef]
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 indentation test evaluation of a reliable qualitative control for layered compounds. J. Mater. Process. Technol. 2003, 143–144, 481–485. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, C.; Han, B.; Geng, H.; Liu, L. Preparation of in situ TiC@TiN core–shell and Ti2N–Al4C3 nanoparticles and their effects on Al–Zn–Mg–Cu alloy. J. Mater. Sci. 2021, 56, 17011–17027. [Google Scholar] [CrossRef]
- Ahlgren, M.; Blomqvist, H. Influence of bias variation on residual stress and texture in TiAlN PVD coatings. Surf. Coat. Technol. 2005, 200, 157–160. [Google Scholar] [CrossRef]
- Hörling, A.; Hultman, L.; Odén, M.; Sjölén, J.; Karlsson, L. Thermal stability of arc evaporated high aluminum-content thin films. J. Vac. Sci. Technol. A 2002, 20, 1815–1823. [Google Scholar] [CrossRef]
- Qiu, R. Electron Microscopy Investigation of Detailed Microstructures of CVD TiAlN and TiN Coatings: Effects of Gas Flow and Substrate on Coating Microstructure. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2020. Available online: https://research.chalmers.se/publication/516330/file/516330_Fulltext.pdf (accessed on 17 September 2025).
- Bartosik, M.; Rumeau, C.; Hahn, R.; Zhang, Z.L.; Mayrhofer, P.H. Fracture toughness and structural evolution in the TiAlN system upon annealing. Sci. Rep. 2017, 7, 16476. [Google Scholar] [CrossRef]
- Abrikosov, I.A.; Knutsson, A.; Alling, B.; Tasnádi, F.; Lind, H.; Hultman, L.; Odén, M. Phase Stability and Elasticity of TiAlN. Materials 2011, 4, 1599–1618. [Google Scholar] [CrossRef] [PubMed]
- Elmkhah, H.; Zhang, T.F.; Abdollah-zadeh, A.; Kim, K.H.; Mahboubi, F. Surface characteristics for the TiAlN coatings deposited by high power impulse magnetron sputtering technique at the different bias voltages. J. Alloys Comp. Part A 2016, 688, 820–827. [Google Scholar] [CrossRef]
- Matei, A.A.; Turcu, R.N.; Pencea, I.; Herghelegiu, E.; Petrescu, M.I.; Niculescu, F. Comparative Characterization of the TiN and TiAlN Coatings Deposited on a New WC-Co Tool Using a CAE-PVD Technique. Crystals 2023, 13, 112. [Google Scholar] [CrossRef]
- Long, T.; Zeng, J.; Yu, D.; Wu, S. Microstructure of TiAlN and CrAlN coatings and cutting performance of coated silicon nitride inserts in cast iron turning. Ceram. Intern. 2014, 40, 9889–9894. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Mahne, N.; Čekada, M.; Panjan, M. Surface Topography of PVD Hard Coatings. Coatings 2021, 11, 1387. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yuan, H.; Qiu, M.; Zhang, X.; Liao, B.; Zhang, F.; Ouyang, X. Tribological Behaviors of Super-Hard TiAlN Coatings Deposited by Filtered Cathode Vacuum Arc Deposition. Materials 2022, 15, 2236. [Google Scholar] [CrossRef]
- Anders, A. Unfiltered and filtered cathodic arc deposition. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2010; pp. 466–531. [Google Scholar]
- Vlasveld, A.C.; Harris, S.G.; Doyle, E.D.; Lewis, D.B.; Münz, W.D. Characterisation and performance of partially filtered arc TiAlN coatings. Surf. Coat. Technol. 2002, 149, 217–224. [Google Scholar] [CrossRef]
- Martin, P.J.; Bendavid, A.; Netterfield, R.P.; Kinder, T.J.; Jahan, F.; Smith, G.B. Plasma deposition of tribological and optical thin film materials with a filtered cathodic arc source. Surf. Coat. Technol. 1999, 112, 257–260. [Google Scholar] [CrossRef]
- Adesina, A.Y.; Gasem, Z.M.; Mohammed, A.S. Comparative investigation and characterization of the scratch and wear resistance behavior of TiN, CrN, AlTiN and AlCrN cathodic arc PVD coatings. Arab. J. Sci. Eng. 2019, 44, 10355–10371. [Google Scholar] [CrossRef]
- Muhammed, M.; Javidani, M.; Ebrahimi Sadrabadi, T.; Heidari, M.; Levasseur, T.; Jahazi, M. A Comprehensive Review of Cathodic Arc Evaporation Physical Vapour Deposition (CAE-PVD) Coatings for Enhanced Tribological Performance. Coatings 2024, 14, 246. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Ratajski, J.; Kuklinski, Z.; Rochowicz, J. An analysis of macroparticle-related defects on CrCN and CrN coatings in dependence of the substrate bias voltage. Vacuum 2012, 86, 1235–1239. [Google Scholar] [CrossRef]
- Guruvenket, S.; Rao, G.M. Effect of ion bombardment and substrate orientation on structure and properties of titanium nitride films deposited by unbalanced magnetron sputtering. J. Vac. Sci. Technol. A 2002, 20, 678–682. [Google Scholar] [CrossRef]
- Chen, L.; Du, Y.; Wang, A.J.; Wang, S.Q.; Zhou, S.Z. Effect of Al content on microstructure and mechanical properties of Ti–Al–Si–N nanocomposite coatings. Int. J. Ref. Met. Hard Mater. 2009, 27, 718–721. [Google Scholar] [CrossRef]
- Cao, H.; Ouyang, X.; Wu, X.; Chen, L.; Wu, J.; Wu, J.; Wang, J.; Liao, B. Mechanical and Electrochemical Properties of Titanium Aluminum Nitride Coatings with Different Nitrogen Flow Rates on CrMnSi Steel by Filter Cathode Vacuum Arc Technology. Coatings 2025, 15, 379. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-C.; Liu, K.-T.; Duh, J.-G.; Chang, K.-W.; Chung, K.-H. Effect of nitride film coatings on cell compatibility. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2008, 24, 986–993. [Google Scholar] [CrossRef]
- Akahori, T.; Niinomi, M.; Nakai, M.; Nishimura, H.; Takei, Y.; Fukui, H.; Ogawa, M. Wear and Mechanical Properties, and Cell Viability of Gas-Nitrided Beta-Type Ti-Nb-Ta-Zr System Alloy for Biomedical Applications. Mater. Trans. 2008, 49, 166–174. [Google Scholar] [CrossRef]
- Van Trinh, T.; Le, H.T.; Le Thi, B. Fabrication of Titanium Nitride Thin Film on Titanium Using Cathodic Arc Plasma Evaporation for Biomedical Application. Eur. J. Dent. 2025. [Google Scholar] [CrossRef] [PubMed]
- Shum, P.W.; Tam, W.C.; Li, K.Y.; Zhou, Z.F.; Shen, Y.G. Mechanical and tribological properties of titanium–aluminium–nitride films deposited by reactive close-field unbalanced magnetron sputtering. Wear 2004, 257, 1030–1040. [Google Scholar] [CrossRef]
- Grenadyorov, A.; Oskirko, V.; Zakharov, A.; Oskomov, K.; Rabotkin, S.; Semenov, V.; Solovyev, A.; Shmakov, A. Properties of TiAlN coatings obtained by Dual-HiPIMS with short pulses. Materials 2023, 16, 1348. [Google Scholar] [CrossRef]
- Prajapati, J.; Bhatt, P.M.; Patel, N.S. Force and wear analysis of PVD coated cutting tool—A review. Int. J. Adv. Res. Innov. Ideas Educ. 2015, 1, 9–16. [Google Scholar]
- Chang, Y.Y.; Lai, H.M. Wear behavior and cutting performance of CrAlSiN and TiAlSiN hard coatings on cemented carbide cutting tools for Ti alloys. Surf. Coat. Technol. 2014, 259, 152–158. [Google Scholar] [CrossRef]
- Hagarová, M.; Jakubéczyová, D.; Baranová, G.; Eliáš, M. Adhesion determination of thin wear resistant coatings. Mater. Sci. Forum 2019, 952, 107–113. [Google Scholar] [CrossRef]
- Estrada-Martínez, F.F.; Gómez-Vargas, O.A.; Vega-Moron, R.C.; Melo-Maximo, D.V. Adhesion and wear study on 316L steel coated with titanium nitride by PVD. J. Technol. Innov. 2022, 9, 9–25. [Google Scholar] [CrossRef]
- Fernandes, L.; Silva, F.J.G.; Alexandre, R. Study of TiAlN PVD coating on stamping dies used in tinplate food package production. Micromachines 2019, 10, 182. [Google Scholar] [CrossRef]
- Elmkhah, H.; Mahboubi, F.; Abdollah-Zadeh, A.; Rouhaghdam, A.R.S. A new approach to improve the surface properties of H13 steel for metal forming applications by applying the TiAlN multi-layer coating. J. Manuf. Process. 2018, 32, 873–877. [Google Scholar] [CrossRef]
- Parreira, P.; Magalhães, A.; Gonçalves, I.C.; Gomes, J.; Vidal, R.; Reis, C.A.; Leckband, D.E.; Martins, M.C.L. Effect of surface chemistry on bacterial adhesion, viability, and morphology. J. Biomed. Mater. Res. A 2011, 99, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Trubiani, O.; Piattelli, M.; Capogreco, M.; Murmura, G. Antibacterial activity of titanium nitride coating: A mini review. Ital. J. Anat. Embryol. 2023, 127, 73–76. [Google Scholar] [CrossRef]
- Cunliffe, D.; Smart, C.A.; Alexander, C.; Vulfson, E.N. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 1999, 65, 4995–5002. [Google Scholar] [CrossRef]
- Ji, M.-K.; Park, S.-W.; Lee, K.; Kang, I.-C.; Yun, K.-D.; Kim, H.-S.; Lim, H.-P. Evaluation of antibacterial activity and osteoblast-like cell viability of TiN, ZrN and (Ti1−xZrx)N coating on titanium. J. Adv. Prosthodont. 2015, 7, 166–171. [Google Scholar] [CrossRef]
- Größner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.; Briedigkeit, H.; Göbel, U.B.; Lange, K. Plaque formation on surface modified dental implants: An in vitro study. Clin. Oral Implant. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Izman, S.; Ismail, S.N.F.; Ayu, H.M.; Kob, C.G.C.; Daud, R.; Kadir, M.R.A. The Influence of Ultrasonic Vibration Frequency on the Properties of TiN Coated Biomedical Ti–13Zr–13Nb. Metals 2018, 8, 317. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Cao, Y.; Su, B.; Chinnaraj, S.; Jana, S.; Bowen, L.; Charlton, S.; Duan, P.; Jakubovics, N.S.; Chen, J. Nanostructured titanium surfaces exhibit recalcitrance towards Staphylococcus epidermidis biofilm formation. Sci. Rep. 2018, 8, 1071. [Google Scholar] [CrossRef] [PubMed]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and Interpretation of Bacterial Viability by Using the LIVE/DEAD BacLight Kit in Combination with Flow Cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, T.; Wei, Q.; Cai, H.; Cheng, Y.; Tian, Y.; Leng, H.; Wang, C.; Feng, S.; Liu, Z. Surface treatment of 3D printed porous Ti6Al4V implants by ultraviolet photofunctionalization for improved osseointegration. Bioact. Mater. 2021, 7, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Spiro, R.C.; Jain, H.; Falk, M.M. Effects of Titanium Implant Surface Topology on Bone Cell Attachment and Proliferation in vitro. Med. Devices 2022, 15, 103–119. [Google Scholar] [CrossRef]
- Anderson, S.; Affi, J.; Yuli, Y.; Niinomi, M.; Akahori, T.; Gunawarman. Improving the Corrosion Resistance of TNTZ in Hanks’ Solution after Thermomechanical Treatment. J. Hunan Univ. Nat. Sci. 2022, 49, 96–109. [Google Scholar] [CrossRef]
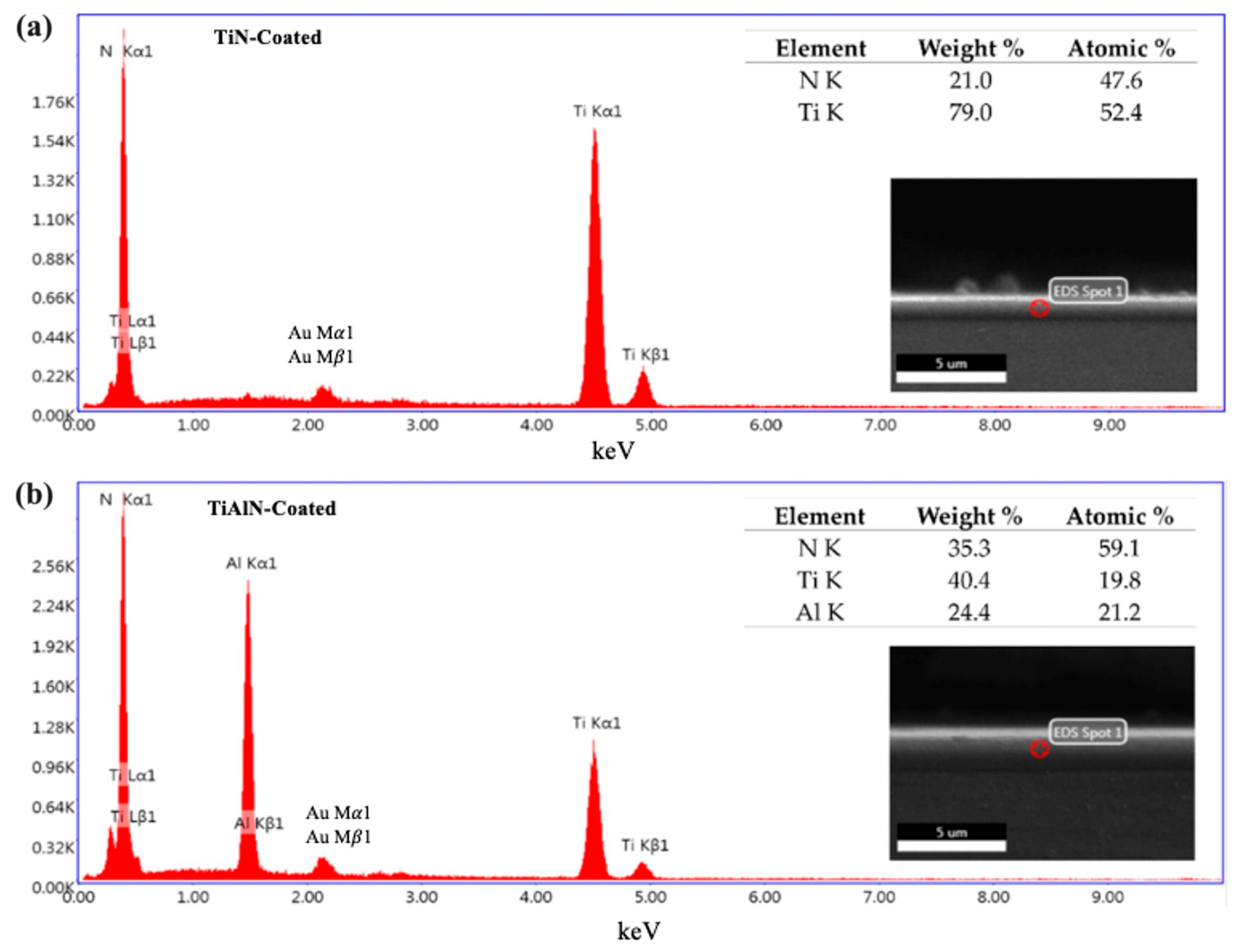
| Nb | Ta | Zr | Fe | C | N | O | H | Ti |
|---|---|---|---|---|---|---|---|---|
| 28.6 | 12.3 | 4.75 | 0.22 | 0.02 | 0.01 | 0.09 | 0.04 | Balance |
| Samples | 2θ | FHWM (Bobs) | FHWM (β) | Crystalite Size (D) |
|---|---|---|---|---|
| TiN (111) | 36.46 | ≈0.86° | ≈0.015 rad | 9.74 nm |
| TiAlN (200) (Figure 2) | 43.36° | ≈1.33° | ≈0.023 rad. | 6.39 nm |
| TiAlN (200) (Figure 3) | 43.36° | ≈0.82° | ≈0.014 rad. | 10.39 nm |
| AFM Topographical Parameters (nm) | Uncoated TNTZ | TiN Coated | TiAlN Coated |
|---|---|---|---|
| Arithmetic roughness (Ra) | 3.05 | 5.23 | 4.02 |
| Maximum height (Rz) | 29.73 | 52.50 | 44.16 |
| Ten-point mean roughness (Rzjis) | 14.82 | 25.28 | 21.88 |
| root-mean-squared roughness (Rq) | 3.96 | 6.58 | 5.29 |
| Maximum profile peak height (Rp) | 14.37 | 27.32 | 27.83 |
| Maximum profile valley depth (Rv) | 15.37 | 25.18 | 16.33 |
| Coating (Substrate) | Deposition Method | Coating Thickness (µm) | Hardness (HV) | Contact Angle (°) | Biological Properties | Refs. |
|---|---|---|---|---|---|---|
| TiN (TNTZ) | Arc-PVD | 1.06 ± 0.04 | 1059 ± 15 | 82 ± 2 | Inhibition zone: 18.02 mm (E. coli) | This study |
| TiAlN (TNTZ) | Arc-PVD | 1.73 ± 0.05 | 1468 ± 20 | 102 ± 2 | Inhibition zone: 19.09 mm (E. coli) | This study |
| TiN (Ti-13Nb-13Zr) | Arc-PVD | ~2 | ~1800 | ~68 | Increased osteoblast proliferation; reduced bacterial adhesion (S. aureus) | [28] |
| TiN (Ti-13Nb-13Zr) | ultrasonic vibrations assisted Arc-PVD. | ~1.58 to 1.86. | n.r. | ~75 | Improved corrosion properties | [74] |
| TiN, TiON and TiAlN (pure titanium) | DC magnetron sputter deposition | n.r. | n.r. | n.r. | TiAlN exhibited superior corrosion resistance, acceptable cytotoxicity | [75] |
| TiAlN (316L SS) | Arc-PVD | ~2 | 2500 | ~93 | Reduced bacterial adhesion; improved wear resistance | [24] |
| TiAlN (M2 steel) (AISI 321 steel) | Magnetron sputtering | 2–3 | 2300–2500 | ~90 | Maintained cell viability; reduced biofilm | [26] |
| TiN and Ti2N, (TNTZ) | Gas nitriding | A few | Over 600 | n.r. | Better cell viability | [59] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmazer, H. Physical Vapor Deposited TiN and TiAlN on Biomedical β-Type Ti-29Nb-13Ta-4.6Zr: Microstructural Characteristics, Surface Hardness Enhancement, and Antibacterial Activity. Coatings 2025, 15, 1126. https://doi.org/10.3390/coatings15101126
Yilmazer H. Physical Vapor Deposited TiN and TiAlN on Biomedical β-Type Ti-29Nb-13Ta-4.6Zr: Microstructural Characteristics, Surface Hardness Enhancement, and Antibacterial Activity. Coatings. 2025; 15(10):1126. https://doi.org/10.3390/coatings15101126
Chicago/Turabian StyleYilmazer, Hakan. 2025. "Physical Vapor Deposited TiN and TiAlN on Biomedical β-Type Ti-29Nb-13Ta-4.6Zr: Microstructural Characteristics, Surface Hardness Enhancement, and Antibacterial Activity" Coatings 15, no. 10: 1126. https://doi.org/10.3390/coatings15101126
APA StyleYilmazer, H. (2025). Physical Vapor Deposited TiN and TiAlN on Biomedical β-Type Ti-29Nb-13Ta-4.6Zr: Microstructural Characteristics, Surface Hardness Enhancement, and Antibacterial Activity. Coatings, 15(10), 1126. https://doi.org/10.3390/coatings15101126







