Developments in Dental Implant Surface Modification
Abstract
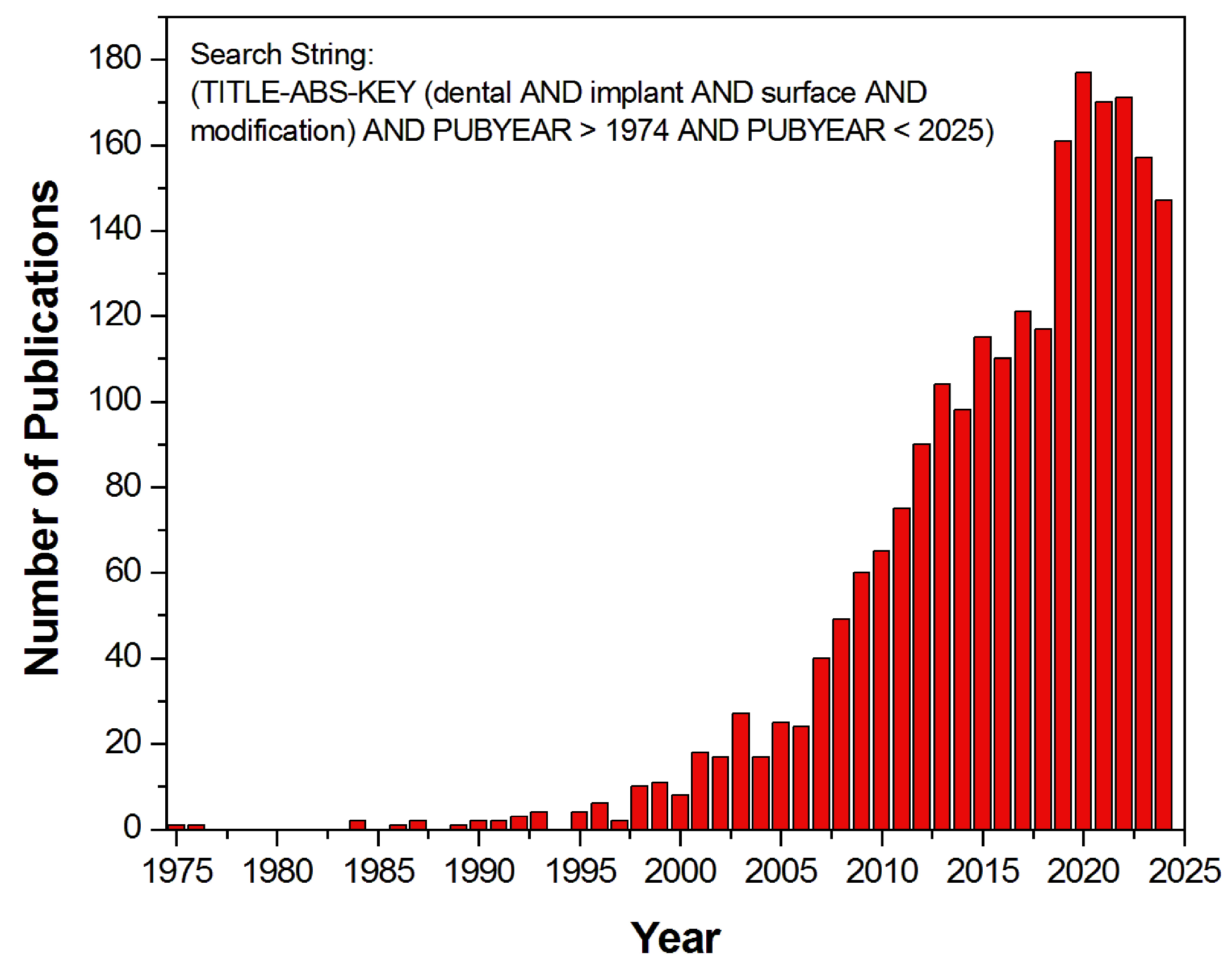
1. Introduction
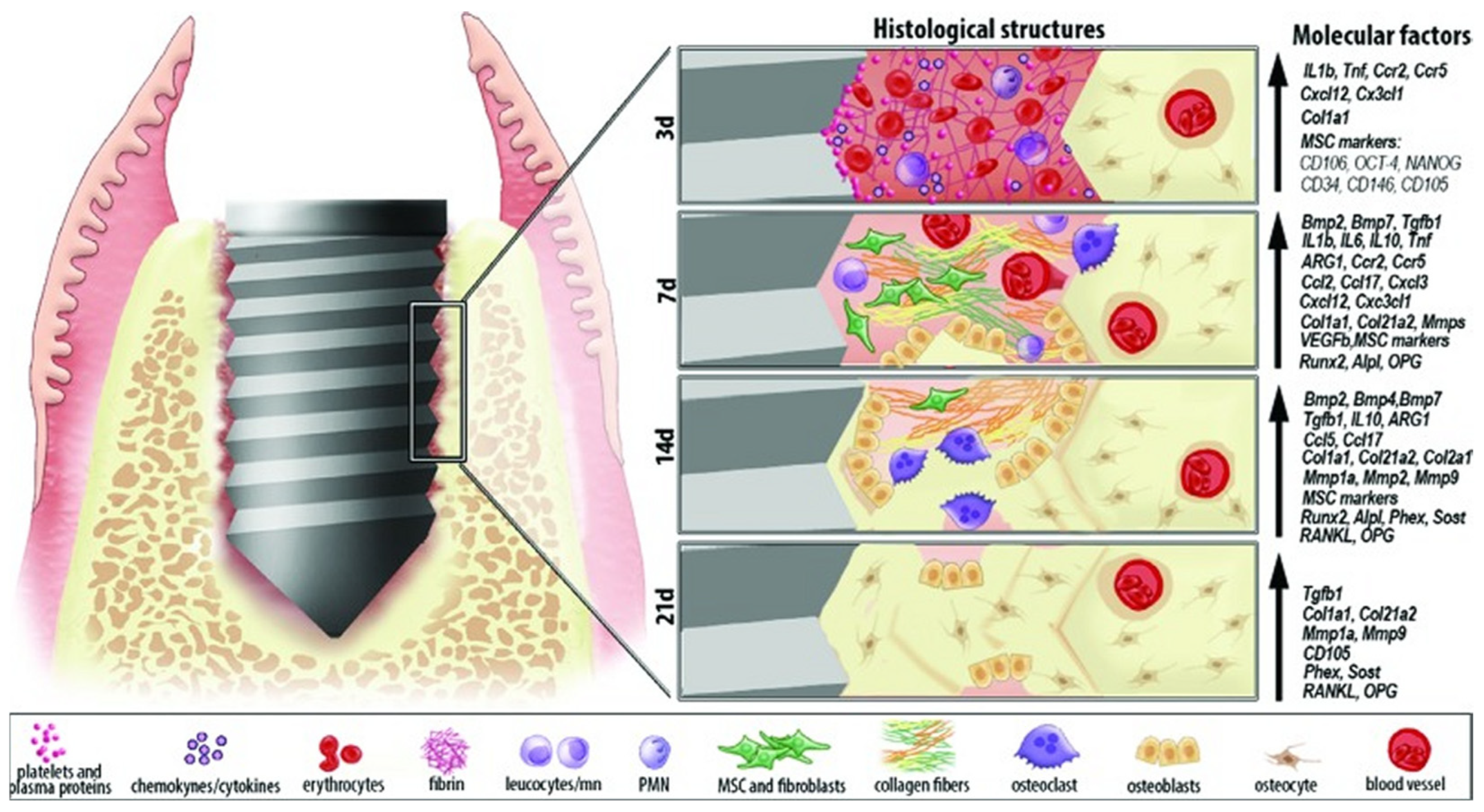
2. Mechanism of the Osseointegration Process
3. Types of Dental Implant Surface
3.1. Machined Surface
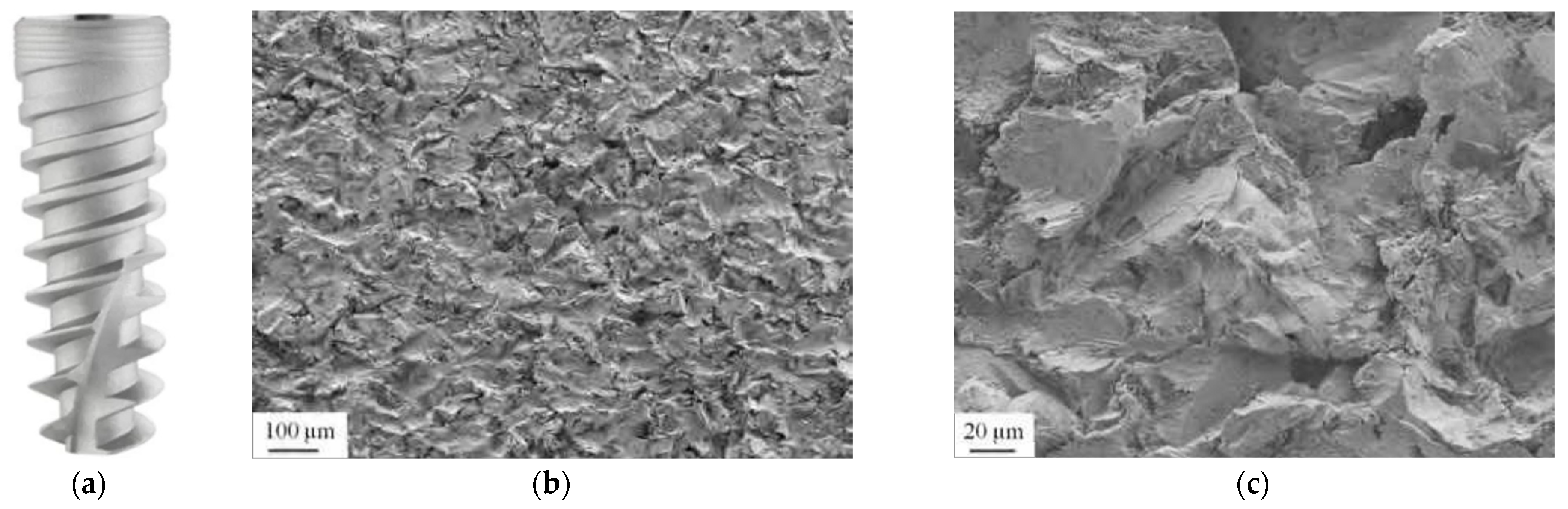
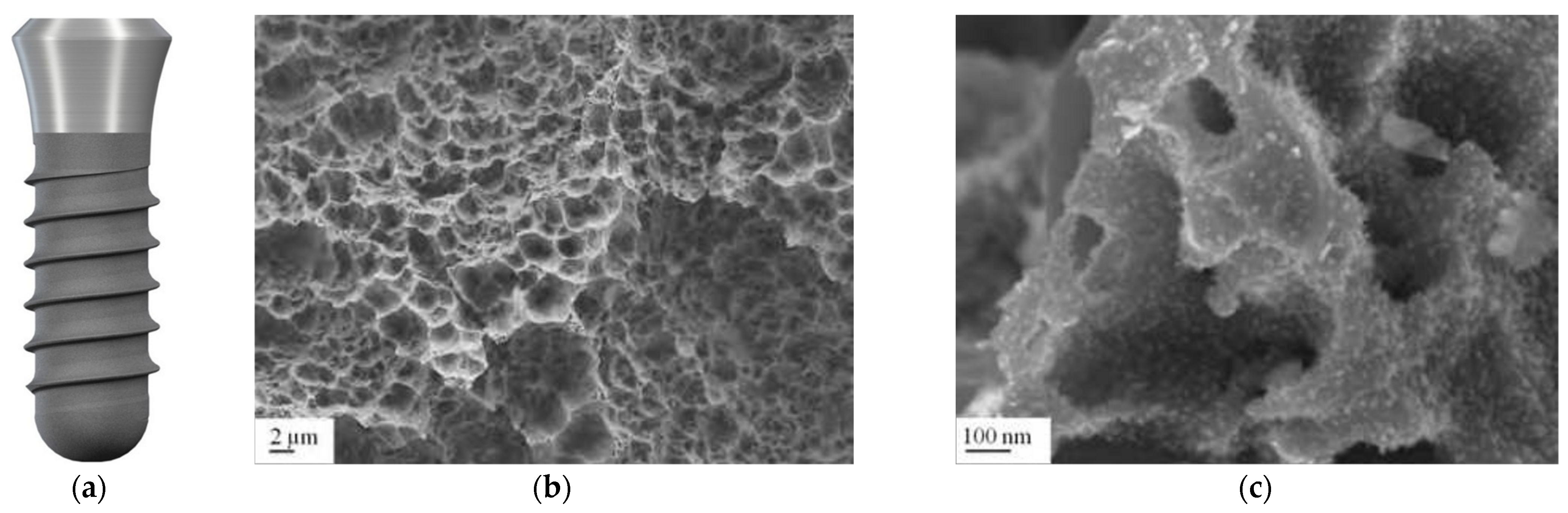
3.2. Titanium Plasma Surface
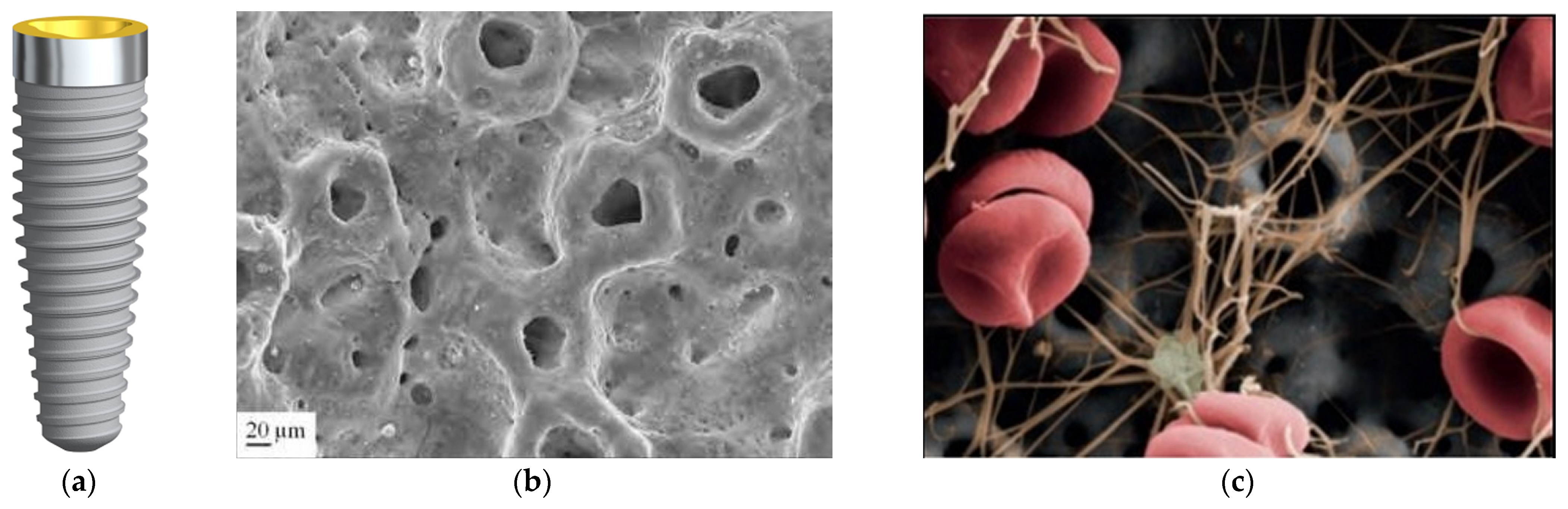
3.3. Sandblasted Surface
3.4. Hydroxyapatite Surface
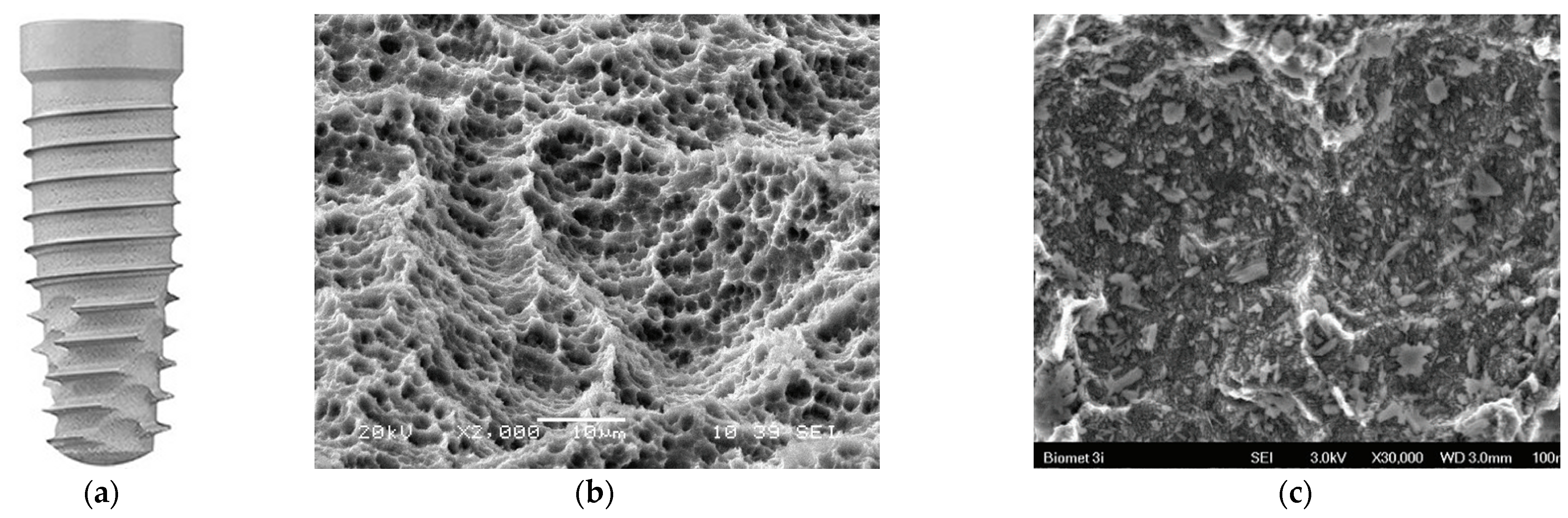
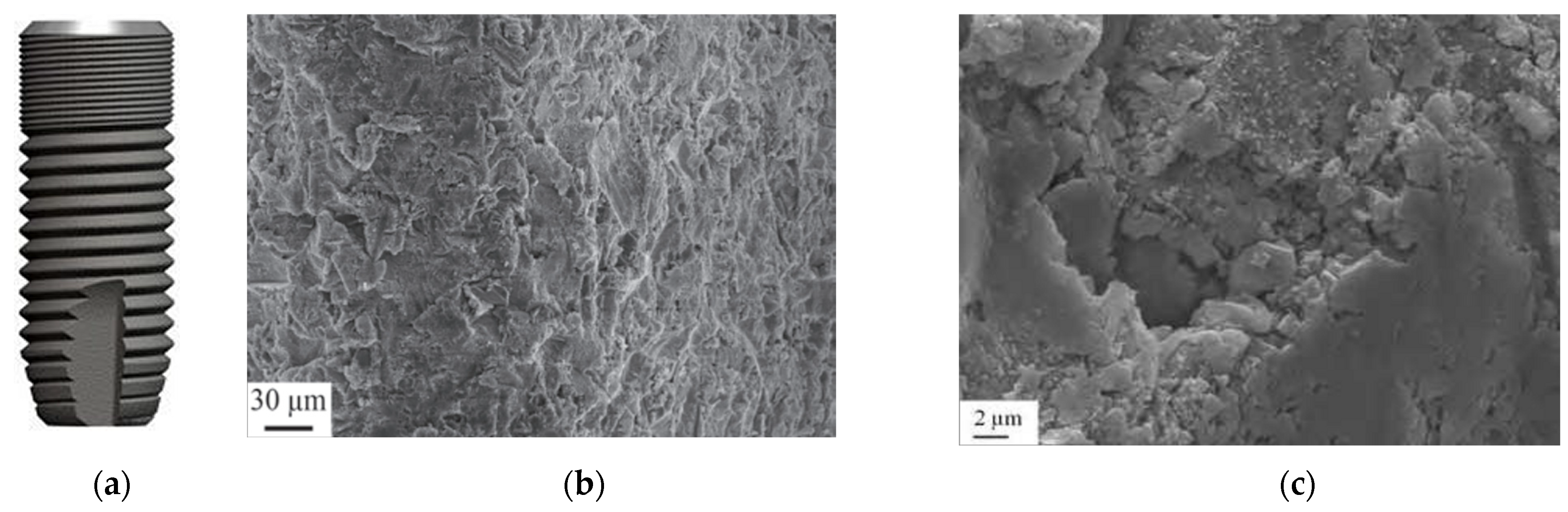
3.5. Double-Etched Surface
3.6. Sandblasted and Etched Surface
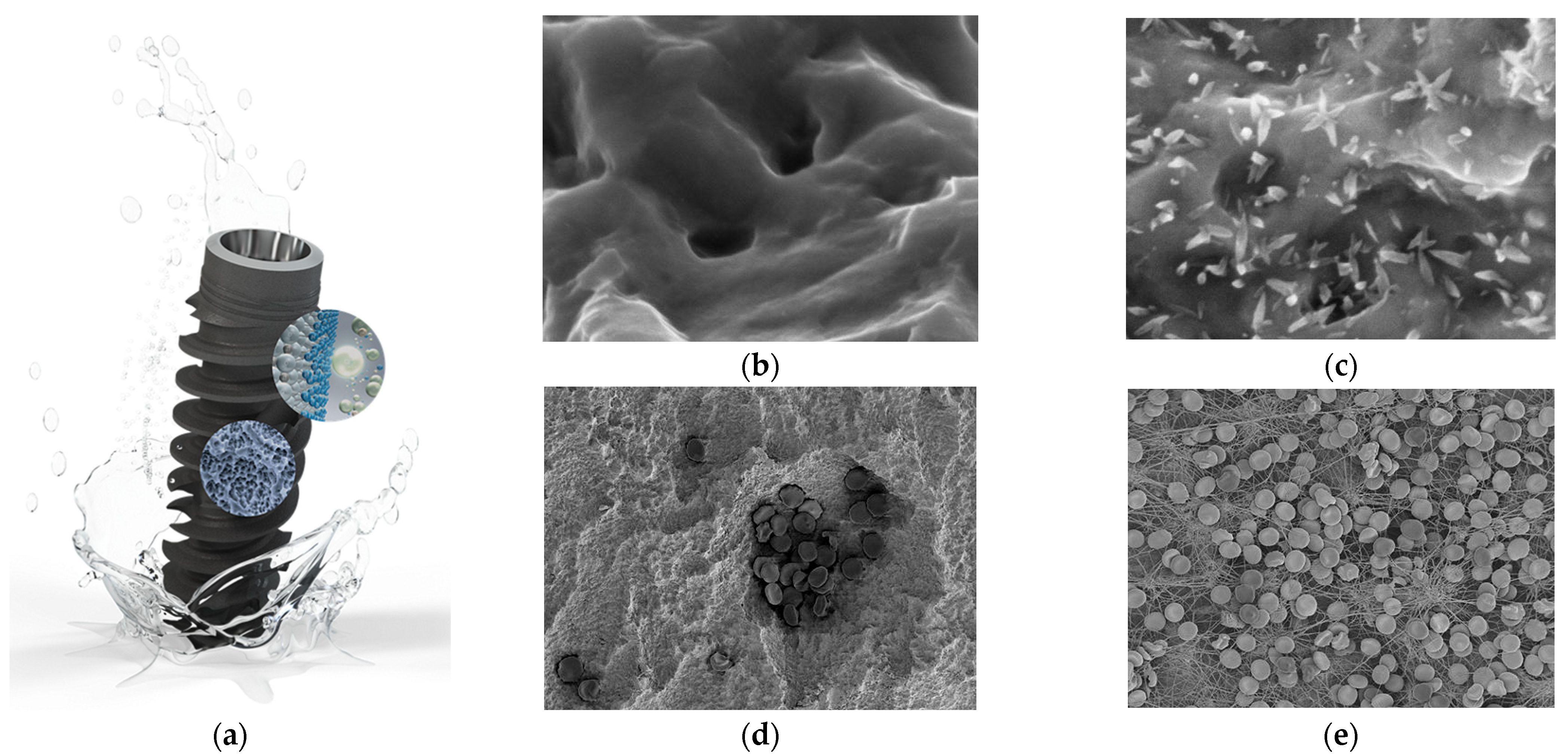
3.7. Hydrophilic Surface
3.8. Oxidized Surface
3.9. Biologically Active Surface
3.10. Hybrid Surface
3.11. Laser-Structured Surface
4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waghmare, G.; Waghmare, K.; Bagde, S.; Deshmukh, M.; Kashyap, D.N.; Shahu, V.T. Materials Evolution in Dental Implantology: A Comprehensive Review. J. Adv. Res. Appl. Mech. 2024, 123, 75–100. [Google Scholar] [CrossRef]
- Lanis, A.; Peña-Cardelles, J.F.; Negreiros, W.M.; Hamilton, A.; Gallucci, G.O. Impact of digital technologies on implant surgery in fully edentulous patients: A scoping review. Clin. Oral Implants Res. 2024, 35, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Shivgotra, R.; Soni, B.; Kaur, M.; Thakur, S. Advancement in Biomaterials in the Form of Implants. In Engineered Biomaterials. Engineering Materials; Malviya, R., Sundram, S., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Nitschke, I.; Krüger, K.; Jockusch, J. Age-related knowledge deficit and attitudes towards oral implants: Survey-based examination of the correlation between patient age and implant therapy awareness. BMC Oral Health 2024, 24, 403. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kim, B. The Effects of the Expansion of Dental Care Coverage for the Elderly. Healthcare 2024, 12, 1949. [Google Scholar] [CrossRef]
- Kim, H.-J.; Sung, I.-Y. Analysis of Dental Prosthetic Treatment in Patients with Cancer Aged 65 Years and Older after Expanded Health Insurance Coverage: A Retrospective Clinical Study. Medicina 2024, 60, 1509. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Ortega-Martínez, J.; Salomó-Coll, O.; López-Boado, A.P.; Paternostro-Betancourt, D.; Hernández-Alfaro, F. Mouth opening limitation and influence of age and surgical location for static fully guided dental implant placement: An observational, cross-sectional clinical study. Int. J. Oral Max. Surg. 2024, 53, 526–532. [Google Scholar] [CrossRef]
- Dave, M.; Tattar, R.; Patel, N. Medical considerations in the ageing implant patient. Oral Surgery 2024, 17, 59–66. [Google Scholar] [CrossRef]
- Karlsson, K.; Derks, J.; Wennström, J.L.; Petzold, M.; Berglundh, T. Health economic aspects of implant-supported restorative therapy. Clin Oral Implants Res. 2022, 33, 221–230. [Google Scholar] [CrossRef]
- Brägger, U.; Krenander, P.; Lang, N.P. Economic aspects of single-tooth replacement. Clin Oral Implants Res. 2005, 16, 335–341. [Google Scholar] [CrossRef]
- Massa, L.O.; Fraunhofer, J.A. Economics of Dental Implants. In The ADA Practical Guide to Dental Implants; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 139–143. [Google Scholar] [CrossRef]
- Losenická, J.; Gajdoš, O.; Kamenský, V. Cost-utility analysis of an implant treatment in dentistry. BMC Oral Health 2021, 21, 433. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Li, S.; Cai, Y.J.; Wei, T.; Ye, P. Smoking in relation to early dental implant failure: A systematic review and meta-analysis. J. Dent. 2024, 151, 105396. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Ofec, R.; Levin, L. Long term clinical performance of 10 871 dental implants with up to 22 years of follow-up: A cohort study in 4247 patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Dental Implants Market Growth & Trends. Available online: https://www.grandviewresearch.com/press-release/global-dental-implants-market (accessed on 28 October 2024).
- O’ Dwyer, S.; Riordain, R.N. The patient experience of dental implant surgery: A literature review of pertinent qualitative studies. Ir. J. Med. Sci. 2021, 190, 835–842. [Google Scholar] [CrossRef]
- Chakaipa, S.; Prior, S.J.; Pearson, S.; van Dam, P.J. Improving Patient Experience through Meaningful Engagement: The Oral Health Patient’s Journey. Oral 2023, 3, 499–510. [Google Scholar] [CrossRef]
- Improve competitive advantage, job satisfaction and the patient experience. Br. Dent. J. 2022, 232, 347. [CrossRef]
- Shrivastava, R.; Luxenberg, R.; Sutton, E.; Emami, E. Patients experience and satisfaction with immediate loading of implant-supported overdentures—A qualitative study. J. Dent. 2023, 137, 104644. [Google Scholar] [CrossRef]
- Stróż, A.; Dercz, G.; Chmiela, B.; Stróż, D.; Łosiewicz, B. Electrochemical Formation of Second Generation TiO2 Nanotubes on Ti13Nb13Zr Alloy for Biomedical Applications. Acta Phys. Pol. A 2016, 130, 1079–1080. [Google Scholar] [CrossRef]
- Smołka, A.; Dercz, G.; Rodak, K.; Łosiewicz, B. Evaluation of corrosion resistance of nanotubular oxide layers on the Ti13Zr13Nb alloy in physiological saline solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Smołka, A.; Rodak, K.; Dercz, G.; Dudek, K.; Łosiewicz, B. Electrochemical Formation of Self-Organized Nanotubular Oxide Layers on Ti13Zr13Nb Alloy for Biomedical Applications. Acta Phys. Pol. A 2014, 125, 932–935. [Google Scholar] [CrossRef]
- Szklarska, M.; Dercz, G.; Rak, J.; Simka, W.; Łosiewicz, B. The influence of passivation type on corrosion resistance of Ti15Mo alloy in simulated body fluids. Arch. Metall. Mater. 2015, 60, 2687–2693. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Stróż, A.; Kubisztal, J.; Osak, P.; Zubko, M. EIS and LEIS Study on In Vitro Corrosion Resistance of Anodic Oxide Nanotubes on Ti–13Zr–13Nb Alloy in Saline Solution. Coatings 2023, 13, 875. [Google Scholar] [CrossRef]
- Hosseini-Faradonbeh, S.A.; Katoozian, H.R. Biomechanical evaluations of the long-term stability of dental implant using finite element modeling method: A systematic review. J. Adv. Prosthodont. 2022, 14, 182–202. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhou, Y.J.; Jiang, Y.B.; Tam, M.S.; Cheang, L.H.; Wang, H.J.; Zha, Z.G.; Zheng, X.F. Effect of Diabetes Mellitus on Implant Osseointegration of Titanium Screws: An Animal Experimental Study. Orthop Surg. 2022, 14, 1217–1228. [Google Scholar] [CrossRef]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Ratajczak, P.; Łosiewicz, B. Long-Term Assessment of the In Vitro Corrosion Resistance of Biomimetic ACP Coatings Electrodeposited from an Acetate Bath. J. Funct. Biomater. 2021, 12, 12. [Google Scholar] [CrossRef]
- Shayeb, M.A.; Elfadil, S.; Abutayyem, H.; Shqaidef, A.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Bioactive surface modifications on dental implants: A systematic review and meta-analysis of osseointegration and longevity. Clin. Oral Investig. 2024, 28, 592. [Google Scholar] [CrossRef]
- Canullo, L.; Menini, M.; Pesce, P.; Iacono, R.; Sculean, A.; Del Fabbro, M. Nano-superhydrophilic and bioactive surface in poor bone environment. Part 1: Transition from primary to secondary stability. A controlled clinical trial. Clin. Oral Investig. 2024, 28, 372. [Google Scholar] [CrossRef]
- Meng, H.W.; Chien, E.Y.; Chien, H.H. Dental implant bioactive surface modifications and their effects on osseointegration: A review. Biomark. Res. 2016, 4, 24. [Google Scholar] [CrossRef]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Macedo de Sousa, B.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Katić, J.; Šarić, A.; Despotović, I.; Matijaković, N.; Petković, M.; Petrović, Ž. Bioactive Coating on Titanium Dental Implants for Improved Anticorrosion Protection: A Combined Experimental and Theoretical Study. Coatings 2019, 9, 612. [Google Scholar] [CrossRef]
- Munisamy, S.; Vaidyanathan, T.K.; Vaidyanathan, J. A bone-like precoating strategy for implants: Collagen immobilization and mineralization on pure titanium implant surface. J. Oral Implantol. 2008, 34, 67–75. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.C.S.; Valverde, T.M.; Martins, T.M.d.M.; Oliveira, F.d.P.; Cunha, P.d.S.; Tavares, M.A.B.; Rodrigues, E.M.; Albergaria, J.D.S.; Vieira, G.M.; Gomes, D.A.; et al. Enhanced osteogenic response by collagen type I coating on surface-modified titanium bone implants. Mater. Today Commun. 2024, 39, 108535. [Google Scholar] [CrossRef]
- Belloni, A.; Argentieri, G.; Orilisi, G.; Notarstefano, V.; Giorgini, E.; D’Addazio, G.; Orsini, G.; Caputi, S.; Sinjari, B. New insights on collagen structural organization and spatial distribution around dental implants: A comparison between machined and laser-treated surfaces. J. Transl. Med. 2024, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Erturk, P.A.; Altuntas, S.; Irmak, G.; Buyukserin, F. Bioinspired Collagen/Gelatin Nanopillared Films as a Potential Implant Coating Material. ACS Appl. Bio Mater. 2022, 5, 4913–4921. [Google Scholar] [CrossRef]
- Petrović, Ž.; Šarić, A.; Despotović, I.; Katić, J.; Peter, R.; Petravić, M.; Ivanda, M.; Petković, M. Surface Functionalisation of Dental Implants with a Composite Coating of Alendronate and Hydrolysed Collagen: DFT and EIS Studies. Materials 2022, 15, 5127. [Google Scholar] [CrossRef]
- O’Neill, L.; Twomey, B.; Tan, F.; O’Donoghue, J.; Junt, J.A. Collagen Coating of Titanium Implants Using Non-thermal Plasma. Plasma Med. 2020, 11, 63–79. [Google Scholar] [CrossRef]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2024, 15, 15. [Google Scholar] [CrossRef]
- Lee, S.W.; Hahn, B.D.; Kang, T.Y.; Lee, M.J.; Choi, J.Y.; Kim, M.K.; Kim, S.G. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2-coated implants, hydroxyapatite only coated implants, and uncoated implants. J. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Kolarovszki, B.; Ficsor, S.; Frank, D.; Katona, K.; Soos, B.; Turzo, K. Unlocking the potential: Laser surface modifications for titanium dental implants. Lasers Med. Sci. 2024, 39, 162. [Google Scholar] [CrossRef]
- Saran, R.; Ginjupalli, K.; George, S.D.; Chidangil, S.; Unnikrishnan, V.K. LASER as a tool for surface modification of dental biomaterials: A review. Heliyon 2023, 9, e17457. [Google Scholar] [CrossRef]
- Santos, A.F.P.; da Silva, R.C.; Hadad, H.; de Jesus, L.K.; Pereira-Silva, M.; Nímia, H.H.; Oliveira, S.H.P.; Guastaldi, A.C.; Queiroz, T.P.; Poli, P.P.; et al. Early Peri-Implant Bone Healing on Laser-Modified Surfaces with and without Hydroxyapatite Coating: An In Vivo Study. Biology 2024, 13, 533. [Google Scholar] [CrossRef] [PubMed]
- The Use of Lasers for Dental Implant Surgery. Available online: https://www.deserthillsdental.com/dental-implants-and-laser-dentistry/ (accessed on 28 October 2024).
- Papa, S.; Maalouf, M.; Claudel, P.; Sedao, X.; Maio, Y.D.; Hamzeh-Cognasse, H.; Thomas, M.; Guignandon, A.; Dumas, V. Key topographic parameters driving surface adhesion of Porphyromonas gingivalis. Sci. Rep. 2023, 13, 15893. [Google Scholar] [CrossRef] [PubMed]
- Fenelon, T.; Bakr, M.; Walsh, L.J.; George, R. Effects of lasers on titanium dental implant surfaces: A narrative review. Laser Dent. Sci. 2022, 6, 153–167. [Google Scholar] [CrossRef]
- Luczak, W.; Reiner-Rozman, C.; Muck, M.; Heitz, J.; Mitov, G.; Pfaffeneder, F.; See, C.; Hassel, A.W.; Kleber, C. Laser Treatment of Dental Implants toward an Optimized Osseointegration: Evaluation via Tapping-Mode Atomic Force Microscopy and Scanning Electron Microscopy. Phys. Status Solidi Appl. Mater. Sci. 2023, 220, 2200605. [Google Scholar] [CrossRef]
- Alamoudi, A. Nanoengineering and Surface Modifications of Dental Implants. Cureus 2024, 16, e51526. [Google Scholar] [CrossRef]
- Gulati, K. Nano-Engineering Solutions for Dental Implant Applications. Nanomaterials 2022, 12, 272. [Google Scholar] [CrossRef]
- Nagamoto, K.; Nakanishi, K.; Akasaka, T.; Abe, S.; Yoshihara, K.; Nakamura, M.; Hayashi, H.; Takemoto, S.; Tamura, M.; Kitagawa, Y.; et al. Investigation of a new implant surface modification using phosphorylated pullulan. Front. Bioeng. Biotechnol. 2024, 12, 1378039. [Google Scholar] [CrossRef]
- Karthik, K.; Thangaswamy, V. Evaluation of implant success: A review of past and present concepts. J. Pharm. Bioallied. Sci. 2013, 5 (Suppl. S1), S117–S119. [Google Scholar] [CrossRef]
- Han, W.; Fang, S.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Cooper, L.F.; Shirazi, S. Osseointegration—The biological reality of successful dental implant therapy: A narrative review. Front. Oral Maxillofac. Med. 2022, 4, 39. [Google Scholar] [CrossRef]
- Lechner, J.; von Baehr, V.; Notter, F.; Schick, F. Osseointegration and osteoimmunology in implantology: Assessment of the immune sustainability of dental implants using advanced sonographic diagnostics: Research and case reports. J. Int. Med. Res. 2024, 52, 3000605231224161. [Google Scholar] [CrossRef] [PubMed]
- Simão, B.S., Jr.; Costa, D.D.; Cangussu, M.C.T.; Sotto-Maior, B.S.; Devita, R.L.; de Carvalho, J.J.; da Silva Brum, I. Observational Study on the Success Rate of Osseointegration: A Prospective Analysis of 15,483 Implants in a Public Health Setting. BioMed 2022, 2, 422–430. [Google Scholar] [CrossRef]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Zarb, G.A.; Albrekson, T. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Publishing Company: Batavia, IL, USA, 1985. [Google Scholar]
- Gill, T.; Kühl, S.; Rawlinson, S.; Pippenger, B.; Bellon, B.; Shahdad, S. Primary stability and osseointegration comparing a novel tapered design tissue-level implant with a parallel design tissue-level implant. An experimental in vivo study. Clin. Oral Implants Res. 2024, 35, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Lioubavina-Hack, N.; Lang, N.P.; Karring, T. Significance of primary stability for osseointegration of dental implants. Clin. Oral Implants Res. 2006, 17, 244–250. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef]
- Xu, L.; Jacobs, R.; Cao, Y.; Sun, X.; Qin, X. Tissue-engineered bone construct promotes early osseointegration of implants with low primary stability in oversized osteotomy. BMC Oral Health 2024, 24, 69. [Google Scholar] [CrossRef]
- Lee, J.; Lim, Y.-J.; Ahn, J.-S.; Kim, B.; Baek, Y.-W.; Lim, B.-S. Correlation of two different devices for the evaluation of primary implant stability depending on dental implant length and bone density: An in vitro study. PLoS ONE 2024, 19, e0290595. [Google Scholar] [CrossRef]
- Barikani, H.; Rashtak, S.; Akbari, S.; Badri, S.; Daneshparvar, N.; Rokn, A. The effect of implant length and diameter on the primary stability in different bone types. J. Dent. 2013, 10, 449–455. [Google Scholar]
- Stoilov, M.; Shafaghi, R.; Stark, H.; Marder, M.; Kraus, D.; Enkling, N. Influence of Implant Macro-Design, -Length, and -Diameter on Primary Implant Stability Depending on Different Bone Qualities Using Standard Drilling Protocols-An In Vitro Analysis. J. Funct. Biomater. 2023, 14, 469. [Google Scholar] [CrossRef]
- Gómez-Polo, M.; Ortega, R.; Gómez-Polo, C.; Martín, C.; Celemín, A.; del Río, J. Does Length, Diameter, or Bone Quality Affect Primary and Secondary Stability in Self-Tapping Dental Implants? J. Oral Maxillofac. Surgery 2016, 74, 1344–1353. [Google Scholar] [CrossRef]
- Cucinelli, C.; Pereira, M.S.; Borges, T.; Figueiredo, R.; Leitão-Almeida, B. The Effect of Increasing Thread Depth on the Initial Stability of Dental Implants: An In Vitro Study. Surgeries 2024, 5, 817–825. [Google Scholar] [CrossRef]
- Hiranmayi, V.K. Factors influencing implant stability. J. Dent. Implants 2018, 8, 69–76. [Google Scholar] [CrossRef]
- Huang, S.; Murphy, L.; Xu, W. Genes and functions from breast cancer signatures. BMC Cancer 2018, 18, 473. [Google Scholar] [CrossRef]
- El-Anwar, M.I.; El-Zawahry, M.M.; El-Mofty, M. Load Transfer on Dental Implants and Surrounding Bones. Aust. J. Basic Appl. Sci. 2012, 6, 551–560. [Google Scholar]
- Hansson, S.; Norton, M. The relation between surface roughness and interfacial shear strength for bone-anchored implants. A mathematical model. J. Biomech. 1999, 32, 829–836. [Google Scholar] [CrossRef]
- Bianchi, A.E.; Dolci, G., Jr.; Sberna, M.T.; Sanfilippo, F. Factors affecting bone response around loaded titanium dental implants: A literature review. J. Appl. Biomater. Biomech. 2005, 3, 135–140. [Google Scholar]
- Stanford, C.M. Surface modifications of dental implants. Aust. Dent. J. 2008, 53, S26–S33. [Google Scholar]
- Skalak, R.; Zhao, Y. Interaction of force-fitting and surface roughness of implants. Clin. Implant Dent. Relat. Res. 2000, 2, 219–224. [Google Scholar] [CrossRef]
- Barfeie, A.; Wilson, J.; Rees, J. Implant surface characteristics and their effect on osseointegration. Br. Dent. J. 2015, 218, E9. [Google Scholar] [CrossRef]
- Romero, M.; Herrero-Climent, M.; Ríos-Carrasco, B.; Brizuela, A.; Romero, M.M.; Gil, J. Investigation of the Influence of Roughness and Dental Implant Design on Primary Stability via Analysis of Insertion Torque and Implant Stability Quotient: An In Vitro Study. J. Clin. Med. 2023, 12, 4190. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layeolle, P.; Amourinq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.H.; Kazemian, M.; Ghorbanzadeh, S. A brief overview of cellular and molecular mechanisms of osseointegration. Int. J. Contemp. Dent. Med. Rev. 2015, 12, 13. [Google Scholar]
- Yu, M.; Yang, H.; Li, B.; Wang, R.; Han, Y. Molecular mechanisms of interrod spacing-mediated osseointegration via modulating inflammatory response and osteogenic differentiation. Chem. Eng. J. 2023, 454, 140141. [Google Scholar] [CrossRef]
- Nishimura, I. Genetic Networks in Osseointegration. J. Dent. Res. 2013, 92 (Suppl. 12), 109S–118S. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, S.; Wang, H.; Chen, X.; Shuai, Y.; Wang, H.; Mao, Y.; He, F. Mesenchymal stem cells and dental implant osseointegration during aging: From mechanisms to therapy. Stem. Cell Res. Ther. 2023, 14, 382. [Google Scholar] [CrossRef]
- Trindade, R.; Albrektsson, T.; Galli, S.; Prgomet, Z.; Tengvall, P.; Wennerberg, A. Osseointegration and foreign body reaction: Titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin. Implant Dent. Relat. Res. 2018, 20, 82–91. [Google Scholar] [CrossRef]
- Yin, X.; Yang, C.; Wang, Z.; Zhang, Y.; Li, Y.; Weng, J.; Feng, B. Alginate/chitosan modified immunomodulatory titanium implants for promoting osteogenesis in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112087. [Google Scholar] [CrossRef]
- Wu, J.; Chen, M.; Xiao, Y.; Yang, H.; Wang, G.; Zhang, X.; Dai, L.; Yuan, Z. The Bioactive Interface of Titanium Implant with Both Anti-Oxidative Stress and Immunomodulatory Properties for Enhancing Osseointegration under Diabetic Condition. Adv. Healthc. Mater. 2024, 13, e2401974. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Qiu, J.; Zhang, X.; Liu, X.; Qiao, Y.; Liu, X. Synergistic effects of immunoregulation and osteoinduction of ds-block elements on titanium surface. Bioact. Mater. 2020, 6, 191–207. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Y.; Yu, L.; Liu, K.; Fei, Y.; Guo, C.; Zhou, Y.; Hu, J.; Shi, L.; Ji, H. Inhibition of Inflammatory Response and Promotion of Osteogenic Activity of Zinc-Doped Micro-Arc Titanium Oxide Coatings. ACS Omega 2022, 7, 14920–14932. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, W.; Zhou, W.; Zhang, S.; Li, M.; Li, N.; Pan, G.; Zhang, X.; Bai, J.; Zhu, C. Immunomodulatory biomaterials for implant-associated infections: From conventional to advanced therapeutic strategies. Biomater. Res. 2022, 26, 72. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.L.; Yun, S.; Lee, H.; Cao, H.L.; Woo, S.H.; Jeong, Y.H.; Jung, T.G.; Kim, C.M.; Choung, P.H. Osseointegration of 3D-printed titanium implants with surface and structure modifications. Dent. Mater. 2022, 38, 1648–1660. [Google Scholar] [CrossRef] [PubMed]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface modification techniques of titanium and titanium alloys for biomedical dental applications: A review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Wennerberg, A.; Lindhe, J. Bone and soft tissue integration to titanium implants with different surface topography: An experimental study in the dog. Int. J. Oral Maxillofac. Implant. 2001, 16, 323–332. [Google Scholar]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239. [Google Scholar] [CrossRef]
- Ikeda, E.; Tsuji, T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin. Biol. Ther. 2008, 8, 735–744. [Google Scholar] [CrossRef]
- Biguetti, C.C.; Cavalla, F.; Silveira, E.M.; Fonseca, A.C.; Vieira, A.E.; Tabanez, A.P.; Rodrigues, D.C.; Trombone, A.P.F.; Garlet, G.P. Oral implant osseointegration model in C57Bl/6 mice: Microtomographic, histological, histomorphometric and molecular characterization. J. Appl. Oral Sci. 2017, 1, e20170601. [Google Scholar] [CrossRef]
- Perlman, R.L. Mouse models of human disease: An evolutionary perspective. Evol. Med. Public Health 2016, 1, 170–176. [Google Scholar] [CrossRef]
- Setiawati, R.; Rahardjo, P. Bone Development and Growth; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Inchingolo, F.; Tari, S.R. Animal Models for Investigating Osseointegration: An Overview of Implant Research over the Last Three Decades. J. Funct. Biomater. 2024, 15, 83. [Google Scholar] [CrossRef]
- Pazzaglia, U.E. Periosteal and endosteal reaction to reaming and nailing: The possible role of revascularization on the endosteal anchorage of cementless stems. Biomaterials 1996, 17, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Quarterman, J.C.; Phruttiwanichakun, P.; Fredericks, D.C.; Salem, A.K. Zoledronic Acid Implant Coating Results in Local Medullary Bone Growth. Mol. Pharm. 2022, 19, 4654–4664. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, A.J.; Anderson, D.E.; Samii, V.F.; Weisbrode, S.E.; Johnson, J.K.; Noon, M.S.; Tomasko, D.L.; Lannutti, J.L. Effects of orthopedic implants with a polycaprolactone polymer coating containing bone morphogenetic protein-2 on osseointegration in bones of sheep. Am. J. Vet. Res. 2009, 70, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. Bone Healing in the Presence of Orthopaedic Implants. In Handbook of Orthopaedic Trauma Implantology; Banerjee, A., Biberthaler, P., Shanmugasundaram, S., Eds.; Springer: Singapore, 2023; pp. 869–904. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef]
- Gupta, S. Bone Healing in the Presence of Orthopedic Implants. In Handbook of Orthopaedic Trauma Implantology; Banerjee, A., Biberthaler, P., Shanmugasundaram, S., Eds.; Springer: Singapore, 2022; pp. 1–36. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials Regulating Bone Hematoma for Osteogenesis. Adv. Healthc. Mater. 2020, 9, e2000726. [Google Scholar] [CrossRef]
- Shiu, H.T.; Leung, P.C.; Ko, C.H. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J. Tissue Eng. Regen. Med. 2018, 12, e1911–e1925. [Google Scholar] [CrossRef]
- Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials 2021, 14, 6642. [Google Scholar] [CrossRef]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of blood clot on biomaterial implants influences bone healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Pathak, U.S.; Balasubramanian, A.; Beilan, J.A.; Butaney, M.; Tatem, A.J.; Thirumavalavan, N.; Lipshultz, L.I. Vasoepididymostomy: An insight into current practice patterns. Transl. Androl. Urol. 2019, 8, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Proctor, A.R.; Ren, J.; Benoit, D.S.W.; Choe, R. Temporal blood flow changes measured by diffuse correlation tomography predict murine femoral graft healing. PLoS ONE 2018, 13, e0197031. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Stevens, R.; McGrath, K.M. Towards the Development of Artificial Bone Grafts: Combining Synthetic Biomineralisation with 3D Printing. J. Funct. Biomater. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Naito, H.; Iba, T.; Takakura, N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int. Immunol. 2020, 32, 295–305. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis-From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and Molecular Aspects of Bone Remodeling. Front. Oral Biol. 2016, 18, 9–16. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis. Res. Ther. 2007, 9 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Yun, J.H.; Han, S.H.; Choi, S.H.; Lee, M.H.; Lee, S.J.; Song, S.U.; Oh, N. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: Preliminary study in canine three-wall intrabony defects. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1021–1030. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 254. [Google Scholar] [CrossRef]
- Fernandes, G.; Yang, S. Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering. Bone Res. 2016, 4, 16036. [Google Scholar] [CrossRef]
- Aniołek, K.; Łosiewicz, B.; Kubisztal, J.; Osak, P.; Stróż, A.; Barylski, A.; Kaptacz, S. Mechanical Properties, Corrosion Resistance and Bioactivity of Oxide Layers Formed by Isothermal Oxidation of Ti-6Al-7Nb Alloy. Coatings 2021, 11, 505. [Google Scholar] [CrossRef]
- Alla, R.K.; Ginjupalli, K.; Upadhya, N.; Mohammed, S.; Sekar, R.; Ravi, R. Surface Roughness of Implants: A Review. Trends Biomater. Artif. Organs 2011, 25, 112–118. [Google Scholar]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Stach, S. Effect of Autoclaving Time on Corrosion Resistance of Sandblasted Ti G4 in Artificial Saliva. Materials 2020, 13, 4154. [Google Scholar] [CrossRef]
- Stróż, A.; Maszybrocka, J.; Goryczka, T.; Dudek, K.; Osak, P.; Łosiewicz, B. Influence of Anodizing Conditions on Biotribological and Micromechanical Properties of Ti–13Zr–13Nb Alloy. Materials 2023, 16, 1237. [Google Scholar] [CrossRef] [PubMed]
- Osak, P.; Maszybrocka, J.; Kubisztal, J.; Łosiewicz, B. Effect of amorphous calcium phosphate coatings on tribological properties of titanium grade 4 in protein-free artificial saliva. Biotribology 2022, 32, 100219. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Maszybrocka, J.; Kubisztal, J.; Bogunia, S.; Ratajczak, P.; Aniołek, K. Effect of Temperature on Electrochemically Assisted Deposition and Bioactivity of CaP Coatings on CpTi Grade 4. Materials 2021, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- Cylindrical Dental Implant MACHINED WINSIX®. Available online: https://www.medicalexpo.com/prod/biosaf/product-124601-1082647.html (accessed on 28 October 2024).
- Ballo, A.M.; Omar, O.; Xia, W.; Palmquist, A. Dental Implant Surfaces—Physicochemical Properties, Biological Performance, and Trends; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef][Green Version]
- Cochran, D.L. A comparison of endosseous dental implant surfaces. J. Periodontol. 1999, 70, 1523–1539. [Google Scholar] [CrossRef]
- Hong, D.G.K.; Oh, J.H. Recent advances in dental implants. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 33. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Pham, M.H.; Haugen, H.J.; Reseland, J.E. Fluoride Modification of Titanium Surfaces Enhance Complement Activation. Materials 2020, 13, 684. [Google Scholar] [CrossRef]
- Addy, L. An introduction to dental implants. Br. Dent. J. 2024, 236, 753–757. [Google Scholar] [CrossRef]
- Vörös, J.; Wieland, M.; Ruiz-Taylor, L.; Textor, M.; Brunette, D.M. Characterization of Titanium Surfaces. In Titanium in Medicine. Engineering Materials; Brunette, D.M., Tengvall, P., Textor, M., Thomson, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; p. 114. [Google Scholar] [CrossRef]
- Machined Surface Coronal (MSc) Dental Implants. Available online: https://southernimplants.com/solutions/innovative-products/msc-implants/ (accessed on 28 October 2024).
- Brånemark System® Mk III. Available online: https://store.nobelbiocare.com/us/en/media/eifu/IFU1014_EN_US_00.pdf (accessed on 28 October 2024).
- Bredent Medical. Available online: https://www.medicalexpo.com.cn/prod/bredent-medical/product-71642-1088938.html (accessed on 28 October 2024).
- Fousová, M.; Vojtech, D.; Jablonska, E.; Fojt, J.; Lipov, J. Novel Approach in the Use of Plasma Spray: Preparation of Bulk Titanium for Bone Augmentations. Materials 2017, 10, 987. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioact. Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef] [PubMed]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray Tech 2018, 27, 1251–1279. [Google Scholar] [CrossRef]
- IMZ Original. Available online: https://www.spotimplant.com/en/dental-implants/imz/imz-original (accessed on 28 October 2024).
- Seidling, R.; Lehmann, L.J.; Lingner, M.; Mauermann, E.; Obertacke, U.; Schwarz, M.L.R. Analysis of the osseointegrative force of a hyperhydrophilic and nanostructured surface refinement for TPS surfaces in a gap healing model with the Göttingen minipig. J. Orthop. Surg. Res. 2016, 11, 119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Simmons, C.A.; Valiquette, N.; Pillar, R.M. Osseointegration of Sintered Porous-surfaced and Plasma Spray–Coated Implants: An Animal Model Study of Early Postimplantation Healing Response and Mechanical Stability. J. Biomed. Mater. Res. 1999, 47, 127–138. [Google Scholar] [CrossRef]
- Hadzik, J.; Jurczyszyn, K.; Gębarowski, T.; Trytek, A.; Gedrange, T.; Kozakiewicz, M.; Dominiak, M.; Kubasiewicz-Ross, P.; Trzcionka-Szajna, A.; Szajna, E.; et al. An Experimental Anodized and Low-Pressure Oxygen Plasma-Treated Titanium Dental Implant Surface-Preliminary Report. Int. J. Mol. Sci. 2023, 24, 3603. [Google Scholar] [CrossRef]
- Rodriguez y Baena, R.; Rizzo, S.; Manzo, L.; Lupi, S.M. Nanofeatured Titanium Surfaces for Dental Implantology: Biological Effects, Biocompatibility, and Safety. J. Nanomater. 2017, 2017, 6092895. [Google Scholar] [CrossRef]
- Vasilev, O.; Hayles, A.; Campbell, D.; Jaarsma, R.; Johnson, L.; Vasilev, K. Nanoscale antibacterial coatings incorporating silver nanoparticles derived by plasma techniques—A state-of-the-art perspective. Mater. Today Chem. 2024, 41, 102341. [Google Scholar] [CrossRef]
- Ten Good Reasons for IMZ®-TwinPlus—DENTSPLY Friadent. Available online: https://www.yumpu.com/en/document/view/9007132/ten-good-reasons-for-imzr-twinplus-dentsply-friadent (accessed on 28 October 2024).
- Bruggenkate, C.M.; Sutter, F.; Schroeder, A.; Oosterbeek, H.S. Explantation procedure in the F-type and Bonefit ITI implant system. Int. J. Oral Maxillofac. Surg. 1991, 20, 155–158. [Google Scholar] [CrossRef]
- Lifecore Dental in the Restore TPS System. In Brief: Lifecore. Available online: https://insights.citeline.com/MT003637/In-Brief-Lifecore/ (accessed on 28 October 2024).
- Steri-Oss®. Available online: https://www.spotimplant.com/en/dental-implants/steri-oss (accessed on 28 October 2024).
- Fintová, S.; Kuběna, I.; Palán, J.; Mertová, K.; Duchek, M.; Hutař, P.; Pastorek, F.; Kunz, L. Influence of Sandblasting and Acid Etching on Fatigue Properties of Ultra-Fine Grained Ti Grade 4 for Dental Implants. J. Mech. Behav. Biomed. Mater. 2020, 111, 104016. [Google Scholar] [CrossRef]
- Kim, H.-K.; Ahn, B. Effect of Al2O3 Sandblasting Particle Size on the Surface Topography and Residual Compressive Stresses of Three Different Dental Zirconia Grades. Materials 2021, 14, 610. [Google Scholar] [CrossRef]
- Gil, F.; Pérez, R.; Olmos, J.; Herraez-Galindo, C.; Gutierrez-Pérez, J.; Torres-Lagares, D. The Effect of Using Al2O3 and TiO2 in Sandblasting of Titanium Dental Implants. J. Mater. Res. 2022, 37, 2604–2613. [Google Scholar] [CrossRef]
- Guo, C.Y.; Matinlinna, J.P.; Tang, A.T. Effects of surface charges on dental implants: Past, present, and future. Int. J. Biomater. 2012, 2012, 381535. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewska-Kuska, M.; Leda, B.; Gajdus, P.; Hedzelek, W. Evaluation of modified titanium surfaces physical and chemical characteristics. Nucl. Instrum. Methods Phys. Res. B 2017, 411, 94–99. [Google Scholar] [CrossRef]
- Kasemo, B.; Lausmaa, J. Surface science aspects on inorganic biomaterials. Crit. Rev. Biocompat. 1986, 2, 335–380. [Google Scholar]
- Prima Plus 4.1 (RD). Available online: https://osseosource.com/prima-plus-4-1-rd-/p-3167.html (accessed on 28 October 2024).
- Osteoplant Hex. Available online: https://www.spotimplant.com/en/dental-implants/osteoplant/osteoplant-hex (accessed on 28 October 2024).
- Collaert, B.; De Bruyn, H. Immediate functional loading of TiOblast dental implants in full-arch edentulous mandibles: A 3-year prospective study. Clin. Oral Implants Res. 2008, 19, 1254–1260. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Kämmerer, P.W.; Morbach, T.; Ladwein, C.; Wegener, J.; Wagner, W. Ten-Year Retrospective Follow-Up Study of the TiOblast™ Dental Implant. Clin. Implant Dent. Rel. Res. 2012, 14, 127–134. [Google Scholar] [CrossRef]
- Ferguson, R. Renova Internal Hex Implant System: Surgical and restorative versatility. Dent. Implantol. Update 2005, 16, 49–54. [Google Scholar]
- Che Isa, N.N.; Mohd, Y.; Yury, N. Electrochemical Deposition and Characterization of Hydroxyapatite (HAp) on Titanium Substrate. APCBEE Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef]
- Usinskas, P.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-Gel Derived Porous and Hydrophilic Calcium Hydroxyapatite Coating on Modified Titanium Substrate. Surf. Coat. Technol. 2016, 307 Pt A, 935–940. [Google Scholar] [CrossRef]
- Jaafar, A.; Schimpf, C.; Mandel, M.; Hecker, C.; Rafaja, D.; Krüger, L.; Arki, P.; Joseph, Y. Sol–gel derived hydroxyapatite coating on titanium implants: Optimization of sol–gel process and engineering the interface. J. Mater. Res. 2022, 37, 2558–2570. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Świeczko-Żurek, B.; Bartmański, M. Investigations of Titanium Implants Covered with Hydroxyapatite Layer. Adv. Mater. Sci. 2016, 16, 78–86. [Google Scholar] [CrossRef]
- Kuroda, K.; Okido, M. Hydroxyapatite coating of titanium implants using hydroprocessing and evaluation of their osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012, 730693. [Google Scholar] [CrossRef]
- Baltatu, M.S.; Sandu, A.V.; Nabialek, M.; Vizureanu, P.; Ciobanu, G. Biomimetic Deposition of Hydroxyapatite Layer on Titanium Alloys. Micromachines 2021, 12, 1447. [Google Scholar] [CrossRef]
- Park, Y.S.; Yi, K.Y.; Lee, I.S.; Han, C.H.; Jung, Y.C. The Effects of Ion Beam– Assisted Deposition of Hydroxyapatite on the Grit-Blasted Surface of Endosseous Implants in Rabbit Tibiae. Int. J. Oral Maxillofac. Implants 2005, 20, 31–38. [Google Scholar]
- 3i T3 Implant. Available online: https://www.dentalproductshopper.com/implants-edentulous-solutions/implants/3i-t3-implant (accessed on 28 October 2024).
- Mautsch, C.; Wolfart, S.; Mautsch, W.; Rittich, A.B. Long-term outcome of the IMZ implant system: A retrospective clinical study with a follow-up between 23 and 34 years. Int. J. Implant Dent. 2022, 8, 54. [Google Scholar] [CrossRef]
- Kallus, T.; Bessing, C.; Homsi, G.; Eklund, I. Five-year evaluation of Lifecore Restore implants: A retrospective comparison with Nobel Biocare MK II implants. Clin. Implant Dent. Relat. Res. 2009, 11, 167–177. [Google Scholar] [CrossRef]
- Nobel Replace External Hex (Steri-Oss). Available online: https://www.spotimplant.com/en/dental-implants/nobel-biocare/nobel-replace-external-hex-steri-oss (accessed on 28 October 2024).
- Petrini, M.; Giuliani, A.; Di Campli, E.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; D’Ercole, S. The Bacterial Anti-Adhesive Activity of Double-Etched Titanium (DAE) as a Dental Implant Surface. Int. J. Mol. Sci. 2020, 21, 8315. [Google Scholar] [CrossRef]
- Xie, Y.; Zuo, J.; Zhou, B.; Ma, L.; Yu, Z.M.; Wei, Q.; Tang, Z.G. Sandblast-free double-etched titanium for dental implants application. Mater. Lett. 2016, 176, 74–77. [Google Scholar] [CrossRef]
- Giner, L.; Mercadé, M.; Torrent, S.; Punset, M.; Pérez, R.A.; Delgado, L.M.; Gil, F.J. Double acid etching treatment of dental implants for enhanced biological properties. J. Appl. Biomater. Funct. Mater. 2018, 16, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Santos Marino, J.; Cortés-Bretón Brinkmann, J.; García-Gil, I.; Martínez-Rodríguez, N.; Fraile, J.F.; Barona Dorado, C.; Martínez-González, J.M. Clinical Evaluation of Dental Implants with a Double Acid-Etched Surface Treatment: A Cohort Observational Study with Up to 10-Year Follow-Up. Materials 2021, 14, 6483. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.S.; David, S.R.N.; Zulhilmi, N.R.; Dhaliwal, S.K.S.; Knights, J.; Junior, R.F.d.A. Contamination of titanium dental implants: A narrative review. SN. Appl. Sci. 2020, 2, 1011. [Google Scholar] [CrossRef]
- The Osseotite® Dental Implant System. Available online: https://www.zimvie.com/en/dental/dental-implant-systems/3i-osseotite-implant.html (accessed on 28 October 2024).
- Osseotite® Implant Reference List. Available online: https://www.biomax.it/wp-content/uploads/2020/10/ZB0120_OsseotiteImplantReference_EN.pdf (accessed on 28 October 2024).
- The Osseotite® Implant. Available online: https://www.biomet3i.cz/userFiles/pdf/zb0067_rev_a_osseotite_implant_brochure_final_secured.pdf (accessed on 28 October 2024).
- del Olmo, R.; Czerwiński, M.; Santos-Coquillat, A.; Dubey, V.; Dhoble, S.J.; Michalska-Domańska, M. Nano-scale Surface Modification of Dental Implants: Fabrication. In Surface Modification of Titanium Dental Implants; Gulati, K., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Rungcharassaeng, K.; Kan, J.Y.K. Fabricating a stable record base for completely edentulous patients treated with osseointegrated implants using healing abutments. J. Prosthet. Dent. 1999, 81, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.H.; Herr, Y.; Shin, S.; Chung, J.H. Effect of Erbium-Doped: Yttrium, Aluminium and Garnet Laser Irradiation on the Surface Microstructure and Roughness of Sand-Blasted, Large Grit, Acid-Etched Implants. J. Periodontal Implant. Sci. 2011, 41, 135–142. [Google Scholar] [CrossRef]
- Velasco-Ortega, E.; Ortiz-Garcia, I.; Jiménez-Guerra, A.; Núñez-Márquez, E.; Moreno-Muñoz, J.; Rondón-Romero, J.L.; Cabanillas-Balsera, D.; Gil, J.; Muñoz-Guzón, F.; Monsalve-Guil, L. Osseointegration of Sandblasted and Acid-Etched Implant Surfaces. A Histological and Histomorphometric Study in the Rabbit. Int. J. Mol. Sci. 2021, 22, 8507. [Google Scholar] [CrossRef]
- Muhammed, H.A.; Mahmoud, E.M.; Fahmy, A.E.; Nasr, D.M. The effect of sandblasting versus acid etching on the surface roughness and biaxial flexural strength of CAD/CAM resin-matrix ceramics (In vitro study). BMC Oral Health 2023, 23, 169. [Google Scholar] [CrossRef]
- Patcas, R.; Zinelis, S.; Eliades, G.; Eliades, T. Surface and interfacial analysis of sandblasted and acid-etched enamel for bonding orthodontic adhesives. Am. J. Orthod. Dentofacial Orthop. 2015, 147 (Suppl. 4), S64–S75. [Google Scholar] [CrossRef]
- Bok, W.M.; Kim, S.Y.; Lee, S.J.; Shin, G.-S.; Park, J.-M.; Lee, M.-H. Surface characteristics and bioactivation of sandblasted and acid-etched (SLA) Ti-10Nb-10Ta alloy for dental implant. Int. J. Precis. Eng. Manuf. 2015, 16, 2185–2192. [Google Scholar] [CrossRef]
- Dental News®. Implant Standard. Available online: https://dentalnews.pl/produkt/implant-standard/ (accessed on 28 October 2024).
- Stafford, G.L. Review Found Little Difference between Sandblasted and Acid-etched (SLA) Dental Implants and Modified Surface (SLActive) Implants. Evid. Based Dent. 2014, 15, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 Particles on Titanium Dental Implant Systems following Sandblasting and Acid-Etching Process. Int. J. Biomater. 2019, 1, 6318429. [Google Scholar] [CrossRef] [PubMed]
- Spiral (SPI) By Alpha Bio Tec®. Available online: https://www.spotimplant.com/en/dental-implants/alpha-bio-tec/spi (accessed on 28 October 2024).
- About the DFI Implant. Available online: https://info.alpha-bio.net/dfi-implant (accessed on 28 October 2024).
- Rocci, M.; Rocci, A.; Martignoni, M.; Albrektsson, T.; Barlattani, A.; Gargari, M. Comparing the TiOblast and Osseospeed surfaces. Histomorphometric and histological analysis in humans. Oral Implantol. 2008, 1, 34–42. [Google Scholar]
- Straumann Group. Available online: https://www.straumann.com/group/en/home/about/our-history.html (accessed on 22 December 2024).
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef]
- Zinelis, S.; Silikas, N.; Thomas, A.; Syres, K.; Eliades, G. Surface characterization of SLActive dental implants. Eur. J. Esthet. Dent. 2012, 7, 72–92. [Google Scholar]
- SLActive. Available online: http://www.schmidt-dental.pl/wp-content/uploads/2015/11/Straumann_SLActive_Studies.pdf (accessed on 28 October 2024).
- Romero-Ruiz, M.M.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a Novel Surface of Bioactive Implants on Osseointegration: A Comparative and Histomorfometric Correlation and Implant Stability Study in Minipigs. Int. J. Mol. Sci. 2019, 20, E2307. [Google Scholar] [CrossRef]
- Straumann® SLActive®. Beyond Hydrophilicity—The Science of High Performance. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Distinct Nano-Structures Present on the SLActive® Surface27,28. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Advanced In-Vitro Research Shows Nano-Structure Support Early Osseointegration23,24. Available online: https://www.straumann.com/en/discover/slactive.html (accessed on 28 October 2024).
- Baier, R.E.; Meyer, A.E. Future directions in surface preparation of dental implants. J. Dent. Educ. 1988, 52, 788–791. [Google Scholar] [CrossRef]
- Şener, I.; Yamaner, G.; Sertgoz, A. Clinical Outcomes of Patients Treated with SLA and SLActive Implants. In Proceedings of the IADR/PER General Session 2010, Barcelona, Spain, 14–17 July 2010. [Google Scholar]
- Birch, J.; Burleigh, T. Oxides Formed on Titanium by Polishing, Etching, Anodizing, or Thermal Oxidizing. Corrosion 2000, 56, 1233–1241. [Google Scholar] [CrossRef]
- Huang, Y.H.; Xiropaidis, A.; Sorensen, R.; Hall, J.; Wikesjö, U. Bone Formation at Titanium Porous Oxide (TiUnite (TM)) Oral Implants in Type IV Bone. Clin. Oral Implants Res. 2005, 16, 105–111. [Google Scholar] [CrossRef]
- Badekas, H.; Panagopoulos, C. Titanium anodization under constant voltage conditions. Surf. Coat. Technol. 1987, 31, 381–388. [Google Scholar] [CrossRef]
- Nowińska, D.; Osak, P.; Maszybrocka, J.; Łosiewicz, B. Anodic Production and Characterization of Biomimetic Oxide Layers on Grade 4 Titanium for Medical Applications. J. Funct. Biomater. 2024, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Ouyang, J.; Chen, X.; Xu, Z.; Sun, X. Bioactivity and Osteogenic Cell Response of TiO2 Nanotubes Coupled with Nanoscale Calcium Phosphate via Ultrasonification-Assisted Electrochemical Deposition. Appl. Surf. Sci. 2014, 305, 24–32. [Google Scholar] [CrossRef]
- Kahar, S.; Singh, A.; Patel, V.; Kanetkar, U. Anodizing of Ti and Ti Alloys for Different Applications: A Review. Int. J. Sci. Res. Dev. 2020, 8, 272–276. [Google Scholar]
- Replace Select Tapered TiUnite RP 4.3 × 13 mm. Available online: https://store.nobelbiocare.com/us/en/replace-select-tapered-tiunite-rp-4-3-x-13-mm (accessed on 28 October 2024).
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef]
- Product Catalog 2017/18 Complete Assortment. Available online: https://www.nobelbiocare.com/sites/g/files/wdvifx201/files/81206_ProdCatalog2017-18_GB.pdf (accessed on 28 October 2024).
- Maló, P.; de Araújo Nobre, M.; Gonçalves, Y.; Lopes, A.; Ferro, A. Immediate Function of Anodically Oxidized Surface Implants (TiUnite™) for Fixed Prosthetic Rehabilitation: Retrospective Study with 10 Years of Follow-Up. BioMed Res. Int. 2016, 2016, 2061237. [Google Scholar] [CrossRef]
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252. [Google Scholar] [CrossRef]
- Karl, M.; Albrektsson, T. Clinical performance of dental implants with a moderately rough (TiUnite) surface: A meta-analysis of prospective clinical studies. Int. J. Oral Maxillofac. Implants. 2017, 32, 717–734. [Google Scholar] [CrossRef]
- Jungner, M.; Lundqvist, P.; Lundgren, S. Oxidized titanium implants (Nobel Biocare TiUnite) compared with turned titanium implants (Nobel Biocare mark III) with respect to implant failure in a group of consecutive patients treated with early functional loading and two-stage protocol. Clin. Oral Implants Res. 2005, 16, 308–312. [Google Scholar] [CrossRef]
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773. [Google Scholar] [CrossRef]
- Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-Arc Oxidation in Titanium and Its Alloys: Development and Potential of Implants. Coatings 2023, 13, 2064. [Google Scholar] [CrossRef]
- Wen, X.; Liu, Y.; Xi, F.; Zhang, X.; Kang, Y. Micro-arc oxidation (MAO) and its potential for improving the performance of titanium implants in biomedical applications. Front. Bioeng. Biotechnol. 2023, 11, 1282590. [Google Scholar] [CrossRef] [PubMed]
- Friedemann, A.E.R.; Thiel, K.; Haßlinger, U.; Ritter, M.; Gesing, T.M.; Plagemann, P. Investigations into the Structure of PEO-Layers for Understanding of Layer Formation. Appl. Surf. Sci. 2018, 443, 467–474. [Google Scholar] [CrossRef]
- Sikdar, S.; Menezes, P.V.; Maccione, R.; Jacob, T.; Menezes, P.L. Plasma Electrolytic Oxidation (PEO) Process—Processing, Properties, and Applications. Nanomaterials 2021, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Biomimetic Implants. Available online: https://implantsystem.avinent.com/wp-content/uploads/sites/4/2019/06/biomimetic-implants-avinent-eng.pdf (accessed on 28 October 2024).
- Łosiewicz, B.; Stróż, A.; Osak, P.; Maszybrocka, J.; Gerle, A.; Dudek, K.; Balin, K.; Łukowiec, D.; Gawlikowski, M.; Bogunia, S. Production, Characterization and Application of Oxide Nanotubes on Ti–6Al–7Nb Alloy as a Potential Drug Carrier. Materials 2021, 14, 6142. [Google Scholar] [CrossRef]
- Osak, P.; Skwarek, S.; Łukowiec, D.; Przeliorz, G.; Łosiewicz, B. Preparation and Characterization of Oxide Nanotubes on Titanium Surface for Use in Controlled Drug Release Systems. Materials 2024, 17, 3753. [Google Scholar] [CrossRef]
- Stróż, A.; Gawlikowski, M.; Balin, K.; Osak, P.; Kubisztal, J.; Zubko, M.; Maszybrocka, J.; Dudek, K.; Łosiewicz, B. Biological Activity and Thrombogenic Properties of Oxide Nanotubes on the Ti-13Nb-13Zr Biomedical Alloy. J. Funct. Biomater. 2023, 14, 375. [Google Scholar] [CrossRef]
- Tran, C.; Walsh, L.J. Novel Models to Manage Biofilms on Microtextured Dental Implant Surfaces; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Lutz, R.; Srour, S.; Nonhoff, J.; Weisel, T.; Damien, C.J.; Schlegel, K.A. Biofunctionalization of Titanium Implants with a Biomimetic Active Peptide (P-15) Promotes Early Osseointegration. Clin. Oral Implants Res. 2010, 21, 726–734. [Google Scholar] [CrossRef]
- Fu, L.; Omi, M.; Sun, M.; Cheng, B.; Mao, G.; Liu, T.; Mendonça, G.; Averick, S.E.; Mishina, Y.; Matyjaszewski, K. Covalent Attachment of P15 Peptide to Ti Alloy Surface Modified with Polymer to Enhance Osseointegration of Implants. ACS Appl. Mater. Interfaces 2019, 11, 38531–38536. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ho, K.-N.; Feng, S.-W.; Huang, H.-M.; Chang, C.-H.; Lin, C.-T.; Teng, N.-C.; Pan, Y.-H.; Chang, W.-J. Fibronectin-Grafted Titanium Dental Implants: An In Vivo Study. BioMed Res. Int. 2016, 2016, 2414809. [Google Scholar] [CrossRef][Green Version]
- Lo, V.; I-Chun Lai, J.; Sunde, M. Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials. Adv. Exp. Med. Biol. 2019, 1174, 161–185. [Google Scholar] [CrossRef]
- Pawar, V.; Bulbake, U.; Khan, W.; Srivastava, R. Chitosan Sponges as a Sustained Release Carrier System for the Prophylaxis of Orthopedic Implant-Associated Infections. Int. J. Biol. Macromol. 2019, 134, 100–112. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Osak, P.; Kubisztal, J. The effect of a composite chitosan/copper(II) ion coating on the corrosion resistance of grade 4 titanium in saline: Preliminary results. Prog. Chem. Appl. Chitin Deriv. 2023, 28, 89–102. [Google Scholar] [CrossRef]
- Szklarska, M.; Łosiewicz, B.; Dercz, G.; Maszybrocka, J.; Rams-Baron, M.; Stach, S. Electrophoretic deposition of chitosan coatings on the Ti15Mo biomedical alloy from a citric acid solution. RSC Adv. 2020, 10, 13386–13393. [Google Scholar] [CrossRef] [PubMed]
- OsseoSpeed TX S (Astra Tech). Available online: https://stg.spotimplant.com/en/dental-implants/dentsply-implants/osseospeed-tx-s (accessed on 28 October 2024).
- Homa, K.; Zakrzewski, W.; Dobrzyński, W.; Piszko, P.J.; Piszko, A.; Matys, J.; Wiglusz, R.J.; Dobrzyński, M. Surface Functionalization of Titanium-Based Implants with a Nanohydroxyapatite Layer and Its Impact on Osteoblasts: A Systematic Review. J. Funct. Biomater. 2024, 15, 45. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, Y.; Lu, Y.; Quan, K.; Mao, Z.; Zheng, Y.; Qin, L.; Xia, D. UiO-66/AgNPs Coating for Dental Implants in Preventing Bacterial Infections. J. Dent. Res. 2024, 103, 516–525. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Z.; Liu, Z.; Shen, X.; Cai, C.; Li, M.; Luo, Z. Functionally Tailored Metal-Organic Framework Coatings for Mediating Ti Implant Osseointegration. Adv. Sci. 2023, 10, e2303958. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, S.; Xie, W.; Xue, Y.; Qiao, M.; Yang, X.; Zhang, X.; Wan, Q.; Wang, J.; Chen, J.; et al. Surface modification of the Ti surface with nanoscale bio-MOF-1 for improving biocompatibility and osteointegration in vitro and in vivo. Mater. Chem. B 2022, 10, 8535–8548. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Kazemzad, M. Recent advances in surface-mounted metal-organic framework thin film coatings for biomaterials and medical applications: A review. Biomater. Res. 2023, 27, 115. [Google Scholar] [CrossRef]
- Kaur, G. Apatites: A Mark of BioactivityBioactivity. In Bioactive Glasses: Potential Biomaterials for Future Therapy; Kaur, G., Ed.; Series in BioEngineering; Springer International Publishing: Cham, Switzerland, 2017; pp. 145–172. ISBN 978-3-319-45716-1. [Google Scholar]
- Tang, W.; Fischer, N.G.; Kong, X.; Sang, T.; Ye, Z. Hybrid coatings on dental and orthopedic titanium implants: Current advances and challenges. BMEMat 2024, 2, e12105. [Google Scholar] [CrossRef]
- Bravo, E.; Serrano, B.; Ribeiro-Vidal, H.; Virto, L.; Sánchez, I.S.; Herrera, D.; Sanz, M. Biofilm formation on dental implants with a hybrid surface microtopography: An in vitro study in a validated multispecies dynamic biofilm model. Clin. Oral Implant. Res. 2023, 34, 475–485. [Google Scholar] [CrossRef]
- Serrano, B.; Sanz-Sánchez, I.; Serrano, K.; Montero, E.; Sanz, M. One-year outcomes of dental implants with a hybrid surface macro-design placed in patients with history of periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Liu, T.T.; Wang, Q.Q.; Zhang, J.; Cao, M.S. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef]
- IMAX NHSI. Available online: https://www.spotimplant.com/en/dental-implants/ires/imax-nhsi-internal-hex-c (accessed on 28 October 2024).
- Morra, M.; Cassinelli, C.; Torre, E.; Iviglia, G. Permanent wettability of a novel, nanoengineered, clinically available, hyaluronan-coated dental implant. Clin. Exp. Dent. Res. 2018, 4, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P. Dental implants in periodontal care. Curr. Opin. Periodontol. 1993, 157, 157–162. [Google Scholar]
- Leesungbok, R.; Hong, S.O.; Lee, S.W.; Htay, P.E.E.; Choi, J.J.; Park, J.J. An eight-year retrospective study on the clinical outcomes of laser surface-treated implants. Int. J. Implant. Dent. 2024, 10, 38. [Google Scholar] [CrossRef]
- Lackington, W.A.; Schweizer, P.; Khokhlova, M.; Cancellieri, C.; Guimond, S.; Chopard-Lallier, A.-L.; Hofstetter, J.; Schmutz, P.; Maeder, X.; Rottmar, M. Femtosecond Laser-Texturing the Surface of Ti-Based Implants to Improve Their Osseointegration Capacity. Adv. Mater. Interfaces 2022, 9, 2201164. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef]
- Veiko, V.; Karlagina, Y.; Zernitckaia, E.; Egorova, E.; Radaev, M.; Yaremenko, A.; Chernenko, G.; Romanov, V.; Shchedrina, N.; Ivanova, E.; et al. Laser-Induced µ-Rooms for Osteocytes on Implant Surface: An In Vivo Study. Nanomaterials 2022, 12, 4229. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sakr, H. Implant Surface Topography Following Different Laser Treatments: An In Vitro Study. Cureus 2023, 15, e38731. [Google Scholar] [CrossRef]
- Shapoff, C.A.; Lahey, B.; Wasserlauf, P.; Kim, D. Radiographic Analysis of Crestal Bone Levels on Laser-Lok® Collar Dental Implants. Int. J. Periodontics Restor. Dent. 2010, 30, 129–137. [Google Scholar]
- Laser Etching: Everything You Need to Know. Available online: https://www.laserax.com/blog/laser-etching (accessed on 28 October 2024).
- Tapered Internal Plus (4.5). Available online: https://osseosource.com/tapered-internal-plus-4-5-/p-2495.html (accessed on 18 October 2024).
- Laser-Lok Microchannels. Clinical Overview. Available online: https://www.laser-lok.com (accessed on 18 October 2024).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łosiewicz, B.; Osak, P.; Nowińska, D.; Maszybrocka, J. Developments in Dental Implant Surface Modification. Coatings 2025, 15, 109. https://doi.org/10.3390/coatings15010109
Łosiewicz B, Osak P, Nowińska D, Maszybrocka J. Developments in Dental Implant Surface Modification. Coatings. 2025; 15(1):109. https://doi.org/10.3390/coatings15010109
Chicago/Turabian StyleŁosiewicz, Bożena, Patrycja Osak, Delfina Nowińska, and Joanna Maszybrocka. 2025. "Developments in Dental Implant Surface Modification" Coatings 15, no. 1: 109. https://doi.org/10.3390/coatings15010109
APA StyleŁosiewicz, B., Osak, P., Nowińska, D., & Maszybrocka, J. (2025). Developments in Dental Implant Surface Modification. Coatings, 15(1), 109. https://doi.org/10.3390/coatings15010109





