Study on Microstructure and Mechanical Properties Modification of Cu-Ti3AlC2 Composites by Ni Element
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
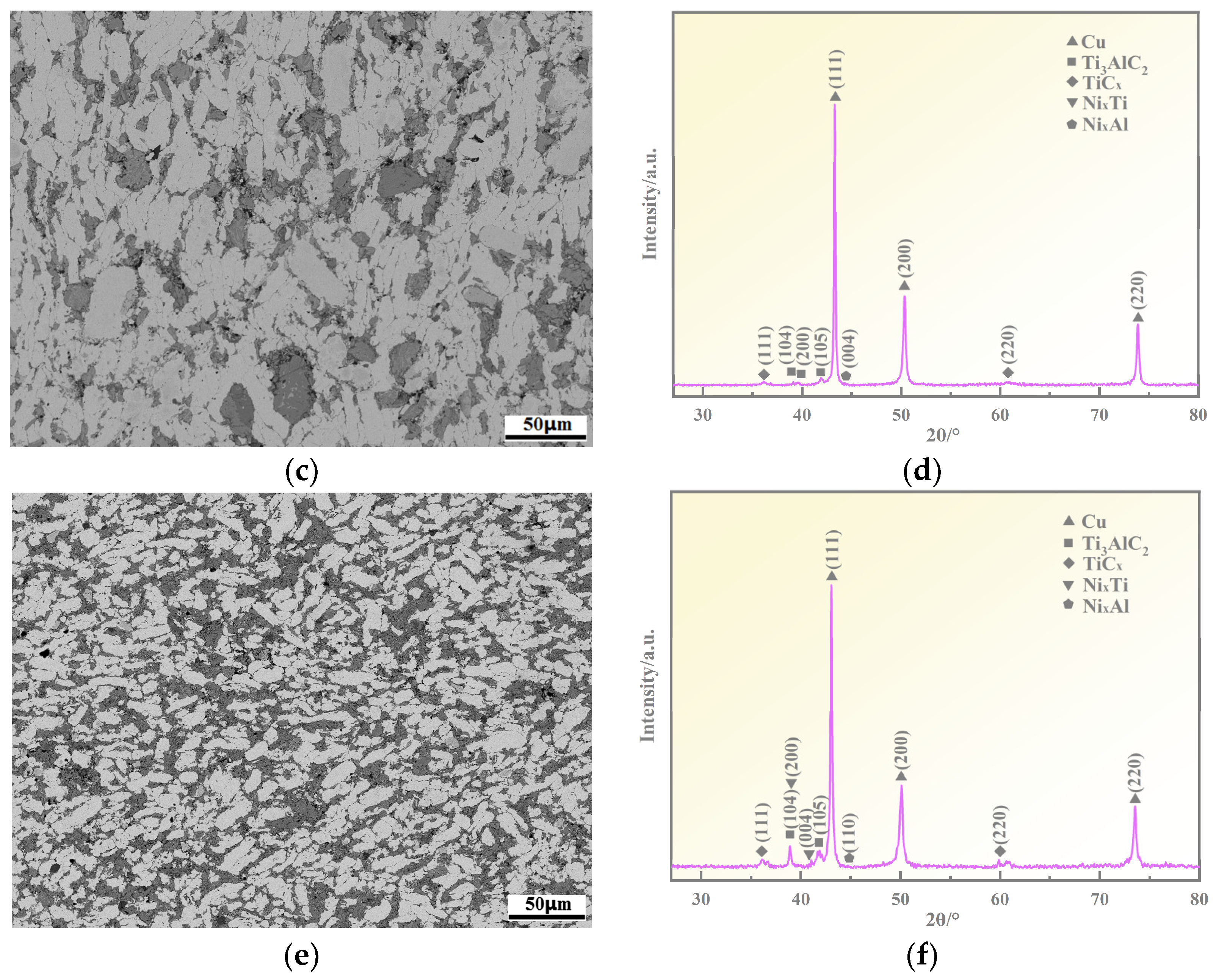
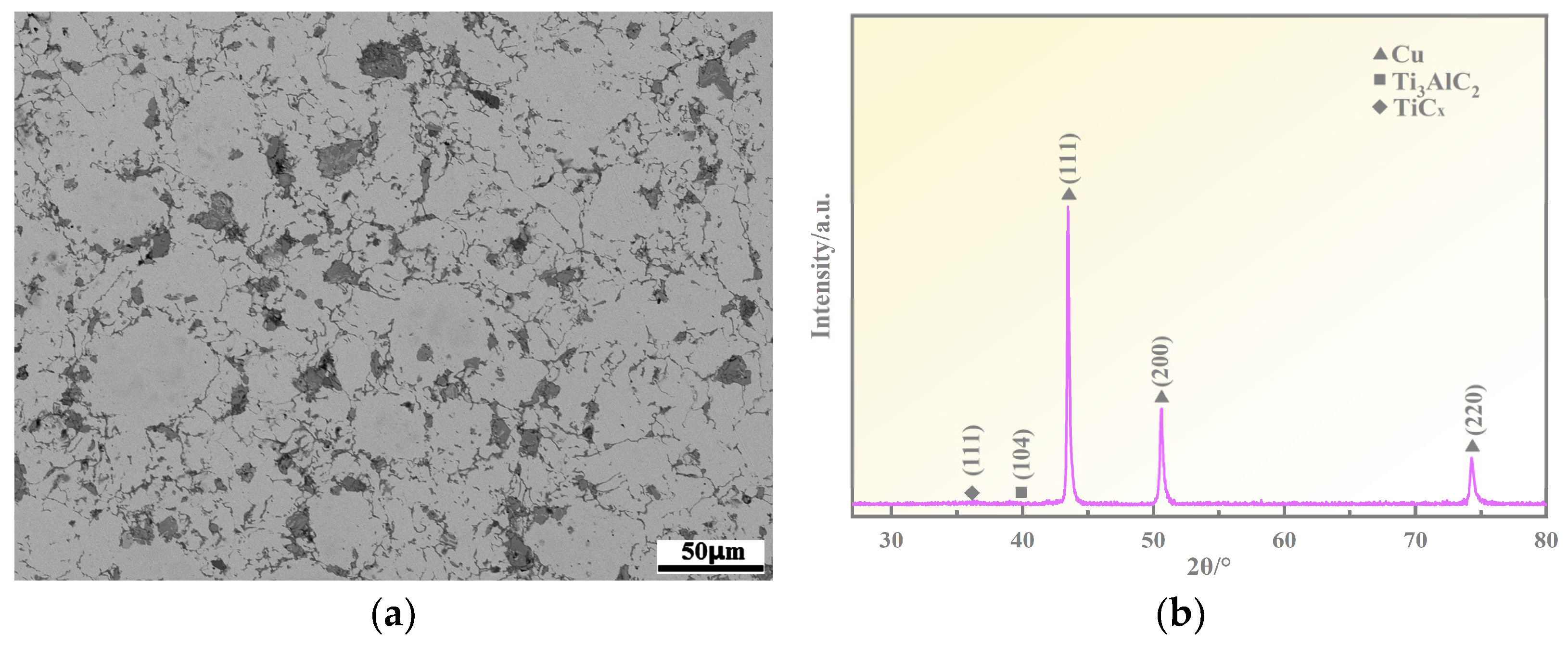
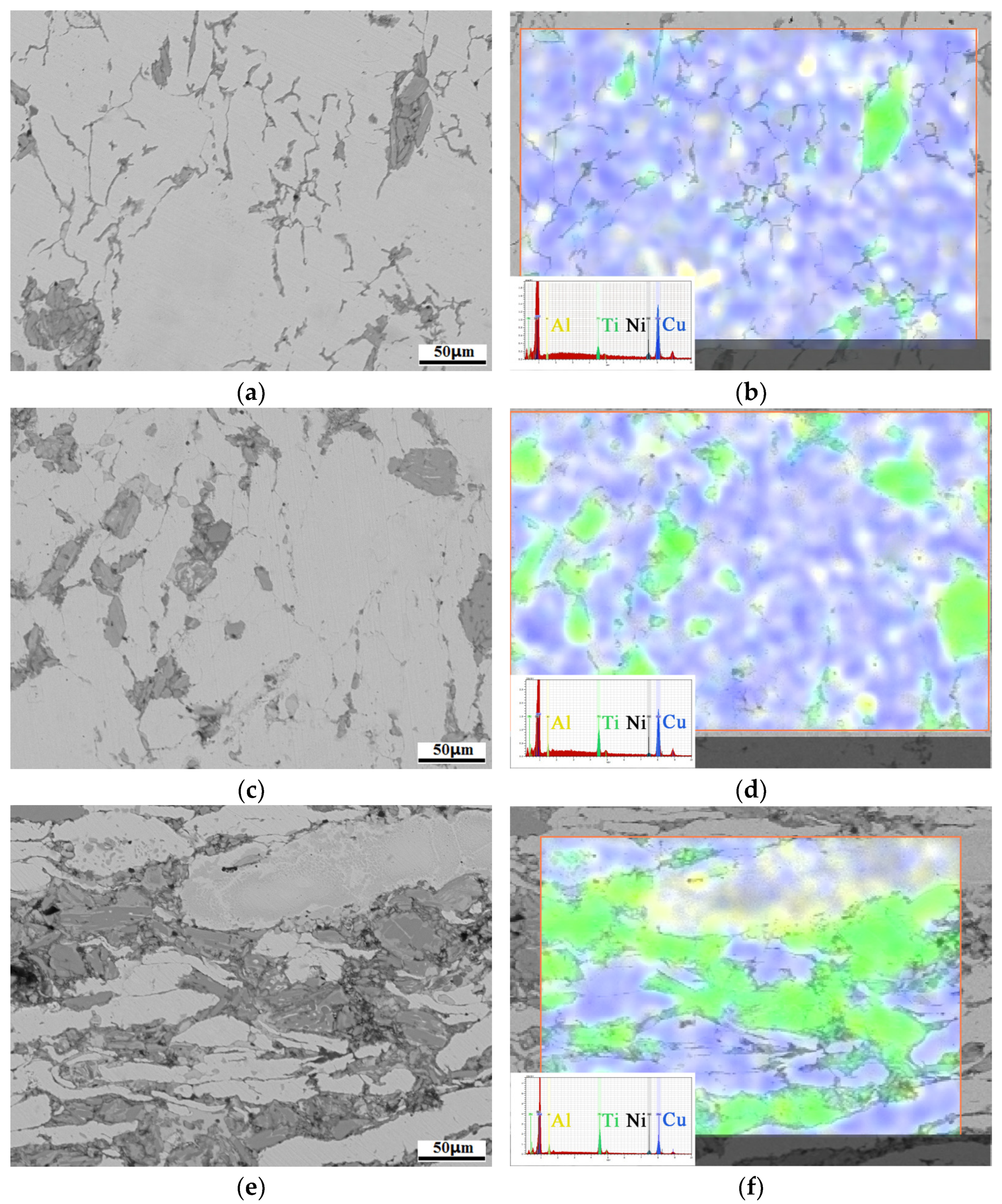
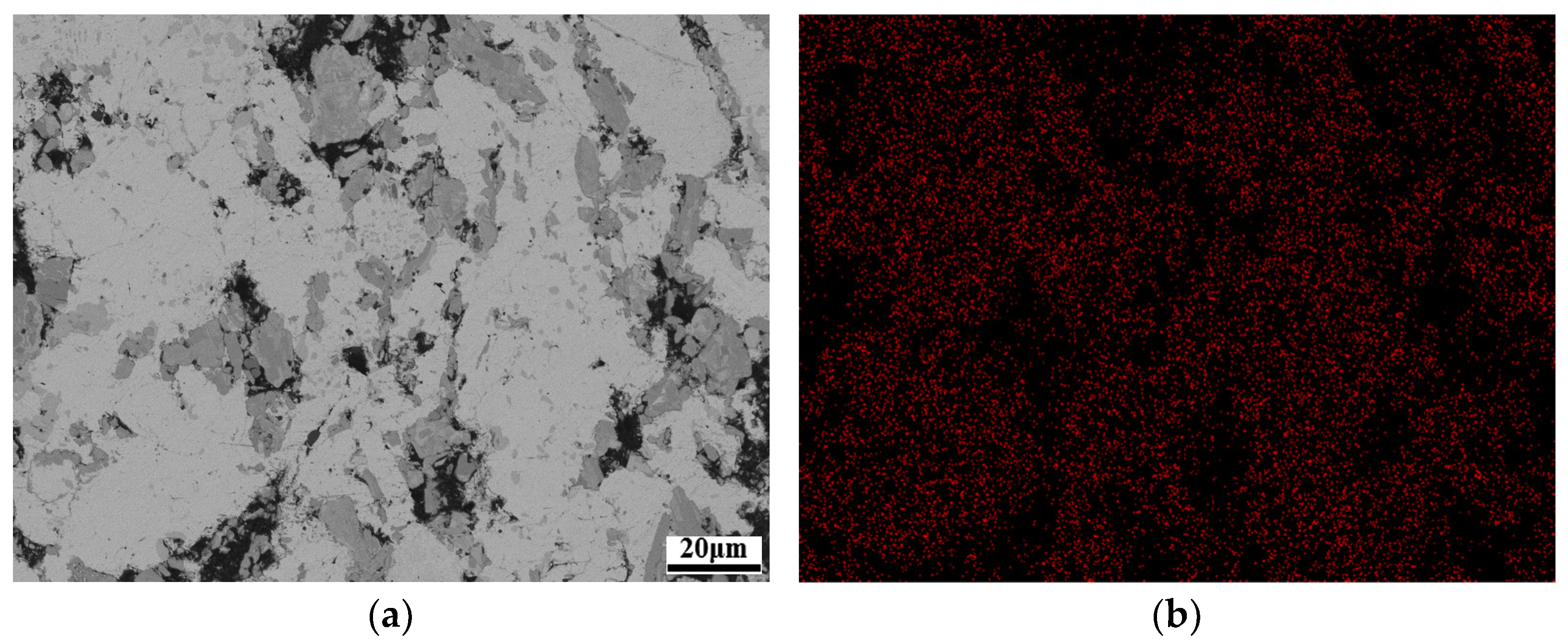
3.1. Microstructure Analyses
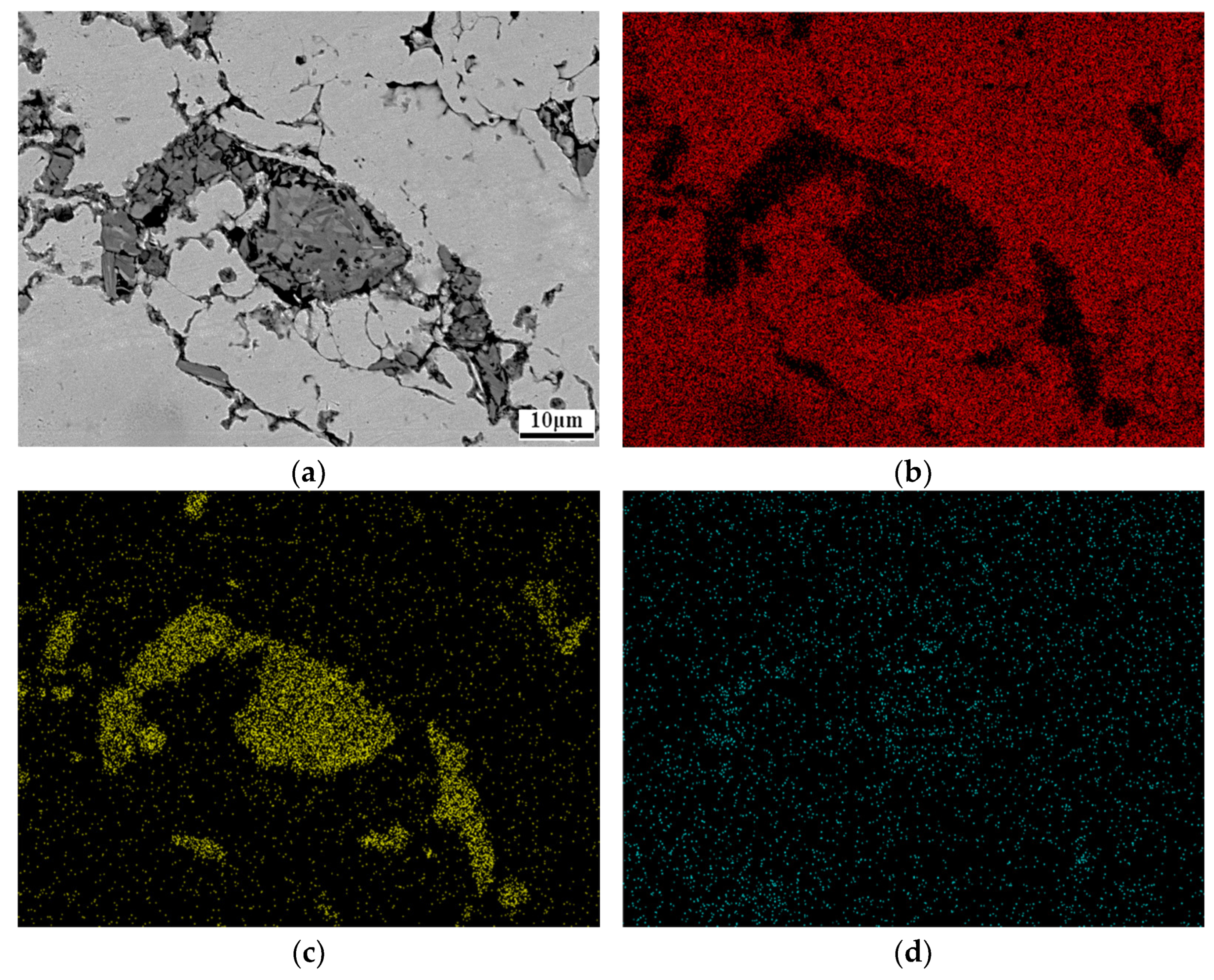
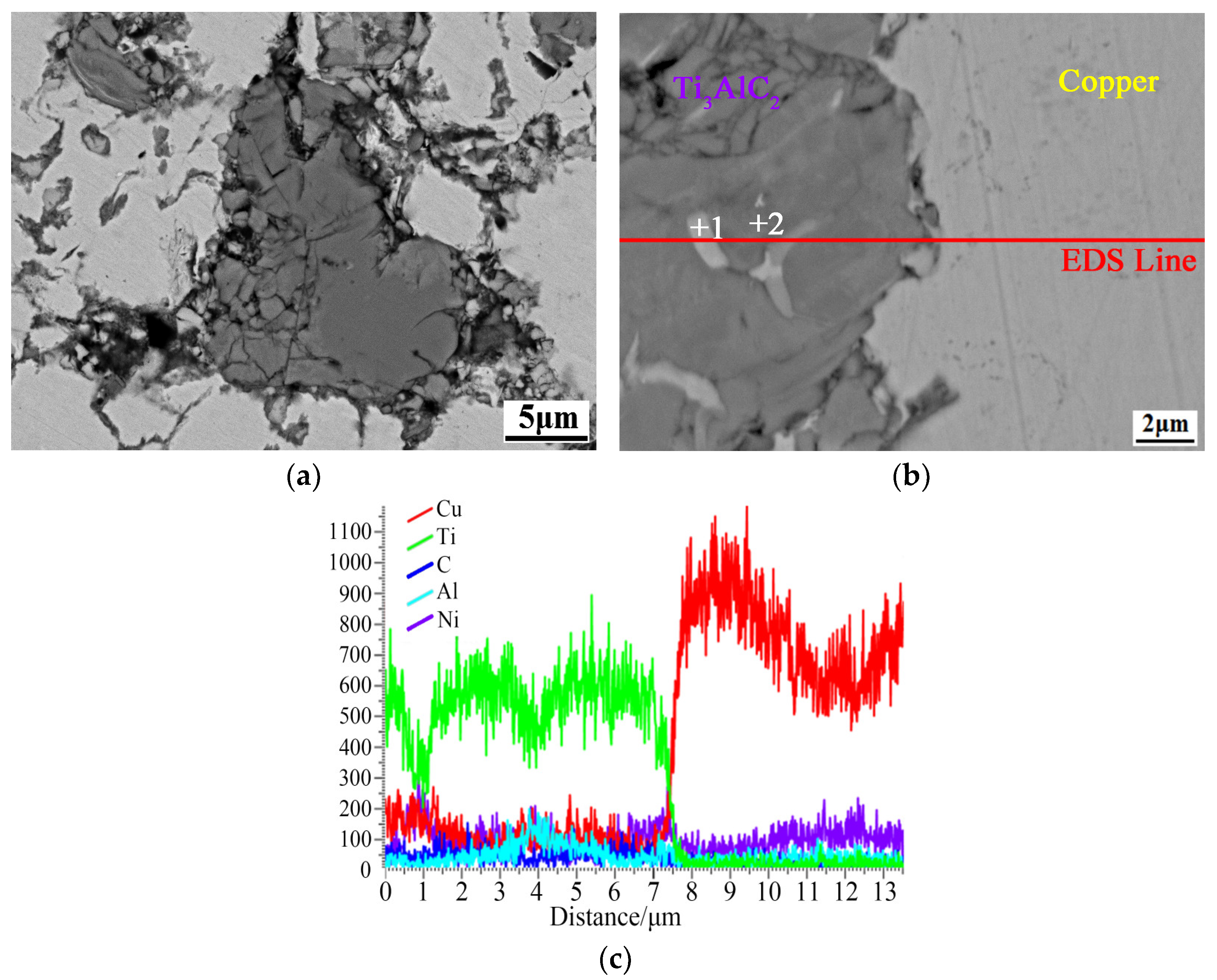
3.2. Ti3AlC2 Reinforcement Phase and the Interface
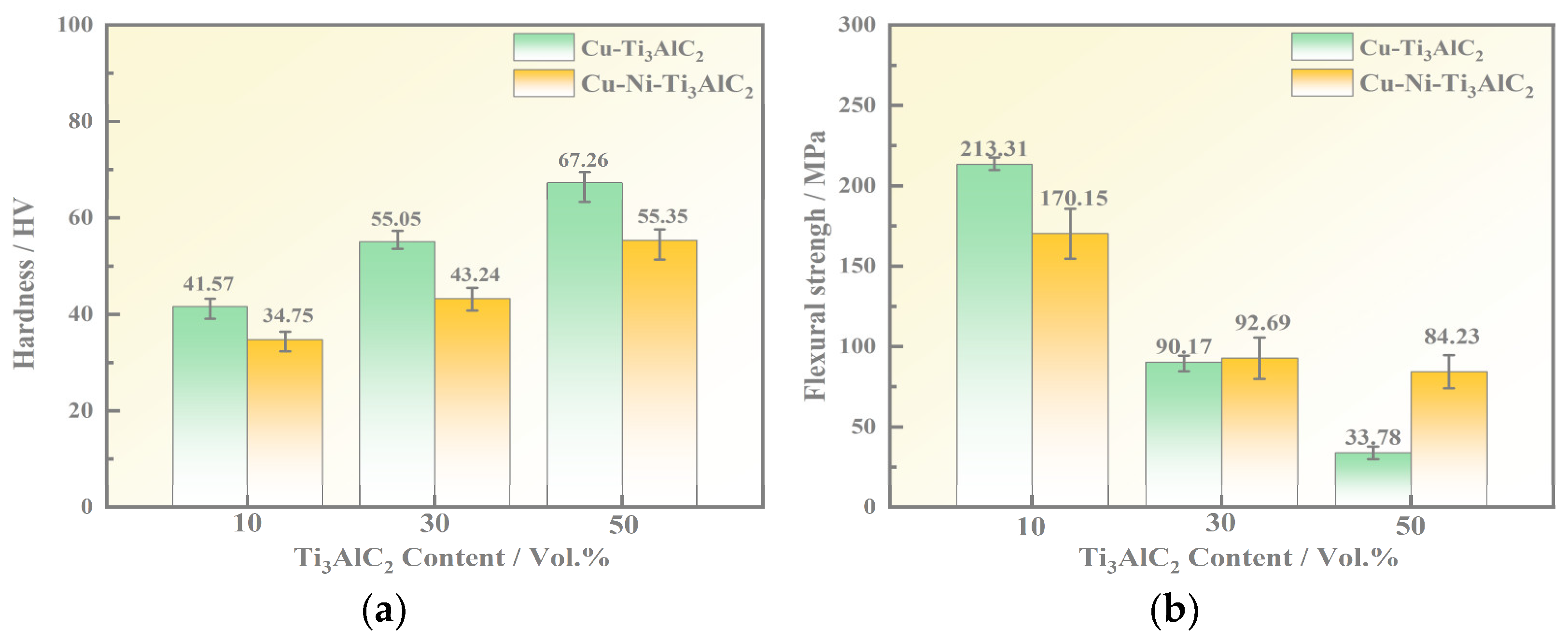
3.3. Mechanical Properties
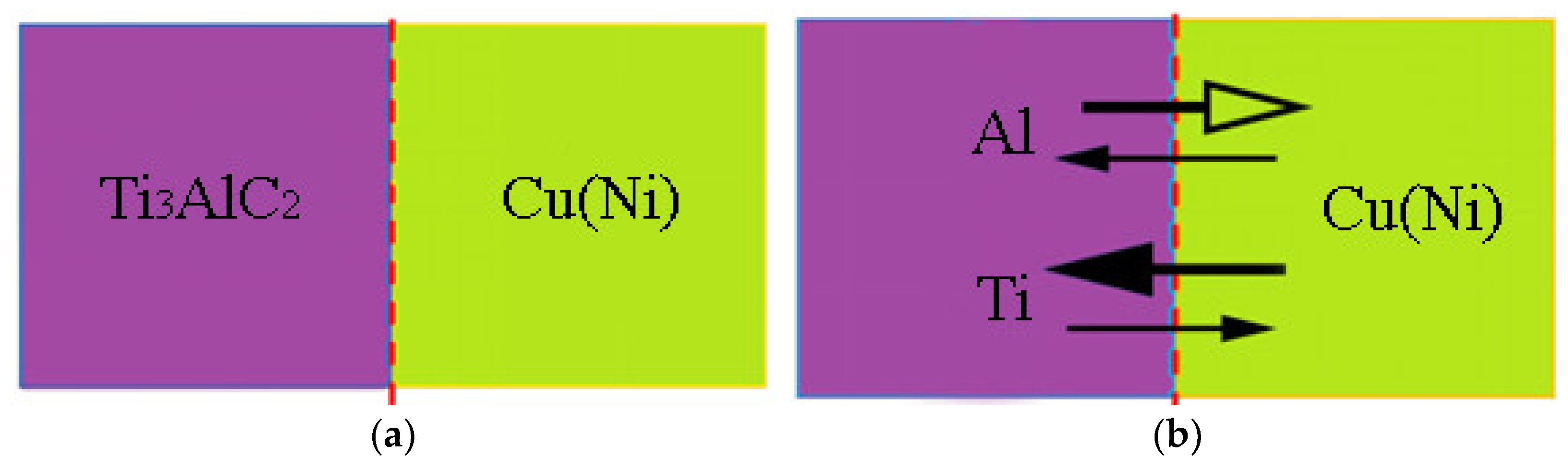
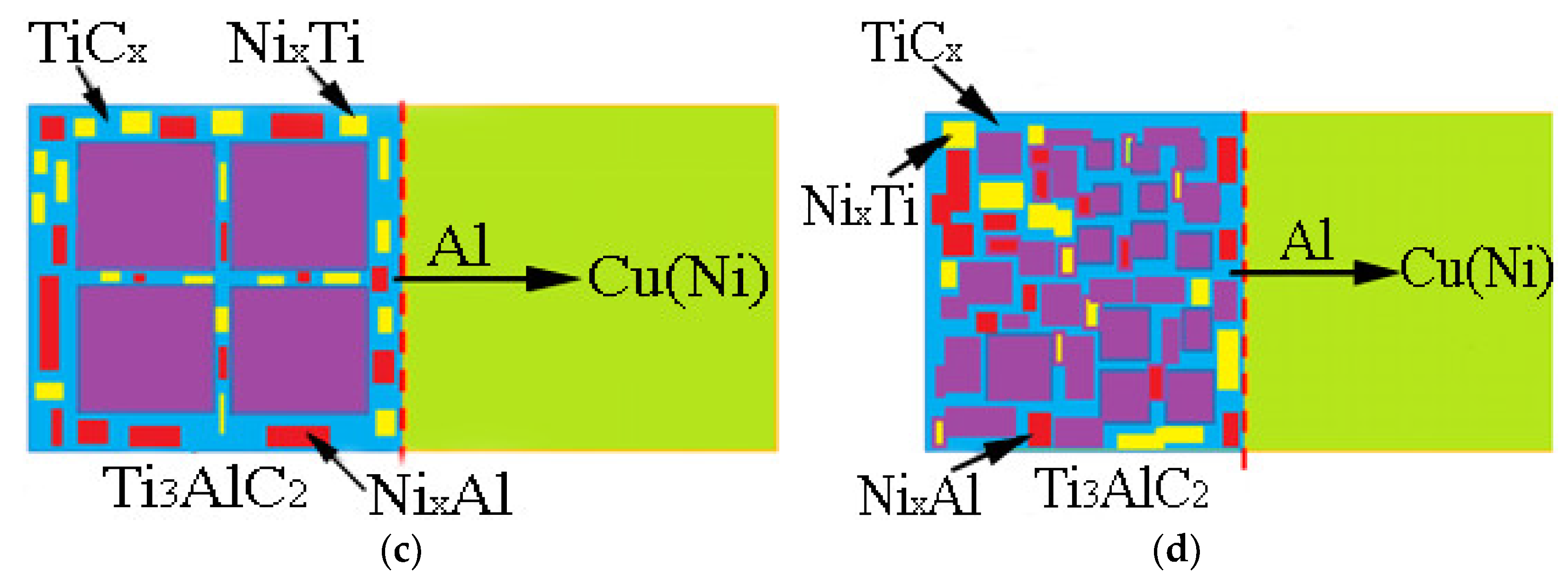
3.4. Mechanism of Anti-Decomposition of Ni Element
4. Conclusions
- The addition of Ni elements results in a microstructure consisting of Ti3AlC2, TiCx, NixAl, NixTi, and a Cu(Ni) matrix in the Cu-Ni-Ti3AlC2 composites. As the volume fraction of Ti3AlC2 particles increases, the morphology changes from a finely dispersed reinforcement phase to a continuous network. This leads to a reduction in the number and volume of pores compared to Cu-Ti3AlC2 composites. This reduction is particularly significant when the volume fraction of Ti3AlC2 exceeds 50%.
- The formation of NixAl and NixTi compounds at the grain boundary of the reinforcement phase, after the addition of Ni elements, restricts the diffusion of Al elements. This mechanism contributes to the anti-decomposition effect of Ni in Cu-Ni-Ti3AlC2 composites.
- The addition of Ni elements improves the mechanical properties of the composites. In terms of hardness, composites with low Ti3AlC2 content experience enhanced hardness, while those with high Ti3AlC2 content may exhibit a slight decrease. Regarding bending strength, composites with low Ti3AlC2 content show a decrease, while those with high content show a significant improvement. Notably, composites with a Ti3AlC2 content of 50% experience a significant increase in flexural strength, rising from 33.78 MPa to 84.23 MPa upon the addition of Ni elements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; He, L.; Zhou, Y. Highly conductive and strengthened copper matrix composite reinforced by Zr2Al3C4 particulates. Scr. Mater. 2009, 60, 976–979. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Li, Y.; Zhai, W.; Sun, L.; Zhang, C. Review on preparation and application of copper-steel bimetal composites. Emerg. Mater. Res. 2019, 8, 538–551. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Zheng, B.; Wang, S.; Yi, Y.; Zhang, Y.; Li, W. On the tribological behaviors of Cu matrix composites with different Cu-coated graphite content. J. Mater. Res. Technol. 2023, 25, 83–94. [Google Scholar] [CrossRef]
- Kumar, N.; Kumaran, S.; Kumaraswamidhas, L. High temperature investigation on EDM process of Al2618 alloy reinforced with Si3N4, AlN and ZrB2 in-situ composites. J. Alloys Compd. 2016, 663, 755–768. [Google Scholar] [CrossRef]
- Moazami-Goudarzi, M.; Akhlaghi, F. Wear behavior of Al5252 alloy reinforced with micrometric and nanometric SiC particles. Tribol. Int. 2016, 102, 28–37. [Google Scholar] [CrossRef]
- Manory, R. A novel electrical contact material with improved self-lubrication for railway current collectors. Wear 2001, 249, 626–636. [Google Scholar]
- Tang, Y.; Liu, H.; Zhao, H.; Liu, L.; Wu, Y. Friction and wear properties of copper matrix composites reinforced with short carbon fibers. Mater. Des. 2008, 29, 257–261. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, G.; Gautam, R.; Mohan, A.; Mohan, S. Wear, friction and profilometer studies of in-situ AA5052/ZrB2 composites. Tribol. Int. 2016, 97, 313–326. [Google Scholar] [CrossRef]
- Gu, D.; Shen, Y. Influence of reinforcement weight fraction on microstructure and properties of submicron WC–Cop/Cu bulk MMCs prepared by direct laser sintering. J. Alloys Compd. 2007, 431, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Li, Y.; Zhang, C.; Sun, L.; Zhai, W. Research on nickel modified graphite/Cu composites interface. Surf. Coat. Technol. 2017, 328, 70–79. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Li, Y.; Li, M.; Sun, L.; Zhai, W.; Li, K. Research on synergistic lubrication effect of silver modified Cu–Ni-graphite composite. Wear 2020, 444–445, 203140. [Google Scholar] [CrossRef]
- Rajkovic, V. Dispersion hardened Cu-Al2O3 produced by high energy milling. Int. J. Powder Metall. 2000, 36, 45–49. [Google Scholar]
- Sun, D.; Jiang, X.; Sun, H.; Song, T.; Luo, Z. Microstructure and mechanical properties of Cu-ZTA cermet prepared by vacuum hot pressing sintering. Mater. Res. Exp. 2020, 7, 026530. [Google Scholar] [CrossRef]
- Deshpande, P.; Lin, R. Wear resistance of WC particle reinforced copper matrix composites and the effect of porosity. Mater. Sci. Eng. A 2006, 418, 137–145. [Google Scholar] [CrossRef]
- Deshpande, P.; Li, J.; Lin, R. Infrared processed Cu composites reinforced with WC particles. Mater. Sci. Eng. A 2006, 429, 58–65. [Google Scholar] [CrossRef]
- Froumin, N.; Frage, N.; Aizenshtein, M.; Dariel, M. Ceramic-metal interaction and wetting phenomena in the B4C/Cu system. J. Eur. Ceram. Soc. 2003, 23, 2821–2828. [Google Scholar] [CrossRef]
- Zarrinfar, N.; Shipway, P.; Kennedy, A.; Saidi, A. Carbide stoichiometry in TiCx and Cu-TiCx produced by self-propagating high-temperature synthesis. Scr. Mater. 2002, 46, 121–126. [Google Scholar] [CrossRef]
- Zarrinfar, N.; Kennedy, A.; Shipway, P. Reaction synthesis of Cu-TiCx master-alloys for the production of copper-matrix composites. Scr. Mater. 2004, 50, 949–952. [Google Scholar] [CrossRef]
- Rado, C.; Drevet, B.; Eustathopoulos, N. The role of compound formation in reactive wetting: The Cu/SiC system. Acta Mater. 2000, 48, 4483–4491. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, J.H.; Park, Y.H.; Cho, K.M.; Choi, I.D.; Park, I.M. Manufacturing of Cu-TiB2 composites by turbulent in situ mixing process. Mater. Sci. Eng. A 2007, 449–451, 1018–1021. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Takahashi, J.; Wan, Y.; Li, M.; Xiao, B.; Zhang, Y.; He, X. Investigation of modification of Cu-Ni-graphite composite by silver. Mater. Chem. Phys. 2019, 239, 121990. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Takahashi, J.; Wan, Y.; Xiao, B.; Zhang, Y.; He, X.; Li, J. Titanium-modified graphite reinforced Cu-Ni composite by multi-arc ion plating technology. Vacuum 2019, 168, 108829. [Google Scholar] [CrossRef]
- Eklund, P.; Beckers, M.; Jansson, U.; Högberg, H.; Hultman, L. The Mn+1AXn phases: Materials science and thin-film processing. Thin Solid Film. 2010, 518, 1851–1878. [Google Scholar] [CrossRef]
- Sun, Z. Progress in research and development on MAX phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Hu, L.; Kothalkar, A.; Proust, G.; Karaman, I.; Radovic, M. Fabrication and characterization of NiTi/Ti3SiC2 and NiTi/Ti2AlC composites. J. Alloys Compd. 2014, 610, 635–644. [Google Scholar] [CrossRef]
- Mahesh, K.; Balanand, S.; Raimond, R.; Mohamed, A.P.; Ananthakumar, S. Polyaryletherketone polymer nanocomposite engineered with nanolaminated Ti3SiC2 ceramic fillers. Mater. Des. 2014, 63, 360–367. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Luo, F.; Zhou, W.; Zhu, D.; Li, Z. Enhanced mechanical, dielectric and microwave absorption properties of cordierite based ceramics by adding Ti3SiC2 powders. J. Alloys Compd. 2015, 619, 854–860. [Google Scholar]
- Zhou, Y.; Wang, X.; Sun, Z.; Chen, S. Electronic and structural properties of the layered ternary carbide Ti3AlC2. J. Mater. Chem. 2001, 11, 2335–2339. [Google Scholar] [CrossRef]
- Huang, Z.; Bonneville, J.; Zhai, H.; Gauthier-Brunet, V.; Dubois, S. Microstructural characterization and compression properties of TiC0.61/Cu(Al) composite synthesized from Cu and Ti3AlC2 powders. J. Alloys Compd. 2014, 602, 53–57. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhou, Y. Structure stability of TiAlC in Cu and microstructure evolution of Cu-TiAlC composites. Acta Mater. 2007, 55, 4381–4390. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y. Microstructure, mechanical, and electrical properties of Cu-Ti3AlC2 and in-situ Cu-TiCx composites. J. Mater. Res. 2008, 23, 924–932. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, H.; Chen, L.; Huang, Z.; Bei, G.; Greil, P. Preparation and mechanical properties of TiCx-(NiCu)3Al-CuNi2Ti-Ni hybrid composites by reactive pressureless sintering pre-alloyed Cu/Ti3AlC2, and Ni as precursor. Mater. Sci. Eng. A 2016, 670, 351–356. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, H.; Chen, L.; Zhou, Y.; Huang, Z.; Bei, G.; Greil, P. Sintering and properties of mechanical alloyed Ti3AlC2-Cu composites. Mater. Sci. Eng. A 2017, 685, 154–158. [Google Scholar] [CrossRef]
- Wang, W.J.; Gauthier-Brunet, V.; Bei, G.P.; Laplanche, G.; Bonneville, J.; Joulain, A.; Dubois, S. Powder metallurgy processing and compressive properties of Ti3AlC2/Al composites. Mater. Sci. Eng. A 2011, 530, 168–173. [Google Scholar] [CrossRef]
- Ho, J.; Hamdeh, H.; Barsoum, M.; El-Raghy, T. Low temperature heat capacities of Ti3Al1.1C1.8, Ti4AlN3, and Ti3SiC2. J. Appl. Phys. 1999, 86, 3609–3611. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y. Effect of Ti in inhibiting the decomposition of Ti3SiC2 in copper matrix composites. Foundry Technol. 2022, 43, 410–416. [Google Scholar]





| Sample | Ti3AlC2/Vol.% | Ni/wt.% | Cu/wt.% |
|---|---|---|---|
| Ni-Al10 | 10 | 8 | Bal. |
| Ni-Al30 | 30 | 8 | Bal. |
| Ni-Al50 | 50 | 8 | Bal. |
| Al10 | 10 | / | Bal. |
| Al30 | 30 | / | Bal. |
| Al50 | 50 | / | Bal. |
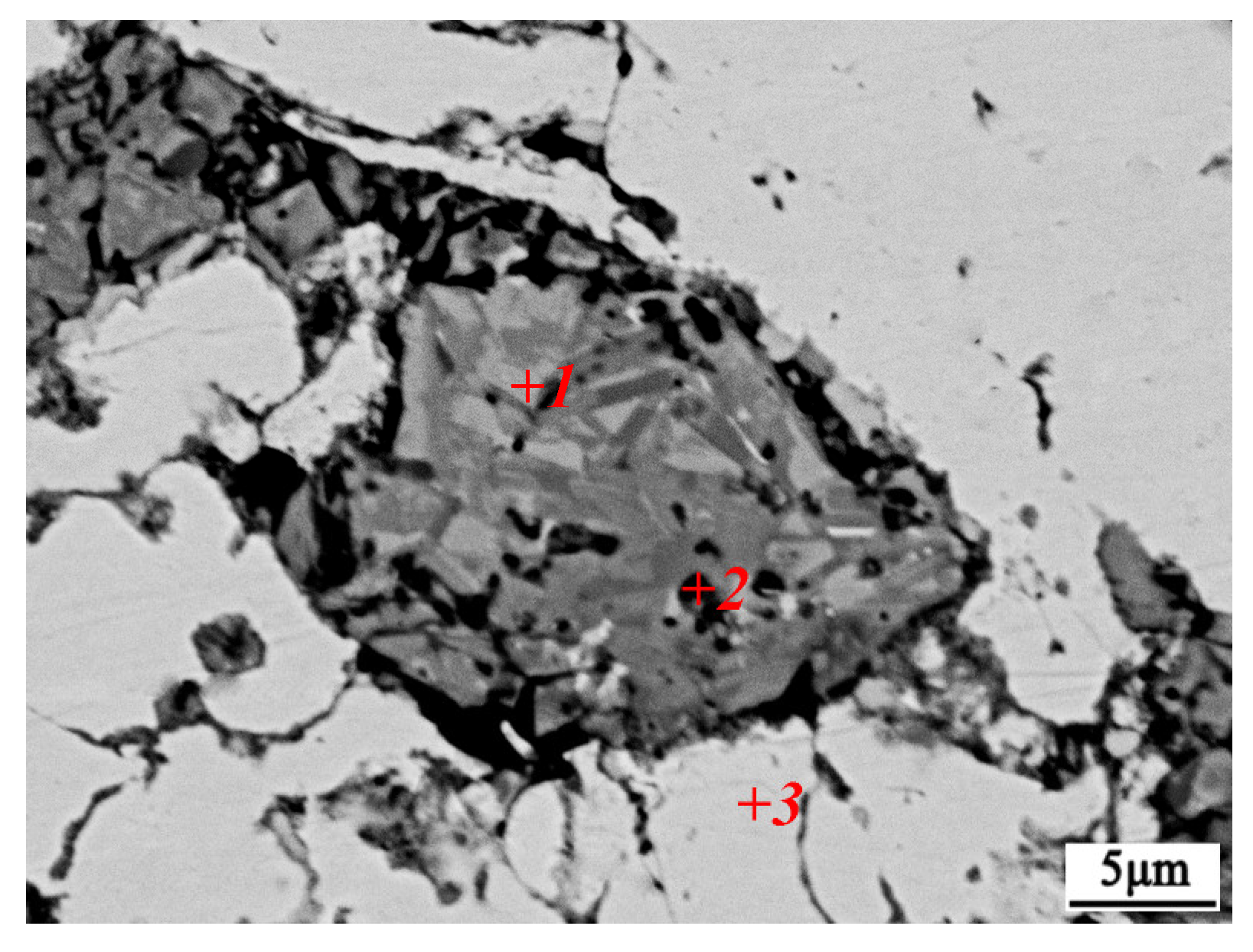
| Point | C | Al | Ti | Ni | Cu | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | |
| 1 | 40.62 | 13.36 | 1.12 | 0.82 | 36.32 | 7.63 | / | / | 21.95 | 38.19 |
| 2 | 29.33 | 8.56 | 10.29 | 6.75 | 20.82 | 4.23 | 5.46 | 7.79 | 34.11 | 52.67 |
| 3 | 27.90 | 6.64 | 1.36 | 0.75 | / | / | 1.29 | 1.55 | 70.25 | 91.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, L.; Li, X. Study on Microstructure and Mechanical Properties Modification of Cu-Ti3AlC2 Composites by Ni Element. Coatings 2023, 13, 1414. https://doi.org/10.3390/coatings13081414
Wang Y, Xu L, Li X. Study on Microstructure and Mechanical Properties Modification of Cu-Ti3AlC2 Composites by Ni Element. Coatings. 2023; 13(8):1414. https://doi.org/10.3390/coatings13081414
Chicago/Turabian StyleWang, Yiran, Liujie Xu, and Xiuqing Li. 2023. "Study on Microstructure and Mechanical Properties Modification of Cu-Ti3AlC2 Composites by Ni Element" Coatings 13, no. 8: 1414. https://doi.org/10.3390/coatings13081414
APA StyleWang, Y., Xu, L., & Li, X. (2023). Study on Microstructure and Mechanical Properties Modification of Cu-Ti3AlC2 Composites by Ni Element. Coatings, 13(8), 1414. https://doi.org/10.3390/coatings13081414









