Physicochemical, Microstructural and Biological Evaluation of Dressing Materials Made of Chitosan with Different Molecular Weights
Abstract
1. Introduction
2. Materials and Methods
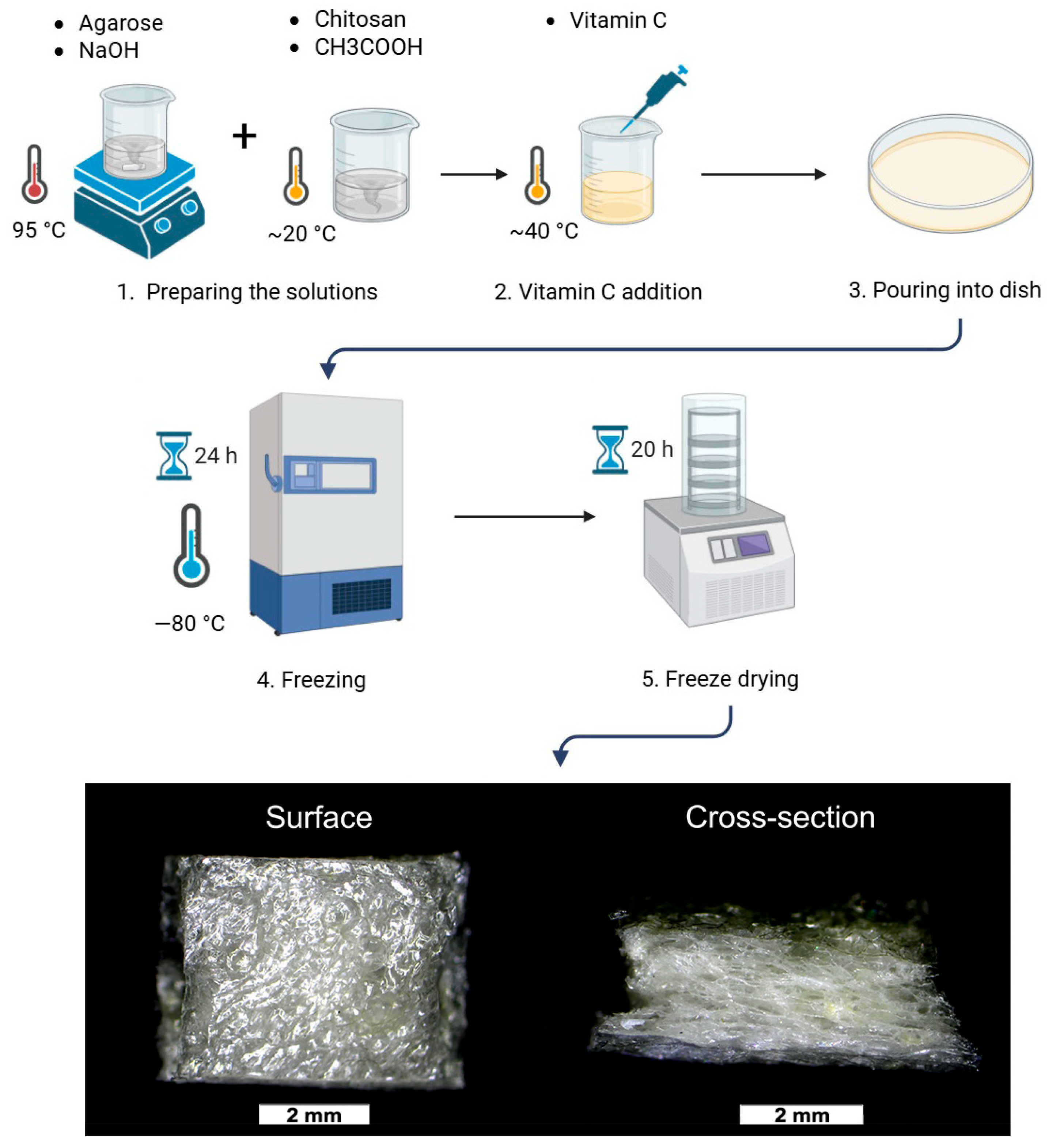
2.1. Preparation of the Foams
2.2. Cell Culture Tests
2.2.1. Cytotoxicity Evaluation
2.2.2. Live/Dead Assay
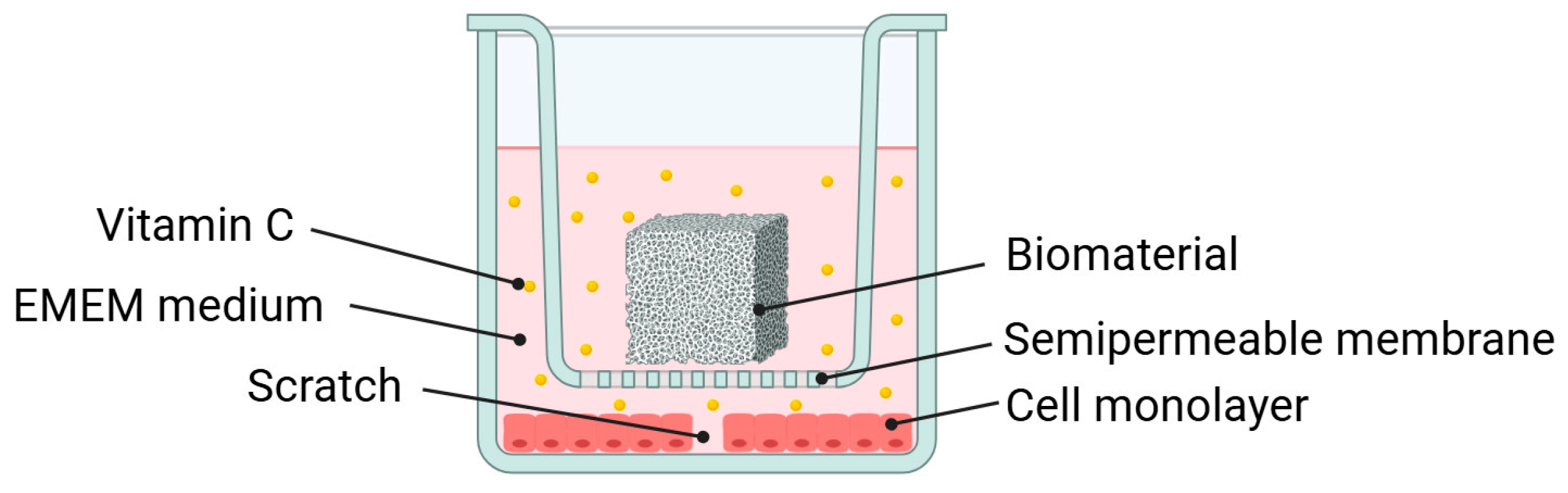
2.3. Wound Healing Assay
2.4. Surface Roughness Analysis
2.5. Wettability Evaluation
2.6. Mechanical Properties Analysis
2.7. Absorption Capacity Test
2.8. Vitamin C Release
2.9. Statistical Analyses
3. Results and Discussion
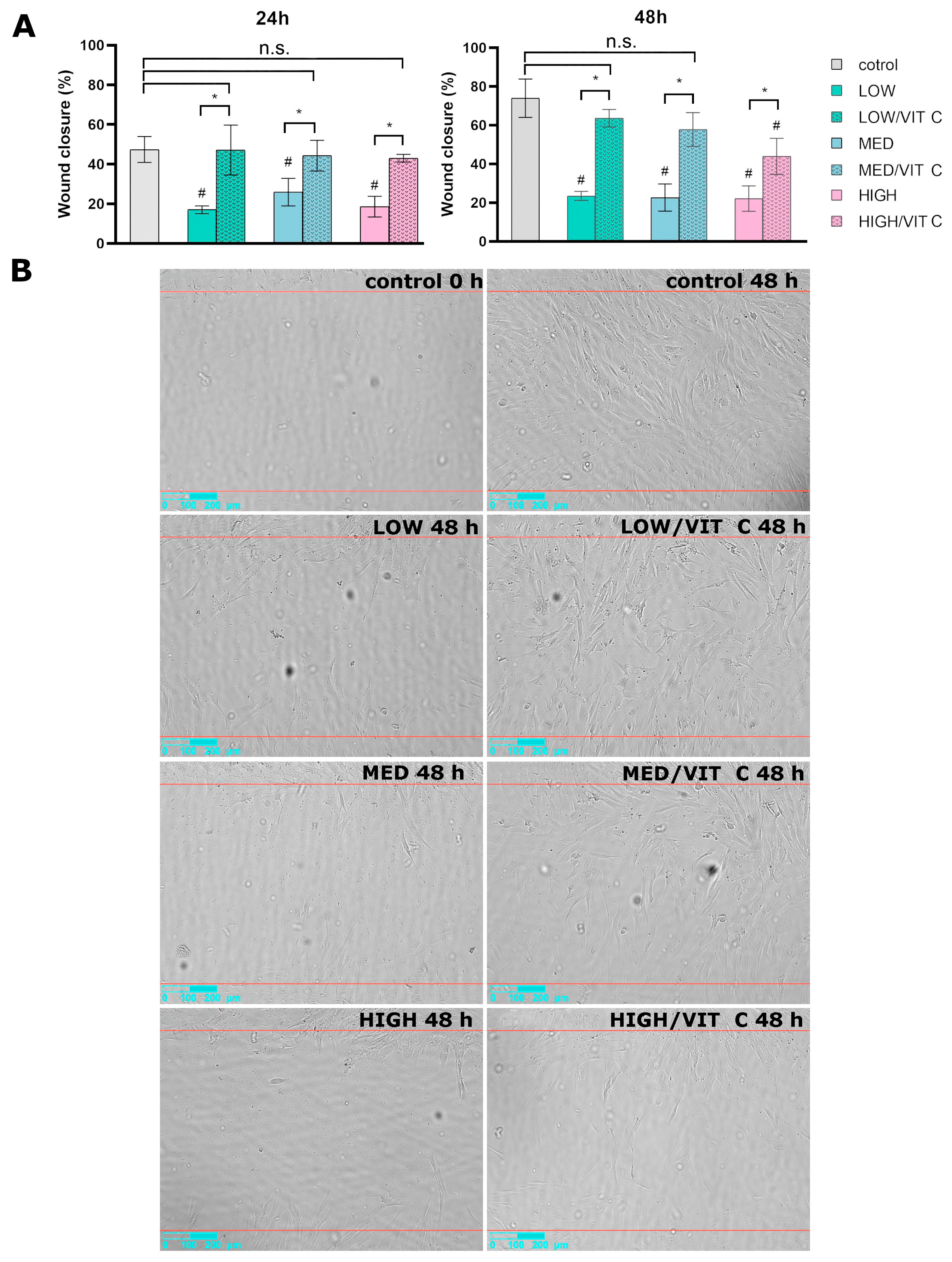
3.1. Cell Culture Tests
3.2. Surface Roughness Analysis
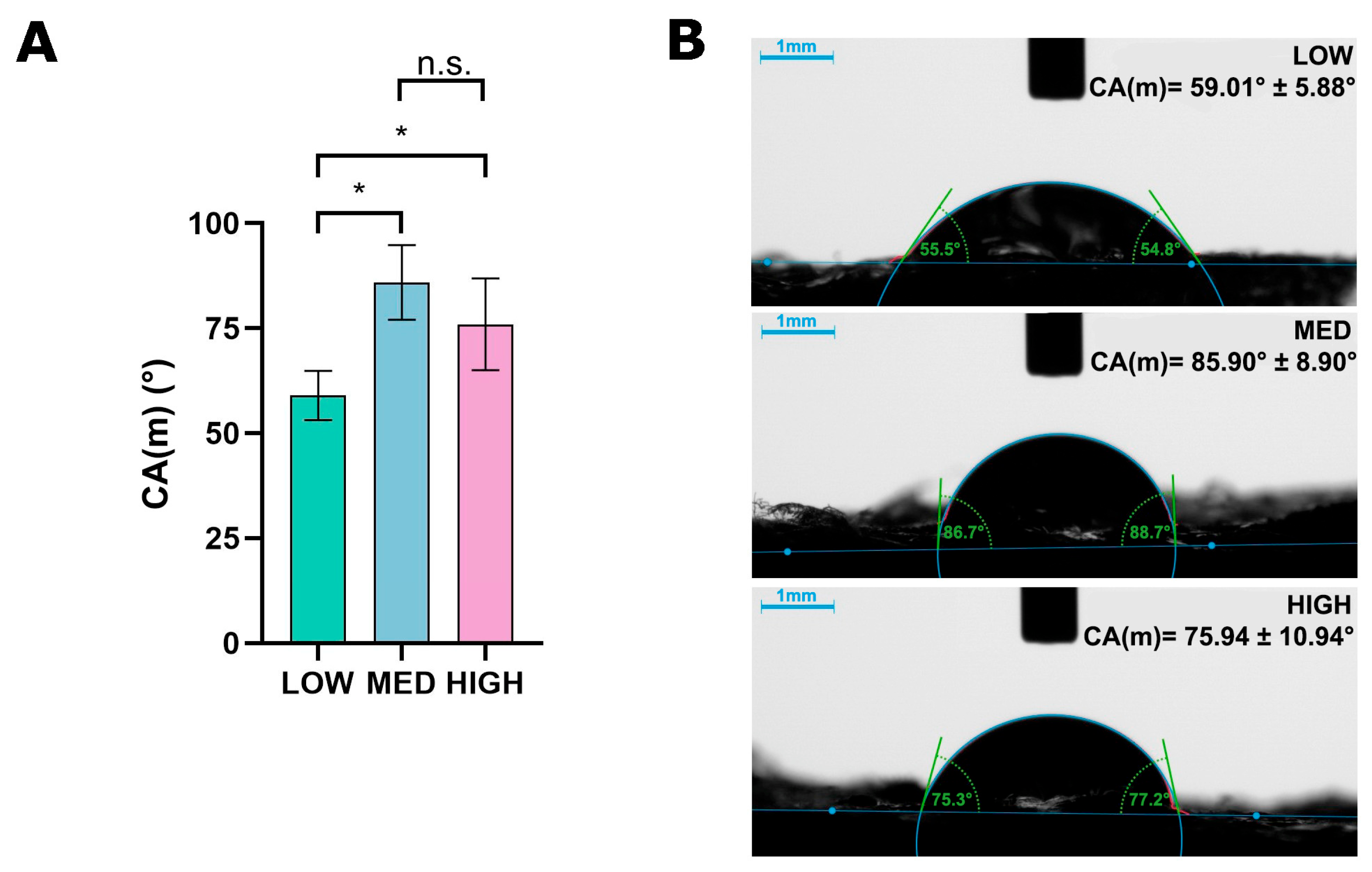
3.3. Wettability Evaluation
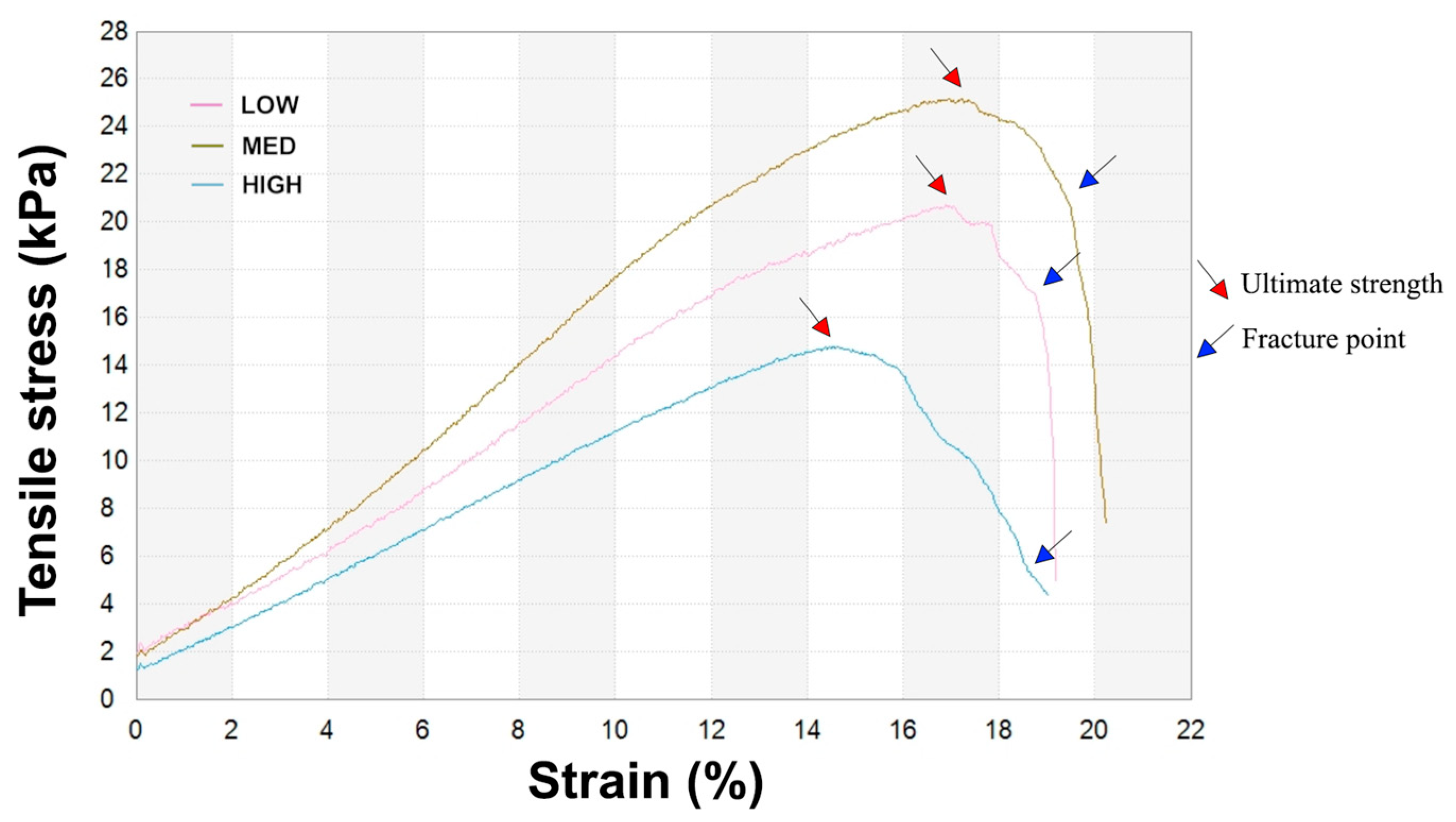
3.4. Mechanical Test
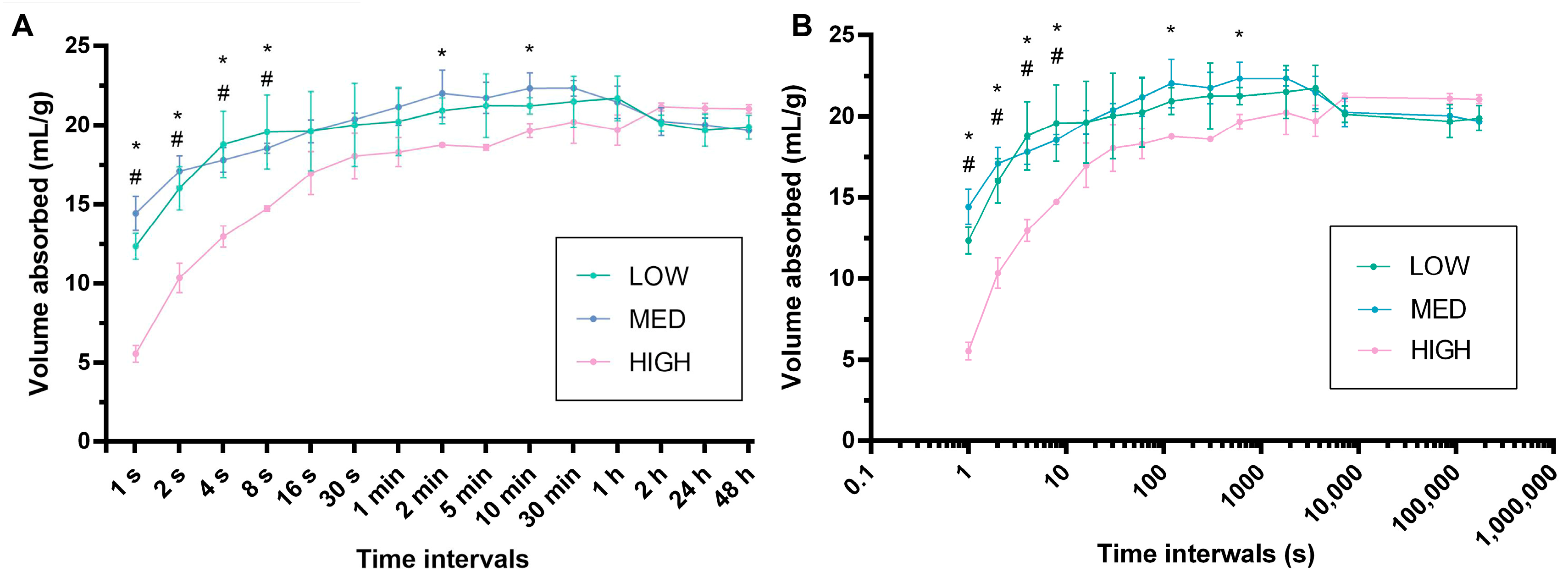
3.5. Absorption Capacity Evaluation
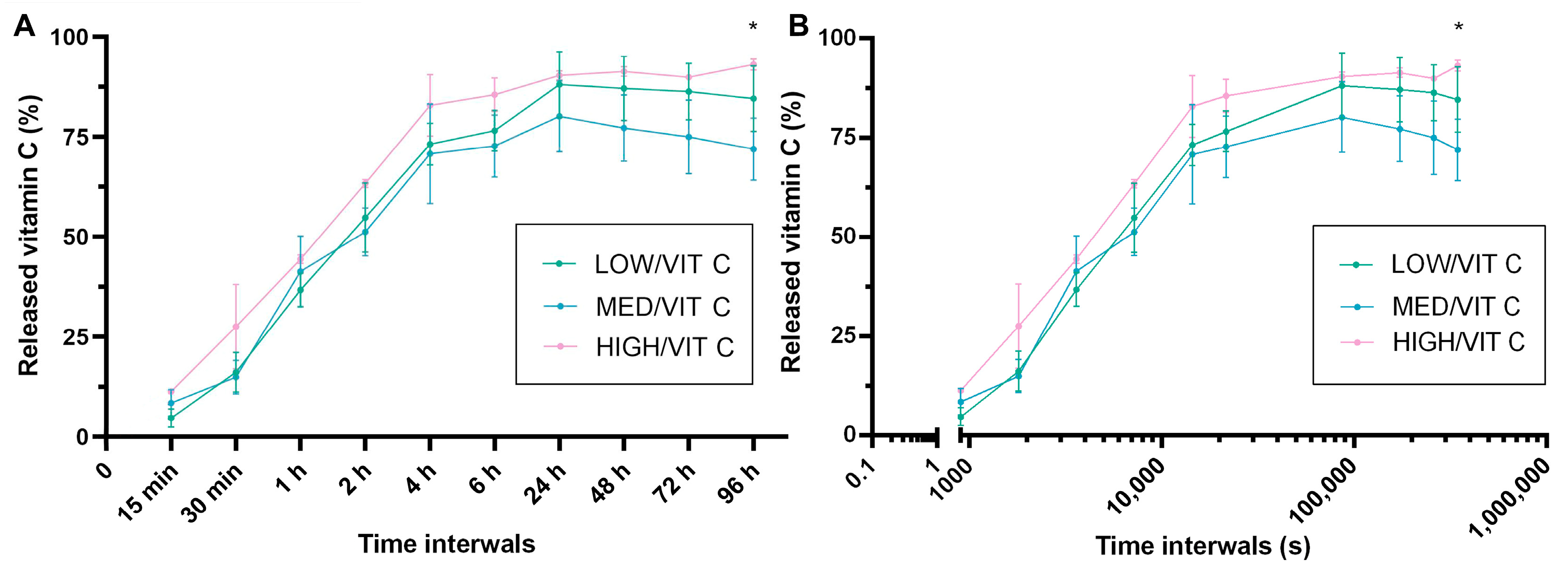
3.6. Vitamin C Release Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, Y.; Cui, C.; Zhang, F.; Su, T.; Guo, K.; Chen, T. Clinical efficacy of wet dressing combined with chitosan wound dressing in the treatment of deep second-degree burn wounds: A prospective, randomised, single-blind, positive control clinical trial. Int. Wound J. 2023, 20, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Stricker-Krongrad, A.-H.; Alikhassy, Z.; Matsangos, N.; Sebastian, R.; Marti, G.; Lay, F.; Harmon, J.W. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. Eplasty 2018, 18, e14. [Google Scholar]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan use in dentistry: A systematic review of recent clinical studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, H. Clinical efficacy of chitosan-based hydrocolloid dressing in the treatment of chronic refractory wounds. Int. Wound J. 2022, 19, 2012–2018. [Google Scholar] [CrossRef]
- Mo, X.; Cen, J.; Gibson, E.; Wang, R.; Percival, S.L. An open multicenter comparative randomized clinical study on chitosan. Wound Repair Regen. 2015, 23, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Unnithan, A.R.; Ghavami Nejad, A.; Sasikala, A.R.K.; Thomas, R.G.; Jeong, Y.Y.; Murugesan, P.; Nasseri, S.; Wu, D.; Park, C.H.; Kim, C.S. Electrospun zwitterionic nanofibers with in situ decelerated epithelialization property for non-adherent and easy removable wound dressing application. Chem. Eng. J. 2016, 287, 640–648. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Shen, Z.; Chen, H. Marine polysaccharides for wound dressings application: An overview. Pharmaceutics 2021, 13, 1666. [Google Scholar] [CrossRef]
- Kumar, A.; Agrawal, A.; Mavely, L.; Devadasu, V. Innovations in Advanced Wound Care from India. Int. J. Health Technol. Innov. 2023, 2, 9–14. [Google Scholar] [CrossRef]
- Davidson, E.; Pereira, J.; Gan Giannelli, G.; Murphy, Z.; Anagnostopoulos, V.; Santra, S. Multi-Functional Chitosan Nanovesicles Loaded with Bioactive Manganese for Potential Wound Healing Applications. Molecules 2023, 28, 6098. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E. Chitosan and trimethyl chitosan (TMC) as drug absorption enhancers: Moving forward toward clinical acceptance. In Encyclopedia of Marine Biotechnology; Wiley: Hoboken, NJ, USA, 2020; pp. 2773–2795. [Google Scholar] [CrossRef]
- Wiegand, C.; Winter, D.; Hipler, U.C. Molecular-weight-dependent toxic effects of chitosans on the human keratinocyte cell line HaCaT. Skin Pharmacol. Physiol. 2010, 23, 164–170. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-based hydrogel wound dressing: From mechanism to applications, a review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef] [PubMed]
- Guariento, S.C.F.; Rodrigues, L.I.; Basilio, L.B.B.; Freitas, L.D.; de Oliveira, R.J.; Cardoso, M.A.; Pereira, M.L.S.; Passos, M.F.; Santos, T.M.; da Cruz Ferraz Dutra, J.; et al. Systematic review on chitosan dressings for diabetic and burn wound healing: Preclinical outcomes and limitations. Acad. Mater. Sci. 2025, 2, 1–26. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; Guedes, G.M.d.M.; da Silva, M.L.Q.; Castelo-Branco, D.S.C.M.; Sidrim, J.J.C.; Cordeiro, R.d.A.; Rocha, M.F.G.; Vieira, R.S.; Brilhante, R.S.N. Effect of the molecular weight of chitosan on its antifungal activity against Candida spp. in planktonic cells and biofilm. Carbohydr. Polym. 2018, 195, 662–669. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Benko, A.; Palka, K.; Wojcik, M.; Przekora, A. Elastic and biodegradable chitosan/agarose film revealing slightly acidic pH for potential applications in regenerative medicine as artificial skin graft. Int. J. Biol. Macromol. 2020, 164, 172–183. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/75769.html (accessed on 10 July 2023).
- Mohammadi, P.; Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Foong, S.Y.; Yusoff, M.A.; Shahbeik, H.; Abdul Razak, N.N.; Lam, S.S.; Sheikh Ahmad Tajuddin, S.A.F.; Aghbashlo, M.; et al. Comparative environmental impact of chitosan extraction methods from shrimp waste. J. Environ. Chem. Eng. 2025, 13, 115124. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly Porous and Superabsorbent Biomaterial Made of Marine-Derived Polysaccharides and Ascorbic Acid as an Optimal Dressing for Exuding Wound Management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef]
- Weeks, B.S.; Fu, R.; Zaidi, M. Vitamin C Promotes Wound Healing: The Use of In Vitro Scratch Assays to Assess Re-Epithelialization; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Murad, S.; Grove, D.; Lindberg, K.A.; Reynolds, G.; Sivarajah, A.; Pinnell, S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA 1981, 78, 2879–2882. [Google Scholar] [CrossRef]
- Jurak, M.; Wiącek, A.E.; Ładniak, A.; Przykaza, K.; Szafran, K. What affects the biocompatibility of polymers? Adv. Colloid Interface Sci. 2021, 294, 102451. [Google Scholar] [CrossRef]
- Kayati, F.N.; Purnomo, C.W.; Kusumastuti, Y.; Rochmadi. The optimization of hydrogel strength from cassava starch using oxidized sucrose as a crosslinking agent. Green Process. Synth. 2023, 12, 2855–2879. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Huang, H.; Bakry, A.M.; Wu, W.; Liu, X.; Liu, F. Advances in enhancing the mechanical properties of biopolymer hydrogels via multi-strategic approaches. Int. J. Biol. Macromol. 2024, 272, 132583. [Google Scholar] [CrossRef]
- Hwang, K.T.; Kim, J.T.; Jung, S.T.; Cho, G.S.; Park, H.J. Properties of chitosan-based biopolymer films with various degrees of deacetylation and molecular weights. J. Appl. Polym. Sci. 2003, 89, 3476–3484. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Wahlsten, A.; Stracuzzi, A.; Lüchtefeld, I.; Restivo, G.; Lindenblatt, N.; Giampietro, C.; Ehret, A.E.; Mazza, E. Multiscale mechanical analysis of the elastic modulus of skin. Acta Biomater. 2023, 170, 155–168. [Google Scholar] [CrossRef]
- Kalra, A.; Lowe, A. An Overview of Factors Affecting the Skins Youngs Modulus. J. Aging Sci. 2016, 4, 1000156. [Google Scholar] [CrossRef]
- Ji, M.; Li, J.; Wang, Y.; Li, F.; Man, J.; Li, J.; Zhang, C.; Peng, S.; Wang, S. Advances in chitosan-based wound dressings: Modifications, fabrications, applications and prospects. Carbohydr. Polym. 2022, 297, 120058. [Google Scholar] [CrossRef] [PubMed]
- Adderley, U.J. Managing wound exudate and promoting healing. Br. J. Community Nurs. 2013, 15 (Suppl. 3), 15–20. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2020, 10, 685–698. [Google Scholar] [CrossRef]
- Fulton, J.A.; Blasiole, K.N.; Cottingham, T.; Tornero, M.; Graves, M.; Smith, L.G.; Mirza, S.; Mostow, E.N. Wound dressing absorption: A comparative study. Adv. Ski. Wound Care 2012, 25, 315–320. [Google Scholar] [CrossRef]
- Kumar, S.; Charitatos, V. Influence of surface roughness on droplet evaporation and absorption: Insights into experiments from lubrication-theory-based models. Langmuir 2022, 38, 15889–15904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; An, M.; Sun, Y.; Yu, Z.; Huang, H. Factors Influencing the Capillary Water Absorption Characteristics of Concrete and Their Relationship to Pore Structure. Appl. Sci. 2022, 12, 2211. [Google Scholar] [CrossRef]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. In Expert Opinion on Drug Delivery; Taylor and Francis Ltd.: Oxford, NY, USA, 2015; pp. 1895–1909. [Google Scholar] [CrossRef]


| Designation | Composition |
|---|---|
| LOW | 2% (w/v) agarose, 1.5% (w/v) low molecular weight chitosan |
| MED | 2% (w/v) agarose, 1.5% (w/v) medium molecular weight chitosan |
| HIGH | 2% (w/v) agarose, 1.5% (w/v) high molecular weight chitosan |
| LOW/VIT C | 2% (w/v) agarose, 1.5% (w/v) low molecular weight chitosan, vitamin C (200 µg per 1 mL of prepared homogeneous mass) |
| MED/VIT C | 2% (w/v) agarose, 1.5% (w/v) medium molecular weight chitosan, vitamin C (200 µg per 1 mL of prepared homogeneous mass) |
| HIGH/VIT C | 2% (w/v) agarose, 1.5% (w/v) high molecular weight chitosan, vitamin C (200 µg per 1 mL of prepared homogeneous mass) |
| Sample | Young’s Modulus (kPa) | Ultimate Tensile Strength (UTS) (kPa) | Elongation at Break (%) |
|---|---|---|---|
| LOW | 105.4 ± 10.6 | 20.3 ± 1.2 | 20.9 ± 2.4 |
| MED | 128.1 ± 21.3 * | 24.1 ± 4.0 * | 19.6 ± 0.8 |
| HIGH | 87.4 ± 7.2 | 15.4 ± 1.2 | 23.6 ± 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płonkowska, Z.; Wójcik, A.; Vivcharenko, V. Physicochemical, Microstructural and Biological Evaluation of Dressing Materials Made of Chitosan with Different Molecular Weights. Coatings 2025, 15, 1116. https://doi.org/10.3390/coatings15101116
Płonkowska Z, Wójcik A, Vivcharenko V. Physicochemical, Microstructural and Biological Evaluation of Dressing Materials Made of Chitosan with Different Molecular Weights. Coatings. 2025; 15(10):1116. https://doi.org/10.3390/coatings15101116
Chicago/Turabian StylePłonkowska, Zofia, Alicja Wójcik, and Vladyslav Vivcharenko. 2025. "Physicochemical, Microstructural and Biological Evaluation of Dressing Materials Made of Chitosan with Different Molecular Weights" Coatings 15, no. 10: 1116. https://doi.org/10.3390/coatings15101116
APA StylePłonkowska, Z., Wójcik, A., & Vivcharenko, V. (2025). Physicochemical, Microstructural and Biological Evaluation of Dressing Materials Made of Chitosan with Different Molecular Weights. Coatings, 15(10), 1116. https://doi.org/10.3390/coatings15101116






