Control of Cell Adhesion and Growth on Polysaccharide-Based Multilayer Coatings by Incorporation of Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Build-Up of PEM Coatings
2.2. Cell Culture and Cell Viability Assays
2.3. In Vitro Cytotoxicity Assay
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Optical Waveguide Lightmode Spectroscopy (OWLS)
2.6. Ellipsometry
3. Results
3.1. Diffusion of Ch Chains in Control HA/Ch and Composite HA/Ch/GO Multilayers
3.2. Thickness and Refractive Index of Composite HA/Ch/GO Films
3.3. Cell Adhesion and Growth on Composite HA/Ch/GO Films
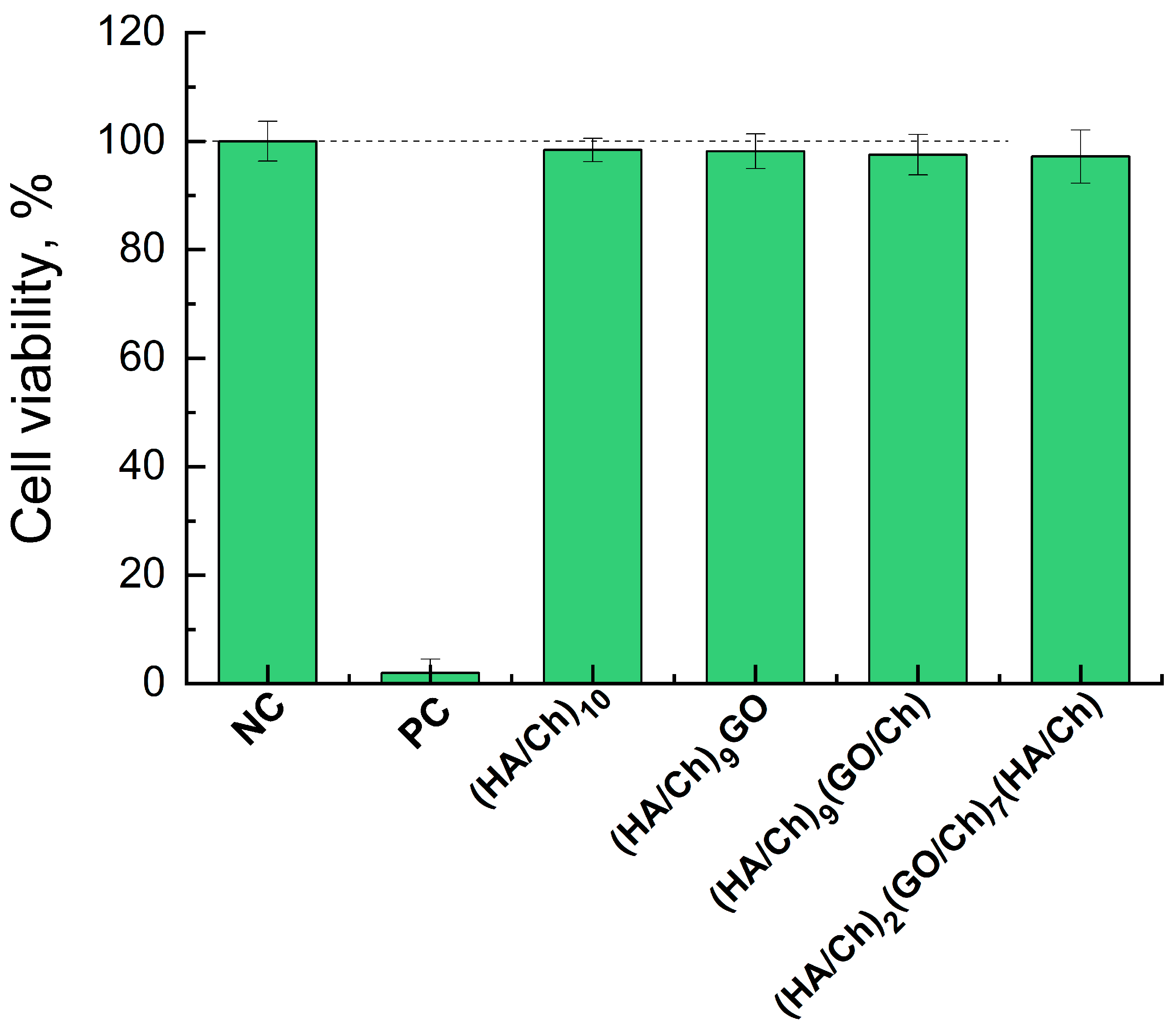
3.4. Cytotoxicity of Composite HA/Ch/GO Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrila, L.M.; Bucatariu, F.; Mihai, M.; Teodosiu, C. Polyelectrolyte Multilayers: An Overview on Fabrication, Properties, and Biomedical and Environmental Applications. Materials 2021, 14, 4152. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Ferri, M.; Tamplenizza, M.; Borghi, F.; Divitini, G.; Ducati, C.; Lenardi, C.; Piazzoni, C.; Merlini, M.; Podestà, A.; et al. Bottom-up engineering of the surface roughness of nanostructured cubic zirconia to control cell adhesion. Nanotechnology 2012, 23, 475101. [Google Scholar] [CrossRef] [PubMed]
- Ranella, A.; Barberoglou, M.; Bakogianni, S.; Fotakis, C.; Stratakis, E. Tuning cell adhesion by controlling the roughness and wettability of 3d micro/nano silicon structures. Acta Biomater. 2010, 6, 2711–2720. [Google Scholar] [CrossRef]
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface 2014, 11, 20140687. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Sun, J.; Dang, Z.; Li, J.; Li, X.; Chen, T. The effects of surface topography of nanostructure arrays on cell adhesion. Phys. Chem. Chem. Phys. 2018, 20, 22946. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C 2019, 104, 109883. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef]
- Kennedy, S.B.; Washburn, N.R.; Simon, C.G.; Amis, E.J. Combinatorial screen of the effect of surface energy on fibronectin-mediated osteoblast adhesion, spreading and proliferation. Biomaterials 2006, 27, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Köstler, S.; Delgado, A.V.; Ribitsch, V. Surface thermodynamic properties of polyelectrolyte multilayers. J. Colloid Interface Sci. 2005, 286, 339. [Google Scholar] [CrossRef]
- Gong, X. Controlling surface properties of polyelectrolyte multilayers by assembly pH. Phys. Chem. Chem. Phys. 2013, 15, 10459. [Google Scholar] [CrossRef] [PubMed]
- Towle, E.G.; Ding, I.; Peterson, A.M. Impact of molecular weight on polyelectrolyte multilayer assembly and surface properties. J. Colloid Interface Sci. 2020, 570, 135. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Dennaoui, H.; Chouery, E.; Rammal, H.; Abdel-Razzak, Z.; Harmouch, C. Chitosan/hyaluronic acid multilayer films are biocompatible substrate for Wharton’s jelly derived stem cells. Stem Cell Investig. 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Hyaluronic acid and chitosan-based nanosystems: A new dressing generation for wound care. Expert Opin. Drug Deliv. 2019, 16, 715. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, H.; Hossfeld, S.; Schlosshauer, B.; Mittnacht, U.; Pêgo, A.P.; Dauner, M.; Doser, M.; Stoll, D.; Krastev, R. Hyaluronic acid/chitosan multilayer coatings on neuronal implants for localized delivery of siRNA nanoplexes. J. Control. Release 2013, 168, 289. [Google Scholar] [CrossRef]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef]
- Correia, C.R.; Moreira-Teixeira, L.S.; Moroni, L.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; Mano, J.F. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng. Part C Methods 2011, 17, 717. [Google Scholar] [CrossRef]
- Schneider, A.; Richert, L.; Francius, G.; Voegel, J.-C.; Picart, C. Elasticity, biodegradability and cell adhesive properties of chitosan/hyaluronan multilayer films. Biomed. Mater. 2007, 2, S45. [Google Scholar] [CrossRef]
- Richert, L.; Lavalle, P.; Payan, E.; Shu, X.Z.; Prestwich, G.D.; Stoltz, J.-F.; Schaaf, P.; Voegel, J.-C.; Picart, C. Layer by layer buildup of polysaccharide films: Physical chemistry and cellular adhesion aspects. Langmuir 2004, 20, 448. [Google Scholar] [CrossRef]
- Jou, C.-H.; Yuan, L.; Lin, S.-M.; Hwang, M.-C.; Chou, W.-L.; Yu, D.-G.; Yang, M.-C. Biocompatibility and antibacterial activity of chitosan and hyaluronic acid immobilized polyester fibers. J. Appl. Polym. Sci. 2007, 104, 220. [Google Scholar] [CrossRef]
- Hoyo-Gallego, S.D.; Pérez-Álvarez, L.; Gómez-Galván, F.; Lizundia, E.; Kuritka, I.; Sedlarik, V.; Laza, J.M.; Vila-Vilela, J.L. Construction of antibacterial poly(ethylene terephthalate) films via layer by layer assembly of chitosan and hyaluronic acid. Carbohydr. Polym. 2016, 143, 35. [Google Scholar] [CrossRef] [PubMed]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. A Blank Slate? Layer-by-Layer Deposition of Hyaluronic Acid and Chitosan onto Various Surfaces. Biomacromolecules 2006, 7, 1610–1622. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Koc, J.; Finlay, J.A.; Clarke, J.L.; Clare, A.S.; Rosenhahn, A. Layer-by-layer constructed hyaluronic acid/chitosan multilayers as antifouling and fouling-release coatings. Biointerphases 2019, 14, 051002. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Etienne, O.; Schneider, A.; Taddei, C.; Richert, L.; Schaaf, P.; Voegel, J.-C.; Egles, C.; Picart, C. Degradability of Polysaccharides Multilayer Films in the Oral Environment: An In Vitro and In Vivo Study. Biomacromolecules 2005, 6, 726–733. [Google Scholar] [CrossRef]
- Schneider, A.; Francius, G.; Obeid, R.; Schwinté, P.; Hemmerlé, J.; Frisch, B.; Schaaf, P.; Voegel, J.-C.; Senger, B.; Picart, C. Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion. Langmuir 2006, 22, 1193. [Google Scholar] [CrossRef]
- Moshnikova, A.B.; Afanasyev, V.N.; Proussakova, O.V.; Chernyshov, S.; Gogvadze, V.; Beletsky, I.P. Cytotoxic activity of 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide is underlain by DNA interchain cross-linking. Cell. Mol. Life Sci. 2006, 63, 229. [Google Scholar] [CrossRef]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of Cell Adhesion by Mechanical Reinforcement of Soft Polyelectrolyte Films with Nanoparticles. Langmuir 2012, 28, 7249. [Google Scholar] [CrossRef]
- Almeida, A.C.; Vale, A.C.; Reis, R.L.; Alves, N.M. Bioactive and adhesive properties of multilayered coatings based on catechol-functionalized chitosan/hyaluronic acid and bioactive glass nanoparticles. Int. J. Biol. Macromol. 2020, 157, 119–134. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, K.; Deng, R.; Ren, X.; Wu, C.; Li, J. Tunable stiffness of graphene oxide/polyacrylamide composite scaffolds regulates cytoskeleton assembly. Chem. Sci. 2018, 9, 6516. [Google Scholar] [CrossRef]
- Abalymov, A.A.; Parakhonskiy, B.V.; Skirtach, A.G. Colloids-at-surfaces: Physicochemical approaches for facilitating cell adhesion on hybrid hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125185. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef]
- Andreeva, T.D.; Stoichev, S.; Taneva, S.G.; Krastev, R. Hybrid graphene oxide/polysaccharide nanocomposites with controllable surface properties and biocompatibility. Carbohydr. Polym. 2018, 181, 78. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, B.-C.; Wang, X.-F.; Ye, B.-C. Interaction of peptides with graphene oxide and its application for real-time monitoring of protease activity. Chem. Commun. 2011, 47, 2399–2401. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, T.D.; Dér, A.; Kelemen, L.; Krastev, R.; Taneva, S.G. Modulation of the internal structure and surface properties of natural and synthetic polymer matrices by graphene oxide doping. Polym. Adv. Technol. 2020, 31, 1562. [Google Scholar] [CrossRef]
- Ormos, P.; Fábián, L.; Oroszi, L.; Wolff, E.K.; Ramsden, J.J.; Dér, A. Protein-based integrated optical switching and modulation. Appl. Phys. Lett. 2002, 80, 4060. [Google Scholar] [CrossRef]
- Fábián, L.; Wolff, E.K.; Oroszi, L.; Ormos, P.; Dér, A. Fast integrated optical switching by the protein bacteriorhodopsin. Appl. Phys. Lett. 2010, 97, 142. [Google Scholar] [CrossRef]
- Tiefenthaler, K.; Lukosz, W. Sensitivity of grating couplers as integrated-optical chemical sensors. J. Opt. Soc. Am. B 1989, 6, 209. [Google Scholar] [CrossRef]
- Andreeva, T.D.; Hartmann, H.; Taneva, S.G.; Krastev, R. Regulation of the growth, morphology, mechanical properties and biocompatibility of natural polysaccharide-based multilayers by Hofmeister anions. J. Mater. Chem. B 2016, 4, 7092. [Google Scholar] [CrossRef]
- Schmiedova, V.; Pospisil, J.; Kovalenko, A.; Ashcheulov, P.; Fekete, L.; Cubon, T.; Kotrusz, P.; Zmeskal, O.; Weiter, M. Physical Properties Investigation of Reduced Graphene Oxide Thin Films Prepared by Material Inkjet Printing. J. Nanomater. 2017, 2017, 3501903. [Google Scholar] [CrossRef]
- Hauser, S.; Jung, F.; Pietzsch, J. Human Endothelial Cell Models in Biomaterial Research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte multilayer assemblies on materials surfaces: From cell adhesion to tissue engineering. Chem. Mater. 2012, 24, 854. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-R.; Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Behaviors of NIH-3T3 Fibroblasts on Graphene/Carbon Nanotubes: Proliferation, Focal Adhesion, and Gene Transfection Studies. ACS Nano 2010, 4, 6587. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and biosafety of graphene oxide wrapped porous CF/PEEK composites as implantable materials: The role of surface structure and chemistry. Dent. Mater. 2020, 36, 1289. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Coll. Int. Sci. 2010, 15, 417. [Google Scholar] [CrossRef]
- Thierry, B.; Winnik, F.M.; Merhi, Y.; Silver, J.; Tabrizian, M. Bioactive Coatings of Endovascular Stents Based on Polyelectrolyte Multilayers. Biomacromolecules 2003, 4, 1564–1571. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557. [Google Scholar] [CrossRef]
- Qi, W.; Xue, Z.; Yuan, W.; Wang, H. Layer-by-layer assembled graphene oxide composite films for enhanced mechanical properties and fibroblast cell affinity. J. Mater. Chem. B 2014, 2, 325. [Google Scholar] [CrossRef] [PubMed]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface Modification of Polymer Substrates for Biomedical Applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Howlett, C.R.; Evans, M.D.M.; Walsh, W.R.; Johnson, G.; Steele, J.G. Mechanism of initial attachment of cells derived from human bone to commonly used prosthetic materials during cell culture. Biomaterials 1994, 15, 213–222. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advance in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971. [Google Scholar] [CrossRef]
- Hoffmann, J.; Haendeler, J.; Aicher, A.; Rössig, L.; Vasa, M.; Zeiher, A.M.; Dimmeler, S. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: Important role of nitric oxide. Circ. Res. 2001, 89, 709. [Google Scholar] [CrossRef] [PubMed]



| Sample | OWLS | Ellipsometry | ||
|---|---|---|---|---|
| Thickness (nm) | Refractive Index | Thickness (nm) | Refractive Index | |
| (HA/Ch)10 | 75.3 ± 0.3 | 1.58 ± 0.01 | 92.0 ± 2.3 | 1.63 ± 0.02 |
| (HA/Ch)9GO | 84.4 ± 3.0 | 1.70 ± 0.03 | ||
| (HA/Ch)9(GO/Ch) | 90.2 ± 2.5 | 1.72 ± 0.04 | ||
| (HA/Ch)2(GO/Ch)7(HA/Ch) | 74.0 ± 1.0 | 1.71 ± 0.01 | 90.0 ± 2.5 | 1.80 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, T.; Rudt, A.; Fábián, L.; Ayaydin, F.; Iliev, I.; Jung, O.; Barbeck, M.; Dér, A.; Krastev, R.; Taneva, S.G. Control of Cell Adhesion and Growth on Polysaccharide-Based Multilayer Coatings by Incorporation of Graphene Oxide. Coatings 2024, 14, 570. https://doi.org/10.3390/coatings14050570
Andreeva T, Rudt A, Fábián L, Ayaydin F, Iliev I, Jung O, Barbeck M, Dér A, Krastev R, Taneva SG. Control of Cell Adhesion and Growth on Polysaccharide-Based Multilayer Coatings by Incorporation of Graphene Oxide. Coatings. 2024; 14(5):570. https://doi.org/10.3390/coatings14050570
Chicago/Turabian StyleAndreeva, Tonya, Alexander Rudt, László Fábián, Ferhan Ayaydin, Ivan Iliev, Ole Jung, Mike Barbeck, Andras Dér, Rumen Krastev, and Stefka G. Taneva. 2024. "Control of Cell Adhesion and Growth on Polysaccharide-Based Multilayer Coatings by Incorporation of Graphene Oxide" Coatings 14, no. 5: 570. https://doi.org/10.3390/coatings14050570
APA StyleAndreeva, T., Rudt, A., Fábián, L., Ayaydin, F., Iliev, I., Jung, O., Barbeck, M., Dér, A., Krastev, R., & Taneva, S. G. (2024). Control of Cell Adhesion and Growth on Polysaccharide-Based Multilayer Coatings by Incorporation of Graphene Oxide. Coatings, 14(5), 570. https://doi.org/10.3390/coatings14050570


.jpg)








