Effect of Quenching Cooling Rate on Hydrogen Embrittlement of Precipitation-Hardened Martensitic Stainless Steels
Abstract
1. Introduction
2. Materials and Methods
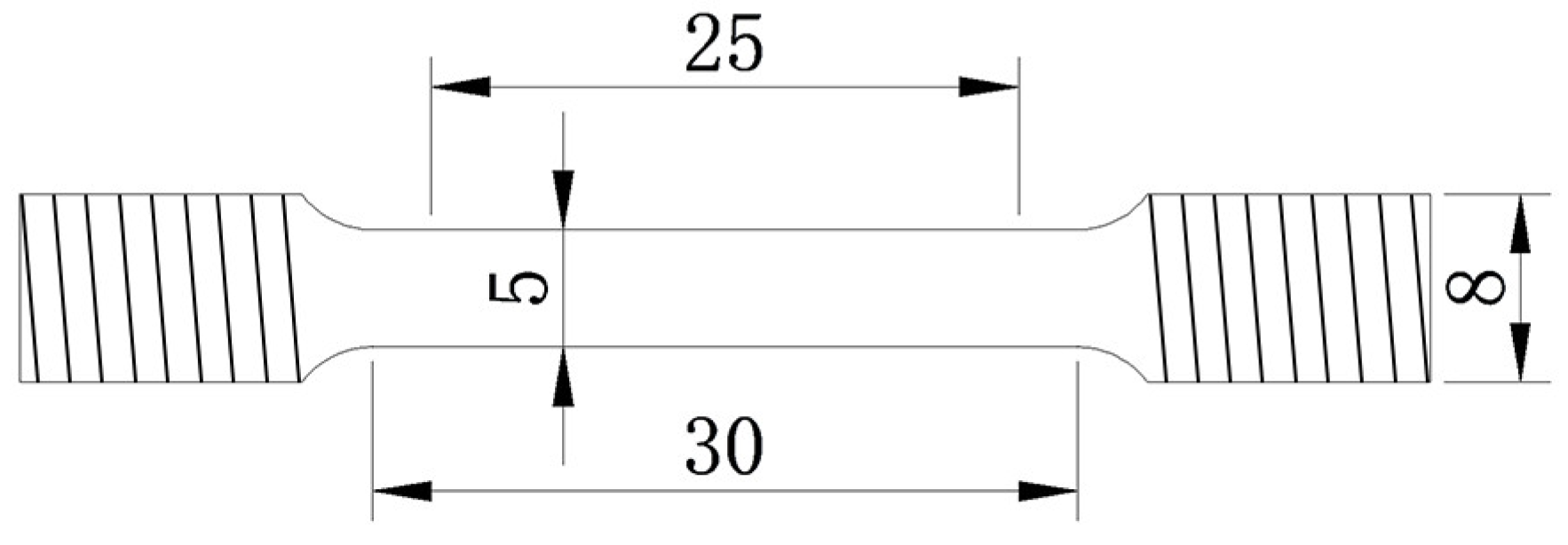
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Hydrogen Embrittlement Testing
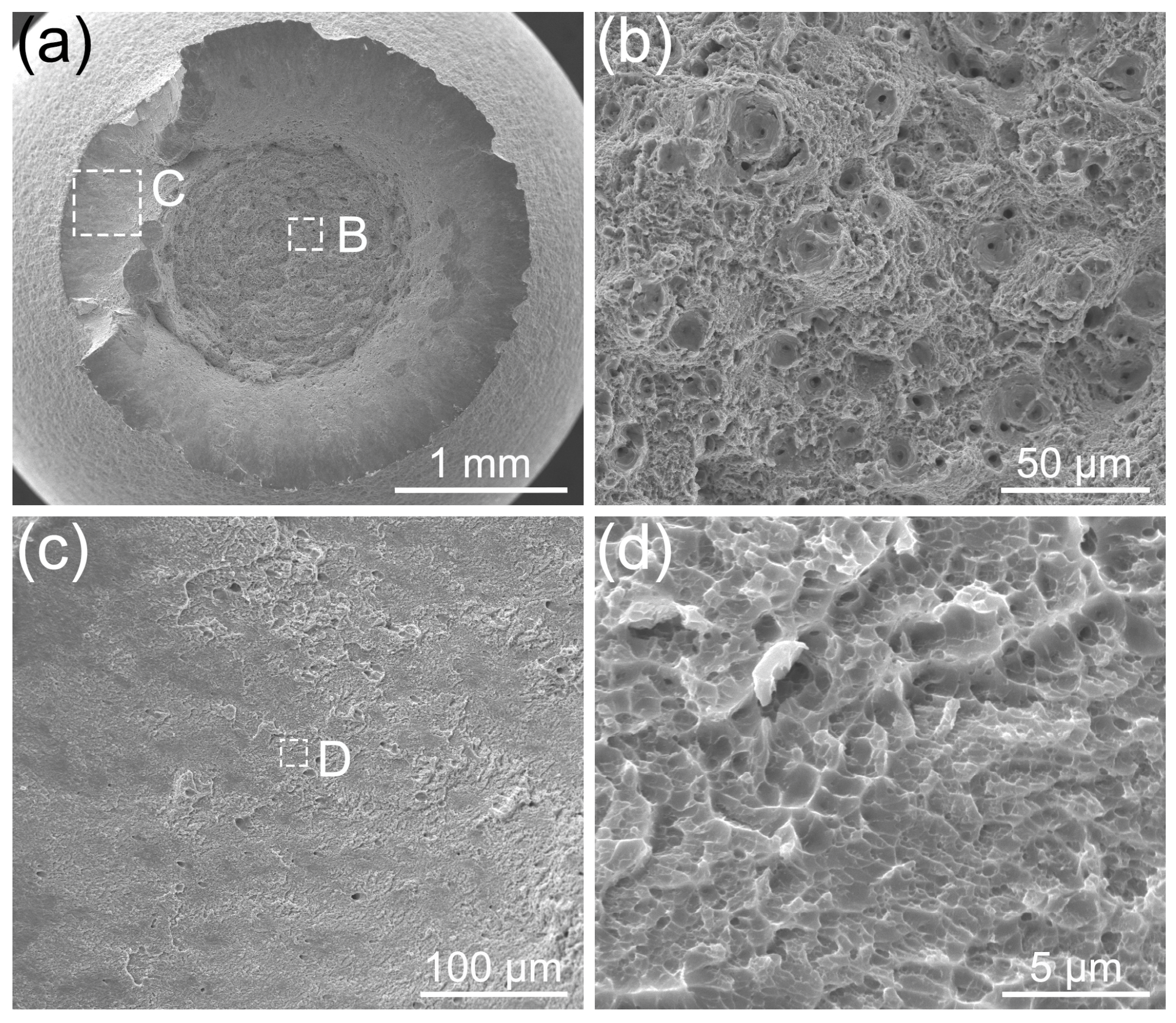
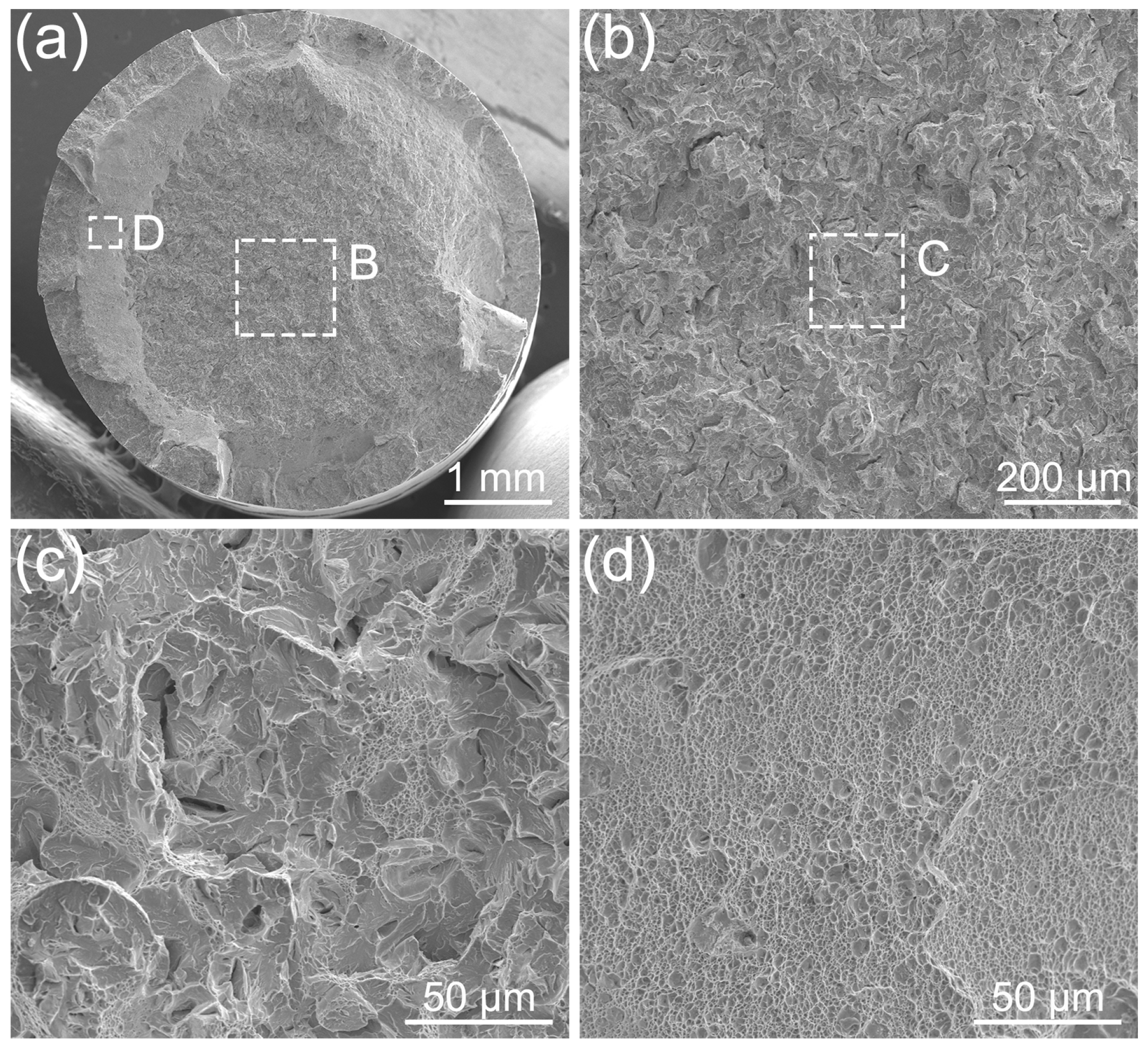
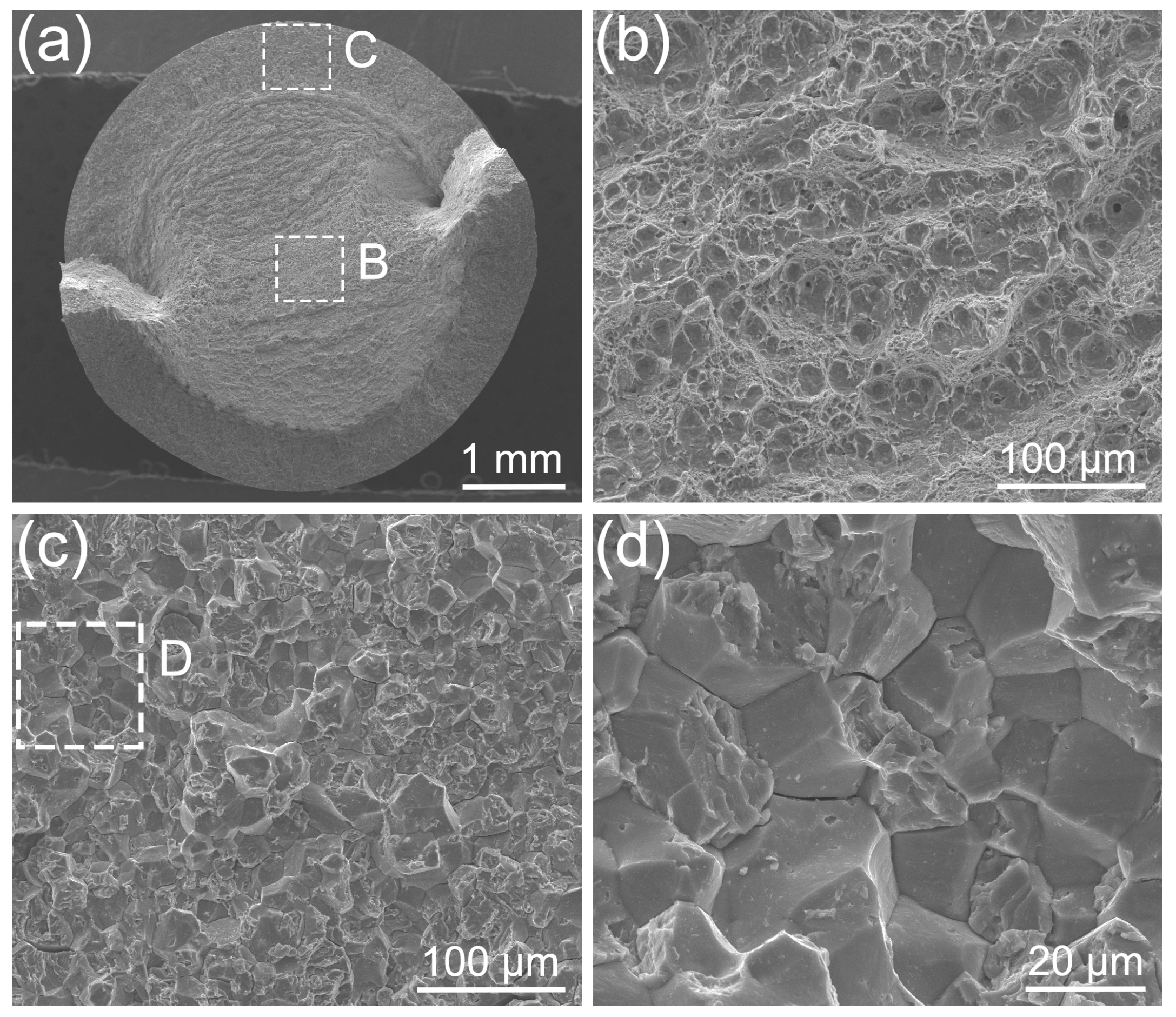
2.4. Fractographic Analysis
3. Results
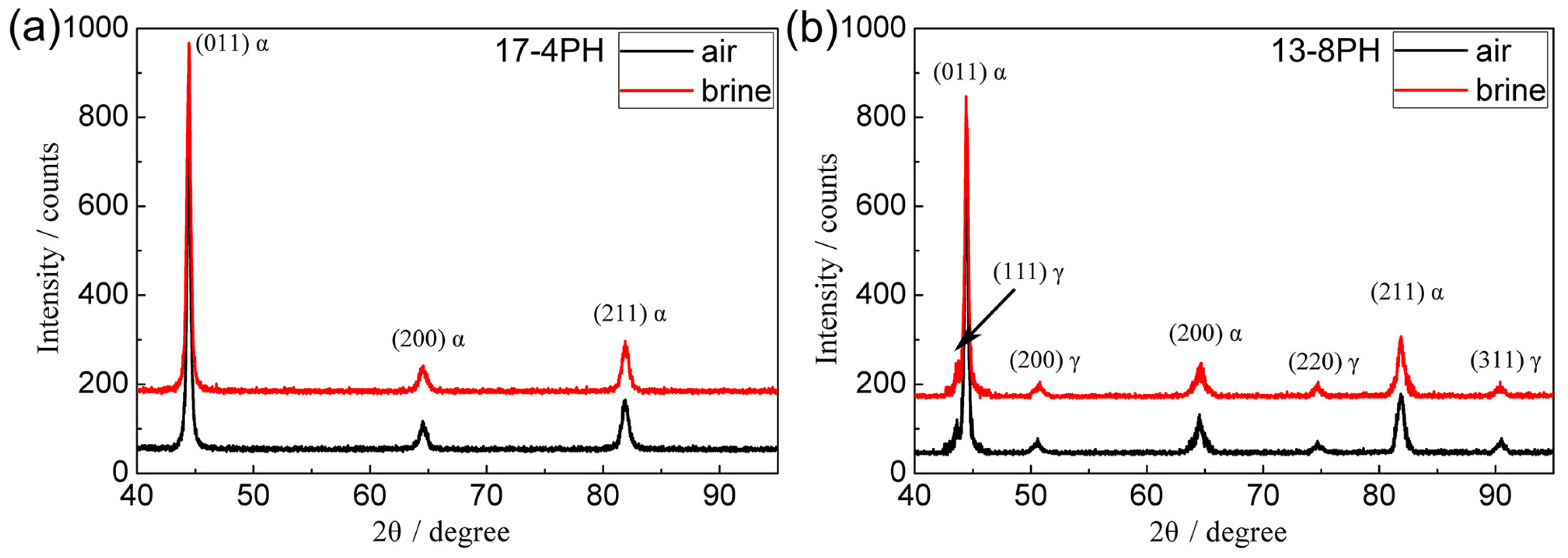
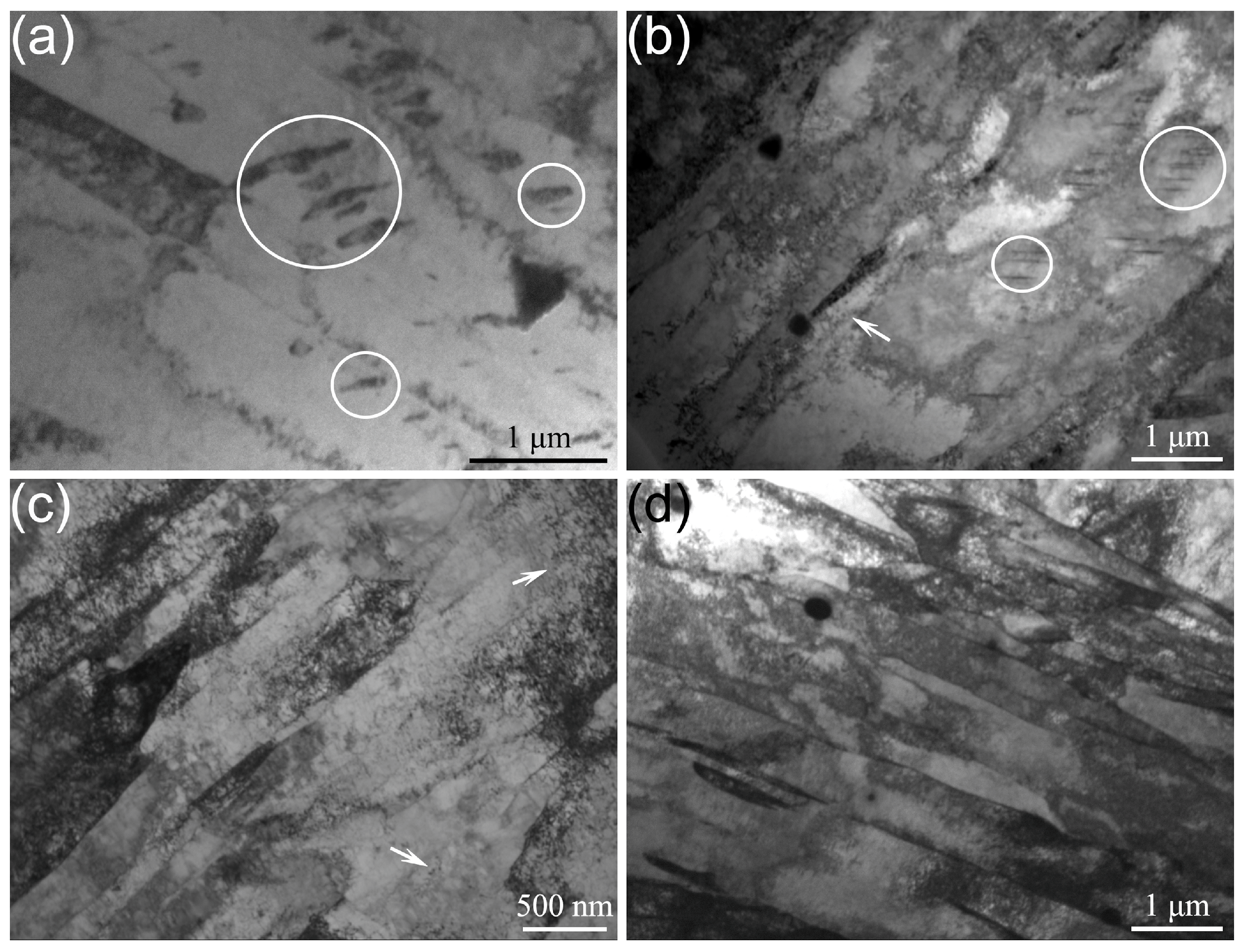
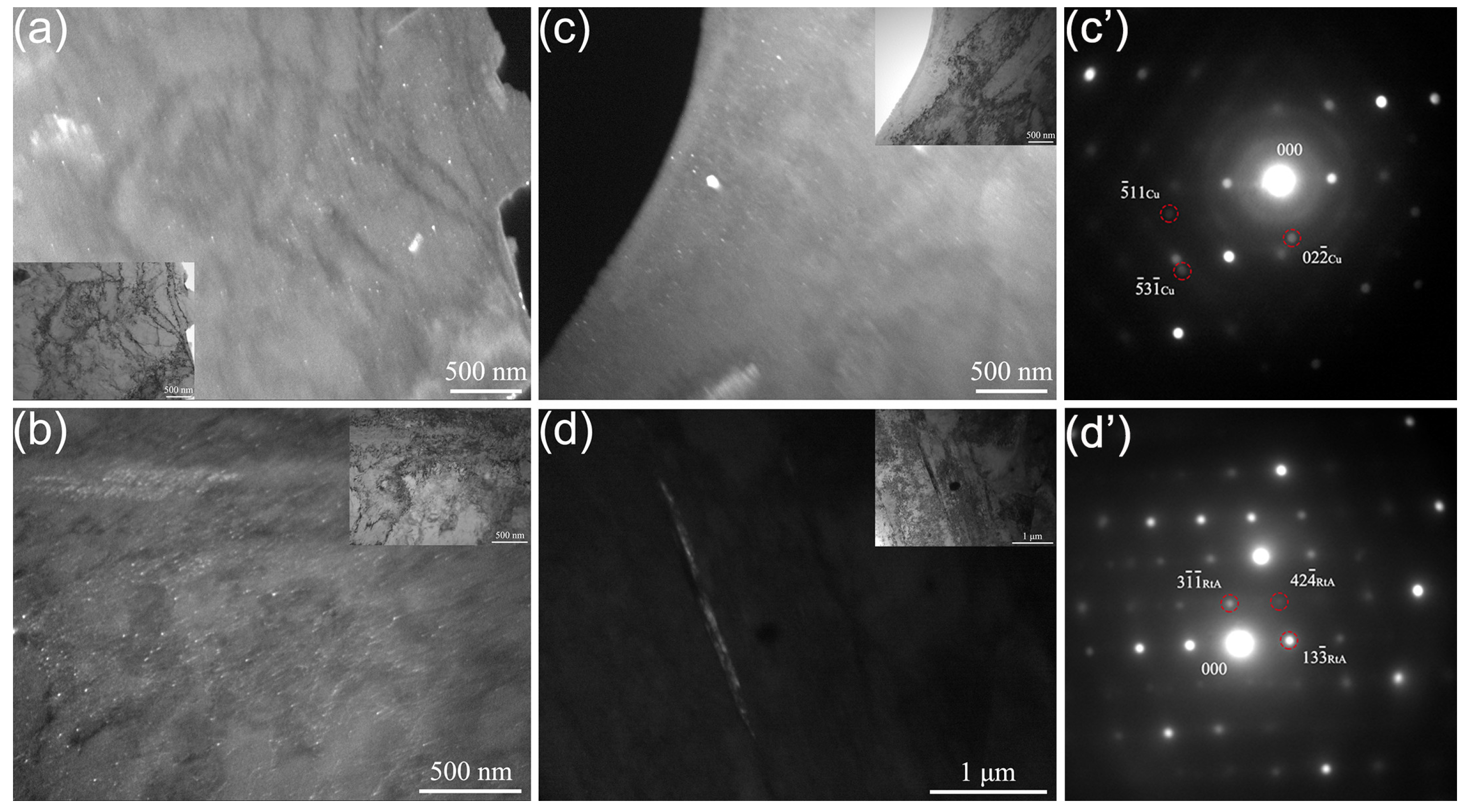
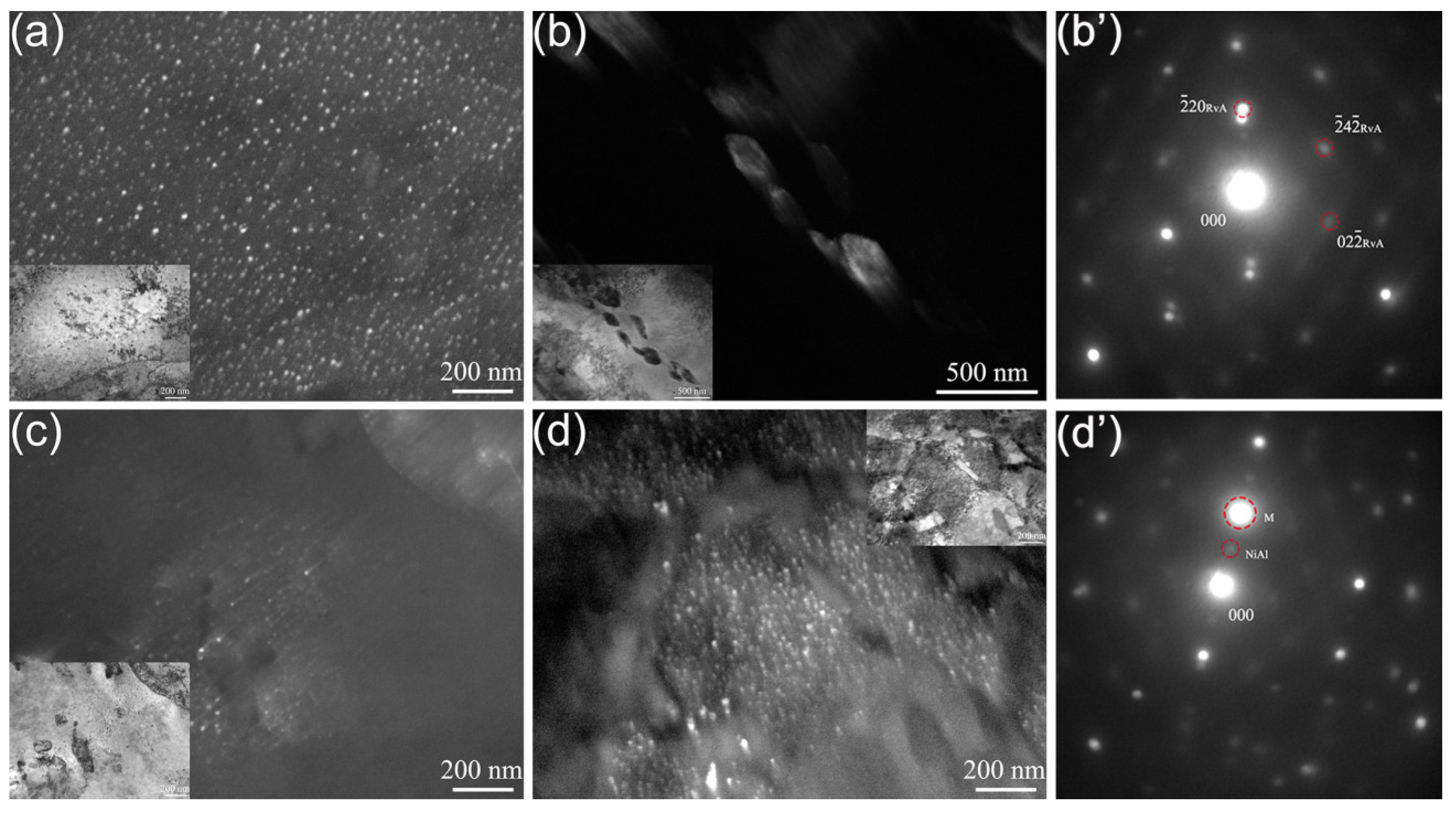
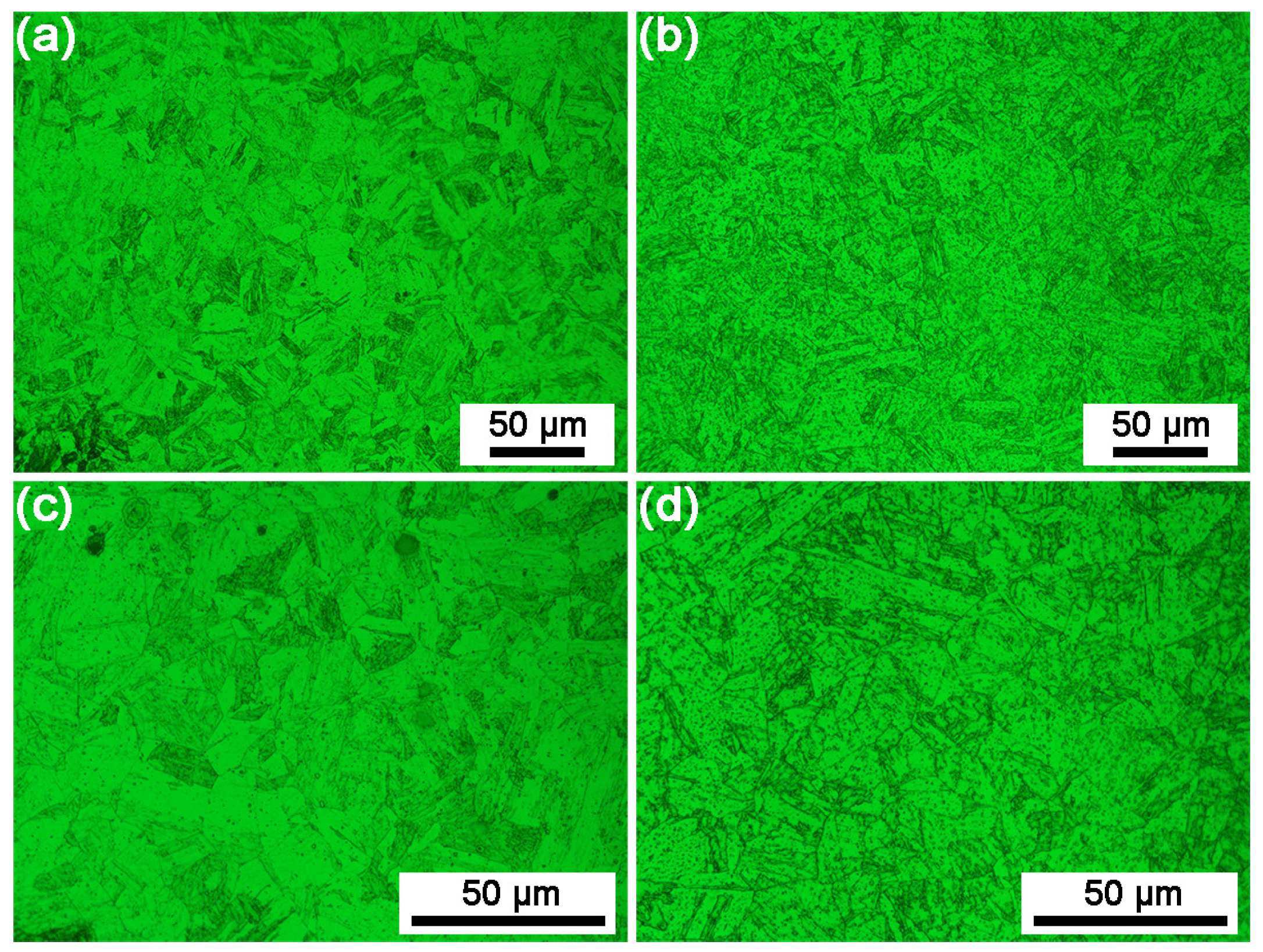
3.1. Microstructure
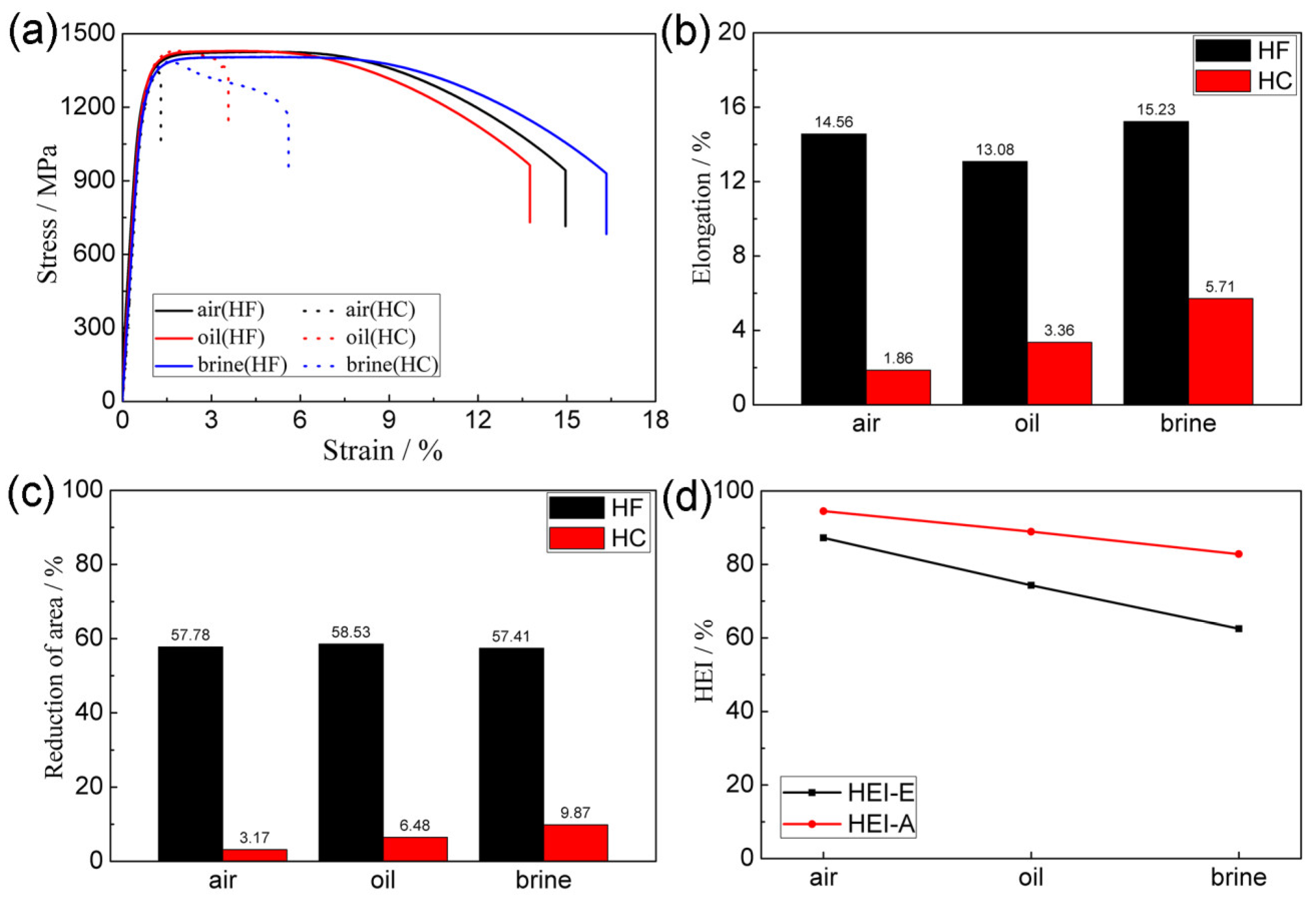
3.2. Tensile Properties
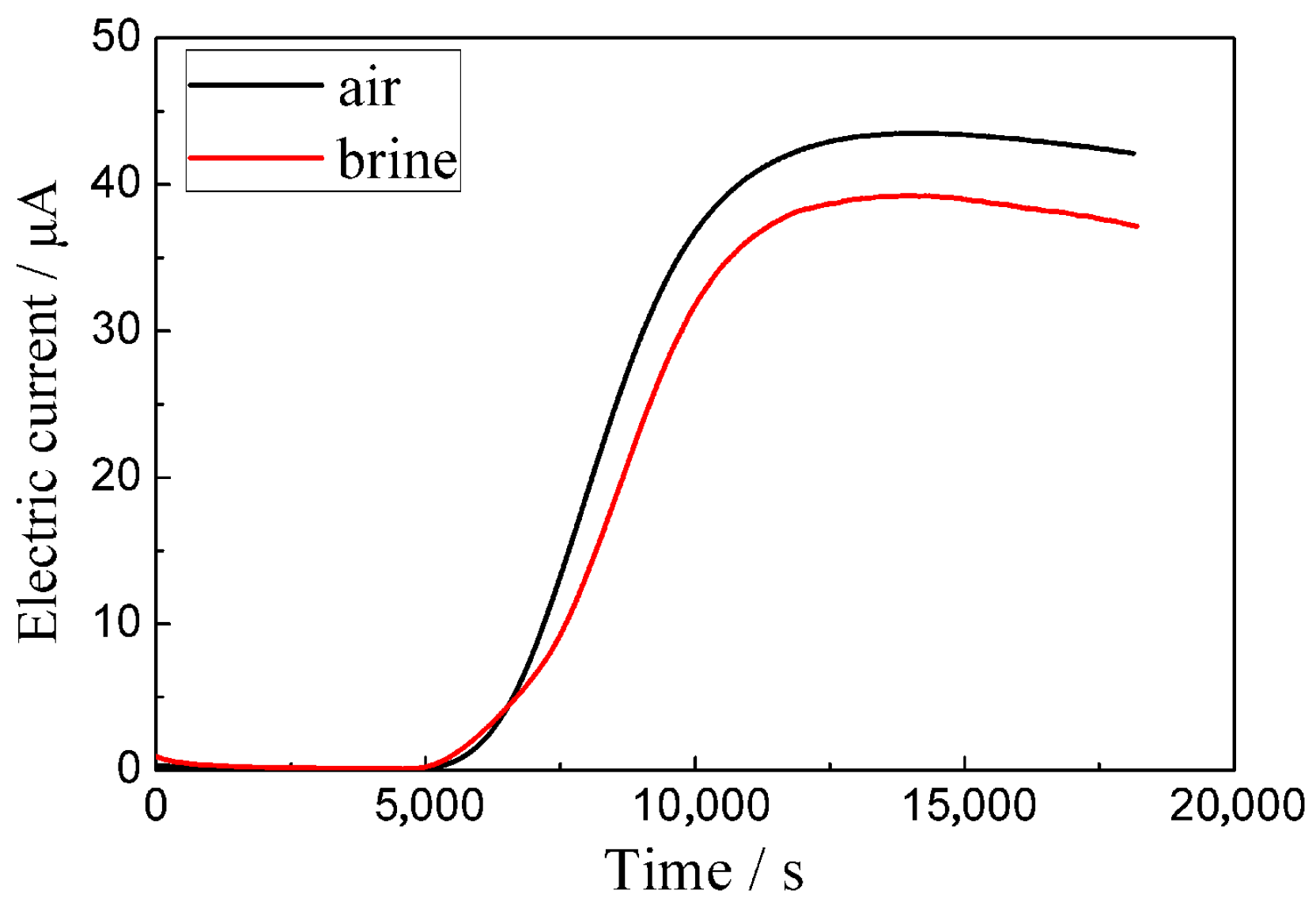
3.3. Fracture Analysis
4. Discussion
4.1. The Effect of the Quenching Cooling Rate on the Microstructure of Precipitation-Hardened Martensitic Stainless Steels
4.2. The Effect of the Quenching Cooling Rate on the Mechanical Properties and Susceptibility to Hydrogen Embrittlement
5. Conclusions
- (1)
- For precipitation-hardened martensitic stainless steels, the quenching cooling rate only affects specific microstructural characteristics. Increasing the quenching cooling rate reduces the number of twin structures and increases the proportion of high-angle grain boundaries;
- (2)
- The mechanical properties of hydrogen-free specimens of precipitation-hardened martensitic stainless steels are not affected by the quenching cooling rate. This is because the quenching cooling rate has little effect on the grain size and precipitates in steels;
- (3)
- The mechanical properties of both 17-4 PH and 13-8 PH are good. After hydrogen charging, both steels exhibited obvious hydrogen embrittlement, which manifested as a substantial loss of plasticity;
- (4)
- The hydrogen embrittlement susceptibility of both 17-4 PH and 13-8 PH decreases as the quenching cooling rate increases. The differences in the twin structure and misorientation angle caused by the quenching cooling rate are the main reasons for the distinct hydrogen embrittlement susceptibility.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Couturier, L.; Geuser, F.D.; Descoins, M.; Deschamps, A. Evolution of the microstructure of a 15-5PH martensitic stainless steel during precipitation hardening heat treatment. Mater. Des. 2016, 107, 416–425. [Google Scholar] [CrossRef]
- Bressan, J.D.; Daros, D.P.; Sokolowski, A.; Mesquita, R.A.; Barbosa, C.A. Influence of hardness on the wear resistance of 17-4 PH stainless steel evaluated by the pin-on-disc testing. J. Mater. Process. Technol. 2008, 205, 353–359. [Google Scholar] [CrossRef]
- Shiue, R.K.; Wu, S.K.; Shiue, J.Y. Infrared brazing of Ti–6Al–4V and 17-4 PH stainless steel with (Ni)/Cr barrier layer(s). Mater. Sci. Eng. A 2008, 488, 186–194. [Google Scholar] [CrossRef]
- Sen, D.; Patra, A.K.; Mazumder, S.; Mittra, J.; Dey, G.K.; De, P.K. Carbide precipitates in solution-quenched PH13-8 Mo stainless steel: A small-angle neutron scattering investigation. Mater. Sci. Eng. A 2005, 397, 370–375. [Google Scholar] [CrossRef]
- Pape, J.A.; Neu, R.W. A comparative study of the fretting fatigue behavior of 4340 steel and PH 13-8 Mo stainless steel. Int. J. Fatigue 2007, 29, 2219–2229. [Google Scholar] [CrossRef]
- Shen, S.; Song, X.; Li, Q.; Li, X.; Zhu, R.; Yang, G. Effect of CrxCy–NiCr coating on the hydrogen embrittlement of 17-4 PH stainless steel using the smooth bar tensile test. J. Mater. Sci. 2019, 54, 7356–7368. [Google Scholar] [CrossRef]
- Wang, S.; Quinn, S.; Starink, M.; Wood, R.; Wharton, J. Tribocorrosion damage of a Jethete M152 type stainless steel. Eng. Fail. Anal. 2008, 15, 903–912. [Google Scholar] [CrossRef][Green Version]
- Hsiao, C.N.; Chiou, C.S.; Yang, J.R. Aging reactions in a 17-4 PH stainless steel. Mater. Chem. Phys. 2002, 74, 134–142. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, W.; Vaumousse, D. Microstructural evolution in a PH13-8 stainless steel after ageing. Acta Mater. 2003, 51, 101–116. [Google Scholar] [CrossRef]
- Ping, D.H.; Ohnuma, M.; Hirakawa, Y.; Kadoya, Y.; Hono, K. Microstructural evolution in 13Cr–8Ni–2.5Mo–2Al martensitic precipitation-hardened stainless steel. Mater. Sci. Eng. A 2005, 394, 285–295. [Google Scholar] [CrossRef]
- Leitner, H.; Schnitzer, R.; Schober, M.; Zinner, S. Precipitate modification in PH13-8 Mo type maraging steel. Acta Mater. 2011, 59, 5012–5022. [Google Scholar] [CrossRef]
- Kakisawa, H.; Minagawa, K.; Kimura, T.; Halada, K. Effect of consolidating temperature on strengthening mechanism in Fe–Cu alloy from rapidly solidified powder. Mater. Sci. Technol. 2003, 19, 743–748. [Google Scholar] [CrossRef]
- Chou, S.L.; Tsai, W.T. Effect of grain size on the hydrogen-assisted cracking in duplex stainless steels. Mater. Sci. Eng. A 1999, 270, 219–224. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Liu, G. Hydrogen trapping in high strength martensitic steel after austenitized at different temperatures. Int. J. Hydrogen Energy 2013, 38, 14364–14368. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Silva, F.J.D.; Scandian, C.; Silva, G.F.D.; Abreu, H.F.G.D. Microstructure and intergranular corrosion resistance of UNS S17400 (17-4PH) stainless steel. Corros. Sci. 2010, 52, 3835–3839. [Google Scholar] [CrossRef]
- Chiang, W.C.; Pu, C.C.; Yu, B.L.; Wu, J.K. Hydrogen susceptibility of 17-4 PH stainless steel. Mater. Lett. 2003, 57, 2485–2488. [Google Scholar] [CrossRef]
- Wu, J.H.; Lin, C.K. Effect of strain rate on high-temperature low-cycle fatigue of 17-4 PH stainless steels. Mater. Sci. Eng. A 2005, 390, 291–298. [Google Scholar] [CrossRef]
- Arisoy, C.F.; Başman, G.; Şeşen, M.K. Failure of a 17-4 PH stainless steel sailboat propeller shaft. Eng. Fail. Anal. 2003, 10, 711–717. [Google Scholar] [CrossRef]
- Atxaga, G.; Pelayo, A.; Irisarri, A.M. Failure analysis of a set of stainless steel disc springs. Eng. Fail. Anal. 2006, 13, 226–234. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Pardal, J.M.; Menezes, L.; Menezes, C.A.B.; D’Ávila, C. Failure analysis of PSV springs of 17-4PH stainless steel. Eng. Fail. Anal. 2009, 16, 1757–1764. [Google Scholar] [CrossRef]
- Ding, Y.S.; Tsay, L.W.; Chiang, M.F.; Chen, C. Gaseous hydrogen embrittlement of PH 13-8 Mo steel. J. Nucl. Mater. 2009, 385, 538–544. [Google Scholar] [CrossRef]
- Tsay, L.W.; Chi, M.Y.; Chen, H.R.; Chen, C. Investigation of hydrogen sulfide stress corrosion cracking of PH 13-8 Mo stainless steel. Mater. Sci. Eng. A 2006, 416, 155–160. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Chen, J.; Shen, S.; Yang, G.; Wang, T.; Song, X. Effect of aging treatment on Hydrogen embrittlement of PH 13-8 Mo martensite stainless steel. Mater. Sci. Eng. A 2015, 651, 474–485. [Google Scholar] [CrossRef]
- Tsay, L.W.; Chi, M.Y.; Wu, Y.F.; Wu, J.K.; Lin, D.Y. Hydrogen embrittlement susceptibility and permeability of two ultra-high strength steels. Corros. Sci. 2006, 48, 1926–1938. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Rong, L. Effect of grain size on the hydrogen embrittlement sensitivity of a precipitation strengthened Fe–Ni based alloy. Mater. Sci. Eng. A 2014, 594, 98–102. [Google Scholar] [CrossRef]
- Shen, S.; Li, X.; Zhang, P.; Nan, Y.; Yang, G.; Song, X. Effect of solution-treated temperature on hydrogen embrittlement of 17-4 PH stainless steel. Mater. Sci. Eng. A 2017, 703, 413–421. [Google Scholar] [CrossRef]
- Fuchigami, H.; Minami, H.; Nagumo, M. Effect of grain size on the susceptibility of martensitic steel to hydrogen-related failure. Philos. Mag. Lett. 2006, 86, 21–29. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Fu, H.; Xie, J. Effect of quench cooling rate on residual stress, microstructure and mechanical property of an Fe–6.5Si alloy. Mater. Sci. Eng. A 2011, 530, 519–524. [Google Scholar] [CrossRef]
- Ammar, H.R.; Moreau, C.; Samuel, A.M.; Samuel, F.H.; Doty, H.W. Influences of alloying elements, solution treatment time and quenching media on quality indices of 413-type Al–Si casting alloys. Mater. Sci. Eng. A 2008, 489, 426–438. [Google Scholar] [CrossRef]
- Nishibata, T.; Kojima, N. Effect of quenching rate on hardness and microstructure of hot-stamped steel. J. Alloys Compd. 2013, 577, S549–S554. [Google Scholar] [CrossRef]
- Bilmes, P.D.; Solari, M.; Llorente, C.L. Characteristics and effects of austenite resulting from tempering of 13Cr–NiMo martensitic steel weld metals. Mater. Charact. 2001, 46, 285–296. [Google Scholar] [CrossRef]
- Schnitzer, R.; Radis, R.; Nöhrer, M.; Schober, M.; Hochfellner, R.; Zinner, S.; Povoden-Karadeniz, E.; Kozeschnik, E.; Leitner, H. Reverted austenite in PH 13-8 Mo maraging steels. Mater. Chem. Phys. 2010, 122, 138–145. [Google Scholar] [CrossRef]
- Maddi, L.; Ballal, A.R.; Peshwe, D.R.; Paretkar, R.K.; Laha, K.; Mathew, M.D. Effect of tempering temperature on the stress rupture properties of Grade 92 steel. Mater. Sci. Eng. A 2015, 639, 431–438. [Google Scholar] [CrossRef]
- Zan, N.; Ding, H.; Guo, X.F.; Tang, Z.Y.; Bleck, W. Effects of grain size on hydrogen embrittlement ina Fe-22Mn-0.6C TWIP steel. Int. J. Hydrogen Energy 2015, 40, 10687–10696. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted failure in a twinning-induced plasticity steel studied under in situ hydrogen charging by electron channeling contrast imaging. Acta Mater. 2013, 61, 4607–4618. [Google Scholar] [CrossRef]
- Oudriss, A.; Creus, J.; Bouhattate, J.; Conforto, E.; Berziou, C.; Savall, C.; Feaugas, X. Grain size and grain-boundary effects on diffusion and trapping of hydrogen in pure nickel. Acta Mater. 2012, 60, 6814–6828. [Google Scholar] [CrossRef]
- Deng, W.; Gao, X.; Qin, X.; Gao, X.; Zhao, D.; Du, L. Effect of cooling rate on microstructure of deformed and undeformed X80 pipeline steels. Acta Metal. Sin. 2010, 46, 959–966. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Liu, S.; Zhang, Y.; Deng, Y. Quench-induced contributions of high angle grain boundary and low angle grain boundary to exfoliation corrosion propagation in an AlZnMgCu alloy. J. Mater. Res. Technol. 2021, 15, 6866–6870. [Google Scholar] [CrossRef]




| C | Cr | Ni | Cu | Nb | Mo | Al | Mn | Si | |
|---|---|---|---|---|---|---|---|---|---|
| 17-4 PH | 0.01 | 16.14 | 4.07 | 3.15 | 0.26 | -- | -- | 0.48 | 0.82 |
| 13-8 PH | 0.04 | 12.58 | 8.26 | -- | -- | 2.25 | 1.07 | 0.01 | 0.02 |
| Air-Quenched (HF) | Oil-Quenched (HF) | Brine-Quenched (HF) | Air-Quenched (HC) | Oil-Quenched (HC) | Brine-Quenched (HC) | |
|---|---|---|---|---|---|---|
| YS (MPa) | 1271 | 1293 | 1265 | 1264 | 1300 | 1276 |
| UTS (MPa) | 1426 | 1430 | 1404 | 1343 | 1429 | 1386 |
| Air-Quenched (HF) | Oil-Quenched (HF) | Brine-Quenched (HF) | Air-Quenched (HC) | Oil-Quenched (HC) | Brine-Quenched (HC) | |
|---|---|---|---|---|---|---|
| YS (MPa) | 1097 | 1095 | 1098 | 1070 | 1071 | 1077 |
| UTS (MPa) | 1194 | 1197 | 1175 | 1111 | 1123 | 1128 |
| J∞L (mol/m s) | Deff (m2/s) | Capp (mol/m3) | |
|---|---|---|---|
| Air-quenched | 7.19 × 10−10 | 1.92 × 10−12 | 375 |
| Brine-quenched | 6.88 × 10−10 | 2.15 × 10−12 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Ma, X.; Song, X.; Zhao, W.; Shen, Y. Effect of Quenching Cooling Rate on Hydrogen Embrittlement of Precipitation-Hardened Martensitic Stainless Steels. Coatings 2024, 14, 572. https://doi.org/10.3390/coatings14050572
Shen S, Ma X, Song X, Zhao W, Shen Y. Effect of Quenching Cooling Rate on Hydrogen Embrittlement of Precipitation-Hardened Martensitic Stainless Steels. Coatings. 2024; 14(5):572. https://doi.org/10.3390/coatings14050572
Chicago/Turabian StyleShen, Sicong, Xingyu Ma, Xiaolong Song, Wenwen Zhao, and Yong Shen. 2024. "Effect of Quenching Cooling Rate on Hydrogen Embrittlement of Precipitation-Hardened Martensitic Stainless Steels" Coatings 14, no. 5: 572. https://doi.org/10.3390/coatings14050572
APA StyleShen, S., Ma, X., Song, X., Zhao, W., & Shen, Y. (2024). Effect of Quenching Cooling Rate on Hydrogen Embrittlement of Precipitation-Hardened Martensitic Stainless Steels. Coatings, 14(5), 572. https://doi.org/10.3390/coatings14050572





