The Influence of Nitrogen Partial Pressure on the Microstructure and Mechanical Properties of HfNbTaTiVZr High-Entropy Nitride Coating Deposited via Direct Current Cathodic Vacuum Arc Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results
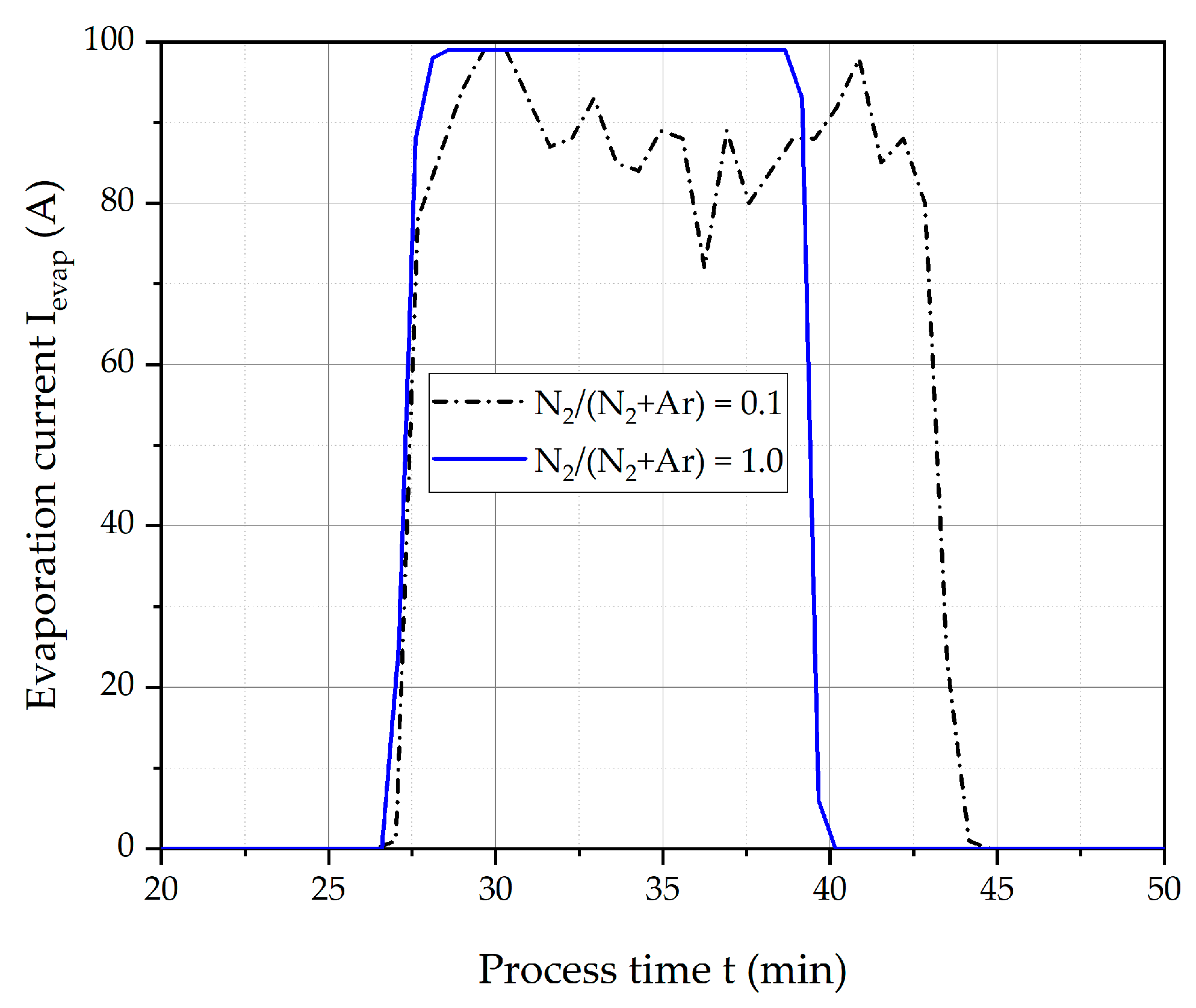
3.1. Deposition Rate
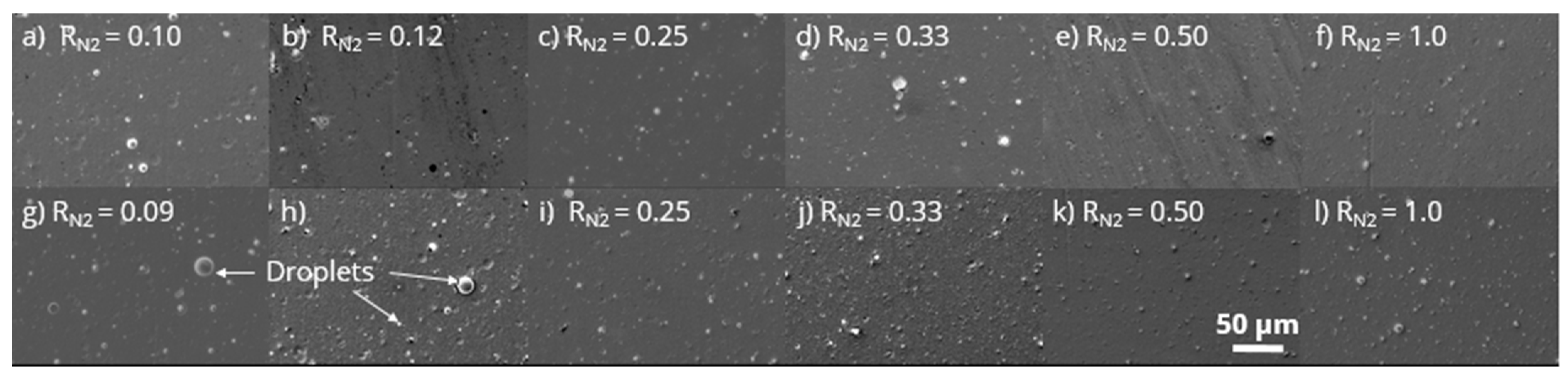
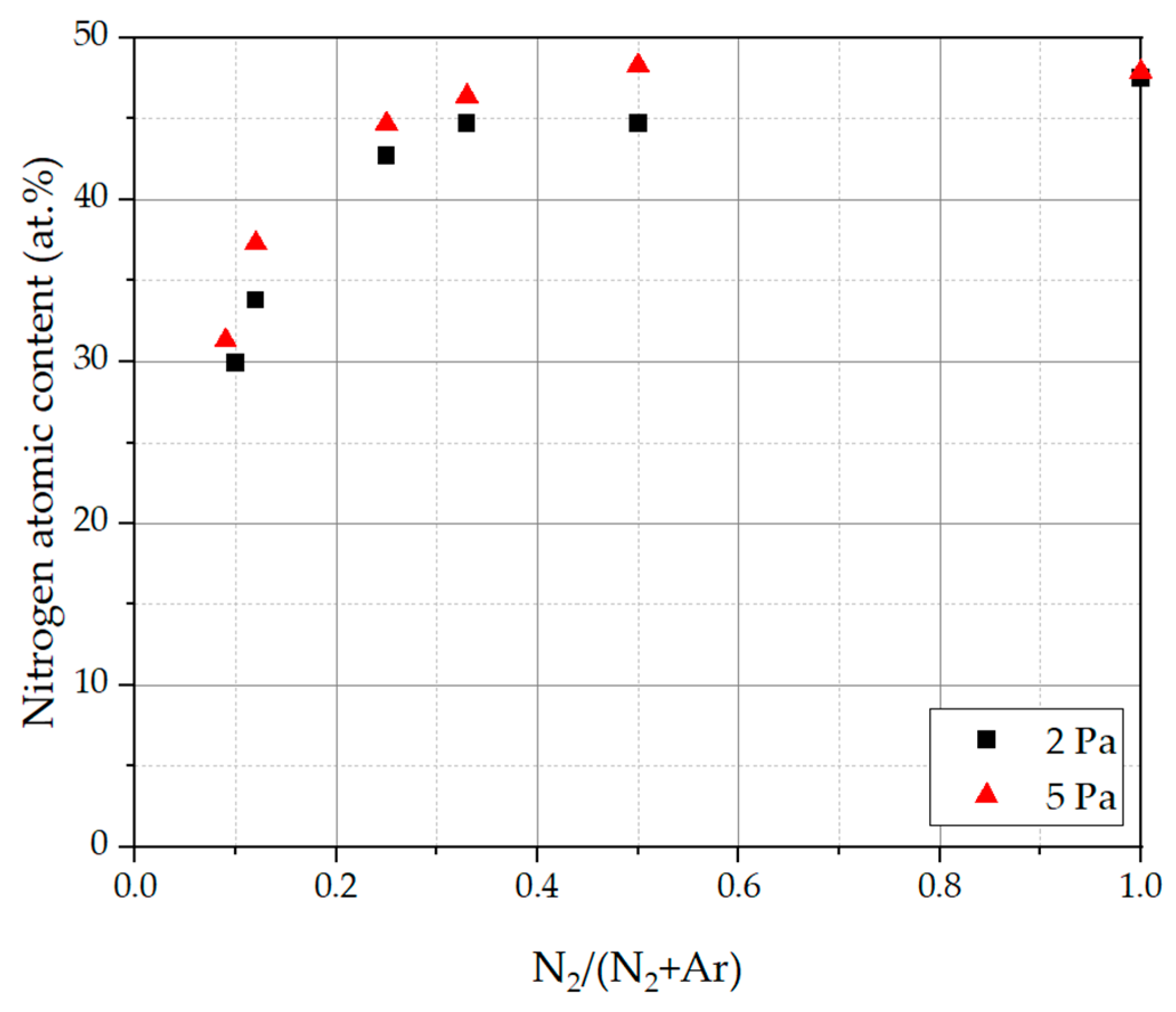
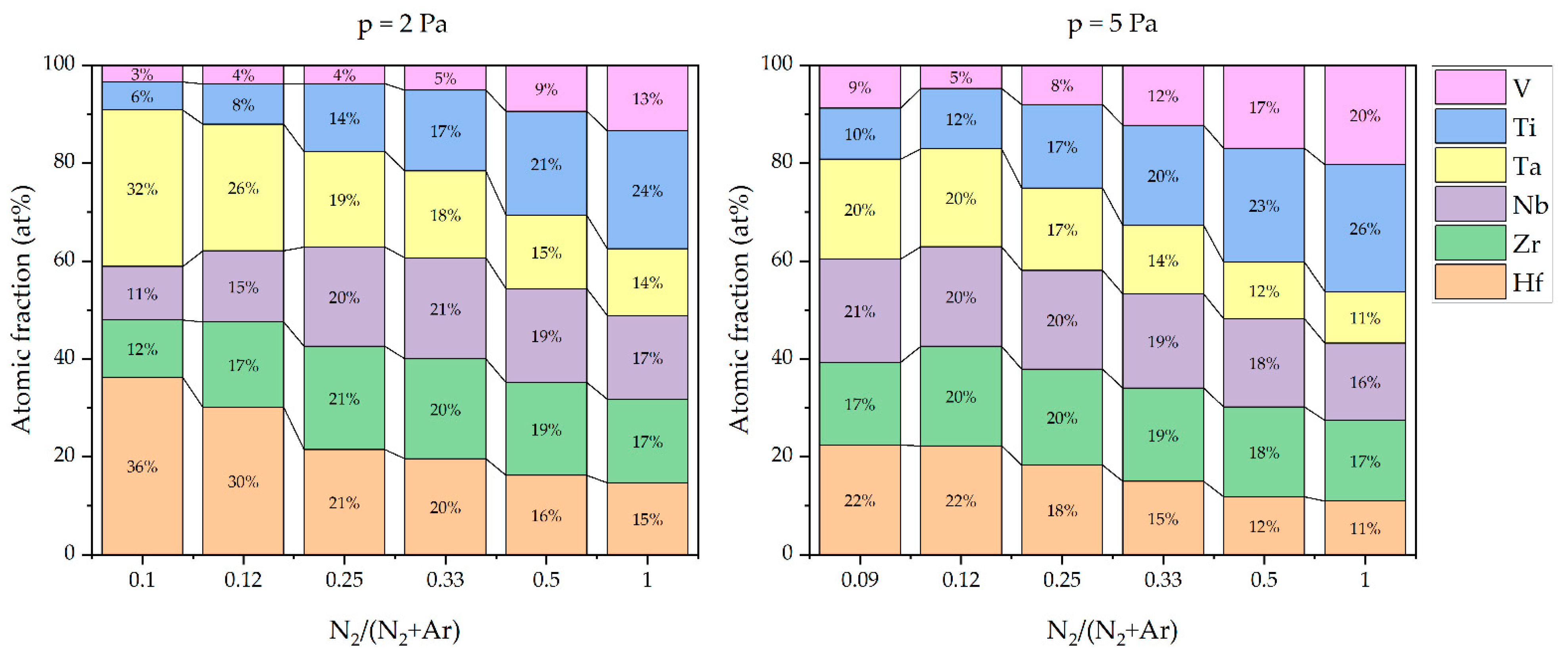
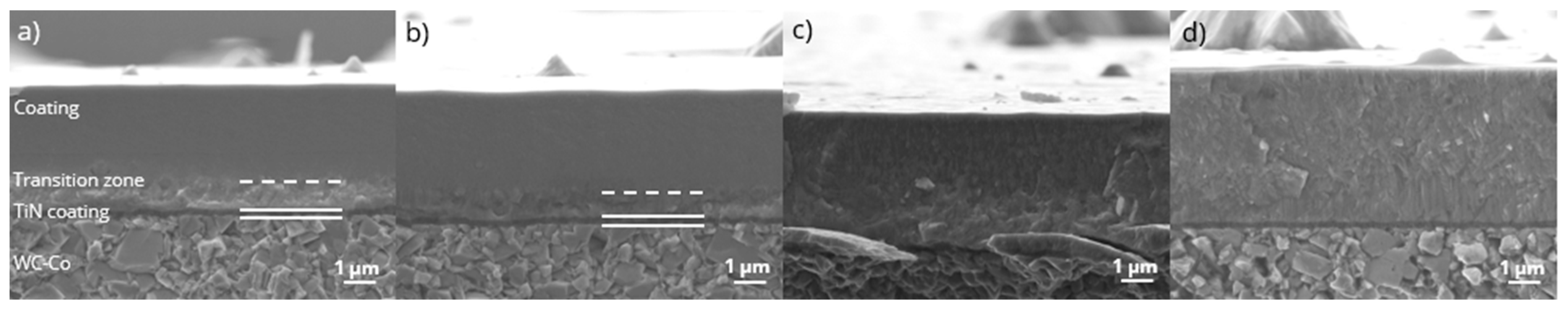
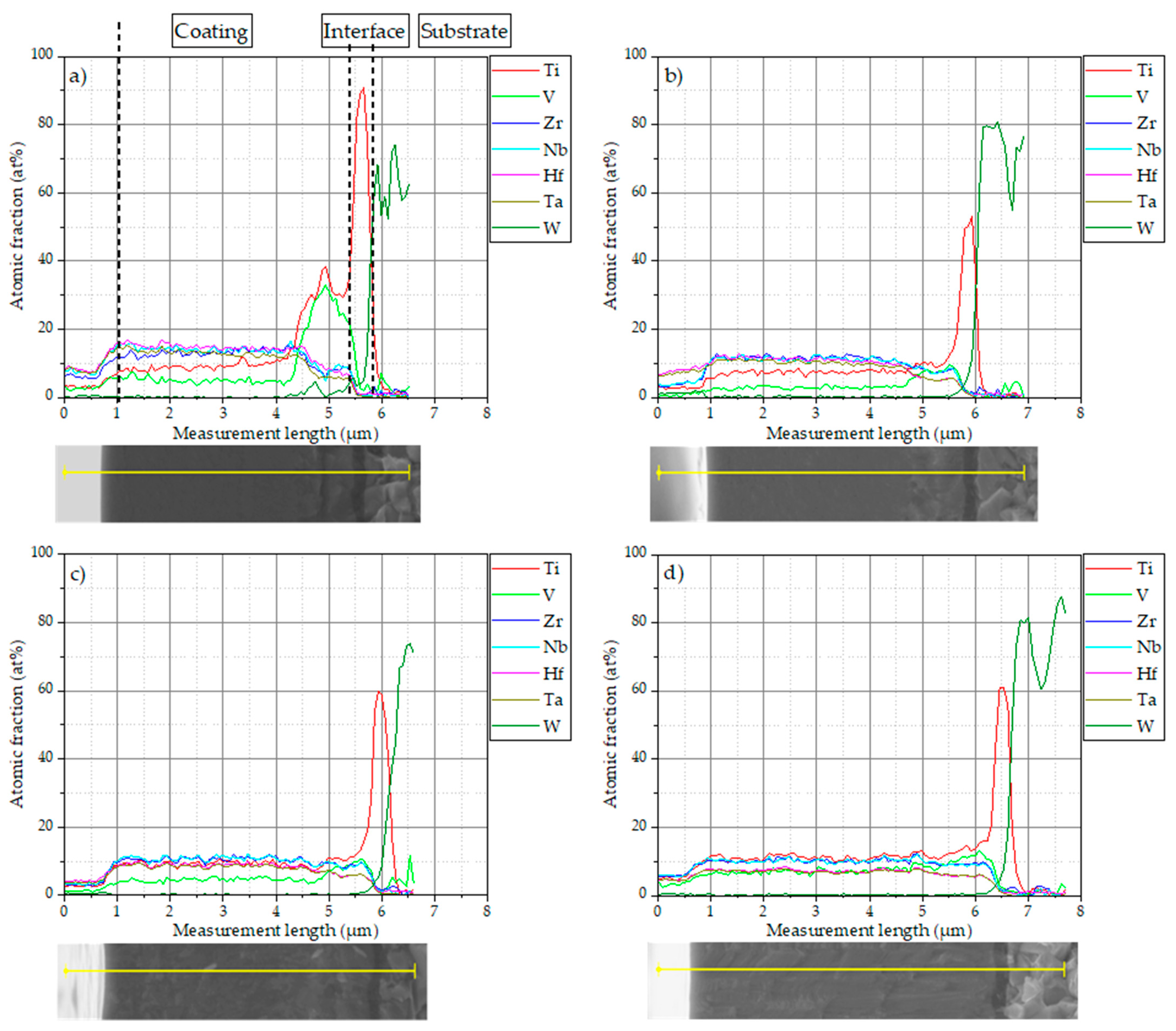
3.2. Coating Morphology and Composition
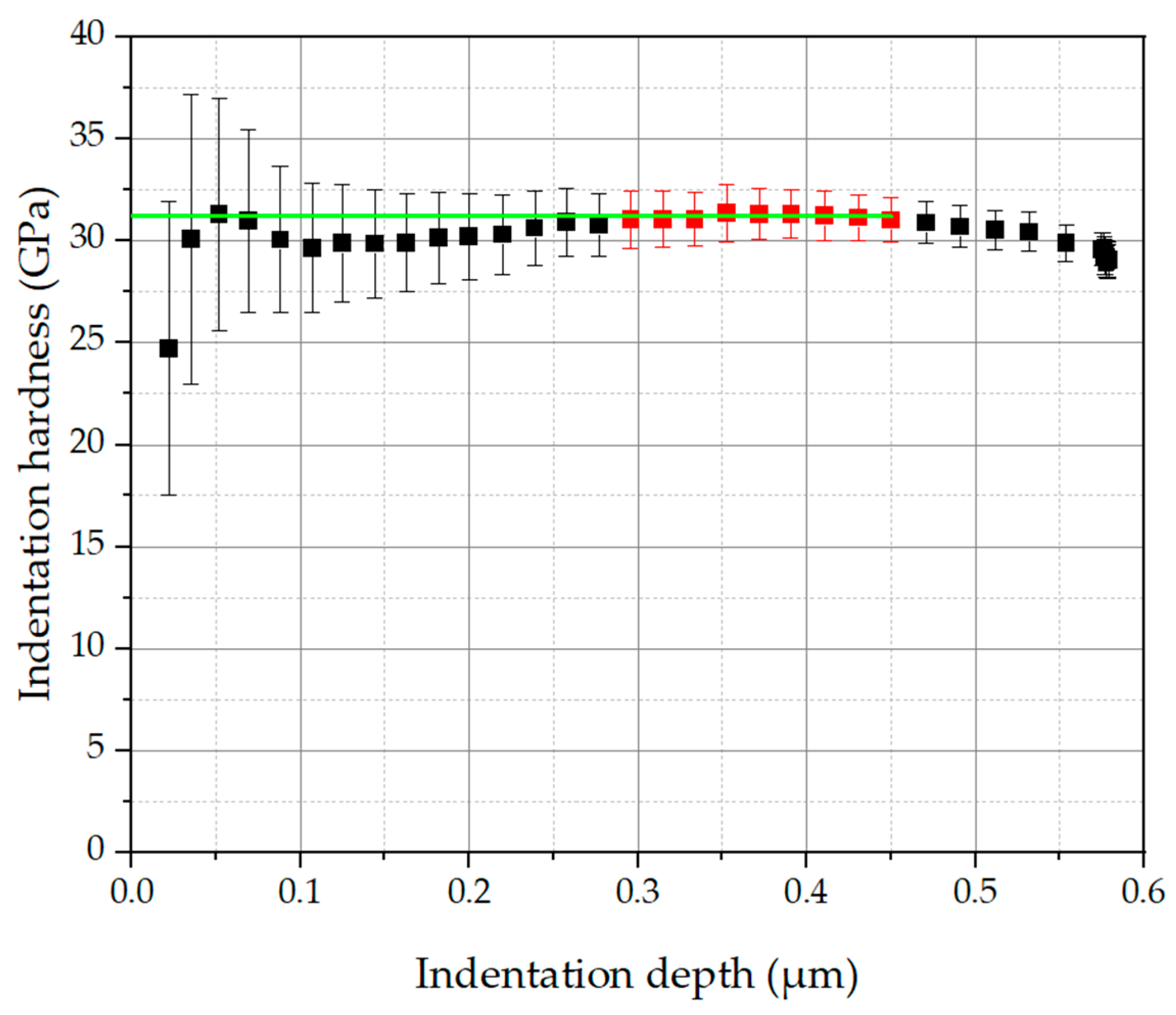
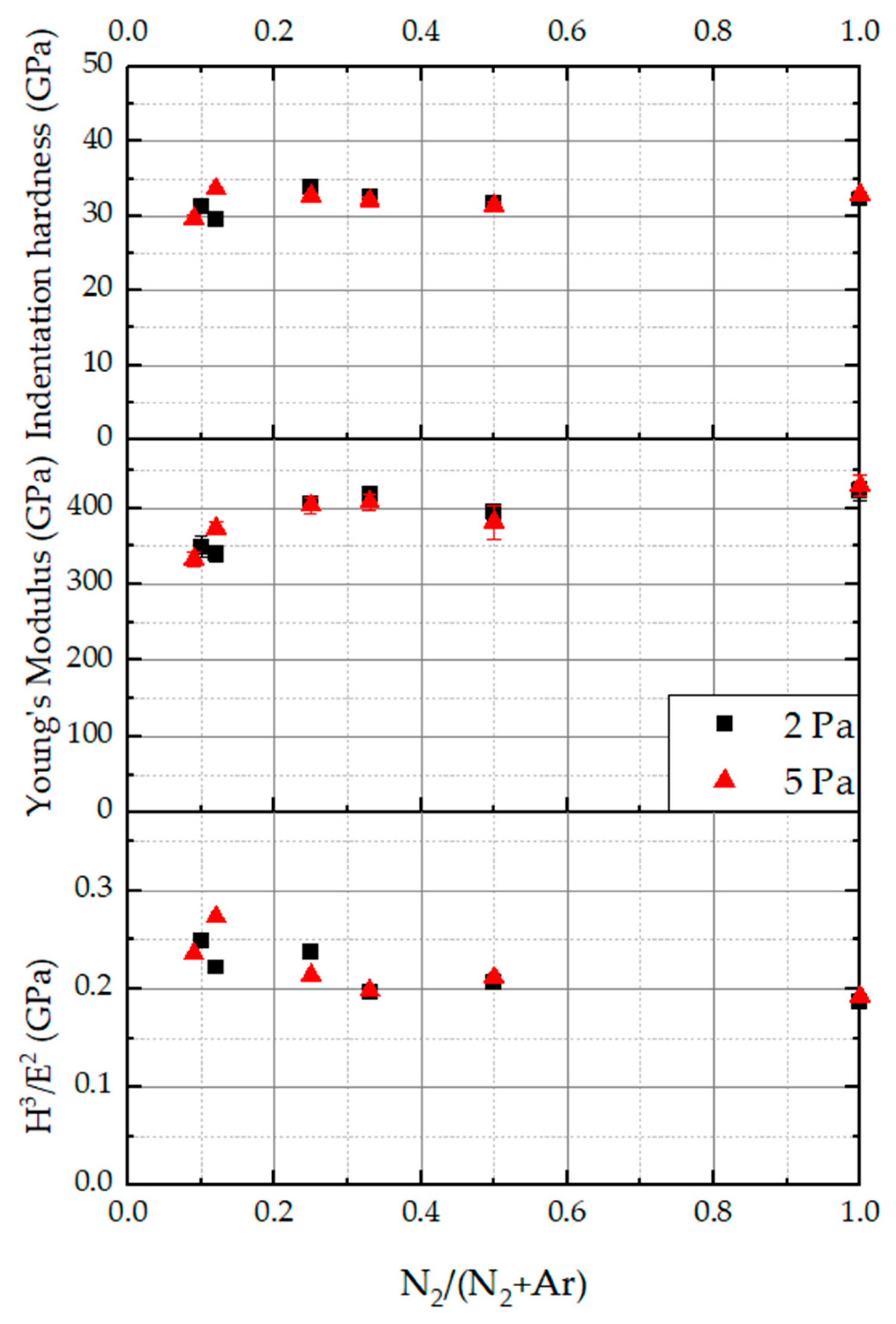
3.3. Mechanical Properties
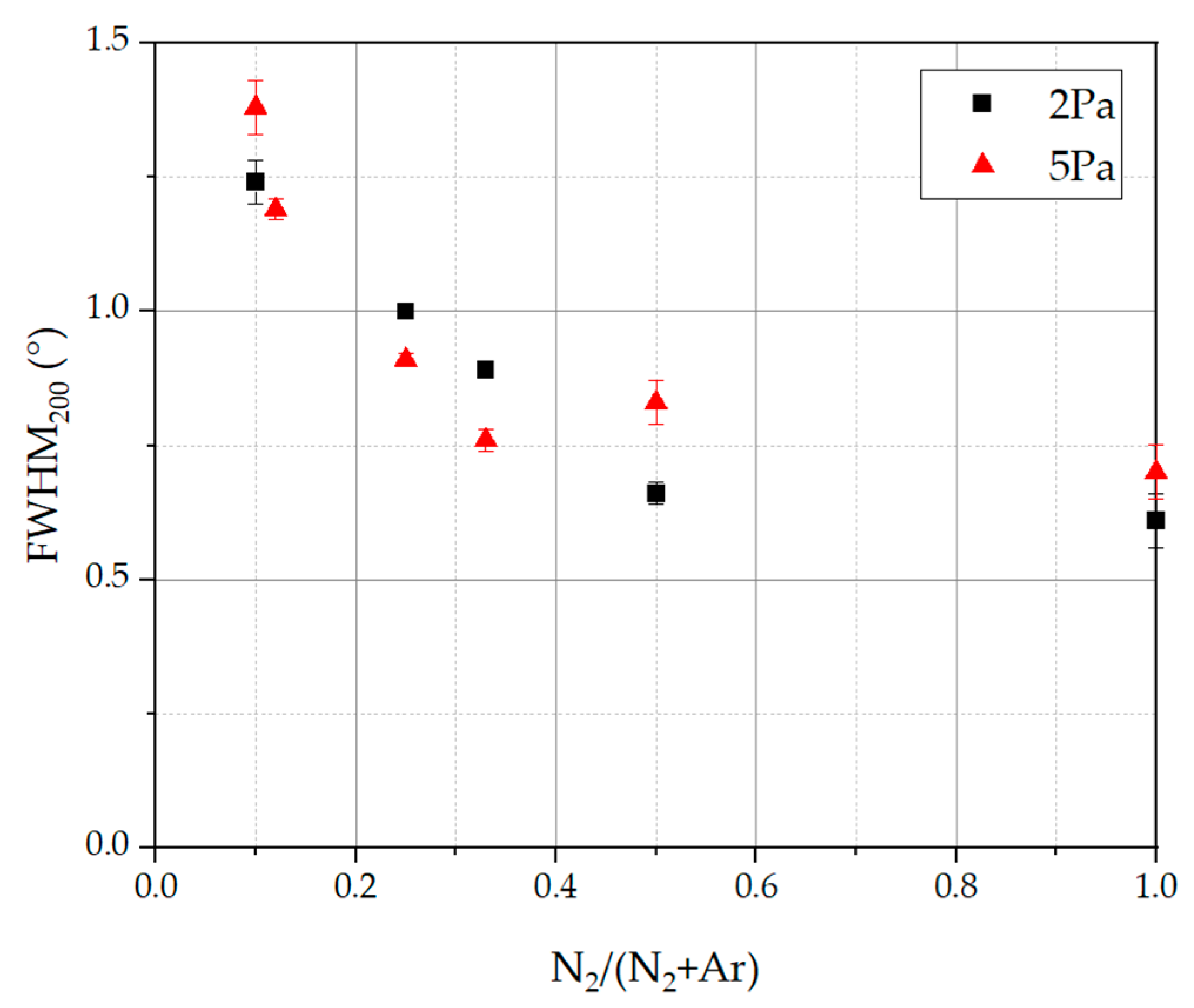
3.4. Structural Analysis
4. Discussion
4.1. Deposition Rate
4.2. Nitrogen Content
4.3. Mechanical Properties
5. Conclusions
- In contrast to magnetron sputtering processes (Figure 1), the deposition rate increases with an increasing N2/(N2 + Ar) ratio, since in DC cathodic vacuum arc deposition target poisoning does not hinder the evaporation of the cathode material.
- An increasing N2/(N2 + Ar) ratio led to an increase in the nitrogen atomic content in the deposited coatings, which could be observed in several studies in the literature. If the N2/(N2 + Ar) ratio and therefore the nitrogen content in the coatings is decreased, a broadening of XRD reflexes could be obtained, leading to a fine-grained and homogeneous microstructure. As the nitrogen content increases, the microstructure becomes coarse and columnar. Despite the change in N2/(N2 + Ar) ratio and nitrogen content, the analyzed coatings showed a single phase fcc structure. This is in contrast to other HEN coating systems in the literature, which could exhibit differing crystal phases or even amorphous phases. For the presented CAE processes with their high deposition rate and energy input, it is probably difficult to reach the amorphous region. This underlines the stability of the CAE process preparing crystalline coatings over a broad variety of nitrogen flow ratios and thus with changing nitrogen contents.
- Although the nitrogen content in the coatings is significantly changed with an increasing N2/(N2 + Ar) ratio, the indentation hardness and the Young’s modulus of the coatings are only slightly affected. This effect could be due to competing effects: solid solution hardening with increasing nitrogen content on the one hand and grain refinement with decreasing nitrogen content on the other hand.
- A transition zone is obtained in the case of low N2/(N2 + Ar) with a higher concentration of Ti and V, which has not been fully understood until now, but can likely be attributed to a change in the gas atmosphere during deposition of the TiN bond coating and the HEN coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Bach, F.-W.; Möhwald, K.; Laarmann, A.; Wenz, T. Modern Surface Technology; Wiley-VCH: Weinheim, Germany, 2006; ISBN 978-3-527-31532-1. [Google Scholar]
- Anders, A. Cathodic Arcs; Springer: New York, NY, USA, 2008; ISBN 978-0-387-79107-4. [Google Scholar]
- Novikov, V.; Stepanov, N.; Zherebtsov, S.; Salishchev, G. Structure and Properties of High-Entropy Nitride Coatings. Metals 2022, 12, 847. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.; Yanov, V.; Saevarsdottir, G. Magnetron Sputtering High-Entropy Alloy Coatings: A Mini-Review. Metals 2022, 12, 319. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.-W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys; Springer International Publishing: Cham, Switzerlands, 2016; ISBN 978-3-319-27011-1. [Google Scholar]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S. High-Entropy Alloys; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-800251-3. [Google Scholar]
- Kuczyk, M.; Krülle, T.; Zawischa, M.; Kaspar, J.; Zimmer, O.; Leonhardt, M.; Leyens, C.; Zimmermann, M. Microstructure and mechanical properties of high entropy alloy nitride coatings deposited via direct current cathodic vacuum arc deposition. Surf. Coat. Technol. 2022, 448, 128916. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, H.J.; Lim, K.S.; Hong, S.H.; Kim, K.B. Structural and Mechanical Properties of AlCoCrNi High Entropy Nitride Films: Influence of Process Pressure. Coatings 2020, 10, 10. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Chen, E.-C.; Chang, Z.-C.; Shieu, F.-S. Effect of Nitrogen Partial Pressure on the Structural, Mechanical, and Electrical Properties of (CrHfNbTaTiVZr)N Coatings Deposited by Reactive Magnetron Sputtering. Coatings 2022, 12, 437. [Google Scholar] [CrossRef]
- Tsai, D.-C.; Huang, Y.-L.; Lin, S.-R.; Liang, S.-C.; Shieu, F.-S. Effect of nitrogen flow ratios on the structure and mechanical properties of (TiVCrZrY)N coatings prepared by reactive magnetron sputtering. Appl. Surf. Sci. 2010, 257, 1361–1367. [Google Scholar] [CrossRef]
- Hsueh, H.-T.; Shen, W.-J.; Tsai, M.-H.; Yeh, J.-W. Effect of nitrogen content and substrate bias on mechanical and corrosion properties of high-entropy films (AlCrSiTiZr)100−xNx. Surf. Coat. Technol. 2012, 206, 4106–4112. [Google Scholar] [CrossRef]
- Stasiak, T.; Soucek, P.; Bursikova, V.; Vasina, P. Magnetron Sputtering Deposition of High Eentropy Nitrides from Chromium-Hafnium-Molybdenum-Tantalum-Wolfram System. In Proceedings of the NANOCON 2021, Brno, Czech Republic, 20–22 October 2021; Tanger Ltd.: Ostrava, Czech Republic, 2021; pp. 98–103. [Google Scholar]
- Shu, R.; Paschalidou, E.-M.; Rao, S.G.; Bakhit, B.; Boyd, R.; Moro, M.V.; Primetzhofer, D.; Greczynski, G.; Nyholm, L.; Le Febvrier, A.; et al. Effect of nitrogen content on microstructure and corrosion resistance of sputter-deposited multicomponent (TiNbZrTa)Nx films. Surf. Coat. Technol. 2020, 404, 126485. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lou, B.-S.; Hung, T.-Y.; Lee, C.-H.; Chen, H.-Y. Effects of nitrogen contents on the phase evolution, microstructure and mechanical properties of high entropy alloy coatings. In Proceedings of the 18th International Conference on Plasma Surface Engineering, Erfurt, Germany, 12–15 September 2022. [Google Scholar]
- Xia, A.; Dedoncker, R.; Glushko, O.; Cordill, M.J.; Depla, D.; Franz, R. Influence of the nitrogen content on the structure and properties of MoNbTaVW high entropy alloy thin films. J. Alloys Compd. 2021, 850, 156740. [Google Scholar] [CrossRef]
- Li, H.; Jiang, N.; Li, J.; Huang, J.; Kong, J.; Xiong, D. Hard and tough (NbTaMoW)Nx high entropy nitride films with sub-stoichiometric nitrogen. J. Alloys Compd. 2021, 889, 161713. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, S.-J.; Yeh, J.-W.; Chang, S.-Y. Preparation and characterization of AlCrTaTiZr multi-element nitride coatings. Surf. Coat. Technol. 2006, 201, 3275–3280. [Google Scholar] [CrossRef]
- Cui, P.; Li, W.; Liu, P.; Zhang, K.; Ma, F.; Chen, X.; Feng, R.; Liaw, P.K. Effects of nitrogen content on microstructures and mechanical properties of (AlCrTiZrHf)N high-entropy alloy nitride films. J. Alloys Compd. 2020, 834, 155063. [Google Scholar] [CrossRef]
- Kirnbauer, A.; Kretschmer, A.; Koller, C.M.; Wojcik, T.; Paneta, V.; Hans, M.; Schneider, J.M.; Polcik, P.; Mayrhofer, P.H. Mechanical properties and thermal stability of reactively sputtered multi-principal-metal Hf-Ta-Ti-V-Zr nitrides. Surf. Coat. Technol. 2020, 389, 125674. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hsu, S.-Y.; Lai, Y.-T.; Kuo, P.-H.; Tsai, S.-Y.; Duh, J.-G. Effect of the N2/(Ar+N2) ratio on mechanical properties of high entropy nitride (Cr0.35Al0.25Nb0.12Si0.08V0.20)Nx films. Mater. Chem. Phys. 2021, 274, 125195. [Google Scholar] [CrossRef]
- Bachani, S.K.; Wang, C.-J.; Lou, B.-S.; Chang, L.-C.; Lee, J.-W. Fabrication of TiZrNbTaFeN high-entropy alloys coatings by HiPIMS: Effect of nitrogen flow rate on the microstructural development, mechanical and tribological performance, electrical properties and corrosion characteristics. J. Alloys Compd. 2021, 873, 159605. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Lai, C.-H.; Yeh, J.-W.; Gan, J.-Y. Effects of nitrogen flow ratio on the structure and properties of reactively sputtered (AlMoNbSiTaTiVZr)N x coatings. J. Phys. D Appl. Phys. 2008, 41, 235402. [Google Scholar] [CrossRef]
- Huang, P.-K.; Yeh, J.-W. Effects of nitrogen content on structure and mechanical properties of multi-element (AlCrNbSiTiV)N coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, X.; Wang, C.; Sui, X.; Wang, Y.; Zhou, H.; Hao, J. Tailoring the microstructure, mechanical and tribocorrosion performance of (CrNbTiAlV)N high-entropy nitride films by controlling nitrogen flow. J. Mater. Sci. Technol. 2022, 107, 172–182. [Google Scholar] [CrossRef]
- Bunshah, R.F. (Ed.) Handbook of Hard Coatings: Deposition Technologies, Properties and Applications; Noyes Publications: Norwich, NY, USA; William Andrew Publishing: Park Ridge, NJ, USA, 2001; ISBN 0815514387. [Google Scholar]
- Mattox, D.M. Particle bombardment effects on thin-film deposition: A review. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 1105–1114. [Google Scholar] [CrossRef]
- Rother, B.; Vetter, J. Plasmabeschichtungsverfahren und Hartstoffschichten, 1st ed.; Dt. Verl. für Grundstoffindustrie: Leipzig, Germany, 1992; ISBN 3342006498. [Google Scholar]
- Zimmer, O. Vacuum arc deposition by using a Venetian blind particle filter. Surf. Coat. Technol. 2005, 200, 440–443. [Google Scholar] [CrossRef]
- Siemroth, P.; Schülke, T. Copper metallization in microelectronics using filtered vacuum arc deposition—principles and technological development. Surf. Coat. Technol. 2000, 133-134, 106–113. [Google Scholar] [CrossRef]
- Krülle, T.; Leonhardt, M.; Kuczyk, M.; Srocke, S.; Zimmer, O.; Leyens, C. Untersuchung des Verdampfungsprozesses und der Schichtabscheidung von nitridischen Hochentropielegierungen mittels PVD-Verdampfung. In Jahrbuch Oberflächentechnik 2023; Sörgel, T., Ed.; Eugen, G. Leuze Verlag: Bad Saulgau, Germany, 2023; ISBN 978-3-87480-385-4. [Google Scholar]
- DIN EN ISO 26423:2016-11; Hochleistungskeramik—Bestimmung der Schichtdicke mit dem Kalottenschleifverfahren. Beuth Verlag GmbH: Berlin, Germany, 2016.
- DIN EN ISO 14577-1:2015-11; Metallische Werkstoffe—Instrumentierte Eindringprüfung zur Bestimmung der Härte und anderer Werkstoffparameter—Teil 1: Prüfverfahren. Beuth Verlag GmbH: Berlin, Germany, 2015.
- Präßler, F.; Grimm, W.; Chudoba, T. Properties of ta-C Films for Tools and Machinery Parts. Plasma Process. Polym. 2009, 6, S468–S472. [Google Scholar] [CrossRef]
- Lorenz, L.; Chudoba, T.; Makowski, S.; Zawischa, M.; Schaller, F.; Weihnacht, V. Indentation modulus extrapolation and thickness estimation of ta-C coatings from nanoindentation. J. Mater. Sci. 2021, 19, 18740–18748. [Google Scholar] [CrossRef]
- ZwickRoell GmbH Co. KG. Available online: https://www.zwickroell.com/industries/academia/nanoindentation/ (accessed on 16 March 2024).
- Wang, Z.; Wang, C.; Zhao, Y.-L.; Huang, T.-H.; Li, C.-L.; Kai, J.-J.; Liu, C.-T.; Hsueh, C.-H. Growth, microstructure and mechanical properties of CoCrFeMnNi high entropy alloy films. Vacuum 2020, 179, 109553. [Google Scholar] [CrossRef]
- Wriedt, H.A.; Murray, J.L. The N-Ti (Nitrogen-Titanium) system. Bull. Alloy Phase Diag. 1987, 8, 378–388. [Google Scholar] [CrossRef]
- Zawischa, M.; Weihnacht, V.; Kaspar, J.; Zimmermann, M. Effect of doping elements to hydrogen-free amorphous carbon coatings on structure and mechanical properties with special focus on crack resistance. Mater. Sci. Eng. A 2022, 857, 144086. [Google Scholar] [CrossRef]



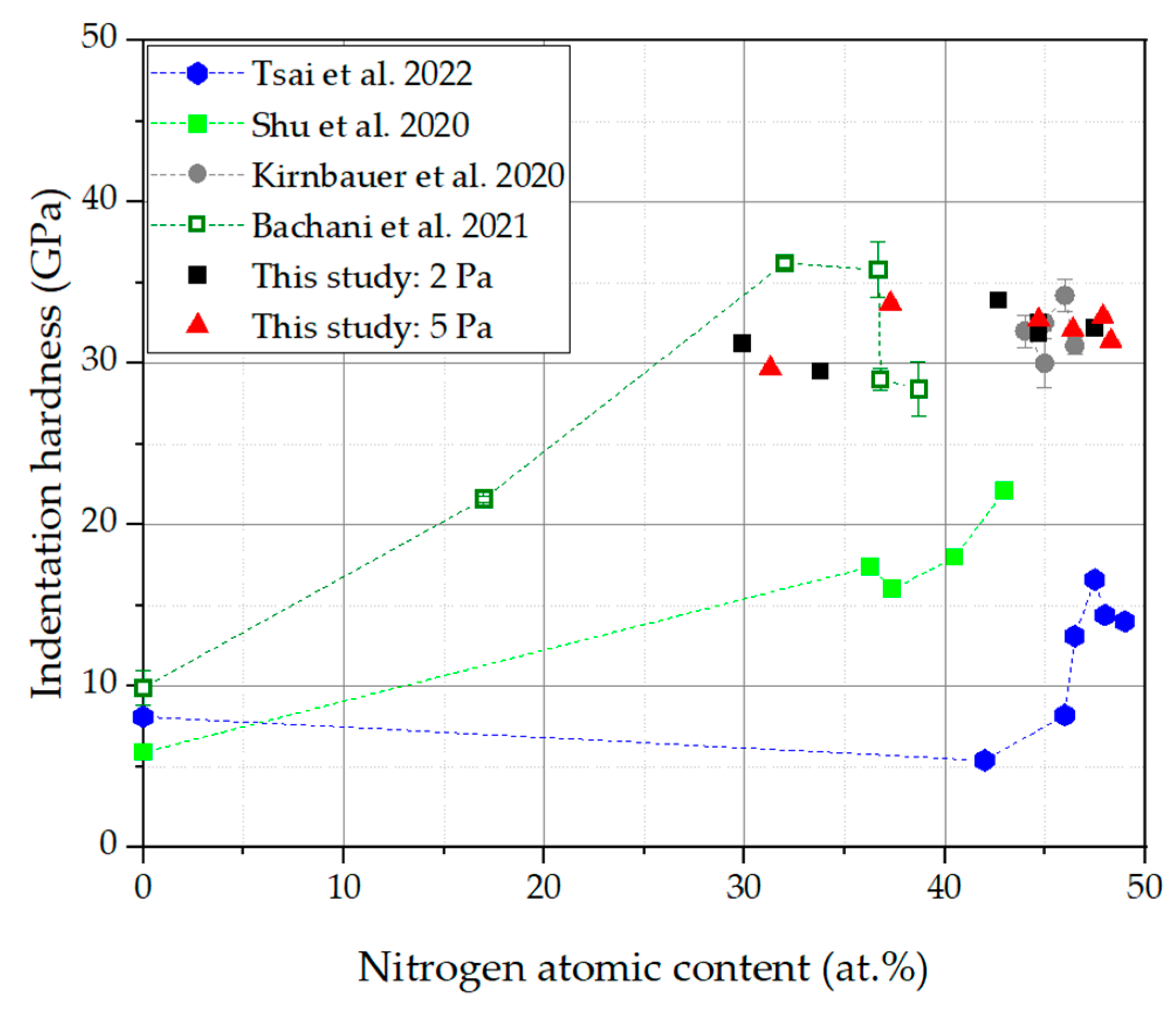
| HEAN System | Deposition Process | Utilized Targets | Studied Process Influences | Described Maximum Indentation Hardness at Pressure and RN2 | Reference |
|---|---|---|---|---|---|
| (AlCoCrNi)N | DCMS | PM target | Influence of process pressure on structure and mechanical properties | Hmax = 8.2 GPa at p = 1.33 Pa and RN2 = 0.25 | [8] |
| (CrHfNbTaTiVZr)N | DCMS | Equimolar VA target | Effect of N2 partial pressure on structural, mechanical and electrical properties | Hmax = 16.6 GPa at p = nc and RN2 = 0.6 | [9] |
| (TiVCrZrY)N | DCMS | Equimolar PM target | Effect of N2 flow ratio on structure and mechanical properties | Hmax = 17.5 GPa at p = nc and RN2 = 1.0 | [10] |
| (AlCrSiTiZr)N | MS | Equimolar VA target | Effect of N2 content and substrate bias on mech. and corrosion properties | Hmax = 17 GPa at p = nc and RN2 = 0.05 | [11] |
| (CrHfMoTaW)N | MS | Cr and segmented Mo/Hf and Ta/W targets | Influence of N2 flow and deposition temperature on microstructure and mechanical properties | Hmax(RT) = 21.9 GPa at p = nc and RN2 = nc; Hmax(700 °C) = 26.6 GPa at p = nc and RN2 = nc | [12] |
| (TiNbZrTa)N | MS | Segmented Nb/Zr and Ti/Ta targets | Effect of N2 content on microstructure | Hmax = 22.1 GPa at p = nc and RN2 = 0.25 and 43 at% nitrogen atomic content (coating) | [13] |
| (VNbMoTaWTiAl)N | HiPIMS | PM target | Effect of N2 flow rate on microstructure, mechanical and tribological performance | Hmax = 22.9 GPa at p = nc and RN2 = nc and 13.1 at% nitrogen atomic content (coating) | [14] |
| (VNbMoTaW)N | HiPIMS | PM target | Hmax = 26.8 GPa at p = nc and RN2 = nc and 22 at% nitrogen atomic content (coating) | ||
| (MoNbTaVW)N | CAE and DCMS | Equimolar PM target (CAE) and equimolar CIP target (DCMS) | Influence of N2 content on structure and mechanical properties | Hmax(CAE) = 30 GPa at p = nc and RN2 = 0.25 and 20 at% nitrogen atomic content (coating); Hmax(DCMS) = 32 GPa at p = nc and RN2 = 0.05; 25 at% nitrogen atomic content (coating) | [15] |
| (NbTaMoW)N | DCMS | Equimolar PM target | Hmax = 30.8 GPa at p = nc and RN2 = 0.1 | [16] | |
| (AlCrTaTiZr)N | RF MS | Equimolar VA target | Influence of N2 flow ratio on chemical composition, microstructure and mechanical properties | Hmax = 32.0 GPa at p = nc and RN2 = 0.14 | [17] |
| (AlCrTiZrHf)N | MS | Equimolar target | Effect of N2 content on microstructure and mechanical properties | Hmax = 33.1 GPa at p = nc and RN2 = 0.56 | [18] |
| (HfTaTiVZr)N | MS | Equimolar PM target | Effect of N2 flow rate on microstructure and mechanical properties | Hmax = 34.0 GPa at p = nc and RN2 = 0.5 | [19] |
| (CrAlNbSiV)N | RF MS | Cr0.35Al0.25Nb0.12Si0.08V0.20 target | Effect of N2 flow ratio on crystal structure, chemical composition, deposition rate, residual stress and mechanical properties | Hmax = 35.0 GPa at p = nc and RN2 = 0.33 | [20] |
| (TiZrNbTaFe)N | HiPIMS | Equimolar target | Effect of N2 flow rate on microstructure, mechanical and tribological performance | Hmax = 36.2 GPa at p = nc and RN2 = 0.1 and 32.0 at% at% nitrogen atomic content (coating) | [14,21] |
| (AlMoNbSiTaTiVZr)N | MS | Equimolar target | Effect of N2 flow ratio on structure and properties | Hmax = 37.0 GPa at p = nc and RN2 = 0.5 | [22] |
| (AlCrNbSiTiV)N | RF UMS | Equimolar VA target | Effects of N2 content on structure and mechanical properties | Hmax = 41 GPa at p = nc and RN2 = 0.2–0.3 | [23] |
| (CrNbTiAlV)N | MS | Cr and CrNbTiAlV targets | Effect of N2 flow on microstructure and tribocorrosion | Hmax = 49.95 GPa at p = nc and RN2 = nc and 35.7 at% nitrogen atomic content (coating) | [24] |
| Target Components | Determined Target Composition (at%) |
|---|---|
| HfNbTaTiVZr | 17.5:17.5:16.8:16.3:15.8:16.0 |
| Process Parameter | Value Setting |
|---|---|
| Working pressure p (Pa) | 2; 5 |
| N2/(N2 +Ar) flow ratio | 0.1; 0.25; 0.33; 0.5; 0.66; 1.0 |
| Bias voltage Ubias (V) | −200 |
| Evaporation current Ievap (A) | 100 |
| Electrical charge Q (Ah) | 20 |
| Deposition time (h) Distance target-sample s (mm) | 0.2 170 |
| Sample movement | Fixed in front of the target |
| Substrate temperature Tsubs (°C) | ≤550 °C |
| Characterization Method | Device | Aim of Analysis |
|---|---|---|
| Calo test | KSG110 (Inovap HEF Group, Andrézieux-Bouthéon, France) | Coating thickness and deposition rate |
| Scanning Electron Microscopy (SEM) | JSM-7800-F (JEOL, Tokyo, Japan) | Structural analysis on as-deposited surfaces, fracture surfaces and cross sections |
| Energy Dispersive X Ray Spectrocopy (EDS) | X-max 80 (Oxford Instruments, Abingdon, UK) | Coating composition |
| X Ray Diffraction (XRD) | D5005 (Siemens, Munich, Germany) | Structural analysis |
| Instrumented Nanoindentation | ZHN-1 (ZwickRoell, Ulm, Germany) | Indentation hardness and Young’s modulus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krülle, T.; Kuczyk, M.; Leonhardt, M.; Zimmer, O.; Leyens, C. The Influence of Nitrogen Partial Pressure on the Microstructure and Mechanical Properties of HfNbTaTiVZr High-Entropy Nitride Coating Deposited via Direct Current Cathodic Vacuum Arc Deposition. Coatings 2024, 14, 398. https://doi.org/10.3390/coatings14040398
Krülle T, Kuczyk M, Leonhardt M, Zimmer O, Leyens C. The Influence of Nitrogen Partial Pressure on the Microstructure and Mechanical Properties of HfNbTaTiVZr High-Entropy Nitride Coating Deposited via Direct Current Cathodic Vacuum Arc Deposition. Coatings. 2024; 14(4):398. https://doi.org/10.3390/coatings14040398
Chicago/Turabian StyleKrülle, Tim, Martin Kuczyk, Michael Leonhardt, Otmar Zimmer, and Christoph Leyens. 2024. "The Influence of Nitrogen Partial Pressure on the Microstructure and Mechanical Properties of HfNbTaTiVZr High-Entropy Nitride Coating Deposited via Direct Current Cathodic Vacuum Arc Deposition" Coatings 14, no. 4: 398. https://doi.org/10.3390/coatings14040398
APA StyleKrülle, T., Kuczyk, M., Leonhardt, M., Zimmer, O., & Leyens, C. (2024). The Influence of Nitrogen Partial Pressure on the Microstructure and Mechanical Properties of HfNbTaTiVZr High-Entropy Nitride Coating Deposited via Direct Current Cathodic Vacuum Arc Deposition. Coatings, 14(4), 398. https://doi.org/10.3390/coatings14040398






